The Adsorption Effect of Methane Gas Molecules on Monolayer PbSe with and without Vacancy Defects: A First-Principles Study
Abstract
1. Introduction
2. Computational Method and Model
3. Results and Discussion
3.1. Adsorption of Methane Molecules on Pristine 2D PbSe
3.2. Adsorption of Methane Molecules on VSe
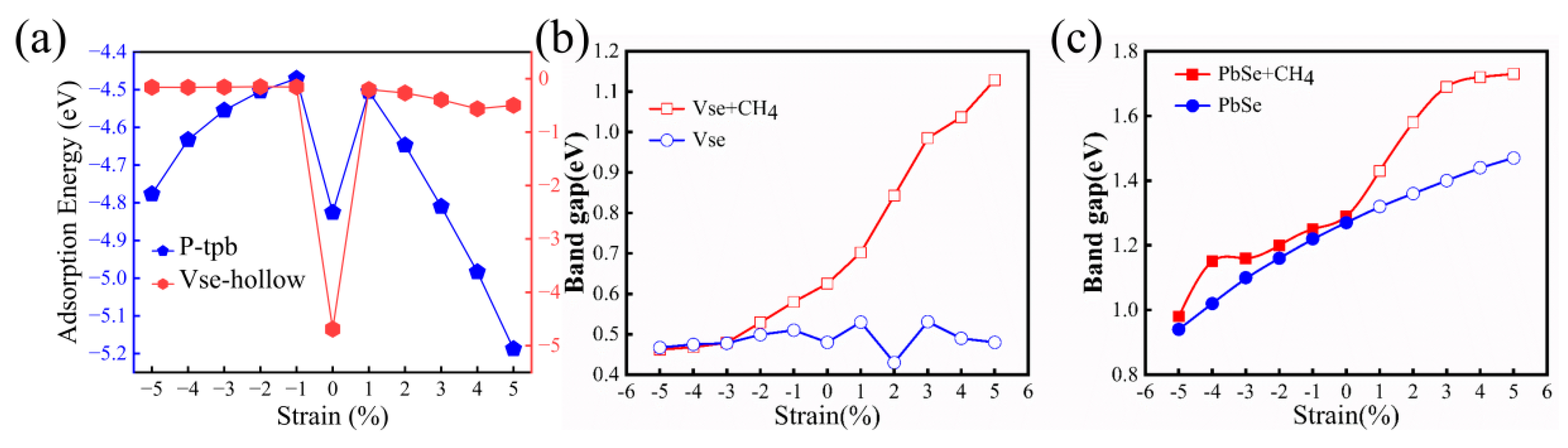
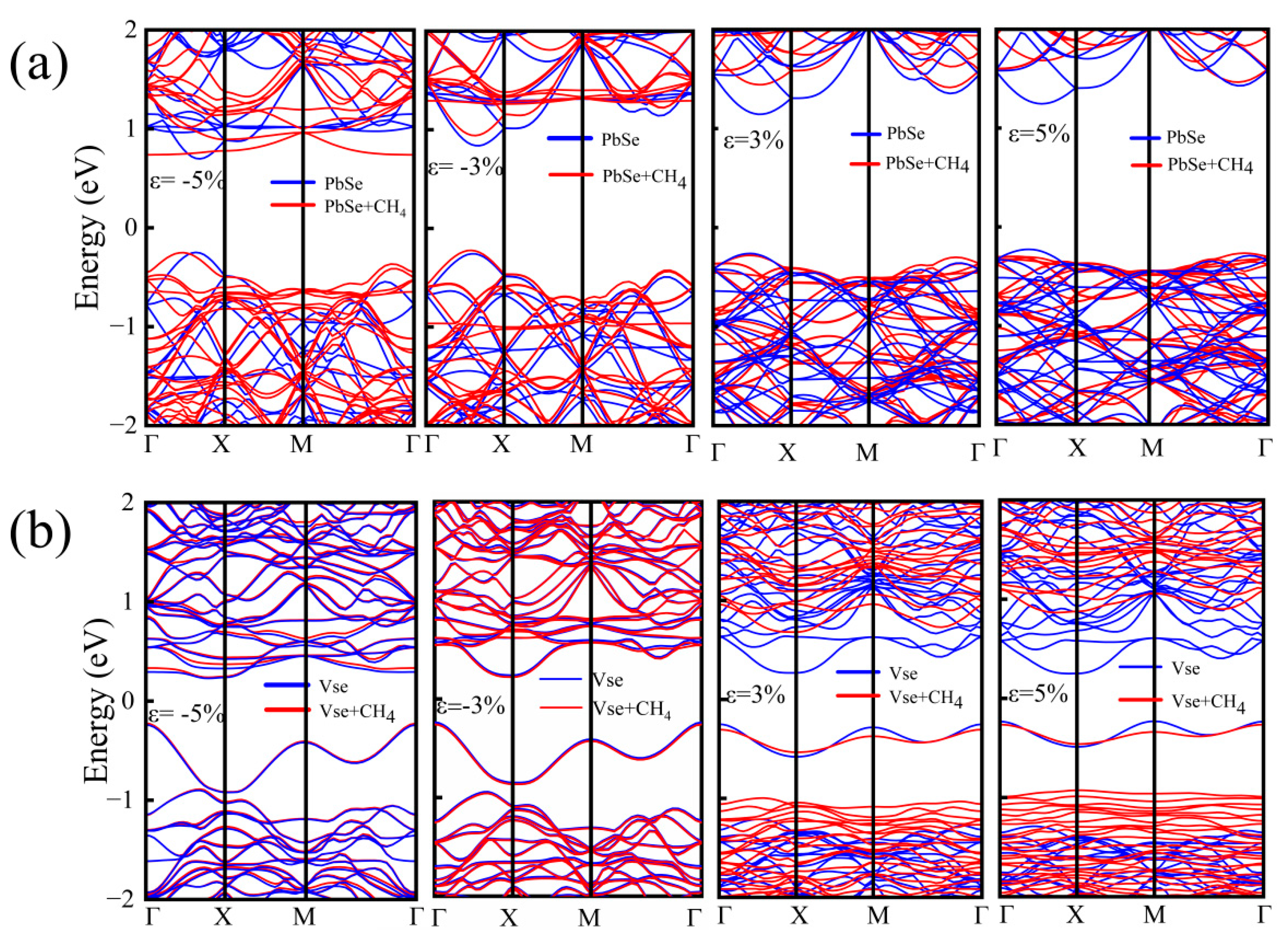
3.3. Adsorbtion Methane Molecules on the PbSe Monolayer under Biaxial Strain
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Han, J.; Chen, X.; Wang, X. Design strategies for two-dimensional material photodetectors to enhance device performance. InfoMat 2019, 1, 33–53. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.A.; Kumar, M. Recent advances in UV photodetectors based on 2D materials: A review. J. Phys. D Appl. Phys. 2021, 55, 133002. [Google Scholar] [CrossRef]
- Liu, C.; Guo, J.; Yu, L.; Li, J.; Zhang, M.; Li, H.; Shi, Y.; Dai, D. Silicon/2D-material photodetectors: From near-infrared to mid-infrared. Light Sci. Appl. 2021, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Wei, Z.; Wei, X.; Lv, Q.; Zhu, W.; Wang, K. Toward High-Performance Photodetectors Based on 2D Materials: Strategy on Methods. Small Methods 2018, 2, 1700349. [Google Scholar] [CrossRef]
- Gao, F.; Yang, H.; Hu, P. Interfacial Engineering for Fabricating High-Performance Field-Effect Transistors Based on 2D Materials. Small Methods 2018, 2, 1700384. [Google Scholar] [CrossRef]
- Ahmad, S.; Fu, P.; Yu, S.; Yang, Q.; Liu, X.; Wang, X.; Wang, X.; Guo, X.; Li, C. Dion-Jacobson Phase 2D Layered Perovskites for Solar Cells with Ultrahigh Stability. Joule 2019, 3, 794–806. [Google Scholar] [CrossRef]
- Shao, M.; Bie, T.; Yang, L.; Gao, Y.; Jin, X.; He, F.; Zheng, N.; Yu, Y.; Zhang, X. Over 21% Efficiency STable 2D Perovskite Solar Cells. Adv. Mater. 2022, 34, 2107211. [Google Scholar] [CrossRef]
