Ligand Engineering of Inorganic Lead Halide Perovskite Quantum Dots toward High and Stable Photoluminescence
Abstract
1. Introduction
2. The Basic Structure and Instability of CsPbX3 PQDs
2.1. Crystal Structure
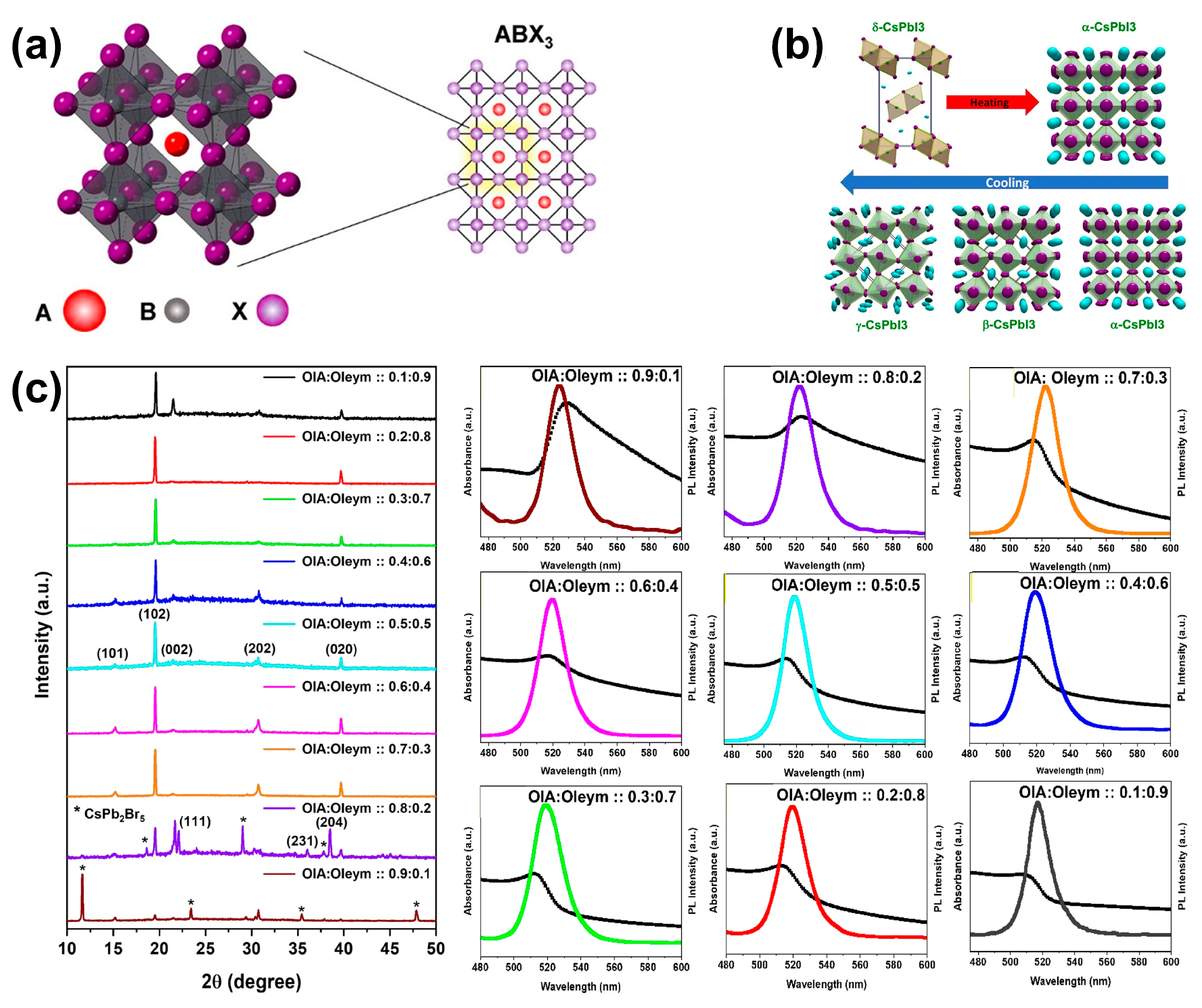
2.2. Ligand
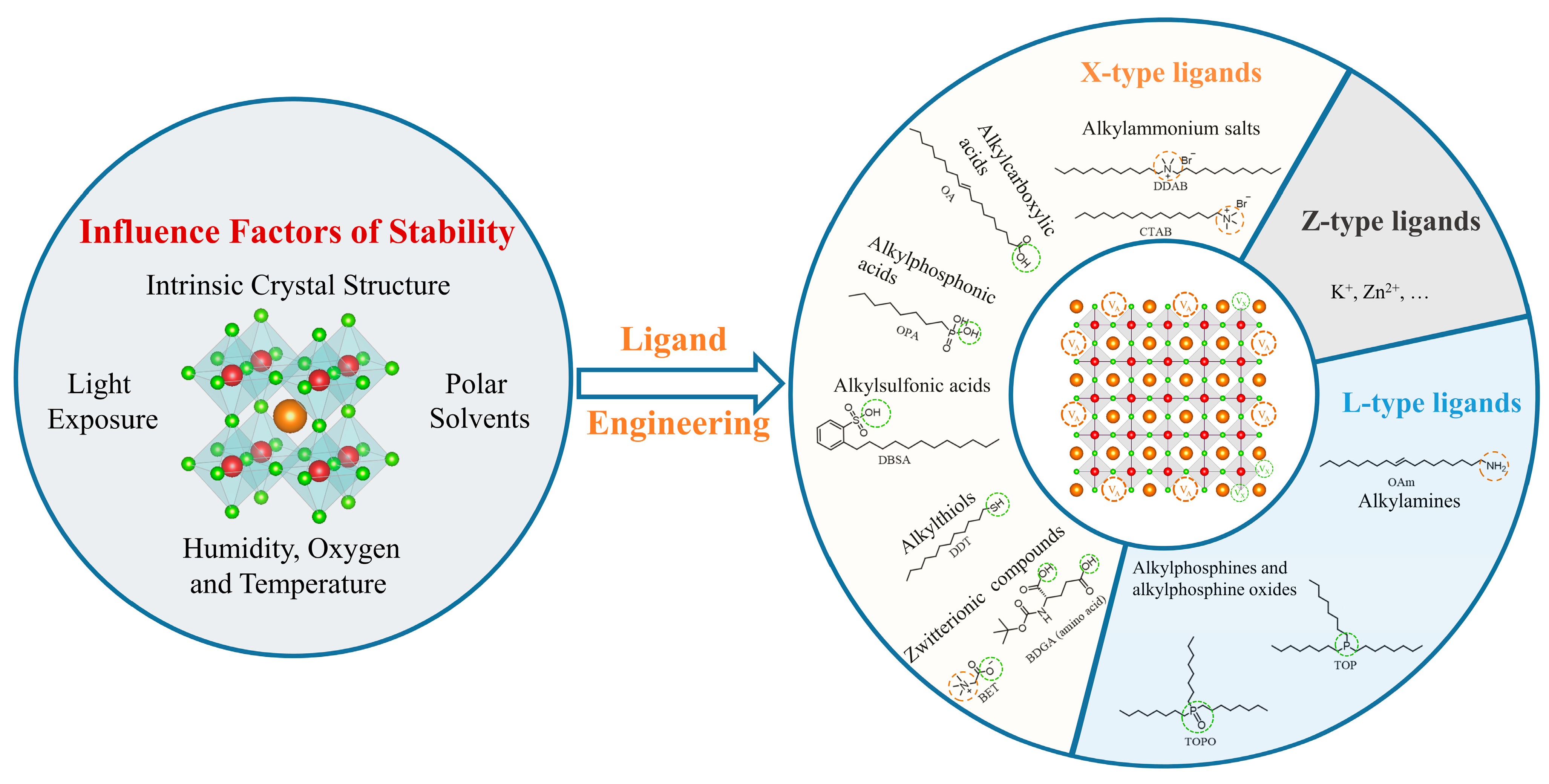
2.3. Influence Factors of Stability
2.3.1. Intrinsic Crystal Structure
2.3.2. Effects of Humidity, Oxygen, and Temperature
2.3.3. Effects of Light Exposure
2.3.4. Polar Solvents
3. Ligand Engineering
3.1. Synthesis Methods
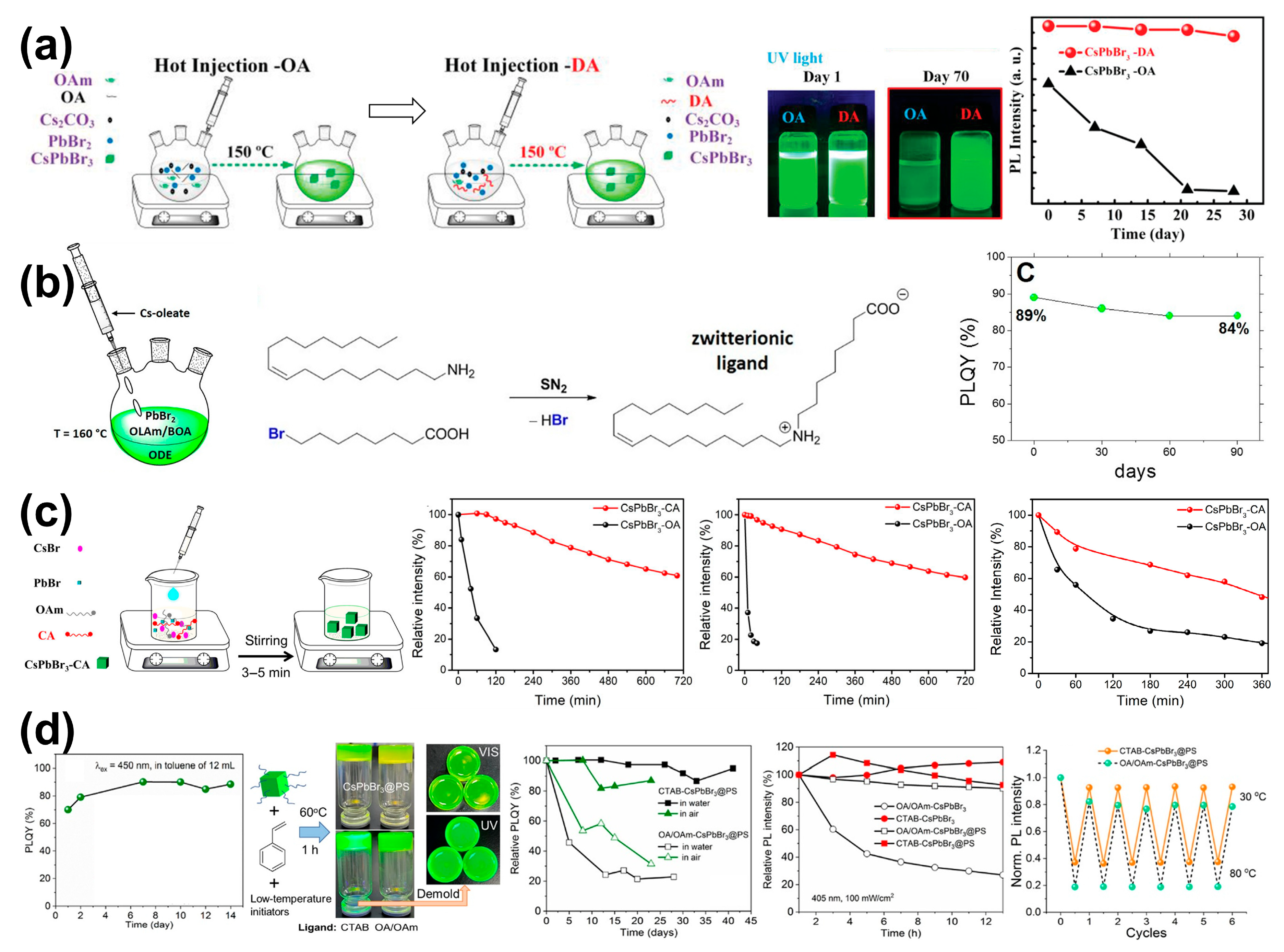
3.1.1. In Situ Ligand Engineering
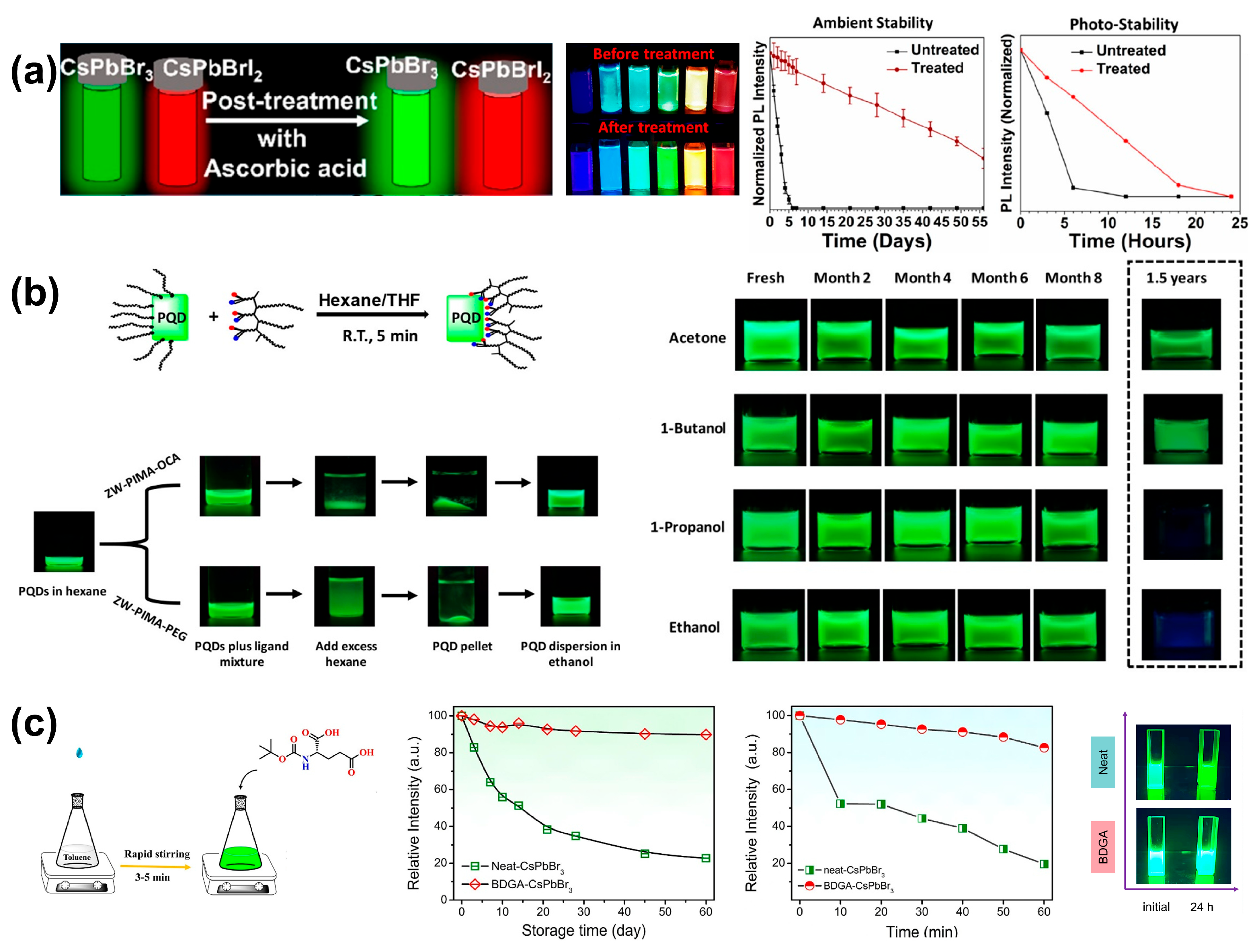
3.1.2. Post-Synthesis Ligand Engineering
3.2. Classification of Ligands
3.3. X-Type Ligands
3.3.1. Alkylammonium Salts
3.3.2. Alkylcarboxylic Acids
3.3.3. Alkylphosphonic Acids
3.3.4. Alkylsulfonic Acids
3.3.5. Alkylthiols
3.3.6. Zwitterionic Compounds
3.4. L-Type Ligands
3.4.1. Alkylamines
3.4.2. Alkylphosphines and Alkylphosphine Oxides
3.5. Z-Type Ligands
4. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Veldhuis, S.A.; Ng, Y.F.; Ahmad, R.; Bruno, A.; Jamaludin, N.F.; Damodaran, B.; Mathews, N.; Mhaisalkar, S.G. Crown ethers enable room-temperature synthesis of CsPbBr3 quantum dots for light-emitting diodes. ACS Energy Lett. 2018, 3, 526–531. [Google Scholar] [CrossRef]
- Dutt, V.G.V.; Akhil, S.; Singh, R.; Palabathuni, M.; Mishra, N. Year-long stability and near-unity photoluminescence quantum yield of CsPbBr3 perovskite nanocrystals by benzoic acid post-treatment. J. Phys. Chem. C 2022, 126, 9502–9508. [Google Scholar] [CrossRef]
- Shi, Y.; Yuan, L.; Liu, Z.; Lu, Y.; Yuan, B.; Shen, W.; Xue, B.; Zhang, Y.; Qian, Y.; Li, F.; et al. In situ growth of strained matrix on CsPbI3 perovskite quantum dots for balanced conductivity and stability. ACS Nano 2022, 16, 10534–10544. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Cohen-Kleinstein, B.; Zhang, X.; Zhao, C.; Zhang, Y.; Ling, X.; Guo, J.; Ko, D.-H.; Xu, B.; Yuan, J.; et al. In situ iodide passivation toward efficient CsPbI3 perovskite quantum dot solar cells. Nanomicro Lett. 2023, 15, 163. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Weng, K.; Lu, S.; Li, F.; Abudukeremu, H.; Zhang, L.; Yang, Y.; Hou, J.; Qiu, H.; Fu, Z.; et al. Direct optical patterning of perovskite nanocrystals with ligand cross-linkers. Sci. Adv. 2022, 8, eabm8433. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Weng, K.; Zhao, H.; Wang, S.; Qiu, H.; Luo, X.; Lu, S.; Duan, L.; Bai, S.; Zhang, H.; et al. Nondestructive direct optical patterning of perovskite nanocrystals with carbene-based ligand cross-linkers. ACS Nano 2024, 18, 6896–6907. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Seo, K.; Lee, S.; Kim, K.; Kim, C.; Lee, J. All-in-one process for color tuning and patterning of perovskite quantum dot light-emitting diodes. Adv. Sci. 2022, 9, e2200073. [Google Scholar] [CrossRef] [PubMed]
- Azar, M.H.; Mohammadi, M.; Rezaei, N.T.; Aynehband, S.; Simchi, A. Effect of silica encapsulation on the stability and photoluminescence emission of FAPbI3 nanocrystals for white-light-emitting perovskite diodes. J. Alloys Compd. 2022, 907, 164465. [Google Scholar] [CrossRef]
- Cao, M.; Xu, Y.; Li, P.; Zhong, Q.; Yang, D.; Zhang, Q. Recent advances and perspectives on light emitting diodes fabricated from halide metal perovskite nanocrystals. J. Mater. Chem. C 2019, 7, 14412–14440. [Google Scholar] [CrossRef]
- Yang, D.; Li, X.; Wu, Y.; Wei, C.; Qin, Z.; Zhang, C.; Sun, Z.; Li, Y.; Wang, Y.; Zeng, H. Surface halogen compensation for robust performance enhancements of CsPbX3 perovskite quantum dots. Adv. Opt. Mater. 2019, 7, 1900276. [Google Scholar] [CrossRef]
- Du, X.; Wu, G.; Cheng, J.; Dang, H.; Ma, K.; Zhang, Y.-W.; Tan, P.-F.; Chen, S. High-quality CsPbBr3 perovskite nanocrystals for quantum dot light-emitting diodes. RSC Adv. 2017, 7, 10391–10396. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum dot light-emitting diodes based on inorganic perovskite cesium lead halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, A.; Marshall, A.R.; Sanehira, E.M.; Chernomordik, B.D.; Moore, D.T.; Christians, J.A.; Chakrabarti, T.; Luther, J.M. Quantum dot-induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Best Research-Cell Efficiencies (NREL). Available online: https://www.nrel.gov/pv/assets/images/cell-pv-eff-emergingpv.jpg (accessed on 14 June 2024).
