Design Strategies of Hydrogen Evolution Reaction Nano Electrocatalysts for High Current Density Water Splitting
Abstract
1. Introduction
2. Challenges of Electrocatalysts Faced for HERs under High Current Densities
3. Design Strategies of Electrocatalysts for HERs under High Current Densities
3.1. Tuning Electronic Structure and Crystal Phase of the Catalyst for Enhancing Intrinsic Activity

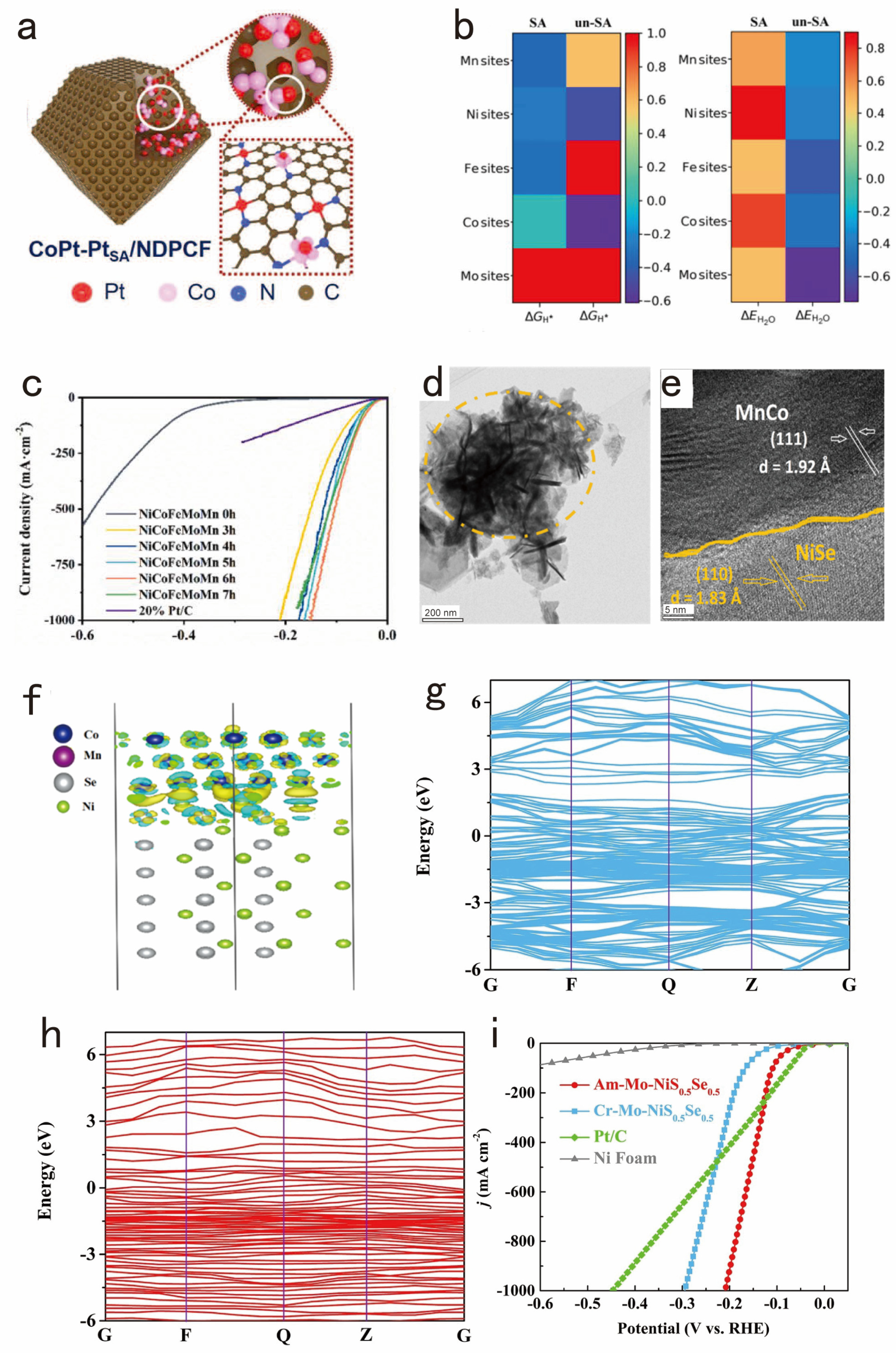
3.2. Designing the Interface of the Electrocatalysts for Exposing a Large Number of Active Sites

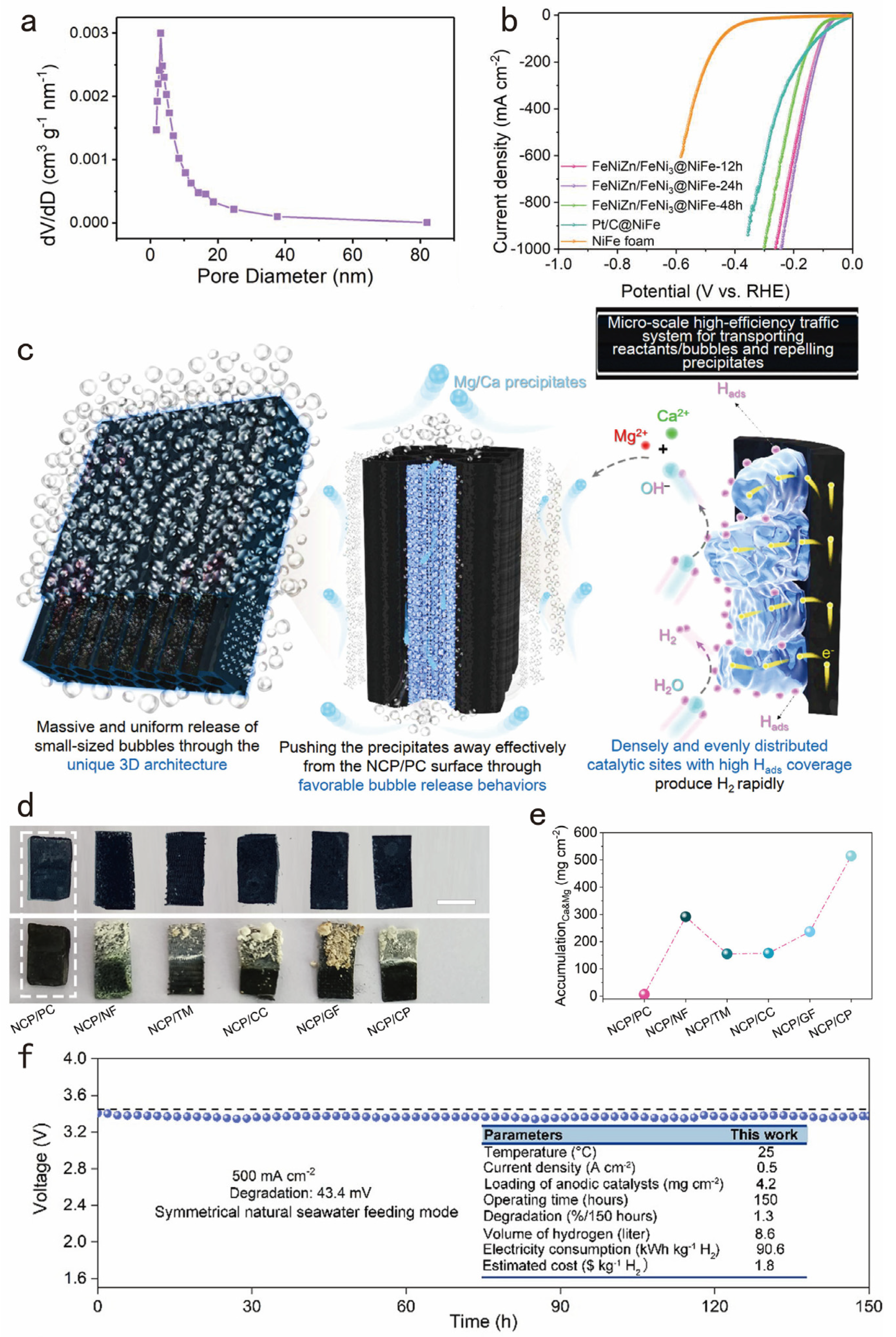
3.3. Designing Superwetting Porous Structure for Accelerating Bubble Detachment

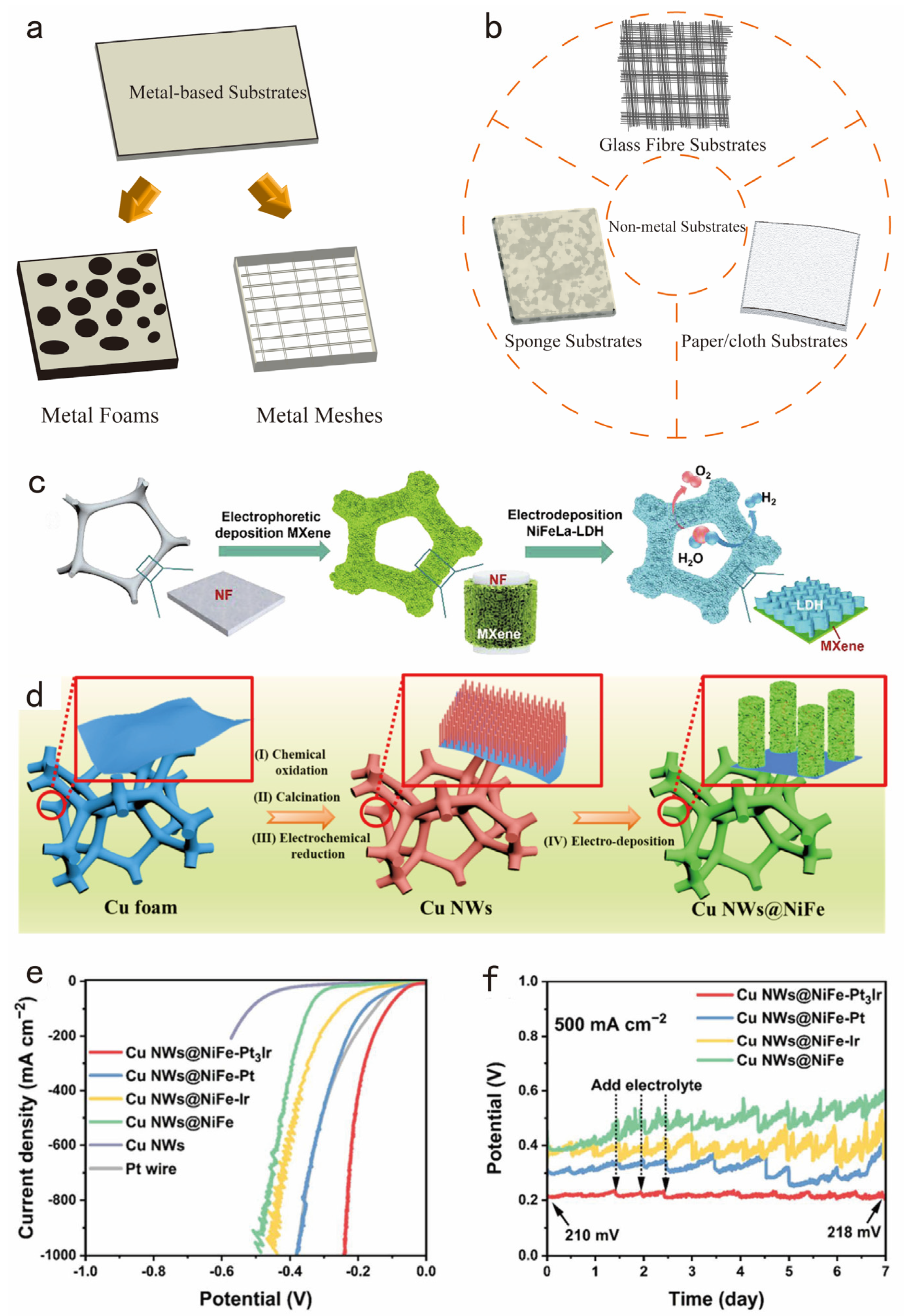
3.4. Modulating Structure of Electrocatalysts for Enhancing the Chemical/Mechanical Strength

4. Conclusions and Outlooks
| Design Strategy | Electrocatalyst | Electrolyte | Activity (mV@mA cm−2) | Stability (h@mA cm−2) | Ref. |
|---|---|---|---|---|---|
| Electronic structure modulation | Ce-CoP/Ni3P | 1 M KOH | 225@1000 | 200@500 | [61] |
| Bi2O3-OV | 1 M KOH | 310@300 | / | [64] | |
| HSD-PtNi/NF | 1 M KOH | 63@1000 | 300@1000 | [65] | |
| MnCo/NiSe/NF | 1 M KOH | 211.6@1000 | 150@500 | [70] | |
| Am-Mo-NiS0.5Se0.5 | 1 M PBS | 209@1000 | / | [71] | |
| MoO2@Ru | 1 M KOH | 131@1000 | 100@1000 | [95] | |
| Pt/NiOx-OV | 1 M KOH | ≈180@500 | 100@1000 | [96] | |
| Ru-NiSe2 | 1 M KOH | 180.8@1000 | 90@1000 | [97] | |
| Ru-Ni3N/NiO | 1 M KOH | 190@1000 | 1000@500 | [98] | |
| Ru/Ni@C | 1 M KOH | 309@1000 | 100@1000 | [99] | |
| Co/NC-HP@Si-NW | 0.5 M H2SO4 | 440@500 | 24@500 | [100] | |
| MoS2-P2 | 1 M KOH | 332@500 | 240@500 | [101] | |
| Crystal phase modulation | MnO/CoP/NF | 1 M KOH | 259.5@1000 | 100@500 | [102] |
| Fe2P/Co2N | 1 M KOH | 131@500 | 40@500 | [103] | |
| Mo2N/Ni3Mo3N | 1 M KOH | 123@500 | 120@500 | [104] | |
| (Fe, Ni)2P@Ni2P | 1 M KOH | 255@1000 | 120@1000 | [105] | |
| RuCo@RuSACoSA-NMC | 1 M KOH | 291@1500 | 576@1000 | [106] | |
| PtNiMg | 1 M KOH | / | 100@2000 | [107] | |
| Ni2.86Te2/NF | 1 M KOH | 348@200 | / | [108] | |
| (WO2-Ni17W3)/ NiFe(OH)X/NF | 1 M KOH | 258@1000 | 120@1000 | [109] | |
| Superwetting structure | Ni(OH)x/Ni3S2 | 1 M KOH | 238@1000 | 1000@320 | [78] |
| NCP/PC | 1 M KOH | 145@1000 | 1000@1000 | [81] | |
| MoNi/NiMoOx | 1 M KOH | 139@1900 | 100@600 | [110] | |
| HW-NiMoN/NF-2h | 1 M KOH | 107@1000 | 100@500 | [111] | |
| Pt-Ni@NiMoN/NF | 1 M KOH | 90@500 | 120@1000 | [112] | |
| Mo2N-Mo2C/N-CW | 1 M KOH | 311@500 | / | [113] | |
| Self-supported electrodes | Cu NWs@NiFe-Pt3Ir | 1 M KOH | 239@1000 | 168@500 | [94] |
| FeCoCrCuOx@CF | 1 M KOH | / | 160@500 | [114] | |
| NMFSOH | 1 M KOH | 200@1000 | 300@500 | [115] | |
| Fe0.01-Ni&Ni0.2Mo0.8N | 1 M KOH | 135@500 | 100@400 | [116] | |
| NiFe-LDH@NiMo-H2@NF | 1 M KOH | 73@500 | 400@500 | [117] | |
| IrNi-FeNi3/NF | 1 M KOH | 288.8@1000 | 124@1000 | [118] | |
| Ni5P4-Co2P/NCF | 1 M KOH | 267@1000 | 100@250 | [119] | |
| MnOX/NiFeP/NF | 1 M KOH | 255@500 | 120@500 | [120] |
| Two-Electrode System | Condition | Activity (V@mA cm−2) | Stability (h@mA cm−2) |
