Flexible and Disposable Hafnium Nitride Extended Gates Fabricated by Low-Temperature High-Power Impulse Magnetron Sputtering
Abstract
1. Introduction
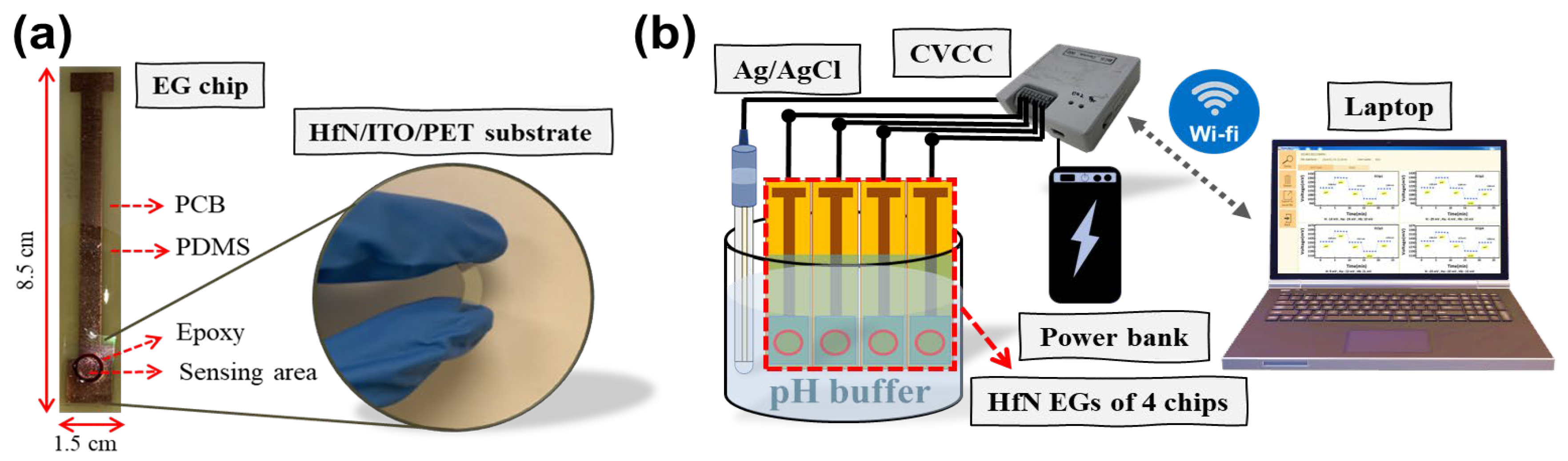
2. Materials and Methods
3. Results
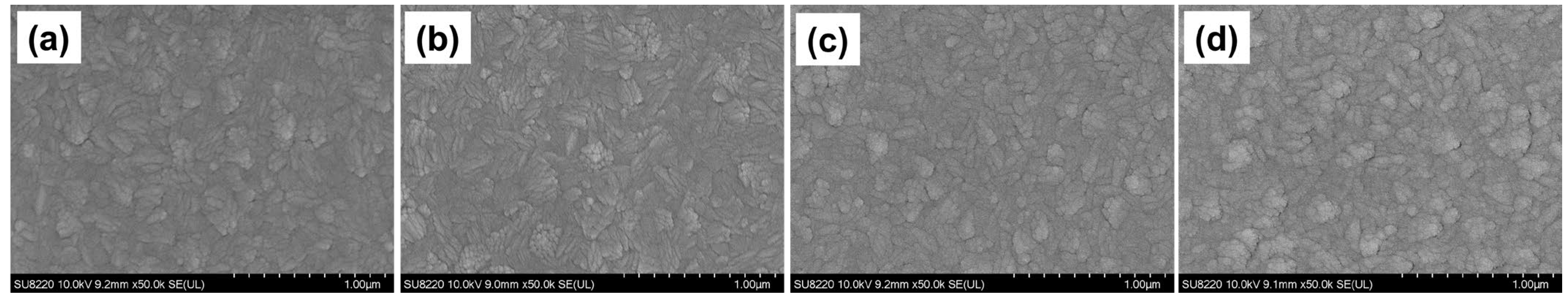
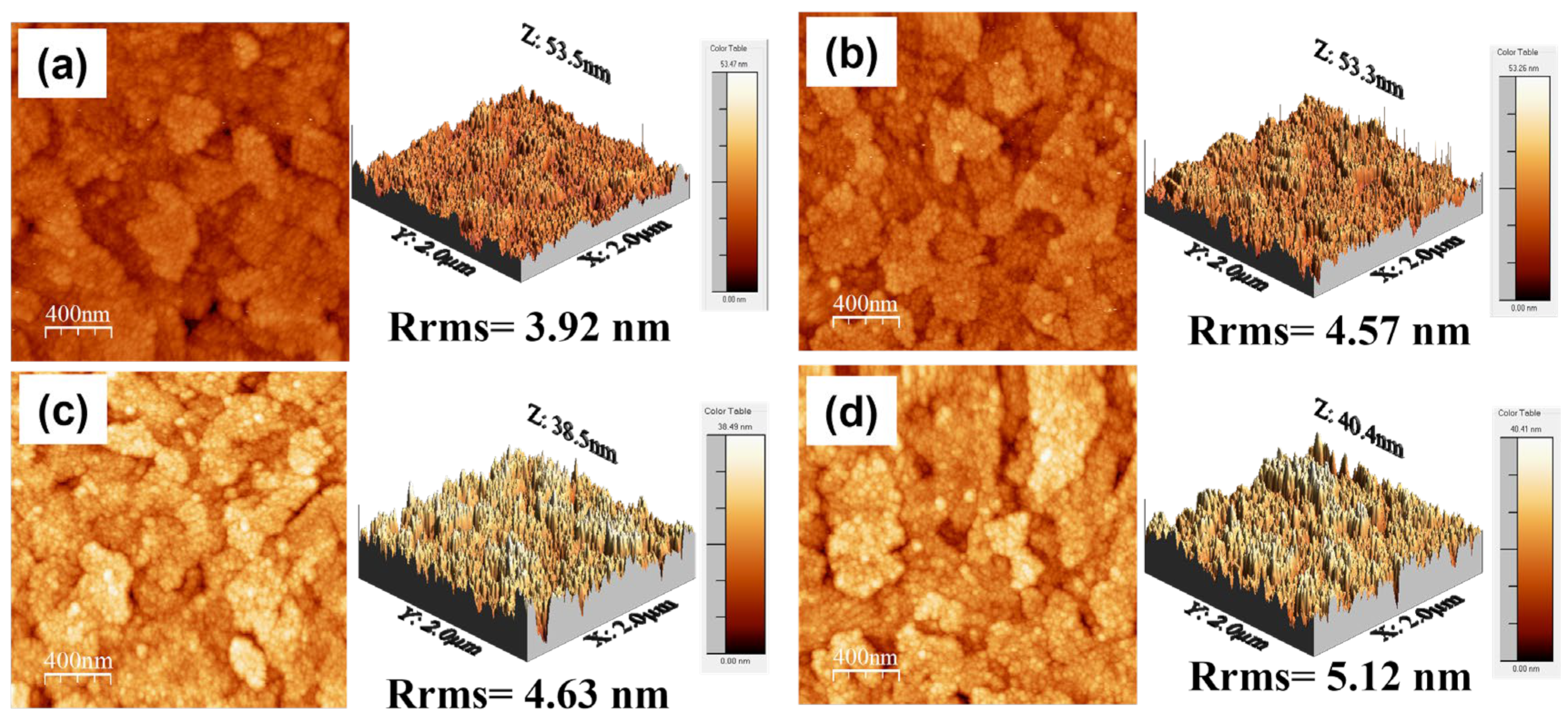
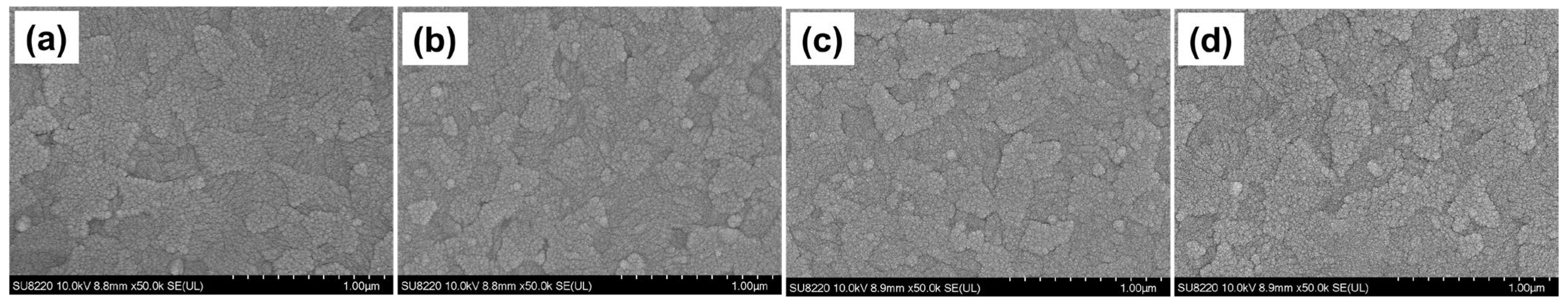
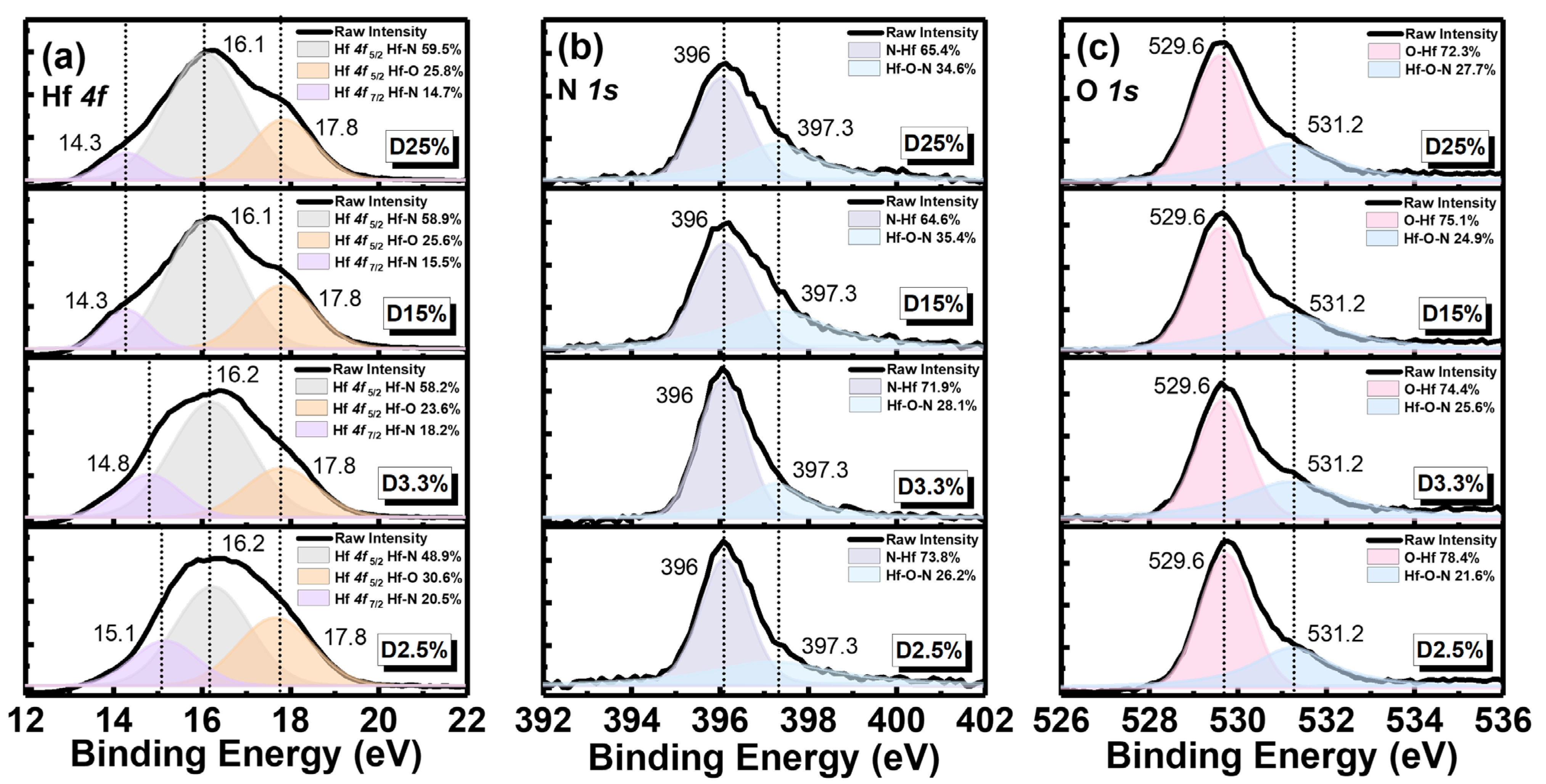
3.1. Surface Structure and Chemical State Analysis
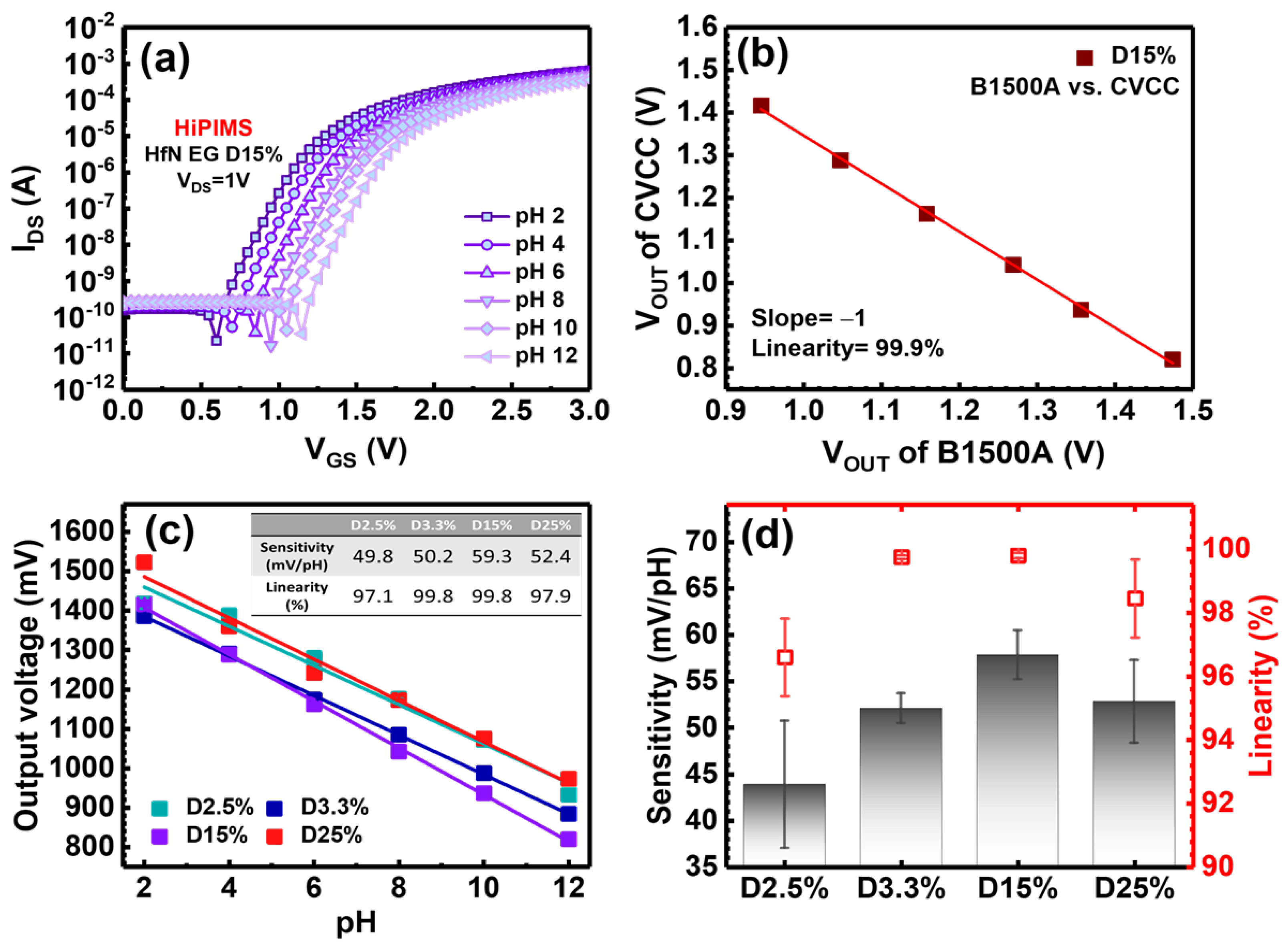
3.2. pH Sensing Characterization of HfN EGs
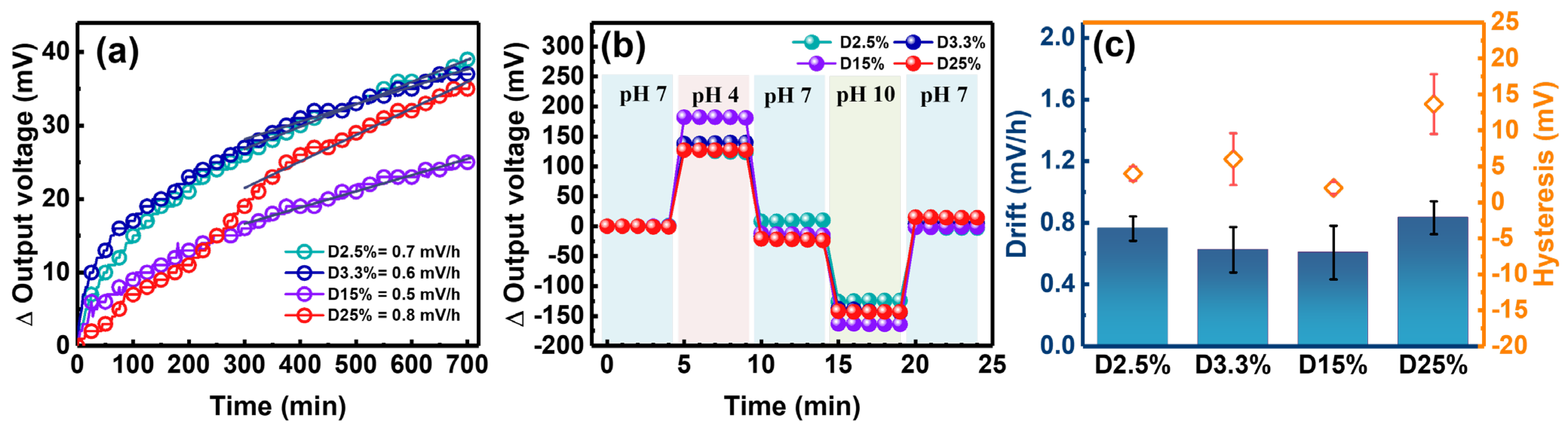
3.3. Reliability Analysis of the Bending Cycles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peter, A.B.; Wang, C.; Zhang, D.; Hernandez, A.; Nagle, D.C.; Mueller, T.; Spicer, J.B. Reactive laser synthesis of ultra-high-temperature ceramics HfC, ZrC, TiC, HfN, ZrN, and TiN for additive manufacturing. Ceram. Int. 2023, 49, 11204–11229. [Google Scholar] [CrossRef]
- Dai, Y.; Zeng, F.; Liu, H.; Gao, Y.; Yang, Q.; Chen, M.; Huang, R.; Gu, Y. Controlled nitrogen content synthesis of hafnium carbonitride powders by carbonizing hafnium nitride for enhanced ablation properties. Ceram. Int. 2023, 49, 33265–33274. [Google Scholar] [CrossRef]
- Squire, T.H.; Marschall, J. Material property requirements for analysis and design of UHTC components in hypersonic applications. J. Eur. Ceram. Soc. 2010, 30, 2239–2251. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; Hilmas, G.E. Ultra-high temperature ceramics: Materials for extreme environments. Scr. Mater. 2017, 129, 94–99. [Google Scholar] [CrossRef]
- Parthasarathy, T.A.; Petry, M.D.; Cinibulk, M.K.; Mathur, T.; Gruber, M.R. Thermal and oxidation response of UHTC leading edge samples exposed to simulated hypersonic flight conditions. J. Am. Ceram. Soc. 2013, 96, 907–915. [Google Scholar] [CrossRef]
