The Structures and Compositions Design of the Hollow Micro–Nano-Structured Metal Oxides for Environmental Catalysis
Abstract
1. Introduction
2. The Application of HSMO Catalysts in Environmental Catalysis
2.1. Automobile and Stationary Sources Emission Control
2.1.1. Catalytic Oxidation of CO
- Pure CeO2 hollow structure
- The composite binary or multiple CeO2 hollow structure
- The multi-element Ce-based hollow structure
- Ce-based hollow structure doped with noble metals
- Other HSMOs
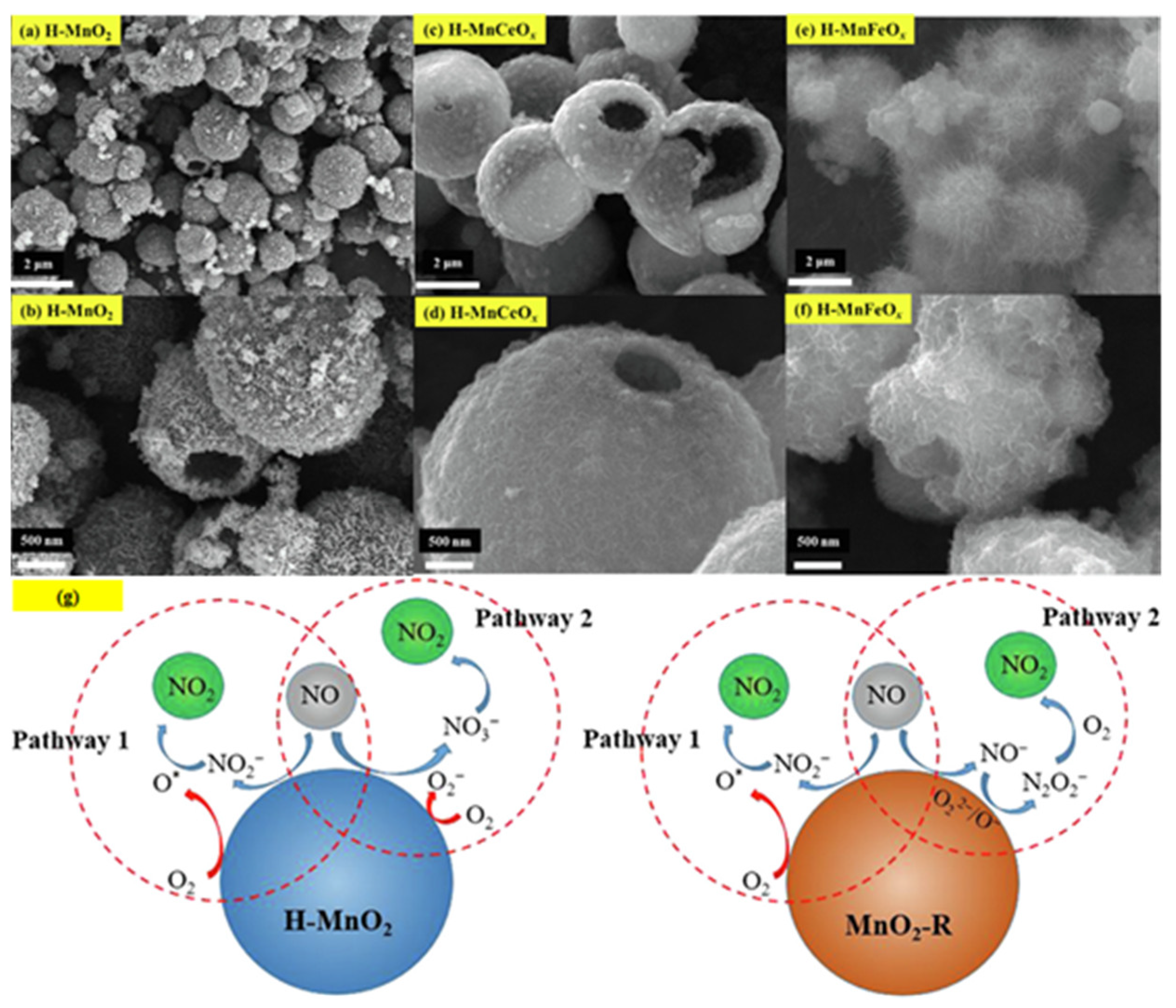
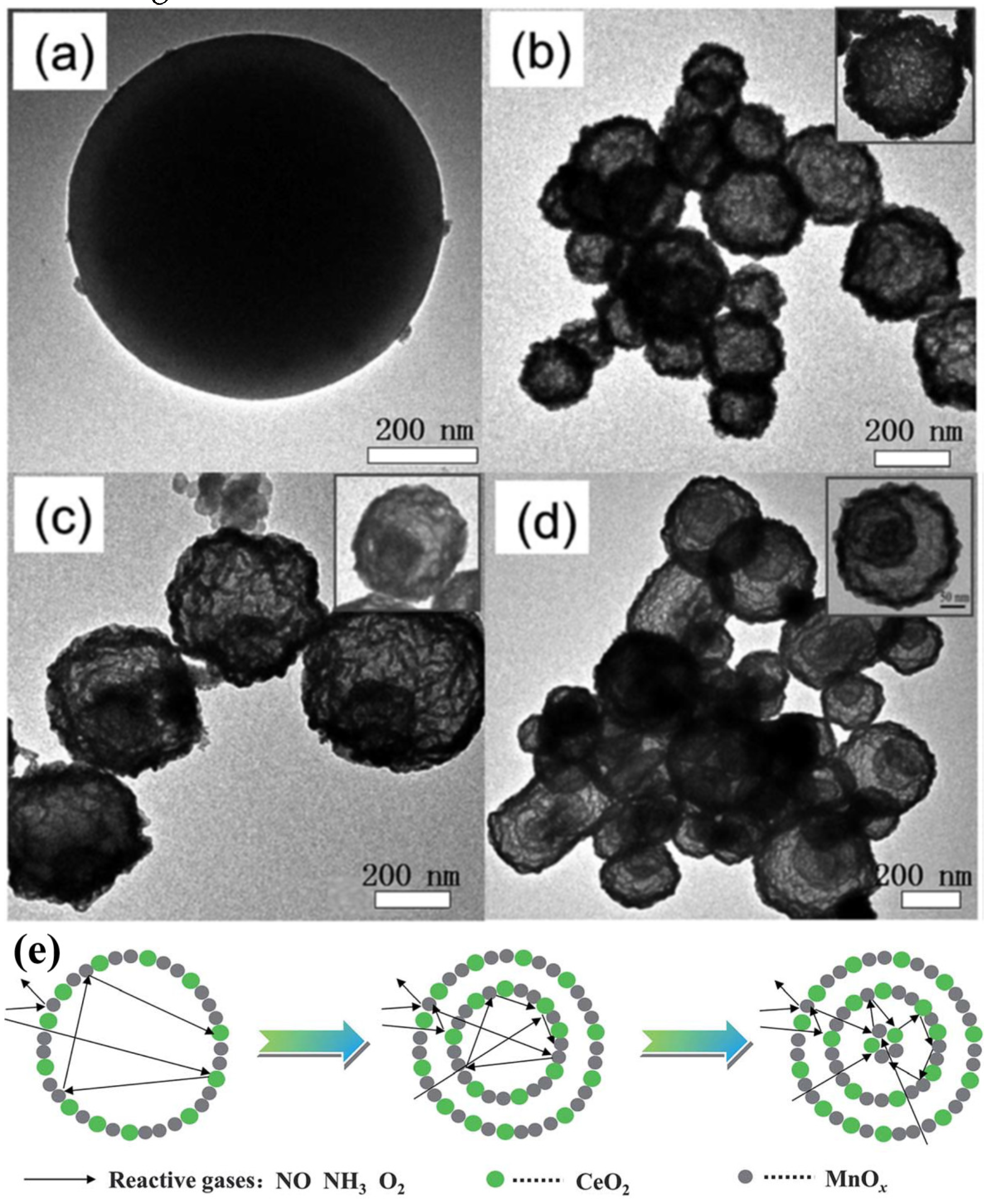
2.1.2. NH3-SCR Removal of NOx
- Single-component HSMOs catalysts
- Multi-component HSMOs catalysts
2.1.3. Catalyst for Automobile Three-Way Catalytic (TWC) Reaction and Diesel Oxidation Catalytic (DOC) Reaction
2.2. Volatile Organic Compounds Emission Control
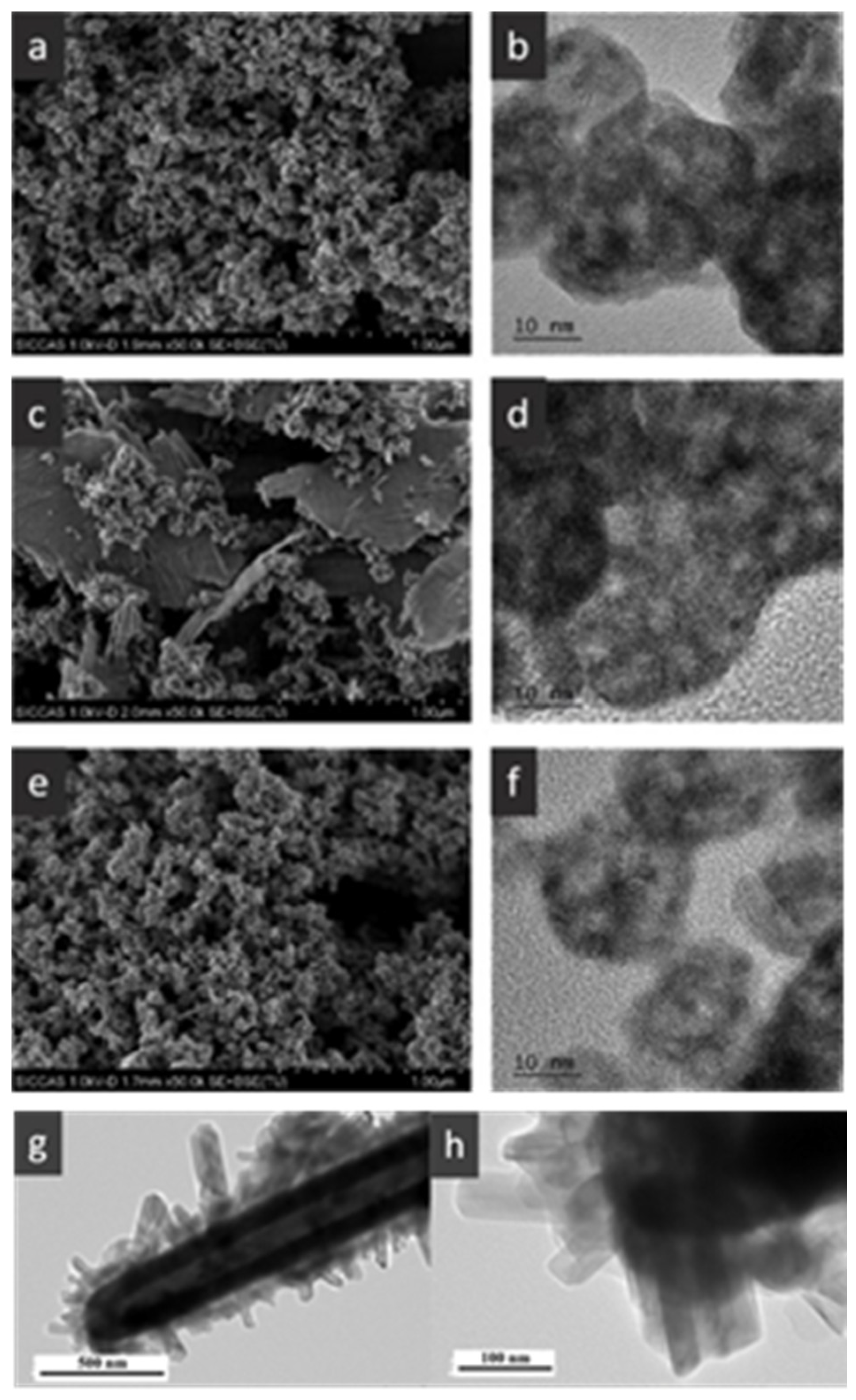
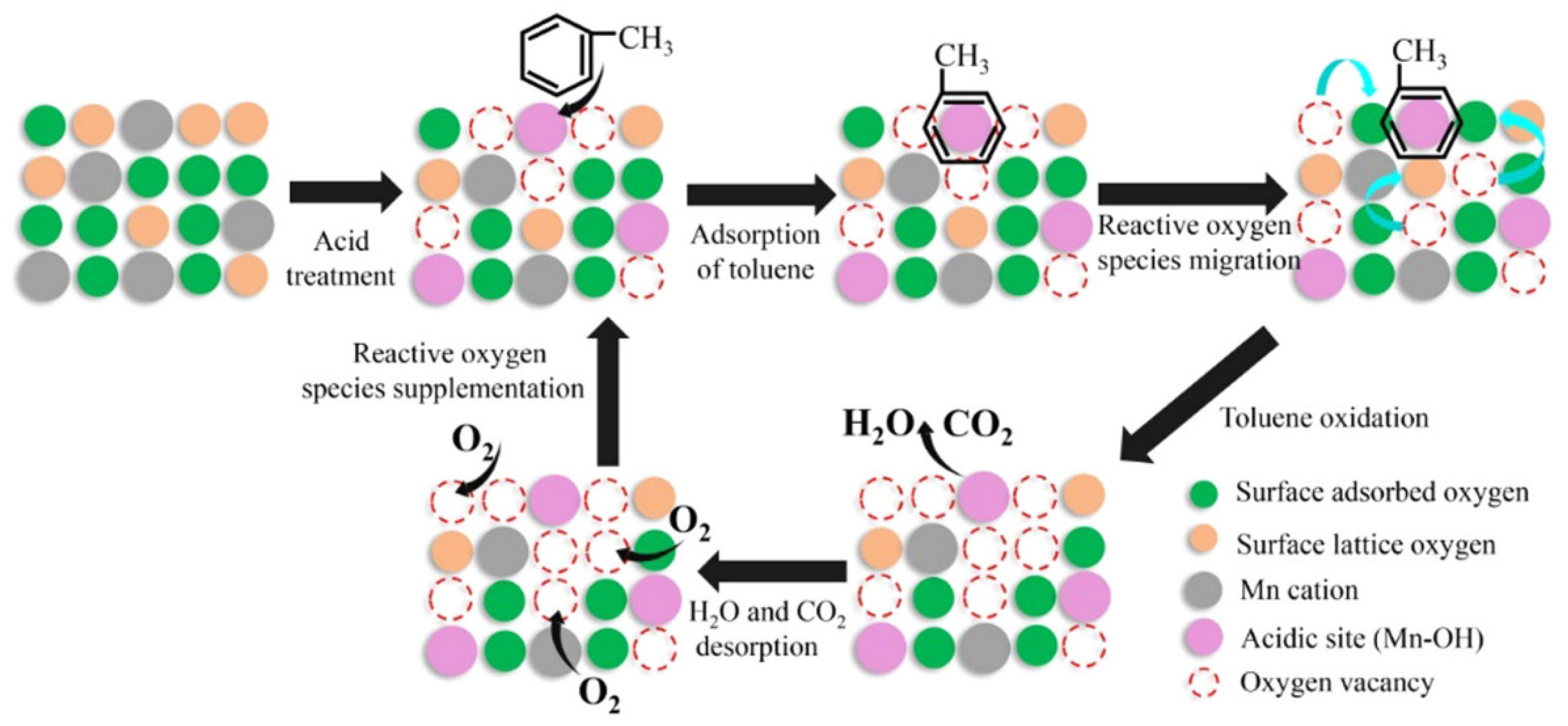
2.2.1. Catalytic Elimination of Toluene
- Single-component metal oxide catalysts with hollow structure
- Hollow-structured metal oxides supported catalysts
- Hollow-structured binary metal oxide catalysts
2.2.2. Removal of Other Volatile Organic Compounds (VOCs)
- Hollow nanospheres
- Other hollow-structured metal oxides
2.3. Removal of Other Pollutants
2.3.1. Catalytic Conversion of CO2
2.3.2. Catalytic Conversion of CH4
2.3.3. Removal of Organic Compounds
3. Conclusions and Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Binas, V.; Venieri, D.; Kotzias, D.; Kiriakidis, G. Modified TiO2 based photocatalysts for improved air and health quality. J. Mater. 2017, 3, 3–16. [Google Scholar]
- Sun, D.; Wageh, S.; Al-Ghamdi, A.A.; Le, Y.; Yu, J.; Jiang, C. Pt/C@MnO2 composite hierarchical hollow microspheres for catalytic formaldehyde decomposition at room temperature. Appl. Surf. Sci. 2019, 466, 301–308. [Google Scholar] [CrossRef]
- Gong, M.; Zhang, J.; Luo, J.-L.; Wang, C.-A. Au/CeO2 hollow nanospheres with enhanced catalytic activity for CO oxidation. Int. J. Appl. Ceram. Technol. 2017, 14, 908–914. [Google Scholar] [CrossRef]
- Lin, J.; Huang, J.; Chen, X.; Zheng, Y.; Xiao, Y.; Zheng, Y.; Jiang, L. Key factors for methane combustion over palladium-based catalysts revealed by enhanced and depressed catalytic performance. Appl. Catal. B 2024, 340, 123283. [Google Scholar] [CrossRef]
- Xu, W.; He, H.; Yu, Y. Deactivation of a Ce/TiO2 Catalyst by SO2 in the Selective Catalytic Reduction of NO by NH3. J. Phys. Chem. C 2009, 113, 4426–4432. [Google Scholar] [CrossRef]
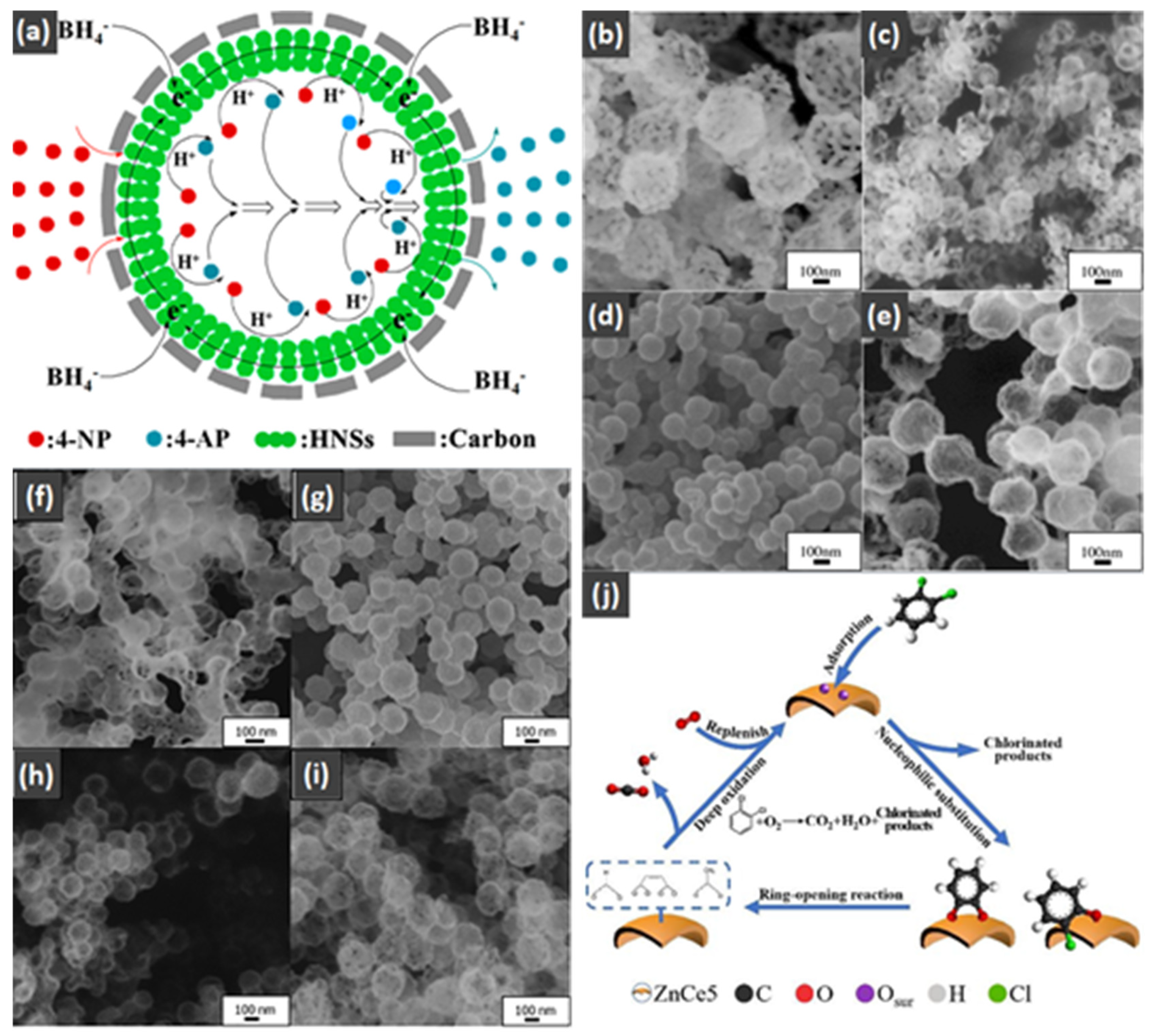
- Qu, J.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Construction of Pd-Modified NiCoOx Hollow Nanospheres with Surface Hydroxyls and Oxygen Vacancies for Highly Enhanced Catalytic Toluene Oxidation Activity. ACS Sustain. Chem. Eng. 2020, 8, 10581–10587. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Qin, L.; Mu, J.; Kang, S.-Z. Co3O4/CoP composite hollow polyhedron: A superior catalyst with dramatic efficiency and stability for the room temperature reduction of 4-nitrophenol. Appl. Surf. Sci. 2018, 434, 967–974. [Google Scholar] [CrossRef]
- Kim, S.; Park, C.; Lee, J. Reduction of polycyclic compounds and biphenyls generated by pyrolysis of industrial plastic waste by using supported metal catalysts: A case study of polyethylene terephthalate treatment. J. Hazard. Mater. 2020, 392, 122464. [Google Scholar] [CrossRef] [PubMed]
- Khalifeh, R.; Sorouri, M.; Damirchi, E.K.; Rajabzadeh, M. Efficient and selective CO2 and CS2 conversion to cyclic carbonates and trithiocarbonates by using multishell hollow CoAl2O4 microsphere as a unique catalyst under solventless condition. J. Taiwan Inst. Chem. Eng. 2020, 115, 229–241. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, L.; Chen, M.; Lv, C.; Lian, X.; Wu, C.-E.; Yang, B.; Miao, Z.; Wang, F.; Hu, X. CO Oxidation over Metal Oxide (La2O3, Fe2O3, PrO2, Sm2O3, and MnO2) Doped CuO-Based Catalysts Supported on Mesoporous Ce0.8Zr0.2O2 with Intensified Low-Temperature Activity. Catalysts 2019, 9, 724. [Google Scholar] [CrossRef]
- Hou, Z.; Pei, W.; Zhang, X.; Zhang, K.; Liu, Y.; Deng, J.; Jing, L.; Dai, H. Rare earth oxides and their supported noble metals in application of environmental catalysis. J. Rare Earths 2020, 38, 819–839. [Google Scholar] [CrossRef]
- Liu, B.; Yu, S.; Wang, Q.; Hu, W.; Jing, P.; Liu, Y.; Jia, W.; Liu, Y.; Liu, L.; Zhang, J. Hollow mesoporous ceria nanoreactors with enhanced activity and stability for catalytic application. Chem. Commun. 2013, 49, 3757–3759. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gan, L.; Si, R. Effect of tungsten oxide on ceria nanorods to support copper species as CO oxidation catalysts. J. Rare Earths 2021, 39, 43–50. [Google Scholar] [CrossRef]
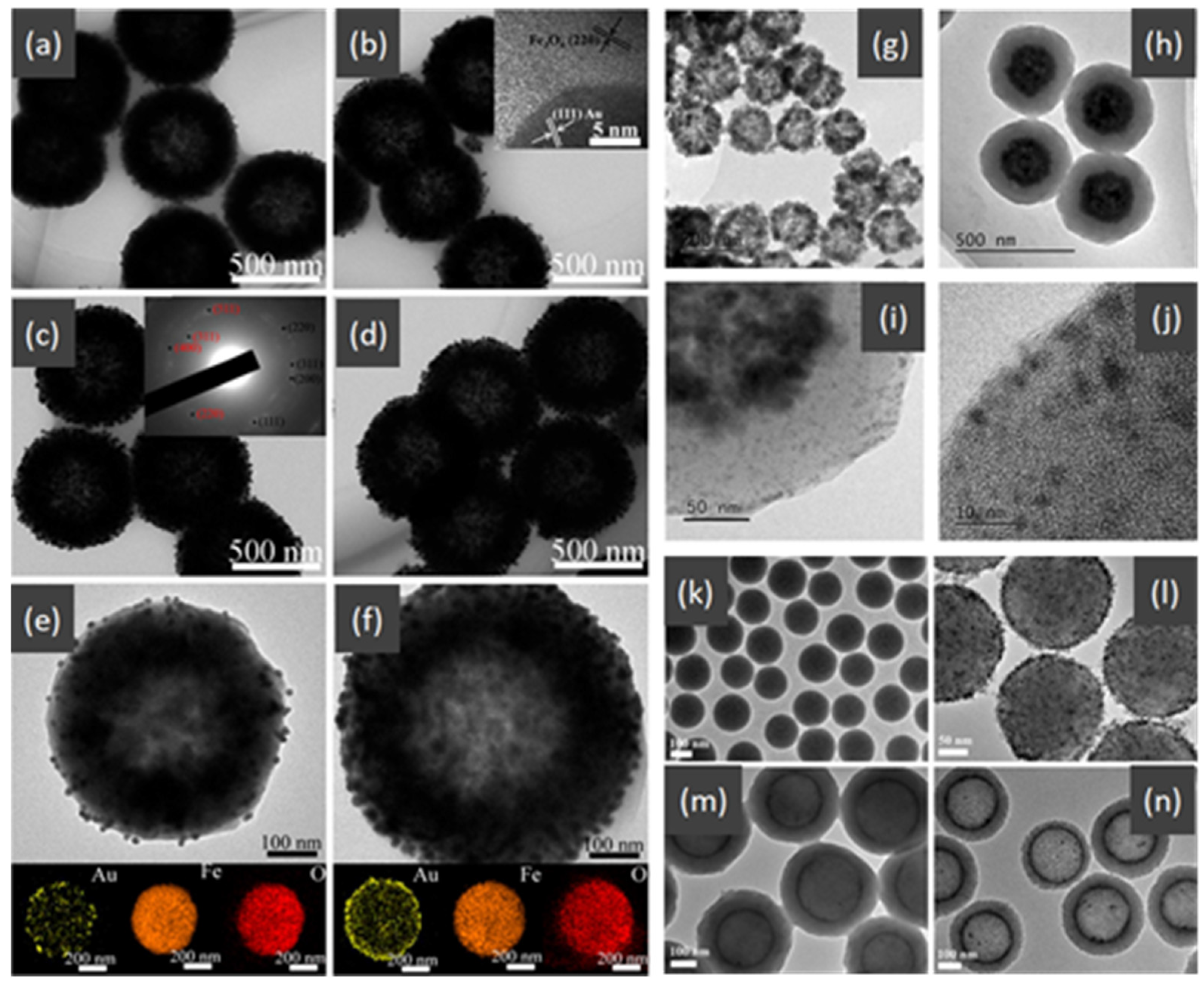
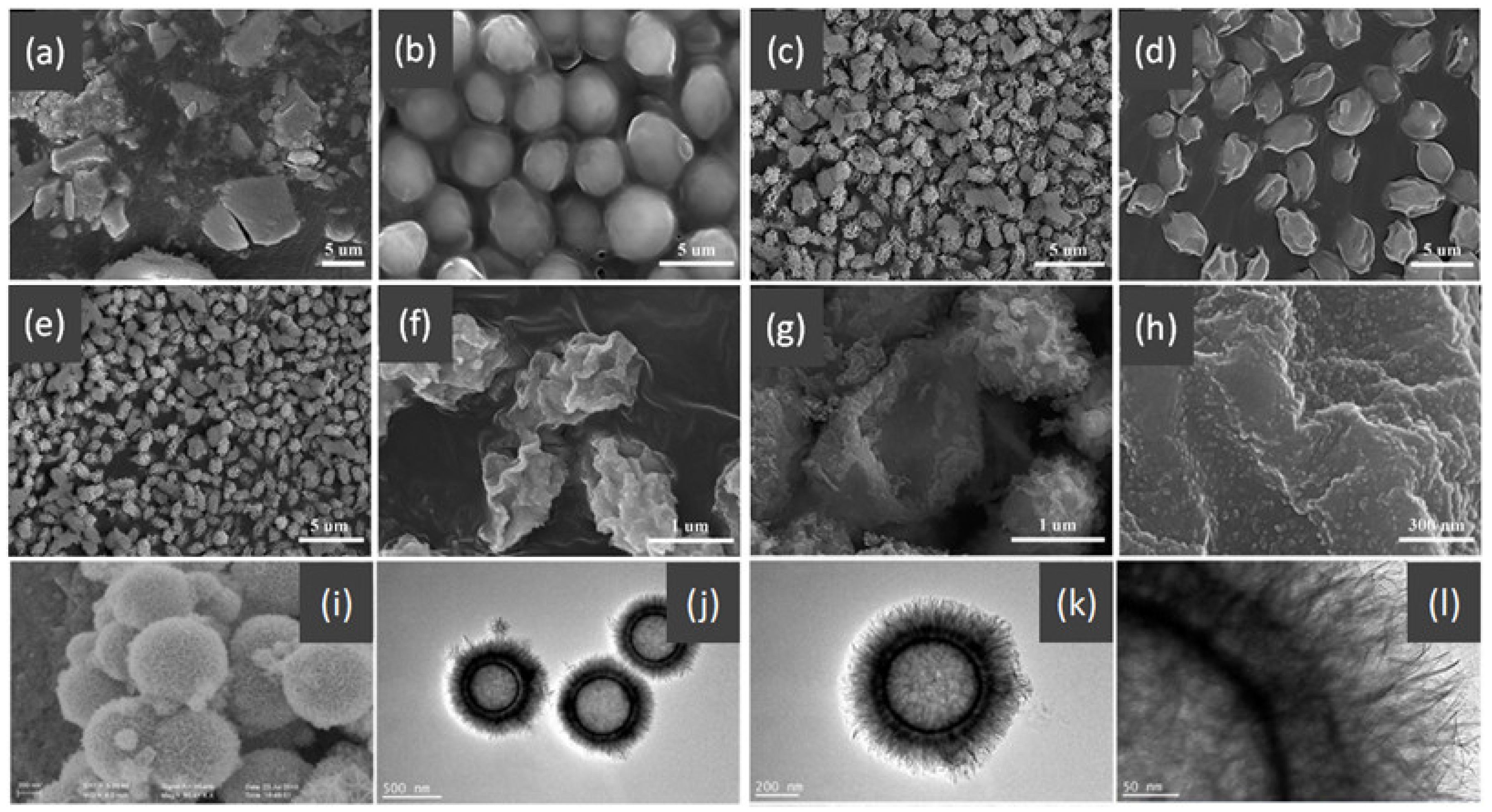
- Wang, X.; Feng, J.; Bai, Y.; Zhang, Q.; Yin, Y. Synthesis, Properties, and Applications of Hollow Micro-/Nanostructures. Chem. Rev. 2016, 116, 10983–11060. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Lian, K.; Wang, L.; Gu, F.; Han, D.; Wang, Z. High specific surface area LaMO3 (M=Co, Mn) hollow spheres:synthesis, characterization and catalytic properties in methane combustion. RSC Adv. 2014, 4, 58699–58707. [Google Scholar] [CrossRef]
- Zhao, H.; Yao, S.; Zhang, M.; Huang, F.; Fan, Q.; Zhang, S.; Liu, H.; Ma, D.; Gao, C. Ultra-Small Platinum Nanoparticles Encapsulated in Sub-50 nm Hollow Titania Nanospheres for Low-Temperature Water-Gas Shift Reaction. ACS Appl. Mater. Interfaces 2018, 10, 36954–36960. [Google Scholar] [CrossRef] [PubMed]
- Jeyavani, V.; Kondhekar, D.; Bhati, M.; Dev, S.; Joshi, K.; Devi, R.N.; Mukherjee, S.P. Remarkable SO2 and H2S Resistant Ability on CO Oxidation by Unique Pd/WO3 3D Hollow Sphere Nanocatalyst: Correlating Structure–Activity Relationships on SO2 Exposure. ACS Appl. Energy Mater. 2024, 7, 1476–1487. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, Y.; Qi, J.; Wan, J.; Wang, Z.; Yu, R.; Wang, D. Mass Transfer Modulation by Hollow Multi-Shelled Structures for High Space-Time Yield Synthesis of Light Olefins from Syngas. Adv. Funct. Mater. 2024, 34, 2316547. [Google Scholar] [CrossRef]
- Xiao, J.; Cheng, K.; Xie, X.; Wang, M.; Xing, S.; Liu, Y.; Hartman, T.; Fu, D.; Bossers, K.; van Huis, M.A.; et al. Tandem catalysis with double-shelled hollow spheres. Nat. Mater. 2022, 21, 572–579. [Google Scholar] [CrossRef]
- Wang, M.M.; Chen, D.Y.; Li, N.; Xu, Q.F.; Li, H.; He, J.H.; Lu, J.M. Nanocage-Shaped Co3−xZrxO4 Solid-Solution Supports Loaded with Pt Nanoparticles as Effective Catalysts for the Enhancement of Toluene Oxidation. Small 2020, 16, 2005715. [Google Scholar] [CrossRef]
- Luo, L.; Huang, R.; Hu, W.; Yu, Z.; Tang, Z.; Chen, L.; Zhang, Y.; Zhang, D.; Xiao, P. Metal–Organic Framework-Derived Hollow CoMn2O4 Nanocube Catalysts for Deep Toluene Oxidation. ACS Appl. Nano Mater. 2022, 5, 8232–8242. [Google Scholar] [CrossRef]
- Ma, X.D.; Wen, J.X.; Guo, H.W.; Ren, G.B. Facile template fabrication of Fe-Mn mixed oxides with hollow microsphere structure for efficient and stable catalytic oxidation of 1,2-dichlorobenzene. Chem. Eng. J. 2020, 382, 122940. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Rochard, G.; Giraudon, J.-M.; Liu, J.; Lamonier, J.-F. Mesoporous MnO2 hollow spheres for enhanced catalytic oxidation of formaldehyde. SM&T 2019, 20, e00091. [Google Scholar]
- Wei, W.; Wang, Z.; Liu, Z.; Liu, Y.; He, L.; Chen, D.; Umar, A.; Guo, L.; Li, J. Metal oxide hollow nanostructures: Fabrication and Li storage performance. J. Power Sources 2013, 238, 376–387. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, L.; Lou, X.W.D. Metal oxide hollow nanostructures for lithium-ion batteries. Adv. Mater. 2012, 24, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Liu, S.B.; Meng, F.L.; Liu, J.Y.; Jin, Z.; Kong, L.T.; Liu, J.H. Metal oxide nanostructures and their gas sensing properties: A review. Sensors 2012, 12, 2610–2631. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Halpert, J.E.; Wang, D. Recent advances in micro-/nano-structured hollow spheres for energy applications: From simple to complex systems. Energy Environ. Sci. 2012, 5, 5604–5618. [Google Scholar] [CrossRef]
