Removal of Diclofenac and Heavy Metal Ions from Aqueous Media Using Composite Sorbents in Dynamic Conditions
Abstract
:1. Introduction
- Conducting experiments in dynamic conditions with four novel composite materials obtained through coacervate deposition. Experimental conditions were conceived to investigate the influence of specific process parameters (e.g., continuous column flow) on the sorption process effectiveness (sorption capacities at breakthrough point), in conditions similar to those used in wastewater treatment plants;
- Using multi-component aqueous solutions in sequential and simultaneous conditions. The multi-component solutions were made up of three heavy metal ions and an organic pollutant at concentrations that would simulate real wastewater conditions;
- Studying the influence of the polyanion nature on composite materials’ performance in immobilizing these pollutants. For this, several approaches were tested during the one-pot syntheses: (a) poly(acrylic acid) (PAA) and poly(sodium methacrylate) (PMAA) were compared as complexation agents for PEI while maintaining the poly(ethyleneimine) (PEI) concentration constant, and (b) using two PEI chain cross-linking degrees (r = 0.1 and r = 1.0).
2. Materials and Methods
2.1. Materials
2.2. Composite Materials’ Synthesis
2.3. Dynamic Sorption Tests
2.4. Adsorption Data Modeling
3. Results
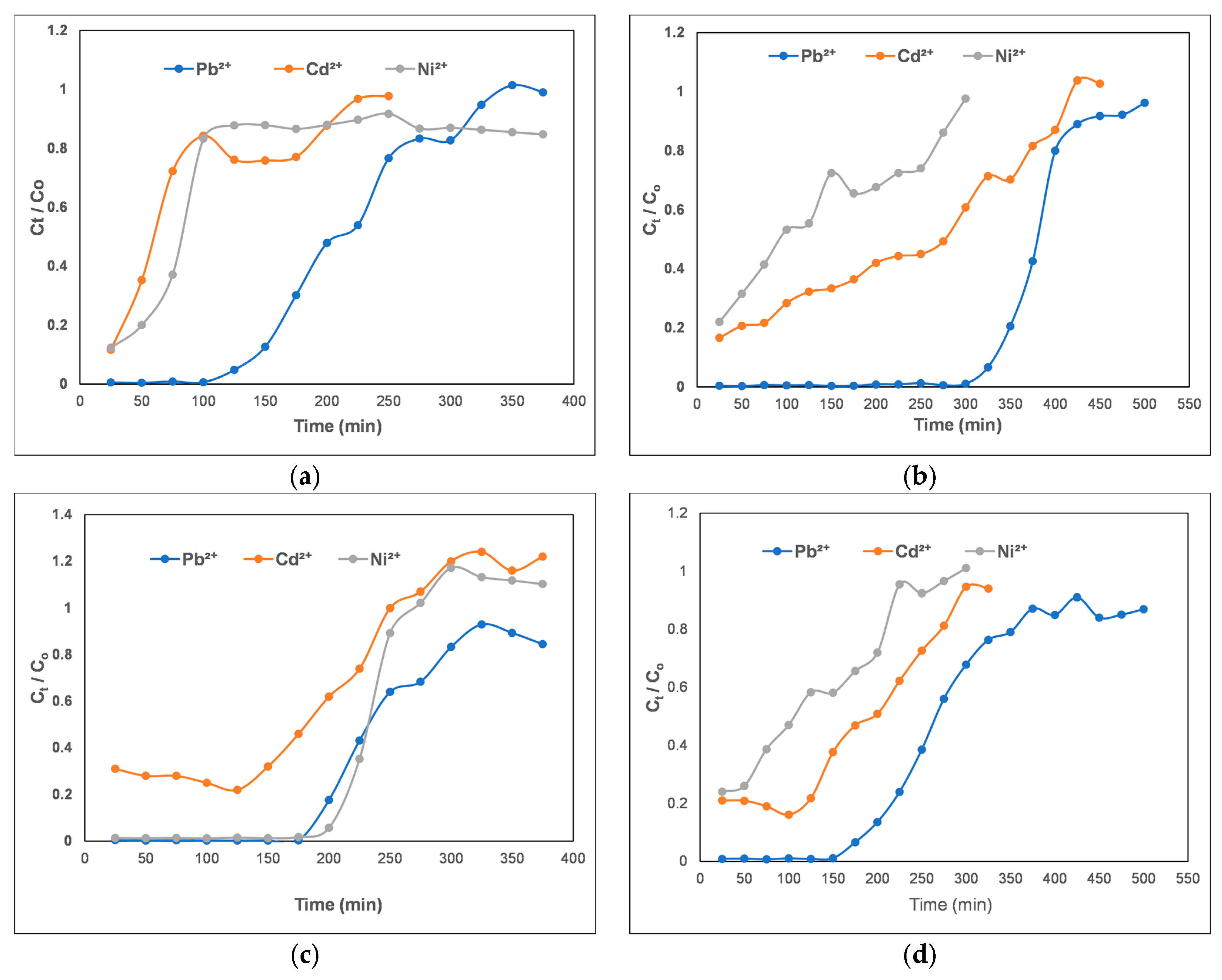
3.1. Breakthrough Curves’ Analysis
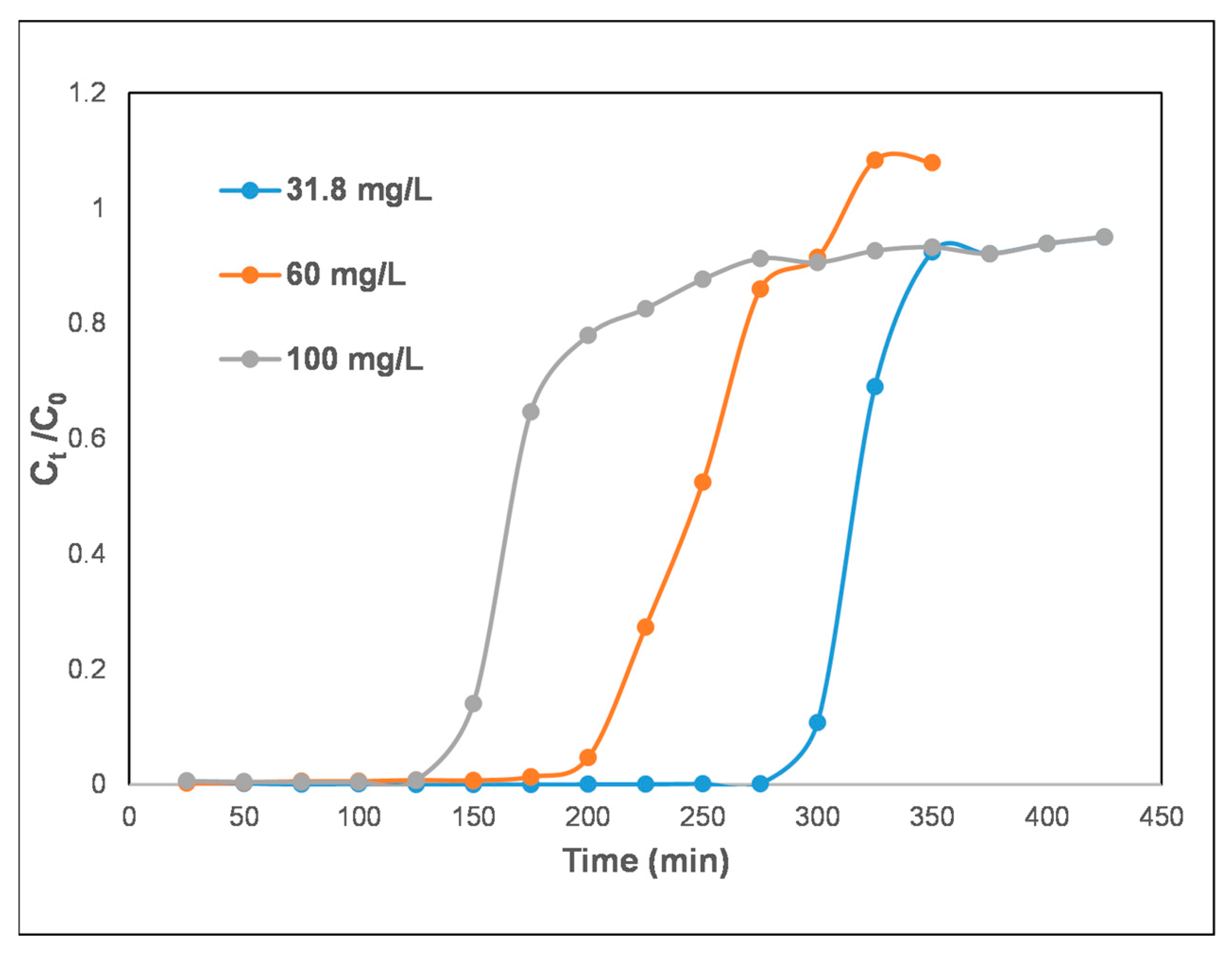
3.2. Initial DCF-Na Concentration Influence
3.3. Column Kinetics Study
3.4. Column Experiments for Multiple Pollutants’ Sorption onto the Same Silica/Polyelectrolyte Composites
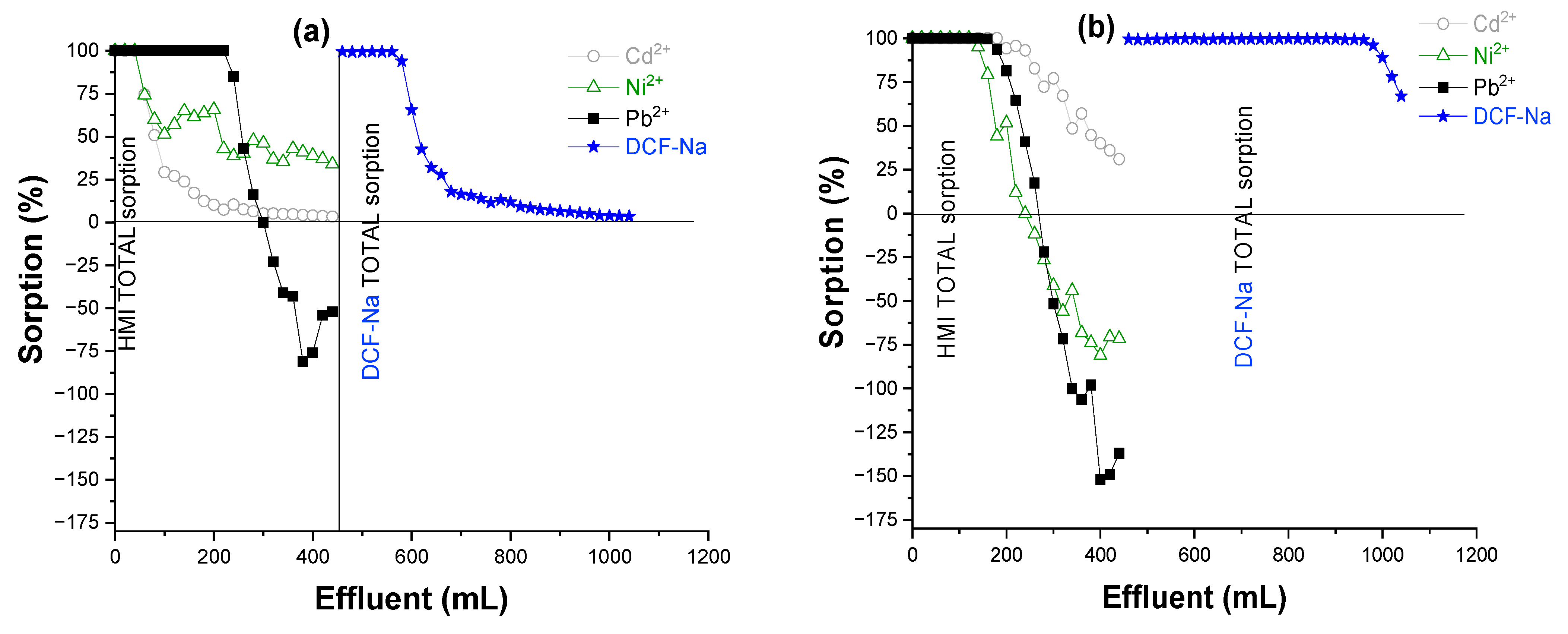
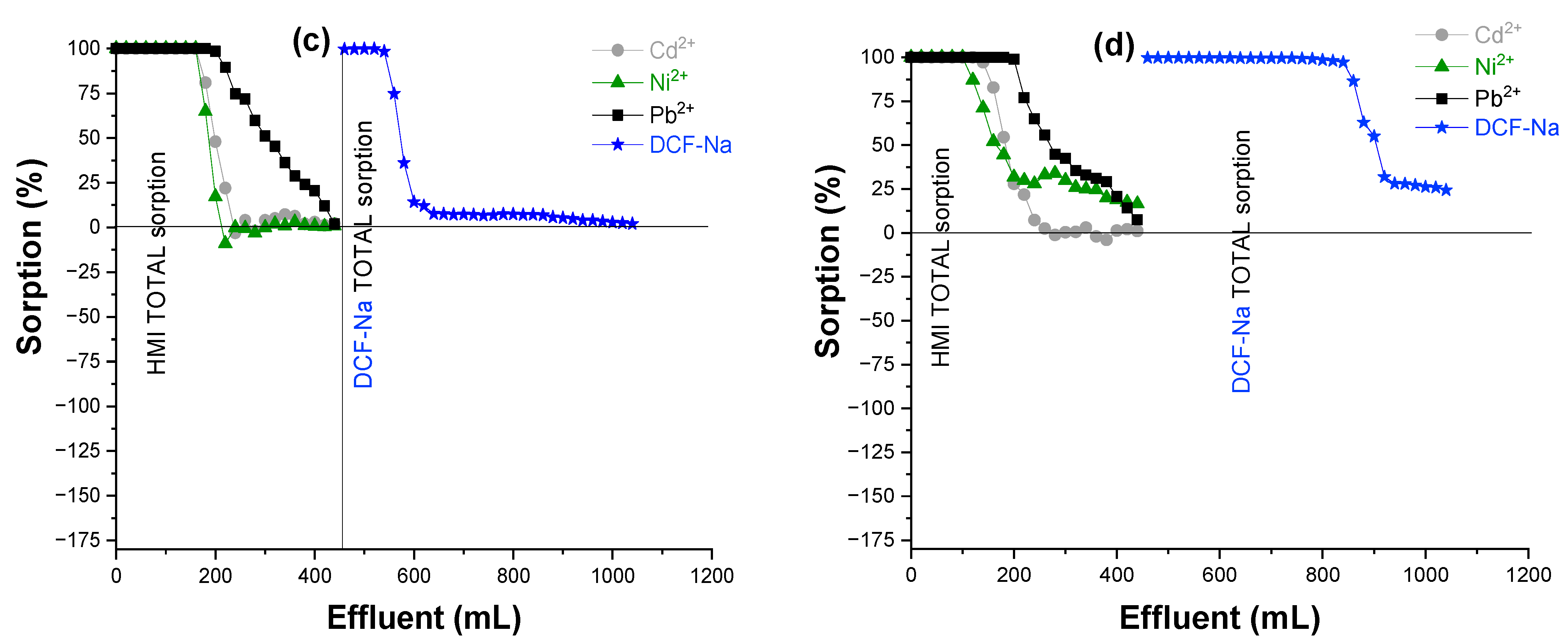
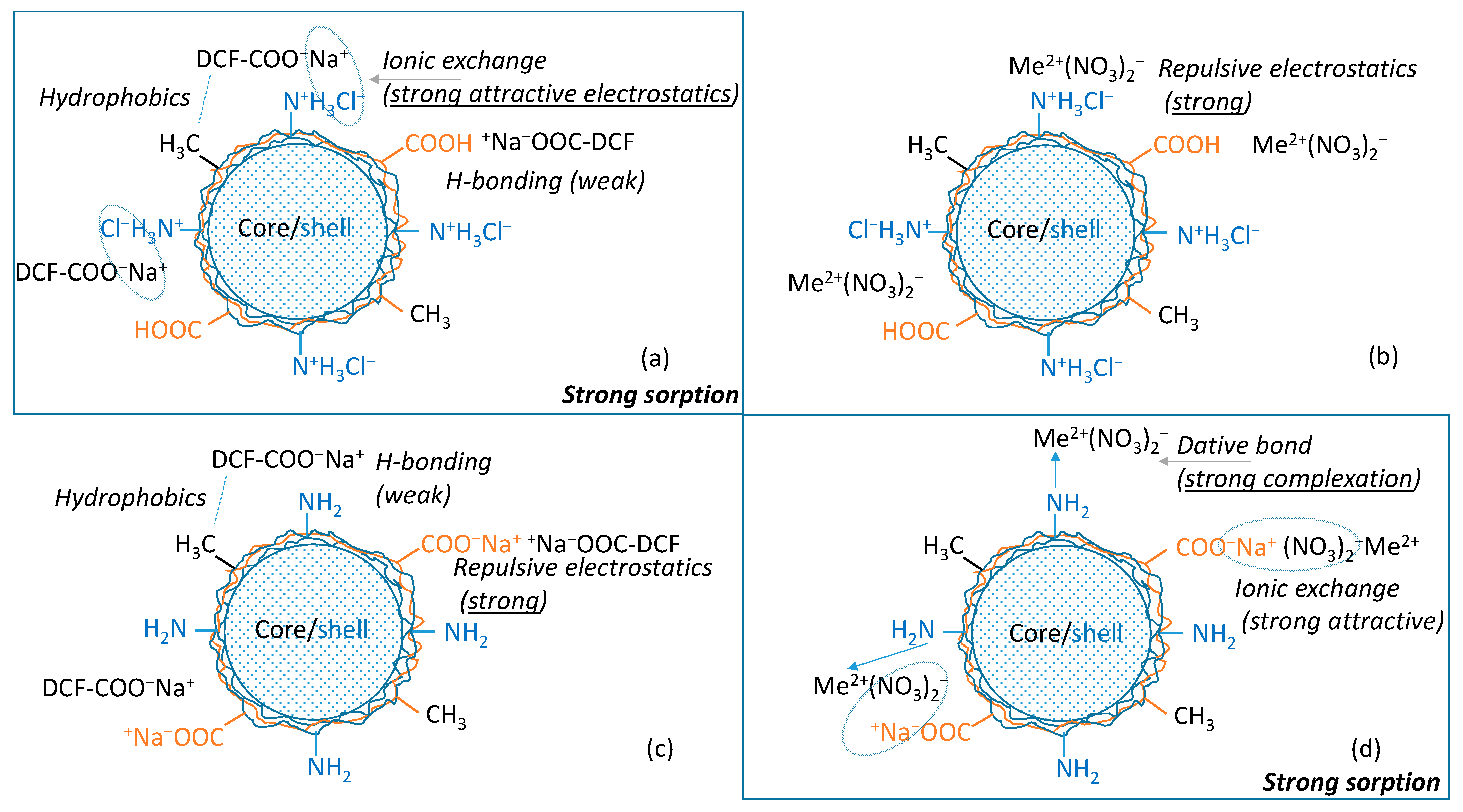
3.5. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barjoveanu, G.; Teodosiu, C.; Morosanu, I.; Ciobanu, R.; Bucatariu, F.; Mihai, M. Life Cycle Assessment as Support Tool for Development of Novel Polyelectrolyte Materials Used for Wastewater Treatment. Nanomaterials 2023, 13, 840. [Google Scholar] [CrossRef] [PubMed]
- Rathi, B.S.; Kumar, P.S.; Vo, D.V.N. Critical Review on Hazardous Pollutants in Water Environment: Occurrence, Monitoring, Fate, Removal Technologies and Risk Assessment. Sci. Total Environ. 2021, 797, 149134. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Kumar, P.S.; Rozbu, M.R.; Chowdhury, A.T.; Nuzhat, S.; Rafa, N.; Mahlia, T.M.I.; Ong, H.C.; Mofijur, M. Heavy Metal Toxicity, Sources, and Remediation Techniques for Contaminated Water and Soil. Environ. Technol. Innov. 2022, 25, 102114. [Google Scholar] [CrossRef]