- Wuchina, E.; Opeka, M.; Causey, S.; Buesking, K.; Spain, J.; Cull, A.; Routbort, J.; Guitierrez Mora, F. Designing for ultrahigh-temperature applications: The mechanical and thermal properties of HfB2, HfCx, HfNx and αHf(N). J. Mater. Sci. 2004, 39, 5939–5949. [Google Scholar] [CrossRef]
- Feilden, E.; Glymond, D.; Saiz, E.; Vandeperre, L. High temperature strength of an ultra-high temperature ceramic produced by additive manufacturing. Ceram. Int. 2019, 45, 18210–18214. [Google Scholar] [CrossRef]
- Gao, Z.; Wu, Z.; Zhao, S.; Zhang, T.; Wang, Q. Enhanced capacitive property of HfN film electrode by plasma etching for supercapacitors. Mater. Lett. 2019, 235, 148–152. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, B.; Wu, Z.; Qi, Z.; Wang, Z. A comparative study on the corrosion behaviour of Al, Ti, Zr and Hf metallic coatings deposited on AZ91D magnesium alloys. Surf. Coat. Technol. 2016, 303, 94–102. [Google Scholar] [CrossRef]
- Patidar, J.; Sharma, A.; Zhuk, S.; Lorenzin, G.; Cancellieri, C.; Sarott, M.F.; Trassin, M.; Thorwarth, K.; Michler, J.; Siol, S. Improving the crystallinity and texture of oblique-angle-deposited AlN thin films using reactive synchronized HiPIMS. Surf. Coat. Technol. 2023, 468, 129719. [Google Scholar] [CrossRef]
- Macak, K.; Kouznetsov, V.; Schneider, J.; Helmersson, U.; Petrov, I. Ionized sputter deposition using an extremely high plasma density pulsed magnetron discharge. J. Vac. Sci. Technol. A Vac. Surf. Film. 2000, 18, 1533–1537. [Google Scholar] [CrossRef]
- Kouznetsov, V.; Mac´ak, K.; Schneider, J.M.; Helmersson, U.; Petrov, I. A novel pulsed magnetron sputter technique utilizing very high target power densities. Surf. Coatings Technol. 1999, 122, 290–293. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Kuang, X. Effect of substrate bias on microstructure and mechanical properties of WC-DLC coatings deposited by HiPIMS. Surf. Coatings Technol. 