MoO3 with the Synergistic Effect of Sulfur Doping and Oxygen Vacancies: The Influence of S Doping on the Structure, Morphology, and Optoelectronic Properties
Abstract
1. Introduction
2. Experimental Procedures
2.1. Synthesis of S-MoO3−x and MoO3−x
2.2. Characterization
2.3. Current–Voltage Curve Testing
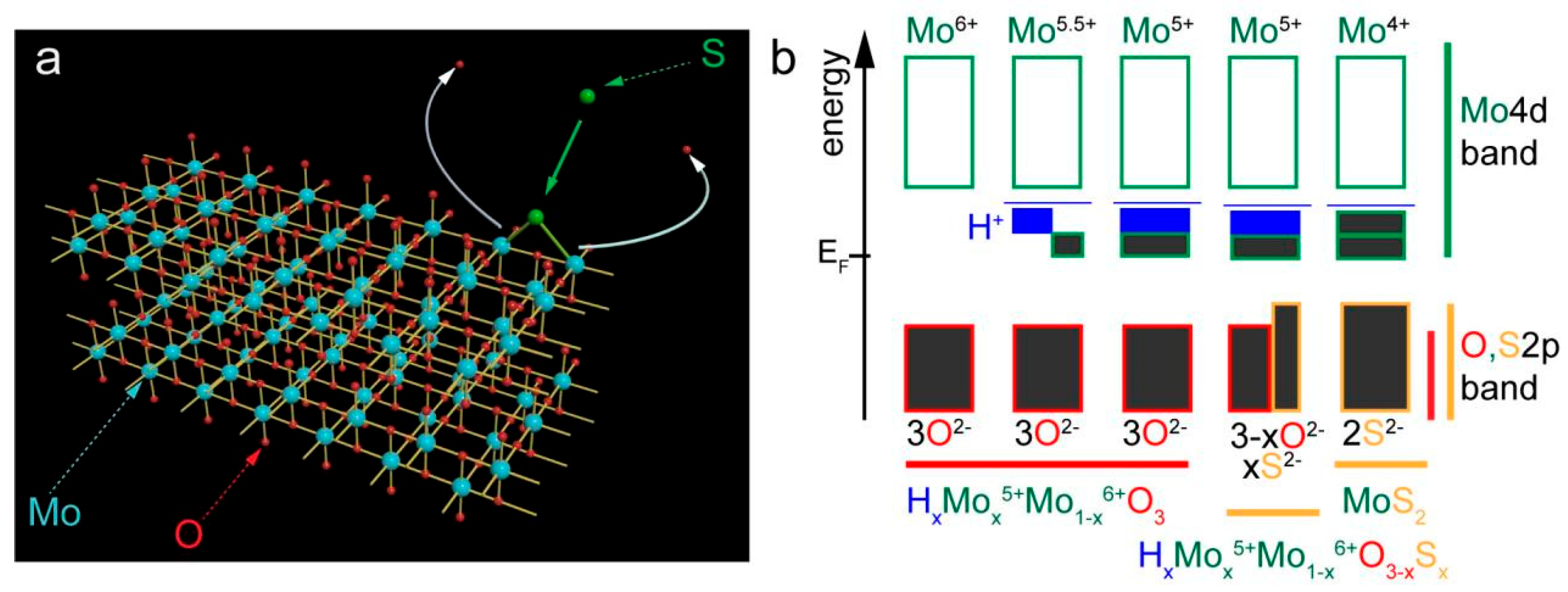
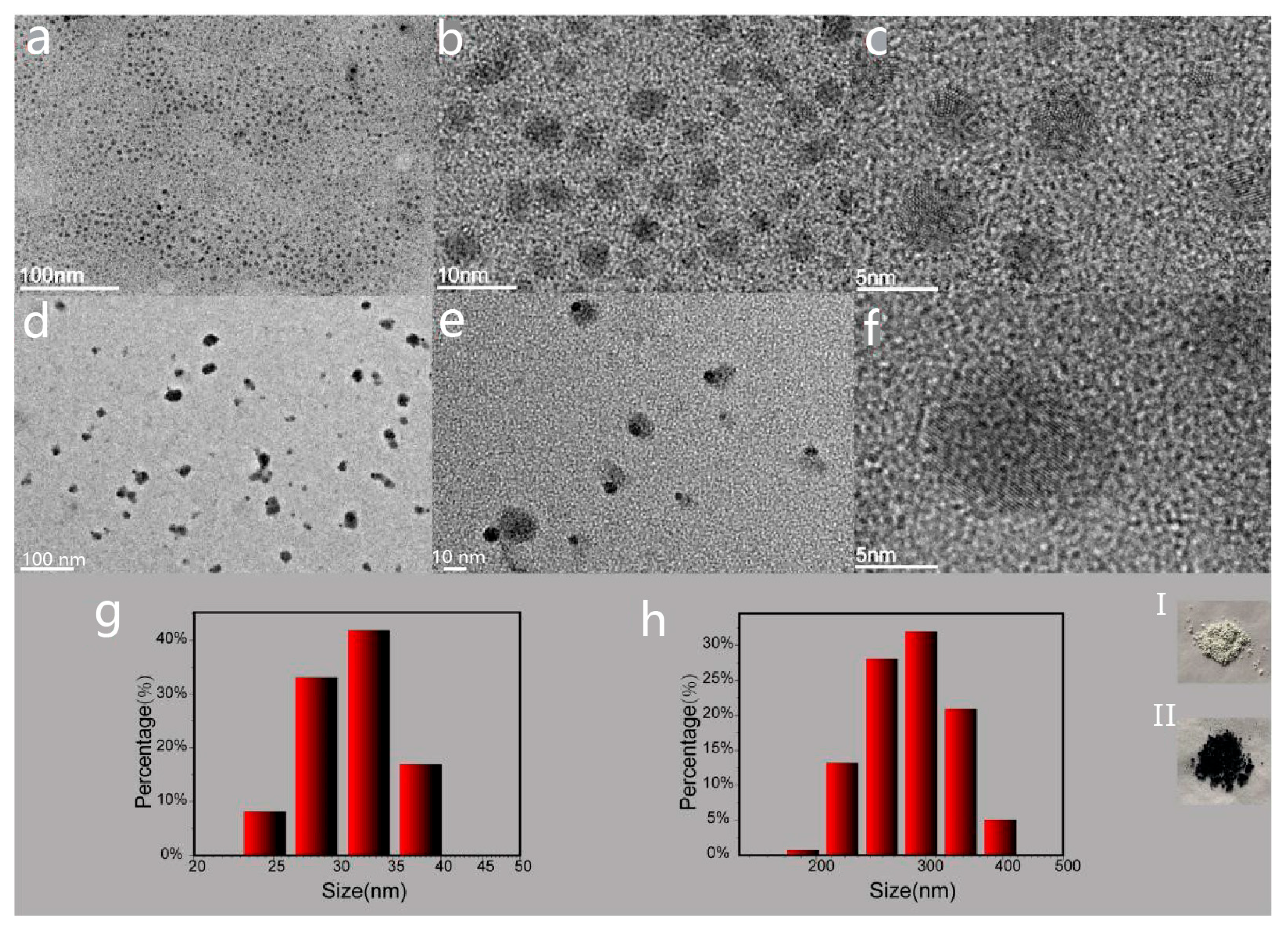
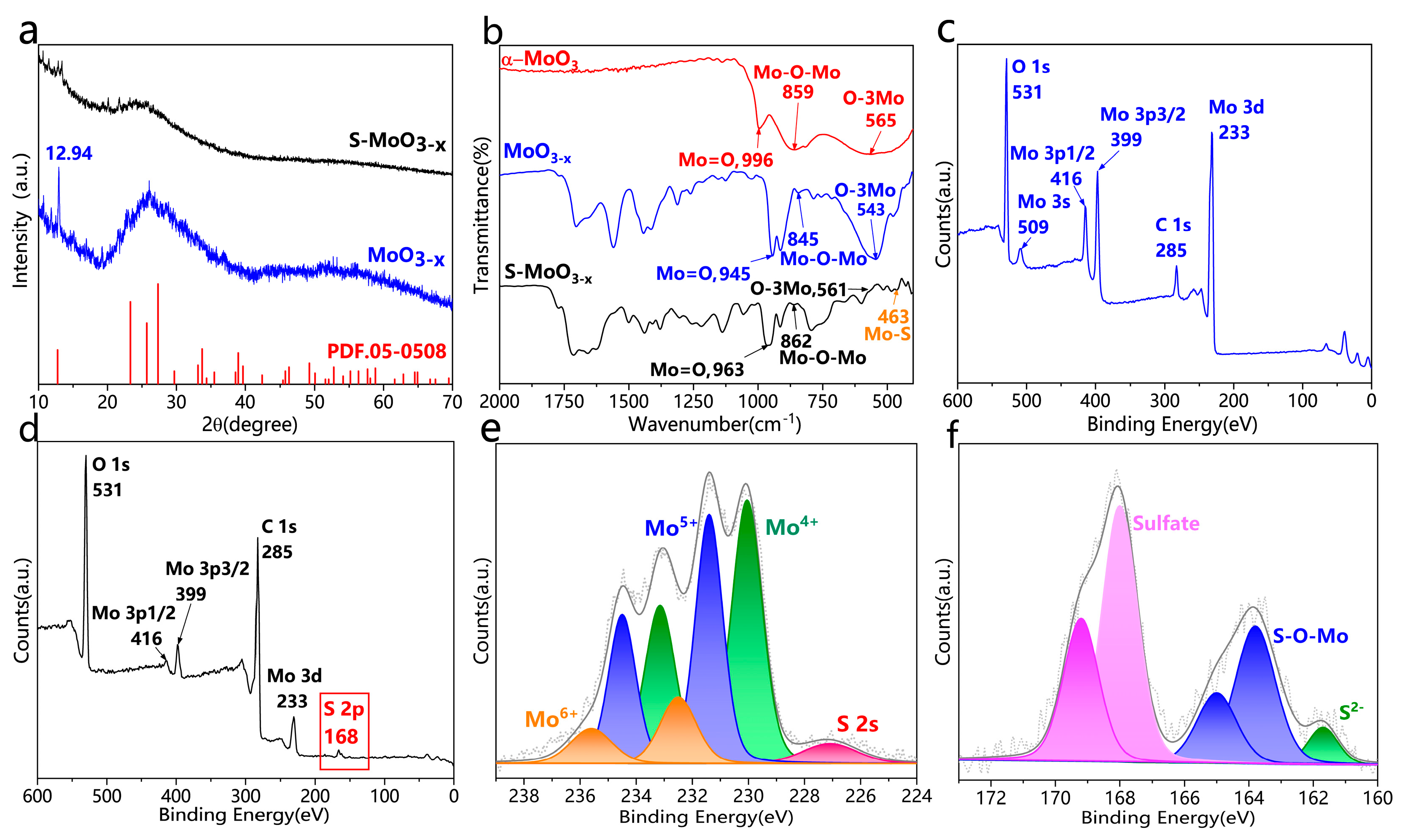
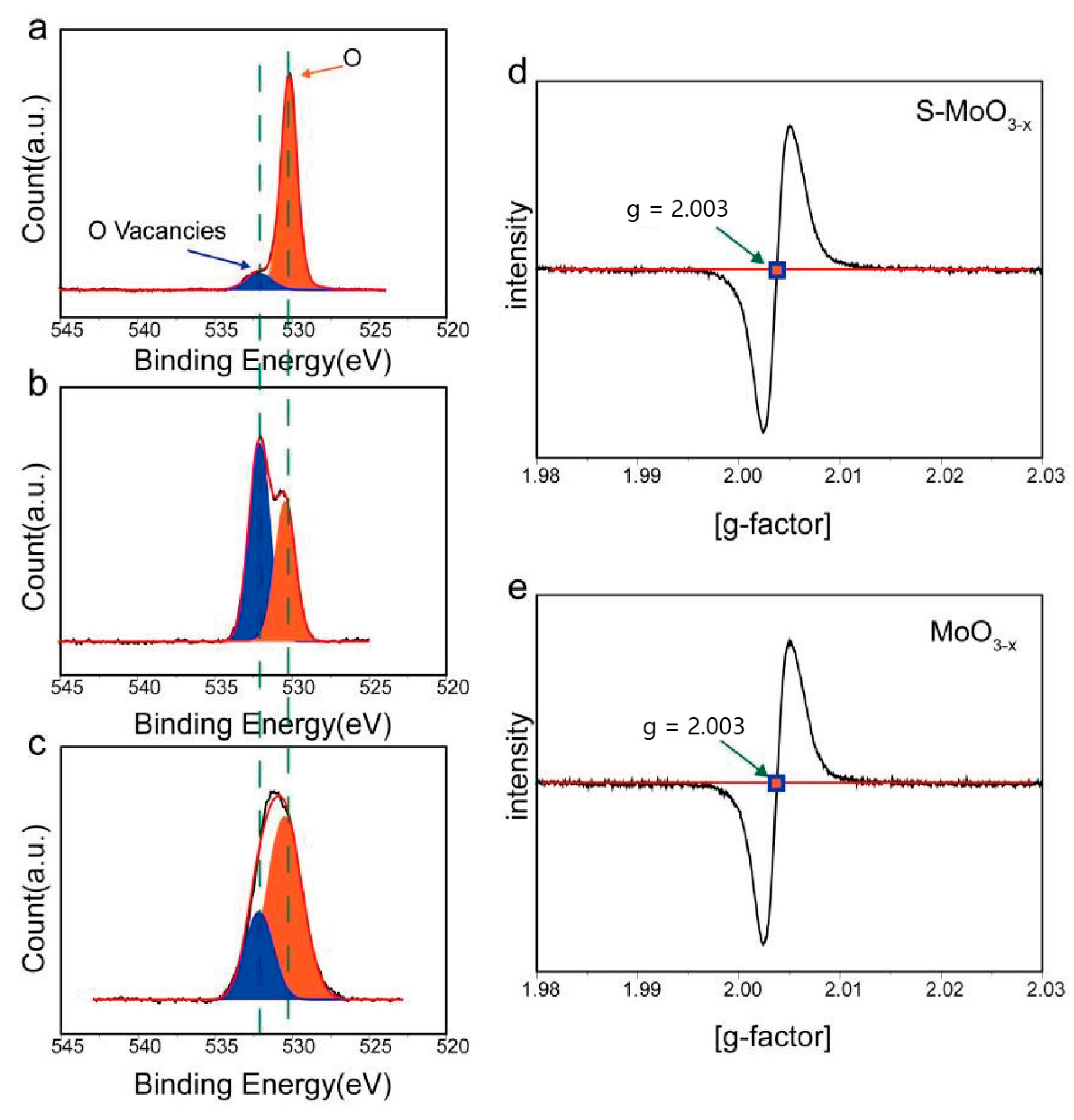
3. Results and Discussion
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, J.; Shen, J.; Yu, X.; Tang, H.; Zulfiqar; Liu, Q. Construction of LSPR-Enhanced 0D/2D CdS/MoO3−x S-Scheme Heterojunctions for Visible-Light-Driven Photocatalytic H2 Evolution. Chin. J. Catal. 2021, 42, 87–96. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Wang, Q.; Wang, Q.; Zhu, K.; Ye, K.; Wang, G.; Cao, D.; Yan, J.; Zhang, Q. High-Capacity and Kinetically Accelerated Lithium Storage in MoO3 Enabled by Oxygen Vacancies and Heterostructure. Adv. Energy Mater. 2021, 11, 2101712. [Google Scholar] [CrossRef]
- Hou, F.; Su, Z.; Jin, F.; Yan, X.; Wang, L.; Zhao, H.; Zhu, J.; Chu, B.; Li, W. Efficient and Stable Planar Heterojunction Perovskite Solar Cells with an MoO3/PEDOT:PSS Hole Transporting Layer. Nanoscale 2015, 7, 9427–9432. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xiao, X.; Chen, P.; Zhu, K.; Cheng, K.; Ye, K.; Wang, G.; Cao, D.; Yan, J. Creating Oxygen-Vacancies in MoO3−x Nanobelts toward High Volumetric Energy-Density Asymmetric Supercapacitors with Long Lifespan. Nano Energy 2019, 58, 455–465. [Google Scholar] [CrossRef]
- Prasomsri, T.; Shetty, M.; Murugappan, K.; Román-Leshkov, Y. Insights into the Catalytic Activity and Surface Modification of MoO3 during the Hydrodeoxygenation of Lignin-Derived Model Compounds into Aromatic Hydrocarbons under Low Hydrogen Pressures. Energy Environ. Sci. 2014, 7, 2660–2669. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Zhang, W.L.; Lv, F.; Guo, S. Recent Progress in Two-Dimensional Inorganic Quantum Dots. Chem. Soc. Rev. 2018, 47, 586–625. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lau, K.Y.; Zheng, J.; Dong, J.; Wang, L.; Wang, W.; Xu, B.; Qiu, J.; Liu, X. Coupled Femtosecond Laser Assisted Doping and Fragmentation of MoO3 Nanosheets Generates Plasmonic QDs with Strong NLO Response. Adv. Opt. Mater. 2023, 11, 2202900. [Google Scholar] [CrossRef]
