Facile Fabrication of TiO2 Quantum Dots-Anchored g-C3N4 Nanosheets as 0D/2D Heterojunction Nanocomposite for Accelerating Solar-Driven Photocatalysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
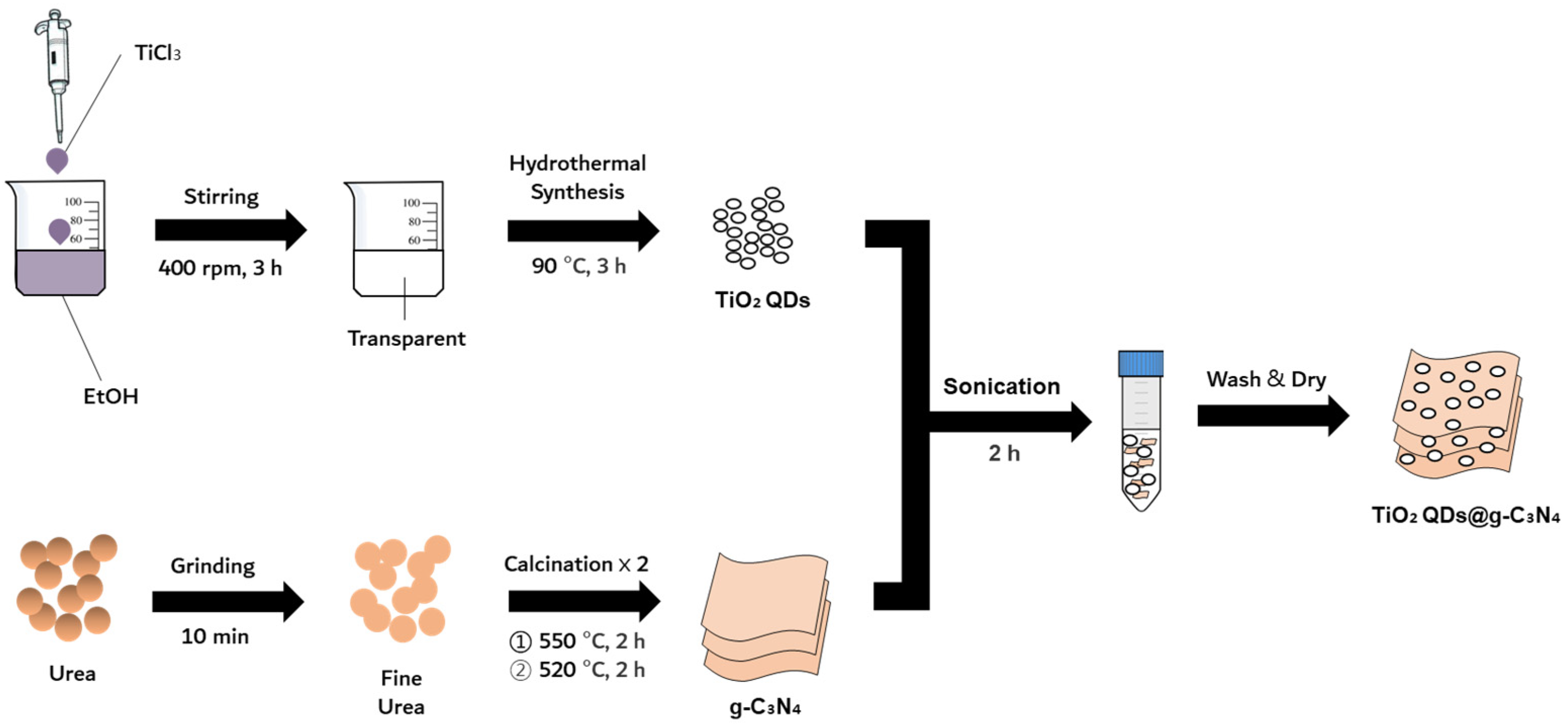
2.2. Synthesis of TiO2 QDs and g-C3N4 NSs
2.3. Synthesis of the TiO2 QDs@g-C3N4 Heterojunction
2.4. Photocatalytic Test
3. Results and Discussion
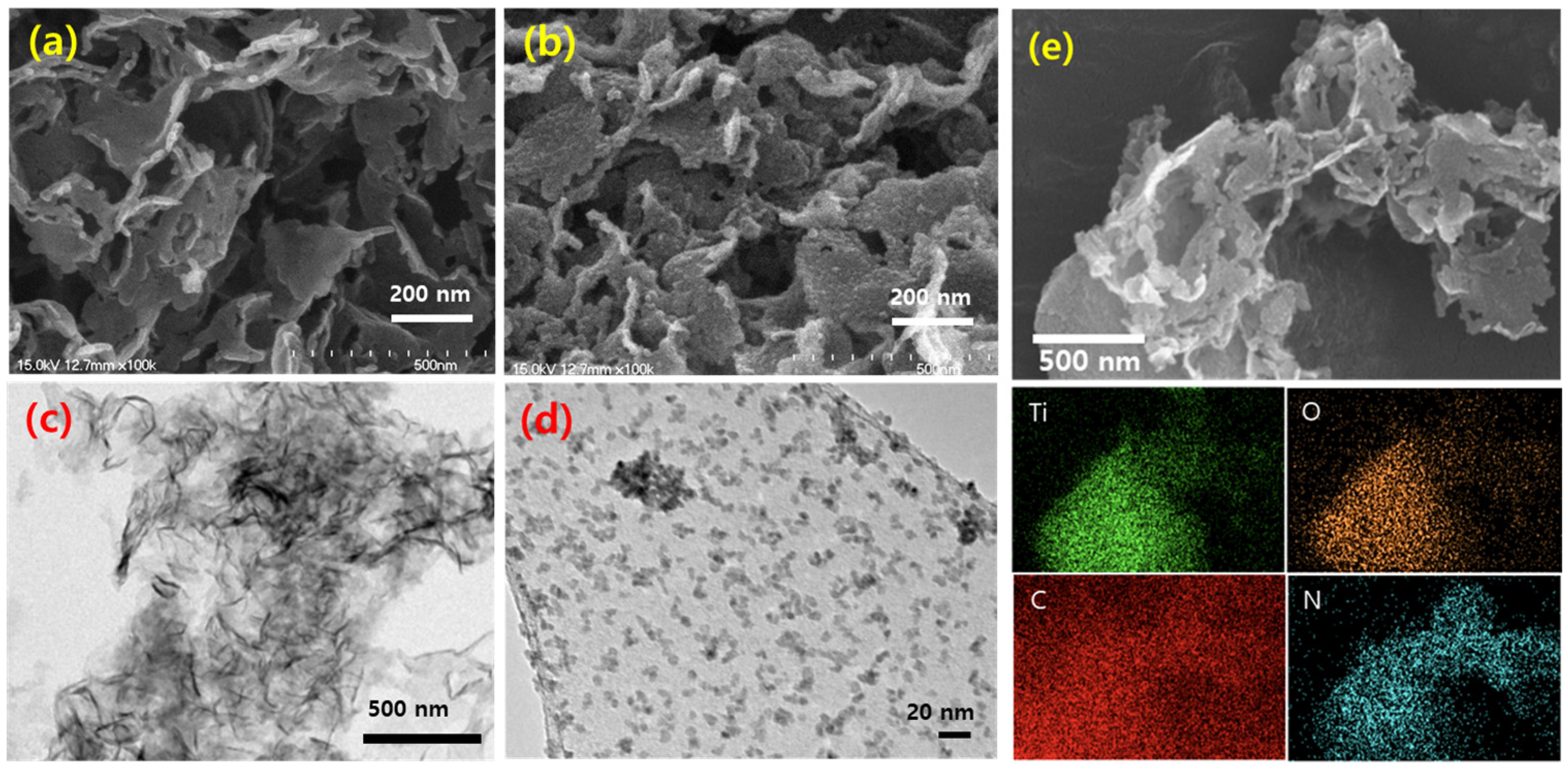
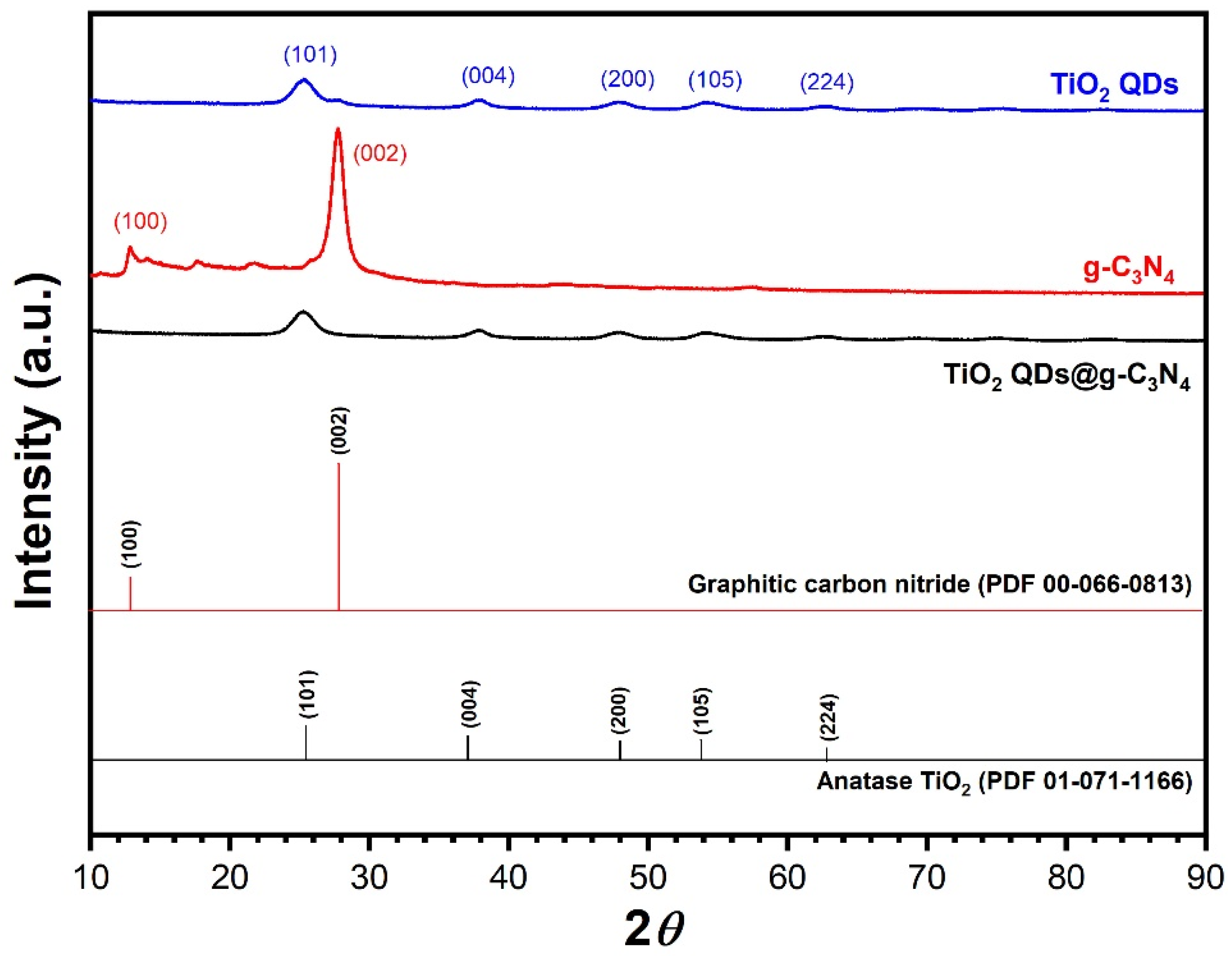
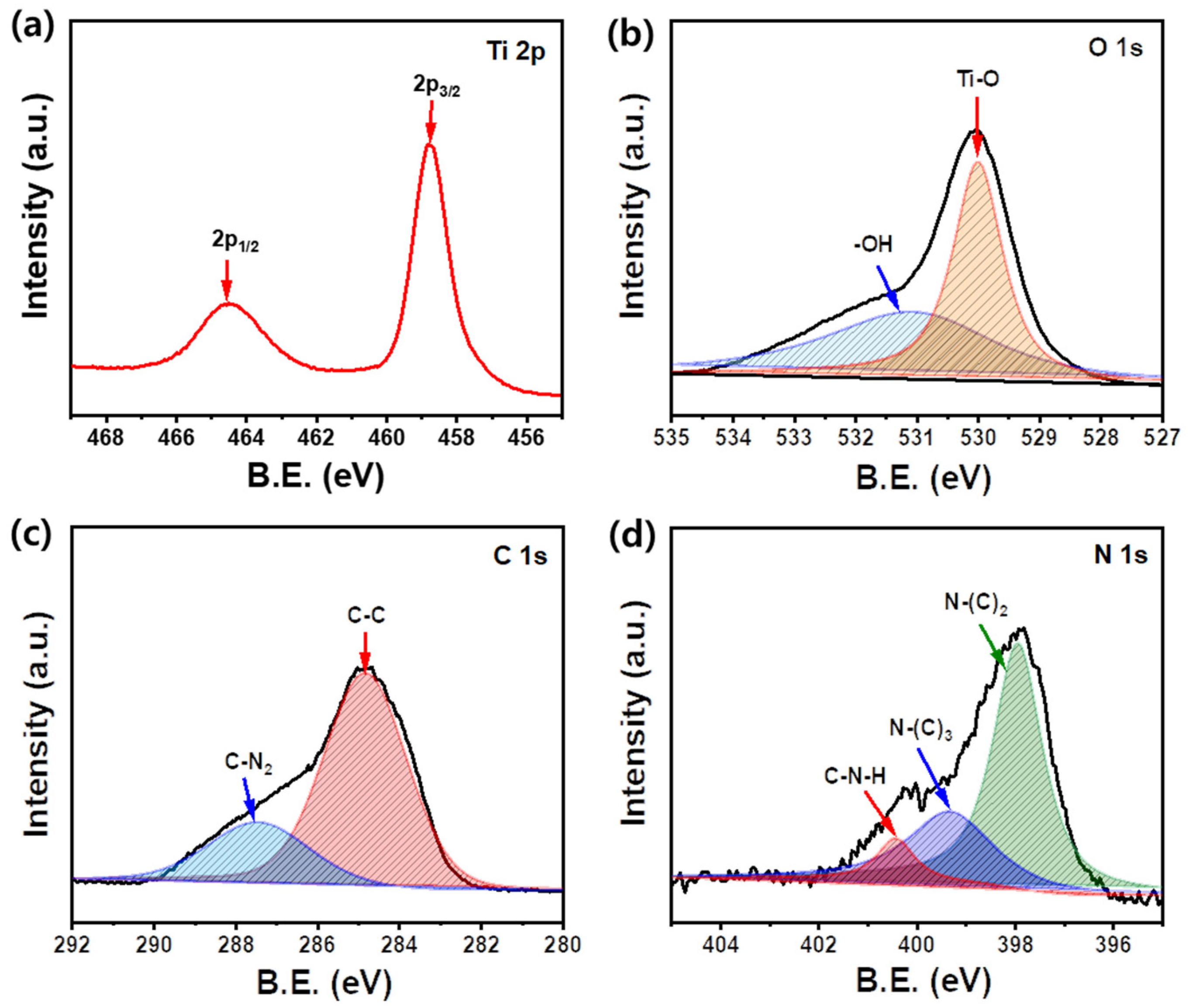
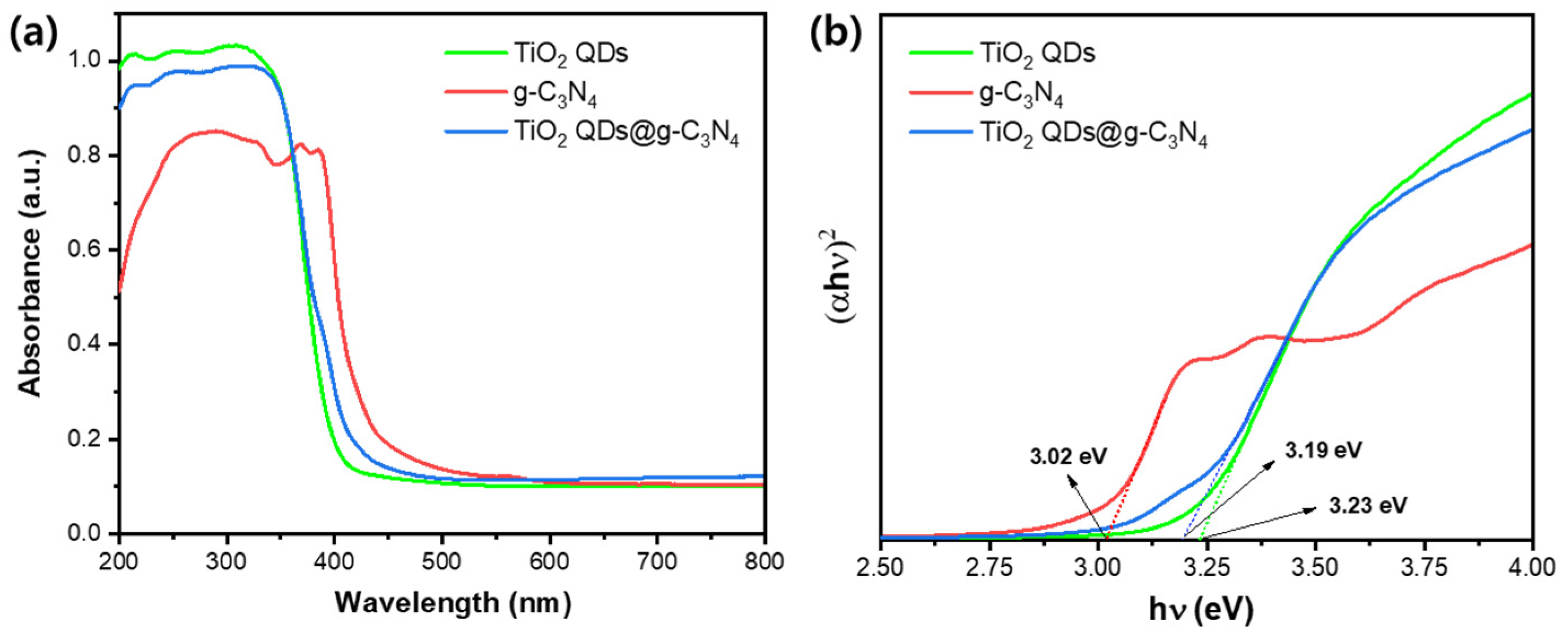
3.1. Preparation and Characterization of Samples
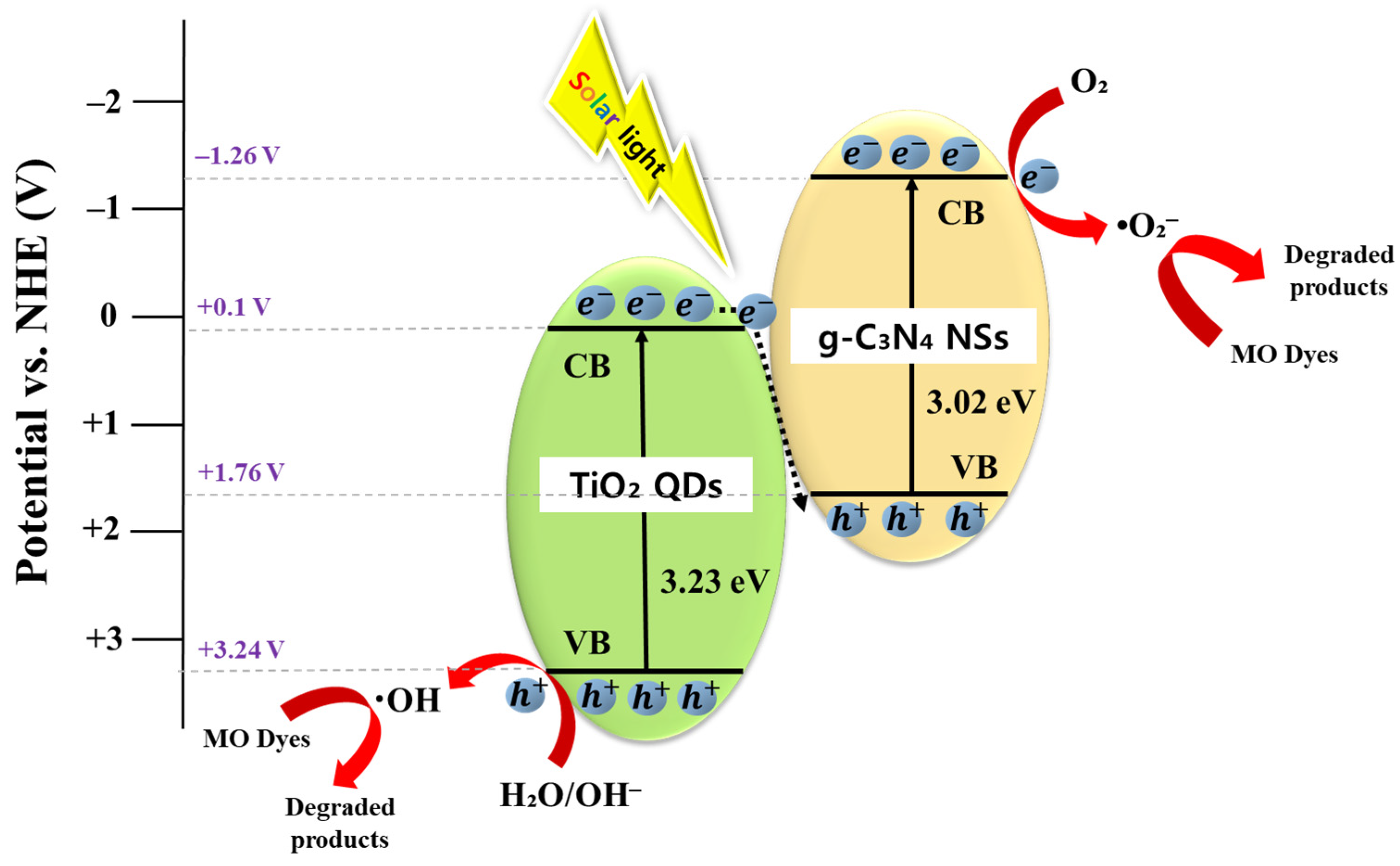
3.2. Photocatalytic Activity and Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic Water Splitting-The Untamed Dream: A Review of Recent Advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under UV/visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef]
- Jaison, A.; Mohan, A.; Lee, Y.-C. Recent Developments in Photocatalytic Nanotechnology for Purifying Air Polluted with Volatile Organic Compounds: Effect of Operating Parameters and Catalyst Deactivation. Catalysts 2023, 13, 407. [Google Scholar] [CrossRef]
- Mao, W.-X.; Lin, X.-J.; Zhang, W.; Chi, Z.-X.; Lyu, R.-W.; Cao, A.-M.; Wan, L.-J. Core–shell structured TiO2@ polydopamine for highly active visible-light photocatalysis. Chem. Commun. 2016, 52, 7122–7125. [Google Scholar] [CrossRef] [PubMed]
