Application of Inorganic Nanomaterials in Cultural Heritage Conservation, Risk of Toxicity, and Preventive Measures
Abstract
1. Introduction
2. Deterioration of Cultural Heritage Materials
Background of the Nanotechnology in Cultural Heritage Conservation
3. Risk of Toxicity during Handling with Nanomaterials in Conservation Procedures
4. Interaction of Nanomaterials with the Human Body
4.1. Critical Particle Properties
4.1.1. Particle Properties
4.1.2. Differences in Surface Roughness
4.1.3. Nanofibers
4.1.4. Physical Chemical Properties
4.1.5. Structure and Defect
4.2. Deposition Mechanisms of Nanomaterials
4.2.1. Access through the Nasal Route
4.2.2. Access to Circulatory and Cardiovascular Systems
4.2.3. Access to the Brain through the Olfactory Way
4.2.4. Access through the Eyes and Tear Ducts
4.2.5. Effects of Nanoparticles in the Eyes
4.2.6. Oral Access and Gastrointestinal Region Interactions
Access to the Liver
4.2.7. Access to the Urinary Track
4.2.8. Access through the Skin
| Access Route | Translocation Mechanisms | Affected Organs |
|---|---|---|
| Nasal |
| Nose, pharynx, larynx, trachea, lungs: bronchiolar and alveolar region [270,276] |
| Access to circulatory and cardiovascular systems |
| Hearth, cardio-pulmonary organs, lymphatic system, placentary blood vessels, and fetus body in pregnant women [197]. |
| Access through the olfactory way | Translocation through the olfactory nerves to the brain | Brain [279,280] |
| Access through the eyes and tear ducts |
| Eyes: Retina and cornea [283] Brain and central nervous system [281,282] |
| Oral and gastrointestinal region interactions |
| Gastrointestinal organs Stomach, liver, pancreas, large and small intestine [287] |
| Access to the urinary track |
| Kidneys, urinary bladder [289] |
| Access through the skin | Internalization through the hair follicles pores and wounds | Epidermis, dermis, sweat gland [292] |
5. Diagnostic Tools
5.1. Particle Size Measurement by Optical Methods
5.2. Morphological, Chemical, and Structural Properties by Microscopic Techniques
6. Main Applications of Nanomaterials in Protection and Restoration Processes and Risks of Toxicity
6.1. Consolidant Nanomaterials
6.1.1. Calcium Hydroxide
6.1.2. Magnesium Hydroxide
6.1.3. Calcium Phosphates
Calcium Carbonate Phosphates and Other Ion Substitutions
6.2. Protective Treatments Using Hydrophobic Coatings
Amorphous Nanosilica
6.3. Self-Cleaning and Biocides
6.3.1. Titanium Dioxide
6.3.2. Zinc Oxide
6.3.3. Silver
6.3.4. Gold
6.3.5. Platinum
6.3.6. Copper
6.4. Multifunctional Properties of Carbon Compounds (Nanotubes, Nanowires, Nanorods)
6.5. Fire Retardants
6.5.1. Magnesium Hydroxide–CNT Combinations
6.5.2. Nano Aluminum Hydroxide
6.5.3. Nanoclays
6.6. Hybrid Nanomaterials and Nanocomposites
| Product | Properties | Reported Toxicity | |
|---|---|---|---|
| Consolidants (artworks, calcareous materials, mortars) | Ca(OH)2 | Stone: [1,15,16,91,94,95,96,323] Artworks [4] | Dermatitis, skin burns [333], eye injuries [334], DNA damage [336], Lung diseases [338]. |
| Mg(OH)2 | [444] | Skin burns and eye injuries [342] | |
| Mg(OH)2 CNT | [118] | Not reported | |
| Stony materials consolidants | Hydroxiapatite Brushite Calcium carbonate phosphates (with or without metallic derivatives) | [348] [358] [357,358] | Kidney stones, nephroliiasis [359] Ectopic calcifications and an increase in arthritis and arteriosclerosis [359] Kidney stones, hyperlipidemia [359] |
| Hydrophobic/consolidant | Amorphous SiO2 | [1,10,12,15,16,18,153] | Inflammatory processes in the lung submucosal cells [364,365,366] |
| Biocides: self cleaning | TiO2 | [369,371,372] | DNA damage, lung diseases, carcinogenic by inhalation, fetus damage [45,48,49,50,378,379] |
| Zn oxide | [107,381,382] | Neurotoxicity [386], hepatic/embryonic cytotoxicity, genotoxicity [387] | |
| Biocides: self cleaning | Silver | [12,401,402,403,404,405] | Diabetes, hyperlipidemia, hypertension [289,407,408] |
| Gold | [411,412] | Oxidative stress in the liver, leukemia, lung fibroblasts, or spermatozoa modifications [416] | |
| TiO2-SiO2-Au | [413] | Not reported | |
| Au-HAP | [414] | Not reported | |
| Platinum | [417,418] | Hepatotoxicity, nephrotoxicity, DNA damage [422]. | |
| Pt/MWCNT | [1] | Not reported | |
| Platinum/silver | [421] | Not reported | |
| Copper | [1,15,16,424,425,426] | Neurodegenerative disorders [427] | |
| Hydrophobic, antimicrobial, consolidant strengthener | Carbon compounds (nanotubes, nanowires, nanorods) | Super-hydrophobic [1] Mechanical properties strengthener [1] Gas permeable membranes [1] | Atherosclerosis, blood alteration [439] Heart, alveolar, and intra-tracheal damage [439], Renal and liver damage [439] Inflammation, apoptosis, and oxidative stress in the brain [442] DNA damage, oxidative stress, or chromosome alterations [443] |
| Hydrophilic | Magnesium hydroxide | [1,2,13,15,16] | Skin burns and eye injuries [342] |
| Mg(OH)2/CNT | [118,449] | Not reported | |
| Aluminum hydroxide | [176,450] | Embryo/fetal toxicity [451], dermal damage [452] | |
| Nanoclays with polymers | [176,450] | Pulmonar inflammation [453] | |
| Montmorillonite | [176] | Cytotoxicity [454], intestinal damage [455] | |
| Protective treatments: hydrophobicity and self-cleaning | Hybrid nanomaterials and nanocomposites | [8,176,456,457,458] | Ecosystem damage [465] |
| TEOS/FOTCS/TiO2 [457] | Not reported | ||
| Silver/TiO2 nanocomposites [105]. | Not reported | ||
| Silver/TiO2/SiO2 [460] | Not reported | ||
| Citrate-stabilized silver/TiO2 nanocomposites [103]. | Not reported |
7. Role of International Organizations in the Control of Exposure to Nanomaterials and the Assessment of the Degree of Toxicity
- Danish Environmental Protection Agency Denmark (NANORISKCAT NRC) [478]
- France (French Agency for Food, Environmental, and Occupational Health & Safety ANSES 2008-INRS) [479]
- Germany Bundesanstalt für Arbeitsschutz und Arbeitsmedizin BAUA, German institute for Standardization -DIN eV, -Federal Institute for Materials Research and Testing (BAM)) [480]
- Italy (INAIL 2011), Italian National Institute for Occupational Safety and Prevention, Department of Occupational Medicine Italy [481]
- Switzerland Bundesamt für Gesundheit (BAG) (INFONANO), nanotechnology [484]
7.1. Qualitative Evaluation Methods
7.1.1. Control Banding Nanotool
7.1.2. Stoffenmanager Nano
7.2. Semiquantitative Method: NEAT
7.3. Dose Control
8. Recommendations for the Proper Handling and Storage of Nanomaterials: State of the Art
- Identify sources of potential ENM exposures
- Establish similar exposure groups by area or job tasks where workers may be exposed
- Characterize exposures of all potentially exposed workers
- Assess the effectiveness of engineering controls, work practices, personal protective Equipment (PPE), training, and other factors used to reduce or eliminate potential exposures.
- Develop an exposure assessment strategy.
- Identify areas and tasks that are more likely to emit engineered nanomaterials, such as handling dry powders or the sonication of liquids. The use of direct reading instruments may assist with identifying these work areas.
- Collect personal breathing zone (PBZ) samples for the worker’s full shift to determine adherence to the applicable REL.
- Collect area samples using filter-based samples at indoor locations both in near proximity to and removed from the use of the engineered nanomaterials of interest to determine product migration and the extent of any cross-contamination (from production to non-production work areas) from work practices or improperly designed high vacuum or other ventilation systems.
- Use task-specific short-term PBZ and area sampling to identify those tasks that are more likely to emit engineered nanomaterials.
- Consult with the analytical laboratory to evaluate detection limits and sample time/volumes to achieve a sensitive enough measurement.
- Any situation in which nanomaterials may become airborne, such as the loading and unloading of nanomaterials or chemicals containing nanomaterials into/from milling or mixing equipment, the filling of chemicals into containers, the sampling of manufactured chemicals, and the opening of systems for product retrieval.
- The cleaning and maintenance of installations (including closed production systems) and of risk reduction equipment, such as filters in local exhaust ventilation systems.
- The research and development of nanomaterial-containing substances, such as composite materials.
- Handling powders and spraying mixtures containing nanomaterials. Powders are likely to have an increased risk of explosion, self-ignition, and electrostatic charging, giving rise to safety concerns. In addition, they may form dust clouds, leading to inhalation exposure.
- Mechanical or thermal treatment of items containing nanomaterials that could release because of these processes (e.g., laser treatment, grinding, or cutting).
- Waste treatment operations involving items containing nanomaterials.
- Prevent inhalation exposure
- Prevent dermal exposure
- Prevent laboratory contamination
- Prevent exposure during spills
- Obtain current toxicity information on nanomaterials in use.
8.1. Personal Protective Equipment (PPE)
8.1.1. Protective Clothing
8.1.2. Respiratory Protective Masks
8.1.3. Hand and Arm Protection
8.1.4. Eye Protection
8.2. Laboratory Adaptation for Nanomaterials Processing and Storage
8.2.1. Ventilation in Workplaces
- (1)
- Extraction cabin.
- (2)
- Conduit that transports the contaminant along the extraction tube.
- (3)
- Fan that moves air through the exhaust system.
- (4)
- Smoke outlet where the system discharges the air.