- Kothavale, V.P.; Sharma, A.; Dhavale, R.P.; Chavan, V.D.; Shingte, S.R.; Selyshchev, O.; Dongale, T.D.; Park, H.H.; Zahn, D.R.T.; Salvan, G.; et al. Carboxyl and Thiol-Functionalized Magnetic Nanoadsorbents for Efficient and Simultaneous Removal of Pb(II), Cd(II), and Ni(II) Heavy Metal Ions from Aqueous Solutions: Studies of Adsorption, Kinetics, and Isotherms. J. Phys. Chem. Solids 2023, 172, 111089. [Google Scholar] [CrossRef]
- Kim, K.H.; Keller, A.A.; Yang, J.K. Removal of Heavy Metals from Aqueous Solution Using a Novel Composite of Recycled Materials. Colloids Surf. A Physicochem. Eng. Asp. 2013, 425, 6–14. [Google Scholar] [CrossRef]
- Jaspal, D.; Malviya, A. Composites for Wastewater Purification: A Review. Chemosphere 2020, 246, 125788. [Google Scholar] [CrossRef] [PubMed]
- Morosanu, I.; Teodosiu, C.; Tofan, L.; Fighir, D.; Paduraru, C. Valorization of Rapeseed Waste Biomass in Sorption Processes for Wastewater Treatment. In Environmental Issues and Sustainable Development; IntechOpen: London, UK, 2020; p. 25. ISBN 0000957720. [Google Scholar]
- Bucatariu, F.; Teodosiu, C.; Morosanu, I.; Fighir, D.; Ciobanu, R.; Petrila, L.M.; Mihai, M. An Overview on Composite Sorbents Based on Polyelectrolytes Used in Advanced Wastewater Treatment. Polymers 2021, 13, 3963. [Google Scholar] [CrossRef]
- Klimenko, V.V.; Ratner, S.V.; Tereshin, A.G. Constraints Imposed by Key-Material Resources on Renewable Energy Development. Renew. Sustain. Energy Rev. 2021, 144, 111011. [Google Scholar] [CrossRef]
- Morosanu, I.; Paduraru, C.; Bucatariu, F.; Fighir, D.; Mihai, M.; Teodosiu, C. Shaping Polyelectrolyte Composites for Heavy Metals Adsorption from Wastewater: Experimental Assessment and Equilibrium Studies. J. Environ. Manag. 2022, 321, 115999. [Google Scholar] [CrossRef]
- Di Natale, F.; Gargiulo, V.; Alfè, M. Adsorption of Heavy Metals on Silica-Supported Hydrophilic Carbonaceous Nanoparticles (SHNPs). J. Hazard. Mater. 2020, 393, 122374. [Google Scholar] [CrossRef]