- Kadam, A.; Dhabbe, R.; Kokate, M.; Gaikwad, Y.; Garadkar, K. Preparation of N doped TiO2 via microwave-assisted method and its photocatalytic activity for degradation of Malathion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 669–676. [Google Scholar] [CrossRef]
- Thompson, T.L.; Yates, J.T. Surface science studies of the photoactivation of TiO2 new photochemical processes. Chem. Rev. 2006, 106, 4428–4453. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhuang, C.; Li, Y.; Gao, C.; Jiang, W.; Sun, Z.; Qi, K. Anchoring ultra-small TiO2 quantum dots onto ultra-thin and large-sized Mxene nanosheets for highly efficient photocatalytic water splitting. Ceram. Int. 2021, 47, 21769–21776. [Google Scholar] [CrossRef]
- Geng, R.; Yin, J.; Zhou, J.; Jiao, T.; Feng, Y.; Zhang, L.; Chen, Y.; Bai, Z.; Peng, Q. In Situ Construction of Ag/TiO2/g-C3N4 Heterojunction Nanocomposite Based on Hierarchical Co-Assembly with Sustainable Hydrogen Evolution. Nanomaterials 2019, 10, 1. [Google Scholar] [CrossRef]
- Daulbayev, C.; Sultanov, F.; Bakbolat, B.; Daulbayev, O. 0D, 1D and 2D nanomaterials for visible photoelectrochemical water splitting. A review. Int. J. Hydrogen Energy 2020, 45, 33325–33342. [Google Scholar] [CrossRef]
- Li, B.; Cao, Z.; Wang, S.; Wei, Q.; Shen, Z. BiVO4 quantum dot-decorated BiVO4 nanorods 0D/1D heterojunction for enhanced visible-light-driven photocatalysis. Dalton Trans. 2018, 47, 10288–10298. [Google Scholar] [CrossRef] [PubMed]
