Impact of Surface Trap States on Electron and Energy Transfer in CdSe Quantum Dots Studied by Femtosecond Transient Absorption Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis Methods
2.3. Treatment with 9-Anthracenecarboxylic Acid (ACA)
2.4. Characterization Methods
3. Results
3.1. Characterization of Surface Trap States
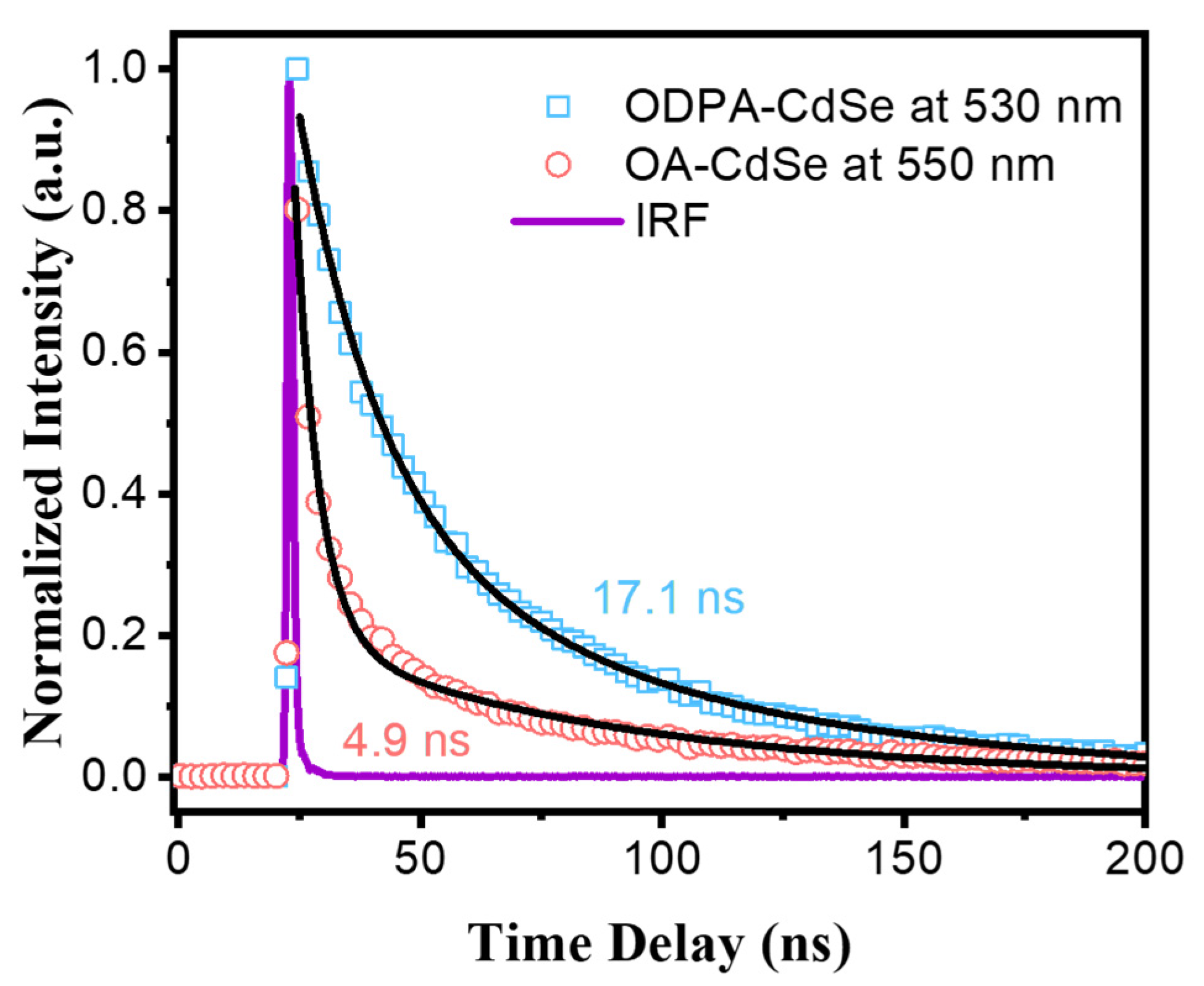
3.2. Time-Resolved Emission Spectroscopy Characterization
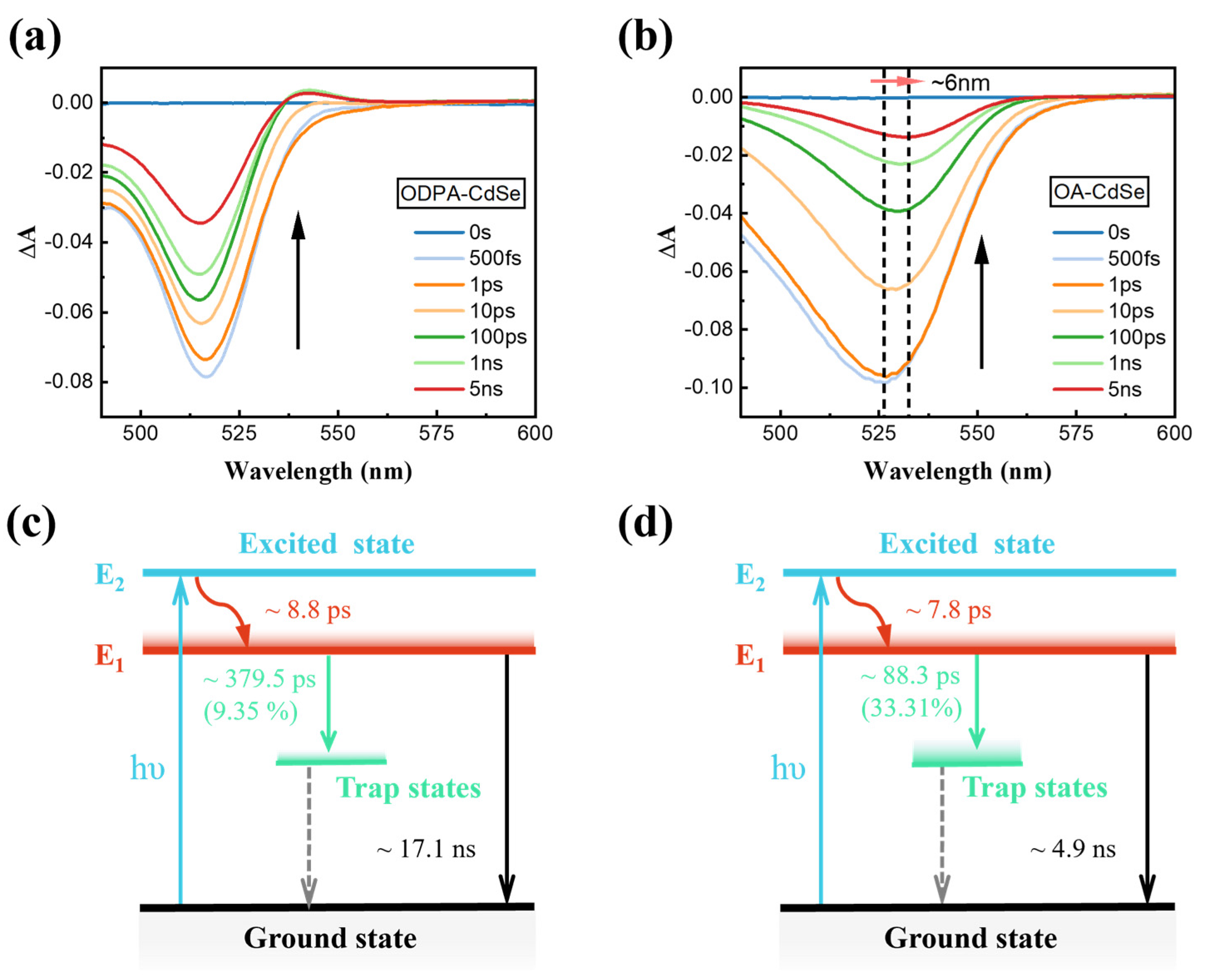
3.3. Transient Absorption Spectroscopy Characterization
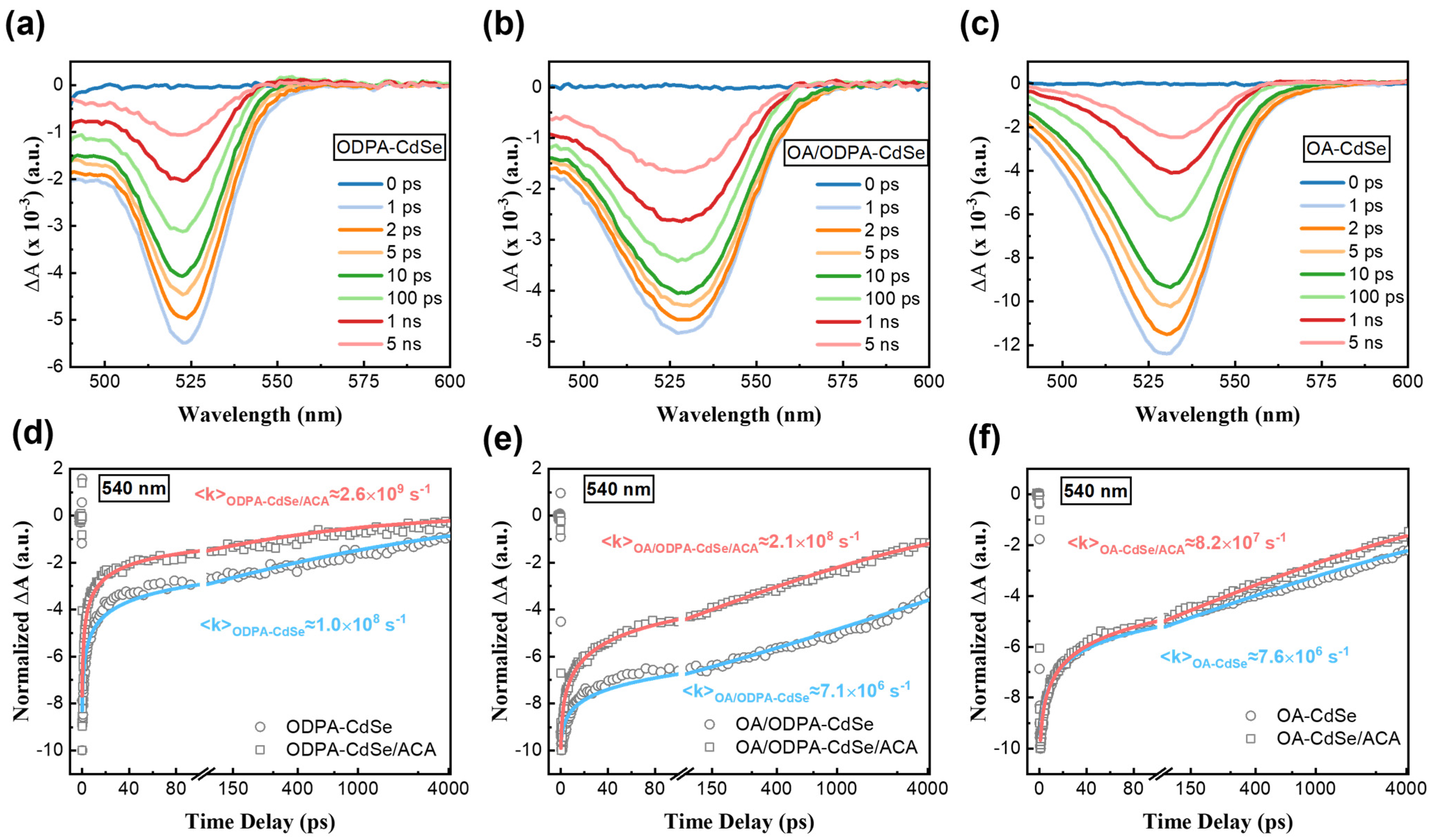
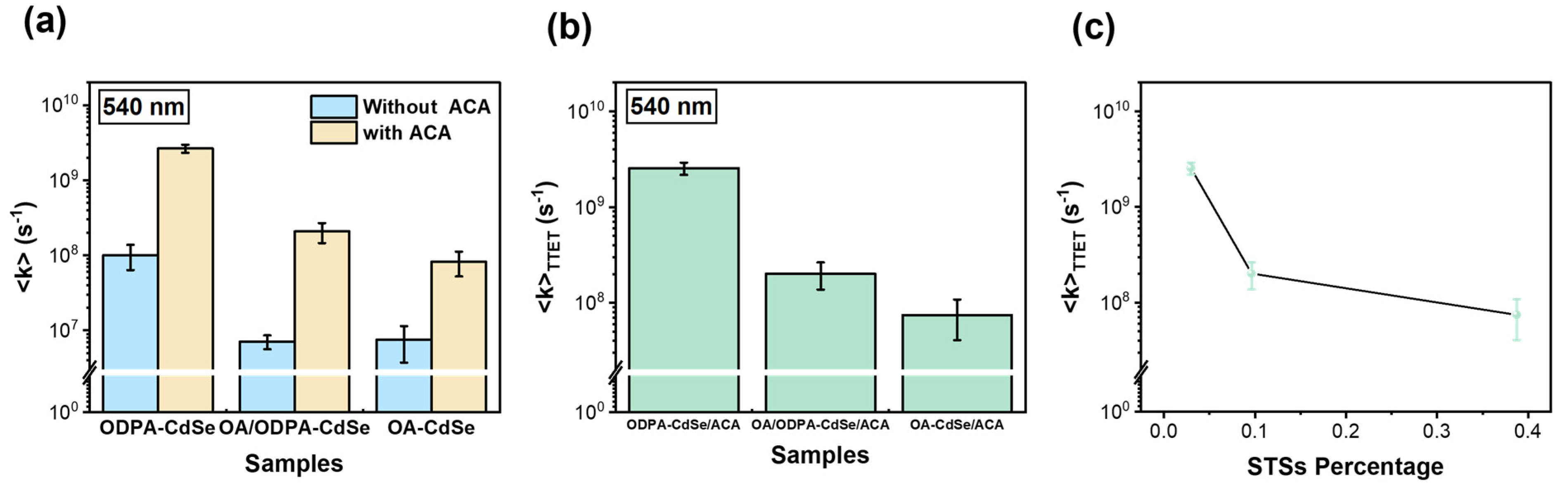
3.4. Influence of STSs on Triplet-Triplet Energy Transfer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, W.W.; Qu, L.; Guo, W.; Peng, X. Experimental Determination of the Extinction Coefficient of CdTe, CdSe, and CdS Nanocrystals. Chem. Mater. 2003, 15, 2854–2860. [Google Scholar] [CrossRef]
- Xie, R.; Kolb, U.; Li, J.; Basche, T.; Mews, A. Synthesis and Characterization of Highly Luminescent CdSe-Core CdS/Zn0.5Cd0.5S/ZnS Multishell Nanocrystals. J. Am. Chem. Soc. 2005, 127, 7480–7488. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Gong, Y.; Li, Z.; Gao, M. Enhancement Effect of Illumination on the Photoluminescence of Water-Soluble CdTe Nanocrystals: Toward Highly Fluorescent CdTe/CdS Core-Shell Structure. Chem. Mater. 2004, 16, 3853–3859. [Google Scholar] [CrossRef]
- Smith, A.M.; Duan, H.; Rhyner, M.N.; Ruan, G.; Nie, S. A Systematic Examination of Surface Coatings on the Optical and Chemical Properties of Semiconductor Quantum Dots. Phys. Chem. Chem. Phys. 2006, 8, 3895–3903. [Google Scholar] [CrossRef] [PubMed]