2018, 352, 33–41. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, D.; Ke, P.; Liu, X.; Wang, A. Influence of substrate negative Bias on structure and properties of TiN coatings prepared by hybrid HIPIMS method. J. Mater. Sci. Technol. 2015, 31, 37–42. [Google Scholar] [CrossRef]
- Huang, W.D.; Cao, H.; Deb, S.; Chiao, M.; Chiao, J.C. A flexible pH sensor based on the iridium oxide sensing film. Sens. Actuators A Phys. 2011, 169, 1–11. [Google Scholar] [CrossRef]
- Yao, X.; Vepsalainen, M.; Isa, F.; Martin, P.; Munroe, P.; Bendavid, A. Advanced RuO2 thin films for pH sensing application. Sensors 2020, 22, 6432. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Zhu, A.; Tian, Y. In vivo electrochemical biosensors: Recent advances in molecular design, electrode materials, and electrochemical devices. Anal. Chem. 2023, 95, 388–406. [Google Scholar] [CrossRef]
- Wrege, R.; Peter, C.; Wesling, B.N.; Rambo, C.R.; Schneider, M.C.; Montoro, C.G. A CMOS Test Chip with Simple Post-Processing Steps for Dry Characterization of ISFET Arrays. IEEE Sens. J. 2021, 21, 4755–4763. [Google Scholar] [CrossRef]
- Kalofonou, M.; Toumazou, C. Semiconductor technology for early detection of DNA methylation for cancer: From concept to practice. Sens. Actuators B 2013, 178, 572–580. [Google Scholar] [CrossRef]
- Nemeth, B.; Piechocinski, M.S.; Cumming, D.R.S. High-resolution real-time ion-camera system using a CMOS-based chemical sensor array for proton imaging. Sens. Actuators B 2012, 171, 747–752. [Google Scholar] [CrossRef]
- Van Der Spiegel, J.; Lauks, I.; Chan, P.; Babic, D. The extended gate chemically sensitive field effect transistor as multi-species microprobe. Sens. Actuators 1983, 4, 291–298. [Google Scholar] [CrossRef]