- Popovic, A.L.; Rusmirovic, J.D.; Velickovic, Z.; Kovacevic, T.; Jovanovic, A.; Cvijetic, I.; Marinkovic, A.D. Kinetics and Column Adsorption Study of Diclofenac and Heavy-Metal Ions Removal by Amino-Functionalized Lignin Microspheres. J. Ind. Eng. Chem. 2021, 93, 302–314. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S. Application of Adsorption Process for Effective Removal of Emerging Contaminants from Water and Wastewater. Environ. Pollut. 2021, 280, 116995. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Yoom, H.; Son, H.; Seo, C.; Kim, K.; Lee, Y.; Kim, Y.M. Removal Efficiency of Organic Micropollutants in Successive Wastewater Treatment Steps in a Full-Scale Wastewater Treatment Plant: Bench-Scale Application of Tertiary Treatment Processes to Improve Removal of Organic Micropollutants Persisting after Secon. Chemosphere 2022, 288, 132629. [Google Scholar] [CrossRef] [PubMed]
- Sellaoui, L.; Abdulaziz, F.; Chebaane, S.; Manai, L.; Azhary, A.; Alsehli, A.H.; Alsowayigh, M.M.; Piscitelli, A.; Erto, A. Adsorption of Pharmaceutical Pollutants on Activated Carbon: Physicochemical Assessment of the Adsorption Mechanism via Advanced Modelling. J. Mol. Liq. 2023, 389, 122929. [Google Scholar] [CrossRef]
- Mubarak, M.F.; Zayed, A.M.; Ahmed, H.A. Activated Carbon/Carborundum@Microcrystalline Cellulose Core Shell Nano-Composite: Synthesis, Characterization and Application for Heavy Metals Adsorption from Aqueous Solutions. Ind. Crops Prod. 2022, 182, 114896. [Google Scholar] [CrossRef]
- Smiljanić, D.; de Gennaro, B.; Izzo, F.; Langella, A.; Daković, A.; Germinario, C.; Rottinghaus, G.E.; Spasojević, M.; Mercurio, M. Removal of Emerging Contaminants from Water by Zeolite-Rich Composites: A First Approach Aiming at Diclofenac and Ketoprofen. Microporous Mesoporous Mater. 2020, 298, 110057. [Google Scholar] [CrossRef]
- Senguttuvan, S.; Janaki, V.; Senthilkumar, P.; Kamala-Kannan, S. Polypyrrole/Zeolite Composite—A Nanoadsorbent for Reactive Dyes Removal from Synthetic Solution. Chemosphere 2022, 287, 132164. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of Emerging Contaminants from Water and Wastewater by Modified Biochar: A Review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Moreira, V.R.; Lebron, Y.A.R.; Lange, L.C.; Santos, L.V.S. Simultaneous Biosorption of Cd(II), Ni(II) and Pb(II) onto a Brown Macroalgae Fucus Vesiculosus: Mono- and Multi-Component Isotherms, Kinetics and Thermodynamics. J. Environ. Manag. 2019, 251, 109587. [Google Scholar] [CrossRef]
- Morosanu, I.; Teodosiu, C.; Fighir, D.; Paduraru, C. Simultaneous Biosorption of Micropollutants from Aqueous Effluents by Rapeseed Waste. Process Saf. Environ. Prot. 2019, 132, 231–239. [Google Scholar] [CrossRef]