- Li, Y.; Mi, Y.; Liu, Z.; Liu, Y.; Zhang, W.; Qiu, S.; Ramos, M.A.; Hu, T.S.; Xu, Q. MoO3−x Quantum Dots-Based Hydrogel with Excellent Light-Triggered Self-Healing Efficiency and Pressure Sensitive Photoluminescence for Accurate Remote Force Measurement. Mater. Today Phys. 2022, 27, 100807. [Google Scholar] [CrossRef]
- Zheng, B.; Fan, J.; Chen, B.; Qin, X.; Wang, J.; Wang, F.; Deng, R.; Liu, X. Rare-Earth Doping in Nanostructured Inorganic Materials. Chem. Rev. 2022, 122, 5519–5603. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Q.; Wei, Z.; Xu, L.; Zeng, W. Experimental and Theoretical Studies of Zn-Doped MoO3 Hierarchical Microflower with Excellent Sensing Performances to Carbon Monoxide. Ceram. Int. 2020, 46 Pt A, 29222–29232. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Salama, T.M.; Morsy, M.; Shahba, R.M.A.; Mohamed, S.H. Facile Strategy of Synthesizing α-MoO3−x Nanorods Boosted as Traced by 1% Graphene Oxide: Efficient Visible Light Photocatalysis and Gas Sensing Applications. Sens. Actuators B Chem. 2019, 299, 126960. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, W.; Chao, S.; Liang, Y.; Zhao, X.; Liu, C.; Xu, J. Advanced Oxygen-Vacancy Ce-Doped MoO3 Ultrathin Nanoflakes Anode Materials Used as Asymmetric Supercapacitors with Ultrahigh Energy Density. Adv. Energy Mater. 2022, 12, 2200101. [Google Scholar] [CrossRef]
- Chen, K.; Wang, J.; Zhang, H.; Ma, D.; Chu, K. Self-Tandem Electrocatalytic NO Reduction to NH3 on a W Single-Atom Catalyst. Nano Lett. 2023, 23, 1735–1742. [Google Scholar] [CrossRef]
- Ijeh, R.O.; Nwanya, A.C.; Nkele, A.C.; Madiba, I.G.; Bashir, A.K.H.; Ekwealor, A.B.C.; Osuji, R.U.; Maaza, M.; Ezema, F. Optical, Electrical and Magnetic Properties of Copper Doped Electrodeposited MoO3 Thin Films. Ceram. Int. 2020, 46 Pt A, 10820–10828. [Google Scholar] [CrossRef]