- Kadam, A.N.; Babu, B.; Lee, S.W.; Kim, J.; Yoo, K. Morphological guided sphere to dendrite BiVO4 for highly efficient organic pollutant removal and photoelectrochemical performance under solar light. Chemosphere 2022, 305, 135461. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.A.; Nguyen, T.P.; Le, V.T.; Kim, I.T.; Lee, S.-W.; Nguyen, C.T. Excellent photocatalytic activity of ternary Ag@WO3@rGO nanocomposites under solar simulation irradiation. J. Sci. Adv. Mater. Devices 2021, 6, 108–117. [Google Scholar] [CrossRef]
- Nguyen, N.T.A.; Kim, H. Ag3PO4-Deposited TiO2@Ti3C2 Petals for Highly Efficient Photodecomposition of Various Organic Dyes under Solar Light. Nanomaterials 2022, 12, 2464. [Google Scholar] [CrossRef]
- Kwon, D.; Kim, J. Silver-doped ZnO for photocatalytic degradation of methylene blue. Korean J. Chem. Eng. 2020, 37, 1226–1232. [Google Scholar] [CrossRef]
- Yu, J.; Kim, J. Synthesis and Characterization of ZnO Doped with Gold Nanoparticles for Improved Photocatalytic Activity. Sci. Adv. Mater. 2021, 13, 944–948. [Google Scholar] [CrossRef]
- Won, S.; Kim, J.-S. Spinach Extract Derived Carbon Dots Decorated on ZnO Nanorods for Photocatalytic Dye Degradation. Sci. Adv. Mater. 2021, 13, 922–926. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Tran, V.V.; Bui, V.K.H.; Kim, M.; Park, D.; Hur, J.; Kim, I.T.; Lee, H.U.; Ko, S.; Lee, Y.C. A Novel Photocatalyst Composite of Magnesium Aminoclay and TiO2 Immobilized into Activated Carbon Fiber (ACF) Matrix for Pollutant Removal. J. Nanosci. Nanotechnol. 2020, 20, 6844–6849. [Google Scholar] [CrossRef] [PubMed]