- Hines, D.A.; Kamat, P.V. Quantum Dot Surface Chemistry: Ligand Effects and Electron Transfer Reactions. J. Phys. Chem. C 2013, 117, 14418–14426. [Google Scholar] [CrossRef]
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and Characterization of Nearly Monodisperse CdE (E = Sulfur, Selenium, Tellurium) Semiconductor Nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Ning, Z.; Yuan, C.; Tian, H.; Hedström, P.; Sun, L.; Ågren, H. Quantum Rod-Sensitized Solar Cells. Chem. Sus. Chem. 2011, 4, 1741–1744. [Google Scholar] [CrossRef]
- Peng, Z.A.; Peng, X. Formation of High-Quality CdTe, CdSe, and CdS Nanocrystals Using CdO as Precursor. J. Am. Chem. Soc. 2001, 123, 183–184. [Google Scholar] [CrossRef]
- Peng, X.; Manna, L.; Yang, W.; Wickham, J.; Scher, E.; Kadavanich, A.; Alivisatos, A.P. Shape Control of CdSe Nanocrystals. Nature 2000, 404, 59–61. [Google Scholar] [CrossRef]
- Lu, H.; Carroll, G.M.; Neale, N.R.; Beard, M.C. Infrared Quantum Dots: Progress, Challenges, and Opportunities. ACS Nano 2019, 13, 939–953. [Google Scholar] [CrossRef]
- Han, Z.; Qiu, F.; Eisenberg, R.; Holland, P.L.; Krauss, T.D. Robust Photogeneration of H2 in Water Using Semiconductor Nanocrystals and a Nickel Catalyst. Science 2012, 338, 1321–1324. [Google Scholar] [CrossRef]
- Li, Z.-J.; Wang, J.-J.; Li, X.-B.; Fan, X.-B.; Meng, Q.-Y.; Feng, K.; Chen, B.; Tung, C.-H.; Wu, L.-Z. An Exceptional Artificial Photocatalyst, Nih-CdSe/CdS Core/Shell Hybrid, Made In Situ from CdSe Quantum Dots and Nickel Salts for Efficient Hydrogen Evolution. Adv. Mater. 2013, 25, 6613–6618. [Google Scholar] [CrossRef]