- Prieto, G.; Tüysüz, H.; Duyckaerts, N.; Knossalla, J.; Wang, G.-H.; Schüth, F. Hollow Nano- and Microstructures as Catalysts. Chem. Rev. 2016, 116, 14056–14119. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Khalifeh, R.; Amrollahi, M.A. Urchin-like double-shelled Pd–PdO/ZnO hollow sphere as an efficient catalyst for the Suzuki-Miyaura reaction. Mater. Today Chem. 2020, 18, 100353. [Google Scholar] [CrossRef]
- Fu, X.-P.; Yu, W.-Z.; Li, M.-Y.; Si, R.; Ma, C.; Jia, C.-J. Facile Fabrication of CeO2-Al2O3 Hollow Sphere with Atomically Dispersed Fe via Spray Pyrolysis. Inorg. Chem. 2021, 60, 5183–5189. [Google Scholar] [CrossRef]
- Wang, L.; Wan, J.; Wang, J.; Wang, D. Small Structures Bring Big Things: Performance Control of Hollow Multishelled Structures. Small Struct. 2020, 2, 2000041. [Google Scholar] [CrossRef]
- Yu, L.; Yu, X.Y.; Lou, X.W.D. The Design and Synthesis of Hollow Micro-/Nanostructures: Present and Future Trends. Adv. Mater. 2018, 30, e1800939. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yin, Y. Self-Templating Approaches to Hollow Nanostructures. Adv. Mater. 2019, 31, e1802349. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, M.; Fang, X.; Wu, L. Fabrication and application of inorganic hollow spheres. Chem. Soc. Rev. 2011, 40, 5472–5491. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Wang, J.; Tang, X.; Li, J.; Zhang, X.; Liu, B. Low-temperature and stable CO oxidation of in-situ grown monolithic Mn3O4/TiO2 catalysts. J. Alloys Compd. 2021, 855, 157444. [Google Scholar] [CrossRef]
- Xiong, J.; Mei, X.; Liu, J.; Wei, Y.; Zhao, Z.; Xie, Z.; Li, J. Efficiently multifunctional catalysts of 3D ordered meso-macroporous Ce0.3Zr0.7O2-supported PdAu@CeO2 core-shell nanoparticles for soot oxidation: Synergetic effect of Pd-Au-CeO2 ternary components. Appl. Catal. B 2019, 251, 247–260. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, L.; Chen, M.; Lian, X.; Wu, C.-E.; Yang, B.; Miao, Z.; Wang, F.; Hu, X. Facilely fabricating mesoporous nanocrystalline Ce–Zr solid solution supported CuO-based catalysts with advanced low-temperature activity toward CO oxidation. Catal. Sci. Technol. 2019, 9, 5605–5625. [Google Scholar] [CrossRef]
- Sun, H.; Liu, Z.; Chen, S.; Quan, X. The role of lattice oxygen on the activity and selectivity of the OMS-2 catalyst for the total oxidation of toluene. Chem. Eng. J. 2015, 270, 58–65. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Sun, J.; Xiao, G.; Zhang, H.; Qiu, X.; Li, H.; Chen, L. Mesoscale Organization of Nearly Monodisperse Flowerlike Ceria Microspheres. J. Phys. Chem. B 2006, 110, 13445–13452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Du, X.; Shi, L.; Gao, R. Shape-controlled synthesis and catalytic application of ceria nanomaterials. Dalton Trans. 2012, 41, 14455–14475. [Google Scholar] [CrossRef]
- Sun, C.; Li, H.; Chen, L. Nanostructured ceria-based materials: Synthesis, properties, and applications. Energy Environ. Sci. 2012, 5, 8475. [Google Scholar] [CrossRef]
- Yuan, Q.; Duan, H.H.; Li, L.L.; Sun, L.D.; Zhang, Y.W.; Yan, C.H. Controlled synthesis and assembly of ceria-based nanomaterials. J. Colloid. Interface Sci. 2009, 335, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, W.; Chen, Z.; Wang, T. Biogenic synthesis and catalysis of porous CeO2 hollow microspheres. J. Rare Earths 2012, 30, 350–354. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, T.; Hu, Q.; Fang, Z.; Han, K. Study of the preparation and properties of CeO2 single/multiwall hollow microspheres. J. Mater. Res. 2007, 22, 1472–1478. [Google Scholar] [CrossRef]
- Yang, Z.; Han, D.; Ma, D.; Liang, H.; Liu, L.; Yang, Y. Fabrication of Monodisperse CeO2 Hollow Spheres Assembled by Nano-octahedra. Cryst. Growth Des. 2010, 10, 291–295. [Google Scholar] [CrossRef]
- Ma, J.; Qian, K.; Huang, W.; Zhu, Y.; Yang, Q. Facile One-Step Synthesis of Double-Shelled CeO2 Hollow Spheres and Their Optical and Catalytic Properties. Bull. Chem. Soc. Jpn. 2010, 83, 1455–1461. [Google Scholar] [CrossRef]
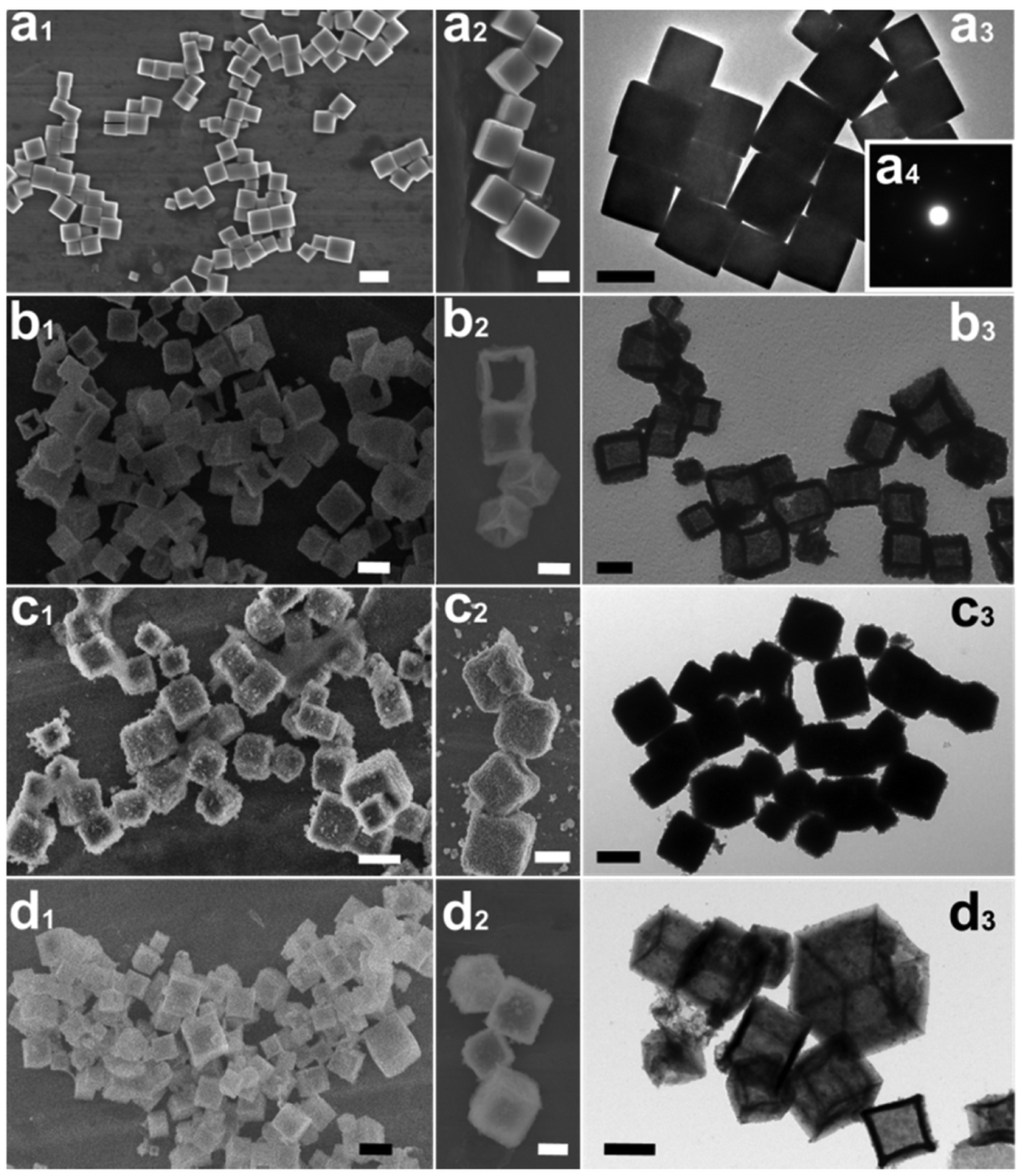
- Chen, G.; Xu, C.; Song, X.; Xu, S.; Ding, Y.; Sun, S. Template-free Synthesis of Single-Crystalline-like CeO2 Hollow Nanocubes. Cryst. Growth Des. 2008, 8, 4449–4453. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, F.; Sun, X.; Sun, S.; Chen, R. Benign synthesis of ceria hollow nanocrystals by a template-free method. CrystEngComm 2011, 13, 2904–2908. [Google Scholar] [CrossRef]
- González-Rovira, L.; Sánchez-Amaya, J.M.; López-Haro, M.; del Rio, E.; Hungría, A.B.; Midgley, P.; Calvino, J.J.; Berna, S.; Botana, F.J. Single-Step Process to Prepare CeO2 Nanotubes with Improved Catalytic Activity. Nano Lett. 2009, 9, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Pan, C.; Shi, L.; Huang, L.; Fang, J.; Fu, H. A highly reactive catalyst for CO oxidation: CeO2 nanotubes synthesized using carbon nanotubes as removable templates. Microporous Mesoporous Mater. 2009, 117, 193–200. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, T.; Pan, C.; Shi, L.; Zhang, J. Carbon nanotube-assisted synthesis and high catalytic activity of CeO2 hollow nanobeads. Mater. Chem. Phys. 2009, 113, 527–530. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, R. Hollow Micro/Nanostructured Ceria-Based Materials: Synthetic Strategies and Versatile Applications. Adv. Mater. 2019, 31, e1800592. [Google Scholar] [CrossRef] [PubMed]
- Devaraju, M.K.; Liu, X.; Yin, S.; Sato, T. A rapid solvothermal synthesis of cerium oxide hollow spheres and characterization. J. Solid. State Chem. 2012, 194, 43–47. [Google Scholar] [CrossRef]
- Chen, G.; Rosei, F.; Ma, D. Interfacial Reaction-Directed Synthesis of Ce-Mn Binary Oxide Nanotubes and Their Applications in CO Oxidation and Water Treatment. Adv. Funct. 2012, 22, 3914–3920. [Google Scholar] [CrossRef]
- Chiu, K.-l.; Kwong, F.-l.; Ng, D.H.L. Enhanced oxidation of CO by using a porous biomorphic CuO/CeO2/Al2O3 compound. Microporous Mesoporous Mater. 2012, 156, 1–6. [Google Scholar] [CrossRef]
- Yoon, K.; Yang, Y.; Lu, P.; Wan, D.; Peng, H.-C.; StammMasias, K.; Fanson, P.T.; Campbell, C.T.; Xia, Y. A highly reactive and sinter-resistant catalytic system based on platinum nanoparticles embedded in the inner surfaces of CeO2 hollow fibers. Angew. Chem. Int. Edit. 2012, 51, 9543–9546. [Google Scholar] [CrossRef]
- Chen, C.; Fang, X.; Wu, B.; Huang, L.; Zheng, N. A Multi-Yolk-Shell Structured Nanocatalyst Containing Sub-10 nm Pd Nanoparticles in Porous CeO2. ChemCatChem 2012, 4, 1578–1586. [Google Scholar] [CrossRef]
- Mai, H.-X.; Sun, L.-D.; Zhang, Y.-W.; Si, R.; Feng, W.; Zhang, H.-P.; Liu, H.-C.; Yan, C.-H. Shape-Selective Synthesis and Oxygen Storage Behavior of Ceria Nanopolyhedra, Nanorods, and Nanocubes. J. Phys. Chem. B 2006, 109, 24380–24385. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Overbury, S.H. On the structure dependence of CO oxidation over CeO2 nanocrystals with well-defined surface planes. J. Catal. 2012, 285, 61–73. [Google Scholar] [CrossRef]
- Tana; Zhang, M.; Li, J.; Li, H.; Li, Y.; Shen, W. Morphology-dependent redox and catalytic properties of CeO2 nanostructures: Nanowires, nanorods and nanoparticles. Catal. Today 2009, 148, 179–183. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, W.; Chang, S.; Huang, W. Morphology Effect of CeO2 Support in the Preparation, Metal–Support Interaction, and Catalytic Performance of Pt/CeO2 Catalysts. ChemCatChem 2013, 5, 3610–3620. [Google Scholar] [CrossRef]
- Han, X.; Li, L.; Wang, C. Template-free synthesis of uniform single-crystal hollow cerium dioxide nanocubes and their catalytic activity. Nanoscale 2013, 5, 7193–7196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, H.; Wang, S.; Liu, W.; Liu, X.; Guo, J.; Yang, Y. Mesoporous CeO2 nanoparticles assembled by hollow nanostructures: Formation mechanism and enhanced catalytic properties. CrystEngComm 2014, 16, 8777–8785. [Google Scholar] [CrossRef]