- Das, G.S.; Bhatnagar, A.; Yli-Pirila, P.; Tripathi, K.M.; Kim, T. Sustainable nitrogen-doped functionalized graphene nanosheets for visible-light-induced photocatalytic water splitting. Chem. Commun. 2020, 56, 6953–6956. [Google Scholar] [CrossRef]
- Park, S.J.; Das, G.S.; Schütt, F.; Adelung, R.; Mishra, Y.K.; Tripathi, K.M.; Kim, T. Visible-light photocatalysis by carbon-nano-onion-functionalized ZnO tetrapods: Degradation of 2,4-dinitrophenol and a plant-model-based ecological assessment. NPG Asia Mater. 2019, 11, 8. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Kim, J. Shape-engineered carbon quantum dots embedded on CdS-nanorods for enhanced visible light harvesting towards photocatalytic application. Appl. Surf. Sci. 2021, 552, 149372. [Google Scholar] [CrossRef]
- Kadam, A.N.; Bathula, C.; Lee, S.-W. In situ growth of 1D/2D CdS–Bi2MoO6 core shell heterostructures for synergistic enhancement of photocatalytic performance under visible light. Chemosphere 2021, 275, 130086. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, Y.; Bathula, C.; Kadam, A.N.; Lee, S.W. A zero-dimensional/two-dimensional Ag-Ag2S-CdS plasmonic nanohybrid for rapid photodegradation of organic pollutant by solar light. Chemosphere 2022, 296, 133973. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, T.; Kadam, A.N.; Bathula, C.; Ghfar, A.A.; Kim, H.; Lee, S.-W. Synergistic photocatalysis of Z-scheme type Fe2O3/g-C3N4 heterojunction coupled with reduced graphene oxide. Surf. Interfaces 2022, 30, 101910. [Google Scholar] [CrossRef]