- Yu, S.; Fan, X.-B.; Wang, X.; Li, J.; Zhang, Q.; Xia, A.; Wei, S.; Wu, L.-Z.; Zhou, Y.; Patzke, G.R. Efficient Photocatalytic Hydrogen Evolution with Ligand Engineered All-Inorganic InP and InP/ZnS Colloidal Quantum Dots. Nat. Commun. 2018, 9, 4009. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Zhang, G.; Chen, H.; Li, H.; Jiang, J.; Long, Y.-T.; Ning, Z. Efficient Defect-Controlled Photocatalytic Hydrogen Generation Based on near-Infrared Cu-In-Zn-S Quantum Dots. Nano Res. 2018, 11, 1379–1388. [Google Scholar] [CrossRef]
- Wang, P.; Yang, Q.; Xu, C.; Wang, B.; Wang, H.; Zhang, J.; Jin, Y. Magic-Sized CdSe Nanoclusters for Efficient Visible-Light-Driven Hydrogen Evolution. Nano Res. 2022, 15, 3106–3113. [Google Scholar] [CrossRef]
- Nozik, A.J.; Beard, M.C.; Luther, J.M.; Law, M.; Ellingson, R.J.; Johnson, J.C. Semiconductor Quantum Dots and Quantum Dot Arrays and Applications of Multiple Exciton Generation to Third-Generation Photovoltaic Solar Cells. Chem. Rev. 2010, 110, 6873–6890. [Google Scholar] [CrossRef] [PubMed]
- Leschkies, K.S.; Divakar, R.; Basu, J.; Enache-Pommer, E.; Boercker, J.E.; Carter, C.B.; Kortshagen, U.R.; Norris, D.J.; Aydil, E.S. Photosensitization of ZnO Nanowires with CdSe Quantum Dots for Photovoltaic Devices. Nano Lett. 2007, 7, 1793–1798. [Google Scholar] [CrossRef]
- Wagner, A.M.; Knipe, J.M.; Orive, G.; Peppas, N.A. Quantum Dots in Biomedical Applications. Acta Biomater. 2019, 94, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, D.; Zhang, G.; Yang, C.; Guo, W.; Han, X.; Bai, X.; Chen, R.; Qin, C.; Hu, J.; et al. The Role of Surface Charges in the Blinking Mechanisms and Quantum-Confined Stark Effect of Single Colloidal Quantum Dots. Nano Res. 2022, 15, 7655–7661. [Google Scholar] [CrossRef]