- Wang, Y.; Lv, Z.; Liao, Q.; Shan, H.; Chen, J.; Zhou, Y.; Zhou, L.; Chen, X.; Roy, V.A.L.; Wang, Z.; et al. Synergies of electrochemical metallization and valance change in all-inorganic perovskite quantum dots for resistive switching. Adv. Mater. 2018, 30, e1800327. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hight-Huf, N.; Kang, J.; Bisnoff, P.; Sundararajan, S.; Thompson, T.; Barnes, M.; Hayward, R.C.; Emrick, T. Polymer zwitterions for stabilization of CsPbBr3 perovskite nanoparticles and nanocomposite films. Angew. Chem. Int. Ed. 2020, 59, 10802–10806. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Wang, X.; Yang, D. Inorganic lead-based halide perovskites: From fundamental properties to photovoltaic applications. Mater. Today 2022, 61, 191–217. [Google Scholar] [CrossRef]
- Yang, X.; Wu, A.; Deng, Z.; Wu, Z.; Zhao, Z.; Hu, Z. The efficient green light-emitting diodes based on low-toxicity Zr-Pb alloy perovskite quantum dots passivated by inorganic ligand. Appl. Mater. Today 2022, 29, 101658. [Google Scholar] [CrossRef]
- Travis, W.; Glover, E.N.K.; Bronstein, H.; Scanlon, D.O.; Palgrave, R.G. On the application of the tolerance factor to inorganic and hybrid halide perovskites: A revised system. Chem. Sci. 2016, 7, 4548–4556. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Kumar, S.; Bär, J.; Bertolotti, F.; Masciocchi, N.; Guagliardi, A.; Grotevent, M.; Shorubalko, I.; Bodnarchuk, M.I.; et al. Dismantling the “red wall” of colloidal perovskites: Highly luminescent formamidinium and formamidinium-cesium lead iodide nanocrystals. ACS Nano 2017, 11, 3119–3134. [Google Scholar] [CrossRef] [PubMed]
- Dunlap-Shohl, W.A.; Zhou, Y.; Padture, N.P.; Mitzi, D.B. Synthetic approaches for halide perovskite thin films. Chem. Rev. 2019, 119, 3193–3295. [Google Scholar] [CrossRef] [PubMed]
- Marronnier, A.; Roma, G.; Boyer-Richard, S.; Pedesseau, L.; Jancu, J.-M.; Bonnassieux, Y.; Katan, C.; Stoumpos, C.C.; Kanatzidis, M.G.; Even, J. Anharmonicity and disorder in the black phases of cesium lead iodide used for stable inorganic perovskite solar cells. ACS Nano 2018, 12, 3477–3486. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Kushwaha, A.K. Capping ligands controlled structural and optoelectronic properties of CsPbBr3 nanocrystals. J. Mater. Sci. Mater. Electron. 2022, 33, 17404–17416. [Google Scholar] [CrossRef]
- Aminzare, M.; Jiang, J.; Mandl, G.A.; Mahshid, S.; Capobianco, J.A.; Courchesne, N.-M.D. Dorval Courchesne. Biomolecules incorporated in halide perovskite nanocrystals: Synthesis, optical properties, and applica-tions. Nanoscale 2023, 15, 2997–3031. [Google Scholar] [CrossRef]
- Wheeler, L.M.; Sanehira, E.M.; Marshall, A.R.; Schulz, P.; Suri, M.; Anderson, N.C.; Christians, J.A.; Nordlund, D.; Sokaras, D.; Kroll, T.; et al. Targeted ligand-exchange chemistry on cesium lead halide perovskite quantum dots for high-efficiency photovoltaics. J. Am. Chem. Soc. 2018, 140, 10504–10513. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, J.; Song, Y.; Chen, F. Enhance luminescence or change morphology: Effect of the doping method on Cu2+-doped CsPbBr3 perovskite nanocrystals. CrystEngComm 2022, 24, 7962–7970. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.; Cao, K.; Wen, Y.; Chen, R.; Shan, B. First-principles study of electronic properties of amine ligand-capped CsPbBr3 surface with organo-metallic alumina precursor treatment. Appl. Surf. Sci. 2022, 600, 154070. [Google Scholar] [CrossRef]
- Wei, Y.; Li, X.; Chen, Y.; Cheng, Z.; Xiao, H.; Li, X.; Ding, J.; Lin, J. In situ light-initiated ligands cross-linking enables efficient all-solution-processed perovskite light-emitting diodes. J. Phys. Chem. Lett. 2020, 11, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Kim, Y.; Wolf, C.; Lee, H.; Lee, T. Improving the stability of metal halide perovskite materials and light-emitting diodes. Adv. Mater. 2018, 30, e1704587. [Google Scholar] [CrossRef] [PubMed]