- Nam, N.N.; Bui, T.L.; Ho, N.T.; Son, S.J.; Joo, S.-W. Controlling Photocatalytic Reactions and Hot Electron Transfer by Rationally Designing Pore Sizes and Encapsulated Plasmonic Nanoparticle Numbers. J. Phys. Chem. C 2019, 123, 23497–23504. [Google Scholar] [CrossRef]
- Rhimi, B.; Wang, C.; Bahnemann, D.W. Latest progress in g-C3N4 based heterojunctions for hydrogen production via photocatalytic water splitting: A mini review. J. Phys. Energy 2020, 2, 042003. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Ghandi, K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)-Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials 2022, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.A.; Shrestha, N.K.; Hussain, S.; Jung, J.; Lee, S.-W.; Bathula, C.; Kadam, A.N.; Im, H.; Kim, H. Catalytic decontamination of organic/inorganic pollutants in water and green H2 generation using nanoporous SnS2 micro-flower structured film. J. Hazard. Mater. 2021, 417, 126105. [Google Scholar] [CrossRef] [PubMed]
- Kadam, A.N.; Moniruzzaman, M.; Lee, S.W. Dual Functional S-Doped g-C3N4 Pinhole Porous Nanosheets for Selective Fluorescence Sensing of Ag+ and Visible-Light Photocatalysis of Dyes. Molecules 2019, 24, 450. [Google Scholar] [CrossRef] [PubMed]