- Voznyy, O.; Sargent, E.H. Atomistic Model of Fluorescence Intermittency of Colloidal Quantum Dots. Phys. Rev. Lett. 2014, 112, 157401. [Google Scholar] [CrossRef]
- Ji, X.; Copenhaver, D.; Sichmeller, C.; Peng, X. Ligand Bonding and Dynamics on Colloidal Nanocrystals at Room Temperature: The Case of Alkylamines on CdSe Nanocrystals. J. Am. Chem. Soc. 2008, 130, 5726–5735. [Google Scholar] [CrossRef]
- Hu, L.; Wu, H. Influence of Size and Surface State Emission on Photoluminescence of CdSe Quantum Dots under UV Irradiation. J. Lumin. 2016, 177, 306–313. [Google Scholar] [CrossRef]
- Giansante, C.; Infante, I. Surface Traps in Colloidal Quantum Dots: A Combined Experimental and Theoretical Perspective. J. Phys. Chem. Lett. 2017, 8, 5209–5215. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.R.; Kamat, P.V. Tuning the Emission of CdSe Quantum Dots by Controlled Trap Enhancement. Langmuir 2010, 26, 11272–11276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, Q.; Liu, M.; Kong, L.; Jiao, M.; Mu, H.; Wang, D.; Wang, H.; Chen, J.; Yang, C. Temperature and Wavelength Dependence of Energy Transfer Process Between Quantized States and Surface States in CdSe Quantum Dots. Nanoscale Res. Lett. 2017, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wang, X.; Ye, S.; Xu, Y.; Feng, Z.; Li, C. Charge-Transfer Dynamics Promoted by Hole Trap States in CdSe Quantum Dots−Ni2+ Photocatalytic System. J. Phys. Chem. C 2017, 121, 17112–17120. [Google Scholar] [CrossRef]