- Fu, W.; Wang, J.; Zuo, L.; Gao, K.; Liu, F.; Ginger, D.S.; Jen, A.K.-Y. Two-Dimensional Perovskite Solar Cells with 14.1% Power Conversion Efficiency and 0.68% External Radiative Efficiency. ACS Energy Lett. 2018, 3, 2086–2093. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Flexible and low power CO gas sensor with Au-functionalized 2D WS2 nanoflakes. Sens. Actuators B Chem. 2020, 313, 128040. [Google Scholar] [CrossRef]
- Buckley, D.J.; Black, N.C.G.; Castanon, E.G.; Melios, C.; Hardman, M.; Kazakova, O. Frontiers of graphene and 2D material-based gas sensors for environmental monitoring. 2D Mater. 2020, 7, 32002. [Google Scholar] [CrossRef]
- Tang, H.; Sacco, L.N.; Vollebregt, S.; Ye, H.; Fan, X.; Zhang, G. Recent advances in 2D/nanostructured metal sulfide-based gas sensors: Mechanisms, applications, and perspectives. J. Mater. Chem. A 2020, 8, 24943–24976. [Google Scholar] [CrossRef]
- Rad, A.S. First principles study of Al-doped graphene as nanostructure adsorbent for NO2 and N2O: DFT calculations. Appl. Surf. Sci. 2015, 357, 1217–1224. [Google Scholar] [CrossRef]
- Jelmy, E.J.; Thomas, N.; Mathew, D.T.; Louis, J.; Padmanabhan, N.T.; Kumaravel, V.; John, H.; Pillai, S.C. Impact of structure, doping and defect-engineering in 2D materials on CO2 capture and conversion. React. Chem. Eng. 2021, 6, 1701–1738. [Google Scholar] [CrossRef]
- Son, J.; Hashmi, A.; Hong, J. Manipulation of n and p type dope black phosphorene layer: A first principles study. Curr. Appl. Phys. 2016, 16, 506–514. [Google Scholar] [CrossRef]
- Liu, H.; Qu, M.; Du, A.; Sun, Q. N/P-Doped MoS2 Monolayers as Promising Materials for Controllable CO2 Capture and Separation under Reduced Electric Fields: A Theoretical Modeling. J. Phys. Chem. C 2021, 126, 203–211. [Google Scholar] [CrossRef]
