A Hybrid Structure to Improve Electrochemical Performance of SiO Anode Materials in Lithium-Ion Battery
Abstract
1. Introduction
2. Materials and Methods
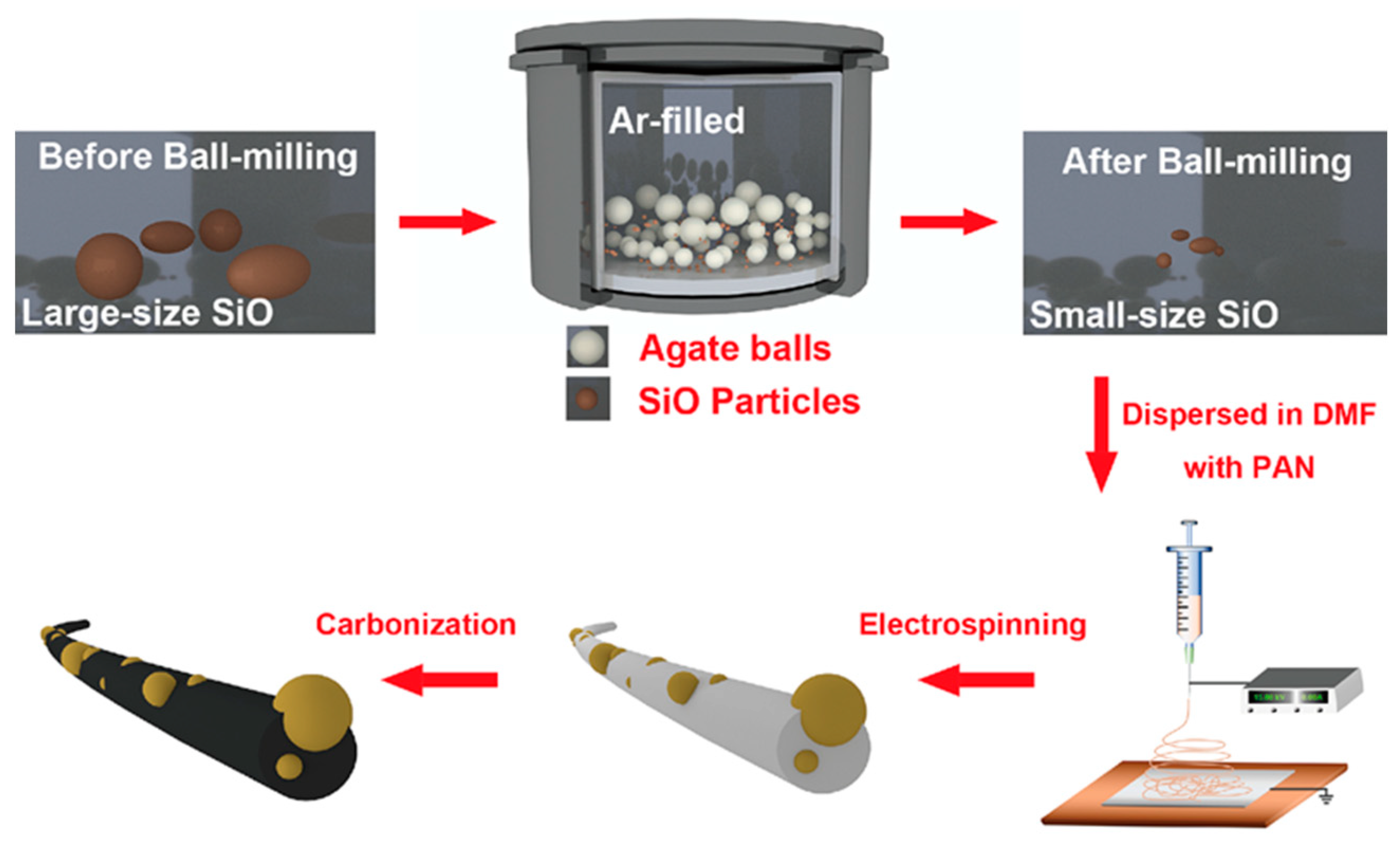
2.1. Materials Preparation
2.2. Synthesis of SiO/CNF
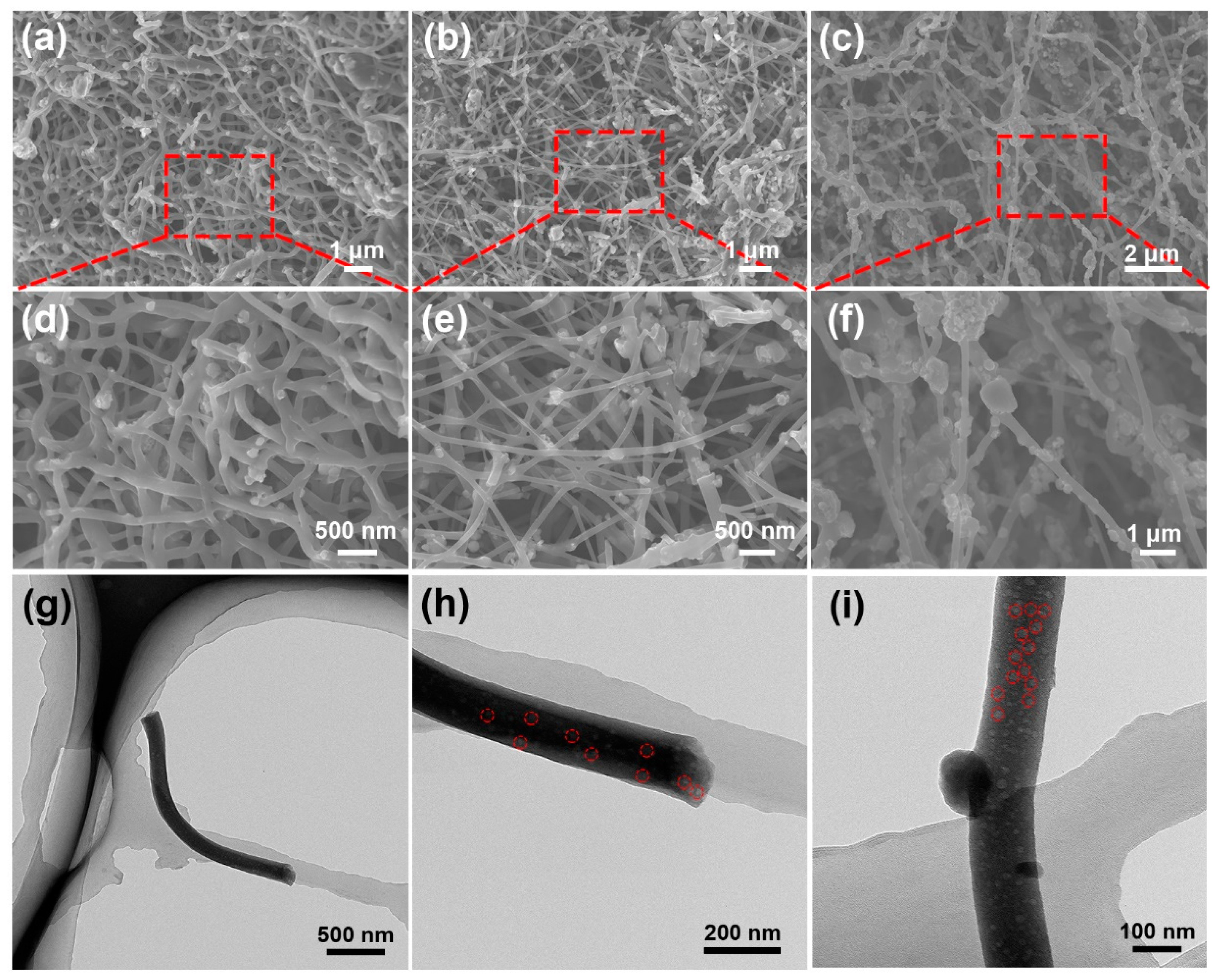
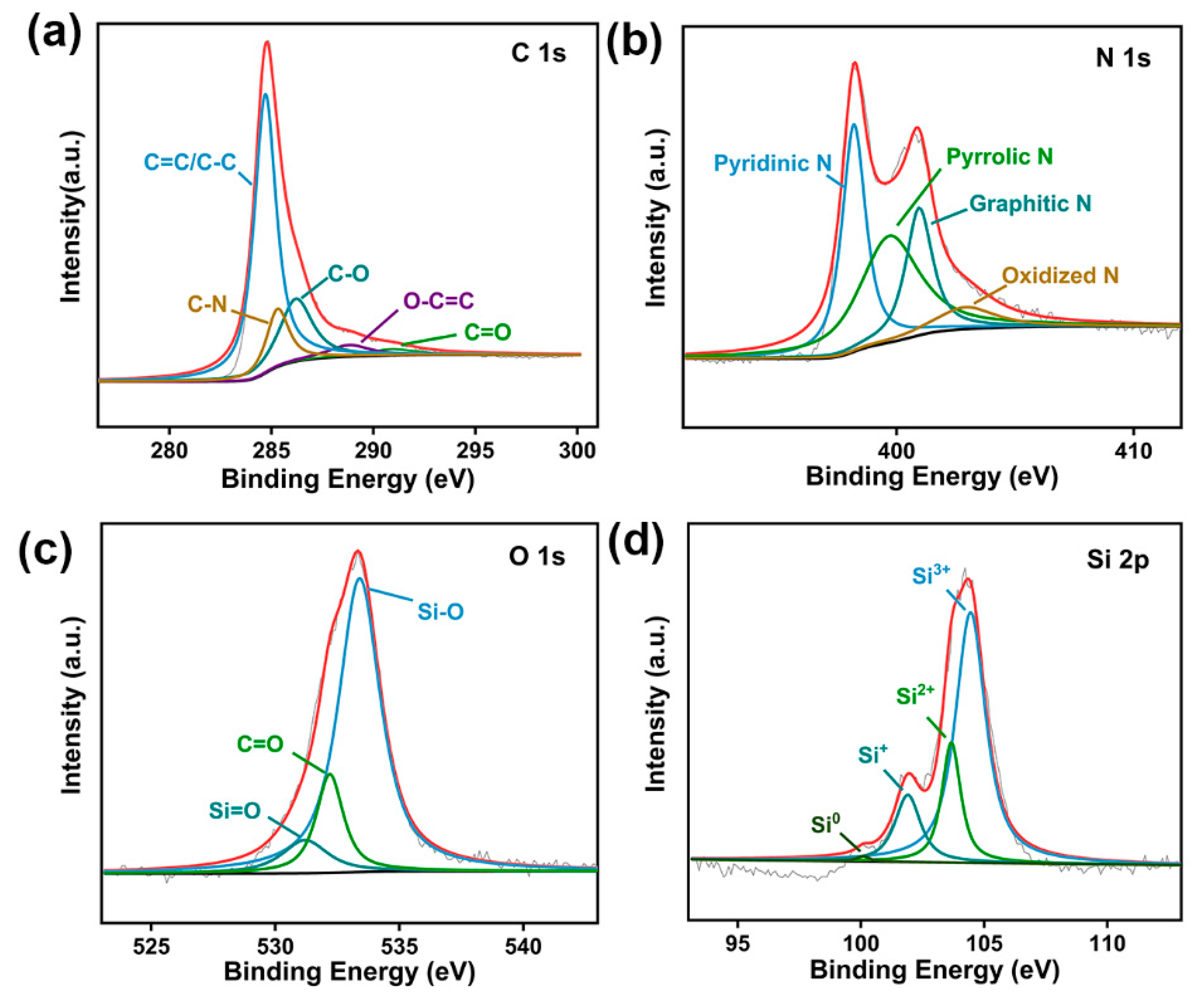
2.3. Structural Characterization
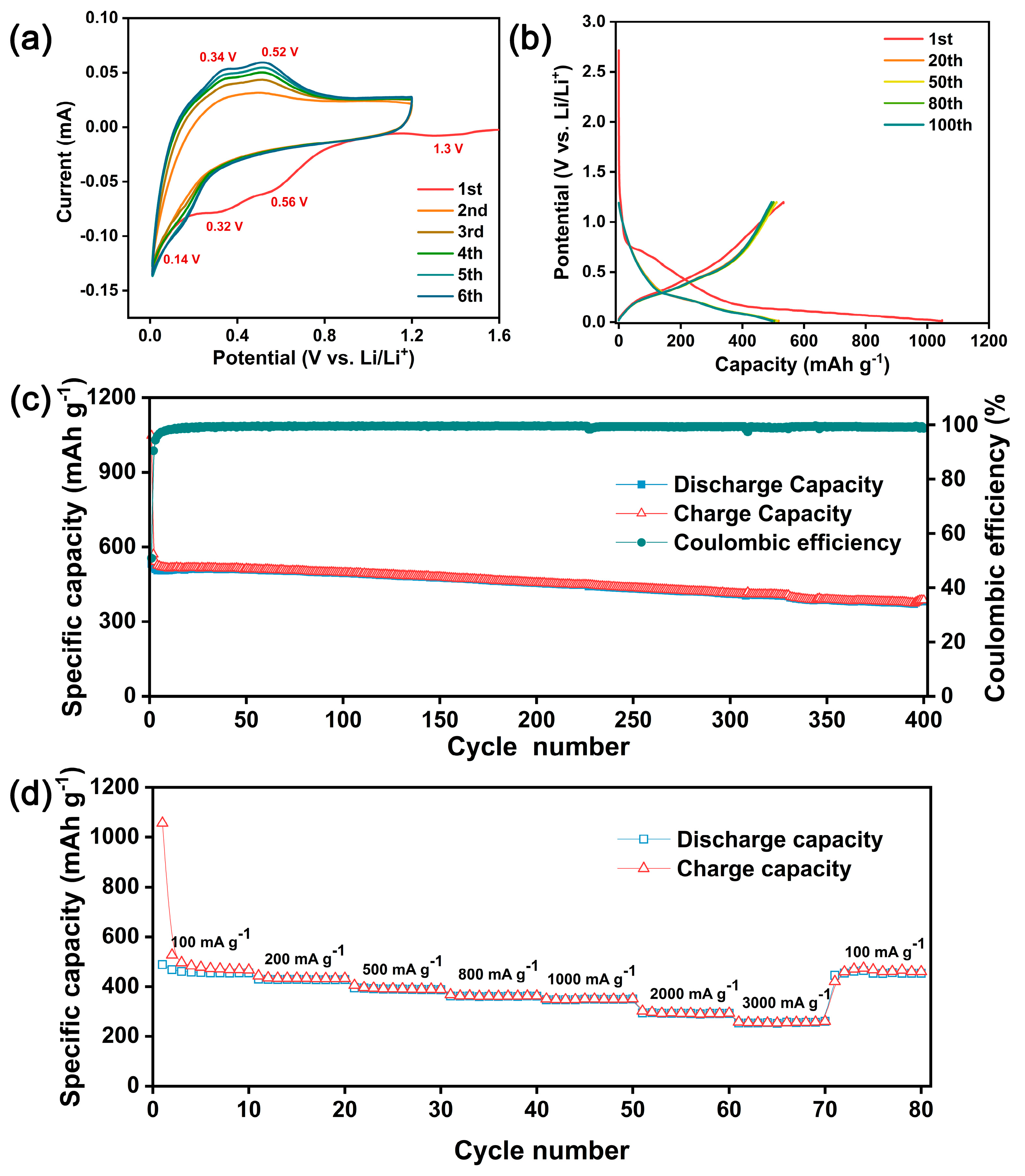
2.4. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.; Xi, B.; Xiong, S.; Qian, Y. Carbon coated SiO nanoparticles embedded in hierarchical porous N-doped carbon nanosheets for enhanced lithium storage. Inorg. Chem. Front. 2021, 8, 4282–4290. [Google Scholar] [CrossRef]
- Li, Y.B.; Ma, G.L.; Shao, H.; Xiao, P.; Lu, J.; Xu, J.; Hou, J.R.; Chen, K.; Zhang, X.; Li, M.; et al. Electrochemical Lithium Storage Performance of Molten Salt Derived V2SnC MAX Phase. Nano-Micro Lett. 2021, 13, 158. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Yan, S.X.; Ali, M.; Mahmood, F.; Zheng, Y.; Li, G.C.; Liu, J.F.; Song, X.H.; Wang, Y. Innovative Solutions for High-Performance Silicon Anodes in Lithium-Ion Batteries: Overcoming Challenges and Real-World Applications. Nano-Micro Lett. 2024, 16, 179. [Google Scholar] [CrossRef] [PubMed]
- Small, L.J.; Eccleston, A.; Lamb, J.; Read, A.C.; Robins, M.; Meaders, T.; Ingersoll, D.; Clem, P.G.; Bhavaraju, S.; Spoerke, E.D. Next generation molten NaI batteries for grid scale energy storage. J. Power Sources 2017, 360, 569–574. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Zan, G.T.; Li, S.Q.; Chen, P.; Dong, K.Z.; Wu, Q.S.; Wu, T. Mesoporous Cubic Nanocages Assembled by Coupled Monolayers with 100% Theoretical Capacity and Robust Cycling. Acs Cent. Sci. 2024, 10, 1283–1294. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Q.; Li, J.; Li, K.; Zhao, Y.; Zhang, Y.G.; Gosselink, D.; Chen, P. SiO2/Cu/polyacrylonitrile-C composite as anode material in lithium ion batteries. J. Power Sources 2013, 240, 659–666. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, G.Y.; Liu, N.A.; Carney, T.J.; Yang, Y.; Cui, Y. Engineering Empty Space between Si Nanoparticles for Lithium-Ion Battery Anodes. Nano Lett. 2012, 12, 904–909. [Google Scholar] [CrossRef]
- Chae, S.; Choi, S.H.; Kim, N.; Sung, J.; Cho, J. Integration of Graphite and Silicon Anodes for the Commercialization of High-Energy Lithium-Ion Batteries. Angew. Chem.-Int. Ed. 2020, 59, 110–135. [Google Scholar] [CrossRef]
- Cao, C.T.; Abate, I.I.; Sivonxay, E.; Shyam, B.; Jia, C.J.; Moritz, B.; Devereaux, T.P.; Persson, K.A.; Steinruck, H.G.; Toney, M.F. Solid Electrolyte Interphase on Native Oxide-Terminated Silicon Anodes for Li-Ion Batteries. Joule 2019, 3, 762–781. [Google Scholar] [CrossRef]
