Growth of α-Ga2O3 from Gallium Acetylacetonate under HCl Support by Mist Chemical Vapor Deposition
Abstract
1. Introduction
2. Experimental Methods
3. Results and Discussions
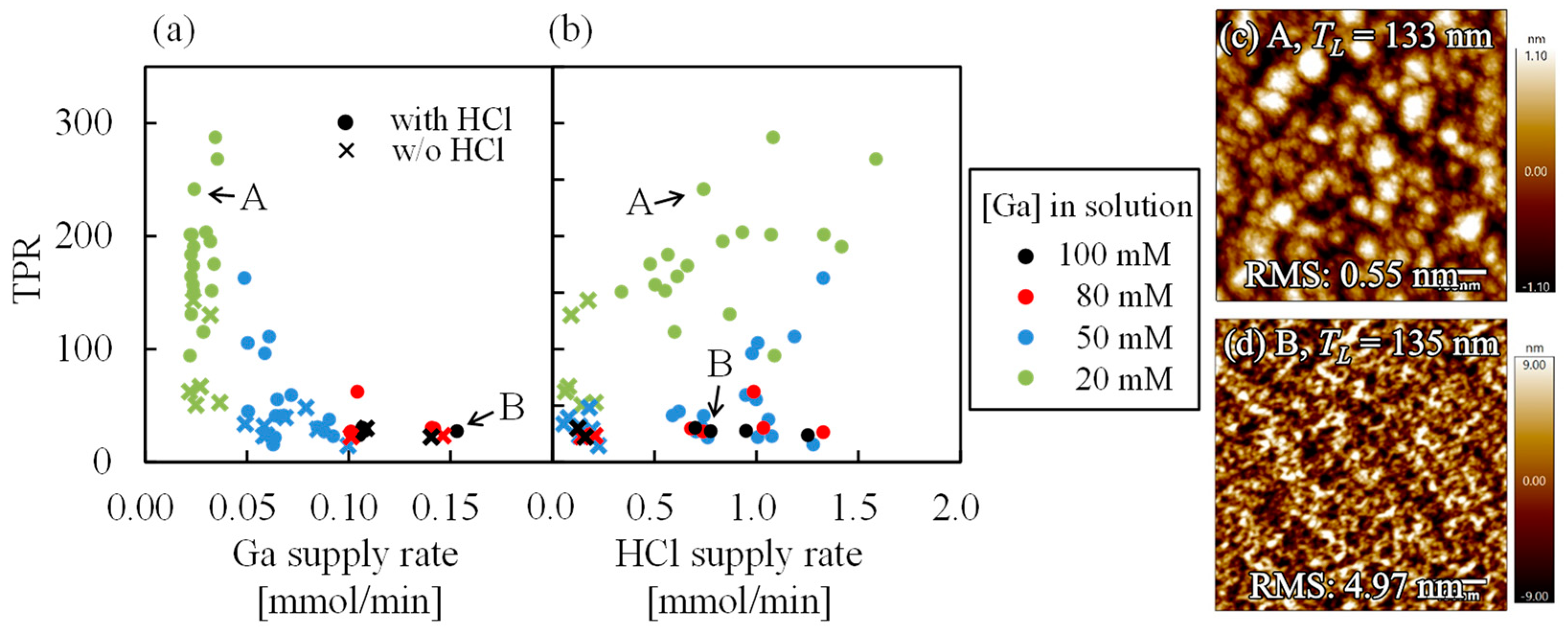
3.1. α-Ga2O3 Film Thicknesses Estimated from Ellipsometry and from Laue Fringes in XRD Spectra
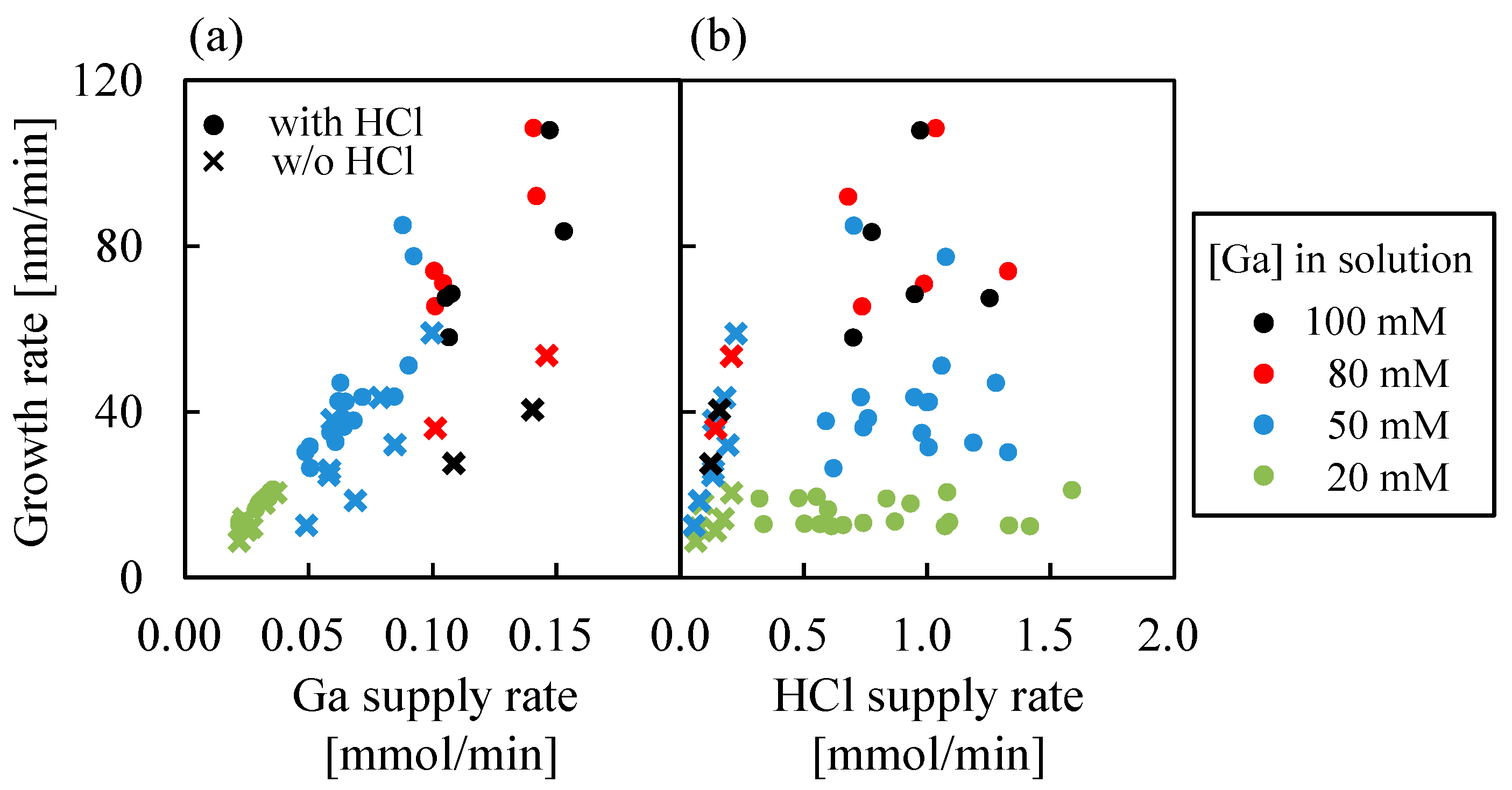
3.2. Effect of HCl on α-Ga2O3 Growth Using Ga(acac)3 as a Starting Material
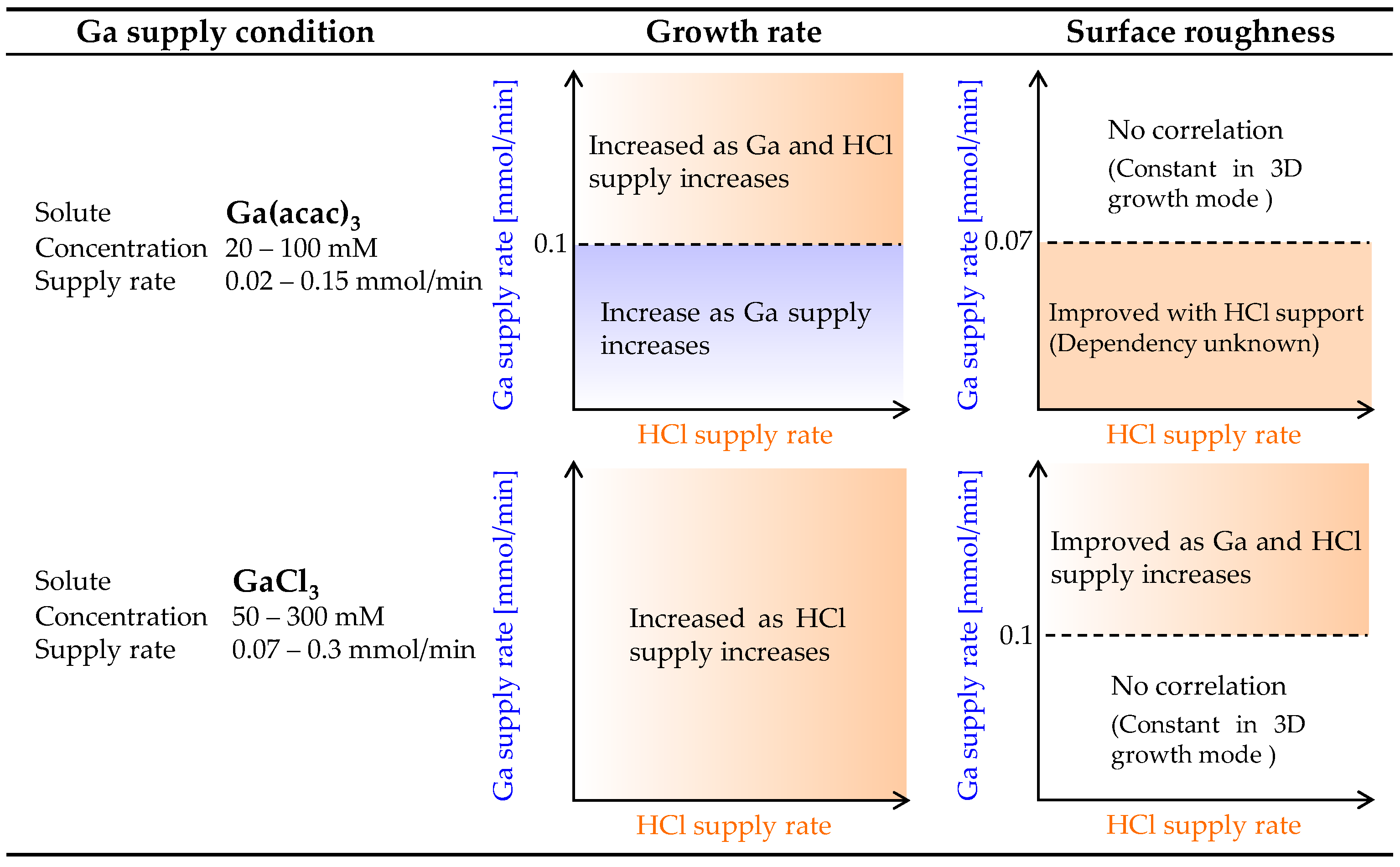
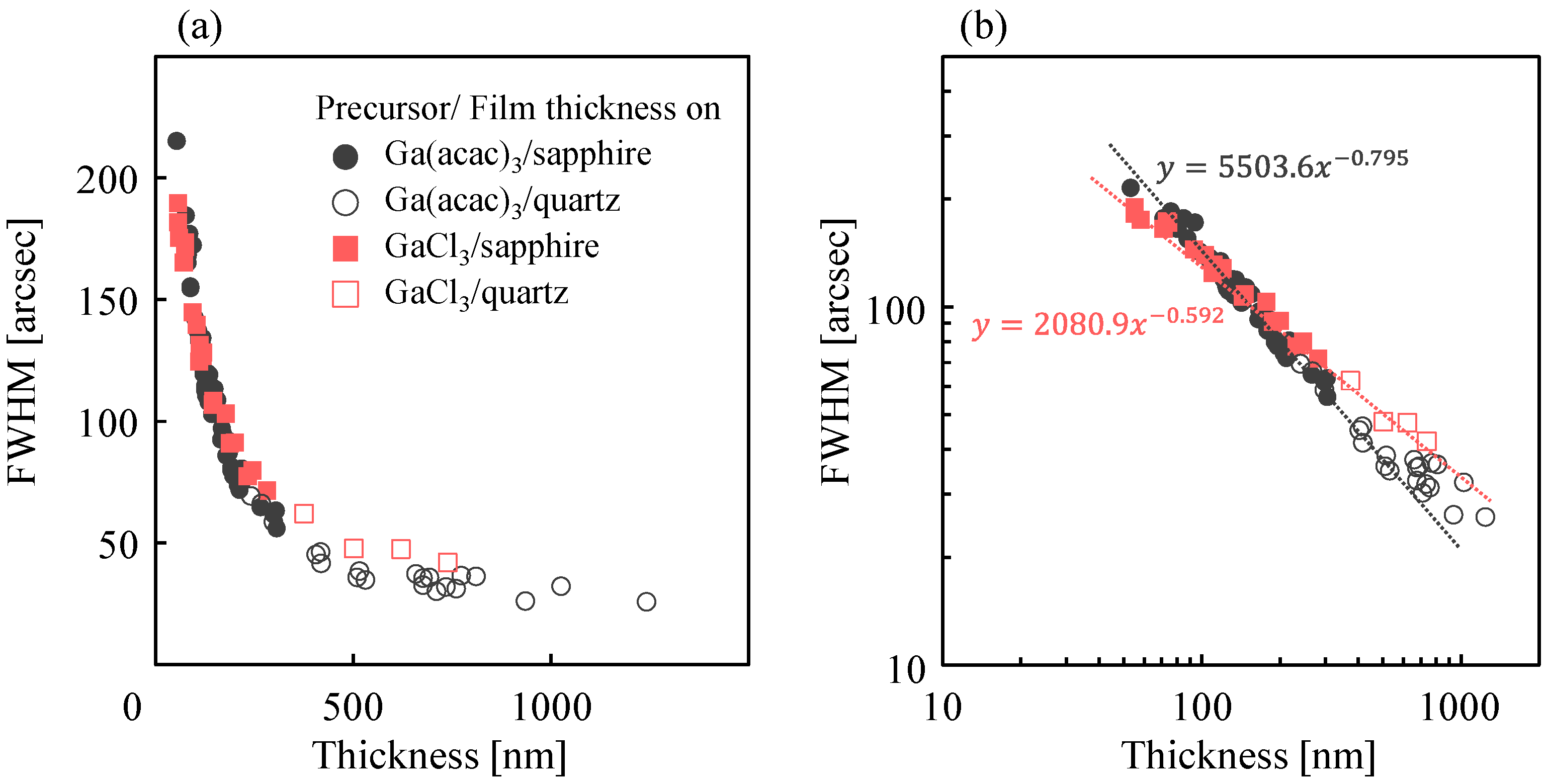
3.3. Growth Characteristics of α-Ga2O3 for HCl Support in Each Starting Material
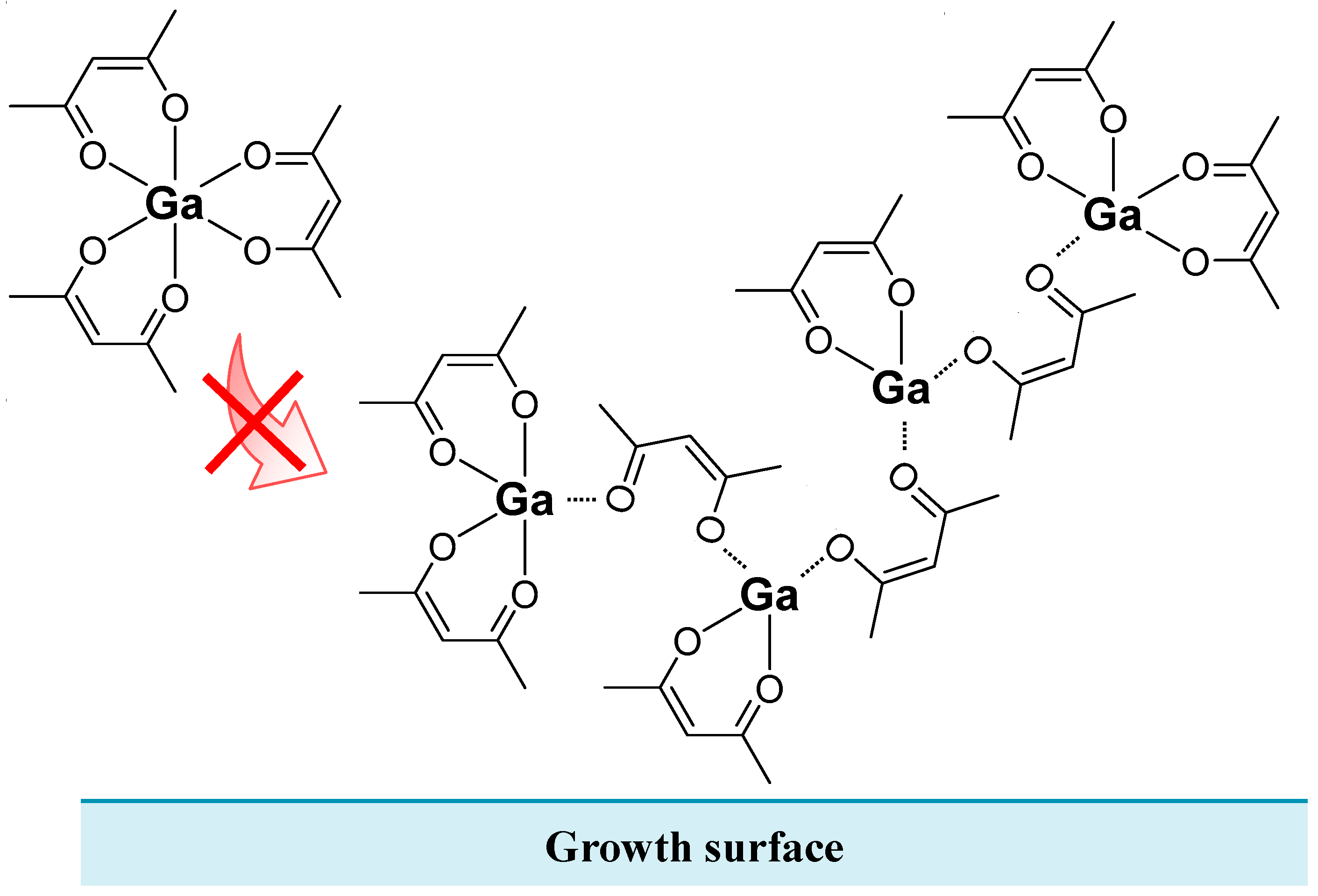
3.4. Facts Revealed in α-Ga2O3 Grown by Mist CVD
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Playford, H.Y.; Hannon, A.C.; Barney, E.R.; Walton, R.I. Structures of Uncharacterised Polymorphs of Gallium Oxide from Total Neutron Diffraction. Chem.—Eur. J. 2013, 19, 2803–2813. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Hill, V.G.; Osborn, E.F. Polymorphism of Ga2O3 and the System Ga2O3—H2O. J. Am. Chem. Soc. 1952, 74, 719–722. [Google Scholar] [CrossRef]
- Orita, M.; Ohta, H.; Hirano, M.; Hosono, H. Deep-Ultraviolet Transparent Conductive β-Ga2O3 Thin Films. Appl. Phys. Lett. 2000, 77, 4166–4168. [Google Scholar] [CrossRef]
- Onuma, T.; Saito, S.; Sasaki, K.; Masui, T.; Yamaguchi, T.; Honda, T.; Higashiwaki, M. Valence Band Ordering in β-Ga2O3 Studied by Polarized Transmittance and Reflectance Spectroscopy. Jpn. J. Appl. Phys. 2015, 54, 112601. [Google Scholar] [CrossRef]