- Kachhap, S.; Singh, S.; Singh, A.K.; Singh, S.K. Lanthanide-doped inorganic halide perovskites (CsPbX3): Novel properties and emerging applications. J. Mater. Chem. C 2022, 10, 3647–3676. [Google Scholar] [CrossRef]
- Bari, M.; Bokov, A.A.; Leach, G.W.; Ye, Z.-G. Ferroelastic domains and effects of spontaneous strain in lead halide perovskite CsPbBr3. Chem. Mater. 2023, 35, 6659–6670. [Google Scholar] [CrossRef]
- Lee, S.M.; Moon, C.J.; Lim, H.; Lee, Y.; Choi, M.Y.; Bang, J. Temperature-dependent photoluminescence of cesium lead halide perovskite quantum dots: Splitting of the photoluminescence peaks of CsPbBr3 and CsPb(Br/I)3 quantum dots at low temperature. J. Phys. Chem. C 2017, 121, 26054–26062. [Google Scholar] [CrossRef]
- Huang, S.; Li, Z.; Wang, B.; Zhu, N.; Zhang, C.; Kong, L.; Zhang, Q.; Shan, A.; Li, L. Morphology evolution and degradation of CsPbBr3 nanocrystals under blue light-emitting diode illumination. ACS Appl. Mater. Interfaces 2017, 9, 7249–7258. [Google Scholar] [CrossRef] [PubMed]
- Diroll, B.T.; Nedelcu, G.; Kovalenko, M.V.; Schaller, R.D. High-temperature photoluminescence of CsPbX3 (X = Cl, Br, I) nanocrystals. Adv. Funct. Mater. 2017, 27, 1606750. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Gao, X. Effect of structure change on luminescent properties of CsPbBr2I perovskite nanocrystals after heat treatment. Aust. J. Chem. 2019, 72, 663–668. [Google Scholar] [CrossRef]
- Seth, S.; Mondal, N.; Patra, S.; Samanta, A. Fluorescence blinking and photoactivation of all-inorganic perovskite nanocrystals CsPbBr3 and CsPbBr2I. J. Phys. Chem. Lett. 2016, 7, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Moyen, E.; Kanwat, A.; Cho, S.; Jun, H.; Aad, R.; Jang, J. Ligand removal and photo-activation of CsPbBr3 quantum dots for enhanced optoelectronic devices. Nanoscale 2018, 10, 8591–8599. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, H.; Zhu, K.; Ye, W.; She, L.; Gao, X.; Ji, W.; Zeng, Q. Research on the influence of polar solvents on CsPbBr3 perovskite QDs. RSC Adv. 2021, 11, 27333–27337. [Google Scholar] [CrossRef] [PubMed]
- Sanjayan, C.G.; Jyothi, M.S.; Balakrishna, R.G. Stabilization of CsPbBr3 quantum dots for photocatalysis, imaging and optical sensing in water and biological medium: A review. J. Mater. Chem. C 2022, 10, 6935–6956. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, Z.; Lin, J. An overview on enhancing the stability of lead halide perovskite quantum dots and their applications in phosphor-converted LEDs. Chem. Soc. Rev. 2019, 48, 310–350. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shen, X.; Tang, C.; Li, X.; Hu, J.; Zhu, J.; Yu, W.W.; Song, H.; Bai, X. Efficient and stable CF3PEAI-passivated CsPbI3 QDs toward red LEDs. ACS Appl. Mater. Interfaces 2022, 14, 8235–8242. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.C.; Lee, S.; Song, J.K.; Yang, H.; Do, Y.R. Efficient and stable CsPbBr3 quantum-dot powders passivated and encapsulated with a mixed silicon nitride and silicon oxide inorganic polymer matrix. ACS Appl. Mater. Interfaces 2018, 10, 11756–11767. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Hossain, M.; Samanta, A. Stable and intense violet-emitting CsPbCl3 nanocrystals for light-emitting diodes: Directly obtained by L-type surface passivation. ACS Appl. Nano Mater. 2023, 6, 4812–4820. [Google Scholar] [CrossRef]
- Lv, W.; Li, L.; Xu, M.; Hong, J.; Tang, X.; Xu, L.; Wu, Y.; Zhu, R.; Chen, R.; Huang, W. Improving the stability of metal halide perovskite quantum dots by encapsulation. Adv. Mater. 2019, 31, e1900682. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhang, H.; Hu, M.; Guo, Y.; Yang, L. Highly crystalline CsPbBr3 perovskite nanoparticles for liquid crystal displays. ACS Appl. Nano Mater. 2023, 6, 3974–3980. [Google Scholar] [CrossRef]