|---|---|---|---|
| Ce0.2-CoP/Ni3P@NF||Ce0.2-FePX/Ni3P | 25 wt% KOH, 50 °C | 1.718@500 | 200@500 |
| HSD-PtNi/NF||NiFe LDH | 1 M KOH, RT | 1.72@500 | / |
| MnCo/NiSe||MnCo/NiSe | 1 M KOH, RT | 1.88@1000 | 200@500 |
| Am-Mo-NiS0.5Se0.5||Am-Mo-NiS0.5Se0.5 | 1 M PBS, RT | 1.98@1000 | / |
| MoO2@Ru NT||NiFe LDH | 1 M KOH, RT | 1.78@1000 | 200@1000 |
| R-NF-Pt||NiFe-LDH | 1 M KOH, RT | 1.776@1000 | 400@1000 |
| Ru-Ni3N/NiO||Ru-Ni3N/NiO | 1 M KOH, RT | 1.74@1000 | 1000@500 |
| MnO-CoP/NF||MnO-CoP/NF | 1 M KOH, RT | 1.76@500 | 100@500 |
| Fe2P/Co2N||Fe2P/Co2N | 1 M KOH, RT | 1.663@500 | 120@500 |
| (Fe, Ni)2P@Ni2P||(Fe, Ni)2P@Ni2P | 1 M KOH, RT | 1.933@1000 | 12@1000 |
| RuCo@RuSACoSA-NMC||RuO2 | 1 M KOH, RT | 2.15@1000 | 7200@1000 |
| Ni(OH)x/Ni3S2/NF||NiFe LDH/NF | 1 M KOH, RT | 1.83@1000 | 400@360 |
| MoNi/NiMoOX||Co2(OH)3Cl/FeOOH | 1 M KOH, RT | 3.05@500 | 1600@200 |
| FeCoCrCuOX/CF||FeCoCrCuOX/CF | 1 M KOH, RT | 2.95@500 | 100@500 |
| Fe0.01-Ni&Ni0.2Mo0.8N||Fe0.01&Mo-NiO | 6 M KOH, 60 °C | 1.539@1000 | 80@425 |
| NiFe-LDH@NiMo-H2@NF|| NiFe-LDH@NiMo-H2@NF | 1 M KOH, RT | 1.61@500 | 200@500 |
| IrNi-FeNi3/NF||IrNi-FeNi3/NF | 1 M KOH, 30 °C | 1.78@500 | 100@500 |
| MnOX/NiFeP/NF||MnOX/NiFeP/NF | 1 M KOH, RT | 1.828@1000 | 120@500 |
- (1)
- Scale-up production and synthesis of electrocatalysts: In high-current-density water splitting, although some excellent electrocatalysts exhibit good stability, they are complex to synthesize and difficult to scale up, limiting their use in commercial alkaline electrolyzers. These electrolyzers typically require large-area electrodes, which are difficult to meet with traditional laboratory-scale solvent heating and electrodeposition methods. To overcome these challenges, experimental designs need to consider the scalability of the synthesis methods. In addition, the development of new synthesis techniques, such as wet chemical methods and 3D printing, is also seen as a powerful way to achieve scale-up [121]. While pursuing technical feasibility, the economic and environmental impacts of the catalysts need to be considered to ensure the cost-effectiveness of the electrodes and the environmental sustainability of their production.
- (2)
- Advances in in situ and operando characterization techniques: In electrocatalytic studies, phase characterization of catalysts is usually only possible in their stable final state [122], which limits our in-depth understanding of the micro-mechanisms of catalytic reactions. The development of in situ and operando characterization techniques is crucial for monitoring phase changes during catalysis, especially when it comes to unraveling the mechanism of HER. Although there are still current controversies regarding the mechanisms of HERs, in situ techniques can help resolve these controversies by providing direct evidence about the reaction intermediates.
- (3)