- Chase, A.B. Growth of β-Ga2O3 by the Verneuil Technique. J. Am. Chem. Soc. 1964, 47, 470. [Google Scholar]
- Kuramata, A.; Koshi, K.; Watanabe, S.; Yamaoka, Y.; Masui, T.; Yamakoshi, S. High-Quality β-Ga2O3 Single Crystals Grown by Edge-Defined Film-Fed Growth. Jpn. J. Appl. Phys. 2016, 55, 1202A2. [Google Scholar] [CrossRef]
- Hoshikawa, K.; Ohba, E.; Kobayashi, T.; Yanagisawa, J.; Miyagawa, C.; Nakamura, Y. Growth of β-Ga2O3 Single Crystals Using Vertical Bridgman Method in Ambient Air. J. Cryst. Growth 2016, 447, 36–41. [Google Scholar] [CrossRef]
- Galazka, Z.; Uecker, R.; Klimm, D.; Irmscher, K.; Naumann, M.; Pietsch, M.; Kwasniewski, A.; Bertram, R.; Ganschow, S.; Bickermann, M. Scaling-Up of Bulk β-Ga2O3 Single Crystals by the Czochralski Method. ECS J. Solid State Sci. Technol. 2017, 6, Q3007–Q3011. [Google Scholar] [CrossRef]
- Green, A.J.; Speck, J.; Xing, G.; Moens, P.; Allerstam, F.; Gumaelius, K.; Neyer, T.; Arias-Purdue, A.; Mehrotra, V.; Kuramata, A.; et al. β-Gallium Oxide Power Electronics. APL Mater. 2022, 10, 029201. [Google Scholar] [CrossRef]
- Guo, D.; Guo, Q.; Chen, Z.; Wu, Z.; Li, P.; Tang, W. Review of Ga2O3-based optoelectronic devices. Mater. Today Phys. 2019, 11, 100157. [Google Scholar] [CrossRef]
- Shinohara, D.; Fujita, S. Heteroepitaxy of Corundum-Structured α-Ga2O3 Thin Films on α-Al2O3 Substrates by Ultrasonic Mist Chemical Vapor Deposition. Jpn. J. Appl. Phys. 2008, 47, 7311–7313. [Google Scholar] [CrossRef]