- Ersan, F.; Gökçe, A.G.; Aktürk, E. Point defects in hexagonal germanium carbide monolayer: A first-principles calculation. Appl. Surf. Sci. 2016, 389, 1–6. [Google Scholar] [CrossRef]
- Lin, L.; Chen, R.; Huang, J.; Wang, P.; Zhu, L.; Yao, L.; Hu, C.; Tao, H.; Zhang, Z. Adsorption of CO, H2S and CH4 molecules on SnS2 monolayer: A first-principles study. Mol. Phys. 2021, 119, e1856429. [Google Scholar] [CrossRef]
- Zheng, H.; Meng, X.; Chen, J.; Que, M.; Wang, W.; Liu, X.; Yang, L.; Zhao, Y. In Situ phase evolution of TiO2/Ti3C2T heterojunction for enhancing adsorption and photocatalytic degradation. Appl. Surf. Sci. 2021, 545, 149031. [Google Scholar] [CrossRef]
- Zhang, R.; Jian, W.; Yang, Z.-D.; Bai, F.-Q. Insights into the photocatalytic mechanism of the C4N/MoS2 heterostructure: A first-principle study. Chin. Chem. Lett. 2020, 31, 2319–2324. [Google Scholar] [CrossRef]
- Jin, Y.; Ding, J.; Chen, H.; Fu, H.; Peng, J. Enhanced adsorption properties of ZnO/GaN heterojunction for CO and H2S under external electric field. Comput. Theor. Chem. 2021, 1206, 113495. [Google Scholar] [CrossRef]
- Pham, K.D.; Dinh, P.C.; Diep, D.V.; Luong, H.L.; Vu, T.V.; Hoang, D.-Q.; Khyzhun, O.Y.; Ngoc, H.V. Effects of electric field and biaxial strain on the (NO2, NO, O2, and SO2) gas adsorption properties of Sc2CO2 monolayer. Micro Nanostructures 2022, 163, 107135. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, C.; Tsai, H.-S.; Zhou, J.; Zhang, Y.; Wang, T.; Ma, G.; Qi, C.; Huo, M. The strain and transition metal doping effects on monolayer Cr2O3 for hydrogen evolution reaction: The first principle calculations. Int. J. Hydrogen Energy 2022, 47, 37429–37437. [Google Scholar] [CrossRef]
- Zhang, H.-P.; Kou, L.; Jiao, Y.; Du, A.; Tang, Y.; Ni, Y. Strain engineering of selective chemical adsorption on monolayer black phosphorous. Appl. Surf. Sci. 2020, 503, 144033. [Google Scholar] [CrossRef]