- Yadlapalli, B.K.; Chou, H.Y.; Chiang, J.L.; Wuu, D.S. Morphological investigation and pH sensing properties of β-Ga2O3 EGFET-pH sensor. Mater. Sci. Eng. B 2024, 300, 117113. [Google Scholar] [CrossRef]
- Smith, J.T.; Katchman, B.A.; Kullman, D.E.; Obahiagbon, U.; Lee, Y.K.; O’Brien, B.P.; Raupp, G.B.; Anderson, K.S.; Christen, J.B. Application of Flexible OLED Display Technology to Point-of-Care Medical Diagnostic Testing. J. Disp. Technol. 2016, 12, 273–280. [Google Scholar] [CrossRef]
- Wang, S.Q.; Chinnasamy, T.; Lifson, M.A.; Inci, F.; Demirci, U. Flexible substrate-based devices for point-of-care diagnostics. Trends Biotechnol. 2016, 34, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Economou, A.; Kokkinos, C.; Prodromidis, M. Flexible plastic, paper and textile lab-on-a chip platforms for electrochemical biosensing. Lab Chip 2018, 18, 1812–1830. [Google Scholar] [CrossRef] [PubMed]
- Lue, C.E.; Wang, I.S.; Huang, C.H.; Shiao, Y.T.; Wang, H.C.; Yang, C.M.; Hsu, S.H.; Chang, C.Y.; Wang, W.; Lai, C.S. pH sensing reliability of flexible ITO/PET electrodes on EGFETs prepared by a roll-to-roll process. Microelectron. Reliab. 2012, 52, 1651–1654. [Google Scholar] [CrossRef]
- Agnes, P.; Tian, Y.C.; Gardin MA, S.; Briliant, A.P.; Liu, H.L.; Yang, C.M.; Lai, C.S. Gold Nanoframe Array Electrode for Straightforward Detection of Hydrogen Peroxide. Chemosensors 2021, 9, 37. [Google Scholar] [CrossRef]
- Lai, C.S.; Yang, C.M.; Lu, T.F. pH Sensitivity Improvement on 8 nm Thick Hafnium Oxide by Post Deposition Annealing. Electrochem. Solid-State Lett. 2006, 9, G90–G92. [Google Scholar] [CrossRef]
- Chang, C.L.; Shih, S.G.; Chen, P.H.; Chen, W.C.; Ho, C.T.; Wu, W.Y. Effect of duty cycles on the deposition and characteristics of high-power impulse magnetron sputtering deposited TiN thin films. Surf. Coat. Technol. 2014, 259, 232–237. [Google Scholar] [CrossRef]