- Mu, G.; Wei, Q.; Huang, Y. Facile fabrication of CeO2 hollow microspheres with yeast as bio-templates. J. Rare Earths 2015, 33, 1329–1334. [Google Scholar] [CrossRef]
- Li, B.X.; Shao, X.K.; Hao, Y.G.; Zhao, Y. Ultrasonic-Spray-Assisted Synthesis of Metal Oxide Hollow/Mesoporous Microspheres for Catalytic CO Oxidation. RSC Adv. 2015, 5, 85640–85645. [Google Scholar] [CrossRef]
- Shen, G.; Liu, H.; Wang, Q.; Wang, Z.; Chen, Y. Self-template hydrothermal synthesis of CeO2 hollow nanospheres. J. Nanopart Res. 2012, 14, 954. [Google Scholar] [CrossRef]
- Li, Z.; Han, F.; Li, C.; Jiao, X.; Chen, D. Hollow CeO2 dodecahedrons: One-step template synthesis and enhanced catalytic performance. RSC Adv. 2016, 6, 60975–60982. [Google Scholar] [CrossRef]
- Wei, J.; Wang, S.; Sun, S.; Yang, Z.; Yang, Y. Formation of catalytically active CeO2 hollow nanoparticles guided by oriented attachment. Mater. Lett. 2012, 84, 77–80. [Google Scholar] [CrossRef]
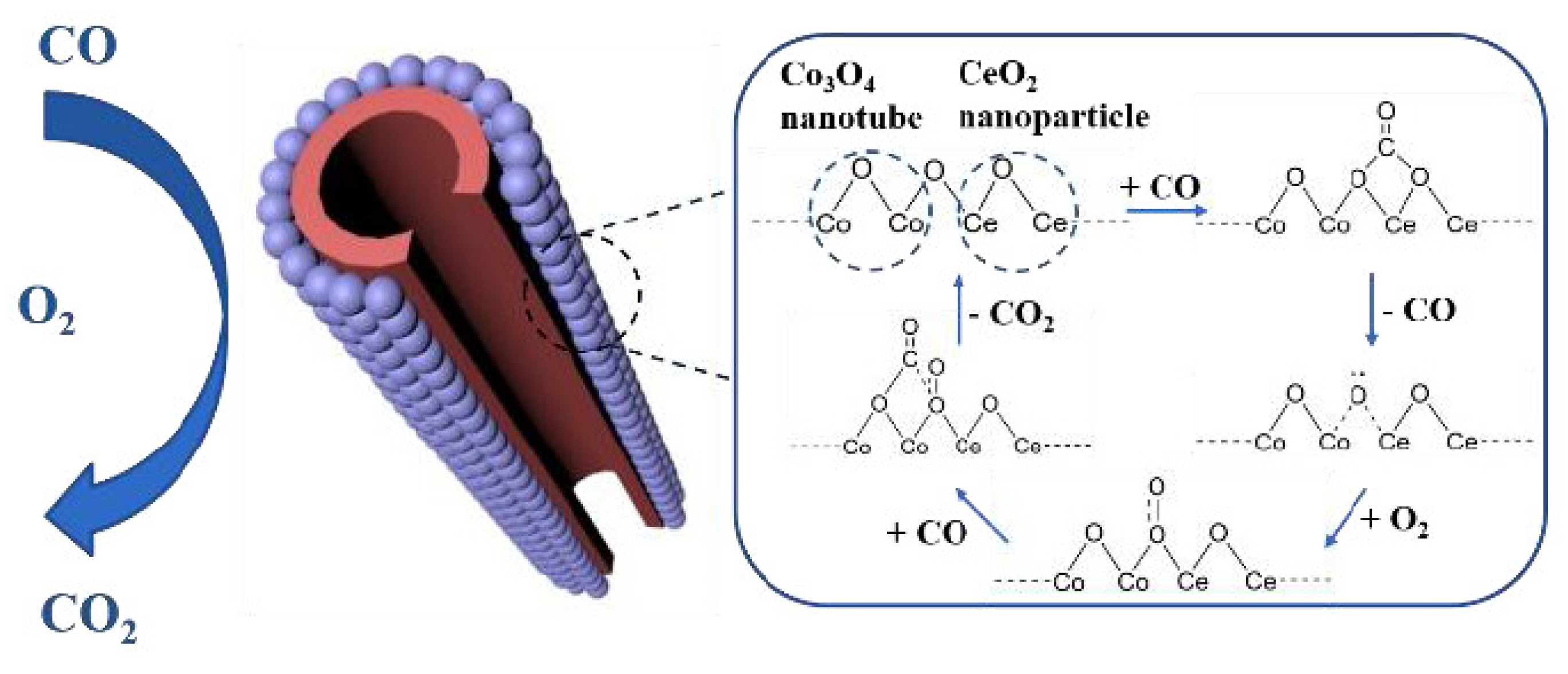
- Wang, H.; Mao, D.; Qi, J.; Zhang, Q.; Ma, X.; Song, S.; Gu, L.; Yu, R.; Wang, D. Hollow Multishelled Structure of Heterogeneous Co3O4–CeO2−x Nanocomposite for CO Catalytic Oxidation. Adv. Funct. 2019, 29, 1806588. [Google Scholar] [CrossRef]
- He, J.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Hollow Mesoporous Co3O4-CeO2 Composite Nanotubes with Open Ends for Efficient Catalytic CO Oxidation. ChemSusChem 2019, 12, 1084–1090. [Google Scholar] [CrossRef]
- Liu, L.; Shi, J.; Wang, R. General fabrication of MCo2O4@CeO2 (M = Ni, Cu, Zn, Mn) core@shell nanospheres and their catalytic performances in CO oxidation. Funct. Mater. Lett. 2017, 10, 1850001. [Google Scholar] [CrossRef]
- Liu, W.; Wang, W.; Tang, K.; Guo, J.; Ren, Y.; Wang, S.; Feng, L.; Yang, Y. The promoting influence of the nickel species in the controllable synthesis and catalytic property of the nickel-ceria catalysts. Catal. Sci. Technol. 2016, 6, 2427–2434. [Google Scholar] [CrossRef]
- Liu, W.; Liu, X.; Feng, L.; Guo, J.; Xie, A.; Wang, S.; Zhang, J.; Yang, Y. The synthesis of CeO2 nanospheres with different hollowness and size induced by copper doping. Nanoscale 2014, 6, 10693–10700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gong, M.; Cao, Y.; Wang, C.-A. Facile synthesis of well-dispersed CeO2–CuOX composite hollow spheres with superior catalytic activity for CO oxidation. RSC Adv. 2015, 5, 95133–95139. [Google Scholar] [CrossRef]
- Li, W.; Hu, Y.; Jiang, H.; Jiang, N.; Bi, W.; Li, C. Litchi-peel-like hierarchical hollow copper-ceria microspheres: Aerosol-assisted synthesis and high activity and stability for catalytic CO oxidation. Nanoscale 2018, 10, 22775–22786. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Feng, X.; Zhang, Z.; Jin, X.; Liu, D.; Zhang, Y. A Controllable Surface Etching Strategy for Well-Defined Spiny Yolk@Shell CuO@CeO2 Cubes and Their Catalytic Performance Boost. Adv. Funct. 2018, 28, 1802559. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, S.; Ning, D.; Zhang, Q.; Han, W.; Wang, Y. The Template-Free Synthesis of CuO@CeO2 Nanospheres: Facile Strategy, Structure Optimization, and Enhanced Catalytic Activity toward CO Oxidation. Eur. J. Inorg. Chem. 2018, 2018, 2927–2934. [Google Scholar] [CrossRef]
- Song, X.-Z.; Su, Q.-F.; Li, S.-J.; Liu, S.-H.; Zhang, N.; Meng, Y.-L.; Chen, X.; Tan, Z. Triple-shelled CuO/CeO2 hollow nanospheres derived from metal–organic frameworks as highly efficient catalysts for CO oxidation. New J. Chem. 2019, 43, 16096–16102. [Google Scholar] [CrossRef]
- Chen, G.; Guo, Z.; Zhao, W.; Gao, D.; Li, C.; Ye, C.; Sun, G. Design of Porous/Hollow Structured Ceria by Partial Thermal Decomposition of Ce-MOF and Selective Etching. ACS Appl. Mater. Interfaces 2017, 9, 39594–39601. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shi, J.; Cao, H.; Wang, R.; Liu, Z. Fabrication of CeO2-MOx (M = Cu, Co, Ni) composite yolk-shell nanospheres with enhanced catalytic properties for CO oxidation. Beilstein J. Nanotechnol. 2017, 8, 2425–2437. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Y.; Wang, C.-A.; Ran, R. Design and Preparation of MnO2/CeO2-MnO2 Double-Shelled Binary Oxide Hollow Spheres and Their Application in CO Oxidation. ACS Appl. Mater. Interfaces 2016, 8, 8670–8677. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shi, J.; Cao, H.; Wang, R. Fabrication of CeO2@MnO2 Core–Shell Nanospheres and Their Application in CO Oxidation. Nano 2017, 12, 1750039. [Google Scholar] [CrossRef]
- Chen, G.; Song, G.; Zhao, W.; Gao, D.; Wei, Y.; Li, C. Carbon sphere-assisted solution combustion synthesis of porous/hollow structured CeO2-MnOx catalysts. Chem. Eng. J. 2018, 352, 64–70. [Google Scholar] [CrossRef]
- Liu, L.; Shi, J.; Wang, R. Facile construction of Mn2O3@CeO2 core@shell cubes with enhanced catalytic activity toward CO oxidation. J. Solid. State Chem. 2019, 269, 419–427. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Y.; Gao, D.; Kang, B.; Li, S.; Li, C.; Chen, G. Ce-Mn Coordination Polymer Derived Hierarchical/Porous Structured CeO2-MnOx for Enhanced Catalytic Properties. Nanoscale 2020, 12, 16381–16388. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shi, J.; Wang, R.; Cao, H.; Liu, Z. Fabrication of double-shelled Fe2O3/CeO2 boxes from CeO2-modified Prussian blue and their enhanced performances for CO removal and water treatment. J. Alloys Compd. 2017, 725, 544–556. [Google Scholar] [CrossRef]
- Fuente ORdl Gonzalez-Barrio, M.A.; Navarro, V.; Pabon, B.M.; Palacio, I.; Mascaraque, A. Surface defects and their influence on surface properties. J. Phys. Condens. 2013, 25, 484008. [Google Scholar] [CrossRef]
- Liu, W.; Tang, K.; Lin, M.; June, L.T.O.; Bai, S.-Q.; Young, D.J.; Li, X.; Yang, Y.-Z.; Hor, T.S.A. Multicomponent (Ce, Cu, Ni) oxides with cage and core-shell structures: Tunable fabrication and enhanced CO oxidation activity. Nanoscale 2016, 8, 9521–9526. [Google Scholar] [CrossRef]
- Cheng, Z.; Yu, M.; Yang, G.; Kang, L. Fabrication of NiCo2O4@CeO2 Core@shell Nanotubes with Enhanced Catalytic Performances. CrystEngComm 2016, 18, 6331–6335. [Google Scholar] [CrossRef]
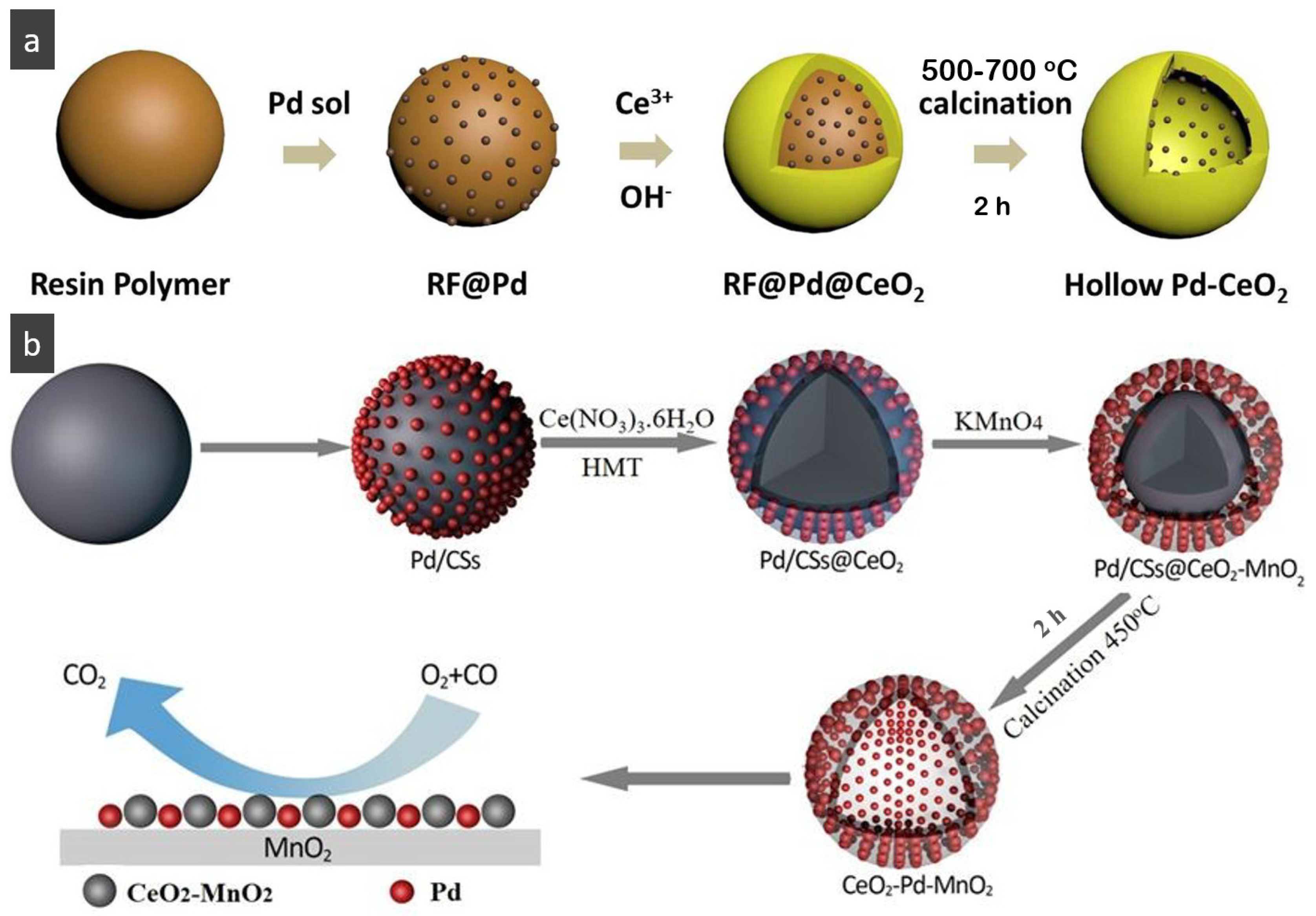
- Du, C.; Guo, Y.; Guo, Y.; Gong, X.-Q.; Lu, G. Polymer-templated synthesis of hollow Pd–CeO2 nanocomposite spheres and their catalytic activity and thermal stability. J. Mater. Chem. A 2015, 3, 23230–23239. [Google Scholar] [CrossRef]
- Guo, H.; He, Y.; Wang, Y.; Liu, L.; Yang, X.; Wang, S.; Huang, Z.; Wei, Q. Morphology-controlled synthesis of cage-bell Pd@CeO2 structured nanoparticle aggregates as catalysts for the low-temperature oxidation of CO. J. Mater. Chem. A 2013, 1, 7494. [Google Scholar] [CrossRef]
- Zhang, J.; Li, T.; Wang, C.-A.; Luo, J.-L. Rational design of sandwich-like MnO2-Pd-CeO2 hollow spheres with enhanced activity and stability for CO oxidation. Nanoscale 2019, 11, 6776–6783. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zhao, Y.; Guo, H.; Lu, A.; Zhang, X.; Wang, L.; Chen, M.-S.; Peng, D.-L. Facile preparation of well-dispersed CeO2-ZnO composite hollow microspheres with enhanced catalytic activity for CO oxidation. ACS Appl. Mater. Interfaces 2014, 6, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wang, F.; Ma, X.; Tang, Q.; Wang, K.; Guo, Y.; Yang, L. Facile one-step synthesis of porous ceria hollow nanospheres for low temperature CO oxidation. Microporous Mesoporous Mater. 2013, 176, 1–7. [Google Scholar] [CrossRef]
- Liu, X.; Yang, H.; Han, L.; Liu, W.; Wang, S.; Zhang, C.; Zhang, X.; Yang, Y. Mesoporous-Shelled CeO2 Hollow Nanospheres Synthesized by a One-pot Hydrothermal Route and its Catalysis Performance. CrystEngComm 2013, 15, 7769–7775. [Google Scholar] [CrossRef]
- Zhu, F.; Chen, G.; Sun, S.; Sun, X. In situ growth of Au@CeO2 core–shell nanoparticles and CeO2nanotubes from Ce(OH)CO3 nanorods. J. Mater. Chem. A 2013, 1, 288–294. [Google Scholar] [CrossRef]