- Morad, V.; Stelmakh, A.; Svyrydenko, M.; Feld, L.G.; Boehme, S.C.; Aebli, M.; Affolter, J.; Kaul, C.J.; Schrenker, N.J.; Bals, S.; et al. Designer phospholipid capping ligands for soft metal halide nanocrystals. Nature 2024, 626, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, M.; Wang, J.; Wang, Y.; Wang, Y.; Liu, W. Stability and spectroscopic analysis of CsPbBr3 quantum dots modified with 2-n-octyl-1-dodecanol. Anal. Methods 2023, 15, 3318–3324. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Shi, T.; Zang, Z.; Zhou, T.; Liu, Z.; Zhang, Z.; Du, J.; Leng, Y.; Tang, X. Ultrastable CsPbBr3 perovskite quantum dot and their enhanced amplified spontaneous emission by surface ligand modification. Small 2019, 15, e1901173. [Google Scholar] [CrossRef] [PubMed]
- Grisorio, R.; Fasulo, F.; Muñoz-García, A.B.; Pavone, M.; Conelli, D.; Fanizza, E.; Striccoli, M.; Allegretta, I.; Terzano, R.; Margiotta, N.; et al. In situ formation of zwitterionic ligands: Changing the passivation paradigms of CsPbBr3 nanocrystals. Nano Lett. 2022, 22, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, G.; She, C.; Liu, S.; Yue, F.; Jing, C.; Cheng, Y.; Chu, J. Room temperature preparation of highly stable cesium lead halide perovskite nanocrystals by ligand modification for white light-emitting diodes. Nano Res. 2021, 14, 2770–2775. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, P.; Bai, W.; Lu, L.; Wang, L.; Chen, X.; Xie, R.-J. Synthesizing bright CsPbBr3 perovskite nanocrystals with high purification yields and their composites with in situ-polymerized styrene for light-emitting diode applications. ACS Sustain. Chem. Eng. 2022, 10, 7385–7393. [Google Scholar] [CrossRef]
- Dutt, V.G.V.; Akhil, S.; Singh, R.; Palabathuni, M.; Mishra, N. High-quality CsPbX3 (X = Cl, Br, or I) perovskite nanocrystals using ascorbic acid post-treatment: Implications for light-emitting applications. ACS Appl. Nano Mater. 2022, 5, 5972–5982. [Google Scholar] [CrossRef]
- Wang, S.; Du, L.; Jin, Z.; Xin, Y.; Mattoussi, H. Enhanced stabilization and easy phase transfer of CsPbBr3 perovskite quantum dots promoted by high-affinity polyzwitterionic ligands. J. Am. Chem. Soc. 2020, 142, 12669–12680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, G.; Hou, G.; Lin, J.; Chen, M.; Liu, S.; Lin, H.; Fang, J.; Jing, C.; Chu, J. Multidentate ligand passivation enabled enhanced photoluminescence and stability of CsPbBr3 nanocrystals for white light-emitting diodes. Chem. Eng. J. 2022, 438, 135270. [Google Scholar] [CrossRef]
- Green, M.L.H. A new approach to the formal classification of covalent compounds of the elements. J. Organomet. Chem. 1995, 500, 127–148. [Google Scholar] [CrossRef]
- Bai, Y.; Hao, M.; Ding, S.; Chen, P.; Wang, L. Surface chemistry engineering of perovskite quantum dots: Strategies, applications, and perspectives. Adv. Mater. 2022, 34, e2105958. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, A.-Y.; Yu, J.C.; Nam, Y.S.; Choi, Y.; Park, J.; Song, M.H. Surface ligand engineering for efficient perovskite nanocrystal-based light-emitting diodes. ACS Appl. Mater. Interfaces 2019, 11, 8428–8435. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.-J.; Shin, M.; Park, M.; Lee, J.-S. In situ tetraalkylammonium ligand engineering of organic–inorganic hybrid perovskite nanoparticles for enhancing long-term stability and optical tunability. Langmuir 2022, 38, 13448–13455. [Google Scholar] [CrossRef] [PubMed]
- Moyen, E.; Jun, H.; Kim, H.-M.; Jang, J. Surface engineering of room temperature-grown inorganic perovskite quantum dots for highly efficient inverted light-emitting diodes. ACS Appl. Mater. Interfaces 2018, 10, 42647–42656. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Li, Z.; Zhang, C.; Wang, B.; Zhang, Q.; Wan, Q.; Kong, L.; Li, L. Stabilizing perovskite nanocrystals by controlling protective surface ligands density. Nano Res. 2019, 12, 1461–1465. [Google Scholar] [CrossRef]