- Maleki, A.; Pajootan, E.; Hayati, B. Ethyl Acrylate Grafted Chitosan for Heavy Metal Removal from Wastewater: Equilibrium, Kinetic and Thermodynamic Studies. J. Taiwan Inst. Chem. Eng. 2015, 51, 127–134. [Google Scholar] [CrossRef]
- Wu, J.; Wang, T.; Wang, J.; Zhang, Y.; Pan, W.P. A Novel Modified Method for the Efficient Removal of Pb and Cd from Wastewater by Biochar: Enhanced the Ion Exchange and Precipitation Capacity. Sci. Total Environ. 2021, 754, 142150. [Google Scholar] [CrossRef] [PubMed]
- Khalfa, L.; Sdiri, A.; Bagane, M.; Cervera, M.L. A Calcined Clay Fixed Bed Adsorption Studies for the Removal of Heavy Metals from Aqueous Solutions. J. Clean. Prod. 2021, 278, 123935. [Google Scholar] [CrossRef]
- Kaur, M.; Kumar, S.; Yusuf, M.; Lee, J.; Malik, A.K.; Ahmadi, Y.; Kim, K.H. Schiff Base-Functionalized Metal-Organic Frameworks as an Efficient Adsorbent for the Decontamination of Heavy Metal Ions in Water. Environ. Res. 2023, 236, 116811. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Bilal, M.; Hassan, A.A.; Nabeel, F.; Bharagava, R.N.; Romanholo Ferreira, L.F.; Tran, H.N.; Iqbal, H.M.N. Environmental Threatening Concern and Efficient Removal of Pharmaceutically Active Compounds Using Metal-Organic Frameworks as Adsorbents. Environ. Res. 2020, 185, 109436. [Google Scholar] [CrossRef]
- de Araújo, T.P.; Quesada, H.B.; dos Santos, D.F.; da Silva Fonseca, B.C.; Barbieri, J.Z.; Bergamasco, R.; de Barros, M.A.S.D. Acetaminophen Removal by Calcium Alginate/Activated Hydrochar Composite Beads: Batch and Fixed-Bed Studies. Int. J. Biol. Macromol. 2022, 203, 553–562. [Google Scholar] [CrossRef]
- Spaolonzi, M.P.; Duarte, E.D.V.; Oliveira, M.G.; Costa, H.P.S.; Ribeiro, M.C.B.; Silva, T.L.; Silva, M.G.C.; Vieira, M.G.A. Green-Functionalized Carbon Nanotubes as Adsorbents for the Removal of Emerging Contaminants from Aqueous Media. J. Clean. Prod. 2022, 373, 133961. [Google Scholar] [CrossRef]
- Nekooei, A.; Miroliaei, M.R.; Shahabi-Nejad, M.; Sheibani, G.; Sheibani, H. Cellulose-Wrapped Graphene Oxide as Efficient Adsorbents for Pharmaceutical Contaminants. Inorg. Chem. Commun. 2023, 154, 110997. [Google Scholar] [CrossRef]
- Al-Yaari, M.; Saleh, T.A. Removal of Lead from Wastewater Using Synthesized Polyethyleneimine-Grafted Graphene Oxide. Nanomaterials 2023, 13, 1078. [Google Scholar] [CrossRef]