- Zheng, X.; Ban, S.; Liu, B.; Chen, G. Strain-controlled graphdiyne membrane for CO2/CH4 separation: First-principle and molecular dynamic simulation. Chin. J. Chem. Eng. 2020, 28, 1898–1903. [Google Scholar] [CrossRef]
- Yu, X.-F.; Li, Y.-C.; Liu, Z.-B.; Li, Q.-Z.; Li, W.-Z.; Yang, X.; Xiao, B. Monolayer Ti2CO2: A promising candidate for NH3 sensor or capturer with high sensitivity and selectivity. ACS Publ. 2015, 7, 13707–13713. [Google Scholar] [CrossRef]
- Gui, Y.; Liu, Z.; Ji, C.; Xu, L.; Chen, X. Adsorption behavior of metal oxides (CuO, NiO, Ag2O) modified GeSe monolayer towards dissolved gases (CO, CH4, C2H2, C2H4) in transformer oil. J. Ind. Eng. Chem. 2022, 112, 134–145. [Google Scholar] [CrossRef]
- Fan, Q.; Zhang, W.; Qing, H.; Yang, J. Exceptional Thermoelectric Properties of Bilayer GeSe: First Principles Calculation. Materials 2022, 15, 971. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, S.-C.; Wang, Y.; Long, M.; Dai, C.-M.; Chen, S.; Zhang, B.; Sun, Z.; Sun, Z.; Hu, C.; et al. In-Plane Optical Anisotropy of Low-Symmetry 2D GeSe. Adv. Opt. Mater. 2019, 7, 1801311. [Google Scholar] [CrossRef]
- Ma, X.-H.; Cho, K.-H.; Sung, Y.-M. Growth mechanism of vertically aligned SnSe nanosheets via physical vapour deposition. CrystEngComm 2014, 16, 5080–5086. [Google Scholar] [CrossRef]
- Zheng, D.; Fang, H.; Long, M.; Wu, F.; Wang, P.; Gong, F.; Wu, X.; Ho, J.C.; Liao, L.; Hu, W. High-Performance Near-Infrared Photodetectors Based on p-Type SnX (X = S, Se) Nanowires Grown via Chemical Vapor Deposition. ACS Nano 2018, 12, 7239–7245. [Google Scholar] [CrossRef]
- Jagani, H.S.; Gupta, S.U.; Bhoraniya, K.; Navapariya, M.; Pathak, V.M.; Solanki, G.K.; Patel, H. Photosensitive Schottky barrier diodes based on Cu/p-SnSe thin films fabricated by thermal evaporation. Mater. Adv. 2022, 3, 2425–2433. [Google Scholar] [CrossRef]