- E10 > 85% efficiency, <15% penetration (integral value)
- E11 > 95% efficiency, <5% penetration (integral value)
- E12 > 99.5% efficiency, 0.5% penetration (integral value)
- HEPA 13 > 99.95% efficiency, <0.05% penetration (integral values); >99.75% efficiency, <0.25% penetration (local values)
- HEPA 14 > 99.995% retention, <0.005% penetration ((integral values); 99.975% retention, <0.025% penetration (local values)
- U15 > 99.995% efficiency, 0.0005% penetration (integral values); >99.975% efficiency, 0.0025% penetration (local values)
- U16 > 99.9995% efficiency, 0.00005% penetration (integral value); >99.99975% efficiency, 0.00025% penetration (local values)
- U17 > 99.99995% efficiency, 0.000005% penetration (integral value); >99.9999% efficiency, 0.0001% penetration (local values)
- Super-Low-Penetrating Air filter with a minimum efficiency of 99.9999% on 0.12 µm particles (added later).
8.2.2. Organizational Measures in the Workplace: Labeling and Specifications
8.2.3. Nanoparticulate Waste Management
- Classify the waste within the families previously established or create a new one, taking into account the characteristics of the waste both for containing nanomaterials (solid, slurry, liquid) as well as by the composition of the dissolved medium (solvents, epoxies) and its shape.
- A suitable container for the waste, which is required to be unbreakable, allows for an airtight seal; in eventual cases, the recommendation is to provide a second container according to the circumstances [198].
- If the residue consists of easily dispersed dust in the air, it must adopt additional measures, such as the case of filling the container. This process must always be carried out within collective protection that acts on the focus and establishes a minimum time settlement of the dust generated inside the container. It can oscillate between half an hour and two hours, while the other option is to use a single-use container.
- Label the container with the information associated with the risk of the collected waste.
- Mark the container with a pictogram indicating the presence of nanomaterials and the risk associated with hazardous chemical agents.
- Establish a temporary storage point enabled in this regard and comply with the table storage incompatibility established until its withdrawal by the authorized manager.
- Establish the safety conditions and the mandatory PPE for handling and action in emergencies. For instance, in cases of cleaning spillages, there cannot be brushing, compressed air cleaning, or traditional vacuum cleaners aspirating in the workplace. In the last case, the recommendation is always to use vacuum cleaners including HEPA-filters [205].
9. Current Status of Regulations on the Protection of Cultural Heritage
- Nanoform and characteristics that can influence (eco)toxicity and environmental exposure.
- Do not solely use molecular structural similarities to justify grouping different nanoforms together.
- Justify the relevance of the safety information provided for all registered nanoforms.
- Document the safety of all registered nanoforms throughout the life cycle.
- Provide information about test conditions and tested nanoforms.
- Fulfill specific ecotoxicity-related test requirements for different nanoforms depending on their dissolution and solubility.
- Comply with specific testing requirements related to toxicity for different nanoforms depending on their nature and likely route of exposure.
- Consider multiple reporting metrics of results for nanoforms that are hazardous.
- Justify waiving information requirements.
- Propose additional testing and/or comply with ECHA testing requirements.
10. Preventive Measures during Conservation Treatments of Cultural Heritage
11. Final Considerations and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- David, M.E.; Ion, R.-M.; Grigorescu, R.M.; Iancu, L.; Andrei, E.R. Nanomaterials Used in Conservation and Restoration of Cultural Heritage: An Up-to-Date Overview. Materials 2020, 13, 2064. [Google Scholar] [CrossRef] [PubMed]
- Girginova, P.I.; Galacho, C.; Veiga, R.; Silva, A.S.; Candeias, A. Inorganic Nanomaterials for Restoration of Cultural Heritage: Synthesis Approaches towards Nanoconsolidants for Stone and Wall Paintings. Chemsuschem 2018, 11, 4168–4182. [Google Scholar] [CrossRef] [PubMed]
- Mohajerani, A.; Burnett, L.; Smith, J.V.; Kurmus, H.; Milas, J.; Arulrajah, A.; Horpibulsuk, S.; Abdul Kadir, A. Nanoparticles in Construction Materials and Other Applications, and Implications of Nanoparticle Use. Materials 2019, 12, 3052. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, P.; Chelazzi, D.; Giorgi, R.; Poggi, G. Colloid and materials science for the conservation of cultural heritage: Cleaning, consolidation, and deacidification. Langmuir 2013, 29, 5110–5122. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.A.M. Possibilities application of nanoscience and nanotechnology in conservation of archaeological wood: A review. Jokull J. 2013, 63, 9–19. [Google Scholar]
- Shroff, A.; Karolia, A.; Dolez, P.I. Nanotechnology-Based Interventions in Museum Textiles. Handb. Mus. Text. 2022, 2, 345–359. [Google Scholar]
- Fierascu, R.C.; Doni, M.; Fierascu, I. Selected Aspects Regarding the Restoration/Conservation of Traditional Wood and Masonry Building Materials: A Short Overview of the Last Decade Findings. Appl. Sci. 2020, 10, 1164. [Google Scholar] [CrossRef]
- Zarzuela, R.; Luna, M.; Carrascosa, L.A.; Mosquera, M.J. Preserving Cultural Heritage Stone: Innovative Consolidant, Superhydrophobic, Self-Cleaning, and Biocidal Products. In Advanced Materials for the Conservation of Stone; Springer: Cham, Switzerland, 2018; pp. 259–275. [Google Scholar] [CrossRef]
- Cappelletti, G.; Fermo, P. Hydrophobic and superhydrophobic coatings for limestone and marble conservation. In Smart Composite Coatings and Membranes; Woodhead Publishing: Sawston, UK, 2016; pp. 421–452. [Google Scholar] [CrossRef]
- Stefanidou, M.; Matziaris, K.; Karagiannis, G. Hydrophobization by Means of Nanotechnology on Greek Sandstones Used as Building Facades. Geosciences 2013, 3, 30–45. [Google Scholar] [CrossRef]
- Fornari, A.; Rossi, M.; Rocco, D.; Mattiello, L. A Review of Applications of Nanocellulose to Preserve and Protect Cultural Heritage Wood, Paintings, and Historical Papers. Appl. Sci. 2022, 12, 12846. [Google Scholar] [CrossRef]
- Kakakhel, M.A.; Wu, F.; Gu, J.-D.; Feng, H.; Shah, K.; Wang, W. Controlling biodeterioration of cultural heritage objects with biocides: A review. Int. Biodeterior. Biodegrad. 2019, 143, 104721. [Google Scholar] [CrossRef]
- Lazar, S.T.; Kolibaba, T.J.; Grunlan, J.C. Flame-retardant surface treatments. Nat. Rev. Mater. 2020, 5, 259–275. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Price, D. (Eds.) Advances in Fire Retardant Materials; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 9781845695071. [Google Scholar]
- Becerra, J.; Zaderenko, A.P.; Gomez-Moron, M.A.; Ortiz, P. Nanoparticles applied to stone buildings. Int. J. Archit. Herit. 2021, 15, 1320–1335. [Google Scholar] [CrossRef]
- Sierra-Fernandez, A.; Gomez-Villalba, L.S.; Rabanal, M.E.; Fort, R. New Nanomaterials for Applications in Conservation and Restoration of Stony Materials: A Review. Mater. Constr. 2017, 67, 107–125. [Google Scholar] [CrossRef]
- Stefanidou, M.; Tsardaka, E.C.; Pavlidou, E. Influence of nano-silica and nano-alumina in lime-pozzolan and lime-metakaolin binders. Mater. Today Proc. 2017, 4, 6908–6922. [Google Scholar] [CrossRef]
- Ziegenbalg, G.; Drdácký, M.; Dietze, C.; Schuch, D. (Eds.) Nanomaterials in Architecture and Art Conservation; Pan Stanford Publishing: Singapore, 2018. [Google Scholar]
- Carvajal-Perez, A. New Advances in the Use of Multifunctional Nanomaterials in Conservation-Restoration of Artistic and Archaeological Heritage. Solid State Phenom. 2019, 286, 75–94. [Google Scholar] [CrossRef]
- Kotsidi, M.; Gorgolis, G.; Carbone, M.G.P.; Anagnostopoulos, G.; Paterakis, G.; Poggi, G.; Manikas, A.; Trakakis, G.; Baglioni, P.; Galiotis, C. Preventing colour fading in artworks with graphene veils. Nat. Nanotechnol. 2021, 16, 1004–1010. [Google Scholar] [CrossRef]
- Gavrilescu, C.M.; Paraschiv, C.; Horjinec, P.; Sotropa, D.M.; Barbu, R.M. The advantages and disadvantages of nanotechnology. Rom. J. Oral Rehabil. 2018, 10, 153–159. [Google Scholar]