- The environmental adaptability of electrocatalysts: The adaptation of electrocatalysts under different electrolyte conditions is one of the central issues of great interest in the field of electrochemistry today. Although most studies have focused on their performance under alkaline conditions, their behavior under acidic and neutral environments has not been fully appreciated [123]. Understanding the differences in the performance of electrocatalysts under different pH environments is crucial, not only for hydrogen energy technology and water utilization but also for the study of other electrocatalytic reactions. In order to achieve this goal, extensive performance evaluations are needed, including electrocatalytic activity in acidic, neutral, and alkaline electrolytes, as well as stability and efficiency tests under simulated industrial conditions. In addition, standardized catalyst test methods and seawater electrolysis components need to be developed in order to advance electrocatalyst technology toward practical applications. This will help ensure consistent and comparable evaluations and provide a solid foundation for future electrocatalyst design and optimization.
- (4)
- Innovations in electrode design: The key to innovation in electrode design is to optimize the hydrophilic and hydrophobic properties of the electrode and introduce novel structures, such as 3D self-supporting electrodes, to enhance electrolysis efficiency and long-term stability [124]. By adjusting the chemical composition and microstructure of the electrode surface, such as micro- or nano-scale roughness, we can significantly improve the hydrophilicity of the electrode, which helps to increase the contact with the aqueous electrolyte and facilitates ion transport. Meanwhile, the superhydrophobic design, such as through nano-arrays and layered structures, can reduce the adhesion of gas bubbles on the electrode surface and accelerate the gas emission, thus improving the gas transfer efficiency of the overall electrolysis process. In addition, optimal activity and stability can be achieved by optimizing the structure and composition of the electrocatalysts at the atomic, nano, and micro scales. These multiscale design strategies not only improve the performance of electrodes but also help to improve the matching of electrolyzer and electrodes and promote the widespread adoption of electrolysis technology in industrial applications.
- (5)
- Systematic study of temperature and pressure: An in-depth study of catalyst performance under different environmental conditions, especially taking into account temperature and pressure variations [125], is essential for optimizing electrocatalytic processes in industrial applications. In practice, catalysts undergo significant changes in active sites and structure under the influence of high temperatures and pressures, and these changes can significantly affect electrocatalytic performance and reaction kinetics [126]. Therefore, by simulating these harsh industrial conditions and evaluating the catalysts, it can help us to gain a deeper understanding and improve the design and functionality of electrocatalysts to meet industrial standards and efficiency requirements.