- Yan, J.; Deng, S.; Zhu, D.; Bai, H.; Zhu, H. Self-powered SnSe photodetectors fabricated by ultrafast laser. Nano Energy 2022, 97, 107188. [Google Scholar] [CrossRef]
- Beltrán-Bobadilla, P.; Beltrán-Bobadilla, P.; Carrillo-Osuna, A.; Rodriguez-Valverde, J.A.; Acevedo-Juárez, B.; De Los Santos, I.M.; Sánchez-Rodriguez, F.J.; Courel, M.; Carrillo-Osuna, A.; Rodriguez-Valverde, J.A.; et al. SnSe Solar Cells: Current Results and Perspectives. Gen. Chem. 2021, 7, 200012. [Google Scholar] [CrossRef]
- Patel, H.; Patel, K.; Patel, A.; Jagani, H.; Patel, K.D.; Solanki, G.K.; Pathak, V.M. Temperature-Dependent I–V Characteristics of In/p-SnSe Schottky Diode. J. Electron. Mater. 2021, 50, 5217–5225. [Google Scholar] [CrossRef]
- Pandit, B.; Jadhav, C.D.; Chavan, P.G.; Tarkas, H.S.; Sali, J.V.; Gupta, R.B.; Sankapal, B.R. Two-Dimensional Hexagonal SnSe Nanosheets as Binder-Free Electrode Material for High-Performance Supercapacitors. IEEE Trans. Power Electron. 2020, 35, 11344–11351. [Google Scholar] [CrossRef]
- Kasiyan, V.; Dashevsky, Z.; Schwarz, C.M.; Shatkhin, M.; Flitsiyan, E.; Chernyak, L.; Khokhlov, D. Infrared detectors based on semiconductor p-n junction of PbSe. J. Appl. Phys. 2012, 112, 086101. [Google Scholar] [CrossRef]
- Sierra, C.; Torquemada, M.C.; Vergara, G.; Rodrigo, M.T.; Gutiérrez, C.; Pérez, G.; Génova, I.; Catalán, I.; Gómez, L.J.; Villamayor, V.; et al. Multicolour PbSe sensors for analytical applications. Sens. Actuators B Chem. 2014, 190, 464–471. [Google Scholar] [CrossRef]
- Li, M.; Luo, J.; Fu, C.; Kan, H.; Huang, Z.; Huang, W.; Yang, S.; Zhang, J.; Tang, J.; Fu, Y.; et al. PbSe quantum dots-based chemiresistors for room-temperature NO2 detection. Sens. Actuators B Chem. 2018, 256, 1045–1056. [Google Scholar] [CrossRef]