- Chen, W.C.; Wang, Z.Y.; Yu, C.Y.; Liao, B.H.; Lin, M.T. A study of the phase transformation of low temperature deposited tantalum thin films using high power impulse magnetron sputtering and pulsed DC magnetron sputtering. Surf. Coat. Technol. 2022, 436, 128288. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, J.C.; Zhang, B.R.; Chen, J.J.; Tang, C.R.; Zhu, R.Y.; Zheng, J. Effect of duty cycle on microstructure and mechanical properties of AlCrN coatings deposited by HiPIMS. Vacuum 2022, 205, 111409. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Zhou, X.; Guo, P.; Chen, R.; Wei, J.; Wang, A.; Ke, P. Enhanced anti-tribocorrosion property of a-C film under high hydrostatic pressure by high power pulsed magnetron sputter (HiPIMS). J. Mater. Res. Technol. 2024, 28, 3052–3067. [Google Scholar] [CrossRef]
- Slewa, L.H.; Sabah, F.A.; Gozeh, B.A.; Othman, H.O.; Abbas, T.A.; Ahmed, N.M. pH-EGFET Sensor Based on the Surface Modification of MacroPSi with Au-NPs. Silicon 2023, 15, 3035–3047. [Google Scholar] [CrossRef]
- Zhang, D.; Qi ZWei, B.; Shen, H.; Wang, Z. Microstructure and corrosion behaviors of conductive Hf/HfN multilayer coatings on magnesium alloys. Ceram. Int. 2018, 44, 9958–9966. [Google Scholar] [CrossRef]
- Lee, Y.B.; Oh, I.K.; Cho, E.N.; Moon, P.; Kim, H. Characterization of HfOxNy thin film formation by in-situ plasma enhanced atomic layer deposition using NH3 and N2 plasmas. Appl. Surf. Sci. 2015, 349, 757–762. [Google Scholar] [CrossRef]
- Zeng, S.; Muneshwar, T.; Riddell, S.; Manuel, A.P.; Vahidzadeh, E.; Kisslinger, R.; Kumar, P.; Alam, K.M.M.; Kobryn, A.E.; Gusarov, S.; et al. TiO2-HfN Radial Nano-Heterojunction: A Hot Carrier Photoanode for Sunlight-Driven Water-Splitting. Catalysts 2021, 11, 1374. [Google Scholar] [CrossRef]