- Liu, Z.H.; Zhao, Y.L.; He, R.H.; Luo, W.; Meng, J.S.; Yu, Q.; Zhao, D.Y.; Zhou, L.; Mai, L.Q. Yolk@Shell SiOx/C microspheres with semi-graphitic carbon coating on the exterior and interior surfaces for durable lithium storage. Energy Storage Mater. 2019, 19, 299–305. [Google Scholar] [CrossRef]
- Zhao, H.S.; Li, J.B.; Zhao, Q.; Huang, X.B.; Jia, S.Y.; Ma, J.M.; Ren, Y.R. Si-Based Anodes: Advances and Challenges in Li-Ion Batteries for Enhanced Stability. Electrochem. Energy Rev. 2024, 7, 11. [Google Scholar] [CrossRef]
- Szczech, J.R.; Jin, S. Nanostructured silicon for high capacity lithium battery anodes. Energy Environ. Sci. 2011, 4, 56–72. [Google Scholar] [CrossRef]
- Bauer, U.; Welsch, A.M.; Behrens, H.; Rahn, J.; Schmidt, H.; Horn, I. Li Diffusion and the Effect of Local Structure on Li Mobility in Li2O-SiO2 Glasses. J. Phys. Chem. B 2013, 117, 15184–15195. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, J.; Huang, Q.; Xu, K.; Fan, W.; Yu, L.; Xia, Q.; Li, W. Insights into the Interfacial Instability between Carbon-Coated SiO Anode and Electrolyte in Lithium-Ion Batteries. J. Phys. Chem. C 2019, 123, 12902–12909. [Google Scholar] [CrossRef]
- Tang, J.J.; Hou, L.; Hu, T.J.; Fan, S.C.; Zhou, X.Y.; Yang, J. Influence of oxygen content on the electrochemical behavior of SiOx@C anodes for Li-ion battery. Compos. Commun. 2021, 23, 100544. [Google Scholar] [CrossRef]
- Yamamura, H.; Nakanishi, S.; Iba, H. Reduction effect of irreversible capacity on SiO anode material heat-reacted with Fe2O3. J. Power Sources 2013, 232, 264–269. [Google Scholar] [CrossRef]
- Morimoto, H.; Higuchi, D.; Tobishima, S. Characteristics of High-capacity SiO-C Anodes Containing Olivine-type LiMgPO4 Compounds for Lithium Secondary Batteries. Electrochemistry 2015, 83, 813–816. [Google Scholar] [CrossRef][Green Version]
- Kim, T.; Park, S.; Oh, S.M. Solid-state NMR and electrochemical dilatometry study on Li+ uptake/extraction mechanism in SiO electrode. J. Electrochem. Soc. 2007, 154, A1112–A1117. [Google Scholar] [CrossRef]
- Ban, C.M.; Kappes, B.B.; Xu, Q.; Engtrakul, C.; Ciobanu, C.V.; Dillon, A.C.; Zhao, Y.F. Lithiation of silica through partial reduction. Appl. Phys. Lett. 2012, 100, 243905. [Google Scholar] [CrossRef]
- Yom, J.H.; Hwang, S.W.; Cho, S.M.; Yoon, W.Y. Improvement of irreversible behavior of SiO anodes for lithium ion batteries by a solid state reaction at high temperature. J. Power Sources 2016, 311, 159–166. [Google Scholar] [CrossRef]
- Na, Z.Y.; Lai, C.; Zhou, J.; Zhang, H.Z.; Song, D.W.; Shi, X.X.; Zhang, L.Q. Enhancing the reversible capacity and cycle stability of lithium-ion batteries with Li-compensation material Li6CoO4. Sci. China-Mater. 2022, 65, 620–628. [Google Scholar] [CrossRef]