- Maimon, O.; Browarnik, A. NHECD—Nano Health and Environmental Commented Database. In Data Mining and Knowledge Discovery Handbook; Springer: Boston, MA, USA, 2010; pp. 1221–1241. [Google Scholar] [CrossRef]
- Juganson, K.; Ivask, A.; Blinova, I.; Mortimer, M.; Kahru, A. NanoE-Tox: New and in-depth database concerning ecotoxicity of nanomaterials. Beilstein J. Nanotechnol. 2015, 6, 1788–1804. [Google Scholar] [CrossRef]
- Wheeler, R.M.; Lower, S.K. A meta-analysis framework to assess the role of units in describing nanoparticle toxicity. Nanoimpact 2021, 21, 100277. [Google Scholar] [CrossRef]
- Roca, C.P.; Rallo, R.; Fernández, A.; Giralt, F. Nanoinformatics for safe-by-design engineered nanomaterials. In Towards Efficient Designing of Safe Nanomaterials: Innovative Merge of Computational Approaches and Experimental Techniques; Royal Society of Chemistry: London, UK, 2012; pp. 89–107. [Google Scholar]
- Sharma, V.K.; Filip, J.; Zboril, R.; Varma, R.S. Natural inorganic nanoparticles–formation, fate, and toxicity in the environment. Chem. Soc. Rev. 2015, 44, 8410–8423. [Google Scholar] [CrossRef]
- Peyravi, M.; Khalili, S.; Jahanshahi, M.; Zakeritabar, S.F. Ecotoxic Effect of Photocatalytic Active Nanoparticles on Human Health and the Environment. In Microbial Nanobionics; Springer: Cham, Switzerland, 2019; pp. 145–168. [Google Scholar]
- Ray, P.C.; Yu, H.; Fu, P.P. Toxicity and Environmental Risks of Nanomaterials: Challenges and Future Needs. J. Environ. Sci. Health Part C 2009, 27, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Borm, P.J.A. Particle toxicology: From coal mining to nanotechnology. Inhal. Toxicol. 2002, 14, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Khosravi-Katuli, K.; Prato, E.; Lofrano, G.; Guida, M.; Vale, G.; Libralato, G. Effects of nanoparticles in species of aquaculture interest. Environ. Sci. Pollut. Res. 2017, 24, 17326–17346. [Google Scholar] [CrossRef] [PubMed]
- Lehutso, R.; Tancu, Y.; Maity, A.; Thwala, M. Aquatic toxicity of transformed and product-released engineered nanomaterials: An overview of the current state of knowledge. Process Saf. Environ. Prot. 2020, 138, 39–56. [Google Scholar] [CrossRef]
- Turk, J.; Pranjić, A.M.; Hursthouse, A.; Turner, R.; Hughes, J.J. Decision support criteria and the development of a decision support tool for the selection of conservation materials for the built cultural heritage. J. Cult. Heritage 2019, 37, 44–53. [Google Scholar] [CrossRef]
- Batool, M.; Zafar, M.N.; Nazar, M.F. General regulations for safe handling of manufactured nanomaterials. In Nanomaterials Recycling; Elsevier: Amsterdam, The Netherlands, 2022; pp. 61–82. [Google Scholar]
- Suresh, J.I.; Judith, A. Hazardous and Safety and Management of Nanomaterials for the Personal Health and Environment. In Nanotechnology for Environmental Pollution Decontamination; Apple Academic Press: New York, NY, USA, 2023; pp. 487–494. [Google Scholar]
- Hayes, A.W.; Sahu, S.C. Genotoxicity of engineered nanomaterials found in the human environment. Curr. Opin. Toxicol. 2020, 19, 68–71. [Google Scholar] [CrossRef]
- Ren, C.; Hu, X.; Zhou, Q. Influence of environmental factors on nanotoxicity and knowledge gaps thereof. Nanoimpact 2016, 2, 82–92. [Google Scholar] [CrossRef]
- Hu, X.; Li, D.; Gao, Y.; Mu, L.; Zhou, Q. Knowledge gaps between nanotoxicological research and nanomaterial safety. Environ. Int. 2016, 94, 8–23. [Google Scholar] [CrossRef]
- Dugershaw, B.B.; Aengenheister, L.; Hansen, S.S.K.; Hougaard, K.S.; Buerki-Thurnherr, T. Recent insights on indirect mechanisms in developmental toxicity of nanomaterials. Part. Fibre Toxicol. 2020, 17, 1–22. [Google Scholar] [CrossRef]
- Bellagamba, I.; Boccuni, F.; Ferrante, R.; Tombolini, F.; Marra, F.; Sarto, M.S.; Iavicoli, S. Workers’ Exposure Assessment during the Production of Graphene Nanoplatelets in R&D Laboratory. Nanomaterials 2020, 10, 1520. [Google Scholar] [CrossRef]
- Nafisi, S.; Maibach, H.I. Nanotechnology in cosmetics. In Cosmetic Science and Technology: Theoretical Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2017; Volume 337. [Google Scholar]
- Sen, D.; Patil, V.; Smriti, K.; Varchas, P.; Ratnakar, R.; Naik, N.; Shetty, D.K.; Kapoor, S. Nanotechnology and Nanomaterials in Dentistry: Present and Future Perspectives in Clinical Applications. Eng. Sci. 2022, 20, 14–24. [Google Scholar] [CrossRef]
- Chen, Z.; Han, S.; Zhou, S.; Feng, H.; Liu, Y.; Jia, G. Review of health safety aspects of titanium dioxide nanoparticles in food application. Nanoimpact 2020, 18, 100224. [Google Scholar] [CrossRef]
- Al Bakri, H.; Abu Elhaija, W.; Al Zyoud, A. Solar photovoltaic panels performance improvement using active self-cleaning nanotechnology of SurfaShield G. Energy 2021, 223, 119908. [Google Scholar] [CrossRef]
- Boro, B.; Gogoi, B.; Rajbongshi, B.M.; Ramchiary, A. Nano-structured TiO2/ZnO nanocomposite for dye-sensitized solar cells application: A review. Renew. Sustain. Energy Rev. 2018, 81, 2264–2270. [Google Scholar] [CrossRef]
- Luo, Z.; Li, Z.; Xie, Z.; Sokolova, I.M.; Song, L.; Peijnenburg, W.J.G.M.; Hu, M.; Wang, Y. Rethinking nano-TiO2 safety: Overview of toxic effects in humans and aquatic animals. Small 2020, 16, 2002019. [Google Scholar] [CrossRef]
- Nohynek, G.J.; Dufour, E.K. Nano-sized cosmetic formulations or solid nanoparticles in sunscreens: A risk to human health? Arch. Toxicol. 2012, 86, 1063–1075. [Google Scholar] [CrossRef]
- Pelclova, D.; Navratil, T.; Kacerova, T.; Zamostna, B.; Fenclova, Z.; Vlckova, S.; Kacer, P. NanoTiO2 Sunscreen Does Not Prevent Systemic Oxidative Stress Caused by UV Radiation and a Minor Amount of NanoTiO2 is Absorbed in Humans. Nanomaterials 2019, 9, 888. [Google Scholar] [CrossRef]
- Pelclova, D.; Barosova, H.; Kukutschova, J.; Zdimal, V.; Navratil, T.; Fenclova, Z.; Vlckova, S.; Schwarz, J.; Zikova, N.; Kacer, P.; et al. Raman microspectroscopy of exhaled breath condensate and urine in workers exposed to fine and nano TiO2 particles: A cross-sectional study. J. Breath Res. 2015, 9, 036008. [Google Scholar] [CrossRef]
- Hou, J.; Wang, L.; Wang, C.; Zhang, S.; Liu, H.; Li, S.; Wang, X. Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J. Environ. Sci. 2019, 75, 40–53. [Google Scholar] [CrossRef]
- Dréno, B.; Alexis, A.; Chuberre, B.; Marinovich, M. Safety of titanium dioxide nanoparticles in cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 34–46. [Google Scholar] [CrossRef]
- Benavente, D.; De Jongh, M.; Cañaveras, J.C. Weathering Processes and Mechanisms Caused by Capillary Waters and Pigeon Droppings on Porous Limestones. Minerals 2020, 11, 18. [Google Scholar] [CrossRef]
- Pinker, F. The effect of anthropogenic actions on the weathering of porous building stones–examples from the austrian conservation practice. Archeometriai Műhely 2020, 17, 3. [Google Scholar]
- Savković, Ž.; Stupar, M.; Unković, N.; Knežević, A.; Vukojević, J.; Ljaljević Grbić, M. Fungal Deterioration of Cultural Heritage Objects. In Biodegradation Technology of Organic and Inorganic Pollutants; Mendes, K.F., de Sousa, R.N., Mielke, K.C., Eds.; IntechOpen: London, UK, 2021; pp. 267–288. [Google Scholar]
- Bethencourt, M.; Fernández-Montblanc, T.; Izquierdo, A.; González-Duarte, M.M.; Muñoz-Mas, C. Study of the influence of physical, chemical and biological conditions that influence the deterioration and protection of Underwater Cultural Heritage. Sci. Total. Environ. 2018, 613–614, 98–114. [Google Scholar] [CrossRef]
- Burns, G. Deterioration of our cultural heritage. Nature 1991, 352, 658–660. [Google Scholar] [CrossRef]
- Pedersen, N.B.; Matthiesen, H.; Blanchette, R.A.; Alfredsen, G.; Held, B.W.; Westergaard-Nielsen, A.; Hollesen, J. Fungal attack on archaeological wooden artefacts in the Arctic—Implications in a changing climate. Sci. Rep. 2020, 10, 14577. [Google Scholar] [CrossRef] [PubMed]
- Camuffo, D.; Bertolin, C. Unfavorable microclimate conditions in exhibition rooms: Early detection, risk identification, and preventive conservation measures. J. Paleontol. Tech. 2016, 15, 144–161. [Google Scholar]
- Török, Á.; Licha, T.; Simon, K.; Siegesmund, S. Urban and rural limestone weathering; the contribution of dust to black crust formation. Environ. Earth Sci. 2011, 63, 675–693. [Google Scholar] [CrossRef]
- Eyssautier-Chuine, S.; Marin, B.; Thomachot-Schneider, C.; Fronteau, G.; Schneider, A.; Gibeaux, S.; Vazquez, P. Simulation of acid rain weathering effect on natural and artificial carbonate stones. Environ. Earth Sci. 2016, 75, 1–19. [Google Scholar] [CrossRef]