- Phaen-Gam, W.; Prathumsit, J.; Gitgeatpong, G.; Chananonnawathorn, C.; Lertvanithphol, T.; Saekow, B.; Nakajima, H.; Horprathum, M. Effect of Post Annealed Treatment on HfN Thin Films Prepared by DC Reactive Magnetron Sputtering. AIP Conf. Proc. 2020, 2279, 120002. [Google Scholar] [CrossRef]
- Maeng, W.J.; Gu, G.H.; Park, C.G.; Lee, K.; Lee, T.; Kim, H. HfO2/HfOxNy/HfO2 Gate Dielectric Fabricated by In Situ Oxidation of Plasma-Enhanced Atomic Layer Deposition HfN Middle Layer. J. Electrochem. Soc. 2009, 156, G109–G113. [Google Scholar] [CrossRef]
- Bousse, L.; Rooij, N.F.D.; Bergveld, P. Operation of Chemically Sensitive Field-Effect Sensors as a Function of the Insulator-Electrolyte Interface. IEEE Trans. Electron Devices 1983, ED-30, 1263–1270. [Google Scholar] [CrossRef]
- Qin, Y.; Kwon, H.J.; Howlader, M.M.R.; Deen, M.J. Microfabricated electrochemical pH and free chlorine sensors for water quality monitoring: Recent advances and research challenges. RSC Adv. 2015, 5, 69086. [Google Scholar] [CrossRef]
- Keawkusonwiwat, S.; Tunhoo, B.; Onlaor, K.; Thiwawong, T. Preparation of pH Sensor Based on Extended-Gate Field-Effect Transistor with Spinel ZnCo2O4 Thin Films by Electrostatic Spray Deposition. J. Electron. Mater. 2023, 52, 8095–8107. [Google Scholar] [CrossRef]
- Niu, M.N.; Ding, X.F.; Tong, Q.Y. Effect of two types of surface sites on the characteristics of Si3N4-gate pH-ISFETs. Sens. Actuators B 1996, 37, 13–17. [Google Scholar] [CrossRef]
- Fung, C.D.; Cheung, P.W.; Ko, W.H. A Generalized Theory of an Electrolyte-Insulator-Semiconductor Field-Effect Transistor. IEEE Trans. Electron Devices 1986, 33, 8–18. [Google Scholar] [CrossRef]
- Wei, F.; Urashima, S.H.; Nihonyanagi, S.; Tahara, T. Elucidation of the pH-Dependent Electric Double Layer Structure at the Silica/Water Interface Using Heterodyne-Detected Vibrational Sum Frequency Generation Spectroscopy. J. Am. Chem. 2023, 145, 8833–8846. [Google Scholar] [CrossRef] [PubMed]