- Wu, Z.-L.; Ji, S.-B.; Liu, L.-K.; Xie, T.; Tan, L.; Tang, H.; Sun, R.-G. High-performance SiO/C as anode materials for lithium-ion batteries using commercial SiO and glucose as raw materials. Rare Met. 2020, 40, 1110–1117. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, C.M.; Kim, H.; Kim, Y.J.; Sohn, H.J. Electrochemical behavior of SiO anode for Li secondary batteries. J. Electroanal. Chem. 2011, 661, 245–249. [Google Scholar] [CrossRef]
- Liu, Z.H.; Yu, Q.; Zhao, Y.L.; He, R.H.; Xu, M.; Feng, S.H.; Li, S.D.; Zhou, L.; Mai, L.Q. Silicon oxides: A promising family of anode materials for lithium-ion batteries. Chem. Soc. Rev. 2019, 48, 285–309. [Google Scholar] [CrossRef]
- Chen, T.; Wu, J.; Zhang, Q.L.; Su, X. Recent advancement of SiOx, based anodes for lithium-ion batteries. J. Power Sources 2017, 363, 126–144. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, B.; Lu, Z.D.; Liu, N.; Zhu, J. Challenges and Recent Progress in the Development of Si Anodes for Lithium-Ion Battery. Adv. Energy Mater. 2017, 7, 1700715. [Google Scholar] [CrossRef]
- Jung, S.C.; Kim, H.J.; Kim, J.H.; Han, Y.K. Atomic-Level Understanding toward a High-Capacity and High Power Silicon Oxide (SiO) Material. J. Phys. Chem. C 2016, 120, 886–892. [Google Scholar] [CrossRef]
- Shi, L.R.; Pang, C.L.; Chen, S.L.; Wang, M.Z.; Wang, K.X.; Tan, Z.J.; Gao, P.; Ren, J.G.; Huang, Y.Y.; Peng, H.L.; et al. Vertical Graphene Growth on SiO Microparticles for Stable Lithium Ion Battery Anodes. Nano Lett. 2017, 17, 3681–3687. [Google Scholar] [CrossRef]
- Park, D.; Kim, H.S.; Seo, H.; Kim, K.; Kim, J.H. Porous SiO composite tailored by scalable mechanochemical oxidation of Si for Li-ion anodes. Electrochim. Acta 2020, 357, 136862. [Google Scholar] [CrossRef]
- Sepehri-Amin, H.; Ohkubo, T.; Kodzuka, M.; Yamamura, H.; Saito, T.; Iba, H.; Hono, K. Evidence for nano-Si clusters in amorphous SiO anode materials for rechargeable Li-ion batteries. Scr. Mater. 2013, 69, 92–95. [Google Scholar] [CrossRef]
- Fu, K.; Gong, Y.H.; Xu, S.M.; Zhu, Y.Z.; Li, Y.J.; Dai, J.Q.; Wang, C.W.; Liu, B.Y.; Pastel, G.; Xie, H.; et al. Stabilizing the Garnet Solid-Electrolyte/Polysulfide Interface in Li-S Batteries. Chem. Mater. 2017, 29, 8037–8041. [Google Scholar] [CrossRef]
- Lee, J.; Moon, J.; Han, S.A.; Kim, J.; Malgras, V.; Heo, Y.U.; Kim, H.; Lee, S.M.; Liu, H.K.; Dou, S.X.; et al. Everlasting Living and Breathing Gyroid 3D Network in Si@SiOx/C Nanoarchitecture for Lithium Ion Battery. ACS Nano 2019, 13, 9607–9619. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.J.; Liu, Y.N.; Xu, C.; Zhu, J.X.; Wei, X.J.; Zhou, L.; He, L.; Yang, W.; Mai, L.Q. Ultrafine Nickel-Nanoparticle-Enabled SiO2 Hierarchical Hollow Spheres for High-Performance Lithium Storage. Adv. Funct. Mater. 2018, 28, 1704561. [Google Scholar] [CrossRef]