- Grossi, C.M.; Brimblecombe, P. The effect of atmospheric pollution on building materials. Le J. Phys. Colloq. 2002, 12, 197–210. [Google Scholar] [CrossRef]
- Sabbioni, C. Mechanisms of air pollution damage to stone. Eff. Air Pollut. Built Environ. 2003, 2, 63–88. [Google Scholar]
- Prieto-Taboada, N.; Maguregui, M.; Martinez-Arkarazo, I.; Olazabal, M.A.; Arana, G.; Madariaga, J.M. Spectroscopic evaluation of the environmental impact on black crusted modern mortars in urban–industrial areas. Anal. Bioanal. Chem. 2011, 399, 2949–2959. [Google Scholar] [CrossRef]
- Brimblecombe, P. History of air pollution and damage to the cultural heritage of european cities. In Science, Technology and European Cultural Heritage; Butterworth-Heinemann: Oxford, UK, 1991; pp. 51–66. [Google Scholar]
- Saxena, P.; Srivastava, A. (Eds.) Air Pollution and Environmental Health; Springer: Singapore, 2020; pp. 7–253. [Google Scholar]
- Corvo, F.; Reyes, J.; Valdes, C.; Villaseñor, F.; Cuesta, O.; Aguilar, D.; Quintana, P. Influence of air pollution and humidity on limestone materials degradation in historical buildings located in cities under tropical coastal climates. Water Air Soil Pollut. 2010, 205, 359–375. [Google Scholar] [CrossRef]
- Haneef, S.; Johnson, J.; Dickinson, C.; Thompson, G.; Wood, G. Effect of dry deposition of NOx and SO2 gaseous pollutants on the degradation of calcareous building stones. Atmos. Environ. Part A Gen. Top. 1992, 26, 2963–2974. [Google Scholar] [CrossRef]
- Sariisik, A.; Sariisik, G.; Şentürk, A. Characterization of Physical and Mechanical Properties of Natural Stones Affected by Ground Water under Different Ambient Conditions. Ekoloji 2010, 19, 88–96. [Google Scholar] [CrossRef]
- Allen, G.C.; El-Turki, A.; Hallam, K.R.; McLaughlin, D.; Stacey, M. Role of NO2 and SO2 in degradation of limestone. Br. Corros. J. 2000, 35, 35–38. [Google Scholar] [CrossRef]
- Gurnagul, N.; Zou, X. The effect of atmospheric pollutants on paper permanence: A literature review. Tappi J. 1994, 77, 199–204. [Google Scholar]
- Williams, E.L.; Grosjean, D. Exposure of Deacidified and Untreated Paper to Ambient Levels of Sulfur Dioxide and Nitrogen Dioxide: Nature and Yields of Reaction Products. J. Am. Inst. Conserv. 1992, 31, 199–212. [Google Scholar] [CrossRef]
- Daniele, V.; Rosatelli, G.; Macera, L.; Taglieri, G. New aqueous nanolime formulations for fully compatible consolidation treatments of historical mortars for hypogeum environment. Constr. Build. Mater. 2022, 356, 129316. [Google Scholar] [CrossRef]
- Otero, J.; Charola, A.E.; Grissom, C.A.; Starinieri, V. An overview of nanolime as a consolidation method for calcareous substrates. Ge-Conservación 2017, 1, 71–78. [Google Scholar] [CrossRef]
- Ion, R.-M.; Rizescu, C.E.; Vasile, D.A.; Vasilievici, G.; Atkinson, I.; Rusu, A.; Predoana, L.; Miculescu, F. Layered Double Hydroxides (LDHs) as New Consolidants for Cultural Heritage Masonry. Crystals 2022, 12, 490. [Google Scholar] [CrossRef]
- Ion, R.M.; Fierăscu, R.C.; Fierăscu, I.; Bunghez, I.R.; Ion, M.L.; Caruţiu-Turcanu, D.; Rădiţoiu, V. Stone Monuments Consolidation with Nanomaterials. In Key Engineering Materials; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2015; Volume 660, pp. 383–388. [Google Scholar]
- Weththimuni, M.L.; Licchelli, M. Heritage Conservation and Restoration: Surface Characterization, Cleaning and Treatments. Coatings 2023, 13, 457. [Google Scholar] [CrossRef]
- Freire-Lista, D.M.; Fort, R.; Varas-Muriel, M.J. Freeze–thaw fracturing in building granites. Cold Reg. Sci. Technol. 2015, 113, 40–51. [Google Scholar] [CrossRef]
- Doehne, E. Salt weathering: A selective review. Geol. Soc. Lond. Spéc. Publ. 2002, 205, 51–64. [Google Scholar] [CrossRef]
- Warscheid, T.; Braams, J. Biodeterioration of stone: A review. Int. Biodeterior. Biodegrad. 2000, 46, 343–368. [Google Scholar] [CrossRef]
- Lamenti, G.; Tomaselli, L.; Tiano, P. Cyanobacteria and biodeterioration of monumental stones. In Molecular Biology and Cultural Heritage; Sweets & Zeitlinger: Lisse, The Netherlands, 2003; pp. 73–78. [Google Scholar] [CrossRef]
- Vismaya, K.; Snehal, K.; Das, B.B. Impact of Phase Change Materials on the Durability Properties of Cementitious Composites—A Review. In Recent Trends in Construction Technology and Management; Springer Nature: Singapore, 2023; Volume 260, pp. 71–82. [Google Scholar] [CrossRef]
- Schiavon, N. Biodeterioration of calcareous and granitic building stones in urban environments. Geol. Soc. Lond. Spéc. Publ. 2002, 205, 195–205. [Google Scholar] [CrossRef]
- Elgohary, Y.M.; Mansour, M.M.A.; Salem, M.Z.M. Assessment of the potential effects of plants with their secreted biochemicals on the biodeterioration of archaeological stones. In Biomass Conversion and Biorefinery; Springer: Berlin, Germany, 2022; pp. 1–15. [Google Scholar] [CrossRef]
- Sciarretta, F.; Eslami, J.; Beaucour, A.-L.; Noumowé, A. State-of-the-art of construction stones for masonry exposed to high temperatures. Constr. Build. Mater. 2021, 304, 124536. [Google Scholar] [CrossRef]
- Hajpál, M. Changes in Sandstones of Historical Monuments Exposed to Fire or High Temperature. Fire Technol. 2002, 38, 373–382. [Google Scholar] [CrossRef]
- Vasanelli, E.; Quarta, G.; Masieri, M.; Calia, A. High temperature effects on the properties of a high porosity calcareous stone building material. Eur. J. Environ. Civ. Eng. 2022, 26, 6733–6745. [Google Scholar] [CrossRef]
- Gomez-Heras, M.; McCabe, S.; Smith, B.J.; Fort, R. Impacts of fire on stone-built heritage: An overview. J. Archit. Conserv. 2009, 15, 47–58. [Google Scholar] [CrossRef]
- Mariappan, T. Fire retardant coatings. In New Technologies in Protective Coatings; IntechOpen: London, UK, 2017; Volume 28, pp. 101–122. [Google Scholar] [CrossRef]
- Vakhitova, L.N. Fire retardant nanocoating for wood protection. In Nanotechnology in Eco-Efficient Construction; Woodhead Publishing: Sawston, UK, 2019; pp. 361–391. [Google Scholar]
- Baglioni, P.; Giorgi, R. Soft and hard nanomaterials for restoration and conservation of cultural heritage. Soft Matter 2006, 2, 293–303. [Google Scholar] [CrossRef]
- D’Armada, P.; Hirst, E. Nano-Lime for Consolidation of Plaster and Stone. J. Arch. Conserv. 2012, 18, 63–80. [Google Scholar] [CrossRef]
- Daniele, V.; Taglieri, G.; Quaresima, R. The nanolimes in Cultural Heritage conservation: Characterisation and analysis of the carbonatation process. J. Cult. Heritage 2008, 9, 294–301. [Google Scholar] [CrossRef]
- Hemeda, S.; Khalil, M.; Shoeb, A.; El Aziz, A.A. Nanostructured materials for strenthening and preservation of historic structural marble columns. Int. J. Conserv. Sci. 2020, 11, 485–498. [Google Scholar]
- Sánchez, M.; Faria, P.; Ferrara, L.; Horszczaruk, E.; Jonkers, H.M.; Kwiecień, A.; Mosa, J.; Peled, A.; Pereira, A.S.; Snoeck, D.; et al. External treatments for the preventive repair of existing constructions: A review. Constr. Build. Mater. 2018, 193, 435–452. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, P.; Ding, J.; Dong, Y.; Cao, Y.; Dong, W.; Zhao, X.; Li, X.; Camaiti, M. Nano Ca(OH)2: A review on synthesis, properties and applications. J. Cult. Herit. 2021, 50, 25–42. [Google Scholar] [CrossRef]
- Gherardi, F. Current and future trends in protective treatments for stone heritage. In Conserving Stone Heritage: Traditional and Innovative Materials and Techniques; Gherardi, F., Maravelaki, P.N., Eds.; Springer: Cham, Switzerland, 2022; pp. 137–176. [Google Scholar] [CrossRef]
- López-Arce, P.; Gomez-Villalba, L.; Pinho, L.; Fernández-Valle, M.; de Buergo, M.; Fort, R. Influence of porosity and relative humidity on consolidation of dolostone with calcium hydroxide nanoparticles: Effectiveness assessment with non-destructive techniques. Mater. Charact. 2010, 61, 168–184. [Google Scholar] [CrossRef]
- Sierra-Fernández, A.; Sotiriadis, K.; Mácová, P.; Len, A.; Gómez Villalba, L.S.; Rabanal, M.E.; Viani, A.; Fort González, R. Investigation of the effect of CO2 concentration on the carbonation of Mg(OH)2 and Ca(OH)2 nanoparticles applied as stone consolidant agents using neutron and spectroscopic techniques. In Proceedings of the 1st International Conference TMM_CH: Transdisciplinary Multispectral Modelling and Cooperation for the Preservation of Cultural Heritage 2018, Athens, Greek, 13–18 October 2018. [Google Scholar]