- Ong, G.M.C.; Gallegos, A.; Wu, J. Modeling Surface Charge Regulation of Colloidal Particles in Aqueous Solutions. Langmuir 2020, 36, 11918–11928. [Google Scholar] [CrossRef] [PubMed]
- Harame, D.L.; Bousse, L.J.; Shott, J.D.; Meindl, J.D. Ion-Sensing Devices with Silicon Nitride and Borosilicate Glass Insulators. IEEE Trans. Electron Devices 1987, ED-34, 1700–1707. [Google Scholar] [CrossRef]
- Yang, C.M.; Wei, C.H.; Fuad, U.; Chang, J.Y.; Pijanowska, D.G.; Lai, C.S. High pH stability and detection of α-synuclein using an EGFET biosensor with an HfO2 gate deposited by high-power pulsed magnetron sputtering. Sens. Actuators B. Chem. 2024, 416, 136006. [Google Scholar] [CrossRef]
- Liao, Y.H.; Chou, J.C. Fabrication and Characterization of a Ruthenium Nitride Membrane for Electrochemical pH Sensors. Sensors 2009, 9, 2478–2490. [Google Scholar] [CrossRef] [PubMed]
- Sabah, F.A.; Ahmed, N.M.; Hassan, Z.; Almessiere, M.A.; Al-Hardan, N.H. Sensitivity of CuS membrane pH sensor with and without MOSFET. JOM 2017, 69, 1134–1142. [Google Scholar] [CrossRef]
- Jamasb, S.; Collins, D.; Smith, R.L. A Physical Model for Threshold Voltage Instability in Si3N4-Gate H+-Sensitive FET’s (pH ISFET’s). IEEE Trans. Electron Devices 1998, 45, 1239–1245. [Google Scholar] [CrossRef]
- Chou, J.C.; Chiang, J.L.; Wu, C.L. pH and Procaine Sensing Characteristics of Extended-Gate Field-Effect Transistor Based on Indium Tin Oxide Glass. Jpn. J. Appl. Phys. 2005, 44, 4838–4842. [Google Scholar] [CrossRef]
- Molinnus, D.; Iken, H.; Johnen, A.L.; Richstein, B.; Hellmich, L.; Poghossian, A.; Knoch, M.J. Schoning Miniaturized pH-Sensitive Field-Effect Capacitors with Ultrathin Ta2O5 Films Prepared by Atomic Layer Deposition. Phys. Status Solidi A 2022, 219, 2100660. [Google Scholar] [CrossRef]
- Matsuo, T.; Esashi, M. Methods of ISFET fabrication. Sens. Actuators 1981, 1, 77–96. [Google Scholar] [CrossRef]