- Kadam, A.N.; Kim, H.; Lee, S.-W. Low-temperature in situ fabrication of porous S-doped g-C3N4 nanosheets using gaseous-bubble template for enhanced visible-light photocatalysis. Ceram. Int. 2020, 46, 28481–28489. [Google Scholar] [CrossRef]
- Lee, S.-W.; Ahn, K.-S.; Zhu, K.; Neale, N.R.; Frank, A.J. Effects of TiCl4 Treatment of Nanoporous TiO2 Films on Morphology, Light Harvesting, and Charge-Carrier Dynamics in Dye-Sensitized Solar Cells. J. Phys. Chem. C 2012, 116, 21285–21290. [Google Scholar] [CrossRef]
- Girish, Y.R.; Udayabhanu; Alnaggar, G.; Hezam, A.; Nayan, M.B.; Nagaraju, G.; Byrappa, K. Facile and rapid synthesis of solar-driven TiO2/g-C3N4 heterostructure photocatalysts for enhanced photocatalytic activity. J. Sci. Adv. Mater. Devices 2022, 7, 100419. [Google Scholar] [CrossRef]
- Ni, B.; Jiang, H.; Guo, W.; Xu, Q.; Min, Y. Tailoring the oxidation state of metallic TiO through Ti3+/Ti2+ regulation for photocatalytic conversion of CO2 to C2H6. Appl. Catal. B Environ. 2022, 307, 121141. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Bao, J.; Fang, J.; Zhao, S.; Zhang, Y.; Sheng, X.; Chen, W. Structure regulation of ZnS@g-C3N4/TiO2 nanospheres for efficient photocatalytic H2 production under visible-light irradiation. Chem. Eng. J. 2018, 346, 226–237. [Google Scholar] [CrossRef]
- Martha, S.; Nashim, A.; Parida, K.M. Facile synthesis of highly active g-C3N4 for efficient hydrogen production under visible light. J. Mater. Chem. A 2013, 1, 7816. [Google Scholar] [CrossRef]