- Zheng, C.; Luo, N.; Huang, S.; Wu, W.; Huang, H.; Wei, M. Nanocomposite of Mo2N Quantum Dots@MoO3@Nitrogen-Doped Carbon as a High-Performance Anode for Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 10198–10206. [Google Scholar] [CrossRef]
- Gao, P.; Ru, Q.; Pan, Z.; Zhang, J.; Xu, W.; Chi-Chung Ling, F.; Wei, L. Robust Hetero-MoO3/MoO2@N-Doped Carbon Nanobelts Decorated with Oxygen Deficiencies as High-Performance Anodes for Potassium/Sodium Storage. J. Colloid Interface Sci. 2021, 599, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Cook, J.B.; Lin, H.; Ko, J.S.; Tolbert, S.H.; Ozolins, V.; Dunn, B. Oxygen Vacancies Enhance Pseudocapacitive Charge Storage Properties of MoO3−x. Nat. Mater. 2017, 16, 454–460. [Google Scholar] [CrossRef]
- Luo, Z.; Miao, R.; Huan, T.D.; Mosa, I.M.; Poyraz, A.S.; Zhong, W.; Cloud, J.E.; Kriz, D.A.; Thanneeru, S.; He, J.; et al. Mesoporous MoO3−x Material as an Efficient Electrocatalyst for Hydrogen Evolution Reactions. Adv. Energy Mater. 2016, 6, 1600528. [Google Scholar] [CrossRef]
- Yu, M.; Cheng, X.; Zeng, Y.; Wang, Z.; Tong, Y.; Lu, X.; Yang, S. Dual-Doped Molybdenum Trioxide Nanowires: A Bifunctional Anode for Fiber-Shaped Asymmetric Supercapacitors and Microbial Fuel Cells. Angew. Chem. Int. Ed. 2016, 55, 6762–6766. [Google Scholar] [CrossRef]
- Yu, X.; Qiu, P.; Wang, Y.; He, B.; Xu, X.; Zhu, H.; Ding, J.; Liu, X.; Li, Z.; Wang, Y. Defect-Induced Charge Redistribution of MoO3−x Nanometric Wires for Photocatalytic Ammonia Synthesis. J. Colloid Interface Sci. 2023, 640, 775–782. [Google Scholar] [CrossRef]
- Teng, Y.; Zhou, Q.; Gao, P. Applications and Challenges of Elemental Sulfur, Nanosulfur, Polymeric Sulfur, Sulfur Composites, and Plasmonic Nanostructures. Crit. Rev. Environ. Sci. Technol. 2019, 49, 2314–2358. [Google Scholar] [CrossRef]