- Chou, J.C.; Wang, Y.F. Preparation and study on the drift and hysteresis properties of the tin oxide gate ISFET by the sol–gel method. Sens. Actuators B 2002, 86, 58–62. [Google Scholar] [CrossRef]


| Sensing Film | Device | Substrate | Method | Background Solution | Sensitivity (mV/pH) | Linearity (%) | Ref. |
|---|---|---|---|---|---|---|---|
| IrOx | EGFET | Polyimide | Sol-gel | pH 1.5–12 | 51.1 | 95.3 | [15] |
| Si3N4 | ISFET | Undoped silicate glass (USG) | Sputter | pH 4–7–9 | 46 | N/A | [18] |
| ITO | EGFET | PET | Sputter | pH 2–12 | 50.1 | 99 | [26] |
| RuN | EGFET | Si | Sputter | pH 1–13 | 58.03 | N/A | [48] |
| CuS | EGFET | Glass | Spray pyrolysis deposition | pH 2–12 | 24 | 98.18 | [49] |
| HfN | EGFET | ITO/PET | HiPIMS | pH 2–12 | 59.3 | 99.8 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-M.; Wei, C.-H.; Chang, J.-Y.; Lai, C.-S. Flexible and Disposable Hafnium Nitride Extended Gates Fabricated by Low-Temperature High-Power Impulse Magnetron Sputtering. Nanomaterials 2024, 14, 1191. https://doi.org/10.3390/nano14141191
Yang C-M, Wei C-H, Chang J-Y, Lai C-S. Flexible and Disposable Hafnium Nitride Extended Gates Fabricated by Low-Temperature High-Power Impulse Magnetron Sputtering. Nanomaterials. 2024; 14(14):1191. https://doi.org/10.3390/nano14141191
Chicago/Turabian StyleYang, Chia-Ming, Chao-Hui Wei, Jia-Yuan Chang, and Chao-Sung Lai. 2024. "Flexible and Disposable Hafnium Nitride Extended Gates Fabricated by Low-Temperature High-Power Impulse Magnetron Sputtering" Nanomaterials 14, no. 14: 1191. https://doi.org/10.3390/nano14141191
APA StyleYang, C.-M., Wei, C.-H., Chang, J.-Y., & Lai, C.-S. (2024). Flexible and Disposable Hafnium Nitride Extended Gates Fabricated by Low-Temperature High-Power Impulse Magnetron Sputtering. Nanomaterials, 14(14), 1191. https://doi.org/10.3390/nano14141191







