PEDOT-Doped Mesoporous Nanocarbon Electrodes for High Capacitive Aqueous Symmetric Supercapacitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.1.1. Preparation of Poly(3,4-Ethylenedioxythiophene)
2.1.2. Preparation of Functionalized Carbon Nanoparticles (f-CNPs) and PEDOT-Doped Functionalized Carbon Nanoparticles (f-CNP-PEDOT) Nanocomposites
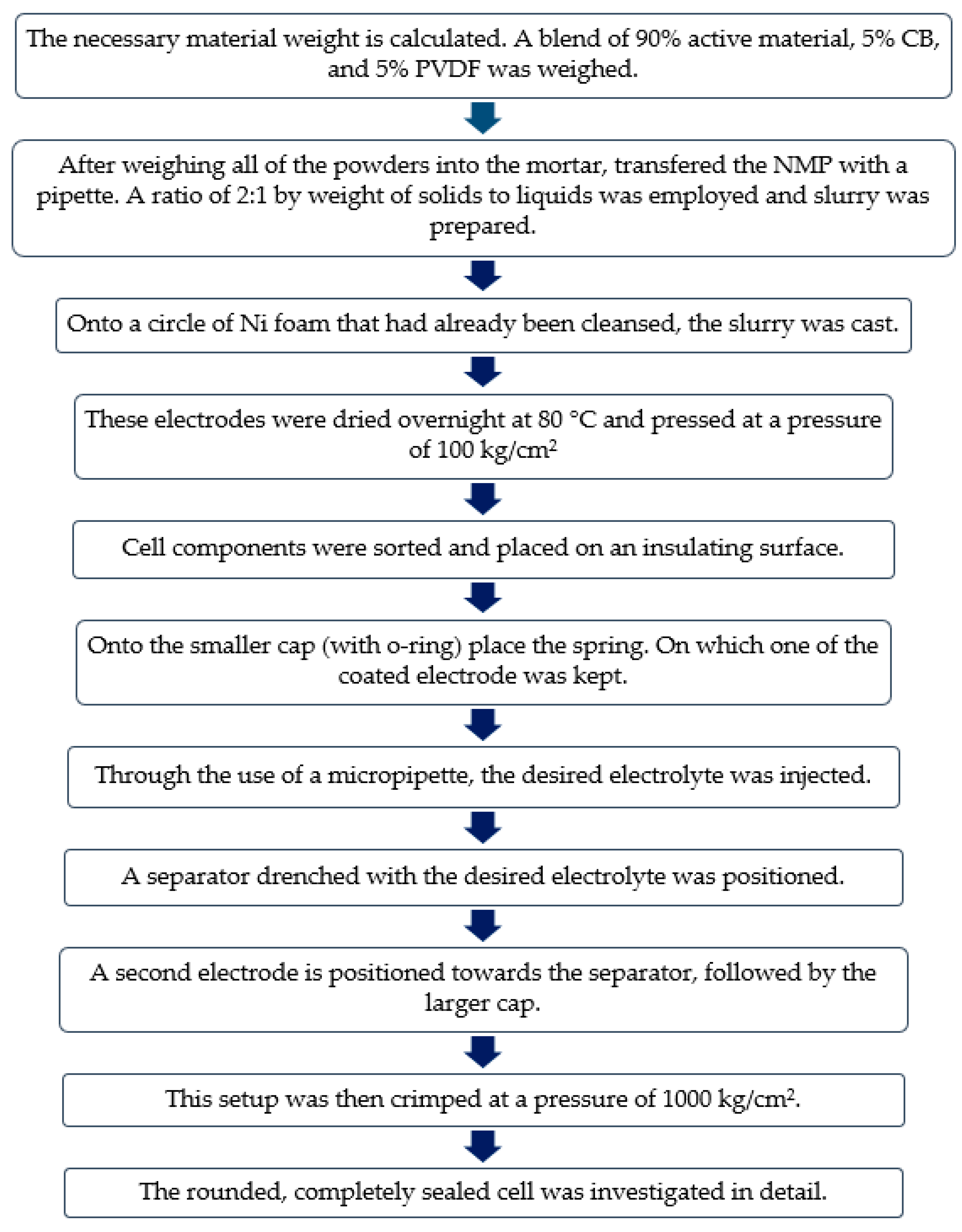
2.2. Electrodes Fabrication
2.3. Characterization Thechniques
3. Results and Discussion
3.1. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
3.2. X-ray Diffraction (XRD) Analysis
3.3. Field-Emission Scanning Electron Microscopy (FESEM) Analysis
3.4. Porosity
3.5. Electrochemical Analysis
3.5.1. Three-Electrode Setup
3.5.2. Aqueous Symmetric Supercapacitor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samui, A.B.; Sivaraman, P. 11—Solid polymer electrolytes for supercapacitors. In Polymer Electrolytes; Sequeira, C., Santos, D., Eds.; Woodhead Publishing: Sawston, UK, 2010; pp. 431–470. [Google Scholar] [CrossRef]
- Wang, W.; Lei, W.; Yao, T.; Xia, X.; Huang, W.; Hao, Q.; Wang, X. One-pot synthesis of graphene/SnO2/PEDOT ternary electrode material for supercapacitors. Electrochim. Acta 2013, 108, 118–126. [Google Scholar] [CrossRef]
- Liang, Y.; Wei, Z.; Wang, H.-E.; Flores, M.; Wang, R.; Zhang, X. Flexible and freestanding PANI: PSS/CNF nanopaper electrodes with enhanced electrochemical performance for supercapacitors. J. Power Sources 2022, 548, 232071. [Google Scholar] [CrossRef]
- Chen, T.; Dai, L. Carbon nanomaterials for high-performance supercapacitors. Mater. Today 2013, 16, 272–280. [Google Scholar] [CrossRef]
- Shrivastav, V.; Sundriyal, S.; Kaur, A.; Tiwari, U.K.; Mishra, S.; Deep, A. Conductive and porous ZIF-67/PEDOT hybrid composite as superior electrode for all-solid-state symmetrical supercapacitors. J. Alloys Compd. 2020, 843, 155992. [Google Scholar] [CrossRef]
- Shaikh, N.S.; Ubale, S.B.; Mane, V.J.; Shaikh, J.S.; Lokhande, V.C.; Praserthdam, S.; Lokhande, C.D.; Kanjanaboos, P. Novel electrodes for supercapacitor: Conducting polymers, metal oxides, chalcogenides, carbides, nitrides, MXenes, and their composites with graphene. J. Alloys Compd. 2022, 893, 161998. [Google Scholar] [CrossRef]
- Meng, Q.; Cai, K.; Chen, Y.; Chen, L. Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 2017, 36, 268–285. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, S.; Jewell, D.; Chen, G.Z. Carbon nanotube and conducting polymer composites for supercapacitors. Prog. Nat. Sci. 2008, 18, 777–788. [Google Scholar] [CrossRef]
- Zhou, Y.; Lachman, N.; Ghaffari, M.; Xu, H.; Bhattacharya, D.; Fattahi, P.; Abidian, M.R.; Wu, S.; Gleason, K.K.; Wardle, B.L.; et al. A high performance hybrid asymmetric supercapacitor via nano-scale morphology control of graphene, conducting polymer, and carbon nanotube electrodes. J. Mater. Chem. A 2014, 2, 9964–9969. [Google Scholar] [CrossRef]