- Gomez Villalba, L.S.; López-Arce Martínez, P.; Zornoza, A.; Álvarez de Buergo, M.; Fort González, R. Evaluation of a consolidation treatment in dolostones by mean of calcium hydroxide nanoparticles in high relative humidity conditions. Bol. La Soc. Esp. Ceram. Y Vidr. 2011, 50, 85–92. [Google Scholar]
- Giorgi, R.; Baglioni, M.; Berti, D.; Baglioni, P. New Methodologies for the Conservation of Cultural Heritage: Micellar Solutions, Microemulsions, and Hydroxide Nanoparticles. Accounts Chem. Res. 2010, 43, 695–704. [Google Scholar] [CrossRef]
- De Filpo, G.; Palermo, A.M.; Rachiele, F.; Nicoletta, F.P. Preventing fungal growth in wood by titanium dioxide nanoparticles. Int. Biodeterior. Biodegrad. 2013, 85, 217–222. [Google Scholar] [CrossRef]
- Gherardi, F.; Maravelaki, P.N. Advances in the application of nanomaterials for natural stone conservation. RILEM Tech. Lett. 2022, 7, 20–29. [Google Scholar] [CrossRef]
- Goffredo, G.B.; Accoroni, S.; Totti, C.; Romagnoli, T.; Valentini, L.; Munafò, P. Titanium dioxide based nanotreatments to inhibit microalgal fouling on building stone surfaces. Build. Environ. 2017, 112, 209–222. [Google Scholar] [CrossRef]
- Becerra, J.; Zaderenko, A.; Karapanagiotis, I.; Ortiz, P. Evaluation of silver nanoparticles effectiveness as biocide by multi-spectral imaging. In Science and Digital Technology for Cultural Heritage; CRC Press: Boca Raton, FL, USA, 2019; pp. 307–311. [Google Scholar]
- Li, Q.; Hu, Y.; Zhang, B. Hydrophilic ZnO Nanoparticle-Based Antimicrobial Coatings for Sandstone Heritage Conservation. ACS Appl. Nano Mater. 2021, 4, 13908–13918. [Google Scholar] [CrossRef]
- Becerra, J.; Zaderenko, A.P.; Ortiz, P. Silver/dioxide titanium nanocomposites as biocidal treatments on limestones. Ge-Conservación 2017, 11, 141–148. [Google Scholar]
- Chobba, M.B.; Weththimuni, M.L.; Messaoud, M.; Urzi, C.; Bouaziz, J.; De Leo, F.; Licchelli, M. Ag-TiO2/PDMS nanocomposite protective coatings: Synthesis, characterization, and use as a self-cleaning and antimicrobial agent. Prog. Org. Coat. 2021, 158, 106342. [Google Scholar] [CrossRef]
- Sierra-Fernandez, A.; De la Rosa-García, S.C.; Gomez-Villalba, L.S.; Gómez-Cornelio, S.; Rabanal, M.E.; Fort, R.; Quintana, P. Synthesis, Photocatalytic, and Antifungal Properties of MgO, ZnO and Zn/Mg Oxide Nanoparticles for the Protection of Calcareous Stone Heritage. ACS Appl. Mater. Interfaces 2017, 9, 24873–24886. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Fernandez, A.; De la Rosa-García, S.C.; Yañez-Macías, R.; Guerrero-Sanchez, C.; Gomez-Villalba, L.S.; Gómez-Cornelio, S.; Rabanal, M.E.; Schubert, U.S.; Fort, R.; Quintana, P. Sol–gel synthesis of Mg(OH)2 and Ca(OH)2 nanoparticles: A comparative study of their antifungal activity in partially quaternized p(DMAEMA) nanocomposite films. J. Sol.-Gel. Sci. Technol. 2019, 89, 310–321. [Google Scholar] [CrossRef]
- Tyagi, P.; Verma, R.K.; Jain, N. Fungal degradation of cultural heritage monuments and management options. Curr. Sci. 2021, 121, 00113891. [Google Scholar] [CrossRef]
- Zhu, C.; Li, Q.; Wang, X.; Hu, Y.; Zhang, B. Biocides for the Control of Mosses on Stone Cultural Relics. In Studies in Conservation; Taylor & Francis: London, UK, 2022; pp. 1–10. [Google Scholar] [CrossRef]
- Zuena, M.; Ruggiero, L.; Caneva, G.; Bartoli, F.; Della Ventura, G.; Ricci, M.A.; Sodo, A. Assessment of Stone Protective Coatings with a Novel Eco-Friendly Encapsulated Biocide. Coatings 2021, 11, 1109. [Google Scholar] [CrossRef]
- Dresler, C.; Saladino, M.L.; Demirbag, C.; Caponetti, E.; Martino, D.F.C.; Alduina, R. Development of controlled release systems of biocides for the conservation of cultural heritage. Int. Biodeterior. Biodegrad. 2017, 125, 150–156. [Google Scholar] [CrossRef]
- Pyzik, A.; Ciuchcinski, K.; Dziurzynski, M.; Dziewit, L. The Bad and the Good—Microorganisms in Cultural Heritage Environments—An Update on Biodeterioration and Biotreatment Approaches. Materials 2021, 14, 177. [Google Scholar] [CrossRef]
- Colangiuli, D.; Calia, A.; Bianco, N. Novel multifunctional coatings with photocatalytic and hydrophobic properties for the preservation of the stone building heritage. Constr. Build. Mater. 2015, 93, 189–196. [Google Scholar] [CrossRef]
- Luna, M.; Delgado, J.J.; Romero, I.; Montini, T.; Gil, M.A.; Martínez-López, J.; Fornasiero, P.; Mosquera, M.J. Photocatalytic TiO2 nanosheets-SiO2 coatings on concrete and limestone: An enhancement of de-polluting and self-cleaning properties by nanoparticle design. Constr. Build. Mater. 2022, 338, 127349. [Google Scholar] [CrossRef]
- Qiu, L.; Xie, R.; Ding, P.; Qu, B. Preparation and characterization of Mg(OH)2 nanoparticles and flame-retardant property of its nanocomposites with EVA. Compos. Struct. 2003, 62, 391–395. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Parisi, F.; Riela, S.; Milioto, S. Nanoclays for Conservation. In Nanotechnologies and Nanomaterials for Diagnostic Conservation and Restoration of Cultural Heritage; Elsevier: Amsterdam, The Netherlands, 2019; pp. 149–170. [Google Scholar]
- Knight, C.C.; Ip, F.; Zeng, C.; Zhang, C.; Wang, B. A highly efficient fire-retardant nanomaterial based on carbon nanotubes and magnesium hydroxide. Fire Mater. 2013, 37, 91–99. [Google Scholar] [CrossRef]
- He, W.; Song, P.; Yu, B.; Fang, Z.; Wang, H. Flame retardant polymeric nanocomposites through the combination of nanomaterials and conventional flame retardants. Prog. Mater. Sci. 2020, 114, 100687. [Google Scholar] [CrossRef]
- Ielo, I.; Giacobello, F.; Castellano, A.; Sfameni, S.; Rando, G.; Plutino, M.R. Development of Antibacterial and Antifouling Innovative and Eco-Sustainable Sol–Gel Based Materials: From Marine Areas Protection to Healthcare Applications. Gels 2022, 8, 26. [Google Scholar] [CrossRef]
- Salazar-Hernández, C.; Alquiza, M.J.P.; Salgado, P.; Cervantes, J. TEOS-colloidal silica-PDMS-OH hybrid formulation used for stone consolidation. Appl. Organomet. Chem. 2010, 24, 481–488. [Google Scholar] [CrossRef]
- Kim, E.K.; Won, J.; Do, J.-Y.; Kim, S.D.; Kang, Y.S. Effects of silica nanoparticle and GPTMS addition on TEOS-based stone consolidants. J. Cult. Heritage 2009, 10, 214–221. [Google Scholar] [CrossRef]
- Manoudis, P.; Papadopoulou, S.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Panayiotou, C. Polymer-Silica nanoparticles composite films as protective coatings for stone-based monuments. J. Phys. Conf. Ser. 2007, 61, 1361–1365. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Kolinkeová, B.; Panayiotou, C. Superhydrophobic films for the protection of outdoor cultural heritage assets. Appl. Phys. A 2009, 97, 351–360. [Google Scholar] [CrossRef]
- Ershad-Langroudi, A.; Fadaei, H.; Ahmadi, K. Application of polymer coatings and nanoparticles in consolidation and hydrophobic treatment of stone monuments. Iran. Polym. J. 2019, 28, 1–19. [Google Scholar] [CrossRef]
- Mosquera, M.J.; Santos, D.M.D.L.; Rivas, T. Surfactant-Synthesized Ormosils with Application to Stone Restoration. Langmuir 2010, 26, 6737–6745. [Google Scholar] [CrossRef] [PubMed]
- Helmi, F.M.; Hefni, Y.K. Using nanocomposites in the consolidation and protection of sandstone. Int. J. Conserv. Sci. 2016, 7, 29–40. [Google Scholar]
- Torrisi, A. Pulsed laser cleaning (PLC) applied to samples in cultural heritage field. Radiat. Eff. Defects Solids 2022, 177, 27–39. [Google Scholar] [CrossRef]
- Fotakis, C.; Anglos, D.; Zafiropulos, V.; Georgiou, S.; Tornari, V. Lasers in the Preservation of Cultural Heritage: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Ciliberto, E.; Condorelli, G.; La Delfa, S.; Viscuso, E. Nanoparticles of Sr(OH)2: Synthesis in homogeneous phase at low temperature and application for cultural heritage artefacts. Appl. Phys. A 2008, 92, 137–141. [Google Scholar] [CrossRef]
- Saoud, K.M.; Ibala, I.; El Ladki, D.; Ezzeldeen, O.; Saeed, S. Microwave Assisted Preparation of Calcium Hydroxide and Barium Hydroxide Nanoparticles and Their Application for Conservation of Cultural Heritage. In Proceedings of the Euro-Mediterranean Conference, Limassol, Cyprus, 3–8 November 2014; Springer: Cham, Switzerland, 2014; pp. 342–352. [Google Scholar] [CrossRef]
- Papadaki, D.; Kiriakidis, G.; Tsoutsos, T. Applications of nanotechnology in construction industry. In Fundamentals of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2018; pp. 343–370. [Google Scholar]