- Jiao, M.L.; Wang, Y.F.; Ye, C.L.; Wang, C.Y.; Zhang, W.K.; Liang, C. High-capacity SiOx (0 ≤ x ≤ 2) as promising anode materials for next-generation lithium-ion batteries. J. Alloys Compd. 2020, 842, 155774. [Google Scholar] [CrossRef]
- Tabuchi, T.; Yasuda, H.; Yamachi, M. Li-doping process for LixSiO-negative active material synthesized by chemical method for lithium-ion cells. J. Power Sources 2005, 146, 507–509. [Google Scholar] [CrossRef]
- Ding, X.L.; Liang, D.W.; Zhao, H.D. Enhanced Electrochemical Performance Promoted by Tin in Silica Anode Materials for Stable and High-Capacity Lithium-Ion Batteries. Materials 2021, 14, 1071. [Google Scholar] [CrossRef]
- Yuan, M.J.; Guo, X.T.; Liu, Y.; Pang, H. Si-based materials derived from biomass: Synthesis and applications in electrochemical energy storage. J. Mater. Chem. A 2019, 7, 22123–22147. [Google Scholar] [CrossRef]
- Rehman, W.U.; Wang, H.F.; Manj, R.Z.A.; Luo, W.; Yang, J.P. When Silicon Materials Meet Natural Sources: Opportunities and Challenges for Low-Cost Lithium Storage. Small 2021, 17, 1904508. [Google Scholar] [CrossRef]
- Zeng, S.Z.; Niu, Y.; Zou, J.Z.; Zeng, X.R.; Zhu, H.O.; Huang, J.T.; Wang, L.Y.; Kong, L.B.; Han, P.G. Green and scalable preparation of disproportionated SiO anode materials with cocoon-like buffer layer. J. Power Sources 2020, 466, 228234. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Zhang, J.; Bao, T.Z.; Xie, X.H.; Xia, B.J. Electrochemical and stress characteristics of SiO/Cu/expanded graphite composite as anodes for lithium ion batteries. J. Power Sources 2017, 348, 16–20. [Google Scholar] [CrossRef]
- Zhao, J.; Lee, H.W.; Sun, J.; Yan, K.; Liu, Y.Y.; Liu, W.; Lu, Z.D.; Lin, D.C.; Zhou, G.M.; Cui, Y. Metallurgically lithiated SiOx anode with high capacity and ambient air compatibility. Proc. Natl. Acad. Sci. USA 2016, 113, 7408–7413. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Baek, S.H.; Park, J.S.; Jeong, Y.M.; Kim, J.H. Improved electrochemical performance of boron-doped SiO negative electrode materials in lithium-ion batteries. J. Power Sources 2015, 299, 25–31. [Google Scholar] [CrossRef]
- Yang, Z.M.; Ding, Y.H.; Jiang, Y.H.; Zhang, P.; Jin, H.B. Hierarchical C/SiOx/TiO2 ultrathin nanobelts as anode materials for advanced lithium ion batteries. Nanotechnology 2018, 29, 405602. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.J.; Gordin, M.L.; Chen, S.R.; Xu, T.; Song, J.X.; Lv, D.P.; Wang, D.H. Enhanced performance of SiO/Fe2O3 composite as an anode for rechargeable Li-ion batteries. Electrochem. Commun. 2013, 28, 79–82. [Google Scholar] [CrossRef]