- Ates, M.; Fernandez, C. Ruthenium oxide–carbon-based nanofiller-reinforced conducting polymer nanocomposites and their supercapacitor applications. Polym. Bull. 2019, 76, 2601–2619. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Park, S.-J.; Son, Y.-R.; Heo, Y.-J. Chapter 6—Prospective Synthesis Approaches to Emerging Materials for Supercapacitor. In Emerging Materials for Energy Conversion and Storage; Cheong, K.Y., Impellizzeri, G., Fraga, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 185–208. [Google Scholar] [CrossRef]
- Zhang, M.; Nautiyal, A.; Du, H.; Wei, Z.; Zhang, X.; Wang, R. Electropolymerization of polyaniline as high-performance binder free electrodes for flexible supercapacitor. Electrochim. Acta 2021, 376, 138037. [Google Scholar] [CrossRef]
- Zhou, H.; Zhi, X.; Zhai, H.-J. A strategy to boost electrochemical properties of the graphene oxide–poly(3,4-ethylenedioxythiophene) composites for supercapacitor electrodes. J. Mater. Sci. 2018, 53, 5216–5228. [Google Scholar] [CrossRef]
- Taj, M.; Manohara, S.R.; Hanagodimath, S.M.; Gerward, L. Novel conducting poly(3,4-ethylenedioxythiophene)—Graphene nanocomposites with gigantic dielectric properties and narrow optical energy band gap. Polym. Test. 2020, 90, 106650. [Google Scholar] [CrossRef]
- Asadian, E.; Ghalkhani, M.; Shahrokhian, S. Electrochemical sensing based on carbon nanoparticles: A review. Sens. Actuators B Chem. 2019, 293, 183–209. [Google Scholar] [CrossRef]
- Lawrence, K.; Baker, C.L.; James, T.D.; Bull, S.D.; Lawrence, R.; Mitchels, J.M.; Opallo, M.; Arotiba, O.A.; Ozoemena, K.I.; Marken, F. Functionalized Carbon Nanoparticles, Blacks and Soots as Electron-Transfer Building Blocks and Conduits. Chem. Asian J. 2014, 9, 1226–1241. [Google Scholar] [CrossRef] [PubMed]
- Bhat, V.S.; Supriya, S.; Hegde, G. Biomass Derived Carbon Materials for Electrochemical Sensors. J. Electrochem. Soc. 2019, 167, 037526. [Google Scholar] [CrossRef]
- Bhat, V.S.; Toghan, A.; Hegde, G.; Varma, R.S. Capacitive dominated charge storage in supermicropores of self-activated carbon electrodes for symmetric supercapacitors. J. Energy Storage 2022, 52, 104776. [Google Scholar] [CrossRef]
- Chaithra, K.P.; Bhat, V.S.; Akshaya, K.B.; Maiyalagan, T.; Hegde, G.; Varghese, A.; George, L. Unique Host Matrix to Disperse Pd Nanoparticles for Electrochemical Sensing of Morin: Sustainable Engineering Approach. ACS Biomater. Sci. Eng. 2020, 6, 5264–5273. [Google Scholar] [CrossRef]
- Mathew, A.T.; Bhat, V.S.; Supriya, S.; Maiyalagan, T.; Varghese, A.; Hegde, G. TEMPO mediated electrocatalytic oxidation of pyridyl carbinol using palladium nanoparticles dispersed on biomass derived porous nanoparticles. Electrochim. Acta 2020, 354, 136624. [Google Scholar] [CrossRef]