- Paul, S.C.; van Rooyen, A.S.; van Zijl, G.P.; Petrik, L.F. Properties of cement-based composites using nanoparticles: A comprehensive review. Constr. Build. Mater. 2018, 189, 1019–1034. [Google Scholar] [CrossRef]
- Kawashima, S.; Hou, P.; Corr, D.J.; Shah, S.P. Modification of cement-based materials with nanoparticles. Cem. Concr. Compos. 2013, 36, 8–15. [Google Scholar] [CrossRef]
- Reches, Y. Nanoparticles as concrete additives: Review and perspectives. Constr. Build. Mater. 2018, 175, 483–495. [Google Scholar] [CrossRef]
- Jayaseelan, R.; Pandalu, G.; Selvam, S. Investigation on the performance characteristics of concrete incorporating nanoparticles. Jordan J. Civ. Eng. 2019, 13, 351–360. [Google Scholar]
- Arizzi, A.; Gomez-Villalba, L.S.; Lopez-Arce, P.; Cultrone, G.; Fort, R. Lime mortar consolidation with nanostructured calcium hydroxide dispersions: The efficacy of different consolidating products for heritage conservation. Eur. J. Miner. 2015, 27, 311–323. [Google Scholar] [CrossRef]
- Ergenc, D.; Sierra-Fernandez, A.; M del Mar Barbero-Barrera, M.; Gomez-Villalba, L.S.; Fort, R. Assessment on the performances of air lime-ceramic mortars with nano-Ca(OH)2 and nano-SiO2 additions. Constr. Build. Mater. 2020, 232, 117163. [Google Scholar] [CrossRef]
- Barbero-Barrera, M.M.; Gomez-Villalba, L.S.; Ergenç, D.; Sierra-Fernández, A.; Fort, R. Influence of curing conditions on the mechanical and hydric performance of air-lime mortars with nano-Ca(OH)2 and nano-SiO2 additions. Cement Concr. Compos. 2022, 132, 104631. [Google Scholar] [CrossRef]
- Sneh, A.; Gupta, M.; Vishwakarma, A.; Gupta, A.; Marieneni, L.R. Acoustic Shielding of Nano-Particle Reinforced Composites. Int. J. Eng. Res. Technol. (IJERT) 2020, 9, 342–345. [Google Scholar] [CrossRef]
- Park, H.C.; Lee, J.H.; Kim, I.H.; Sajjad, M.; Ahn, K.H.; Kim, K.S. Effects of Nano-Porous Materials and Inert Gas on Sound Proof Properties of Double Layer Acryl Plate. Mater. Sci. Forum 2015, 804, 89–92. [Google Scholar] [CrossRef]
- Alhilo, E.A.; Kuba, S.A.; Dirweesh, A.F. Nanotechnology use to preserve the durability of archaeological brick buildings in Al-Najaf city. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1067, 012044. [Google Scholar] [CrossRef]
- Artesani, A.; Di Turo, F.; Zucchelli, M.; Traviglia, A. Recent Advances in Protective Coatings for Cultural Heritage–An Overview. Coatings 2020, 10, 217. [Google Scholar] [CrossRef]
- Tsardaka, E.-C.; Stefanidou, M. The role of nano-modified coverings against salt attack. J. Build. Eng. 2022, 57, 104845. [Google Scholar] [CrossRef]
- Ming, W.; Jiang, Z.; Luo, G.; Xu, Y.; He, W.; Xie, Z.; Shen, D.; Li, L. Progress in Transparent Nano-Ceramics and Their Potential Applications. Nanomaterials 2022, 12, 1491. [Google Scholar] [CrossRef]
- Giordano, A.; Barresi, G.; Rotolo, V.; Schiavone, S.; Palla, F. The conservation of contemporary paintings: From dry-cleaning to microemulsions. In Nanotechnologies and Nanomaterials for Diagnostic Conservation and Restoration of Cultural Heritage; Elsevier: Amsterdam, The Netherlands, 2019; pp. 277–298. [Google Scholar]
- Kanth, A.P.; Soni, A.K. Application of nanocomposites for conservation of materials of cultural heritage. J. Cult. Heritage 2023, 59, 120–130. [Google Scholar] [CrossRef]
- Fruth, V.; Todan, L.; Codrea, C.I.; Poenaru, I.; Petrescu, S.; Aricov, L.; Ciobanu, M.; Jecu, L.; Ion, R.M.; Predoana, L. Multifunctional Composite Coatings Based on Photoactive Metal-Oxide Nanopowders (MgO/TiO2) in Hydrophobic Polymer Matrix for Stone Heritage Conservation. Nanomaterials 2021, 11, 2586. [Google Scholar] [CrossRef]
- Gherardi, F.; Roveri, M.; Goidanich, S.; Toniolo, L. Photocatalytic Nanocomposites for the Protection of European Architectural Heritage. Materials 2018, 11, 65. [Google Scholar] [CrossRef]
- Afsharpour, M.; Imani, S. Preventive protection of paper works by using nanocomposite coating of zinc oxide. J. Cult. Heritage 2017, 25, 142–148. [Google Scholar] [CrossRef]
- Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite Nanotubes: Interfacial Properties and Applications in Cultural Heritage. Langmuir 2020, 36, 3677–3689. [Google Scholar] [CrossRef] [PubMed]
- Markevicius, T.; Meyer, H.; Saborowski, K.; Olsson, N.; Furferi, R. Carbon nanotubes in art conservation. Int. Mag. Conserv. Sci. 2013, 4, 633–646. [Google Scholar]
- David, M.E.; Ion, R.-M.; Grigorescu, R.M.; Iancu, L.; Constantin, M.; Stirbescu, R.M.; Gheboianu, A.I. Wood Surface Modification with Hybrid Materials Based on Multi-Walled Carbon Nanotubes. Nanomaterials 2022, 12, 1990. [Google Scholar] [CrossRef] [PubMed]
- Carfagni, M.; Furferi, R.; Governi, L.; Volpe, Y.; Hegelbach, R.; Markevicius, T.; Meyer, H.; Olsson, N.; Saborowski, K.; Seymour, K. Application of carbon nanotubes–based coating in the field of art conservation: The IMAT project and the development of new mild heat transfer technology. In Handbook of Modern Coating Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 81–133. [Google Scholar]
- Gharib, A.; Maher, M.A.; Ismail, S.H.; Mohamed, G.G. Effect Titanium Dioxide/Paraloid B.72 Nanocomposite Coating on Protection of Treated Cu-Zn Archaeological Alloys. Int. J. Archaeol. 2019, 7, 47. [Google Scholar] [CrossRef]
- Chatzigrigoriou, A.; Manoudis, P.N.; Karapanagiotis, I. Fabrication of Water Repellent Coatings Using Waterborne Resins for the Protection of the Cultural Heritage. Macromol. Symp. 2013, 331–332, 158–165. [Google Scholar] [CrossRef]
- Chatzigrigoriou, A.; Karapanagiotis, I.; Poulios, I. Superhydrophobic Coatings Based on Siloxane Resin and Calcium Hydroxide Nanoparticles for Marble Protection. Coatings 2020, 10, 334. [Google Scholar] [CrossRef]
- Sutar, R.S.; Patil, P.B.; Bhosale, A.K.; Nagappan, S.; Shinde, S.R.; Chikode, P.P.; Patil, C.E.; Kadam, S.S.; Kadam, P.M.; Bobade, C.R.; et al. Photocatalytic and Superhydrophilic TiO2-SiO2 Coatings on Marble for Self-Cleaning Applications. In Macromolecular Symposia; Wiley-VCH GmbH: Weinheim, Germany, 2021; Volume 400, p. 2100083. [Google Scholar]
- Crupi, V.; Fazio, B.; Gessini, A.; Kis, Z.; La Russa, M.F.; Majolino, D.; Masciovecchio, C.; Ricca, M.; Rossi, B.; Ruffolo, S.A.; et al. TiO2–SiO2–PDMS nanocomposite coating with self-cleaning effect for stone material: Finding the optimal amount of TiO2. Constr. Build. Mater. 2018, 166, 464–471. [Google Scholar] [CrossRef]
- Rangasamy, M. Nano technology: A review. J. Appl. Pharm. Sci. 2011, 1, 8–16. [Google Scholar]
- Otero, J.; Pozo-Antonio, J.S.; Montojo, C. Influence of application method and number of applications of nanolime on the effectiveness of the Doulting limestone treatments. Mater. Struct. 2021, 54, 1–19. [Google Scholar] [CrossRef]
- Pinto, A.F.; Rodrigues, J.D. Stone consolidation: The role of treatment procedures. J. Cult. Heritage 2008, 9, 38–53. [Google Scholar] [CrossRef]
- Daniele, V.; Taglieri, G. Nanolime suspensions applied on natural lithotypes: The influence of concentration and residual water content on carbonatation process and on treatment effectiveness. J. Cult. Heritage 2010, 11, 102–106. [Google Scholar] [CrossRef]
- López-Arce, P.; Zornoza-Indart, A.; Gomez-Villalba, L.S.; Fort, R. Short- and Longer-Term Consolidation Effects of Portlandite (CaOH)2 Nanoparticles in Carbonate Stones. J. Mater. Civ. Eng. 2013, 25, 1655–1665. [Google Scholar] [CrossRef]
- Capitelli, F.; Dida, B.; Ventura, G.D.; Baldassarre, F.; Capelli, D.; Senesi, G.S.; Mele, A.; Siliqi, D. Functional nano-hydroxyapatite for applications in conservation of stony monuments of cultural heritage. Multidiscip. Digit. Publ. Inst. Proc. 2021, 62, 11. [Google Scholar]
- Waked, A.M. Nano materials applications for conservation of cultural heritage. WIT Trans. Built Environ. 2011, 118, 577–588. [Google Scholar] [CrossRef]
- Ruffolo, S.A.; Ricca, M.; Macchia, A.; La Russa, M.F. Antifouling coatings for underwater archaeological stone materials. Prog. Org. Coat. 2017, 104, 64–71. [Google Scholar] [CrossRef]
- Ricca, M.; Ruffolo, S.A.; La Russa, M.F.; Rispoli, C.; Grifa, C.; Sierra-Fernández, A.; Fort, R.; Randazzo, L. Antifouling Mortars for Underwater Restoration. Nanomaterials 2022, 12, 1498. [Google Scholar] [CrossRef]
- Lee, D.-K.; Yoo, J.; Kim, H.; Kang, B.-H.; Park, S.-H. Electrical and Thermal Properties of Carbon Nanotube Polymer Composites with Various Aspect Ratios. Materials 2022, 15, 1356. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Carbon nanotubes: Synthesis, properties and engineering applications. Carbon Lett. 2019, 29, 419–447. [Google Scholar] [CrossRef]