- Jiang, J.; Cheng, R.; Yin, L.; Wen, Y.; Wang, H.; Zhai, B.; Liu, C.; Shan, C.; He, J. Van der Waals epitaxial growth of two-dimensional PbSe and its high-performance heterostructure devices. Sci. Bull. 2022, 67, 1659–1668. [Google Scholar] [CrossRef]
- Díaz-Torres, E.; Flores-Conde, A.; Ávila-García, A.; Ortega-López, M. Electronic transport study of PbSe pellets prepared from self-assembled 2D-PbSe nanostructures. Curr. Appl. Phys. 2018, 18, 226–230. [Google Scholar] [CrossRef]
- Díaz-Torres, E.; Ortega-López, M.; Matsumoto, Y.; Santoyo-Salazar, J. Simple synthesis of PbSe nanocrystals and their self-assembly into 2D ‘flakes’ and 1D ‘ribbons’ structures. Mater. Res. Bull. 2016, 80, 96–101. [Google Scholar] [CrossRef]
- Geuchies, J.J.; Van Overbeek, C.; Evers, W.H.; Goris, B.; De Backer, A.; Gantapara, A.P.; Rabouw, F.T.; Hilhorst, J.; Peters, J.L.; Konovalov, O. In Situ study of the formation mechanism of two-dimensional superlattices from PbSe nanocrystals. Nat. Mater. 2016, 15, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Hennig, R. Computational prediction of two-dimensional group-IV mono-chalcogenides. Appl. Phys. Lett. 2014, 105, 042103. [Google Scholar] [CrossRef]
- Xiong, F.; Zhang, X.; Lin, Z.; Chen, Y. Ferroelectric engineering of two-dimensional group-IV monochalcogenides: The effects of alloying and strain. J. Mater. 2018, 4, 139–143. [Google Scholar] [CrossRef]
- Zhang, J.; Pang, J.; Chen, H.; Wei, G.; Yan, J. Theoretical Study and Application of Doped 2D PbSe for Toxic Gases. Phys. Status Solidi 2023, 260, 2200250. [Google Scholar] [CrossRef]
- Zhang, J.; Pang, J.; Chen, H.; Wei, G.; Wei, S.; Yan, J.; Jin, S. Study on SO2 and Cl2 sensor application of 2D PbSe based on first principles calculations. RCS Adv. 2022, 12, 8530–8535. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, S.; Wang, L.; Jiao, Z. A novel highly selective and sensitive NH3 gas sensor based on monolayer Hf2CO2. Appl. Surf. Sci. 2019, 492, 116–124. [Google Scholar] [CrossRef]