- Kanagavalli, P.; Pandey, G.R.; Bhat, V.S.; Veerapandian, M.; Hegde, G. Nitrogenated-carbon nanoelectrocatalyst advertently processed from bio-waste of Allium sativum for oxygen reduction reaction. J. Nanostructure Chem. 2021, 11, 343–352. [Google Scholar] [CrossRef]
- Ali, G.A.; Divyashree, A.; Supriya, S.; Chong, K.F.; Ethiraj, A.S.; Reddy, M.V.; Algarni, H.; Hegde, G. Carbon nanospheres derived from Lablab purpureus for high performance supercapacitor electrodes: A green approach. Dalton Trans. 2017, 46, 14034–14044. [Google Scholar] [CrossRef] [PubMed]
- Bhat, V.S.; Kanagavalli, P.; Sriram, G.; John, N.S.; Veerapandian, M.; Kurkuri, M.; Hegde, G. Low cost, catalyst free, high performance supercapacitors based on porous nano carbon derived from agriculture waste. J. Energy Storage 2020, 32, 101829. [Google Scholar] [CrossRef]
- Bhat, V.S.; Krishnan, S.G.; Jayeoye, T.J.; Rujiralai, T.; Sirimahachai, U.; Viswanatha, R.; Khalid, M.; Hegde, G. Self-activated ‘green’ carbon nanoparticles for symmetric solid-state supercapacitors. J. Mater. Sci. 2021, 56, 13271–13290. [Google Scholar] [CrossRef]
- Bhat, V.S.; Jayeoye, T.J.; Rujiralai, T.; Sirimahachai, U.; Chong, K.F.; Hegde, G. Acacia auriculiformis–Derived Bimodal Porous Nanocarbons via Self-Activation for High-Performance Supercapacitors. Front. Energy Res. 2021, 9, 744133. [Google Scholar] [CrossRef]
- Zhang, M.; Du, H.; Wei, Z.; Zhang, X.; Wang, R. Ultrafast microwave synthesis of nickel-cobalt sulfide/graphene hybrid electrodes for high-performance asymmetrical supercapacitors. ACS Appl. Energy Mater. 2021, 4, 8262–8274. [Google Scholar] [CrossRef]
- Bhat, V.S.; Hegde, G.; Nasrollahzadeh, M. A sustainable technique to solve growing energy demand: Porous carbon nanoparticles as electrode materials for high-performance supercapacitors. J. Appl. Electrochem. 2020, 50, 1243–1255. [Google Scholar] [CrossRef]
- Supriya, S.; Sriram, G.; Ngaini, Z.; Kavitha, C.; Kurkuri, M.; De Padova, I.P.; Hegde, G. The Role of Temperature on Physical–Chemical Properties of Green Synthesized Porous Carbon Nanoparticles. Waste Biomass Valorization 2020, 11, 3821–3831. [Google Scholar] [CrossRef]
- Krishnamurthy, G.; Namitha, R. Synthesis of structurally novel carbon micro/nanospheres by low temperature-hydrothermal process. J. Chil. Chem. Soc. 2013, 58, 1930–1933. [Google Scholar] [CrossRef]
- Supriya, S.; Bhat, V.S.; Jayeoye, T.J.; Rujiralai, T.; Chong, K.F.; Hegde, G. An investigation on temperature-dependant surface properties of porous carbon nanoparticles derived from biomass. J. Nanostructure Chem. 2021, 12, 495–511. [Google Scholar] [CrossRef]
- Taj, M.; Manohara, S.R. Significantly enhanced electrical and dielectric properties, and thermal stability of poly(3,4-ethylenedioxythiophene) coated on functionalized multiwalled carbon nanotubes. Synth. Met. 2020, 269, 116572. [Google Scholar] [CrossRef]