- Du, C.; Guo, Y.; Guo, Y.; Gong, X.-Q.; Lu, G. Synthesis of hollow structured core-shell Au@CeO2-ZrO2 nanocatalyst and its excellent catalytic performance. J. Mater. Chem. A 2017, 5, 5601–5611. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, J.; Zhao, K.; Zong, L.; Tang, Z.; Wang, L.; Yu, R. Controlled synthesis of highly active Au/CeO2 nanotubes for CO oxidation. Mater. Chem. Front. 2017, 1, 1629–1634. [Google Scholar] [CrossRef]
- Wang, Y.; Song, G.; Xu, Z.; Rosei, F.; Ma, D.; Chen, G. Interfacial Reaction-Directed Synthesis of Ceria Nanotube-embedded Ultra-small Pt Nanoparticle Catalyst with High Catalytic Activity and Thermal Stability. J. Mater. Chem. A 2016, 4, 14148–14154. [Google Scholar] [CrossRef]
- Wu, K.; Zhou, L.; Jia, C.-J.; Sun, L.-D.; Yan, C.-H. Pt-embedded-CeO2 hollow spheres for enhancing CO oxidation performance. Mater. Chem. Front. 2017, 1, 1754–1763. [Google Scholar] [CrossRef]
- Chen, G.; Yang, Y.; Guo, Z.; Gao, D.; Zhao, W.; Yan, H.; Wang, W.-W.; Jia, C.-J.; Sun, G. Thermally Stable and Highly Active Pt/CeO2@SiO2 Catalysts with a Porous/Hollow Structure. Catal. Sci. Technol. 2018, 8, 4413–4419. [Google Scholar] [CrossRef]
- Chen, G.Z.; Wang, Y.; Wei, Y.W.; Zhao, W.; Gao, D.W.; Yang, H.X.; Li, C.C. Successive Interfacial Reaction-Directed Synthesis of CeO2@Au@CeO2-MnO2 Environmental Catalyst with Sandwich Hollow Structure. ACS Appl. Mater. Interfaces 2018, 10, 11595–11603. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Chen, J.; Li, G.; Li, S.; Gao, Y.; Tang, Z. Facile synthesis of core–shell Au@CeO2 nanocomposites with remarkably enhanced catalytic activity for CO oxidation. Energy Environ. Sci. 2012, 5, 8937. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Liang, Q.; Chen, L.; Zhan, W.; Li, Y. Structure-induced hollow Co3O4 nanoparticles with rich oxygen vacancies for efficient CO oxidation. Sci. China Mater. 2019, 63, 267–275. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.; Xu, G.; Ma, X.; Xu, J.; Zhang, L.; Qi, C.; Xie, Y.; Sun, Z.; Jia, D. Hollow and Core-Shell Nanostructure Co3O4 Derived from a Metal Formate Framework toward High Catalytic Activity of CO Oxidation. ACS Appl. Nano Mater. 2018, 1, 800–806. [Google Scholar] [CrossRef]
- Zeng, L.; Li, K.; Wang, H.; Yu, H.; Zhu, X.; Wei, Y.; Ning, P.; Shi, C.; Luo, Y. CO Oxidation on Au/α-Fe2O3-Hollow Catalysts: General Synthesis and Structural Dependence. J. Phys. Chem. C 2017, 121, 12696–12710. [Google Scholar] [CrossRef]
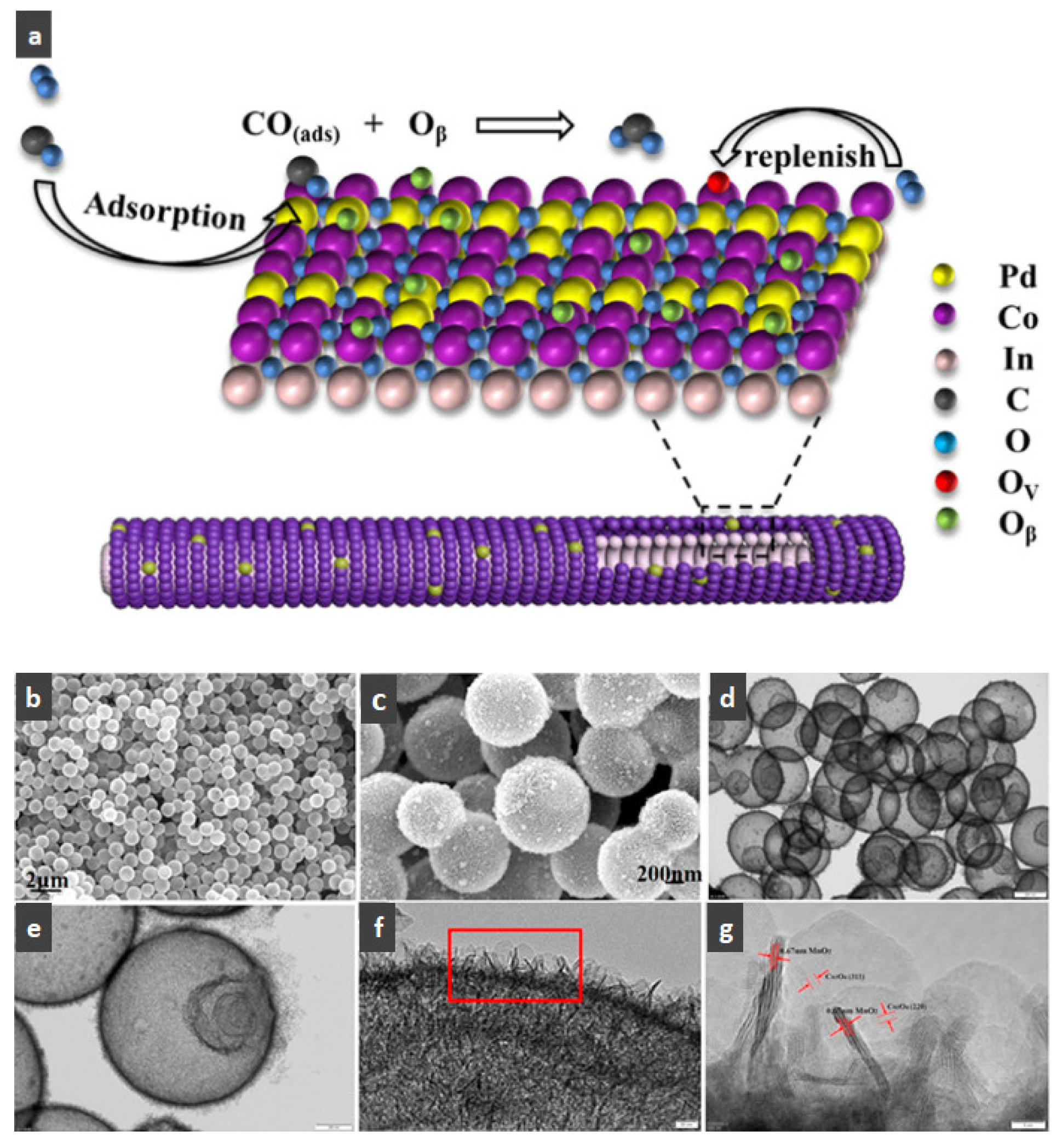
- Du, X.; Dong, F.; Tang, Z.; Zhang, J. The synthesis of hollow In2O3@Pd-Co3O4 core/shell nanofibers with ultra-thin shell for the low-temperature CO oxidation reaction. Appl. Surf. Sci. 2020, 505, 144471. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, F.; Wang, C.-A. Facile synthesis of multi-shelled MnO2-Co3O4 hollow spheres with superior catalytic activity for CO oxidation. Ceram. Int. 2021, 47, 18411–18416. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, D.; Wang, D. Mesoscience in Hollow Multi-Shelled Structures. Adv. Sci. 2023, 11, 2305408. [Google Scholar] [CrossRef]
- Barth, J.O.; Jentys, A.; Lercher, J.A. Elementary Reactions and Intermediate Species Formed during the Oxidative Regeneration of Spent Fluid Catalytic Cracking Catalysts. Ind. Eng. Chem. Res. 2004, 43, 3097–3104. [Google Scholar] [CrossRef]
- Ma, K.; Guo, K.; Li, L.; Zou, W.; Tang, C.; Dong, L. Cavity size dependent SO2 resistance for NH3-SCR of hollow structured CeO2-TiO2 catalysts. Catal. Commun. 2019, 128, 105719. [Google Scholar] [CrossRef]
- Shao, J.; Lin, F.; Huang, Y.; Wang, Z.; Li, Y.; Chen, G.; Cen, K. MnOx fabrication with rational design of morphology for enhanced activity in NO oxidation and SO2 resistance. Appl. Surf. Sci. 2020, 503, 144064. [Google Scholar] [CrossRef]
- Shi, Y.; Yi, H.; Gao, F.; Zhao, S.; Xie, Z.; Tang, X. Facile synthesis of hollow nanotube MnCoOx catalyst with superior resistance to SO2 and alkali metal poisons for NH3-SCR removal of NOx. Sep. Purif. 2021, 265, 118517. [Google Scholar] [CrossRef]
- Katare, S.R.; Patterson, J.E.; Laing, P.M. Diesel Aftertreatment Modeling: A Systems Approach to NOx Control. Ind. Eng. Chem. Res. 2007, 46, 2445–2454. [Google Scholar] [CrossRef]
- Vélez, R.P.; Ellmers, I.; Huang, H.; Bentrup, U.; Schünemann, V.; Grünert, W.; Brückner, A. Identifying active sites for fast NH3-SCR of NO/NO2 mixtures over Fe-ZSM-5 by operando EPR and UV–vis spectroscopy. J. Catal. 2014, 316, 103–111. [Google Scholar] [CrossRef]
- Li, C.; Tang, X.; Yi, H.; Wang, L.; Cui, X.; Chu, C.; Li, J.; Zhang, R.; Yu, Q. Rational design of template-free MnOx-CeO2 hollow nanotube as de-NOx catalyst at low temperature. Appl. Surf. Sci. 2018, 428, 924–932. [Google Scholar] [CrossRef]
- Xie, C.; Yang, S.; Shi, J.-W.; Niu, C. Constructing hollow silkworm structure in MnOx–TiO2 catalysts for improving the performance in selective catalytic reduction of NO by NH3. React. Kinet. Mech. Catal. 2019, 128, 681–693. [Google Scholar] [CrossRef]
- Liu, C.; Gao, G.; Shi, J.-W.; He, C.; Li, G.; Bai, N.; Niu, C. MnOx-CeO2 shell-in-shell microspheres for NH3-SCR de-NOx at low temperature. Catal. Commun. 2016, 86, 36–40. [Google Scholar] [CrossRef]
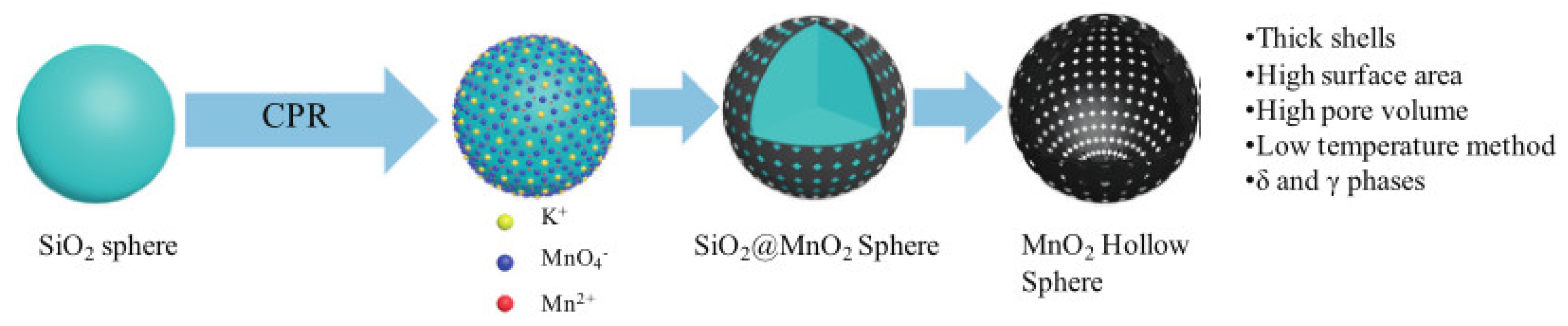
- Guo, R.-T.; Chen, Q.-L.; Ding, H.-L.; Wang, Q.-S.; Pan, W.-G.; Yang, N.-Z.; Lu, C.-Z. Preparation and characterization of CeOx@MnOx core–shell structure catalyst for catalytic oxidation of NO. Catal. Commun. 2015, 69, 165–169. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, L.; Sun, N.; Wang, H.; Zhong, L.; He, C.; Wei, W.; Sun, Y. Hollow MnOx-CeO2 mixed oxides as highly efficient catalysts in NO oxidation. Chem. Eng. J. 2017, 322, 46–55. [Google Scholar] [CrossRef]
- Ma, K.; Zou, W.; Zhang, L.; Li, L.; Yu, S.; Tang, C.; Gao, F.; Dong, L. Construction of hybrid multi-shell hollow structured CeO2–MnOx materials for selective catalytic reduction of NO with NH3. RSC Adv. 2017, 7, 5989–5999. [Google Scholar] [CrossRef]
- Han, Y.; Mu, J.; Li, X.; Gao, J.; Fan, S.; Tan, F.; Zhao, Q. Triple-Shelled NiMn2O4 Hollow Spheres as an Efficient Catalyst for Low-Temperature Selective Catalytic Reduction of NOx with NH3. Chem. Commun. 2018, 54, 9797–9800. [Google Scholar] [CrossRef]
- Cheng, S.; Shao, J.; Huang, B.; Guan, J.; Zhou, L. Promotion effect of urchin-like MnOx@PrOx hollow core-shell structure catalysts for the low-temperature selective catalytic reduction of NO with NH3. RSC Adv. 2020, 10, 13855–13865. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Du, Y.; Liu, J.; Zhao, Z.; Cheng, K.; Chen, Y.; Wei, Y.; Song, W.; Zhang, X. Design of MoFe/Beta@CeO2 catalysts with a core−shell structure and their catalytic performances for the selective catalytic reduction of NO with NH3. Appl. Catal. B 2017, 203, 704–714. [Google Scholar] [CrossRef]
- Zhan, S.; Shi, Q.; Zhang, Y.; Li, Y.; Tian, Y. Preparation of novel CeMo(x) hollow microspheres for low-temperature SCR removal of NOx with NH3. RSC Adv. 2016, 6, 59185–59194. [Google Scholar] [CrossRef]
- Hao, Z.; Jiao, Y.; Shi, Q.; Zhang, H.; Zhan, S. Improvement of NH3-SCR performance and SO2 resistance over Sn modified CeMoOx electrospun fibers at low temperature. Catal. Today 2019, 327, 37–46. [Google Scholar] [CrossRef]
- Zhao, Y.; Cai, W.; Chen, M.; Bu, Y. Turning the activity of Cr–Ce mixed oxide towards thermocatalytic NO oxidation and photocatalytic CO2 reduction via the formation of yolk shell structure hollow microspheres. J. Alloys Compd. 2020, 829, 154508. [Google Scholar] [CrossRef]
- Chi, B.; Qu, H.; Xing, X.; Zhong, Q. Assembly of hollow CeO2@Fe-ZSM-5 and SCR performance. J. Alloys Compd. 2017, 726, 906–912. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Q.; Wu, Q.; Luo, Z.; Zhao, W.; Chen, J.; Li, J. A hollow structure WO3@CeO2 catalyst for NH3-SCR of NOx. Catal. Commun. 2021, 149, 106252. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, R. Rational design of porous CexNb1−x oxide hollow nanospheres as a novel NH3-SCR catalyst. J. Mater. Chem. A 2022, 10, 12269–12277. [Google Scholar] [CrossRef]
- Gong, P.; Xie, J.; Fang, D.; He, F.; Li, F.; Qi, K. Enhancement of the NH3-SCR property of Ce-Zr-Ti by surface and structure modification with P. Appl. Surf. Sci. 2020, 505, 144641. [Google Scholar] [CrossRef]