- Li, S.-S.; Li, X.-H.; Cui, X.-H.; Zhang, R.-Z.; Cui, H.-L.J. Effect of the biaxial strain on the electronic structure, quantum capacitance of NH3 adsorption on pristine Hf2CO2 MXene using first-principles calculations. Appl. Surf. Sci. 2022, 575, 151659. [Google Scholar] [CrossRef]
- Ma, S.; Yuan, D.; Wang, Y.; Jiao, Z. Monolayer GeS as a potential candidate for NO2 gas sensors and capturers. J. Mater. Chem. C 2018, 6, 8082–8091. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Wu, Z.; Cohen, R.E. A More Accurate Generalized Gradient Approximation for Solids. Phys. Rev. B 2006, 73, 235116. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Mao, Y.; Wu, R.; Ding, D.; He, F. Tunable optoelectronic properties of two-dimensional PbSe by strain: First-principles study. Comput. Mater. Sci. 2022, 202, 110957. [Google Scholar] [CrossRef]
- Li, X.-B.; Guo, P.; Zhang, Y.-N.; Peng, R.-F.; Zhang, H.; Liu, L.-M. High carrier mobility of few-layer PbX (X = S, Se, Te). J. Mater. Chem. C 2015, 3, 6284–6290. [Google Scholar] [CrossRef]

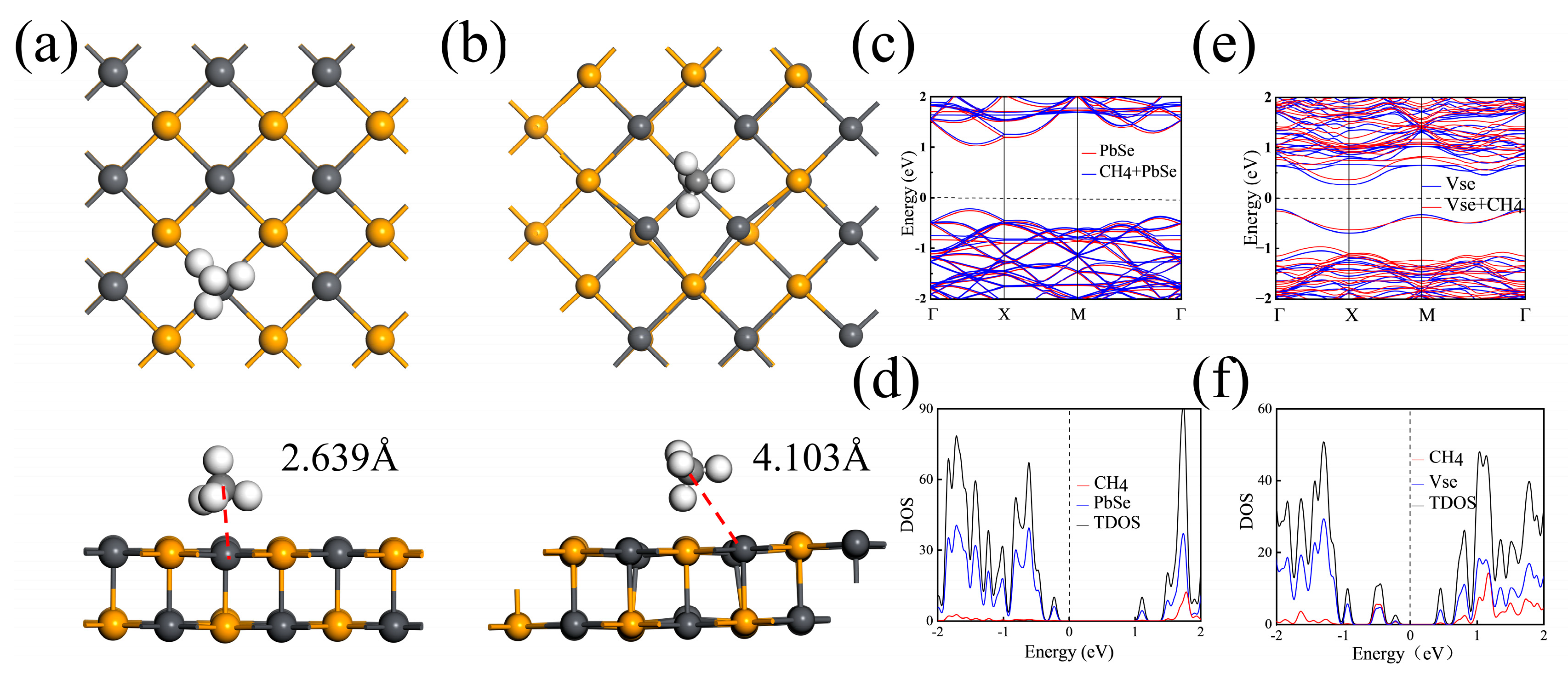
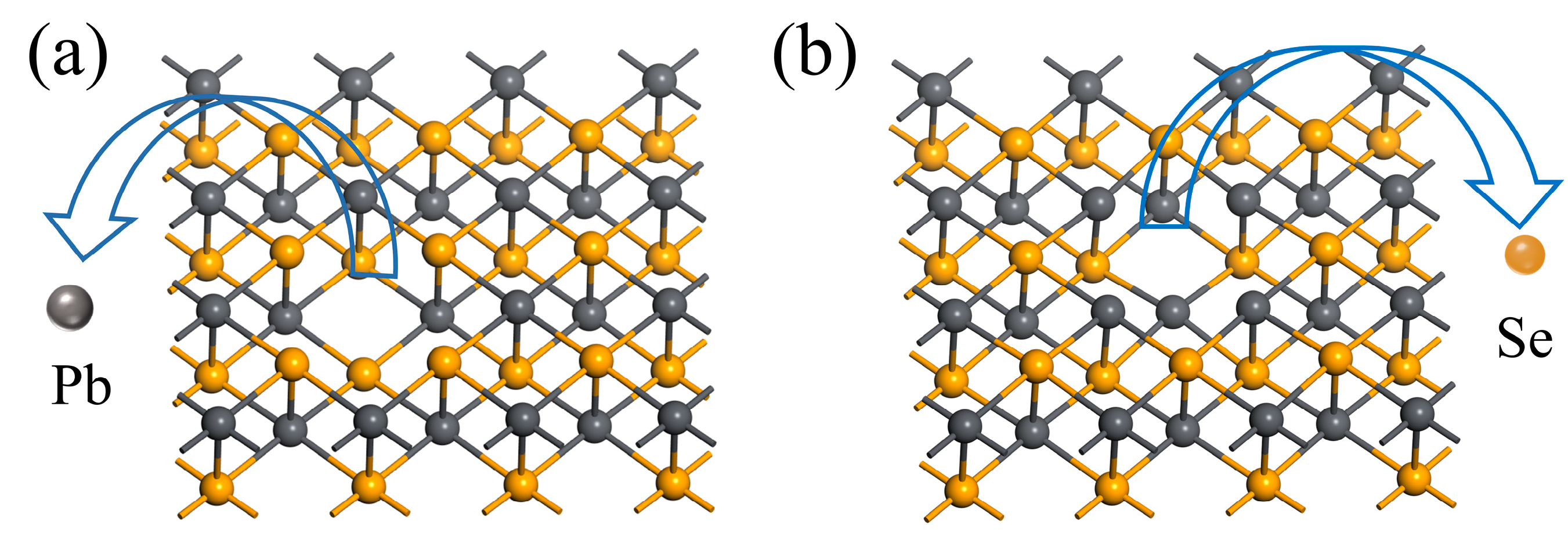
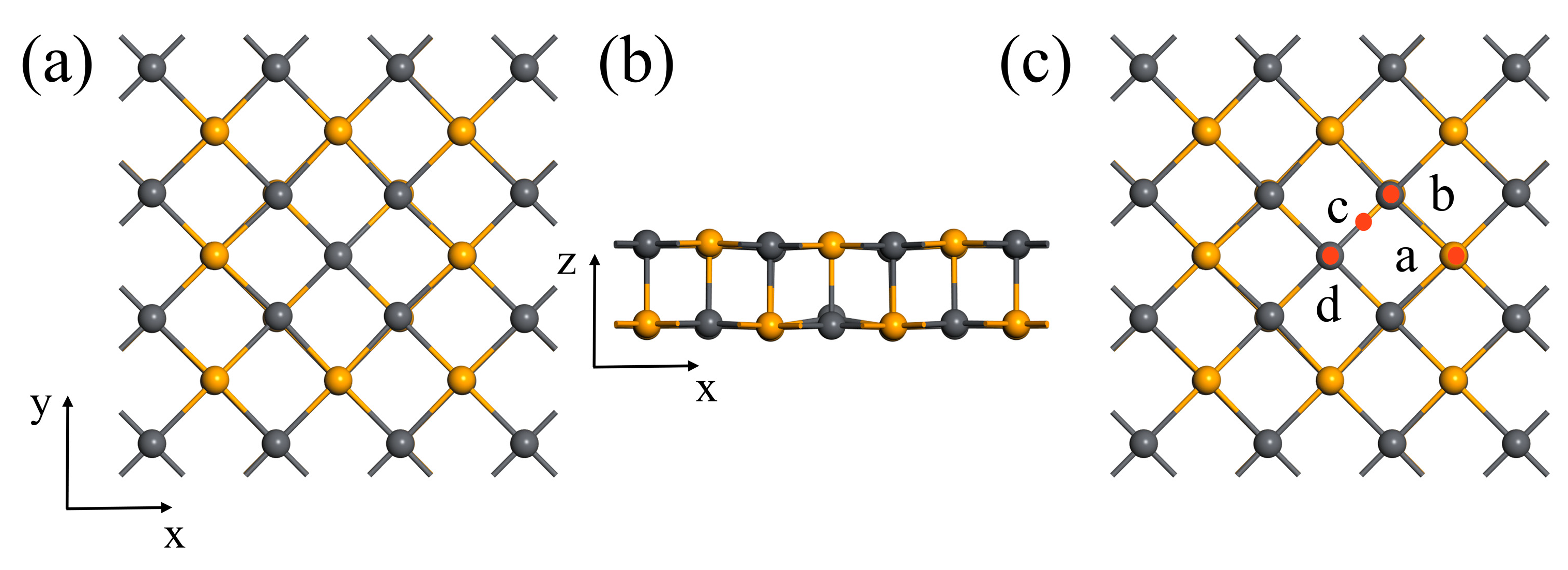

| Site | TPb | TSe | Bridge | Hollow |
|---|---|---|---|---|
| P-PbSe (eV) | −4.8259 | −4.7887 | −4.8158 | −4.8177 |
| VSe-PbSe (eV) | −4.6776 | −4.6506 | −4.6467 | −4.6885 |
| Atom | CH4 (e) | CH4-TPb (e) | Transfer (e) |
|---|---|---|---|
| C | 4.09581 | 4.115225 | +0.019415 |
| H1 | 0.967049 | 0.985145 | +0.018096 |
| H2 | 0.967049 | 0.955066 | −0.011983 |
| H3 | 0.992434 | 0.988498 | −0.003936 |
| H4 | 0.977659 | 0.967289 | −0.01037 |
| Atom | CH4 (e) | CH4-Hollow (e) | Transfer (e) |
|---|---|---|---|
| C | 4.09581 | 4.110206 | +0.014396 |
| H1 | 0.967049 | 0.980938 | +0.013889 |
| H2 | 0.967049 | 0.979328 | +0.012279 |