- Saini, S.; Bhowmick, S.; Shenoy, V.B.; Bagchi, B. Rate of Excitation Energy Transfer between Fluorescent Dyes and Nanoparticles. J. Photochem. Photobiol. A Chem. 2007, 190, 335–341. [Google Scholar] [CrossRef]
- Bender, J.A.; Raulerson, E.K.; Li, X.; Goldzak, T.; Xia, P.; Van Voorhis, T.; Tang, M.L.; Roberts, S.T. Surface States Mediate Triplet Energy Transfer in Nanocrystal–Acene Composite Systems. J. Am. Chem. Soc. 2018, 140, 7543–7553. [Google Scholar] [CrossRef]
- Luo, X.; Han, Y.; Chen, Z.; Li, Y.; Liang, G.; Liu, X.; Ding, T.; Nie, C.; Wang, M.; Castellano, F.N.; et al. Mechanisms of Triplet Energy Transfer across the Inorganic Nanocrystal/Organic Molecule Interface. Nat. Commun. 2020, 11, 28. [Google Scholar] [CrossRef]
- Berera, R.; van Grondelle, R.; Kennis, J.T.M. Ultrafast Transient Absorption Spectroscopy: Principles and Application to Photosynthetic Systems. Photosynth. Res. 2009, 101, 105–118. [Google Scholar] [CrossRef]
- Miao, T.J.; Tang, J. Characterization of Charge Carrier Behavior in Photocatalysis Using Transient Absorption Spectroscopy. J. Chem. Phys. 2020, 152, 194201. [Google Scholar] [CrossRef] [PubMed]
- Mongin, C.; Garakyaraghi, S.; Razgoniaeva, N.; Zamkov, M.; Castellano, F.N. Direct Observation of Triplet Energy Transfer from Semiconductor Nanocrystals. Science 2016, 351, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Mongin, C.; Moroz, P.; Zamkov, M.; Castellano, F.N. Thermally Activated Delayed Photoluminescence from Pyrenyl-Functionalized CdSe Quantum Dots. Nat. Chem. 2018, 10, 225–230. [Google Scholar] [CrossRef]
- Das, S.; Rakshit, S.; Datta, A. Interplay of Multiexciton Relaxation and Carrier Trapping in Photoluminescent CdS Quantum Dots Prepared in Aqueous Medium. J. Phys. Chem. C 2020, 124, 28313–28322. [Google Scholar] [CrossRef]