- Bucatariu, F.; Schwarz, D.; Zaharia, M.; Steinbach, C.; Ghiorghita, C.A.; Schwarz, S.; Mihai, M. Nanostructured Polymer Composites for Selective Heavy Metal Ion Sorption. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125211. [Google Scholar] [CrossRef]
- El-sayed, M.E.A. Nanoadsorbents for Water and Wastewater Remediation. Sci. Total Environ. 2020, 739, 139903. [Google Scholar] [CrossRef] [PubMed]
- Khodakarami, M.; Bagheri, M. Recent Advances in Synthesis and Application of Polymer Nanocomposites for Water and Wastewater Treatment. J. Clean. Prod. 2021, 296, 126404. [Google Scholar] [CrossRef]
- Basheer, A.A. New Generation Nano-Adsorbents for the Removal of Emerging Contaminants in Water. J. Mol. Liq. 2018, 261, 583–593. [Google Scholar] [CrossRef]
- Bucatariu, F.; Ghiorghita, C.A.; Schwarz, D.; Boita, T.; Mihai, M. Layer-by-Layer Polyelectrolyte Architectures with Ultra-Fast and High Loading/Release Properties for Copper Ions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123704. [Google Scholar] [CrossRef]
- Kitsou, I.; Tsetsekou, A. A Water-Based Synthesis of Hybrid Silica/Hyperbranched Poly(Ethylene Imine) Nanopowder for Heavy Metal Sorption from Aqueous Solutions. J. Nanomater. 2019, 2019, 2730317. [Google Scholar] [CrossRef]
- Tiwari, D.; Lalhriatpuia, C.; Lee, S.M. Hybrid Materials in the Removal of Diclofenac Sodium from Aqueous Solutions: Batch and Column Studies. J. Ind. Eng. Chem. 2015, 30, 167–173. [Google Scholar] [CrossRef]
- Mangaleshwaran, L.; Thirulogachandar, A.; Rajasekar, V.; Muthukumaran, C.; Rasappan, K. Batch and Fixed Bed Column Studies on Nickel (II) Adsorption from Aqueous Solution by Treated Polyurethane Foam. J. Taiwan Inst. Chem. Eng. 2015, 55, 112–118. [Google Scholar] [CrossRef]
- Ghasemabadi, S.M.; Baghdadi, M.; Safari, E.; Ghazban, F. Investigation of Continuous Adsorption of Pb(II), As(III), Cd(II), and Cr(VI) Using a Mixture of Magnetic Graphite Oxide and Sand as a Medium in a Fixed-Bed Column. J. Environ. Chem. Eng. 2018, 6, 4840–4849. [Google Scholar] [CrossRef]
- Heidarzadeh-Samani, M.; Behzad, T.; Mehrabani-Zeinabad, A. Development of a Continuous Fixed–Bed Column to Eliminate Cadmium(II) Ions by Starch-g-Poly(Acrylic Acid)/Cellulose Nanofiber Bio-Nanocomposite Hydrogel. Environ. Sci. Pollut. Res. 2021, 28, 57902–57917. [Google Scholar] [CrossRef]
- Jodeh, S.; Abdelwahab, F.; Jaradat, N.; Warad, I.; Jodeh, W. Adsorption of Diclofenac from Aqueous Solution Using Cyclamen Persicum Tubers Based Activated Carbon (CTAC). J. Assoc. Arab Univ. Basic Appl. Sci. 2016, 20, 32–38. [Google Scholar] [CrossRef]