- Segura, A.; Artús, L.; Cuscó, R.; Goldhahn, R.; Feneberg, M. Band Gap of Corundumlike α-Ga2O3 Determined by Absorption and Ellipsometry. Phys. Rev. Mater. 2017, 1, 024604. [Google Scholar] [CrossRef]
- Dang, G.T.; Yasuoka, T.; Tagashira, Y.; Tadokoro, T.; Theiss, W.; Kawaharamura, T. Bandgap Engineering of α-(AlxGa1-x)2O3 by a Mist Chemical Vapor Deposition Two-Chamber System and Verification of Vegard’s Law. Appl. Phys. Lett. 2018, 113, 2–7. [Google Scholar] [CrossRef]
- Jinno, R.; Kaneko, K.; Fujita, S. Thermal Stability of α-(AlxGa1-x)2O3 Films Grown on c-Plane Sapphire Substrates with an Al Composition up to 90%. Jpn. J. Appl. Phys. 2021, 60, SBBD13. [Google Scholar] [CrossRef]
- Jinno, R.; Chang, C.S.; Onuma, T.; Cho, Y.; Ho, S.T.; Rowe, D.; Cao, M.C.; Lee, K.; Protasenko, V.; Schlom, D.G.; et al. Crystal Orientation Dictated Epitaxy of Ultrawide-Bandgap 5.4-to 8.6-eV α-(AlGa)2O3 on m-Plane Sapphire. Sci. Adv. 2021, 7, eabd5891. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Jinno, R.; Takemoto, S.; Kaneko, K.; Fujita, S. Evaluation of Band Alignment of α-Ga2O3/α-(AlxGa1-x)2O3 Heterostructures by X-Ray Photoelectron Spectroscopy. Jpn. J. Appl. Phys. 2018, 57, 6–9. [Google Scholar] [CrossRef]
- Oda, M.; Kaneko, K.; Fujita, S.; Hitora, T. Crack-free thick (~5 µm) α-Ga2O3 films on sapphire substrates with α-(Al,Ga)2O3 buffer layers. Jpn. J. Appl. Phys. 2016, 55, 1202B4. [Google Scholar] [CrossRef]