- Kim, J.H.; Sohn, H.J.; Kim, H.; Jeong, G.; Choi, W. Enhanced cycle performance of SiO-C composite anode for lithium-ion batteries. J. Power Sources 2007, 170, 456–459. [Google Scholar] [CrossRef]
- Liu, W.R.; Yen, Y.C.; Wu, H.C.; Winter, M.; Wu, N.L. Nano-porous SiO/carbon composite anode for lithium-ion batteries. J. Appl. Electrochem. 2009, 39, 1643–1649. [Google Scholar] [CrossRef]
- Park, C.M.; Choi, W.; Hwa, Y.; Kim, J.H.; Jeong, G.; Sohn, H.J. Characterizations and electrochemical behaviors of disproportionated SiO and its composite for rechargeable Li-ion batteries. J. Mater. Chem. 2010, 20, 4854–4860. [Google Scholar] [CrossRef]
- Xu, Q.; Sun, J.K.; Yin, Y.X.; Guo, Y.G. Facile Synthesis of Blocky SiOx/C with Graphite-Like Structure for High-Performance Lithium-Ion Battery Anodes. Adv. Funct. Mater. 2018, 28, 1705235. [Google Scholar] [CrossRef]
- Cao, Z.L.; Xia, B.J.; Xie, X.H.; Zhao, J.B. Influences of co-sputtered carbon on the electrochemical performance of SiO/C thin film anodes for lithium-ion batteries. Electrochim. Acta 2019, 313, 311–320. [Google Scholar] [CrossRef]
- Hou, G.L.; Cheng, B.L.; Cao, Y.B.; Yao, M.S.; Li, B.Q.; Zhang, C.; Weng, Q.H.; Wang, X.; Bando, Y.; Golberg, D.; et al. Scalable production of 3D plum-pudding-like Si/C spheres: Towards practical application in Li-ion batteries. Nano Energy 2016, 24, 111–120. [Google Scholar] [CrossRef]
- Wu, J.X.; Qin, X.Y.; Miao, C.; He, Y.B.; Liang, G.M.; Zhou, D.; Liu, M.; Han, C.P.; Li, B.H.; Kang, F.Y. A honeycomb-cobweb inspired hierarchical core-shell structure design for electrospun silicon/carbon fibers as lithium-ion battery anodes. Carbon 2016, 98, 582–591. [Google Scholar] [CrossRef]
- Fan, X.-m.; Zhang, X.-h.; Hu, G.-r.; Zhang, B.; He, Z.-j.; Li, Y.-j.; Zheng, J.-c. Single-walled carbon nanotube as conductive additive for SiO/C composite electrodes in pouch-type lithium-ion batteries. Ionics 2019, 26, 1721–1728. [Google Scholar] [CrossRef]
- Liu, N.; He, W.; Liao, H.; Li, Z.; Jiang, J.; Zhang, X.; Dou, H. Polydopamine grafted cross-linked polyacrylamide as robust binder for SiO/C anode toward high-stability lithium-ion battery. J. Mater. Sci. 2021, 56, 6337–6348. [Google Scholar] [CrossRef]
- Zhang, C.R.; Yao, L.; Yang, Z.; Kong, E.S.W.; Zhu, X.F.; Zhang, Y.F. Graphene Oxide-Modified Polyacrylonitrile Nanofibrous Membranes for Efficient Air Filtration. Acs Appl. Nano Mater. 2019, 2, 3916–3924. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, C.R.; Wu, W.D.; Cai, Y.K.; Zhang, Y.F. Nodes-connected silicon-carbon nanofibrous hybrids anodes for lithium-ion batteries. Appl. Surf. Sci. 2021, 548, 148944. [Google Scholar] [CrossRef]