- De Castro, I.A.; Datta, R.S.; Ou, J.Z.; Castellanos-Gomez, A.; Sriram, S.; Daeneke, T.; Kalantar-Zadeh, K. Molybdenum Oxides—From Fundamentals to Functionality. Adv. Mater. 2017, 29, 1701619. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, R.; Zhang, Q. Few-Layer MoS2: A Promising Layered Semiconductor. ACS Nano 2014, 8, 4074–4099. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, Z.; Ranjbar, M. Plasmonic MoO3−x Nanosheets by Anodic Oxidation of Molybdenum for Colorimetric Sensing of Hydrogen Peroxide. Anal. Chim. Acta 2022, 1198, 339529. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Li, X.; Ren, X.; Yang, L.; Zhu, J.; Wei, B. Optical and Electrical Enhancement of Hydrogen Evolution by MoS2@MoO3 Core–Shell Nanowires with Designed Tunable Plasmon Resonance. Adv. Funct. Mater. 2018, 28, 1802567. [Google Scholar] [CrossRef]
- Kim, M.; Kwon, B.-H.; Joo, C.W.; Cho, M.S.; Jang, H.; Kim, Y.; Cho, H.; Jeon, D.Y.; Cho, E.N.; Jung, Y.S. Metal Oxide Charge Transfer Complex for Effective Energy Band Tailoring in Multilayer Optoelectronics. Nat. Commun. 2022, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Mai, L.; Chen, W.; Yang, F. From MoO3 Nanobelts to MoO2 Nanorods: Structure Transformation and Electrical Transport. ACS Nano 2009, 3, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.K.; Dutta, S.; Khan, M.R.; Manir, M.S.; Dutta, S.; Al Mortuza, A.; Razia, S.; Hakim, M.A. Characterization and Antibacterial Activity Study of Hydrothermally Synthesized H-MoO3 Nanorods and α-MoO3 Nanoplates. BioNanoScience 2019, 9, 873–882. [Google Scholar] [CrossRef]
- Maheswari, N.; Muralidharan, G. Controlled Synthesis of Nanostructured Molybdenum Oxide Electrodes for High Performance Supercapacitor Devices. Appl. Surf. Sci. 2017, 416, 461–469. [Google Scholar] [CrossRef]
- Lima, C.D.A.; Barbosa Moura, J.V.; de Castro Maciel, A.; Luz-Lima, C.; Sinimbu, L.I.M.; Chaves, J.F.; Oliveira, J.D.S.; Syed, A.R.; Sommer, R.L.; Ghosh, A.; et al. Synthesis and Magnetic Characterization of Iron-Doped Molybdenum Trioxide (α-MoO3:xFe). Solid State Sci. 2023, 145, 107313. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Shao, H.; Xu, J.; Chen, F.; Feng, Y. Preparation of MoS2 Nanofibers by Electrospinning. Mater. Lett. 2012, 73, 223–225. [Google Scholar] [CrossRef]