- Suzuki, N.; Kaneko, K.; Fujita, S. Growth of Corundum-Structured (InxGa1-x)2O3 alloy Thin Films on Sapphire Substrates with Buffer Layers. J. Cryst. Growth 2014, 401, 670–672. [Google Scholar] [CrossRef]
- Williams, M.S.; Alonso-Orts, M.; Schowalter, M.; Karg, A.; Raghuvansy, S.; McCandless, J.P.; Jena, D.; Rosenauer, A.; Eickhoff, M.; Vogt, P. Growth, Catalysis, and Faceting of α-Ga2O3 and α-(InxGa1−x)2O3 on m-Plane α-Al2O3 by Molecular Beam Epitaxy. APL Mater. 2024, 12, 011120. [Google Scholar] [CrossRef]
- Kaneko, K.; Nomura, T.; Kakeya, I.; Fujita, S. Fabrication of Highly Crystalline Corundum-Structured α-(Ga1-xFex)2O3 alloy Thin Films on Sapphire Substrates. Appl. Phys. Express 2009, 2, 3–6. [Google Scholar] [CrossRef]
- Yasuoka, T.; Liu, L.; Ozaki, T.; Asako, K.; Ishikawa, Y.; Fukue, M.; Dang, G.T.; Kawaharamura, T. The Effect of HCl on the α-Ga2O3 Thin Films Fabricated by Third Generation Mist Chemical Vapor Deposition. AIP Adv. 2021, 11, 045123. [Google Scholar] [CrossRef]
- Wakamatsu, T.; Takane, H.; Kaneko, K.; Araki, T.; Tanaka, K. Influence of HCl Concentration in Source Solution and Growth Temperature on Formation of α-Ga2O3 Film via Mist-CVD Process. Jpn. J. Appl. Phys. 2023, 62, SF1024. [Google Scholar] [CrossRef]
- Jinno, R.; Kaneko, K.; Fujita, S. Thermal Stability of α-Ga2O3 films Grown on c-Plane Sapphire Substrates via Mist-CVD. AIP Adv. 2020, 10, 115013. [Google Scholar] [CrossRef]