- Gualdrón-Reyes, A.F.; Fernández-Climent, R.; Masi, S.; Mesa, C.A.; Echeverría-Arrondo, C.; Aiello, F.; Balzano, F.; Uccello-Barretta, G.; Rodríguez-Pereira, J.; Giménez, S.; et al. Efficient ligand passivation enables ultrastable CsPbX3 perovskite nanocrystals in fully alcohol environments. Adv. Opt. Mater. 2023, 11, 2203096. [Google Scholar] [CrossRef]
- Zheng, C.; Bi, C.; Huang, F.; Binks, D.J.; Tian, J. Stable and strong emission CsPbBr3 quantum dots by surface engineering for high-performance optoelectronic films. ACS Appl. Mater. Interfaces 2019, 11, 25410–25416. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Tang, X.; Wangyang, P.; Yao, Z.; Zhou, D.; Chen, F.; Li, S.; Lin, H.; Zeng, F.; Wu, D.; et al. Surface-passivated cesium lead halide perovskite quantum dots: Toward efficient light-emitting diodes with an inverted sandwich structure. Adv. Opt. Mater. 2018, 6, 1800007. [Google Scholar] [CrossRef]
- Sasaki, M.; Hashimoto, S.; Iso, Y.; Oaki, Y.; Isobe, T.; Imai, H. Enhanced and stabilized photoluminescence of perovskite cesium lead bromide nanocubes through ordered assemblies. Nanoscale Adv. 2023, 5, 2553–2557. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Quan, L.N.; Zhao, Y.; Peng, W.; Murali, B.; Sarmah, S.P.; Yuan, M.; Sinatra, L.; Alyami, N.M.; Liu, J.; et al. Highly efficient perovskite-quantum-dot light-emitting diodes by surface engineering. Adv. Mater. 2016, 28, 8718–8725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hou, G.; Wu, Y.; Chen, M.; Dai, Y.; Liu, S.; Zhao, Q.; Lin, H.; Fang, J.; Jing, C.; et al. Surface reconstruction of CsPbBr3 nanocrystals by the ligand engineering approach for achieving high quantum yield and improved stability. Langmuir 2023, 39, 6222–6230. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wan, Q.; Wang, H.; Carulli, F.; Sun, X.; Zheng, W.; Kong, L.; Zhang, Q.; Zhang, C.; Zhang, Q.; et al. Suppression of temperature quenching in perovskite nanocrystals for efficient and thermally stable light-emitting diodes. Nat. Photonics 2021, 15, 379–385. [Google Scholar] [CrossRef]
- Gao, L.; Cheng, T.; Gou, L.; Zhang, Y.; Liu, Y.; Yuan, L.; Zhang, X.; Wang, Y.; Meng, F.; Zhang, J. Eliminating nanocrystal surface light loss and ion migration to achieve bright mixed-halide blue perovskite LEDs. ACS Appl. Mater. Interfaces 2023, 15, 18125–18133. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, M.; Shen, M.; Cai, Y.; Xie, R.-J. Bidentate aliphatic quaternary ammonium ligand-stabilized CsPbBr3 perovskite nanocrystals with high PLQY (92.3%) and superior stability. J. Mater. Chem. C 2022, 10, 8356–8363. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, R.; Wan, W.; Jing, X.; Wen, T.; Ye, S. Stabilizing CsPbBr3 quantum dots with conjugated aromatic ligands and their regulated optical behaviors. Chem. Eng. J. 2020, 389, 124453. [Google Scholar] [CrossRef]
- Yan, D.; Zhao, S.; Wang, H.; Zang, Z. Ultrapure and highly efficient green light emitting devices based on ligand-modified CsPbBr3 quantum dots. Photonics Res. 2020, 8, 1086–1092. [Google Scholar] [CrossRef]
- Zhu, H.; Pan, Y.; Peng, C.; Lian, H.; Lin, J. 4-Bromo-butyric acid-assisted in situ passivation strategy for superstable all-inorganic halide perovskite CsPbX3 Quantum Dots in Polar Media. Angew. Chem. Int. Ed. 2022, 61, e202116702. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Peng, C.; Li, J.; Cao, X.; Pan, Y. A study on the reversible anion-exchange rate in perovskite CsPbX3 nanocrystals. J. Lumin. 2022, 252, 119321. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, B.; Guo, J.; Guo, J.; Zhao, C.; Zhao, C.; Zhang, X.; Zhang, X.; Huang, H.; Huang, H.; et al. Design and synthesis of fluorinated quantum dots for efficient and stable 0D/3D perovskite solar cells. Adv. Funct. Mater. 2023, 33, 2304161. [Google Scholar] [CrossRef]
- Patra, D.; Singh, S.P. 2,2′-Bipyridine-4,4′-dicarboxylic acid-mediated surface engineering of Mn-doped CsPbCl3 perovskite nanocrystals. J. Phys. Chem. C 2023, 127, 9397–9406. [Google Scholar] [CrossRef]
- Zu, Y.; Dai, J.; Li, L.; Yuan, F.; Chen, X.; Feng, Z.; Li, K.; Song, X.; Yun, F.; Yu, Y.; et al. Ultra-stable CsPbBr3 nanocrystals with near-unity photoluminescence quantum yield via postsynthetic surface engineering. J. Mater. Chem. A 2019, 7, 26116–26122. [Google Scholar] [CrossRef]