- Wang, H.; Jin, B.; Wang, H.; Ma, N.; Liu, W.; Weng, D.; Wu, X.; Liu, S. Study of Ag promoted Fe2O3@CeO2 as superior soot oxidation catalysts: The role of Fe2O3 crystal plane and tandem oxygen delivery. Appl. Catal. B 2018, 237, 251–262. [Google Scholar] [CrossRef]
- Li, W.-J.; Wey, M.-Y. Core-shell design and well-dispersed Pd particles for three-way catalysis: Effect of halloysite nanotubes functionalized with Schiff base. Sci. Total Environ. 2019, 675, 397–407. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Dai, H.X.; Au, C.T. An investigation on the utilization of perovskite-type oxides La1−xSrxMO3 (M = Co0.77Bi0.20Pd0.03) as three-way catalysts. Appl. Catal. B 2001, 33, 65–80. [Google Scholar] [CrossRef]
- Zhao, H.; Li, H.; Pan, Z.; Feng, F.; Gu, Y.; Du, J.; Zhao, Y. Design of CeMnCu ternary mixed oxides as soot combustion catalysts based on optimized Ce/Mn and Mn/Cu ratios in binary mixed oxides. Appl. Catal. B 2020, 268, 118422. [Google Scholar] [CrossRef]
- Li, W.-J.; Wey, M.-Y. Sintering-resistant, highly thermally stable and well-dispersed Pd@CeO2/halloysite as an advanced three-way catalyst. Sci. Total Environ. 2020, 707, 136137. [Google Scholar] [CrossRef]
- Zhou, Z.; Ouyang, J.; Yang, H.; Tang, A. Three-way catalytic performances of Pd loaded halloysite-Ce0.5Zr0.5O2 hybrid materials. Appl. Clay Sci. 2016, 121–122, 63–70. [Google Scholar] [CrossRef]
- Feng, N.J.; Zhu, Z.J.; Zhao, P.; Wang, L.; Wan, H.; Guan, G.F. Facile fabrication of trepang-like CeO2@MnO2 nanocomposite with high catalytic activity for soot removal. Appl. Surf. Sci. 2020, 515, 146013. [Google Scholar] [CrossRef]
- Fang, F.; Zhao, P.; Feng, N.; Chen, C.; Li, X.; Liu, G.; Wan, H.; Guan, G. Construction of a hollow structure in La0.9K0.1CoO3−δ nanofibers via grain size control by Sr substitution with an enhanced catalytic performance for soot removal. Catal. Sci. Technol. 2019, 9, 4938–4951. [Google Scholar] [CrossRef]
- Yu, Y.; Ma, Q.; Tan, C.; Wang, H.; Hu, Z.; Zhang, Q.; Zhang, H.; Zhang, B. Preparation of hierarchical hollow structures assembled from porous NiCo2O4 nanosheets for diesel soot elimination. EcoMat 2020, 2, e12041. [Google Scholar] [CrossRef]
- Scire, S.; Liotta, L.F. Supported gold catalysts for the total oxidation of volatile organic compounds. Appl. Catal. B 2012, 125, 222–246. [Google Scholar] [CrossRef]
- Lyu, J.; Zhou, L.; Shao, J.; Zhou, Z.; Gao, J.; Li, J.; Dong, Y.; Wang, Z. Synthesis of TiO2/H2Ti3O7 composite with nanoscale spiny hollow hierarchical structure for photocatalytic mineralization of VOCs. Chem. Eng. J. 2020, 400, 125927. [Google Scholar] [CrossRef]
- Kondratowicz, T.; Drozdek, M.; Michalik, M.; Gac, W.; Gajewska, M.; Kuśtrowski, P. Catalytic activity of Pt species variously dispersed on hollow ZrO2 spheres in combustion of volatile organic compounds. Appl. Surf. Sci. 2020, 513, 145788. [Google Scholar] [CrossRef]
- Qi, L.; Cheng, B.; Yu, J.; Ho, W. High-surface area mesoporous Pt/TiO2 hollow chains for efficient formaldehyde decomposition at ambient temperature. J. Hazard. Mater. 2016, 301, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Levasseur, B.; Kaliaguine, S. Effects of iron and cerium in La1−yCeyCo1−xFexO3 perovskites as catalysts for VOC oxidation. Appl. Catal. B 2009, 88, 305–314. [Google Scholar] [CrossRef]
- Feng, Z.; Ren, Q.; Peng, R.; Mo, S.; Zhang, M.; Fu, M.; Chen, L.; Ye, D. Effect of CeO2 morphologies on toluene catalytic combustion. Catal. Today 2019, 332, 177–182. [Google Scholar] [CrossRef]
- Li, D.; Yang, J.; Tang, W.; Wu, X.; Wei, L.; Chen, Y. Controlled synthesis of hierarchical MnO2 microspheres with hollow interiors for the removal of benzene. RSC Adv. 2014, 4, 26796. [Google Scholar] [CrossRef]
- Fang, W.; Chen, J.; Zhou, X.; Chen, J.; Ye, Z.; Li, J. Zeolitic Imidazolate Framework-67-Derived CeO2@Co3O4 Core–Shell Microspheres with Enhanced Catalytic Activity toward Toluene Oxidation. Ind. Eng. Chem. Res. 2020, 59, 10328–10337. [Google Scholar] [CrossRef]
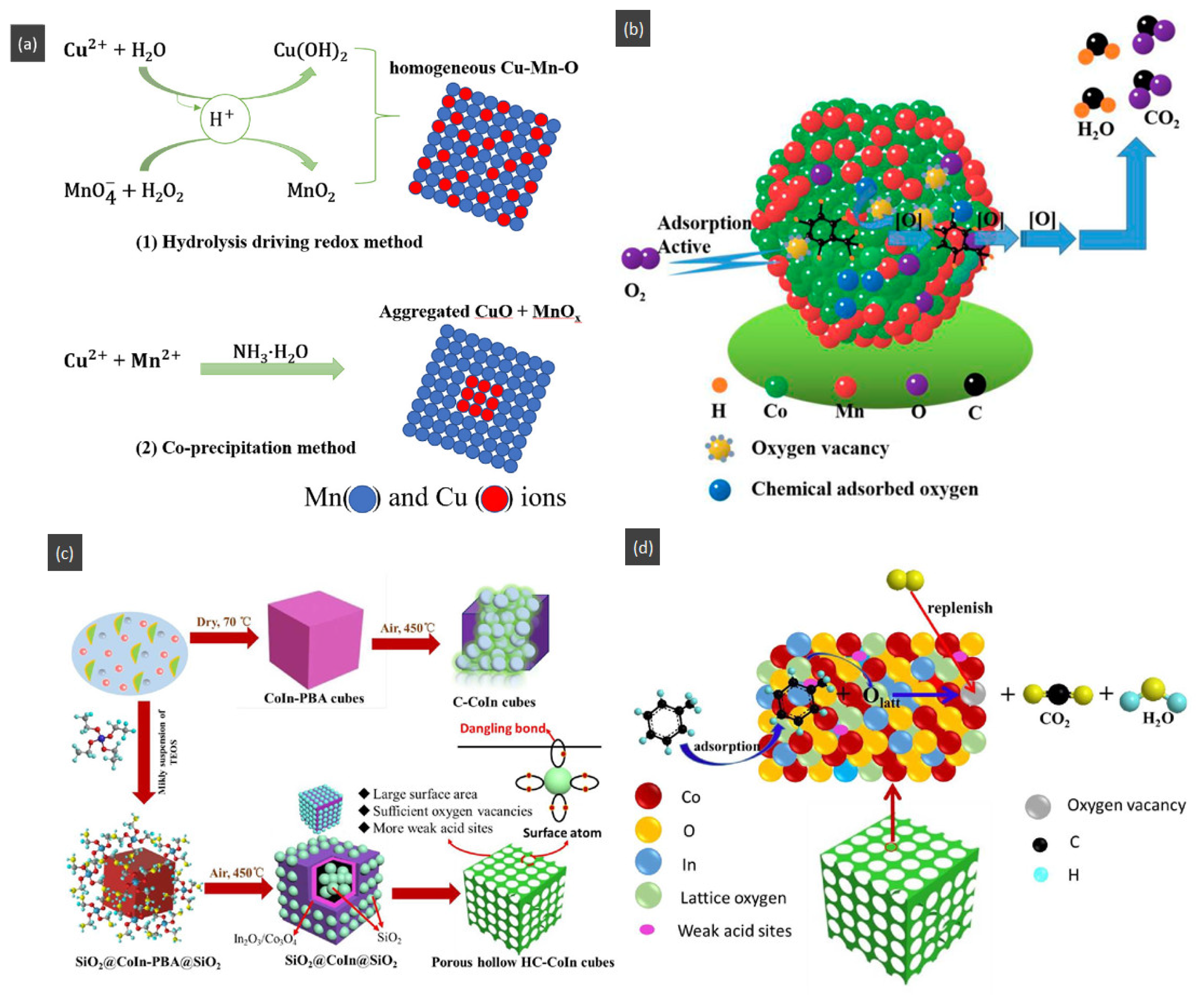
- Dong, F.; Han, W.; Zhao, H.; Zhang, G.; Tang, Z. The Porous hollow CoInOx nanocubes as a highly efficient catalyst for the catalytic combustion of toluene. Nanoscale 2019, 11, 9937–9948. [Google Scholar] [CrossRef]
- Gu, W.X.; Li, C.Q.; Qiu, J.H.; Yao, J.F. Facile fabrication of flower-like MnO2 hollow microspheres as high-performance catalysts for toluene oxidation. J. Hazard. Mater. 2021, 408, 124458. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.H.; Tang, Z.C.; Dong, F.; Zhang, J.Y. Controlled porous hollow Co3O4 polyhedral nanocages derived from metal-organic frameworks (MOFs) for toluene catalytic oxidation. Mol. Catal. 2019, 463, 77–86. [Google Scholar] [CrossRef]
- Liao, Y.N.; Zhang, X.; Peng, R.S.; Zhao, M.Q.; Ye, D.Q. Catalytic properties of manganese oxide polyhedra with hollow and solid morphologies in toluene removal. Appl. Surf. Sci. 2017, 405, 20–28. [Google Scholar] [CrossRef]
- Mo, S.; Zhang, Q.; Zhang, M.; Zhang, Q.; Li, J.; Fu, M.; Wu, J.; Chen, P.; Ye, D. Elucidating the special role of strong metal–support interactions in Pt/MnO2 catalysts for total toluene oxidation. Nanoscale Horiz. 2019, 4, 1425–1433. [Google Scholar] [CrossRef]
- Xiao, Z.; Yang, J.; Ren, R.; Li, J.; Wang, N.; Chu, W. Facile synthesis of homogeneous hollow microsphere Cu-Mn based catalysts for catalytic oxidation of toluene. Chemosphere 2020, 247, 125812. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, C.; Yan, J.; Wang, D.; Peng, Y.; Li, J.; Crittenden, J. Distinctive Bimetallic Oxides for Enhanced Catalytic Toluene Combustion: Insights into the Tunable Fabrication of Mn−Ce Hollow Structure. ChemCatChem 2020, 12, 2872–2879. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Z.; Li, Y.; Leng, X.; Zhang, T.; Yuan, F.; Niu, X.; Zhu, Y. Synthesis of CeaMnOx hollow microsphere with hierarchical structure and its excellent catalytic performance for toluene combustion. Appl. Catal. B 2019, 245, 502–512. [Google Scholar] [CrossRef]
- Zhao, J.H.; Han, W.L.; Tang, Z.C.; Zhang, J.Y. Carefully Designed Hollow MnxCo3−xO4 Polyhedron Derived from in Situ Pyrolysis of Metal–Organic Frameworks for Outstanding Low-Temperature Catalytic Oxidation Performance. Cryst. Growth Des. 2019, 19, 6207–6217. [Google Scholar] [CrossRef]
- Yang, S.L.; Yang, H.C.; Yang, J.Y.; Qi, H.L.; Kong, J.; Bo, Z.; Li, X.D.; Yan, J.H.; Cen, K.F.; Tu, X. Three-dimensional hollow urchin α-MnO2 for enhanced catalytic activity towards toluene decomposition in post-plasma catalysis. Chem. Eng. J. 2020, 402, 126154. [Google Scholar] [CrossRef]
- Xu, Y.; Dhainaut, J.; Rochard, G.; Dacquin, J.-P.; Mamede, A.-S.; Giraudon, J.-M.; Lamonier, J.-F.; Zhang, H.; Royer, S. Hierarchical porous ε-MnO2 from perovskite precursor: Application to the formaldehyde total oxidation. Chem. Eng. J. 2020, 388, 124146. [Google Scholar] [CrossRef]
- Dong, C.; Qu, Z.; Qin, Y.; Fu, Q.; Sun, H.; Duan, X. Revealing the Highly Catalytic Performance of Spinel CoMn2O4 for Toluene Oxidation: Involvement and Replenishment of Oxygen Species Using In Situ Designed-TP Techniques. ACS Catal. 2019, 9, 6698–6710. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, W.; Long, B.; Li, H.; Qiu, W.; Zhao, F.; Tong, Y.; Ji, H. Alkali-modified non-precious metal 3D-NiCo2O4 nanosheets for efficient formaldehyde oxidation at low temperature. J. Mater. Chem. A 2016, 4, 3648–3654. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.; Chen, F.; Xiang, Y.; Yan, J.; Chu, W. Facile fabrication of hollow structured Cu-Ce binary oxides and their catalytic properties for toluene combustion. Catal. Today 2020, 376, 239–246. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Le, Y.; Qi, L.; Wang, C.; Song, S. Hierarchical Pt/WO3 nanoflakes assembled hollow microspheres for room-temperature formaldehyde oxidation activity. Appl. Surf. Sci. 2020, 512, 145763. [Google Scholar] [CrossRef]