- Si, Q.; Hanai, K.; Ichikawa, T.; Phillipps, M.B.; Hirano, A.; Imanishi, N.; Yamamoto, O.; Takeda, Y. Improvement of cyclic behavior of a ball-milled SiO and carbon nanofiber composite anode for lithium-ion batteries. J. Power Sources 2011, 196, 9774–9779. [Google Scholar] [CrossRef]
- Huang, X.; Li, M.Q. Multi-channel and porous SiO@N-doped C rods as anodes for high-performance lithium-ion batteries. Appl. Surf. Sci. 2018, 439, 336–342. [Google Scholar] [CrossRef]
- Ratha, S.; Rout, C.S. Supercapacitor Electrodes Based on Layered Tungsten Disulfide-Reduced Graphene Oxide Hybrids Synthesized by a Facile Hydrothermal Method. Acs Appl. Mater. Interfaces 2013, 5, 11427–11433. [Google Scholar] [CrossRef]
- Wu, N.; She, X.L.; Yang, D.J.; Wu, X.F.; Su, F.B.; Chen, Y.F. Synthesis of network reduced graphene oxide in polystyrene matrix by a two-step reduction method for superior conductivity of the composite. J. Mater. Chem. 2012, 22, 17254–17261. [Google Scholar] [CrossRef]
- Yan, W.C.; Liu, D.; Tan, D.Y.; Yuan, P.; Chen, M. FTIR spectroscopy study of the structure changes of palygorskite under heating. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2012, 97, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Q.; Gu, J.W.; Feng, X.F.; He, H.Y.; Zeng, C.M. Amorphous-silicon@silicon oxide/chromium/carbon as an anode for lithium-ion batteries with excellent cyclic stability. Electrochim. Acta 2015, 164, 163–170. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, C.R.; Hu, N.T.; Zhang, L.Y.; Zhou, Z.H.; Zhang, Y.F. Three-dimensional skeleton networks of reduced graphene oxide nanosheets/vanadium pentoxide nanobelts hybrid for high-performance supercapacitors. Electrochim. Acta 2019, 295, 14–21. [Google Scholar] [CrossRef]
- Yao, L.; Dong, X.W.; Zhang, C.R.; Hu, N.T.; Zhang, Y.F. Metal oxide nanoprism-arrays assembled in N-doped carbon foamy nanoplates that have efficient polysulfide-retention for ultralong-cycle-life lithium-sulfur batteries. J. Mater. Chem. A 2018, 6, 11260–11269. [Google Scholar] [CrossRef]
- Shi, M.J.; Zhang, S.; Jiang, Y.T.; Jiang, Z.M.; Zhang, L.H.; Chang, J.; Wei, T.; Fan, Z.J. Sandwiching Sulfur into the Dents between N, O Co-Doped Graphene Layered Blocks with Strong Physicochemical Confinements for Stable and High-Rate Li-S Batteries. Nano-Micro Lett. 2020, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xu, S.W.; Li, B.; Yin, M.S.; Shao, F.; Li, H.; Xia, T.; Yang, Z.; Su, Y.J.; Zhang, Y.F.; et al. In-plane Defect Engineering Enabling Ultra-Stable Graphene Paper-based Hosts for Lithium Metal Anodes. Chemelectrochem 2021, 8, 3273–3281. [Google Scholar] [CrossRef]