- Morosanu, I.; Teodosiu, C.; Paduraru, C.; Ibanescu, D.; Tofan, L. Biosorption of Lead Ions from Aqueous Effluents by Rapeseed Biomass. N. Biotechnol. 2017, 39, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Harripersadth, C.; Musonge, P. The Dynamic Behaviour of a Binary Adsorbent in a Fixed Bed Column for the Removal of Pb2+ Ions from Contaminated Water Bodies. Sustainability 2022, 14, 7662. [Google Scholar] [CrossRef]
- Thomas, H.G. Chromatography: A Problem in Kinetics. Ann. N. Y. Acad. Sci. 1948, 49, 161–182. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.H.; Nelson, J.H. Application of Gas Adsorption Kinetics—II. A Theoretical Model for Respirator Cartridge Service Life and Its Practical Applications. Am. Ind. Hyg. Assoc. J. 1984, 45, 509–516. [Google Scholar] [CrossRef]
- Lodeiro, P.; Herrero, R.; de Vicente, M.E.S. The Use of Protonated Sargassum Muticum as Biosorbent for Cadmium Removal in a Fixed-Bed Column. J. Hazard. Mater. 2006, 137, 244–253. [Google Scholar] [CrossRef]
- Al-Degs, Y.S.; Khraisheh, M.A.M.; Allen, S.J.; Ahmad, M.N. Adsorption Characteristics of Reactive Dyes in Columns of Activated Carbon. J. Hazard. Mater. 2009, 165, 944–949. [Google Scholar] [CrossRef]
- Fadzail, F.; Hasan, M.; Ibrahim, N.; Mokhtar, Z.; Asih, A.Y.P.; Syafiuddin, A. Adsorption of Diclofenac Sodium Using Low-Cost Activated Carbon in a Fixed-Bed Column. Biointerface Res. Appl. Chem. 2022, 12, 8042–8056. [Google Scholar] [CrossRef]

| Composite Material | qPEI (mg/g Composite) | [CHO]:[NH2] (r) (Cross-Linking Degree) | Cexchange (meq/g Composite) |
|---|---|---|---|
| IS/(PEI-PAA)c-r0.1 | 103.2 | 1:10 | 2.40 |
| IS/(PEI-PMAA)c-r0.1 | 103.2 | 1:10 | 2.40 |
| IS/(PEI-PAA)c-r1 | 103.2 | 1:1 | 2.40 |
| IS/(PEI-PMAA)c-r1 | 103.2 | 1:1 | 2.40 |
| Composite Material | Pollutant | Vb, mL | tb, min | Vs, mL | ts, min | qdyn mg/g | MTZ |
|---|---|---|---|---|---|---|---|
| IS/(PEI-PAA)c-r0.1 | Pb2+ | 282.9 | 141.5 | 630.1 | 315.05 | 22.33 | 2.20 |
| Cd2+ | 50 | 25 | 412.71 | 206.35 | 5.04 | 3.51 | |
| Ni2+ | 50 | 25 | 458 | 229 | 3.466 | 3.11 | |
| DCF-Na | 470.73 | 235.36 | 597.61 | 298.80 | 88.319 | 0.74 | |
| IS/(PEI-PMAA)c-r0.1 | Pb2+ | 661.9 | 331 | 867.1 | 433.6 | 41.44 | 0.95 |
| Cd2+ | 50 | 25 | 808.66 | 404.33 | 17.352 | 3.28 | |
| Ni2+ | 50 | 25 | 556.8 | 278.4 | 4.23 | 3.64 | |
| DCF-Na | 1016.81 | 508.41 | 1471.40 | 735.70 | 230.90 | 0.93 | |
| IS/(PEI-PAA)c-r1 | Pb2+ | 378 | 189 | 625 | 312.5 | 25.73 | 1.58 |