- Wang, C.; Liu, N.; Zhang, C.; Liu, X.; Li, X.; Zhao, X.S. Ruthenium/cobalt binary oxides supported on hollow alumina microspheres as highly efficient catalyst for vinyl chloride oxidation. Appl. Surf. Sci. 2019, 497, 143776. [Google Scholar] [CrossRef]
- Chen, H.M.; He, J.H.; Zhang, C.B.; He, H. Self-Assembly of Novel Mesoporous Manganese Oxide Nanostructures and Their Application in Oxidative Decomposition of Formaldehyde. J. Phys. Chem. C 2007, 111, 18033–18038. [Google Scholar] [CrossRef]
- Pang, G.; Wang, D.; Zhang, Y.; Ma, C.; Hao, Z. Catalytic activities and mechanism of formaldehyde oxidation over gold supported on MnO2 microsphere catalysts at room temperature. Front. Environ. Sci. Eng. 2015, 10, 447–457. [Google Scholar] [CrossRef]
- Nie, L.H.; Meng, A.Y.; Yu Jg Jaroniec, M. Hierarchically macro-mesoporous Pt/γ-Al2O3 composite microspheres for efficient formaldehyde oxidation at room temperature. Sci. Rep. 2013, 3, 3215. [Google Scholar] [CrossRef]
- Qi, L.; Cheng, B.; Ho, W.; Liu, G.; Yu, J. Hierarchical Pt/NiO Hollow Microspheres with Enhanced Catalytic Performance. ChemNanoMat 2015, 1, 58–67. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, Y.; Qin, L.F.; Zeng, C.; Xiao, W. Hollow structured CoSn(OH)6-supported Pt for effective room-temperature oxidation of gaseous formaldehyde. Funct. Mater. Lett. 2017, 09, 1642009. [Google Scholar] [CrossRef]
- Lv, T.; Peng, C.; Zhu, H.; Xiao, W. Heterostructured Fe2O3@SnO2 core–shell nanospindles for enhanced Room-temperature HCHO oxidation. Appl. Surf. Sci. 2018, 457, 83–92. [Google Scholar] [CrossRef]
- Zhang, C.B.; He, H.; Tanaka, K.-i. Perfect catalytic oxidation of formaldehyde over a Pt/TiO2 catalyst at room temperature. Catal. Commun. 2005, 6, 211–214. [Google Scholar] [CrossRef]
- Zhang, C.B.; He, H.; Tanaka, K.-i. Catalytic performance and mechanism of a Pt/TiO2 catalyst for the oxidation of formaldehyde at room temperature. Appl. Catal. B 2006, 65, 37–43. [Google Scholar] [CrossRef]
- Khalifeh, R.; Karimi, M.; Rajabzadeh, M.; Hafizi, A.; Nogorani, F.S. Synthesis and morphology control of nano CuAl2O4 hollow spheres and their application as an efficient and sustainable catalyst for CO2 fixation. J. CO2 Util. 2020, 41, 101233. [Google Scholar] [CrossRef]
- Prasad, D.; Patil, K.N.; Bhanushali, J.T.; Nagaraja, B.M.; Jadhav, A.H. Sustainable fixation of CO2 into epoxides to form cyclic carbonates using hollow marigold CuCo2O4 spinel microspheres as a robust catalyst. Catal. Sci. Technol. 2019, 9, 4393–4412. [Google Scholar] [CrossRef]
- Bian, Y.; Xu, C.; Wen, X.; Xu, L.; Cui, Y.; Wang, S.; Wu, C.-E.; Qiu, J.; Cheng, G.; Chen, M. CO2 methanation over the Ni-based catalysts supported on nano-CeO2 with varied morphologies. Fuel 2023, 331, 125755. [Google Scholar] [CrossRef]
- Cui, Y.; Lian, X.; Xu, L.; Chen, M.; Yang, B.; Wu, C.-E.; Li, W.; Huang, B.; Hu, X. Designing and Fabricating Ordered Mesoporous Metal Oxides for CO2 Catalytic Conversion: A Review and Prospect. Materials 2019, 12, 276. [Google Scholar] [CrossRef]
- Heydari, P.; Hafizi, A.; Rajabzadeh, M.; Karimi, M.; Khalifeh, R.; Rahimpour, M.R. Synthesis and application of nanoporous triple-shelled CuAl2O4 hollow sphere catalyst for atmospheric chemical fixation of carbon dioxide. J. Taiwan Inst. Chem. Eng. 2020, 114, 81–90. [Google Scholar] [CrossRef]
- Ion, A.; Parvulescu, V.; Jacobs, P.; Vos, D.D. Synthesis of symmetrical or asymmetrical urea compounds from CO2 via base catalysis. Green. Chem. 2007, 9, 158–161. [Google Scholar] [CrossRef]
- Leitner, W. Carbon Dioxide as a Raw Material: The Synthesis of Formic Acid and Its Derivatives from CO2. Angew. Chem. Int. Ed. Engl. 1995, 34, 2207–2221. [Google Scholar] [CrossRef]
- Qian, Q.; Zhang, J.; Cui, M.; Han, B. Synthesis of acetic acid via methanol hydrocarboxylation with CO2 and H2. Nat. Commun. 2016, 7, 11481. [Google Scholar] [CrossRef]
- Witoon, T.; Permsirivanich, T.; Donphai, W.; Jaree, A.; Chareonpanich, M. CO2 hydrogenation to methanol over Cu/ZnO nanocatalysts prepared via a chitosan-assisted co-precipitation method. Fuel Process. Technol. 2013, 116, 72–78. [Google Scholar] [CrossRef]
- Tian, H.-F.; Yu, L.; Ding, J.; Zha, F.; Tang, X.-H.; Chang, Y. Synthesis of hollow CuO/ZnO/Al2O3 composite microspheres for catalysing carbon dioxide hydrogenation. Micro Nano Lett. 2019, 14, 932–936. [Google Scholar] [CrossRef]
- Han, X.; Li, M.; Chang, X.; Hao, Z.; Chen, J.; Pan, Y.; Kawi, S.; Ma, X. Hollow structured Cu@ZrO2 derived from Zr-MOF for selective hydrogenation of CO2 to methanol. J. Energy Chem. 2022, 71, 277–287. [Google Scholar] [CrossRef]
- Cui, W.G.; Zhang, Q.; Zhou, L.; Wei, Z.C.; Yu, L.; Dai, J.J.; Zhang, H.; Hu, T.L. Hybrid MOF Template-Directed Construction of Hollow-Structured In2O3@ZrO2 Heterostructure for Enhancing Hydrogenation of CO2 to Methanol. Small 2022, 19, 2204914. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Jaén, J.J.D.; Garrido, J.C.H.; Bakhmutsky, K.; Montini, T.; Gámez, J.J.C.; Gorte, R.J.; Fornasiero, P. Exceptional activity for methane combustion over modular Pd@CeO2 subunits on functionalized Al2O3. Science 2012, 337, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, D.; Shi, L.; Gao, R.; Zhang, J. Morphology Dependence of Catalytic Properties of Ni/CeO2 Nanostructures for Carbon Dioxide Reforming of Methane. J. Phys. Chem. C 2012, 116, 10009–10016. [Google Scholar] [CrossRef]
- Li, Z.W.; Kawi, S. Multi-Ni@Ni phyllosilicate hollow sphere for CO2 reforming of CH4: Influence of Ni precursors on structure, sintering and carbon resistance. Catal. Sci. Technol. 2018, 8, 1915–1922. [Google Scholar] [CrossRef]
- Wang, G.; Liang, Y.; Song, J.; Li, H.; Zhao, Y. Study on High Activity and Outstanding Stability of Hollow-NiPt@SiO2 Core-Shell Structure Catalyst for DRM Reaction. Front. Chem. 2020, 8, 220. [Google Scholar] [CrossRef]
- Sheng, K.F.; Luan, D.; Jiang, H.; Zeng, F.; Wei, B.; Pang, F.; Ge, J.P. NixCoy Nanocatalyst Supported by ZrO2 Hollow Sphere for Dry Reforming of Methane: Synergetic Catalysis by Ni and Co in Alloy. ACS Appl. Mater. Interfaces 2019, 11, 24078–24087. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Wang, Z.; Kawi, S. Oxidative CO2 reforming of methane in La0.6Sr0.4Co0.8Ga0.2O3-delta (LSCG) hollow fiber membrane reactor. Environ. Sci. Technol. 2013, 47, 14510–14517. [Google Scholar] [CrossRef]
- Li, Z.; Sibudjing, K. Facile Synthesis of Multi-Ni-Core@Ni Phyllosilicate@CeO2 Shell Hollow Spheres with High Oxygen Vacancy Concentration for Dry Reforming of CH4. ChemCatChem 2018, 10, 2994–3001. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, X.; Liu, J.; Zhao, L.; Song, X.; Zhang, P.; Gao, L. Hollow hierarchical Ni/MgO-SiO2 catalyst with high activity, thermal stability and coking resistance for catalytic dry reforming of methane. Int. J. Hydrogen Energy 2018, 43, 11056–11068. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, D.; Zhao, Y.; Moyo, P.S.; Zhao, Y.; Wang, S.; Ma, X. Enhanced catalytic performance of Nix-V@HSS catalysts for the DRM reaction: The study of interfacial effects on Ni-VOx structure with a unique yolk-shell structure. J. Catal. 2021, 396, 65–80. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Zhou, Y.; Xiang, S.; Wang, Q.; Zhang, C.; Sheng, X. CeO2 hollow nanospheres synthesized by a one pot template-free hydrothermal method and their application as catalyst support. RSC Adv. 2015, 5, 58237–58245. [Google Scholar] [CrossRef]
- Chen, C.; Li, D.; Wang, A.; Guo, J.; Dong, S.; Chen, D.; Jiao, X.; Xia, Y. Interfacial enhancement for hydrogen radical transfer on hollow Cu2O/rGO nanohybrid with efficient catalytic reduction activity. Appl. Catal. A Gen. 2020, 590, 117331. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wang, C.; Wang, T. Facile approach to synthesize uniform Au@mesoporous SnO2 yolk–shell nanoparticles and their excellent catalytic activity in 4-nitrophenol reduction. J. Nanopart Res. 2015, 18, 2. [Google Scholar] [CrossRef]
- Zhang, S.F.; Zhao, D.Y.; Hou, C.; Liang, C.; Li, H. Facile one-pot synthesis of cellulose nanocrystal-supported hollow CuFe2O4 nanoparticles as efficient catalyst for 4-nitrophenol reduction. J. Nanopart Res. 2018, 20, 161. [Google Scholar] [CrossRef]