- Chen, T.; Yang, Q.; Zhang, P.; Chen, R.; Lin, Y.; Zhou, W.; Sui, L.; Zheng, X.; Chen, G.; Li, F. Stapled ligand for synthesis of highly emissive and stable CsPbBr3 perovskite nanocrystals in polar organic solvent. Inorg. Chem. Front. 2023, 10, 5303–5310. [Google Scholar] [CrossRef]
- Lu, M.; Guo, J.; Sun, S.; Lu, P.; Zhang, X.; Shi, Z.; Yu, W.W.; Zhang, Y. Surface ligand engineering-assisted CsPbI3 quantum dots enable bright and efficient red light-emitting diodes with a top-emitting structure. Chem. Eng. J. 2021, 404, 126563. [Google Scholar] [CrossRef]
- Chen, D.; Xu, K.; Yang, M.; Hu, J.; Li, R.; Huang, D.; Liang, S.; He, K.; Yuan, L.; Wang, S.; et al. Surface chemistry engineering enables polar solvent resistant and highly emissive perovskite nanocrystals for multifunctional applications. Chem. Eng. J. 2023, 471, 144848. [Google Scholar] [CrossRef]
- Ding, S.; Hao, M.; Lin, T.; Bai, Y.; Wang, L. Ligand engineering of perovskite quantum dots for efficient and stable solar cells. J. Energy Chem. 2022, 69, 626–648. [Google Scholar] [CrossRef]
- Yang, D.; Li, X.; Zhou, W.; Zhang, S.; Meng, C.; Wu, Y.; Wang, Y.; Zeng, H. CsPbBr3 quantum dots 2.0: Benzenesulfonic acid equivalent ligand awakens complete purification. Adv. Mater. 2019, 31, e1900767. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Hu, X.; Wei, C.; Xu, B.; Leng, J.; Miao, H.; Zeng, H.; Li, X. Ligands for CsPbBr3 perovskite quantum dots: The stronger the better? Chem. Eng. J. 2023, 453, 139904. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, Y.; Chang, Y.; Guo, X.; Zou, D.; Sun, Y.; Zeng, Q.; Liu, X. Ultra-small-size, highly efficient and stable CsPbBr3 quantum dots synthesized by using a cesium-dodecyl benzene sulfonic acid solution. Chem. Eng. J. 2023, 473, 145213. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Wang, J.; Yang, P. Cubic CsPbI3 nanoarchitectonics with high and stable photoluminescence toward white light-emitting diodes. J. Phys. Chem. C 2023, 127, 3123–3130. [Google Scholar] [CrossRef]
- Baek, S.; Kim, Y.; Kim, S.-W. Highly photo-stable CsPbI3 perovskite quantum dots via thiol ligand exchange and their polymer film application. J. Ind. Eng. Chem. 2020, 83, 279–284. [Google Scholar] [CrossRef]
- Ghorai, A.; Mahato, S.; Singh, S.; Bose, S.; Roy, B.; Jeong, U.; Ray, S.K. Ligand-mediated revival of degraded α-CsPbI3 to stable highly luminescent perovskite. Angew. Chem. Int. Ed. 2023, 62, e202302852. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, Y.; Wang, J.; Ma, J.; Wang, L.; Jiang, W. Ligand-modified synthesis of shape-controllable and highly luminescent CsPbBr3 perovskite nanocrystals under ambient conditions. Inorg. Chem. Front. 2022, 9, 6080–6090. [Google Scholar] [CrossRef]
- Mei, X.; He, K.; Zhuang, R.; Yu, M.; Hua, Y.; Zhang, X. Stabilizing dynamic surface of highly luminescent perovskite quantum dots for light-emitting diodes. Chem. Eng. J. 2023, 453, 139909. [Google Scholar] [CrossRef]
- Lee, A.-Y.; Kim, J.; Lee, D.; Song, M.H. Efficient and stable perovskite nanocrystal light-emitting diodes with sulfobetaine-based ligand treatment. ACS Appl. Electron. Mater. 2023, 5, 5325–5331. [Google Scholar] [CrossRef]
- Mir, W.J.; Alamoudi, A.; Yin, J.; Yorov, K.E.; Maity, P.; Naphade, R.; Shao, B.; Wang, J.; Lintangpradipto, M.N.; Nematulloev, S.; et al. Lecithin capping ligands enable ultrastable perovskite-phase CsPbI3 quantum dots for Rec. 2020 bright-red light-emitting diodes. J. Am. Chem. Soc. 2022, 144, 13302–13310. [Google Scholar] [CrossRef] [PubMed]
- Krieg, F.; Ochsenbein, S.T.; Yakunin, S.; Brinck, S.T.; Aellen, P.; Süess, A.; Clerc, B.; Guggisberg, D.; Nazarenko, O.; Shynkarenko, Y.; et al. Colloidal CsPbX3 (X = Cl, Br, I) nanocrystals 2.0: Zwitterionic capping ligands for improved durability and stability. ACS Energy Lett. 2018, 3, 641–646. [Google Scholar] [CrossRef]
- Jin, H.; Park, G.Y.; Kim, M.K.; Cha, J.; Ham, D.S. Eco-friendly solvent-processible and highly luminescent perovskite nanocrystals with polymer zwitterions for air-stable optoelectronics. Chem. Eng. J. 2023, 459, 141531. [Google Scholar] [CrossRef]