| Cd2+ | 50 | 25 | 481.20 | 240.60 | 8.09 | 3.58 | |
| Ni2+ | 407.2 | 204 | 458.9 | 229 | 6.70 | 0.45 | |
| DCF-Na | 284.94 | 142.47 | 532.69 | 266.34 | 44.41 | 1.86 | |
| IS/(PEI-PMAA)c-r1 | Pb2+ | 374.5 | 187.2 | 841,5 | 420.7 | 29.98 | 2.22 |
| Cd2+ | 50 | 25 | 582.70 | 291 | 10.83 | 3.66 | |
| Ni2+ | 50 | 25 | 438.35 | 219.2 | 3.85 | 3.54 | |
| DCF-Na | 163.47 | 81.74 | 843.25 | 421.63 | 72.09 | 3.22 |
| Composite Material | DCF-Na mg/L | Vb, mL | tb, min | Vs, mL | ts, min | qdyn mg/g | MTZ |
|---|---|---|---|---|---|---|---|
| IS/(PEI-PAA)c-r1 | 31.8 | 559.7 | 279.84 | 694.89 | 347.44 | 26.52 | 0.78 |
| 60 | 411.78 | 205.89 | 600.67 | 300.33 | 41.17 | 1.25 | |
| 100 | 284.93 | 142.46 | 532.68 | 266.34 | 44.41 | 1.86 |
| Composite Material | Pollutants | Kth L/mg·min | qe mg/g | χ2 | R2 |
|---|---|---|---|---|---|
| IS/(PEI-PAA)c-r0.1 | Pb2+ | 6.436 × 10−4 | 25.116 | 1.65 × 10−3 | 0.990 |
| Cd2+ | 1.060 × 10−3 | 4.181 | 1.60 × 10−2 | 0.786 | |
| Ni2+ | 3.660 × 10−3 | 2.725 | 1.31 × 10−2 | 0.820 | |
| DCF-Na | 7.134 × 10−4 | 76.487 | 8.24 × 10−4 | 0.996 | |
| IS/(PEI-PMAA)c-r0.1 | Pb2+ | 1.210 × 10−3 | 44.740 | 4.96 × 10−4 | 0.995 |
| Cd2+ | 4.269 × 10−4 | 14.497 | 5.79 × 10−3 | 0.925 | |
| Ni2+ | 9.158 × 10−4 | 3.746 | 4.22 × 10−3 | 0.913 | |
| DCF-Na | 3.228 × 10−4 | 159.203 | 3.58 × 10−3 | 0.978 | |
| IS/(PEI-PAA)c-r1 | Pb2+ | 7.634 × 10−4 | 28.686 | 4.15 × 10−3 | 0.973 |
| Cd2+ | 1.020 × 10−3 | 10.236 | 3.31 × 10−2 | 0.807 | |
| Ni2+ | 9.010 × 10−3 | 7.900 | 5.69 × 10−3 | 0.979 | |
| DCF-Na (31.8 mg/L) | 3.410 × 10−3 | 29.088 | 9.23 × 10−4 | 0.994 | |
| DCF-Na (60 mg/L) | 9.384 × 10−4 | 42.169 | 1.74 × 10−3 | 0.991 | |
| DCF-Na (100 mg/L) | 5.244 × 10−4 | 49.599 | 6.02 × 10−3 | 0.967 | |
| IS/(PEI-PMAA)c-r1 | Pb2+ | 5.086 × 10−4 | 32.549 | 2.75 × 10−3 | 0.980 |
| Cd2+ | 6.916 × 10−4 | 11.583 | 4.42 × 10−3 | 0.949 | |
| Ni2+ | 1.290 × 10−3 | 3.827 | 3.47 × 10−3 | 0.954 | |
| DCF-Na | 9.015 × 10−5 | 67.289 | 9.04 × 10−3 | 0.898 |
| Composite Material | Pollutants | Yoon–Nelson Model | |||
|---|---|---|---|---|---|
| kYN 1/min | τ, min | χ2 | R2 | ||
| IS/(PEI-PAA)c-r0.1 | Pb2+ | 2.665 × 10−2 | 212.335 | 1.65 × 10−3 | 0.990 |
| Cd2+ | 2.33 × 10−3 | 66.520 | 1.60 × 10−2 | 0.786 | |
| Ni2+ | 4.387 × 10−2 | 79.483 | 1.31 × 10−2 | 0.820 | |
| DCF-Na | 7.135 × 10−2 | 267.703 | 8.24 × 10−4 | 0.996 | |
| IS/(PEI-PMAA)c-r0.1 | Pb2+ | 5.030 × 10−2 | 378.238 | 4.96 × 10−4 | 0.995 |
| Cd2+ | 9.390 × 10−3 | 230.641 | 5.79 × 10−3 | 0.925 | |