- Saleem, S.; Salman, M.; Ali, S.; Ling, Y.; Khan, M. Electrocatalytic Hydrogen Evolution Reaction on Sulfur-Deficient MoS2 Nanostructures. Int. J. Hydrogen Energy 2022, 47, 7713–7723. [Google Scholar] [CrossRef]
- Kondekar, N.P.; Boebinger, M.G.; Woods, E.V.; McDowell, M.T. In Situ XPS Investigation of Transformations at Crystallographically Oriented MoS2 Interfaces. ACS Appl. Mater. Interfaces 2017, 9, 32394–32404. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ang, E.H.; Yang, Y.; Zhang, Y.; Geng, H.; Ye, M.; Li, C.C. Interlayer Engineering of Molybdenum Trioxide toward High-Capacity and Stable Sodium Ion Half/Full Batteries. Adv. Funct. Mater. 2020, 30, 2001708. [Google Scholar] [CrossRef]
- Liu, S.; Xu, C.; Yang, H.; Qian, G.; Hua, S.; Liu, J.; Zheng, X.; Lu, X. Atomic Modulation Triggering Improved Performance of MoO3 Nanobelts for Fiber-Shaped Supercapacitors. Small 2020, 16, 1905778. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Jin, Z.; Bi, Y. Charge Transmission Channel Construction between a MOF and rGO by Means of Co–Mo–S Modification. Catal. Sci. Technol. 2017, 7, 4478–4488. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, J.; Zong, S.; Wang, X.; Chen, K.; Liu, L.; Fang, Y. Fabrication of Graphitic Carbon Nitride/Nonstoichiometric Molybdenum Oxide Nanorod Composite with the Nonmetal Plasma Enhanced Photocatalytic Hydrogen Evolution Activity. J. Colloid Interface Sci. 2022, 606, 848–859. [Google Scholar] [CrossRef]
- Bharathi, M.; Balraj, B.; Sivakumar, C.; Wang, Z.; Shuai, J.; Ho, M.-S.; Guo, D. Effect of Ag Doping on Bipolar Switching Operation in Molybdenum Trioxide (MoO3) Nanostructures for Non-Volatile Memory. J. Alloys Compd. 2021, 862, 158035. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, Y.; Zhang, J.; Chen, K.; Huang, C.; Chen, H.; Qiu, X. Plasmonic MoO3−x Nanosheets with Tunable Oxygen Vacancies as Efficient Visible Light Responsive Photocatalyst. Appl. Surf. Sci. 2019, 490, 395–402. [Google Scholar] [CrossRef]