- Uno, K.; Ohta, M.; Tanaka, I. Growth Mechanism of α-Ga2O3 on a Sapphire Substrate by Mist Chemical Vapor Deposition Using Acetylacetonated Gallium Source Solutions. Appl. Phys. Lett. 2020, 117, 2–7. [Google Scholar] [CrossRef]
- Oshima, Y.; Víllora, E.G.; Shimamura, K. Halide Vapor Phase Epitaxy of Twin-Free α-Ga2O3 on Sapphire (0001) Substrates. Appl. Phys. Express 2015, 8, 4–8. [Google Scholar] [CrossRef]
- Leach, J.H.; Udwary, K.; Rumsey, J.; Dodson, G.; Splawn, H.; Evans, K.R. Halide Vapor Phase Epitaxial Growth of β-Ga2O3 and α-Ga2O3 Films. APL Mater. 2019, 7, 022504. [Google Scholar] [CrossRef]
- Yao, Y.; Okur, S.; Lyle, L.A.M.; Tompa, G.S.; Salagaj, T.; Sbrockey, N.; Davis, R.F.; Porter, L.M. Growth and Characterization of α-, β-, and ϵ-phases of Ga2O3 Using MOCVD and HVPE Techniques. Mater. Res. Lett. 2018, 6, 268–275. [Google Scholar] [CrossRef]
- Sun, H.; Li, K.H.; Castanedo, C.G.T.; Okur, S.; Tompa, G.S.; Salagaj, T.; Lopatin, S.; Genovese, A.; Li, X. HCl Flow-Induced Phase Change of α-, β-, and ϵ-Ga2O3 Films Grown by MOCVD. Cryst. Growth Des. 2018, 18, 2370–2376. [Google Scholar] [CrossRef]
- Bhuiyan, A.F.M.A.U.; Feng, Z.; Huang, H.L.; Meng, L.; Hwang, J.; Zhao, H. Metalorganic Chemical Vapor Deposition of α-Ga2O3 and α-(AlxGa1−x)2O3 Thin Films on m-Plane Sapphire Substrates. APL Mater. 2021, 9, 101109. [Google Scholar] [CrossRef]
- Kracht, M.; Karg, A.; Feneberg, M.; Bläsing, J.; Schörmann, J.; Goldhahn, R.; Eickhoff, M. Anisotropic Optical Properties of Metastable (012) α-Ga2O3 Grown by Plasma-Assisted Molecular Beam Epitaxy. Phys. Rev. Appl. 2018, 10, 1. [Google Scholar] [CrossRef]