| H3 | 0.992434 | 0.961884 | −0.03055 |
| H4 | 0.977659 | 0.999377 | +0.021718 |
| Strain (%) | Ead (eV) | Egap (eV) | Transfer (e) |
|---|---|---|---|
| −5 | −4.767 | 0.99 | 0.0175 |
| −4 | −4.624 | 1.15 | 0.0131 |
| −3 | −4.555 | 1.16 | 0.0123 |
| −2 | −4.504 | 1.21 | 0.0107 |
| −1 | −4.471 | 1.26 | 0.0104 |
| 0 | −4.826 | 1.29 | 0.0112 |
| 1 | −4.505 | 1.43 | 0.0118 |
| 2 | −4.647 | 1.58 | 0.0113 |
| 3 | −4.81 | 1.7 | 0.0108 |
| 4 | −4.984 | 1.72 | 0.0108 |
| 5 | −5.187 | 1.73 | 0.0104 |
| Strain (%) | Ead (eV) | Egap (eV) | Transfer (e) |
|---|---|---|---|
| −5 | −0.161 | 0.46 | 0.028 |
| −4 | −0.161 | 0.47 | 0.028 |
| −3 | −0.157 | 0.48 | 0.029 |
| −2 | −0.15 | 0.53 | 0.031 |
| −1 | −0.155 | 0.58 | 0.031 |
| 0 | −4.689 | 0.63 | 0.03 |
| 1 | −0.202 | 0.7 | 0.03 |
| 2 | −0.265 | 0.84 | 0.029 |
| 3 | −0.394 | 0.99 | 0.029 |
| 4 | −0.563 | 1.04 | 0.228 |
| 5 | −0.498 | 1.13 | 0.027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Mao, Y. The Adsorption Effect of Methane Gas Molecules on Monolayer PbSe with and without Vacancy Defects: A First-Principles Study. Nanomaterials 2023, 13, 1566. https://doi.org/10.3390/nano13091566
Zhou X, Mao Y. The Adsorption Effect of Methane Gas Molecules on Monolayer PbSe with and without Vacancy Defects: A First-Principles Study. Nanomaterials. 2023; 13(9):1566. https://doi.org/10.3390/nano13091566
Chicago/Turabian StyleZhou, Xing, and Yuliang Mao. 2023. "The Adsorption Effect of Methane Gas Molecules on Monolayer PbSe with and without Vacancy Defects: A First-Principles Study" Nanomaterials 13, no. 9: 1566. https://doi.org/10.3390/nano13091566
APA StyleZhou, X., & Mao, Y. (2023). The Adsorption Effect of Methane Gas Molecules on Monolayer PbSe with and without Vacancy Defects: A First-Principles Study. Nanomaterials, 13(9), 1566. https://doi.org/10.3390/nano13091566