Author Contributions
Funding
Conflicts of Interest
References
- Mishra, R.; Kumar, V.; Choi, G.; Ryu, J.; Mane, S.; Shin, J.; Gwag, J. Hexagonal NiO nanosheets on Ni-foam as an electrocatalyst for high-performance water splitting application. Mater. Lett. 2022, 324, 132740. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.; Shao, Z. Designing single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Siddiqui, O.; Dincer, I. A well to pump life cycle environmental impact assessment of some hydrogen production routes. Int. J. Hydrogen Energy 2019, 44, 5773–5786. [Google Scholar] [CrossRef]
- Wen, Q.; Zhao, Y.; Liu, Y.; Li, H.; Zhai, T. Ultrahigh-current-density and long-term-durability electrocatalysts for water splitting. Small 2022, 18, 2104513. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, J.; Jia, J.; Liu, F.; Zhang, Y.; Xiong, G.; Zhang, R.; Yang, R.; Sun, D.; Liu, H. Metallic Ni3Mo3N porous microrods with abundant catalytic sites as efficient electrocatalyst for large current density and superstability of hydrogen evolution reaction and water splitting. Appl. Catal. B 2020, 272, 118956. [Google Scholar] [CrossRef]
- Lv, X.; Wan, S.; Mou, T.; Han, X.; Zhang, Y.; Wang, Z.; Tao, X. Atomic-level surface engineering of nickel phosphide nanoarrays for efficient electrocatalytic water splitting at large current density. Adv. Funct. Mater. 2023, 33, 2205161. [Google Scholar] [CrossRef]
- Qian, G.; Chen, J.; Luo, L.; Yu, T.; Wang, Y.; Jiang, W.; Xu, Q.; Feng, S.; Yin, S. Industrially promising nanowire heterostructure catalyst for enhancing overall water splitting at large current density. ACS Sustain. Chem. Eng. 2020, 8, 12063–12071. [Google Scholar] [CrossRef]
- Wu, T.; Xu, S.; Zhang, Z.; Luo, M.; Wang, R.; Tang, Y.; Wang, J.; Huang, F. Bimetal modulation stabilizing a metallic heterostructure for efficient overall water splitting at large current density. Adv. Sci. Lett. 2022, 9, 2202750. [Google Scholar] [CrossRef]
- Wang, P.; Luo, Y.; Zhang, G.; Wu, M.; Chen, Z.; Sun, S.; Shi, Z. MnOx-Decorated Nickel-Iron Phosphides Nanosheets: Interface Modifications for Robust Overall Water Splitting at Ultra-High Current Densities. Small 2022, 18, 2105803. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, X.; Guo, X.; Zhang, J.; Yuan, A.; Du, Y.; Gao, F. Design of modified MOFs electrocatalysts for water splitting: High current density operation and long-term stability. Appl. Catal. B 2023, 336, 122891. [Google Scholar] [CrossRef]
- Wu, D.; Chen, D.; Zhu, J.; Mu, S. Ultralow Ru incorporated amorphous cobalt-based oxides for high-current-density overall water splitting in alkaline and seawater media. Small 2021, 17, 2102777. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, X.; Dong, Y.; Nan, J.; Sun, Y.; Yu, H.; Zhang, X.; Dong, B.; Chai, Y. Strong ion interaction inducing ultrahigh activity of NiCoP nanowires for overall water splitting at large current density. Appl. Surf. Sci. 2022, 589, 152837. [Google Scholar] [CrossRef]
- Yan, F.; Wang, Y.; Li, K.; Zhu, C.; Gao, P.; Li, C.; Zhang, X.; Chen, Y. Highly Stable Three-Dimensional Porous Nickel-Iron Nitride Nanosheets for Full Water Splitting at High Current Densities. Chemistry 2017, 23, 10187–10194. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; Yuan, L.; Qi, H.; Sun, X.; Zhang, L.; Li, H.; Yuan, H.; Feng, S. Sn-Ni3S2 ultrathin nanosheets as efficient bifunctional water-splitting catalysts with a large current density and low overpotential. ACS Appl. Mater. Interfaces 2018, 10, 40568–40576. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sirisomboonchai, S.; Yoshida, A.; An, X.; Hao, X.; Abudula, A.; Guan, G. Bifunctional CoNi/CoFe2O4/Ni foam electrodes for efficient overall water splitting at a high current density. J. Mater. Chem. A 2018, 6, 19221–19230. [Google Scholar] [CrossRef]
- Wen, Q.; Yang, K.; Huang, D.; Cheng, G.; Ai, X.; Liu, Y.; Fang, J.; Li, H.; Yu, L.; Zhai, T. Schottky heterojunction nanosheet array achieving high-current-density oxygen evolution for industrial water splitting electrolyzers. Adv. Energy Mater. 2021, 11, 2102353. [Google Scholar] [CrossRef]
- Yu, T.; Xu, Q.; Qian, G.; Chen, J.; Zhang, H.; Luo, L.; Yin, S. Amorphous CoOX-decorated crystalline RuO2 nanosheets as bifunctional catalysts for boosting overall water splitting at large current density. ACS Sustain. Chem. Eng. 2020, 8, 17520–17526. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Z.; Yang, F.; Li, J.; Liu, Z.; Ren, W.; Zhang, S.; Liu, B. Stabilized hydroxide-mediated nickel-based electrocatalysts for high-current-density hydrogen evolution in alkaline media. Energy Environ. Sci. 2021, 14, 4610–4619. [Google Scholar] [CrossRef]
- Liang, C.; Zou, P.; Nairan, A.; Zhang, Y.; Liu, J.; Liu, K.; Hu, S.; Kang, F.; Fan, H.J.; Yang, C. Exceptional performance of hierarchical Ni–Fe oxyhydroxide@NiFe alloy nanowire array electrocatalysts for large current density water splitting. Energy Environ. Sci. 2020, 13, 86–95. [Google Scholar] [CrossRef]
- Sun, H.; Tian, C.; Fan, G.; Qi, J.; Liu, Z.; Yan, Z.; Cheng, F.; Chen, J.; Li, C.P.; Du, M. Boosting activity on Co4N porous nanosheet by coupling CeO2 for efficient electrochemical overall water splitting at high current densities. Adv. Funct. Mater. 2020, 30, 1910596. [Google Scholar] [CrossRef]
- Xing, Z.; Liu, Q.; Asiri, A.M.; Sun, X. High-efficiency electrochemical hydrogen evolution catalyzed by tungsten phosphide submicroparticles. ACS Catal. 2015, 5, 145–149. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Chen, Y.; Dou, S.; Chen, X.; Dong, B.; Guo, B.; Liu, D.; Liu, C.; Chai, Y. Hydrogen evolution under large-current-density based on fluorine-doped cobalt-iron phosphides. Chem. Eng. J. 2020, 399, 125831. [Google Scholar] [CrossRef]
- Chai, S.; Zhang, G.; Li, G.; Zhang, Y. Industrial hydrogen production technology and development status in China: A review. Clean Technol. Environ. Policy 2021, 23, 1931–1946. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, W.; Zhao, J.; Dong, B.; Liu, C.; Chai, Y. Recent development on self-supported transition metal-based catalysts for water electrolysis at large current density. Appl. Mater. Today 2021, 22, 100913. [Google Scholar] [CrossRef]