- Nie, S.; Liu, L.; Liu, J.F.; Xie, J.J.; Zhang, Y.; Xia, J.; Yan, H.X.; Yuan, Y.T.; Wang, X.Y. Nitrogen-Doped TiO2-C Composite Nanofibers with High-Capacity and Long-Cycle Life as Anode Materials for Sodium-Ion Batteries. Nano-Micro Lett. 2018, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.F.; Cao, Y.L.; Henderson, W.A.; Sushko, M.L.; Shao, Y.Y.; Xiao, J.; Wang, W.; Engelhard, M.H.; Nie, Z.M.; Liu, J. Hard carbon nanoparticles as high-capacity, high-stability anodic materials for Na-ion batteries. Nano Energy 2016, 19, 279–288. [Google Scholar] [CrossRef]
- Fan, L.L.; Li, X.F.; Yan, B.; Feng, J.M.; Xiong, D.B.; Li, D.J.; Gu, L.; Wen, Y.R.; Lawes, S.; Sun, X.L. Controlled SnO2 Crystallinity Effectively Dominating Sodium Storage Performance. Adv. Energy Mater. 2016, 6, 1502057. [Google Scholar] [CrossRef]
- Dai, C.Y.; Li, C.S.; Huang, H.Y.; Wang, Z.; Zhu, X.X.; Liao, X.B.; Chen, X.; Pan, Y.; Fang, D.N. In situ strain measurements and stress analysis of SiO@C composite electrodes during electrochemical cycling by using digital image correlation. Solid State Ion. 2019, 331, 56–65. [Google Scholar] [CrossRef]
- Liu, Y.C.; Huang, J.Y.; Zhang, X.Q.; Wu, J.W.; Baker, A.; Zhang, H.Y.; Chang, S.; Zhang, X.H. A bm-SiO/Ni/rGO composite as an anode material for lithium-ion batteries. J. Alloys Compd. 2018, 749, 236–243. [Google Scholar] [CrossRef]
- Huang, Z.H.; Dang, G.J.; Jiang, W.P.; Sun, Y.Y.; Yu, M.; Zhang, Q.S.; Xie, J.Y. A Low-Cost and Scalable Carbon Coated SiO-Based Anode Material for Lithium-Ion Batteries. Chemistryopen 2021, 10, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Wang, K.; Tan, Y.; Li, Q.L.; Sun, J.M. Studies on performance of SiO addition to Li4Ti5O12 as anode material for lithium-ion batteries. Chem. Phys. Lett. 2019, 730, 623–629. [Google Scholar] [CrossRef]
- Lee, S.; Go, N.; Ryu, J.H.; Mun, J. Multidimensional Conducting Agents for a High-Energy-Density Anode with SiO for Lithium-Ion Batteries. J. Electrochem. Sci. Technol. 2019, 10, 244–249. [Google Scholar] [CrossRef]
- Sun, M.F.; Ma, J.J.; Xu, M.H.; Yang, H.X.; Zhang, J.Z.; Wang, C.H. A Low-Cost SiOx/C@Graphite Composite Derived from Oat Husk as an Advanced Anode for High-Performance Lithium-lon Batteries. Acs Omega 2022, 7, 15123–15131. [Google Scholar] [CrossRef]

| Materials | Si | SiO | SiO2 |
|---|---|---|---|
| Capacity (mAh g−1) | 4200 [34] | 2600 [35] | 1965 [36] |
| Coulombic Efficiency (%) | 100 [37] | 69 [38] | 52 [39] |
| Charge–Discharge Rate | High | Medium | Low |
| Volume Expansion (%) | 300 | 150 | 100 |
| Safety [40] | Low | Medium | High |
| Cost [41] | High | Medium | Low |