- Zhang, Y.; Ping, X.; Hao, L.; He, Y.; Guo, Y.; Zhao, Q.; Zheng, Z.; Lu, Y. Facile Preparation of Anodized MoO3−x Films and Their Boosted Photocatalytic Activity. J. Environ. Chem. Eng. 2021, 9, 105565. [Google Scholar] [CrossRef]
- Sari, F.N.I.; Ting, J.-M. MoS2/MoOx-Nanostructure-Decorated Activated Carbon Cloth for Enhanced Supercapacitor Performance. ChemSusChem 2018, 11, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhao, Y.; Peng, K.; Yu, L. One-Step Hydrothermal Synthesis of MoO2/MoS2 Nanocomposites as High-Performance Electrode Material for Supercapacitors. ACS Appl. Mater. Interfaces 2022, 14, 49909–49918. [Google Scholar] [CrossRef] [PubMed]
- Kite, S.V.; Kadam, A.N.; Sathe, D.J.; Patil, S.; Mali, S.S.; Hong, C.K.; Lee, S.; Garadkar, K.M. Nanostructured TiO2 Sensitized with MoS2 Nanoflowers for Enhanced Photodegradation Efficiency toward Methyl Orange. ACS Omega 2021, 6, 17071–17085. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Feng, Y.; Wang, F.; Chen, D.; Zhang, Q.; Zeng, Y.; Lv, W.; Liu, G. Construction of Carbon Dots Modified MoO3/g-C3N4 Z-Scheme Photocatalyst with Enhanced Visible-Light Photocatalytic Activity for the Degradation of Tetracycline. Appl. Catal. B Environ. 2018, 229, 96–104. [Google Scholar] [CrossRef]
- Wei, W.; Tian, Q.; Sun, H.; Liu, P.; Zheng, Y.; Fan, M.; Zhuang, J. Efficient Visible-Light-Driven Photocatalytic H2 Evolution over MoO2-C/CdS Ternary Heterojunction with Unique Interfacial Microstructures. Appl. Catal. B Environ. 2020, 260, 118153. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Feng, H.-P.; Niu, C.-G.; Huang, D.-W.; Guo, H.; Liang, C.; Liu, H.-Y.; Chen, S.; Tang, N.; Li, L. Constructing a Plasma-Based Schottky Heterojunction for near-Infrared-Driven Photothermal Synergistic Water Disinfection: Synergetic Effects and Antibacterial Mechanisms. Chem. Eng. J. 2021, 426, 131902. [Google Scholar] [CrossRef]
- Kurnia, F.; Ng, Y.H.; Amal, R.; Valanoor, N.; Hart, J.N. Defect Engineering of ZnS Thin Films for Photoelectrochemical Water-Splitting under Visible Light. Sol. Energy Mater. Sol. Cells 2016, 153, 179–185. [Google Scholar] [CrossRef]