- McCandless, J.P.; Rowe, D.; Pieczulewski, N.; Protasenko1, V.; Alonso-Orts, M.; Williams, M.S.; Eickhoff, M.; Xing, H.G.; Muller, D.A.; Jena, D.; et al. Growth of α-Ga2O3 on α-Al2O3 by Conventional Molecular-beam Epitaxy and Metal-oxide-catalyzed Epitaxy. Jpn. J. Appl. Phys. 2023, 62, SF1013. [Google Scholar] [CrossRef]
- Roberts, J.W.; Jarman, J.C.; Johnstone, D.N.; Midgley, P.A.; Chalker, P.R.; Oliver, R.A.; Massabuau, F.C.P. α-Ga2O3 Grown by Low Temperature Atomic Layer Deposition on Sapphire. J. Cryst. Growth 2018, 487, 23–27. [Google Scholar] [CrossRef]
- Wheeler, V.D.; Nepal, N.; Boris, D.R.; Qadri, S.B.; Nyakiti, L.O.; Lang, A.; Koehler, A.; Foster, G.; Walton, S.G.; Eddy, C.R.; et al. Phase Control of Crystalline Ga2O3 Films by Plasma-Enhanced Atomic Layer Deposition. Chem. Mater. 2020, 32, 1140–1152. [Google Scholar] [CrossRef]
- Lee, S.D.; Kaneko, K.; Fujita, S. Homoepitaxial Growth of Beta Gallium Oxide Films by Mist Chemical Vapor Deposition. Jpn. J. Appl. Phys. 2016, 55, 1202B8. [Google Scholar] [CrossRef]
- Nishinaka, H.; Tahara, D.; Yoshimoto, M. Heteroepitaxial Growth of ε-Ga2O3 Thin Films on Cubic (111) MgO and (111) Yttria-Stablized Zirconia Substrates by Mist Chemical Vapor Deposition. Jpn. J. Appl. Phys. 2016, 55, 1202BC. [Google Scholar] [CrossRef]