- Li, S.; Li, E.; An, X.; Hao, X.; Jiang, Z.; Guan, G. Transition metal-based catalysts for electrochemical water splitting at high current density: Current status and perspectives. Nanoscale 2021, 13, 12788–12817. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Zhang, Y.; Lee, E.; Shankhari, P.; Fokwa, B.P. High-Current-Density HER Electrocatalysts: Graphene-like Boron Layer and Tungsten as Key Ingredients in Metal Diborides. ChemSusChem 2019, 12, 3726–3731. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Zhang, Z.; Cui, Y.; Zhang, R.; Zhang, L.; Zeng, J.; Cheng, D. One-Step Approach for Constructing High-Density Single-Atom Catalysts toward Overall Water Splitting at Industrial Current Densities. Angew. Chem. 2023, 135, e202214259. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, S.; Wei, C.; Fan, J.; Yao, H.; Dai, L.; Wang, G.; Li, H.; Su, B.; Guo, X. Synergistic effect between platinum single atoms and oxygen vacancy in MoO2 boosting pH-universal hydrogen evolution reaction at large current density. Chem. Eng. J. 2022, 427, 131309. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, L.; Zhou, H.; Li, D.; Tang, D.; Yu, F. Innovative strategies in design of transition metal-based catalysts for large-current-density alkaline water/seawater electrolysis. Mater. Today Phys. 2022, 26, 100727. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Huang, Y.; Fu, J. Recent advances in electrocatalysts for neutral and large-current-density water electrolysis. Nano Energy 2021, 80, 105545. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, W.; Lei, T.; Zhou, B.; Jiao, Y.; Yan, Y.; Du, X.; Huang, J.; Wu, C.; Wang, X. Carbon quantum dots–modified interfacial interactions and ion conductivity for enhanced high current density performance in lithium–sulfur batteries. Adv. Energy Mater. 2019, 9, 1802955. [Google Scholar] [CrossRef]
- Yu, L.; Mishra, I.; Xie, Y.; Zhou, H.; Sun, J.; Zhou, J.; Ni, Y.; Luo, D.; Yu, F.; Yu, Y. Ternary Ni2(1−x) Mo2xP nanowire arrays toward efficient and stable hydrogen evolution electrocatalysis under large-current-density. Nano Energy 2018, 53, 492–500. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, Y.; Pan, C.; Wang, C.; Hu, S.; Xiao, W.; Chi, X.; Fang, Y.; Yang, J.; Deng, H. Interfacial sp C-O-Mo hybridization originated high-current density hydrogen evolution. J. Am. Chem. Soc. 2021, 143, 8720–8730. [Google Scholar] [CrossRef] [PubMed]
- Friedl, J.; Bauer, C.M.; Rinaldi, A.; Stimming, U. Electron transfer kinetics of the VO2+/VO2+-Reaction on multi-walled carbon nanotubes. Carbon 2013, 63, 228–239. [Google Scholar] [CrossRef]
- Wu, Z.; Fang, B.; Wang, Z.; Wang, C.; Liu, Z.; Liu, F.; Wang, W.; Alfantazi, A.; Wang, D.; Wilkinson, D.P. MoS2 nanosheets: A designed structure with high active site density for the hydrogen evolution reaction. ACS Catal. 2013, 3, 2101–2107. [Google Scholar] [CrossRef]
- Cheng, F.; Peng, X.; Hu, L.; Yang, B.; Li, Z.; Dong, C.-L.; Chen, J.-L.; Hsu, L.-C.; Lei, L.; Zheng, Q. Accelerated water activation and stabilized metal-organic framework via constructing triangular active-regions for ampere-level current density hydrogen production. Nat. Commun. 2022, 13, 6486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, F.; Zhao, J.; Dong, B.; Wang, F.; Wu, Z.; Wang, L.; Chai, Y.; Liu, C. Tailoring the d-band centers of FeP nanobelt arrays by fluorine doping for enhanced hydrogen evolution at high current density. Fuel 2022, 316, 123206. [Google Scholar] [CrossRef]
- Olesen, A.; Frensch, S.; Kær, S. Towards uniformly distributed heat, mass and charge: A flow field design study for high pressure and high current density operation of PEM electrolysis cells. Electrochim. Acta 2019, 293, 476–495. [Google Scholar] [CrossRef]
- Xiao, C.; Li, Y.; Lu, X.; Zhao, C. Bifunctional porous NiFe/NiCo2O4/Ni foam electrodes with triple hierarchy and double synergies for efficient whole cell water splitting. Adv. Funct. Mater. 2016, 26, 3515–3523. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Yang, Y.; Lan, L.; Liu, R.; Fu, Q.; Zhang, L.; Liao, Q.; Zhu, X. Gradient porous electrode-inducing bubble splitting for highly efficient hydrogen evolution. Appl. Energy 2022, 307, 118278. [Google Scholar] [CrossRef]
- Bae, M.; Kang, Y.; Lee, D.W.; Jeon, D.; Ryu, J. Superaerophobic polyethyleneimine hydrogels for improving electrochemical hydrogen production by promoting bubble detachment. Adv. Energy Mater. 2022, 12, 2201452. [Google Scholar] [CrossRef]
- Kou, T.; Wang, S.; Shi, R.; Zhang, T.; Chiovoloni, S.; Lu, J.; Chen, W.; Worsley, M.; Wood, B.; Baker, S. Periodic porous 3D electrodes mitigate gas bubble traffic during alkaline water electrolysis at high current densities. Adv. Energy Mater. 2020, 10, 2002955. [Google Scholar] [CrossRef]
- Li, Y.; Yang, G.; Yu, S.; Kang, Z.; Mo, J.; Han, B.; Talley, D.; Zhang, F. In-situ investigation and modeling of electrochemical reactions with simultaneous oxygen and hydrogen microbubble evolutions in water electrolysis. Int. J. Hydrogen Energy 2019, 44, 28283–28293. [Google Scholar] [CrossRef]
- Park, S.; Liu, L.; Demirkır, Ç.; van der Heijden, O.; Lohse, D.; Krug, D.; Koper, M.T. Solutal Marangoni effect determines bubble dynamics during electrocatalytic hydrogen evolution. Nat. Chem. 2023, 15, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Xu, T.; Lu, Z.; Wu, X.; Wan, P.; Sun, X.; Jiang, L. Under-water superaerophobic pine-shaped Pt nanoarray electrode for ultrahigh-performance hydrogen evolution. Adv. Funct. Mater. 2015, 25, 1737–1744. [Google Scholar] [CrossRef]
- Shang, L.; Zhao, Y.; Kong, X.; Shi, R.; Waterhouse, G.; Wen, L.; Zhang, T. Underwater superaerophobic Ni nanoparticle-decorated nickel–molybdenum nitride nanowire arrays for hydrogen evolution in neutral media. Nano Energy 2020, 78, 105375. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Z.; Chhowalla, M.; Liu, B. Recent Advances in Design of Electrocatalysts for High-Current-Density Water Splitting. Adv. Mater. 2022, 34, 2108133. [Google Scholar] [CrossRef]
- Wu, T.; Song, E.; Zhang, S.; Luo, M.; Zhao, C.; Zhao, W.; Liu, J.; Huang, F. Engineering metallic heterostructure based on Ni3N and 2M-MoS2 for alkaline water electrolysis with industry-compatible current density and stability. Adv. Mater. 2022, 34, 2108505. [Google Scholar] [CrossRef]
- Qian, G.; Chen, J.; Yu, T.; Luo, L.; Yin, S. N-doped graphene-decorated NiCo alloy coupled with mesoporous NiCoMoO nano-sheet heterojunction for enhanced water electrolysis activity at high current density. Nano-Micro Lett. 2021, 13, 77. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel–iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 2015, 6, 6616. [Google Scholar] [CrossRef]
- Raja, D.; Lin, H.; Lu, S. Synergistically well-mixed MOFs grown on nickel foam as highly efficient durable bifunctional electrocatalysts for overall water splitting at high current densities. Nano Energy 2019, 57, 1–13. [Google Scholar] [CrossRef]
- Ding, J.; Yang, H.; Zhang, S.; Liu, Q.; Cao, H.; Luo, J.; Liu, X. Advances in the Electrocatalytic Hydrogen Evolution Reaction by Metal Nanoclusters-based Materials. Small 2022, 18, 2204524. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Shi, Z.; Peng, J.; Ou, L.; Chen, Y.; Huang, J. Hierarchically Porous Carbonized Wood Decorated with MoNi4-Embedded MoO2 Nanosheets: An Efficient Electrocatalyst for Water Splitting. Adv. Funct. Mater. 2023, 34, 2308337. [Google Scholar] [CrossRef]
- Maleki, M.; Sabour Rouhaghdam, A.; Barati Darband, G.; Han, D.; Shanmugam, S. Highly Active and Durable NiCoSeP Nanostructured Electrocatalyst for Large-Current-Density Hydrogen Production. ACS Appl. Energy Mater. 2022, 5, 2937–2948. [Google Scholar] [CrossRef]