- Chen, O.; Zhao, J.; Chauhan, V.P.; Cui, J.; Wong, C.; Harris, D.K.; Wei, H.; Han, S.; Fukumura, D.; Jain, R.K.; et al. Compact high-quality CdSe-CdS core-shell nanocrystals with narrow emission linewidths and suppressed blinking. Nat. Mater. 2013, 12, 445–451. [Google Scholar] [CrossRef]
- Hanifi, D.A.; Bronstein, N.D.; Koscher, B.A.; Nett, Z.; Swabeck, J.K.; Takano, K.; Schwartzberg, A.M.; Maserati, L.; Vandewal, K.; van de Burgt, Y.; et al. Redefining Near-Unity Luminescence in Quantum Dots with Photothermal Threshold Quantum Yield. Science 2019, 363, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.W.; Peng, X. Formation of High-Quality CdS and Other II–VI Semiconductor Nanocrystals in Noncoordinating Solvents: Tunable Reactivity of Monomers. Angew. Chem. Int. Ed. 2002, 41, 2368–2371. [Google Scholar] [CrossRef]
- Wang, J.; Long, Y.; Zhang, Y.; Zhong, X.; Zhu, L. Preparation of Highly Luminescent CdTe/CdS Core/Shell Quantum Dots. ChemPhysChem 2009, 10, 680–685. [Google Scholar] [CrossRef]
- Xie, R.; Battaglia, D.; Peng, X. Colloidal InP Nanocrystals as Efficient Emitters Covering Blue to Near-Infrared. J. Am. Chem. Soc. 2007, 129, 15432–15433. [Google Scholar] [CrossRef]
- Krause, M.M.; Mack, T.G.; Jethi, L.; Moniodis, A.; Mooney, J.D.; Kambhampati, P. Unraveling Photoluminescence Quenching Pathways in Semiconductor Nanocrystals. Chem. Phys. Lett. 2015, 633, 65–69. [Google Scholar] [CrossRef]
- Babentsov, V.; Sizov, F. Defects in Quantum Dots of IIB–VI Semiconductors. Opto.-Electron. Rev. 2008, 633, 208–225. [Google Scholar] [CrossRef]