- Kato, T.; Nishinaka, H.; Shimazoe, K.; Kanegae, K.; Yoshimoto, M. Demonstration of Bixbyite-Structured δ-Ga2O3 Thin Films Using β-Fe2O3 Buffer Layers by Mist Chemical Vapor Deposition. ACS Appl. Electron. Mater. 2023, 5, 1715–1720. [Google Scholar] [CrossRef]
- Oshima, T.; Nakazono, T.; Mukai, A.; Ohtomo, A. Epitaxial Growth of γ-Ga2O3 Films by Mist Chemical Vapor Deposition. J. Cryst. Growth 2012, 359, 60–63. [Google Scholar] [CrossRef]
- Kawaharamura, T.; Nishi, M.; Liu, L.; Rutthongjan, P.; Ishikawa, Y.; Sakamoto, M.; Yasuoka, T.; Asako, K.; Ozaki, T.; Fukue, M.; et al. Growth Mechanism under the Supply-Limited Regime in Mist Chemical Vapor Deposition: Presumption of Mist Droplet State in High-Temperature Field. Jpn. J. Appl. Phys. 2024, 63, 015502. [Google Scholar] [CrossRef]
- Liu, L.; Kawaharamura, T.; Dang, G.T.; Pradeep, E.K.C.; Sato, S.; Uchida, T.; Fujita, S.; Hiramatsu, T.; Kobayashi, H.; Orita, H. Study on Fabrication of Conductive Antimony Doped Tin Oxide Thin Films (SnO X :Sb) by 3rd Generation Mist Chemical Vapor Deposition. Jpn. J. Appl. Phys. 2019, 58, 025502. [Google Scholar] [CrossRef]
- Dang, G.T.; Kawaharamura, T.; Furuta, M.; Allen, M.W. Mist-CVD Grown Sn-Doped α-Ga2O3 MESFETs. IEEE Trans. Electron. Devices 2015, 62, 3640–3644. [Google Scholar] [CrossRef]
- Dang, G.T.; Yasuoka, T.; Kawaharamura, T. Sub-Μm Features Patterned with Laser Interference Lithography for the Epitaxial Lateral Overgrowth of α-Ga2O3 via Mist Chemical Vapor Deposition. Appl. Phys. Lett. 2021, 119, 041902. [Google Scholar] [CrossRef]


| Solution | Ga Solution | HCl Solution | |