- Aggarwal, P.; Halder, A.; Neelakshi; Ramapanicker, R.; Rao, V.G. Energy funneling from water-dispersed perovskites to chromophores. ACS Energy Lett. 2023, 8, 1520–1528. [Google Scholar] [CrossRef]
- Ahlawat, M.; Neelakshi, R.; Ramapanicker, R.; Rao, V.G. Design principle of a water-dispersed photocatalytic perovskite through ligand deconstruction. ACS Energy Lett. 2023, 8, 2159–2168. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhang, X.; Bing, Q.; Xiong, Y.; Yang, F.; Liu, H.; Liu, J.-Y.; Zhang, H.; Zheng, W.; Rogach, A.L.; et al. Surface stabilization of colloidal perovskite nanocrystals via multi-amine chelating ligands. ACS Energy Lett. 2022, 7, 1963–1970. [Google Scholar] [CrossRef]
- Hu, X.; Xu, Y.; Wang, J.; Ma, J.; Wang, L.; Jiang, W. Scalable synthesis of efficiently luminescent and color-tunable CsPbX3 (X = Cl, Br, I) nanocrystals by regulating the reaction parameters. J. Lumin. 2022, 251, 119191. [Google Scholar] [CrossRef]
- Wang, H.; Sui, N.; Bai, X.; Zhang, Y.; Rice, Q.; Seo, F.J.; Zhang, Q.; Colvin, V.L.; Yu, W.W. Emission recovery and stability enhancement of inorganic perovskite quantum dots. J. Phys. Chem. Lett. 2018, 9, 4166–4173. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Xue, W.; Wang, W.; Zhu, W.; Zhao, L. Highly luminescent and stable CsPbBr3 perovskite quantum dots modified by phosphine ligands. Nano Res. 2019, 12, 785–789. [Google Scholar] [CrossRef]
- Wu, L.; Zhong, Q.; Yang, D.; Chen, M.; Hu, H.; Pan, Q.; Liu, H.; Cao, M.; Xu, Y.; Sun, B.; et al. Improving the stability and size tunability of cesium lead halide perovskite nanocrystals using trioctylphosphine oxide as the capping ligand. Langmuir 2017, 33, 12689–12696. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuan, F.; Dong, Y.; Li, J.; Johnston, A.; Chen, B.; Saidaminov, M.I.; Zhou, C.; Zheng, X.; Hou, Y.; et al. All-inorganic quantum-dot leds based on a phase-stabilized α-CsPbI3 perovskite. Angew. Chem. Int. Ed. 2021, 60, 16164–16170. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Yao, Z.; Hu, J.; Wang, X.; Zhang, M.; Tian, S.; Liu, A.; Lu, Y.; de Leeuw, N.H.; Sui, M.; et al. Suppressing auger recombination of perovskite quantum dots for efficient pure-blue-light-emitting diodes. ACS Energy Lett. 2022, 8, 731–739. [Google Scholar] [CrossRef]


| Type | Ligands | Methods * | PQDs | PLQY | Stability * | Ref. |
|---|---|---|---|---|---|---|
| X | DA | In situ | CsPbBr3 | - | ~94% (F *, in air, 28 d) | [49] |
| X | BOA | In situ | CsPbBr3 | 89% | PLQY: 84% (S *, ambient conditions, 90 d) PLQY: 81% (S, UV, 0.5 h) | [50] |
| X | CA | In situ | CsPbBr3 | 71% | 80% (S, in water, 6 h) 75% (S, in ethanol, 6 h) 50% (S, UV, 6 h) | [51] |
| X | CTAB | In situ | CsPbBr3 | 70% | PLQY: 90% (S, ambient conditions, 7 d) 110% (-, UV, 13 h) | [52] |
| X | AA | Post | CsPbBr3 | 99% | 72% (S, in air, 42 d) 33% (S, UV, 24 h) | [53] |
| X | AA | Post | CsPbI3 | 95% | No phase change (S, in air, 55 d) ~77% (S, UV, 4 h) | [53] |
| X | AA | Post | CsPbBrI2 | 95% | 69% (S, in air, 42 d) 44% (S, UV, 24 h) | [53] |
| X | AA | Post | CsPb(Br/I)3 CsPbCl1.5Br1.5 CsPbClBr2 | >95% 22% 51% | No exact numbers provided | [53] |
| X | ZW-PIMA-PEG | Post | CsPbBr3 | 70–80% | Complete loss of fluorescence (S, in polar solvents,1 month) | [54] |
| X | ZW-PIMA-OCA | Post | CsPbBr3 | 65–75% | No phase change (S, storage, 1.5 years) >85% (S, in acetone or ethanol, 8 months) Strong green fluorescence (P*, in water, 14 d) | [54] |
| X | BDGA | Post | CsPbBr3 | ~100% | ~90% (S, ambient conditions, 60 d) ~95% (S, UV, 24 h) >80% (S, 60 °C, 1 h) No phase change (F, ambient conditions, 120 d) | [55] |
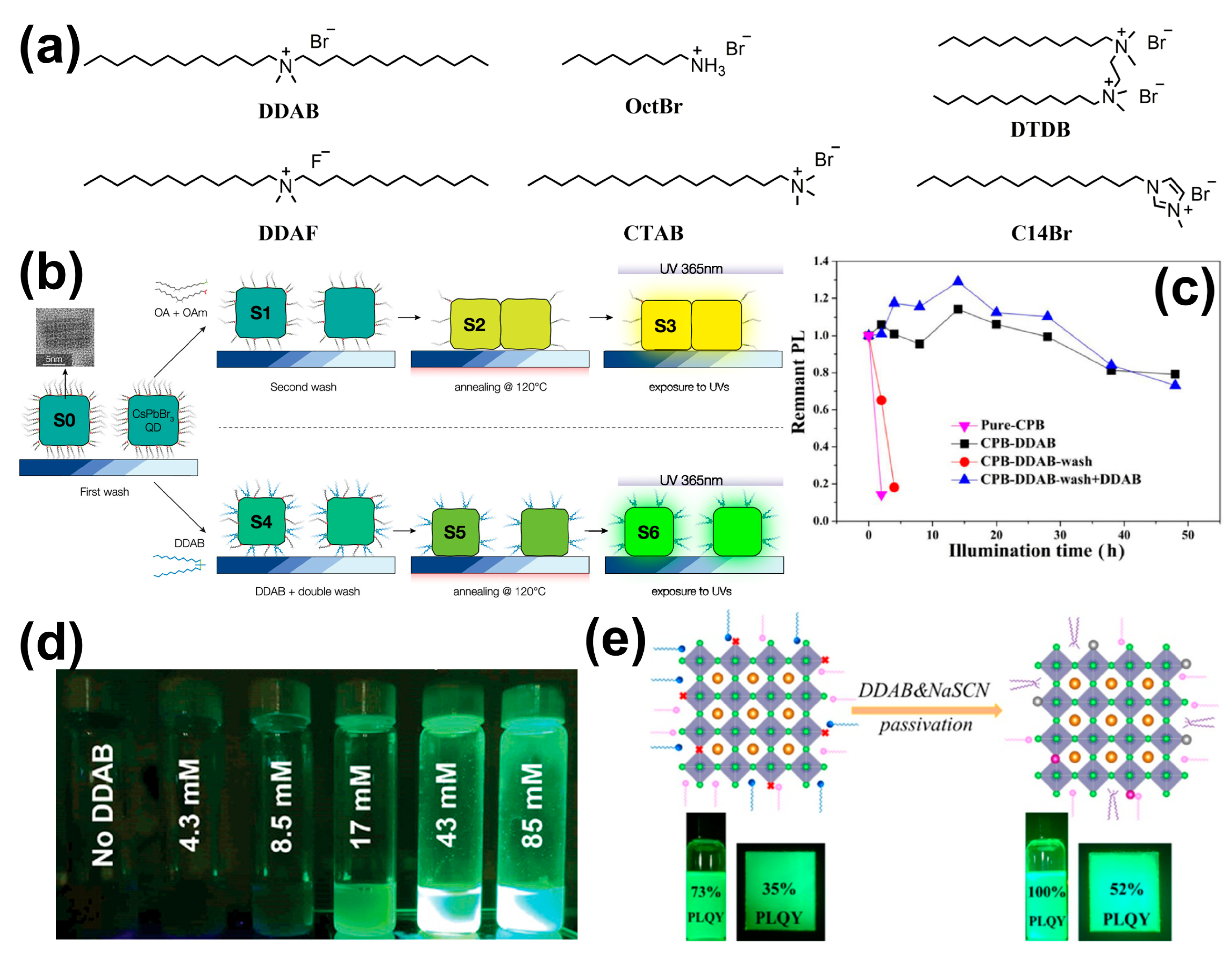
| X | DDAB | Post | CsPbBr3 | ~100% | PL emission is mainly preserved (S, in methanol/butanol, 7 months) | [62] |
| / | DDAB + NaSCN | Post | CsPbBr3 | ~100% (solution) 52% (films) | 100% (-, UV, 1 h) 80% (S, in water, 1.5 h) 60% (F, heat to 200 °C) | [63] |