- Grigoriev, S.; Fateev, V.; Bessarabov, D.; Millet, P. Current status, research trends, and challenges in water electrolysis science and technology. Int. J. Hydrogen Energy 2020, 45, 26036–26058. [Google Scholar] [CrossRef]
- Jin, H.; Liu, X.; Chen, S.; Vasileff, A.; Li, L.; Jiao, Y.; Song, L.; Zheng, Y.; Qiao, S. Heteroatom-doped transition metal electrocatalysts for hydrogen evolution reaction. ACS Energy Lett. 2019, 4, 805–810. [Google Scholar] [CrossRef]
- Ren, J.; Chen, L.; Wang, H.; Tian, W.; Song, X.; Kong, Q.; Yuan, Z. Synergistic Activation of Crystalline Ni2P and Amorphous NiMoOX for Efficient Water Splitting at High Current Densities. ACS Catal. 2023, 13, 9792–9805. [Google Scholar] [CrossRef]
- Pan, Q.; Wang, Y.; Chen, B.; Zhang, X.; Lin, D.; Yan, S.; Han, F.; Zhao, H.; Meng, G. Pt Single-Atoms on Structurally-Integrated 3D N-Doped Carbon Tubes Grid for Ampere-Level Current Density Hydrogen Evolution. Small 2024, 2024, 2309067. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, F.; Liu, W.; Mo, S.; Li, X.; Yang, D.; Liu, Y.; Bao, S. Multifunctional carbon-armored Ni electrocatalyst for hydrogen evolution under high current density in alkaline electrolyte solution. Appl. Catal. B 2023, 321, 122081. [Google Scholar] [CrossRef]
- Yu, J.; Hu, K.; Zhang, Z.; Luo, L.; Liu, Y.; Zhou, D.; Wang, F.; Kuang, Y.; Xu, H.; Li, H.; et al. Interfacial nanobubbles’ growth at the initial stage of electrocatalytic hydrogen evolution. Energy Environ. Sci. 2023, 16, 2068–2079. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Han, W.; Qian, Y.; Qiu, L.; He, Y.; Lei, L.; Zhang, X. The Synergistic Activation of Ce-Doping and CoP/Ni3P Hybrid Interaction for Efficient Water Splitting at Large-Current-Density. Adv. Funct. Mater. 2022, 33, 2212381. [Google Scholar] [CrossRef]
- Liao, P.; Kang, J.; Zhong, Y.; Xiang, R.; Wang, S.; Li, S.; Liu, X.; Li, G. Recent advances of two-dimensional metal-organic frameworks in alkaline electrolysis water for hydrogen production. Sci. China Chem. 2023, 66, 1924–1939. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, Y.; Zhang, K.; Tang, X.; Huang, J.; Yuan, K.; Chen, Y. Coaxial electrospun free-standing and mechanically stable hierarchical porous carbon nanofiber membranes for flexible supercapacitors. Carbon 2020, 160, 80–87. [Google Scholar] [CrossRef]
- Wu, Z.; Liao, T.; Wang, S.; Mudiyanselage, J.; Micallef, A.; Li, W.; O’Mullane, A.; Yang, J.; Luo, W.; Ostrikov, K.; et al. Conversion of Catalytically Inert 2D Bismuth Oxide Nanosheets for Effective Electrochemical Hydrogen Evolution Reaction Catalysis via Oxygen Vacancy Concentration Modulation. Nano-Micro Lett. 2022, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Cheng, C.; Dong, C.; Xiao, L.; Zhao, Y.; Liu, Z.; Zhao, X.; Sasaki, K.; Cheng, H.; Du, X.; et al. Dislocation Network-Boosted PtNi Nanocatalysts Welded on Nickel Foam for Efficient and Durable Hydrogen Evolution at Ultrahigh Current Densities. Adv. Energy Mater. 2022, 13, 2202595. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, F.; Zhu, Q.; Sun, J.; Qin, F.; Yu, L.; Bao, J.; Yu, Y.; Chen, S.; Ren, Z. Water splitting by electrolysis at high current densities under 1.6 volts. Energy Environ. Sci. 2018, 11, 2858–2864. [Google Scholar] [CrossRef]
- Yang, W.; Cheng, P.; Li, Z.; Lin, Y.; Li, M.; Zi, J.; Shi, H.; Li, G.; Lian, Z.; Li, H. Tuning the Cobalt–Platinum Alloy Regulating Single-Atom Platinum for Highly Efficient Hydrogen Evolution Reaction. Adv. Funct. Mater. 2022, 32, 2205920. [Google Scholar] [CrossRef]
- Liu, H.; Qin, H.; Kang, J.; Ma, L.; Chen, G.; Huang, Q.; Zhang, Z.; Liu, E.; Lu, H.; Li, J.; et al. A freestanding nanoporous NiCoFeMoMn high-entropy alloy as an efficient electrocatalyst for rapid water splitting. Chem. Eng. J. 2022, 435, 134898. [Google Scholar] [CrossRef]
- Liu, X.; Yao, Y.; Zhang, H.; Pan, L.; Shi, C.; Zhang, X.; Huang, Z.-F.; Zou, J.-J. In Situ-Grown Cobalt–Iron Phosphide-Based Integrated Electrode for Long-Term Water Splitting under a Large Current Density at the Industrial Electrolysis Temperature. ACS Sustain. Chem. Eng. 2020, 8, 17828–17838. [Google Scholar] [CrossRef]
- Andaveh, R.; Sabour Rouhaghdam, A.; Ai, J.; Maleki, M.; Wang, K.; Seif, A.; Barati Darband, G.; Li, J. Boosting the electrocatalytic activity of NiSe by introducing MnCo as an efficient heterostructured electrocatalyst for large-current-density alkaline seawater splitting. Appl. Catal. B 2023, 325, 122355. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Huang, Z.; Wang, H.; Chen, Z.; Zhang, J.; Zheng, X.; Deng, Y.; Hu, W. Amorphous Mo-doped NiS0.5Se0.5 Nanosheets@Crystalline NiS0.5Se0.5 Nanorods for High Current-density Electrocatalytic Water Splitting in Neutral Media. Angew. Chem. Int. Ed. 2023, 62, e202215256. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, J.; Jin, Y.; Ren, D.; Li, L.; Zhou, K.; Zhang, Q.; Liu, J.; Han, C.; Wang, H. Accelerated mass/electron transfer by self-supporting Ni2P@Cu3P heterostructure array to boost alkaline hydrogen evolution at high current density. Fuel 2023, 350, 128828. [Google Scholar] [CrossRef]
- Chen, W.; Wei, W.; Li, F.; Wang, Y.; Liu, M.; Dong, S.; Cui, J.; Zhang, Y.; Wang, R.; Ostrikov, K.; et al. Tunable Built-In Electric Field in Ru Nanoclusters-Based Electrocatalyst Boosts Water Splitting and Simulated Seawater Electrolysis. Adv. Funct. Mater. 2023, 34, 2310690. [Google Scholar] [CrossRef]
- Li, Q.; Chen, C.; Luo, W.; Yu, X.; Chang, Z.; Kong, F.; Zhu, L.; Huang, Y.; Tian, H.; Cui, X. In Situ Active Site Refreshing of Electro-Catalytic Materials for Ultra-Durable Hydrogen Evolution at Elevated Current Density. Adv. Energy Mater. 2024, 2304099. [Google Scholar] [CrossRef]
- Yu, T.; Wang, Z.; Tan, K.; He, H.; Yin, S. Novel hierarchical FeWO4–FeNi3 heterostructure nanosheets for highly active water electrolysis at large current density. Mater. Today Phys. 2023, 35, 101138. [Google Scholar] [CrossRef]
- Qian, G.; Chen, J.; Yu, T.; Liu, J.; Luo, L.; Yin, S. Three-Phase Heterojunction NiMo-Based Nano-Needle for Water Splitting at Industrial Alkaline Condition. Nano-Micro Lett. 2021, 14, 20. [Google Scholar] [CrossRef]
- Chen, J.; Luo, X.; Zhang, H.; Liang, X.; Xiao, K.; Ouyang, T.; Dan, M.; Liu, Z.-Q. Constructing superhydrophilic CoRu-LDH/PANI nanowires with optimized electronic structure for hydrogen evolution reaction. Electrochim. Acta 2023, 439, 141711. [Google Scholar] [CrossRef]