- Zhang, Y.; Xin, M.; Lin, W.; Yu, Z.; Peng, J.; Xu, K.; Chen, M. Synthesis and characterization of PEDOT particles in aqueous dispersion of π-conjugated polyelectrolyte. Synth. Met. 2014, 193, 8–16. [Google Scholar] [CrossRef]
- Ali, G.A.M.; Supriya, S.; Chong, K.F.; Shaaban, E.R.; Algarni, H.; Maiyalagan, T.; Hegde, G. Superior supercapacitance behavior of oxygen self-doped carbon nanospheres: A conversion of Allium cepa peel to energy storage system. Biomass Convers. Biorefinery 2021, 11, 1311–1323. [Google Scholar] [CrossRef]
- Ma, H.; Chen, Z.; Gao, X.; Liu, W.; Zhu, H. 3D hierarchically gold-nanoparticle-decorated porous carbon for high-performance supercapacitors. Sci. Rep. 2019, 9, 17065. [Google Scholar] [CrossRef] [PubMed]
- Largeot, C.; Portet, C.; Chmiola, J.; Taberna, P.-L.; Gogotsi, Y.; Simon, P. Relation between the Ion Size and Pore Size for an Electric Double-Layer Capacitor. J. Am. Chem. Soc. 2008, 130, 2730–2731. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, M.; Krishna Bhat, D. Activated carbon-polyethylenedioxythiophene composite electrodes for symmetrical supercapacitors. J. Appl. Polym. Sci. 2008, 107, 2165–2170. [Google Scholar] [CrossRef]
- Fleischmann, S.; Mitchell, J.B.; Wang, R.; Zhan, C.; Jiang, D.-e.; Presser, V.; Augustyn, V. Pseudocapacitance: From Fundamental Understanding to High Power Energy Storage Materials. Chem. Rev. 2020, 120, 6738–6782. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.; Xu, C.; Jiang, H.; Li, C.; Zhang, L.; Lin, J.; Shen, Z.X. Advanced Energy Storage Devices: Basic Principles, Analytical Methods, and Rational Materials Design. Adv. Sci. 2018, 5, 1700322. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wei, C.; Obrovac, M.N. Understanding interfacial impedance growth in porous electrodes containing blended active materials. J. Power Sources 2019, 438, 226955. [Google Scholar] [CrossRef]
- Sun, Y.; Xue, J.; Dong, S.; Zhang, Y.; An, Y.; Ding, B.; Zhang, T.; Dou, H.; Zhang, X. Biomass-derived porous carbon electrodes for high-performance supercapacitors. J. Mater. Sci. 2020, 55, 5166–5176. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, D.; She, W.; Wang, J.; Gao, S.; Wang, Y.; Wang, K. Remarkably improving the specific energy of supercapacitor based on a biomass-derived interconnected hierarchical porous carbon by using a newly-developed mixed alkaline aqueous electrolyte with widened operation voltage. J. Power Sources 2021, 492, 229666. [Google Scholar] [CrossRef]
- Yu, F.; Ye, Z.; Chen, W.; Wang, Q.; Wang, H.; Zhang, H.; Peng, C. Plane tree bark-derived mesopore-dominant hierarchical carbon for high-voltage supercapacitors. Appl. Surf. Sci. 2020, 507, 145190. [Google Scholar] [CrossRef]
- Karamanova, B.; Shipochka, M.; Georgiev, M.; Stankulov, T.; Stoyanova, A.; Stoyanova, R. Biomass-Derived Carbonaceous Materials to Achieve High-Energy-Density Supercapacitors. Front. Mater. 2021, 8, 654841. [Google Scholar] [CrossRef]