| / | DDAB + ZnBr2 | Post | CsPbBr3 | 95% | 85% (S, ambient conditions, 14 d) 90% (S, 50 °C, 60 min) 93% (S, UV, 24 h) | [67] |
| X | DDAF | Post | CsPbBr3 | 90% | No obvious change (S, heat to 100 °C) | [68] |
| X | OctBr | Post | CsPbCl1.5Br1.5 | 95% | 80% (F, 380 K) | [69] |
| X | DTDB | In situ | CsPbBr3 | ~92% | 80% (S, UV, 4.5 h) 80% (S, UV, 4.5 h) 95% (S, 80 °C, 4 h) 76% (S, in water, 17 h) | [70] |
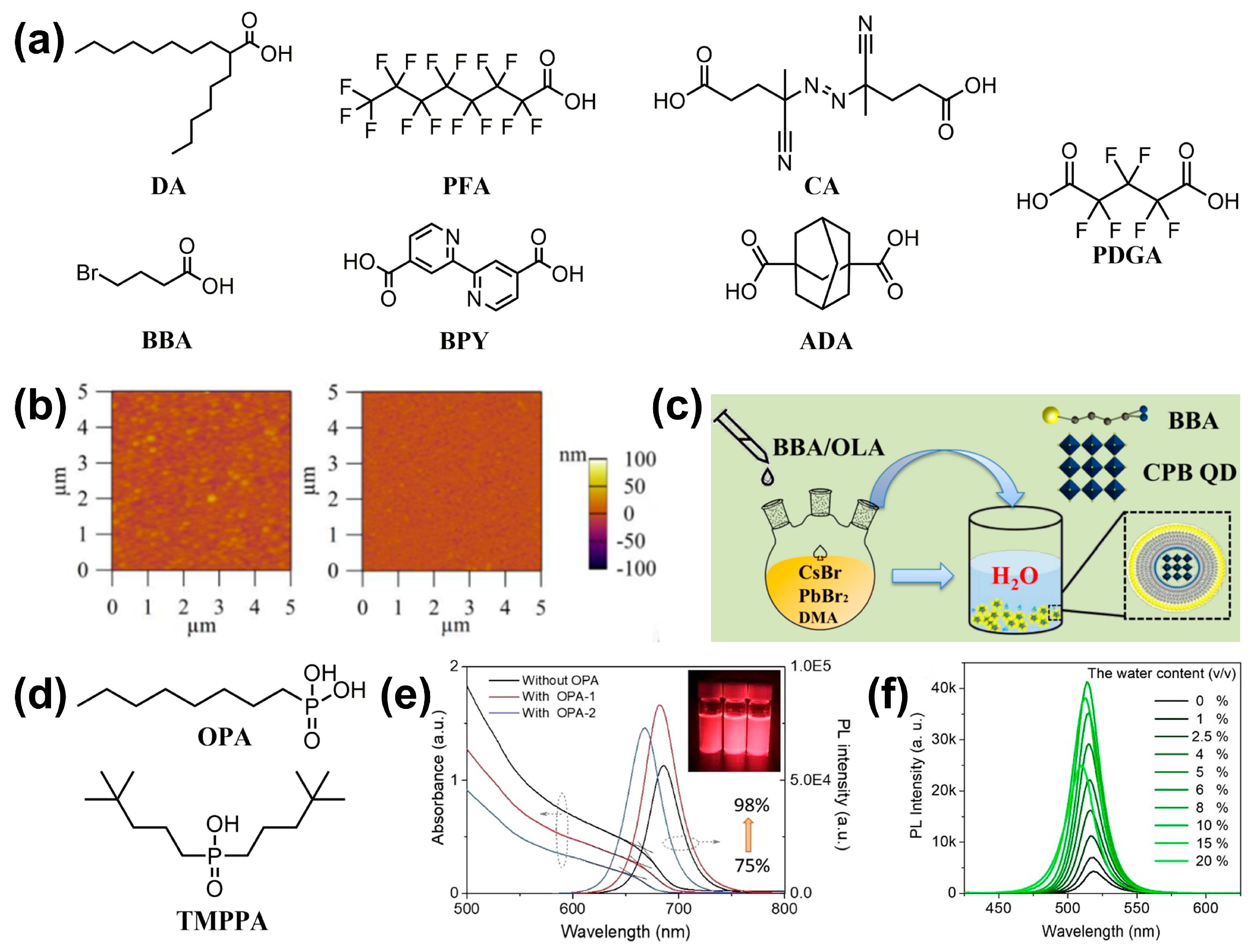
| X | BBA | In situ | CsPbBr3 | ~86% | 79% (S, in water, 72 h) | [73] |
| X | PFA | In situ | CsPbI3 | >80% | 80% (S, ambient conditions, 120 d) | [75] |
| / | ADA + ZnBr2 | Post | CsPbBr3 | ~97% | 93% (S, long-term stability, 65 d) Stronger brightness (P, in water, 15 min) 80% (S, 80 °C, 2 h) | [77] |
| X | PFGA | In situ | CsPbBr3 | 85% | ~100% (S, long-term stability, 60 d) | [78] |
| X | OPA | In situ | CsPbI3 | 98% | 50% (S, in air, 15 d) Better storage stability in nitrogen | [79] |
| X | TMPPA | In situ | CsPbBr3 | ~83% | 86% (S, ambient conditions, 1.5 years) | [80] |
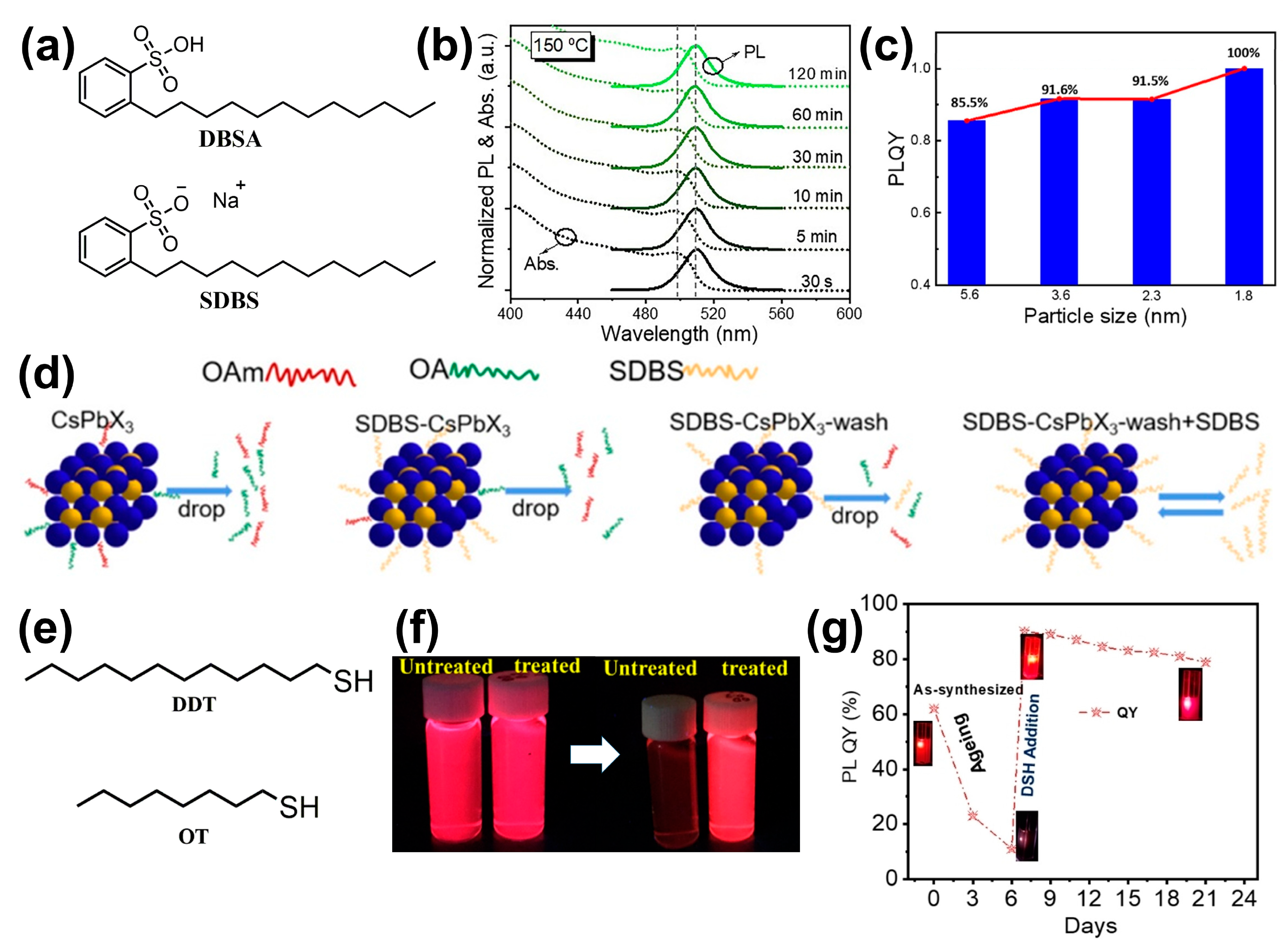
| X | DBSA | In situ | CsPbBr3 | 100% | PLQY: from 91.6% to 90.8% (S, 15 °C, 90 d) ~90% (S, UV, 1 h) | [84] |
| X | SDBS | In situ | CsPbI3 | ~91% | 83% (S, ambient conditions, 60 d) Bright red luminescence (F, in water, 3 h) 72% (S, UV, 3 h) | [85] |
| X | DDT | Post | CsPbI3 | 90% | 90% (S, UV, 3 d) | [86] |
| X | DDT | Post | CsPbI3 | 90% | PLQY: stable (S, ambient conditions, 15 d) | [87] |
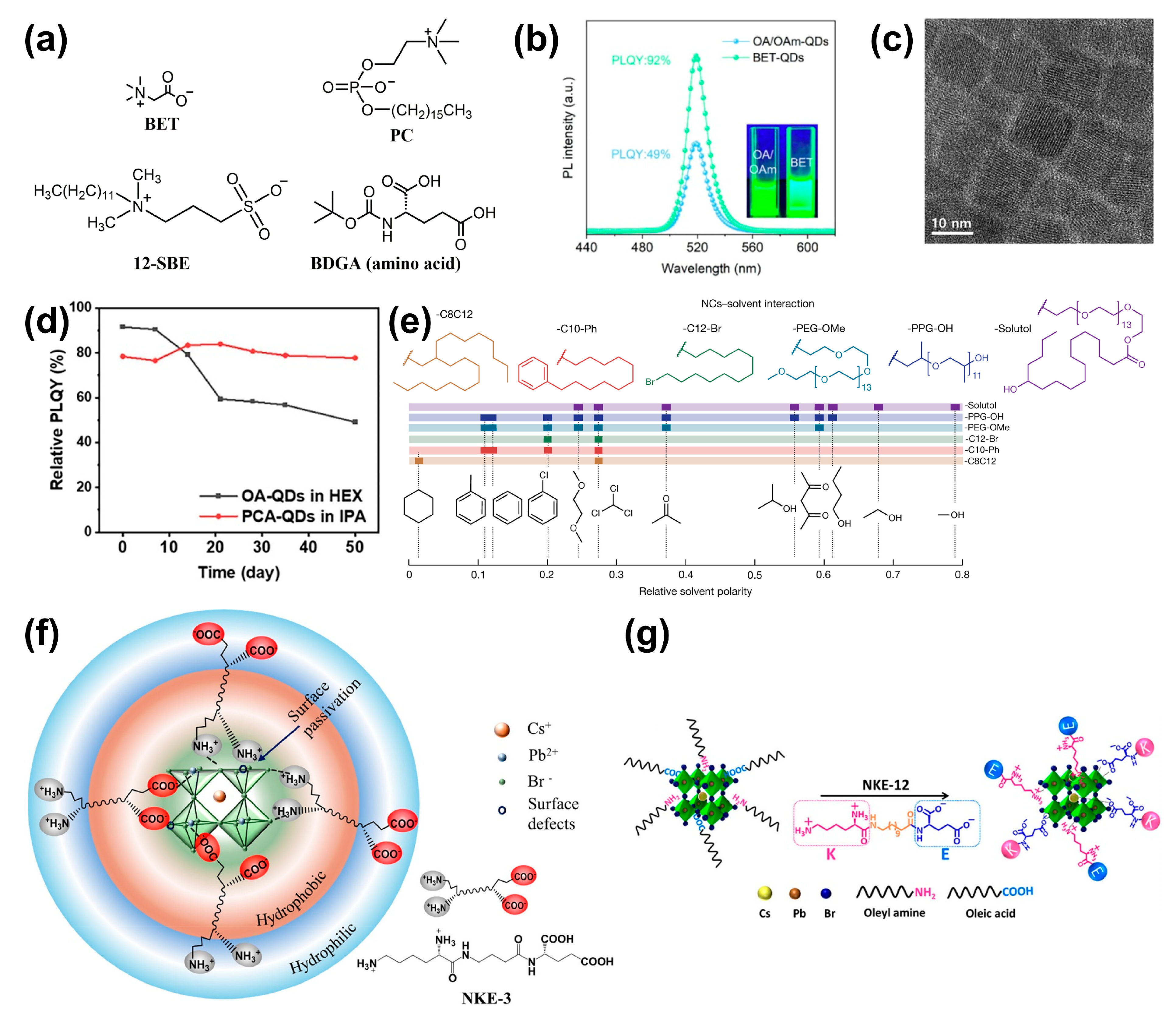
| X | BET | Post | CsPbBr3 | 92% | >75% (S, ambient conditions, 10 d) >50% (F, ambient conditions, 15 d) >80% (S, UV, 1.5 h) | [89] |
| X | S-BET | Post | CsPbBr3 | ~100% | No exact numbers provided | [90] |
| X | PC | In situ | CsPbI3 | ~100% | Increased stability (S, in air, 6 months) | [91] |
| X | NKE-3 | Post | CsPbBr3 | ∼25% (water dispersed) | 29% (S, in water, 72 h) | [94] |
| X | NKE-12 | Post | CsPbBr3 | - | 70% (S, in water, 14 d) | [95] |
| L | AHDA | In situ | CsPbI3 | 64.6% | PLQY: 63.7% (S, in air, 110 d) No phase change (F, 85 °C in air, 20 d) No phase change (F, UV, 500 min) | [96] |
| L | OTAm | In situ | CsPbBr3 | ~85% | >80% (S, 25 °C, 40% RH, 30 d) | [97] |
| L | TOP | Post | CsPbBr1.2I1.8 | - | No change (S, in nitrogen, 14 d) 43% (S, heat from 20 to 90 °C) Increased PL intensity (S, UV, 12 h) No change (S, add ethanol) | [98] |
| L | TOP | In situ | CsPbBr3 | 83% | Bright (S, ambient conditions, 4 months) >50% (S, in ethanol, 30 h) ~70% (S, in water, 50 h) 90% (S, UV, 30 h) Bright (F, ambient conditions, 35 d) | [99] |
| L | DPP | In situ | CsPbBr3 | 81% | Bright (S, ambient conditions, 4 months) >50% (S, in ethanol, 30 h) ~90% (S, in water, 50 h) >80% (S, UV, 30 h) Bright (F, ambient conditions, 35 d) | [99] |
| L | TBP | In situ | CsPbBr3 | 75% | Bright (S, ambient conditions, 4 months) >20% (S, in ethanol, 30 h) ~70% (S, in water, 50 h) >80% (S, UV, 30 h) | [99] |
| L | TOPO | In situ | CsPbBr3 | - | 95% (S, in ethanol, 100 min) | [100] |
| Z | K+ | Post | CsPbI3 | 96% (films) | No phase change (F, 25 °C, 40% RH, 2 months) | [101] |
| Z | Zn2+ | Post | CsPbBr3 | 99% | - | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, C.; Huang, Q.; Fu, Z.; Lu, Y. Ligand Engineering of Inorganic Lead Halide Perovskite Quantum Dots toward High and Stable Photoluminescence. Nanomaterials 2024, 14, 1201. https://doi.org/10.3390/nano14141201
Deng C, Huang Q, Fu Z, Lu Y. Ligand Engineering of Inorganic Lead Halide Perovskite Quantum Dots toward High and Stable Photoluminescence. Nanomaterials. 2024; 14(14):1201. https://doi.org/10.3390/nano14141201
Chicago/Turabian StyleDeng, Changbo, Qiuping Huang, Zhengping Fu, and Yalin Lu. 2024. "Ligand Engineering of Inorganic Lead Halide Perovskite Quantum Dots toward High and Stable Photoluminescence" Nanomaterials 14, no. 14: 1201. https://doi.org/10.3390/nano14141201
APA StyleDeng, C., Huang, Q., Fu, Z., & Lu, Y. (2024). Ligand Engineering of Inorganic Lead Halide Perovskite Quantum Dots toward High and Stable Photoluminescence. Nanomaterials, 14(14), 1201. https://doi.org/10.3390/nano14141201