| Ni2+ | 1.099 × 10−2 | 109.256 | 4.22 × 10−3 | 0.913 | |
| DCF-Na | 3.229 × 10−2 | 557.204 | 3.58 × 10−3 | 0.978 | |
| IS/(PEI-PAA)c-r1 | Pb2+ | 3.161 × 10−2 | 242.515 | 4.15 × 10−3 | 0.973 |
| Cd2+ | 2.240 × 10−2 | 162.849 | 3.31 × 10−2 | 0.807 | |
| Ni2+ | 1.081 × 10−1 | 230.412 | 5.69 × 10−3 | 0.979 | |
| DCF-Na (31.8 mg/L) | 1.083 × 10−1 | 318.23 | 9.75 × 10−4 | 0.994 | |
| DCF-Na (60 mg/L) | 5.623 × 10−2 | 245.98 | 1.74 × 10−3 | 0.99 | |
| DCF-Na (100 mg/L) | 5.245 × 10−2 | 173.597 | 6.02 × 10−3 | 0.967 | |
| IS/(PEI-PMAA)c-r1 | Pb2+ | 2.106 × 10−2 | 275.170 | 2.75 × 10−3 | 0.980 |
| Cd2+ | 1.522 × 10−2 | 184.271 | 4.42 × 10−3 | 0.949 | |
| Ni2+ | 1.543 × 10−2 | 111.622 | 3.47 × 10−3 | 0.954 | |
| DCF-Na | 9.020 × 10−3 | 235.515 | 9.04 × 10−3 | 0.898 | |
| Sorbent | qCd2+ (mg/g) | qNi2+ (mg/g) | qPb2+ (mg/g) | qDCF (mg/g) | qtotal (mg/g) |
|---|---|---|---|---|---|
| IS/(PEI/PAA)c-r0.1 | 0.640 | 0.337 | 6.505 | 25.372 | 32.854 |
| IS/(PEI/PMAA)c-r0.1 | 3.840 | 1.180 | 5.322 | 109.940 | 120.282 |
| IS/(PEI/PAA)c-r1 | 2.560 | 1.348 | 5.914 | 21.142 | 30.964 |
| IS/(PEI/PMAA)c-r1 | 2.241 | 0.842 | 5.914 | 84.57 | 93.567 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fighir, D.; Paduraru, C.; Ciobanu, R.; Bucatariu, F.; Plavan, O.; Gherghel, A.; Barjoveanu, G.; Mihai, M.; Teodosiu, C. Removal of Diclofenac and Heavy Metal Ions from Aqueous Media Using Composite Sorbents in Dynamic Conditions. Nanomaterials 2024, 14, 33. https://doi.org/10.3390/nano14010033
Fighir D, Paduraru C, Ciobanu R, Bucatariu F, Plavan O, Gherghel A, Barjoveanu G, Mihai M, Teodosiu C. Removal of Diclofenac and Heavy Metal Ions from Aqueous Media Using Composite Sorbents in Dynamic Conditions. Nanomaterials. 2024; 14(1):33. https://doi.org/10.3390/nano14010033
Chicago/Turabian StyleFighir, Daniela, Carmen Paduraru, Ramona Ciobanu, Florin Bucatariu, Oana Plavan, Andreea Gherghel, George Barjoveanu, Marcela Mihai, and Carmen Teodosiu. 2024. "Removal of Diclofenac and Heavy Metal Ions from Aqueous Media Using Composite Sorbents in Dynamic Conditions" Nanomaterials 14, no. 1: 33. https://doi.org/10.3390/nano14010033
APA StyleFighir, D., Paduraru, C., Ciobanu, R., Bucatariu, F., Plavan, O., Gherghel, A., Barjoveanu, G., Mihai, M., & Teodosiu, C. (2024). Removal of Diclofenac and Heavy Metal Ions from Aqueous Media Using Composite Sorbents in Dynamic Conditions. Nanomaterials, 14(1), 33. https://doi.org/10.3390/nano14010033