- Jasieniak, J.; Mulvaney, P. From Cd-Rich to Se-Rich—The Manipulation of CdSe Nanocrystal Surface Stoichiometry. J. Am. Chem. Soc. 2007, 129, 2841–2848. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.H.-Y.; Evans, C.M.; Swartz, B.D.; Neukirch, A.J.; Young, J.; Prezhdo, O.V.; Krauss, T.D. Colloidal Semiconductor Quantum Dots with Tunable Surface Composition. Nano Lett. 2012, 12, 4465–4471. [Google Scholar] [CrossRef]
- Schneeweiss, M.A.; Kolb, D.M. The Initial Stages of Copper Deposition on Bare and Chemically Modified Gold Electrodes. Phys. Status Solidi A 1999, 173, 51–71. [Google Scholar] [CrossRef]
- Leung, K.; Whaley, K.B. Surface Relaxation in CdSe Nanocrystals. J. Chem. Phys. 1999, 110, 11012–11022. [Google Scholar] [CrossRef]
- Bawendi, M.; Carroll, P.; Wilson, W.; Brus, L. Luminescence Properties of CdSe Quantum Crystallites: Resonance between Interior and Surface Localized States. J. Chem. Phys. 1992, 96, 946–954. [Google Scholar] [CrossRef]
- Wang, X.; Qu, L.; Zhang, J.; Peng, X.; Xiao, M. Surface-Related Emission in Highly Luminescent CdSe Quantum Dots. Nano Lett. 2003, 3, 1103–1106. [Google Scholar] [CrossRef]
- Patra, S.; Samanta, A. A Fluorescence Correlation Spectroscopy, Steady-State, and Time-Resolved Fluorescence Study of the Modulation of Photophysical Properties of Mercaptopropionic Acid Capped CdTe Quantum Dots upon Exposure to Light. J. Phys. Chem. C 2013, 117, 23313–23321. [Google Scholar] [CrossRef]
- Robel, I.; Kuno, M.; Kamat, P.V. Size-Dependent Electron Injection from Excited CdSe Quantum Dots into TiO2 Nanoparticles. J. Am. Chem. Soc. 2007, 129, 4136–4137. [Google Scholar] [CrossRef]