- He, W.; Zhang, R.; Cao, D.; Li, Y.; Zhang, J.; Hao, Q.; Liu, H.; Zhao, J.; Xin, H. Super-Hydrophilic Microporous Ni(OH)x/Ni3S2 Heterostructure Electrocatalyst for Large-Current-Density Hydrogen Evolution. Small 2022, 19, 2205719. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Z.; Han, N.; Tian, Y.; Kallio, T.; Yu, C.; Jiang, L. Superaerophilic/superaerophobic cooperative electrode for efficient hydrogen evolution reaction via enhanced mass transfer. Sci. Adv. 2023, 9, eadd6978. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, C.; Hou, J.; Ma, W.; Jian, T.; Yan, S.; Liu, H. Duplex Interpenetrating-Phase FeNiZn and FeNi3 Heterostructure with Low-Gibbs Free Energy Interface Coupling for Highly Efficient Overall Water Splitting. Nano-Micro Lett. 2023, 15, 95. [Google Scholar] [CrossRef]
- Liang, J.; Cai, Z.; Li, Z.; Yao, Y.; Luo, Y.; Sun, S.; Zheng, D.; Liu, Q.; Sun, X.; Tang, B. Efficient bubble/precipitate traffic enables stable seawater reduction electrocatalysis at industrial-level current densities. Nat. Commun. 2024, 15, 2950. [Google Scholar] [CrossRef]
- Wang, L.; Ma, M.; Zhang, C.; Chang, H.; Zhang, Y.; Li, L.; Chen, H.; Peng, S. Manipulating the Microenvironment of Single Atoms by Switching Support Crystallinity for Industrial Hydrogen Evolution. Angew. Chem. Int. Ed. 2024, 63, e202317220. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, L.; Khan, U.; Yu, Q.; Cheng, H.; Zou, X.; Liu, B. Morphology and surface chemistry engineering toward pH-universal catalysts for hydrogen evolution at high current density. Nat. Commun. 2019, 10, 269. [Google Scholar] [CrossRef]
- Shi, H.; Sun, X.; Zeng, S.; Liu, Y.; Han, G.; Wang, T.; Wen, Z.; Fang, Q.; Lang, X.; Jiang, Q. Nanoporous Nonprecious High-Entropy Alloys as Multisite Electrocatalysts for Ampere-Level Current-Density Hydrogen Evolution. Small Struct. 2023, 4, 2300042. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, M.; Huang, Z.; Zhu, W.; Zheng, J.; Jiang, Q.; Wang, Z.; Liang, H. Surface Reconstruction of Water Splitting Electrocatalysts. Adv. Energy Mater. 2022, 12, 2201713. [Google Scholar] [CrossRef]
- Yu, W.; Gao, Y.; Chen, Z.; Zhao, Y.; Wu, Z.; Wang, L. Strategies on improving the electrocatalytic hydrogen evolution performances of metal phosphides. Chin. J. Catal. 2021, 42, 1876–1902. [Google Scholar] [CrossRef]
- Yan, S.; Chen, X.; Li, W.; Zhong, M.; Xu, J.; Xu, M.; Wang, C.; Pinna, N.; Lu, X. Highly Active and Stable Alkaline Hydrogen Evolution Electrocatalyst Based on Ir-Incorporated Partially Oxidized Ru Aerogel under Industrial-Level Current Density. Adv. Sci. Lett. 2023, 11, 2307061. [Google Scholar] [CrossRef]
- Yang, C.; Zhong, W.; Shen, K.; Zhang, Q.; Zhao, R.; Xiang, H.; Wu, J.; Li, X.; Yang, N. Electrochemically Reconstructed Cu-FeOOH/Fe3O4 Catalyst for Efficient Hydrogen Evolution in Alkaline Media. Adv. Energy Mater. 2022, 12, 2200077. [Google Scholar] [CrossRef]
- Liu, X.; Yu, Q.; Qu, X.; Wang, X.; Chi, J.; Wang, L. Manipulating Electron Redistribution in Ni2P for Enhanced Alkaline Seawater Electrolysis. Adv. Mater. 2023, 36, 2307395. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Bai, H.; Kao, C.; Jiang, K.; Pan, H.; Lu, Y.; Tan, Y. Platinum–Ruthenium Dual-Atomic Sites Dispersed in Nanoporous Ni0.85Se Enabling Ampere-Level Current Density Hydrogen Production. Small 2024, 20, 2311178. [Google Scholar] [CrossRef]
- Liang, R.; Fan, J.; Guo, Y.; Huang, X.; Lei, F.; Ji, D.; Hao, W. In situ fabrication of sporoid-like flexible electrodes via Fe-regulated electron density for highly efficient and ultra-stable overall seawater splitting. J. Colloid Interface Sci. 2023, 652, 1170–1183. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y.; Luo, L.; Gong, S.; Wei, M.; Li, Y.; Gan, X.; Zhao, Y.; Zhu, Z.; Li, Z. Single-atom and bimetallic nanoalloy supported on nanotubes as a bifunctional electrocatalyst for ultrahigh-current-density overall water splitting. ACS Catal. 2022, 12, 1167–1179. [Google Scholar] [CrossRef]
- Yu, M.; Zheng, J.; Guo, M. La-doped NiFe-LDH coupled with hierarchical vertically aligned MXene frameworks for efficient overall water splitting. J. Energy Chem. 2022, 70, 472–479. [Google Scholar] [CrossRef]
- Ni, Z.; Luo, C.; Cheng, B.; Kuang, P.; Li, Y.; Yu, J. Construction of hierarchical and self-supported NiFe-Pt3Ir electrode for hydrogen production with industrial current density. Appl. Catal. B 2023, 321, 122072. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, C.; Zhu, X.; Qu, K.; Shi, P.; Song, L.; Wang, J.; Lu, Q.; Wang, A. Hetero-Interface Manipulation in MoOx@Ru to Evoke Industrial Hydrogen Production Performance with Current Density of 4000 mA cm−2. Adv. Energy Mater. 2023, 13, 2301492. [Google Scholar] [CrossRef]
- Wang, K.; Wang, S.; Hui, K.; Li, J.; Zha, C.; Dinh, D.; Shao, Z.; Yan, B.; Tang, Z.; Hui, K. Dense Platinum/Nickel Oxide Heterointerfaces with Abundant Oxygen Vacancies Enable Ampere-Level Current Density Ultrastable Hydrogen Evolution in Alkaline. Adv. Funct. Mater. 2022, 33, 2211273. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, H.; Lu, X.; Wang, L.; Zou, Y.; Miao, J.; Qiao, M.; Tang, Y.; Zhu, D. Self-Supported Ru-Incorporated NiSe2 for Ampere-Level Current Density Hydrogen Evolution. Chemistry 2023, 29, 2300205. [Google Scholar] [CrossRef]
- Liu, R.; Sun, M.; Liu, X.; Lv, Z.; Yu, X.; Wang, J.; Liu, Y.; Li, L.; Feng, X.; Yang, W.; et al. Enhanced Metal-Support Interactions Boost the Electrocatalytic Water Splitting of Supported Ruthenium Nanoparticles on a Ni3N/NiO Heterojunction at Industrial Current Density. Angew. Chem. Int. Ed. 2023, 62, e2312644. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Chen, Z.; Dai, Q.; Dong, C.; Yang, B.; Li, Z.; Hu, X.; Lei, L.; Hou, Y. Theory-guided design of electron-deficient ruthenium cluster for ampere-level current density electrochemical hydrogen evolution. Nano Energy 2023, 115, 108694. [Google Scholar] [CrossRef]
- Yang, T.; Chen, Z.; Wang, Y.; Huang, H.; Yin, K.; Liao, F.; Liu, Y.; Kang, Z. In Situ Insertion of Silicon Nanowires into Hollow Porous Co/NC Polyhedra Enabling Large-Current-Density Hydrogen Evolution Electrocatalysis. Small 2023, 20, 2305873. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, D.; Miao, H.; Wu, X.; Wang, Z.; Zhan, T.; Lai, J.; Wang, L. Amorphous/2H-MoS2 nanoflowers with P doping and S vacancies to achieve efficient pH-universal hydrogen evolution at high current density. Sci. China Chem. 2022, 65, 1829–1837. [Google Scholar] [CrossRef]
- Dong, Y.; Deng, Z.; Zhang, H.; Liu, G.; Wang, X. A Highly Active and Durable Hierarchical Electrocatalyst for Large-Current-Density Water Splitting. Nano Lett. 2023, 23, 9087–9095. [Google Scholar] [CrossRef]
- Zhou, X.; Mo, Y.; Yu, F.; Liao, L.; Yong, X.; Zhang, F.; Li, D.; Zhou, Q.; Sheng, T.; Zhou, H. Engineering Active Iron Sites on Nanoporous Bimetal Phosphide/Nitride Heterostructure Array Enabling Robust Overall Water Splitting. Adv. Funct. Mater. 2022, 33, 2209465. [Google Scholar] [CrossRef]
- Zhu, Z.; Luo, L.; He, Y.; Mushtaq, M.; Li, J.; Yang, H.; Khanam, Z.; Qu, J.; Wang, Z.; Balogun, M. High-Performance Alkaline Freshwater and Seawater Hydrogen Catalysis by Sword-Head Structured Mo2N-Ni3Mo3N Tunable Interstitial Compound Electrocatalysts. Adv. Funct. Mater. 2023, 34, 2306061. [Google Scholar] [CrossRef]