- Xia, Q.D.; Fu, S.S.; Ren, G.J.; Chai, F.; Jiang, J.J.; Qu, F.Y. Fabrication of Fe3O4@Au hollow spheres with recyclable and efficient catalytic properties. New J. Chem. 2016, 40, 818–824. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Kou, Q.; Liu, Y.; Han, D.; Wang, D.; Sun, Y.; Zhang, Y.; Wang, Y.; Lu, Z.; et al. Enhanced Catalytic Reduction of 4-Nitrophenol Driven by Fe3O4-Au Magnetic Nanocomposite Interface Engineering: From Facile Preparation to Recyclable Application. Nanomaterials 2018, 8, 353. [Google Scholar] [CrossRef]
- Ma, M.L.; Yang, Y.Y.; Feng, R.J.; Jia, L.; Chen, G.P.; Li, W.T.; Lyu, P. Preparation and characterization of magnetic hollow Fe3O4/P(GMA-EGDMA)-SO3H/Au-PPy recyclable catalyst for catalytic reduction of 4-nitrophenol. Appl. Organomet. Chem. 2018, 32, e4534. [Google Scholar] [CrossRef]
- Cheng, J.C.; Zhao, S.L.; Gao, W.B.; Jiang, P.B.; Li, R. Au/Fe3O4@TiO2 hollow nanospheres as efficient catalysts for the reduction of 4-nitrophenol and photocatalytic degradation of rhodamine B. React. Kinet. Mech. Catal. 2017, 121, 797–810. [Google Scholar] [CrossRef]
- Zhang, D.F.; Zhang, G.Z.; Zhang, L. Multi-shelled FeCo2O4 hollow porous microspheres/CCFs magnetic hybrid and its dual-functional catalytic performance. Chem. Eng. J. 2017, 330, 792–803. [Google Scholar] [CrossRef]
- Gao, Q.; Sun, Z.Q. Facile Fabrication of Uniform MFe2O4 (M = Co, Ni, Cu) Hollow Spheres and Their Recyclable Superior Catalytic Activity Towards 4-Nitrophenol Reduction. J. Nanosci. Nanotechnol. 2018, 18, 5645–5653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Z.; Jin, X.; Xu, Z.H.; Zhang, J.M.; Rendon, U.F.; Razzari, L.; Chaker, M.; Ma, D.L. Plasmonic Au Loaded Hierarchical Hollow Porous TiO2 Spheres: Synergistic Catalysts for Nitroaromatic Reduction. J. Phys. Chem. Lett. 2018, 9, 5317–5326. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Liang, X.; Zhang, L.; Tang, Z.; Al-Mamun, M.; Zhao, H.; Su, X. Fabrication of Highly Stable Metal Oxide Hollow Nanospheres and Their Catalytic Activity toward 4-Nitrophenol Reduction. ACS Appl. Mater. Interfaces 2017, 9, 18207–18214. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Zhang, Q.; Chen, J.Y.; Xia, Y.N. A comparison study of the catalytic properties of Au-based nanocages, nanoboxes, and nanoparticles. Nano Lett. 2010, 10, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.D.; Feng, X.; Guo, J.; Cao, H.Q.; Suo, X.Y.; Sun, H.W.; Zheng, M.H. Catalytic oxidation of 1,2-dichlorobenzene over Ca-doped FeOx hollow microspheres. Appl. Catal. B 2014, 147, 666–676. [Google Scholar] [CrossRef]
- Wen, J.X.; Guo, H.W.; Ma, X.D.; Wei, Z.Z.; He, X.; Zhang, L.L.; Li, B.D.; Wang, T.; Cheng, Y.H. Mesoporous Ce-doped ZnO hollow microspheres for oxidation of 1, 2-dichlorobenzene. Catal. Sci. Technol. 2020, 10, 3739–3747. [Google Scholar] [CrossRef]
- Zhao, B.; Shao, Q.; Hao, L.; Zhang, L.; Liu, Z.; Zhang, B.; Ge, S.; Guo, Z. Yeast-template synthesized Fe-doped cerium oxide hollow microspheres for visible photodegradation of acid orange 7. J. Colloid. Interface Sci. 2018, 511, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.W.; Fan, Q.H.; Gao, H.H.; Huang, Y.S.; Liu, X.; Li, J.X.; Xu, X.J.; Wang, X.K. Retraction: Formation of Fe3O4@MnO2 ball-in-ball hollow spheres as a high performance catalyst for enhanced catalytic performances. J. Mater. Chem. A 2022, 4, 1414–1422. [Google Scholar] [CrossRef]
- Lin, C.J.; Yang, W.-T.; Chou, C.-Y.; Liou, S.Y.H. Hollow mesoporous TiO2 microspheres for enhanced photocatalytic degradation of acetaminophen in water. Chemosphere 2016, 152, 490–495. [Google Scholar] [CrossRef]
- Chen, L.; Zuo, X.; Yang, S.; Cai, T.; Ding, D. Rational design and synthesis of hollow Co3O4@Fe2O3 core-shell nanostructure for the catalytic degradation of norfloxacin by coupling with peroxymonosulfate. Chem. Eng. J. 2019, 359, 373–384. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Wu, B.; Zhang, W.; Zhang, X.; Shan, C.; Liu, Q. CeO2/Co3O4 hollow microsphere: Pollen-biotemplated preparation and application in photo-catalytic degradation. Colloids Surf. 2020, 586, 124193. [Google Scholar] [CrossRef]
- Lee, D.-E.; Moru, S.; Jo, W.-K.; Tonda, S. Porous g-C3N4-encapsulated TiO2 hollow sphere as a high-performance Z-scheme hybrid for solar-induced photocatalytic abatement of environmentally toxic pharmaceuticals. J. Mater. Sci. Technol. 2021, 82, 21–32. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.; Dai, M.; Wang, W.; Lu, D.; Zhang, M.; Chen, Y.; Song, H. Resin microsphere templates for TiO2 hollow structure with uniform mesopores: Preparation and photocatalytic application. Mater. Chem. Phys. 2021, 260, 124158. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, Y.; Liu, Y.; Zhang, W.; Wang, Y.; Guo, X.; Tang, X.; Zhang, Y.; Wang, Z.; Zhang, T. Magnetic Mn-Doped Fe3O4 hollow Microsphere/RGO heterogeneous Photo-Fenton Catalyst for high efficiency degradation of organic pollutant at neutral pH. Mater. Chem. Phys. 2019, 238, 121893. [Google Scholar] [CrossRef]
- Wu, X.; Xia, F.; Nan, Z. Facile synthesis of double-mesoporous-shelled hollow spheres of Cu–CuFe2O4/SiO2 composite as excellent Fenton catalyst. Mater. Chem. Phys. 2020, 242, 122490. [Google Scholar] [CrossRef]
- He, F.; Ji, Y.; Wang, Y.; Zhang, Y. Preparation of bifunctional hollow mesoporous Fe0@C@MnFe2O4 as Fenton-like catalyst for degradation of Tetrabromobisphenol A. J. Taiwan Inst. Chem. Eng. 2017, 80, 553–562. [Google Scholar] [CrossRef]
- Ding, R.-R.; Li, W.-Q.; He, C.-S.; Wang, Y.-R.; Liu, X.-C.; Zhou, G.-N.; Mu, Y. Oxygen vacancy on hollow sphere CuFe2O4 as an efficient Fenton-like catalysis for organic pollutant degradation over a wide pH range. Appl. Catal. B 2021, 291, 120069. [Google Scholar] [CrossRef]



| Items | Details |
|---|---|
| Automobile and stationary sources emission | Catalytic oxidation of CO |
| NH3-SCR removal of NOx | |
| Catalyst for automobile three-way catalytic (TWC) reaction | |
| Catalyst for diesel oxidation catalytic (DOC) reaction | |
| Volatile organic compounds (VOCs) | Catalytic oxidation of toluene |
| Catalytic oxidation of vinyl chloride (VC) | |
| Catalytic oxidation of formaldehyde (HCHO) | |
| Greenhouse gases | Catalytic conversion of CO2 |
| Catalytic conversion of CH4 | |
| Other potential pollutants | Hydrogenation of 4-nitrophenol (4-NP) |
| Catalytic oxidation of 1,2-dichlorobenzene (o-DCB) | |
| Catalytic oxidation of dyes (e.g., acid orange 7(AO7), methylene blue) | |
| Photocatalytic degradation of pharmaceuticals (e.g., aceta-minophen, norfloxacin (NOR), tetracycline (TC), and ciprofloxacin) | |
| Photocatalytic degradation of organic pollutions (e.g., phenol) |
| Synthesis Method | SBET (m2 g−1) | Catalytic Performance | Morphology | Ref. |
|---|---|---|---|---|
| One-pot template-free route | 14.7 | T50 = 280 °C |  | [46] |
| Hydrothermal process | 22.0 | — |  | [47] |
| Template-free method | — | T50 < 270 °C |  | [49] |
| Surfactant-assisted solvothermal synthesis | 74.0 | — |  | [54] |
| Self-template hydrothermal synthesis | 36.7 | — |  | [67] |
| Template-free method | 19.6 | — |  | [69] |
| Template-free method | 106.4 | T80 < 310 °C |  | [63] |
| Solvothermal or hydrothermal route | 147.6 | T95 = 250 °C |  | [64] |
| Ultrasonic-spray-assisted synthesis | 75.8 | T100 = 280 °C |  | [66] |
| Yeast cells as templates | 38.7 | T90 = 372 °C |  | [65] |
| One-step liquid phase reaction | 128.0 | T100 = 170 °C |  | [68] |
| Doped Metals | Material | Synthesis Method | SBET (m2 g−1) | Total CO Conversion Temperature | Morphology | Ref. |
|---|---|---|---|---|---|---|
| Co | Co3O4-CeO2−x | Sequential templating approach | 55.2 | 166.9 °C |  | [70] |
| Co3O4-CeO2 | Self-templating method | 44.8 | 145 °C |  | [71] | |
| Cu | (Cu doping) CeO2 | One-step solvothermal process | 165.5 | 21 °C |  | [74] |
| CeO2-CuOx | Self-assembled approach | 98.7 | 112 °C |  | [75] | |
| CuCe-L | Aerosol-assisted synthesis | 48.0~58.6 | 120 °C |  | [76] | |