- Zhang, Y.; Ram, M.K.; Stefanakos, E.K.; Goswami, D.Y. Synthesis, Characterization, and Applications of ZnO Nanowires. J. Nanomater. 2012, 20, 1–22. [Google Scholar] [CrossRef]
- Zhu, J.; Li, X.; Zhang, Y.; Wang, J.; Wei, B. Graphene-Enhanced Nanomaterials for Wall Painting Protection. Adv. Funct. Mater. 2018, 28, 1803872. [Google Scholar] [CrossRef]
- González-Campelo, D.; Fernández-Raga, M.; Gómez-Gutiérrez, Á.; Guerra-Romero, M.I.; González-Domínguez, J.M. Extraordinary Protective Efficacy of Graphene Oxide over the Stone-Based Cultural Heritage. Adv. Mater. Interfaces 2021, 8, 2101012. [Google Scholar] [CrossRef]
- Mancic, L.; Nikolic, M.; Gomez, L.; Rabanal, M.; Milosevic, O. The processing of optically active functional hierarchical nanoparticles. Adv. Powder Technol. 2017, 28, 3–22. [Google Scholar] [CrossRef]
- Muñoz-Fernandez, L.; Gomez-Villalba, L.; Milošević, O.; Rabanal, M. Influence of nanoscale defects on the improvement of photocatalytic activity of Ag/ZnO. Mater. Charact. 2022, 185, 111718. [Google Scholar] [CrossRef]
- Teles, F.; Martins, G.; Antunes, F. Fire retardancy in nanocomposites by using nanomaterial additives. J. Anal. Appl. Pyrolysis 2022, 163, 105466. [Google Scholar] [CrossRef]
- Olender, J.; Young, C.; Taylor, A. The applicability of gecko-inspired dry adhesives to the conservation of photographic prints. In Proceedings of the Modern Materials and Contemporary Art 2017, ICOM-CC 18th Triennial Conference, Copenhagen, Denmark, 4–8 September 2017. [Google Scholar]
- Hu, S.; Xia, Z.; Dai, L. Advanced gecko-foot-mimetic dry adhesives based on carbon nanotubes. Nanoscale 2013, 5, 475–486. [Google Scholar] [CrossRef]
- Chen, B.; Zhong, G.; Oppenheimer, P.G.; Zhang, C.; Tornatzky, H.; Esconjauregui, S.; Hofmann, S.; Robertson, J. Influence of Packing Density and Surface Roughness of Vertically-Aligned Carbon Nanotubes on Adhesive Properties of Gecko-Inspired Mimetics. ACS Appl. Mater. Interfaces 2015, 7, 3626–3632. [Google Scholar] [CrossRef]
- Martínez Garrido, M.I.; Fort, R.; Gómez Heras, M.; Valles-Iriso, J.; Varas Muriel, M.J. An overview of non-destructive and minimally invasive techniques for moisture control in the cultural heritage. J. Appl. Geophys. 2018, 155, 36–52. [Google Scholar] [CrossRef]
- Martínez-Garrido, M.; Fort, R. Experimental assessment of a wireless communications platform for the built and natural heritage. Measurement 2016, 82, 188–201. [Google Scholar] [CrossRef]
- Sekhaneh, W.; Dahmani, H. Nanosized zinc oxide deposited on single wall carbon nanotubes composites for nitrogen dioxide-sensors in museums and art galleries monitoring. Mediterr. Archaeol. Archaeom. 2014, 14, 25–35. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Wang, W.-M.; Chen, C.-Y.; Lu, T.-H.; Liao, C.-M. Assessing human exposure risk and lung disease burden posed by airborne silver nanoparticles emitted by consumer spray products. Int. J. Nanomed. 2019, 14, 1687. [Google Scholar] [CrossRef]
- Talikka, M.; Belcastro, V.; Gubian, S.; Martin, F.; Peitsch, M.C.; Hoeng, J. Systems toxicology meta-analysis—From aerosol exposure to nanotoxicology. Curr. Opin. Toxicol. 2019, 16, 39–48. [Google Scholar] [CrossRef]
- Riebeling, C.; Luch, A.; Götz, M.E. Comparative modeling of exposure to airborne nanoparticles released by consumer spray products. Nanotoxicology 2016, 10, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Leonida, M.D.; Kumar, I. Nanotoxicity and the Skin. In Bionanomaterials for Skin Regeneration; Springer: Cham, Switzerland, 2016; pp. 131–134. [Google Scholar] [CrossRef]
- Zaiter, T.; Cornu, R.; El Basset, W.; Martin, H.; Diab, M.; Béduneau, A. Toxicity assessment of nanoparticles in contact with the skin. J. Nanoparticle Res. 2022, 24, 149. [Google Scholar] [CrossRef]
- Kim, J.S.; Song, K.S.; Sung, J.H.; Ryu, H.R.; Gil Choi, B.; Cho, H.S.; Lee, J.K.; Yu, I.J. Genotoxicity, acute oral and dermal toxicity, eye and dermal irritation and corrosion and skin sensitisation evaluation of silver nanoparticles. Nanotoxicology 2013, 7, 953–960. [Google Scholar] [CrossRef]
- Evans, S.J.; Vecchiarelli, P.M.; Clift, M.J.D.; Doak, S.H.; Lead, J.R. Overview of Nanotoxicology in Humans and the Environment; Developments, Challenges and Impacts. In Nanotoxicology in Humans and the Environment; Springer: Berlin, Germany, 2021; pp. 1–40. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, M.I.; Patil, G. Nanotoxicity of Occupational Dust Generated in Granite Stone Saw Mill. In Proceedings of the IEEE 2011 International Conference on Nanoscience, Technology and Societal Implications, Bhubaneswar, India, 8–10 December 2011; pp. 1–6. [Google Scholar] [CrossRef]
- Cokic, S.M.; Hoet, P.; Godderis, L.; Wiemann, M.; Asbach, C.; Reichl, F.X.; De Munck, J.; Van Meerbeek, B.; Van Landuyt, K.L. Cytotoxic effects of composite dust on human bronchial epithelial cells. Dent. Mater. 2016, 32, 1482–1491. [Google Scholar] [CrossRef]
- Cypriyana, P.J.; Saigeetha, S.; Lavanya, A.; Samrot, A.V.; Kumar, S.; Ponniah, P.; Chakravarthi, S. Overview on toxicity of nanoparticles, it’s mechanism, models used in toxicity studies and disposal methods—A review. Biocatal. Agric. Biotechnol. 2021, 36, 102117. [Google Scholar]
- Reyes-Estebanez, M.; Ortega-Morales, B.O.; Chan-Bacab, M.; Granados-Echegoyen, C.; Camacho-Chab, J.C.; Pereañez-Sacarias, J.E.; Gaylarde, C. Antimicrobial engineered nanoparticles in the built cultural heritage context and their ecotoxicological impact on animals and plants: A brief review. Heritage Sci. 2018, 6, 52. [Google Scholar] [CrossRef]
- Delgado, G.C. Environmental Risks of Nanotechnology: Nanoparticles and Nanostructures. Rev. Cienc. Ambient. 2006, 31, 34–39. [Google Scholar]
- Griffitt, R.J.; Luo, J.; Gao, J.; Bonzongo, J.-C.; Barber, D.S. Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ. Toxicol. Chem. 2008, 27, 1972–1978. [Google Scholar] [CrossRef]
- Li, X.; Liu, W.; Sun, L.; Aifantis, K.E.; Yu, B.; Fan, Y.; Feng, Q.L.; Cui, F.; Watari, F. Effects of physicochemical properties of nanomaterials on their toxicity. J. Biomed. Mater. Res. Part A 2015, 103, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Simkó, M.; Nentwich, M.; Gazsó, A.; Fiedeler, U. How Nanoparticles Enter the Human Body and Their Effects There; NanoTrust Dossier No. 003en—November 2010; Institute of Technology Assessment of the Austrian Academy of Sciences: Vienna, Austria, 2010. [Google Scholar]
- European Commission. Guidance on the Protection of the Health and Safety of Workers from the Potential Risks Related to the Nanomaterials at Work. Guidance for Employers and Health and Safety Practitioners. 2013. Available online: https://ec.europa.eu/social/BlobServlet?docId=13087&langId=en%2 (accessed on 25 February 2023).
- Luyts, K.; Napierska, D.; Nemery, B.; Hoet, P.H.M. How physico-chemical characteristics of nanoparticles cause their toxicity: Complex and unresolved interrelations. Environ. Sci. Process. Impacts 2013, 15, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Gosens, I.; Post, J.A.; De La Fonteyne, L.J.J.; Jansen, E.H.J.M.; Geus, J.W.; Cassee, F.R.; De Jong, W.H. Impact of agglomeration state of nano- and submicron sized gold particles on pulmonary inflammation. Part. Fibre Toxicol. 2010, 7, 37. [Google Scholar] [CrossRef]
- Choi, S.-J.; Lee, J.K.; Jeong, J.; Choy, J.-H. Toxicity evaluation of inorganic nanoparticles: Considerations and challenges. Mol. Cell. Toxicol. 2013, 9, 205–210. [Google Scholar] [CrossRef]
- Riediker, M.; Zink, D.; Kreyling, W.; Oberdörster, G.; Elder, A.; Graham, U.; Lynch, I.; Duschl, A.; Ichihara, G.; Ichihara, S.; et al. Particle toxicology and health—Where are we? Part. Fibre Toxicol. 2019, 16, 19. [Google Scholar] [CrossRef]
- Gnach, A.; Lipinski, T.; Bednarkiewicz, A.; Rybka, J.; Capobianco, J.A. Upconverting nanoparticles: Assessing the toxicity. Chem. Soc. Rev. 2014, 44, 1561–1584. [Google Scholar] [CrossRef]
- B.S. 2955; Glossary of Terms Relating to Particle Technology. British Standards Institution: London, UK, 1991. Available online: https://standards.globalspec.com/std/174140/BS%202955 (accessed on 25 February 2023).
- UKNSPG UK NanoSafety Partnership Group 2012 Working Safely with Nanomaterials in Research & Development. Available online: https://www.hse.gov.uk/nanotechnology/publications.htm (accessed on 25 February 2023).