- Wu, M.; Yan, J.-M.; Zhang, X.-w.; Zhao, M. Synthesis of g-C3N4 with heating acetic acid treated melamine and its photocatalytic activity for hydrogen evolution. Appl. Surf. Sci. 2015, 354, 196–200. [Google Scholar] [CrossRef]
- Alcudia-Ramos, M.A.; Fuentez-Torres, M.O.; Ortiz-Chi, F.; Espinosa-González, C.G.; Hernández-Como, N.; García-Zaleta, D.S.; Kesarla, M.K.; Torres-Torres, J.G.; Collins-Martínez, V.; Godavarthi, S. Fabrication of g-C3N4/TiO2 heterojunction composite for enhanced photocatalytic hydrogen production. Ceram. Int. 2020, 46, 38–45. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Wang, D.; Li, D.; Ke, J.; Wang, S.; Liu, S.; Xiao, H.; Wang, R. Highly Dispersed NiCo2O4 Nanodots Decorated Three-Dimensional g-C3N4 for Enhanced Photocatalytic H2 Generation. ACS Sustain. Chem. Eng. 2019, 7, 12428–12438. [Google Scholar] [CrossRef]
- Zhu, B.; Xia, P.; Ho, W.; Yu, J. Isoelectric point and adsorption activity of porous g-C3N4. Appl. Surf. Sci. 2015, 344, 188–195. [Google Scholar] [CrossRef]
- Niu, P.; Yin, L.C.; Yang, Y.Q.; Liu, G.; Cheng, H.M. Increasing the visible light absorption of graphitic carbon nitride (melon) photocatalysts by homogeneous self-modification with nitrogen vacancies. Adv. Mater. 2014, 26, 8046–8052. [Google Scholar] [CrossRef] [PubMed]