- Soloviev, V.N.; Eichhöfer, A.; Fenske, D.; Banin, U. Size-Dependent Optical Spectroscopy of a Homologous Series of CdSe Cluster Molecules. J. Am. Chem. Soc. 2001, 123, 2354–2364. [Google Scholar] [CrossRef] [PubMed]



| Sample | Seb/Cdb | Ses/Cds | Ses/Se | Cds/Cd | Se/Cd | Surface Atomic Ratio | |
|---|---|---|---|---|---|---|---|
| Cd | Se | ||||||
| ODPA-CdSe | 1.78 | 0.02 | 0.01 | 0.40 | 1.08 | 0.98 | 0.02 |
| OA-CdSe | 0.98 | 0.54 | 0.28 | 0.41 | 0.8 | 0.65 | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, H.; Yuan, C.; Zhu, R.; Li, L.; Zhang, J.; Weng, T.-C. Impact of Surface Trap States on Electron and Energy Transfer in CdSe Quantum Dots Studied by Femtosecond Transient Absorption Spectroscopy. Nanomaterials 2024, 14, 34. https://doi.org/10.3390/nano14010034
Dou H, Yuan C, Zhu R, Li L, Zhang J, Weng T-C. Impact of Surface Trap States on Electron and Energy Transfer in CdSe Quantum Dots Studied by Femtosecond Transient Absorption Spectroscopy. Nanomaterials. 2024; 14(1):34. https://doi.org/10.3390/nano14010034
Chicago/Turabian StyleDou, Hongbin, Chunze Yuan, Ruixue Zhu, Lin Li, Jihao Zhang, and Tsu-Chien Weng. 2024. "Impact of Surface Trap States on Electron and Energy Transfer in CdSe Quantum Dots Studied by Femtosecond Transient Absorption Spectroscopy" Nanomaterials 14, no. 1: 34. https://doi.org/10.3390/nano14010034
APA StyleDou, H., Yuan, C., Zhu, R., Li, L., Zhang, J., & Weng, T.-C. (2024). Impact of Surface Trap States on Electron and Energy Transfer in CdSe Quantum Dots Studied by Femtosecond Transient Absorption Spectroscopy. Nanomaterials, 14(1), 34. https://doi.org/10.3390/nano14010034