- Li, Y.; Yu, X.; Gao, J.; Ma, Y. Structural and electronic modulation of (Fe,Ni)2P@Ni2P heterostructure for efficient overall water splitting at high current density. Chem. Eng. J. 2023, 470, 144373. [Google Scholar] [CrossRef]
- Wang, X.; Yao, H.; Zhang, C.; Li, C.; Tong, K.; Gu, M.; Cao, Z.; Huang, M.; Jiang, H. Double-Tuned RuCo Dual Metal Single Atoms and Nanoalloy with Synchronously Expedited Volmer/Tafel Kinetics for Effective and Ultrastable Ampere-Level Current Density Hydrogen Production. Adv. Funct. Mater. 2023, 33, 2301804. [Google Scholar] [CrossRef]
- Zheng, Y.; Shang, P.; Pei, F.; Ma, G.; Ye, Z.; Peng, X.; Li, D. Achieving an efficient hydrogen evolution reaction with a bicontinuous nanoporous PtNiMg alloy of ultralow Noble-metal content at an ultrawide range of current densities. Chem. Eng. J. 2022, 433, 134571. [Google Scholar] [CrossRef]
- Xiang, Y.; Xiong, K.; Yu, L.; Zhang, H.; Chen, J.; Xia, M. Tuning of crystal phase of nickel telluride nanosheets to construct superior electrocatalyst for hydrogen evolution. J. Alloys Compd. 2022, 891, 161955. [Google Scholar] [CrossRef]
- Liu, J.; Qian, G.; Yu, T.; Chen, J.; Zhu, C.; Li, Y.; He, J.; Luo, L.; Yin, S. Amorphous-crystalline heterostructure for simulated practical water splitting at high-current–density. Chem. Eng. J. 2022, 431, 134247. [Google Scholar] [CrossRef]
- Shi, X.; Zheng, X.; Wang, H.; Zhang, H.; Qin, M.; Lin, B.; Qi, M.; Mao, S.; Ning, H.; Yang, R.; et al. Hierarchical Crystalline/Amorphous Heterostructure MoNi/NiMoOx for Electrochemical Hydrogen Evolution with Industry-Level Activity and Stability. Adv. Funct. Mater. 2023, 33, 2307109. [Google Scholar] [CrossRef]
- Ning, M.; Wang, Y.; Wu, L.; Yang, L.; Chen, Z.; Song, S.; Yao, Y.; Bao, J.; Chen, S.; Ren, Z. Hierarchical Interconnected NiMoN with Large Specific Surface Area and High Mechanical Strength for Efficient and Stable Alkaline Water/Seawater Hydrogen Evolution. Nano-Micro Lett. 2023, 15, 157. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Z.; Zhang, Y.; Thomas, T.; Du, H.; Huang, K.; Attfield, J.P.; Yang, M. An ultra-low Pt metal nitride electrocatalyst for sustainable seawater hydrogen production. Energy Environ. Sci. 2023, 16, 4584–4592. [Google Scholar] [CrossRef]
- Bang, J.; Moon, I.K.; Kim, Y.K.; Oh, J. Heterostructured Mo2N-Mo2C Nanoparticles Coupled with N-Doped Carbonized Wood to Accelerate the Hydrogen Evolution Reaction. Small Struct. 2023, 4, 2200283. [Google Scholar] [CrossRef]
- Liu, C.; Feng, J.; Zhou, P.; Liu, D.; Qiao, L.; Liu, D.; Cao, Y.; Su, S.; Liu, H.; Pan, H. Multi-metal interaction boosts reconstructed FeCoCrCuOx@CF toward efficient alkaline water electrolysis under large current density. Chem. Eng. J. 2023, 476, 146710. [Google Scholar] [CrossRef]
- Fang, P.; Zhu, M.; Liu, J.; Zhu, Z.; Hu, J.; Xu, X. Making Ternary-Metal Hydroxysulfide Catalyst via Cathodic Reconstruction with Ion Regulation for Industrial-Level Hydrogen Generation. Adv. Energy Mater. 2023, 13, 2301222. [Google Scholar] [CrossRef]
- Ning, M.; Zhang, F.; Wu, L.; Xing, X.; Wang, D.; Song, S.; Zhou, Q.; Yu, L.; Bao, J.; Chen, S.; et al. Boosting efficient alkaline fresh water and seawater electrolysis via electrochemical reconstruction. Energy Environ. Sci. 2022, 15, 3945–3957. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, B.; Yan, M.; Shen, Z.; Chen, Y.; Tian, J.; Xu, F.; Chen, G.; Wang, X.; Yang, L.; et al. Self-supported NiFe-LDH nanosheets on NiMo-based nanorods as high-performance bifunctional electrocatalysts for overall water splitting at industrial-level current densities. Nano Res. 2023, 17, 3769–3776. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, G.; Xu, Q.; Zhang, H.; Shen, F.; Luo, L.; Yin, S. Industrially promising IrNi-FeNi3 hybrid nanosheets for overall water splitting catalysis at large current density. Appl. Catal. B 2021, 286, 119881. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Bai, K.; Xiao, Z.; Fan, S. A flow-through electrode for hydrogen production from water splitting by mitigating bubble induced overpotential. J. Power Sources 2023, 561, 232733. [Google Scholar] [CrossRef]
- Ning, S.; Wu, Q.; Zhu, Y.; Liu, S.; Zhou, W.; Mi, L.; Zhou, K.; Zhao, D.; Zhang, X.; Wang, N. N-doped carbon nanowire array confined cobalt phosphides as efficient bifunctional electrocatalysts for water splitting. Inorg. Chem. Front. 2023, 10, 2145–2153. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Z.; Liu, J.; Sun, F.; Yang, P.; Qiu, J. A hierarchically porous and hydrophilic 3D nickel–iron/MXene electrode for accelerating oxygen and hydrogen evolution at high current densities. Nano Energy 2019, 63, 103880. [Google Scholar] [CrossRef]
- Ren, G.; Zhou, M.; Hu, P.; Chen, J.; Wang, H. Bubble-water/catalyst triphase interface microenvironment accelerates photocatalytic OER via optimizing semi-hydrophobic OH radical. Nat. Commun. 2024, 15, 2346. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Wan, S.; Kuang, P.; Cheng, B.; Yu, L.; Yu, J. Crystalline/amorphous Ni/NixSy supported on hierarchical porous nickel foam for high-current-density hydrogen evolution. Appl. Catal. B 2024, 340, 123195. [Google Scholar] [CrossRef]
- Li, J.; Song, M.; Hu, Y.; Zhu, Y.; Zhang, J.; Wang, D. Hybrid Heterostructure Ni3N|NiFeP/FF Self-Supporting Electrode for High-Current-Density Alkaline Water Electrolysis. Small Methods 2023, 7, e2201616. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, X.; Ding, Y.; Fu, H.; Wang, R.; Mao, F.; Liu, P.; Yang, H. Rational Design of Hydrogen Evolution Reaction Electrocatalysts for Commercial Alkaline Water Electrolysis. Small Struct. 2023, 4, 2200404. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.; Shao, Z. Perovskite for Electrocatalytic Oxygen Evolution at Elevated Temperatures. ChemSusChem 2024, 13, e202301534. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zang, B.; Liu, X.; Gu, C.; Chen, J.; Wang, L.; Zheng, W. Design Strategies of Hydrogen Evolution Reaction Nano Electrocatalysts for High Current Density Water Splitting. Nanomaterials 2024, 14, 1172. https://doi.org/10.3390/nano14141172
Zang B, Liu X, Gu C, Chen J, Wang L, Zheng W. Design Strategies of Hydrogen Evolution Reaction Nano Electrocatalysts for High Current Density Water Splitting. Nanomaterials. 2024; 14(14):1172. https://doi.org/10.3390/nano14141172
Chicago/Turabian StyleZang, Bao, Xianya Liu, Chen Gu, Jianmei Chen, Longlu Wang, and Weihao Zheng. 2024. "Design Strategies of Hydrogen Evolution Reaction Nano Electrocatalysts for High Current Density Water Splitting" Nanomaterials 14, no. 14: 1172. https://doi.org/10.3390/nano14141172
APA StyleZang, B., Liu, X., Gu, C., Chen, J., Wang, L., & Zheng, W. (2024). Design Strategies of Hydrogen Evolution Reaction Nano Electrocatalysts for High Current Density Water Splitting. Nanomaterials, 14(14), 1172. https://doi.org/10.3390/nano14141172