- Kadam, A.N.; Salunkhe, T.T.; Kim, H.; Lee, S.-W. Biogenic synthesis of mesoporous N–S–C tri-doped TiO2 photocatalyst via ultrasonic-assisted derivatization of biotemplate from expired egg white protein. Appl. Surf. Sci. 2020, 518, 146194. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Low, J.; Xiao, W. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 2013, 15, 16883–16890. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Jeong, S.-Y.; Son, Y.-D.; Lee, S.-W. Facile Fabrication of TiO2 Quantum Dots-Anchored g-C3N4 Nanosheets as 0D/2D Heterojunction Nanocomposite for Accelerating Solar-Driven Photocatalysis. Nanomaterials 2023, 13, 1565. https://doi.org/10.3390/nano13091565
Lee J-H, Jeong S-Y, Son Y-D, Lee S-W. Facile Fabrication of TiO2 Quantum Dots-Anchored g-C3N4 Nanosheets as 0D/2D Heterojunction Nanocomposite for Accelerating Solar-Driven Photocatalysis. Nanomaterials. 2023; 13(9):1565. https://doi.org/10.3390/nano13091565
Chicago/Turabian StyleLee, Jin-Hyoek, Sang-Yun Jeong, Young-Don Son, and Sang-Wha Lee. 2023. "Facile Fabrication of TiO2 Quantum Dots-Anchored g-C3N4 Nanosheets as 0D/2D Heterojunction Nanocomposite for Accelerating Solar-Driven Photocatalysis" Nanomaterials 13, no. 9: 1565. https://doi.org/10.3390/nano13091565
APA StyleLee, J.-H., Jeong, S.-Y., Son, Y.-D., & Lee, S.-W. (2023). Facile Fabrication of TiO2 Quantum Dots-Anchored g-C3N4 Nanosheets as 0D/2D Heterojunction Nanocomposite for Accelerating Solar-Driven Photocatalysis. Nanomaterials, 13(9), 1565. https://doi.org/10.3390/nano13091565