| Anode Material | Current Density/Cycles/Cycle Stability | Initial Coulombic Efficiency | Capacity Retention | References |
|---|---|---|---|---|
| Boron-doped SiO | 0.5 C/100/947 mAh g−1 | 65.1% | 90% | [45] |
| LixSi/Li2O | 0.5C/400/961 mAh g−1 | 77.7% | 99.87% | [44] |
| SiO/Fe2O3 | 160 mA/g/50/133 mAh g−1 | 68% | 71% | [47] |
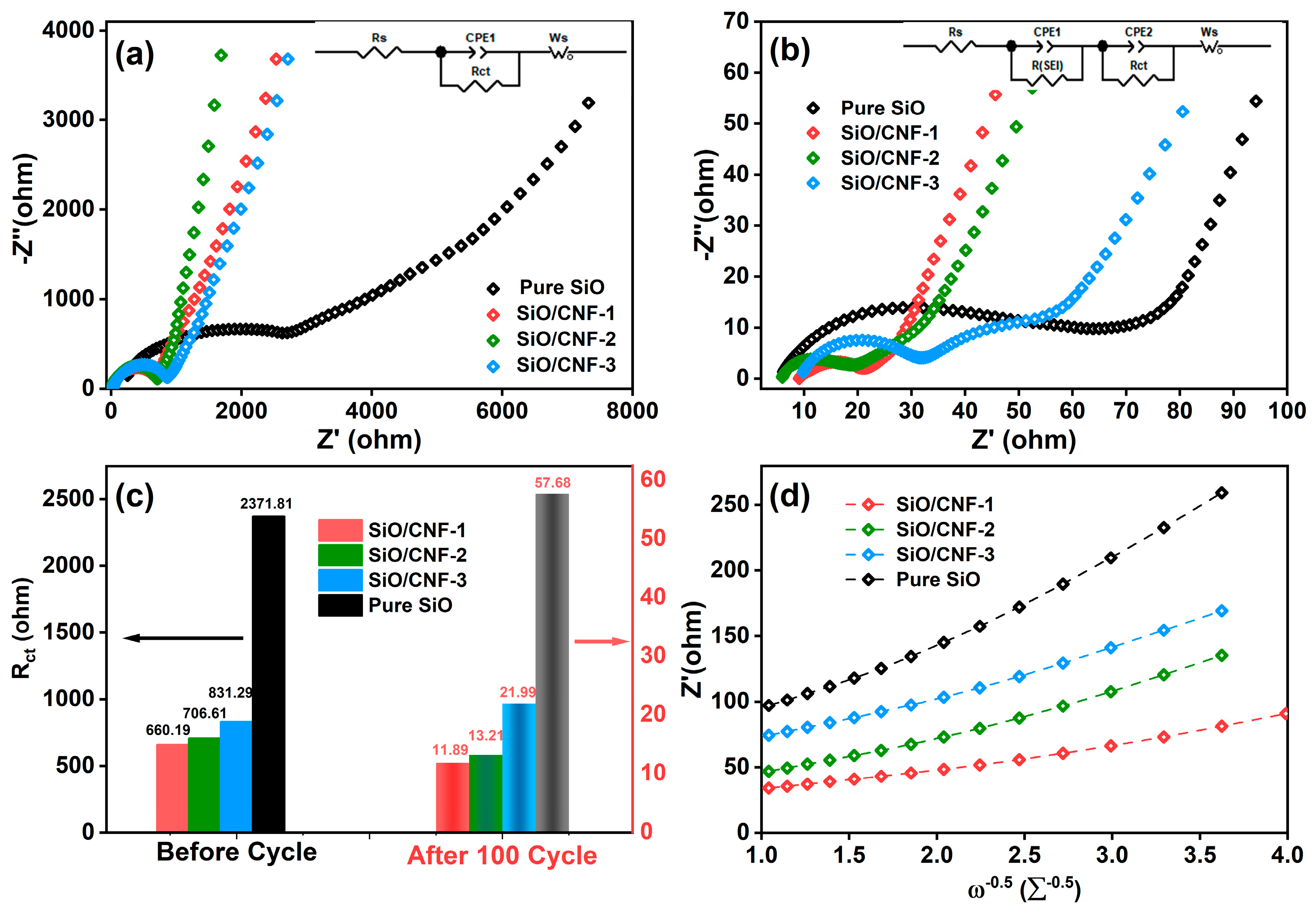
| Samples | Before Cycling | After Cycling | σω (Ω s−0.5) | D (cm2s−1) | ||
|---|---|---|---|---|---|---|
| Rs (Ω) | Rct (Ω) | Rs (Ω) | Rct (Ω) | |||
| SiO/CNF-1 | 20.01 | 660.19 | 9.10 | 11.89 | 18.35 | 8.30 × 10−15 |
| SiO/CNF-2 | 5.59 | 706.61 | 5.95 | 13.21 | 32.42 | 2.66 × 10−15 |
| SiO/CNF-3 | 29.71 | 831.29 | 9.86 | 21.99 | 35.00 | 2.28 × 10−15 |
| SiO | 252.20 | 2371.80 | 6.15 | 57.68 | 62.08 | 0.73 × 10−15 |
| Materials | Charge Capacity (mA h g−1) | ICE(%) | Current Density | Cycling Performance (mA h g−1) | Capacity Retention Rate (%) | Ref. |
|---|---|---|---|---|---|---|
| Bm-SiO/Ni/rGO | 1636.4 | 62.4 | 0.1 A g−1 | 720.3 (100 cycles) | 70.5 | [72] |
| SiO/Fe2O3 | 1893 | 68 | 4.8 A g−1 | ~1335 (50 cycles) | 71 | [46] |
| SiO-C | 1491 | 64.81 | 0.1 C | 1000 (100 cycles) | 67.2 | [73] |
| 7LTO3SiO | 621.3 | 94.82 | 1 A g−1 | 228.7 (100 cycles) | 36.8 | [74] |
| VGCF/Super P | 600.6 | 87.9 | 0.2 C | 403 (40 cycles) | 67.1 | [75] |
| SiOx/C@Graphite | 591.2 | 40.16 | 1 A g−1 | 374.5 (100 cycles) | 63.3 | [76] |
| SiO/CNF | 516.5 | 50.9 | 0.1 A g−1 | 381.7 (400 cycles) | 73.9 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Zhang, C.; Huang, X.; Cao, L.; Wang, A.; Dai, W.; Li, D.; Dai, Y.; Zhou, C.; Zhang, Y.; et al. A Hybrid Structure to Improve Electrochemical Performance of SiO Anode Materials in Lithium-Ion Battery. Nanomaterials 2024, 14, 1223. https://doi.org/10.3390/nano14141223
Yu J, Zhang C, Huang X, Cao L, Wang A, Dai W, Li D, Dai Y, Zhou C, Zhang Y, et al. A Hybrid Structure to Improve Electrochemical Performance of SiO Anode Materials in Lithium-Ion Battery. Nanomaterials. 2024; 14(14):1223. https://doi.org/10.3390/nano14141223
Chicago/Turabian StyleYu, Jian, Chaoran Zhang, Xiaolu Huang, Leifeng Cao, Aiwu Wang, Wanjun Dai, Dikai Li, Yanmeng Dai, Cangtao Zhou, Yaozhong Zhang, and et al. 2024. "A Hybrid Structure to Improve Electrochemical Performance of SiO Anode Materials in Lithium-Ion Battery" Nanomaterials 14, no. 14: 1223. https://doi.org/10.3390/nano14141223
APA StyleYu, J., Zhang, C., Huang, X., Cao, L., Wang, A., Dai, W., Li, D., Dai, Y., Zhou, C., Zhang, Y., & Zhang, Y. (2024). A Hybrid Structure to Improve Electrochemical Performance of SiO Anode Materials in Lithium-Ion Battery. Nanomaterials, 14(14), 1223. https://doi.org/10.3390/nano14141223