- Zhou, J.; Yuan, S.; Lu, C.; Yang, M.; Song, Y. Hierarchical porous carbon microtubes derived from corn silks for supercapacitors electrode materials. J. Electroanal. Chem. 2020, 878, 114704. [Google Scholar] [CrossRef]







| Samples | Elemental Composition (%) | ||
|---|---|---|---|
| C | O | S | |
| Pristine f-CNP | 89.78 | 10.22 | - |
| f-CNP-PEDOT1 | 84.84 | 15.16 | - |
| f-CNP-PEDOT2.5 | 83.89 | 15.95 | 0.16 |
| f-CNP-PEDOT5 | 81.71 | 17.66 | 0.63 |
| f-CNP-PEDOT10 | 88.33 | 11.44 | 0.23 |
| f-CNP-PEDOT20 | 81.96 | 17.24 | 0.80 |
| Circuit Parameters | Values | |
|---|---|---|
| Before 10,000 Cycles | After 10,000 Cycles | |
| Inductance | 7.51 × 10−9 H | 2.816 × 10−7 H |
| Solution resistance (Rs) | 1.045 Ω | 1.081 Ω |
| Charge transfer resistance (RCT) | 5.435 Ω | 9.848 Ω |
| Constant phase element (Diffusion) | 0.0608 Ssn | 0.0936 Ssn |
| n | 0.873 | 0.923 |
| Biomass Precursor | Potential Window (V) | Electrolyte | Energy Density (Wh/kg) | Efficiency Retainment (%) | Capacitance Retention (%) | Reference |
|---|---|---|---|---|---|---|
| Quinoa | 1.0 | 6.0 M KOH | 9.5 | 100 @ 10,000 cycles | ~93 | [41] |
| IHPC | 1.3 | Mixed alkaline electrolyte | 19.7 | 89 | - | [42] |
| Platanus bark | 3.0 | TEABF4/ACN | 34.6 | - | 70.8 @ 5000 cycles | [43] |
| Coconut | 1.8 | LiBF4 in EC/DMC | 91.1 | 89 @ 1000 cycles | 99 @ 1000 cycles | [44] |
| Corn silks | 2.7 | 1.0 M MeEt3NBF4/PC | 16.4 | - | 81.6 @ 10,000 cycles | [45] |
| Acacia auriculiformis pods | 2.3 | 1.0 M TEABF4/ ACN | 16.7 | 100 @ 10,000 cycles | ~93.2 @ 10,000 cycles | [26] |
| f-CNP-PEDOT | 1.4 | 1.0 M KOH | 26.3 | 99.3 @ 10,000 cycles | 86.6 @ 10,000 cycles | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taj, M.; Bhat, V.S.; Sriram, G.; Kurkuri, M.; Manohara, S.R.; Padova, P.D.; Hegde, G. PEDOT-Doped Mesoporous Nanocarbon Electrodes for High Capacitive Aqueous Symmetric Supercapacitors. Nanomaterials 2024, 14, 1222. https://doi.org/10.3390/nano14141222
Taj M, Bhat VS, Sriram G, Kurkuri M, Manohara SR, Padova PD, Hegde G. PEDOT-Doped Mesoporous Nanocarbon Electrodes for High Capacitive Aqueous Symmetric Supercapacitors. Nanomaterials. 2024; 14(14):1222. https://doi.org/10.3390/nano14141222
Chicago/Turabian StyleTaj, Mohsina, Vinay S. Bhat, Ganesan Sriram, Mahaveer Kurkuri, S. R. Manohara, Paola De Padova, and Gurumurthy Hegde. 2024. "PEDOT-Doped Mesoporous Nanocarbon Electrodes for High Capacitive Aqueous Symmetric Supercapacitors" Nanomaterials 14, no. 14: 1222. https://doi.org/10.3390/nano14141222
APA StyleTaj, M., Bhat, V. S., Sriram, G., Kurkuri, M., Manohara, S. R., Padova, P. D., & Hegde, G. (2024). PEDOT-Doped Mesoporous Nanocarbon Electrodes for High Capacitive Aqueous Symmetric Supercapacitors. Nanomaterials, 14(14), 1222. https://doi.org/10.3390/nano14141222