- Hao, X.; Wang, Y.; Zhou, J.; Cui, Z.; Wang, Y.; Zou, Z. Zinc Vacancy-Promoted Photocatalytic Activity and Photostability of ZnS for Efficient Visible-Light-Driven Hydrogen Evolution. Appl. Catal. B Environ. 2018, 221, 302–311. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering Heterogeneous Semiconductors for Solar Water Splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Yi, S.-S.; Yan, J.-M.; Wulan, B.-R.; Li, S.-J.; Liu, K.-H.; Jiang, Q. Noble-Metal-Free Cobalt Phosphide Modified Carbon Nitride: An Efficient Photocatalyst for Hydrogen Generation. Appl. Catal. B Environ. 2017, 200, 477–483. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Niu, C.-G.; Huang, D.-W.; Guo, H.; Feng, H.-P.; Li, L.; Liu, H.-Y.; Fan, Q.-Q.; Qin, M.-Z. Appropriate Oxygen Vacancies and Mo-N Bond Synergistically Modulate Charge Transfer Dynamics of MoO3−x/S-CN for Superior Photocatalytic Disinfection: Unveiling Synergistic Effects and Disinfection Mechanism. J. Hazard. Mater. 2023, 445, 130481. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, K.; Guo, T.; Wu, X.; Zhang, G. Fabrication of Z-Scheme MoO3/Bi2O4 Heterojunction Photocatalyst with Enhanced Photocatalytic Performance under Visible Light Irradiation. Chin. J. Catal. 2020, 41, 161–169. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, S.-J. Facile Construction of MoO3@ZIF-8 Core-Shell Nanorods for Efficient Photoreduction of Aqueous Cr (VI). Appl. Catal. B Environ. 2019, 240, 92–101. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, L.; Wang, A.; Hou, J.; Guo, X. Facile Preparation of Oxygen-Vacancy-Engineered MoOx Nanostructures for Photoreversible Switching Systems. Nanomaterials 2021, 11, 3192. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Prabhakar, R.R.; Tan, J.; Tilley, S.D.; Moon, J. Strategies for Enhancing the Photocurrent, Photovoltage, and Stability of Photoelectrodes for Photoelectrochemical Water Splitting. Chem. Soc. Rev. 2019, 48, 4979–5015. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Q.; Liu, X.; Yang, Q. P-Doped Nanorod MoO3 and Nanoflower NiAl-LDH Construct S-Type Heterojunction for Photocatalytic High-Efficiency Hydrogen Evolution. Surf. Interfaces 2023, 43, 103593. [Google Scholar] [CrossRef]