|---|---|---|---|
| Solute | Ga(acac)3 (a) | GaCl3 (b) | HCl (c) |
| Concentration | 20–100 mM | 50–300 mM | 0.57–1.13 M |
| Solvent (mixing ratio) | DI water, HCl (199:1, 99:1) | DI water | DI water (d) |
| Carrier and dilution gas | N2 | ||
| Substrate | c-plane sapphire | ||
| Growth temperature | 400 °C | ||
| Ultrasonic transducer | 2.4 MHz, 24 V–0.625 A (×3) | ||
| Precursor | Ga Supply Rate | HCl Support | |
|---|---|---|---|
| Surface roughness | ✓ | ✓ | ✓ |
| Crystallinity | ✓ | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasuoka, T.; Liu, L.; Dang, G.T.; Kawaharamura, T. Growth of α-Ga2O3 from Gallium Acetylacetonate under HCl Support by Mist Chemical Vapor Deposition. Nanomaterials 2024, 14, 1221. https://doi.org/10.3390/nano14141221
Yasuoka T, Liu L, Dang GT, Kawaharamura T. Growth of α-Ga2O3 from Gallium Acetylacetonate under HCl Support by Mist Chemical Vapor Deposition. Nanomaterials. 2024; 14(14):1221. https://doi.org/10.3390/nano14141221
Chicago/Turabian StyleYasuoka, Tatsuya, Li Liu, Giang T. Dang, and Toshiyuki Kawaharamura. 2024. "Growth of α-Ga2O3 from Gallium Acetylacetonate under HCl Support by Mist Chemical Vapor Deposition" Nanomaterials 14, no. 14: 1221. https://doi.org/10.3390/nano14141221
APA StyleYasuoka, T., Liu, L., Dang, G. T., & Kawaharamura, T. (2024). Growth of α-Ga2O3 from Gallium Acetylacetonate under HCl Support by Mist Chemical Vapor Deposition. Nanomaterials, 14(14), 1221. https://doi.org/10.3390/nano14141221