| CuO@CeO2 | Surface Etching Strategy | 36.0 | — |  | [77] | |
| CuO@CeO2 | Template-free synthesis | 90.0 | 60 °C |  | [78] | |
| CuO/CeO2-8% | Two-step route | 24.9 | 130 °C |  | [79] | |
| Ce-MOF CeO2-CuO | Assistance of selective etching | 86.7 | 98 °C |  | [80] | |
| CeO2-MOx (M = Cu, Co, Ni) | Wet-chemical approach | — | 160 °C |  | [81] | |
| Mn | Ce–Mn Binary Oxide | Interfacial reaction-directed synthesis | 202.0 | T50 = 120 °C |  | [55] |
| MnO2/CeO2-MnO2 | Sacrificial templates | 103.1 | 206 °C |  | [82] | |
| CeO2@MnO2 | Wet-chemical synthetic strategy | 98.3 | 230 °C |  | [83] | |
| CeO2-MnOx | Hard template-assisted solution combustion | 115.2 | 160 °C |  | [84] | |
| Mn2O3@CeO2 | Wet-chemical process | 54.5 | 220 °C |  | [85] | |
| CeO2-MnOx | Pyrolyzing Ce–Mn coordination polymers | 77.8 | ~250 °C |  | [86] | |
| Fe | Fe2O3/CeO2 | PB-based wet chemical approach | 73.9 | ~230 °C |  | [87] |
| Doped Noble Metal | Material | Synthesis Method | SBET (m2 g−1) | Catalytic Performance | Morphology | Ref. |
|---|---|---|---|---|---|---|
| Pd | Pd@CeO2 | Template-assisted and solvothermal alcoholysis strategy | 73.3 | T90 = 2 °C |  | [92] |
| h-Pd-CeO2 NCSs | Polymer-templated synthesis | 59.3 | T80 = 130 °C |  | [91] | |
| MnO2-Pd-CeO2 | Multi-assembly method | 128.0 | T90 = 90 °C |  | [93] | |
| Au | Au/CeO2-ZnO | Chemical reaction | 32.4 | T100 = 60 °C |  | [94] |
| Au/CeO2 | One-step template-free strategy | 145.0 | T92 = 25 °C |  | [95] | |
| Au/CeO2 | Template-free method | 23.9 | T90 = 185 °C |  | [96] | |
| Au@CeO2 | In situ redox reaction | — | T100 = 21 °C |  | [97] | |
| Au@CeO2-ZrO2 | Electrostatic attraction-induced deposition method | — | T100 = 130 °C |  | [98] | |
| Au/CeO2 | Hard template synthesis method | 77.8 | T100 = 81 °C |  | [3] | |
| Au/CeO2 | Conventional solvothermal+ method auto-redox method | — | T100 = 73 °C |  | [99] | |
| Pt | Ptencap/CeO2 | Template-based procedure | — | — |  | [57] |
| CeO2-Pt | Interfacial reactions | 62.3 | T100 = 93 °C |  | [100] | |
| Pt/CeO2 | One-pot template-free solvothermal method | 190.1 | T100 = 155 °C |  | [101] | |
| Pt/CeO2@SiO2 | Microemulsion method | 146.2 | T100 = 162 °C |  | [102] |
| Materials | Synthesis Method | SBET (m2 g−1) | T100 of 100% CO Conversion | Morphology | Ref. |
|---|---|---|---|---|---|
| α-Fe2O3 hollow microspheres | Ultrasonic-spray-assisted synthesis method | 49.3 | 320 °C |  | [66] |
| Co3O4 hollow microspheres | Ultrasonic-spray-assisted synthesis method | 37.6 | 260 °C |  | [66] |
| H-Co3O4@H-C | Reduction–oxidation pyrolysis process | 104.0 | 130 °C |  | [105] |
| Hollow nanostructure Co3O4 | Self-sacrificial template strategy | 40.6 | 130 °C |  | [106] |
| Core–shell nanostructure Co3O4 | self-sacrificial template strategy | 56.1 | 90 °C |  | [106] |
| Au/α-Fe2O3-Hollow Catalysts | Hydrothermal–thermal decomposition process | 10.9 | — |  | [107] |
| Hollow In2O3@Pd–Co3O4 core/shell nanofibers | Coaxial electrospinning | 30.0 | 57 °C |  | [108] |
| MnO2–Co3O4 hollow spheres | “Kirkendall effect” method | 123.0 | 135 °C |  | [109] |
| Catalysts | Synthesis Method | SBET (m2 g−1) | Reaction Conditions | Catalytic Performance | Morphology | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Toluene Concentration, Weight Hourly Space Velocity (WHSV) | T90 (°C) | T100 (°C) | Ea (kJ mol−1) | |||||
| CeO2 hollow sphere | Hydrothermal methods | 130.2 | 1000 ppm, 48,000 mL g−1 h−1 | 207 | — | 55.0 |  | [147] |
| Hollow Co3O4 polyhedral nanocages | Thermal treatment of ZIF-67 templates | 74.3 | 12,000 ppm, 21,000 mL g−1 h−1 | 259 | 280 | 77.9 |  | [152] |
| Flower-like MnO2 hollow microspheres | Interface reaction method | 214.0 | 3000 ppm, 15,000 mL g−1 h−1 | 237 | — | — |  | [151] |
| Manganese oxide polyhedra with hollow morphologies | Hydrothermal route | 90.0 | 1000 ppm, 32,000 mL g−1 h−1 | — | 240 | — |  | [153] |
| Pt/ZrO2 (0.57) | Modified Stöber process | 285.0 | 1000 ppm in a total air flow of 100 mL min−1 | 172 | — | — |  | [144] |
| Pt/H-MnO2 | Carbon spheres template method | 54.0 | 1000 ppm, 60,000 mL g−1 h−1 | 180 | — | — |  | [154] |
| Nanocage-shaped Co3−xZrxO4 loaded with Pt | Template method | 23.5 | 50 ppm, 36,000 mL g−1 h−1 | 165 | — | 66.2 |  | [20] |
| Pd-Modified NiCoOx hollow nanospheres | Hard template method | 162.1 | 500 ppm, 36,000 mL g−1 h−1 | — | 190 | — |  | [6] |
| hollow microsphere CuMnOx | One-pot preparation | 193.3 | 1000 ppm, 30,000 mL g−1 h−1 | 237 | — | 55.7 |  | [155] |
| MnCeOx–OH hollow structure | Carbon spheres as hard templates | 88.4 | 1000 ppm, 36,000 mL g−1 h−1 | 237 | — | 98.9 |  | [156] |
| Ce0.03MnOx hollow microsphere | Redox co-precipitation method | 51.2 | 1000 ppm, 20,000 mL g−1 h−1 | — | 225 | 90.4 |  | [157] |
| Hollow MnxCo3−xO4 Polyhedron | Controlling heating rates | 59.7 | 3000 ppm, 30,000 mL g−1 h−1 | 188 | 195 | 57.4 |  | [158] |
| Hollow CoInOx nanocube | SiO2 template strategy | 36.0 | 3000 ppm, 30,000 mL g−1 h−1 | 178 | — | 41.6 |  | [150] |
| Catalysts | Synthesis Method | Textural Properties | Reaction Conditions | X (%) | T (°C) | Ref. | |
|---|---|---|---|---|---|---|---|
| SBET (m2g−1) | Pore Volume (cm3g−1) | ||||||
| KxMnO2 hollow nanospheres | Soft chemistry route | 40.7 | 0.09 | 100 ppm HCHO, 20 vol % O2, GHSV = 50,000 h−1 | 80 | 100 | [167] |
| MnO2 hollow spheres | Hard templating method | 104.0~236.0 | 0.40~0.80 | 100 ppmv HCHO in dry air, GHSV 30,000 h−1 | 99.7 | 90 | [23] |
| Au/MnO2 hierarchical hollow microsphere | Hydrothermal method and sol-gel method | 52.3 | 0.16 | 200 ppm HCHO in air, GHSV 30,000 mL⋅g−1cat⋅h−1 | 59.2 | 25 | [168] |
| Pt/C@MnO2 composite hierarchical hollow microspheres | Hydro- thermal method with hollow carbon spheres as a sacrificial template | 153.0 | 0.37 | HCHO solution (38% mass concentration) | 90.5 | __ | [2] |
| Hierarchical Pt/WO3 nanoflakes assembled hollow microspheres | Solution method | 23.0 | 0.12 | HCHO solution (38% mass concentration), 260 ppm HCHO concentration | 97 | __ | [165] |
| Hierarchically macro-mesoporous Pt/γ-Al2O3 composite hollow microspheres | Chemically induced self-transformation method | 114.0 | 0.37 | HCHO solution (38%) | __ | __ | [169] |
| Hierarchical Pt/NiO hollow microspheres | Template-free approach | 50.8 | 0.11 | HCHO solution (38%) | __ | __ | [170] |
| Pt/CoSn(OH)6 hollow nanoboxes | __ | __ | __ | HCHO solution (38%), ~180 ppm HCHO concentration | 80.1 | __ | [171] |
| Hollow chains mesoporous Pt/TiO2 (RPt-nominal were 0.5 wt%) | Microwave–hydrothermal route | 132.0 | 0.29 | HCHO solution (38%) | __ | __ | [145] |
| Fe2O3@SnO2 core–shell nanospindles | __ | 108.0 | 0.18 | HCHO aqueous solution (38 wt%, contains 10–15 wt% methanol) | 95.99 | __ | [172] |
| RuCoOx/Al2O3 hollow microspheres | Soft-template method | 193.0 | 0.39 | Gas containing 0.1% vinyl chloride in air, weight hourly space velocity (WHSV) of 30,000 mL·g−1·h−1 | 90 | 345 | [166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Bian, Y.; Tian, W.; Pan, C.; Wu, C.-e.; Xu, L.; Wu, M.; Chen, M. The Structures and Compositions Design of the Hollow Micro–Nano-Structured Metal Oxides for Environmental Catalysis. Nanomaterials 2024, 14, 1190. https://doi.org/10.3390/nano14141190
Xu J, Bian Y, Tian W, Pan C, Wu C-e, Xu L, Wu M, Chen M. The Structures and Compositions Design of the Hollow Micro–Nano-Structured Metal Oxides for Environmental Catalysis. Nanomaterials. 2024; 14(14):1190. https://doi.org/10.3390/nano14141190
Chicago/Turabian StyleXu, Jingxin, Yufang Bian, Wenxin Tian, Chao Pan, Cai-e Wu, Leilei Xu, Mei Wu, and Mindong Chen. 2024. "The Structures and Compositions Design of the Hollow Micro–Nano-Structured Metal Oxides for Environmental Catalysis" Nanomaterials 14, no. 14: 1190. https://doi.org/10.3390/nano14141190
APA StyleXu, J., Bian, Y., Tian, W., Pan, C., Wu, C.-e., Xu, L., Wu, M., & Chen, M. (2024). The Structures and Compositions Design of the Hollow Micro–Nano-Structured Metal Oxides for Environmental Catalysis. Nanomaterials, 14(14), 1190. https://doi.org/10.3390/nano14141190