- Liu, X.; Luo, Y.; Ling, C.; Shi, Y.; Zhan, G.; Li, H.; Gu, H.; Wei, K.; Guo, F.; Ai, Z.; et al. Rare Earth La Single Atoms Supported MoO3−x for Efficient Photocatalytic Nitrogen Fixation. Appl. Catal. B Environ. 2022, 301, 120766. [Google Scholar] [CrossRef]
- Ye, L.; Han, C.; Ma, Z.; Leng, Y.; Li, J.; Ji, X.; Bi, D.; Xie, H.; Huang, Z. Ni2P Loading on Cd0.5Zn0.5S Solid Solution for Exceptional Photocatalytic Nitrogen Fixation under Visible Light. Chem. Eng. J. 2017, 307, 311–318. [Google Scholar] [CrossRef]
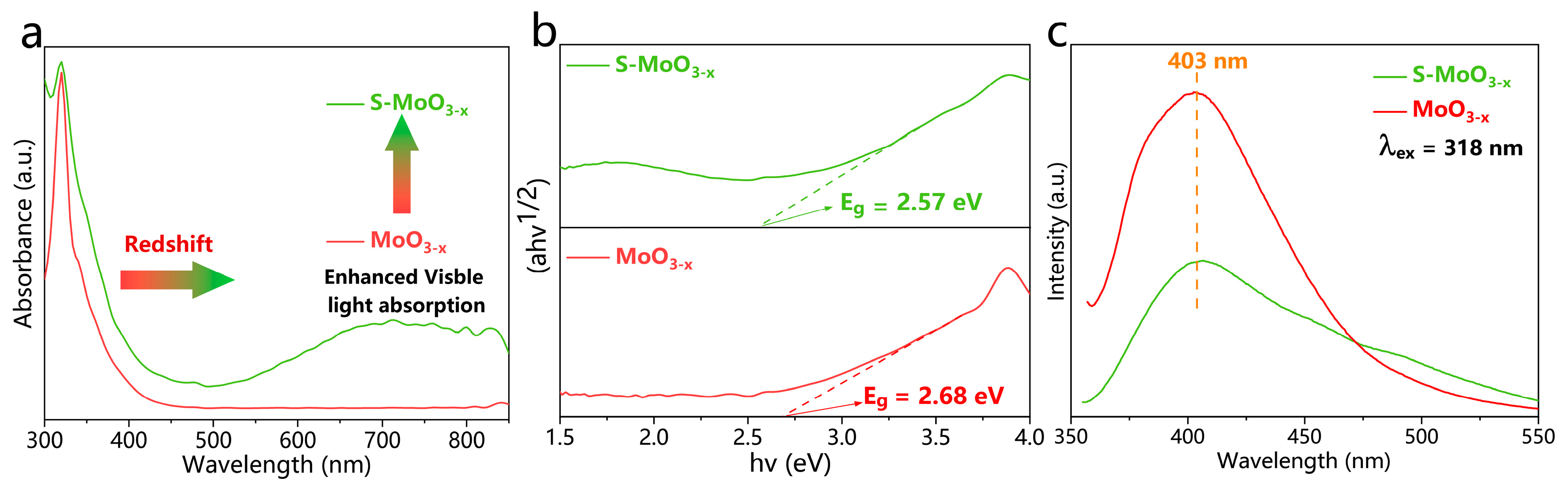
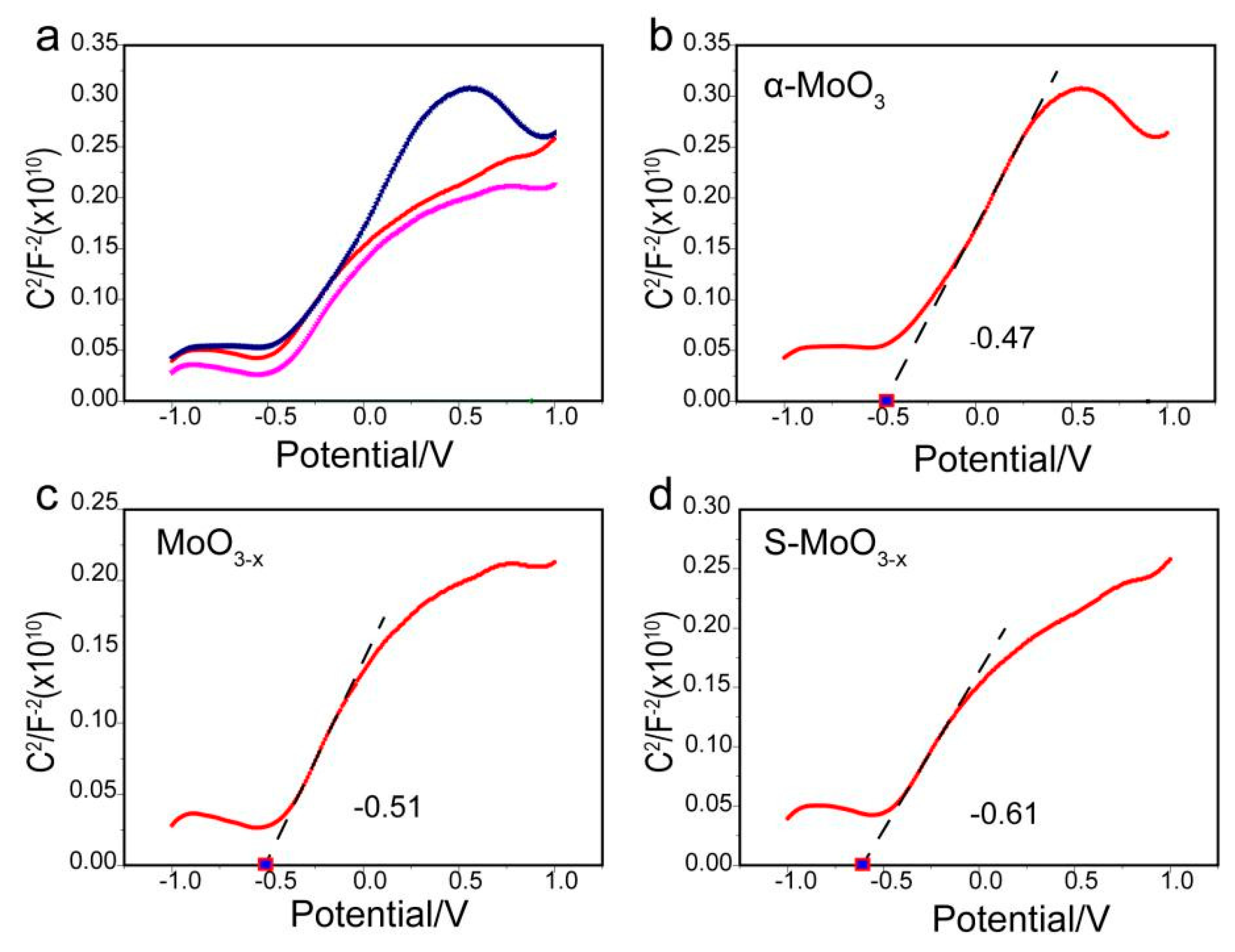
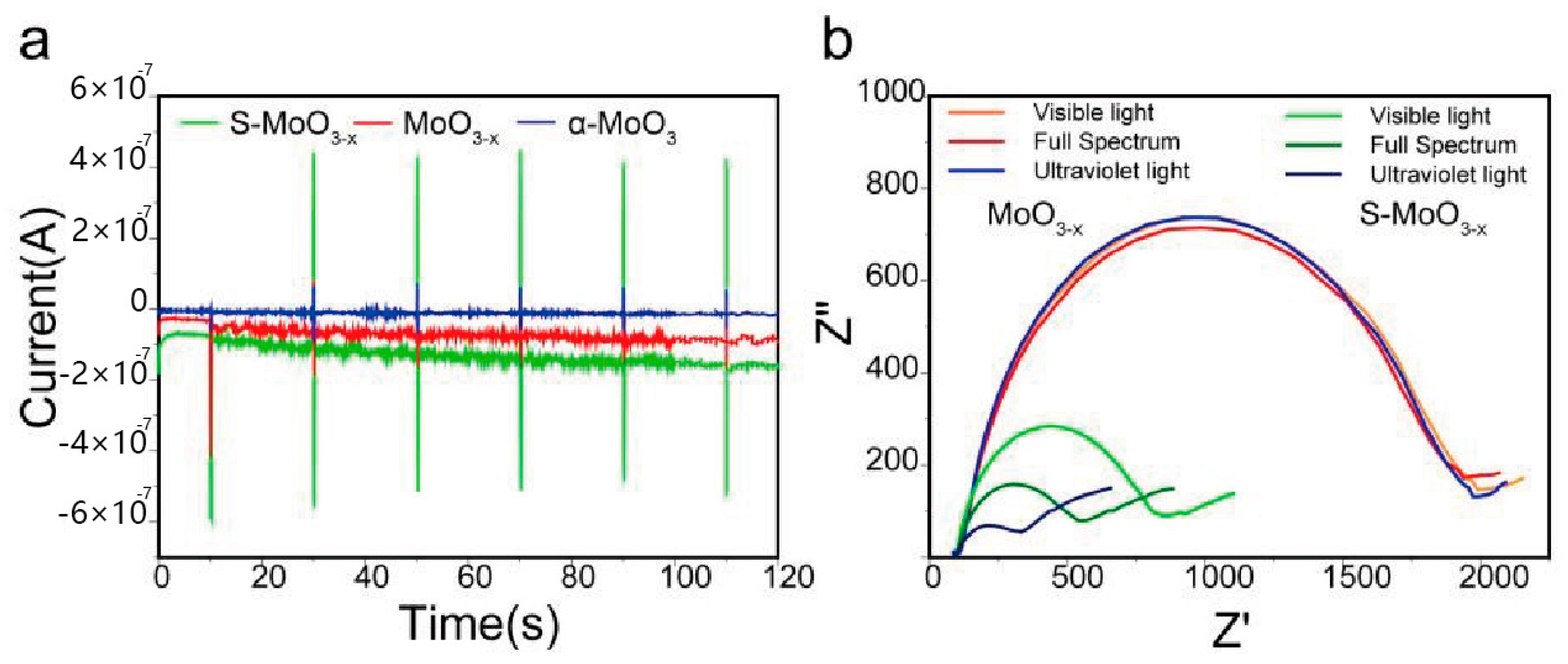

| Semiconductor | Eg (eV) | ECB (eV) | EVB (eV) |
|---|---|---|---|
| MoO3−x | 2.68 | −0.51 | 2.17 |
| S-MoO3−x | 2.57 | −0.61 | 1.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Zheng, Z.; Wang, A.; Humayun, M.; Attia, Y.A. MoO3 with the Synergistic Effect of Sulfur Doping and Oxygen Vacancies: The Influence of S Doping on the Structure, Morphology, and Optoelectronic Properties. Nanomaterials 2024, 14, 1189. https://doi.org/10.3390/nano14141189
Yu J, Zheng Z, Wang A, Humayun M, Attia YA. MoO3 with the Synergistic Effect of Sulfur Doping and Oxygen Vacancies: The Influence of S Doping on the Structure, Morphology, and Optoelectronic Properties. Nanomaterials. 2024; 14(14):1189. https://doi.org/10.3390/nano14141189
Chicago/Turabian StyleYu, Jian, Zhaokang Zheng, Aiwu Wang, Muhammad Humayun, and Yasser A. Attia. 2024. "MoO3 with the Synergistic Effect of Sulfur Doping and Oxygen Vacancies: The Influence of S Doping on the Structure, Morphology, and Optoelectronic Properties" Nanomaterials 14, no. 14: 1189. https://doi.org/10.3390/nano14141189
APA StyleYu, J., Zheng, Z., Wang, A., Humayun, M., & Attia, Y. A. (2024). MoO3 with the Synergistic Effect of Sulfur Doping and Oxygen Vacancies: The Influence of S Doping on the Structure, Morphology, and Optoelectronic Properties. Nanomaterials, 14(14), 1189. https://doi.org/10.3390/nano14141189