- Nichols, G.; Byard, S.; Bloxham, M.J.; Botterill, J.; Dawson, N.J.; Dennis, A.; Diart, V.; North, N.C.; Sherwood, J.D. A Review of the Terms Agglomerate and Aggregate with a Recommendation for Nomenclature Used in Powder and Particle Characterization. J. Pharm. Sci. 2002, 91, 2103–2109. [Google Scholar] [CrossRef]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle Aggregation: Challenges to Understanding Transport and Reactivity in the Environment. J. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef]
- Bae, E.; Lee, B.-C.; Kim, Y.; Choi, K.; Yi, J. Effect of agglomeration of silver nanoparticle on nanotoxicity depression. Korean J. Chem. Eng. 2013, 30, 364–368. [Google Scholar] [CrossRef]
- Klahn, E.; Grosshans, H. Modeling the agglomeration of electrostatically charged particles. J. Phys. Conf. Ser. 2019, 1322, 012026. [Google Scholar] [CrossRef]
- Loza, K.; Epple, M.; Maskos, M. Stability of nanoparticle dispersions and particle agglomeration. In Biological Responses to Nanoscale Particles: Molecular and Cellular Aspects and Methodological Approaches; Springer: Berlin, Germany, 2019; pp. 85–100. [Google Scholar]
- Hartley, P.; Parfitt, G.; Pollack, L. The role of the van der Waals force in the agglomeration of powders containing submicron particles. Powder Technol. 1985, 42, 35–46. [Google Scholar] [CrossRef]
- Bian, Y.; Kim, K.; Ngo, T.; Kim, I.; Bae, O.-N.; Lim, K.-M.; Chung, J.-H. Silver nanoparticles promote procoagulant activity of red blood cells: A potential risk of thrombosis in susceptible population. Part. Fibre Toxicol. 2019, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jun, E.-A.; Lim, K.-M.; Kim, K.; Bae, O.-N.; Noh, J.-Y.; Chung, K.-H.; Chung, J.-H. Silver nanoparticles enhance thrombus formation through increased platelet aggregation and procoagulant activity. Nanotoxicology 2011, 5, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Magdolenova, Z.; Bilaničová, D.; Pojana, G.; Fjellsbø, L.M.; Hudecova, A.; Hasplova, K.; Marcomini, A.; Dusinska, M. Impact of agglomeration and different dispersions of titanium dioxide nanoparticles on the human related in vitro cytotoxicity and genotoxicity. J. Environ. Monit. 2012, 14, 455–464. [Google Scholar] [CrossRef]
- Saleemi, M.A.; Fouladi, M.H.; Yong, P.V.C.; Chinna, K.; Palanisamy, N.K.; Wong, E.H. Toxicity of Carbon Nanotubes: Molecular Mechanisms, Signaling Cascades, and Remedies in Biomedical Applications. Chem. Res. Toxicol. 2020, 34, 24–46. [Google Scholar] [CrossRef]
- Polarz, S. Shape Matters: Anisotropy of the Morphology of Inorganic Colloidal Particles—Synthesis and Function. Adv. Funct. Mater. 2011, 21, 3214–3230. [Google Scholar] [CrossRef]
- Morin, J.; Fujimoto, K.; Preston, A.; Guillen, D.P. Synthesis Methods for Nanoparticle Morphology Control in Energy Applications. In REWAS 2022: Energy Technologies and CO2 Management; Springer: Cham, Switzerland, 2022; Volume II, pp. 21–31. [Google Scholar] [CrossRef]
- Auclair, J.; Gagné, F. Shape-Dependent Toxicity of Silver Nanoparticles on Freshwater Cnidarians. Nanomaterials 2022, 12, 3107. [Google Scholar] [CrossRef]
- Jin, Y.; Lohstreter, S.; Zhao, J.X. Toxicity of Spherical and Anisotropic Nanosilica. Pref. XV List. Contrib. XIX 2009, 25, 32–36. [Google Scholar] [CrossRef]
- Tay, Y.; Li, S.; Boey, F.; Cheng, Y.; Liang, M. Growth mechanism of spherical ZnO nanostructures synthesized via colloid chemistry. Phys. B Condens. Matter 2007, 394, 372–376. [Google Scholar] [CrossRef]
- Lee, J.H.; Ju, J.E.; Kim, B.I.; Pak, P.J.; Choi, E.-K.; Lee, H.-S.; Chung, N. Rod-shaped iron oxide nanoparticles are more toxic than sphere-shaped nanoparticles to murine macrophage cells. Environ. Toxicol. Chem. 2014, 33, 2759–2766. [Google Scholar] [CrossRef]
- Zhao, X.; Ng, S.; Heng, B.C.; Guo, J.; Ma, L.; Tan, T.T.; Ng, K.W.; Loo, S.C. Cytotoxicity of hydroxyapatite nanoparticles is shape and cell dependent. Arch. Toxicol. 2013, 87, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Tilly, T.B.; Kerr, L.L.; Braydich-Stolle, L.K.; Schlager, J.J.; Hussain, S.M. Dispersions of geometric TiO2 nanomaterials and their toxicity to RPMI 2650 nasal epithelial cells. J. Nanoparticle Res. 2014, 16, 1–15. [Google Scholar] [CrossRef]
- Magrez, A.; Horváth, L.; Smajda, R.; Salicio, V.; Pasquier, N.; Forró, L.; Schwaller, B. Cellular Toxicity of TiO2-Based Nanofilaments. ACS Nano 2009, 3, 2274–2280. [Google Scholar] [CrossRef]
- Dien, N.D. Preparation of various morphologies of ZnO nanostructure through wet chemical methods. Adv. Mater. Sci. 2019, 4, 1–5. [Google Scholar] [CrossRef]
- Muñoz-Fernandez, L.; Sierra-Fernandez, A.; Flores-Carrasco, G.; Milošević, O.; Rabanal, M. Solvothermal synthesis of Ag/ZnO micro/nanostructures with different precursors for advanced photocatalytic applications. Adv. Powder Technol. 2017, 28, 83–92. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Delagrammatikas, M.; Papadopoulou, O.; Vassiliou, P. Why Does the Addition of Nano-alumina Improve the Performance of Acrylic Coatings Employed in Cultural Heritage Conservation? In Proceedings of the International Symposium on the Conservation of Monuments in the Mediterranean Basin, Athens, Greece, 20–22 September 2017; Springer: Cham, Switzerland, 2017; pp. 115–125. [Google Scholar]
- Park, E.-J.; Lee, G.-H.; Shim, J.-H.; Cho, M.-H.; Lee, B.-S.; Kim, Y.-B.; Kim, J.-H.; Kim, Y.; Kim, D.-W. Comparison of the toxicity of aluminum oxide nanorods with different aspect ratio. Arch. Toxicol. 2015, 89, 1771–1782. [Google Scholar] [CrossRef]
- Sultana, S.; Djaker, N.; Boca-Farcau, S.; Salerno, M.; Charnaux, N.; Astilean, S.; Hlawaty, H.; de la Chapelle, M.L. Comparative toxicity evaluation of flower-shaped and spherical gold nanoparticles on human endothelial cells. Nanotechnology 2015, 26, 055101. [Google Scholar] [CrossRef]
- Huang, L.-H.; Sun, X.-Y.; Ouyang, J.-M. Shape-dependent toxicity and mineralization of hydroxyapatite nanoparticles in A7R5 aortic smooth muscle cells. Sci. Rep. 2019, 9, 1–18. [Google Scholar] [CrossRef]
- Rao, C.-Y.; Sun, X.-Y.; Ouyang, J.-M. Effects of physical properties of nano-sized hydroxyapatite crystals on cellular toxicity in renal epithelial cells. Mater. Sci. Eng. C 2019, 103, 109807. [Google Scholar] [CrossRef]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of nano/micro scale poly(l-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar] [CrossRef] [PubMed]
- Rana, R.; Gupta, S. Production of Nanofibers, Environmental Challenges and Solutions. In Emerging Technologies for Nanoparticle Manufacturing; Springer: Cham, Switzerland, 2021; pp. 237–260. [Google Scholar]
- de Lima, R.; Mattoso, L.H.C.; Feitosa, L.O.; Maruyama, C.R.; Barga, M.A.; Yamawaki, P.C.; Vieira, I.J.; Teixeira, E.M.; Fraceto, L.F. Evaluation of the genotoxicity of cellulose nanofibers. Int. J. Nanomed. 2012, 7, 3555–3565. [Google Scholar] [CrossRef] [PubMed]
- Mossman, B.T.; Borm, P.J.; Castranova, V.; Costa, D.L.; Donaldson, K.; Kleeberger, S.R. Mechanisms of action of inhaled fibers, particles and nanoparticles in lung and cardiovascular diseases. Part. Fibre Toxicol. 2007, 4, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Pacurari, M.; Lowe, K.; Tchounwou, P.B.; Kafoury, R. A Review on the Respiratory System Toxicity of Carbon Nanoparticles. Int. J. Environ. Res. Public Health 2016, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Delforce, L.; Hofmann, E.; Nardello-Rataj, V.; Aubry, J.-M. TiO2 nanoparticle dispersions in water and nonaqueous solvents studied by gravitational sedimentation analysis: Complementarity of Hansen Parameters and DLVO interpretations. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 628, 127333. [Google Scholar] [CrossRef]
- Panyala, N.R.; Peña-Méndez, E.M.; Havel, J. Silver or silver nanoparticles: A hazardous threat to the environment and human health. J. Appl. Biomed. 2008, 6, 117–129. [Google Scholar] [CrossRef]
- Remzova, M.; Zouzelka, R.; Brzicova, T.; Vrbova, K.; Pinkas, D.; Rőssner, P.; Topinka, J.; Rathousky, J. Toxicity of TiO2, ZnO, and SiO2 Nanoparticles in Human Lung Cells: Safe-by-Design Development of Construction Materials. Nanomaterials 2019, 9, 968. [Google Scholar] [CrossRef]
- Kalyani, V.; Vasile, B.S.; Ianculescu, A.; Testino, A.; Carino, A.; Buscaglia, M.T.; Buscaglia, V.; Nanni, P. Hydrothermal Synthesis of SrTiO3: Role of Interfaces. Cryst. Growth Des. 2015, 15, 5712–5725. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Ohno, T.; Sarukawa, K.; Matsumura, M. Photocatalytic Activities of Pure Rutile Particles Isolated from TiO2 Powder by Dissolving the Anatase Component in HF Solution. J. Phys. Chem. B 2001, 105, 2417–2420. [Google Scholar] [CrossRef]
- Pujalté, I.; Passagne, I.; Daculsi, R.; de Portal, C.; Ohayon-Courtès, C.; L’Azou, B. Cytotoxic effects and cellular oxidative mechanisms of metallic nanoparticles on renal tubular cells: Impact of particle solubility. Toxicol. Res. 2015, 4, 409–422. [Google Scholar] [CrossRef]
- Turney, T.W.; Duriska, M.B.; Jayaratne, V.; Elbaz, A.; O’Keefe, S.J.; Hastings, A.S.; Piva, T.J.; Wright, P.F.A.; Feltis, B.N. Formation of Zinc-Containing Nanoparticles from Zn2+ Ions in Cell Culture Media: Implications for the Nanotoxicology of ZnO. Chem. Res. Toxicol. 2012, 25, 2057–2066. [Google Scholar] [CrossRef]
- Gomez-Villalba, L.S.; Sierra-Fernandez, A.; Quintana, P.; Rabanal, M.E.; Fort, R. Correlation between microstructure and cathodoluminescence properties of Mg(OH)2 (brucite) nanoparticles: Effect of synthesis method. CrystEngComm 2018, 20, 5632–5640. [Google Scholar] [CrossRef]
- Jain, S.; Thakare, V.S.; Das, M.; Godugu, C.; Jain, A.K.; Mathur, R.; Chuttani, K.; Mishra, A.K. Toxicity of Multiwalled Carbon Nanotubes with End Defects Critically Depends on Their Functionalization Density. Chem. Res. Toxicol. 2011, 24, 2028–2039. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Lin, S.; Ji, Z.; Thomas, C.R.; Li, L.; Mecklenburg, M.; Meng, H.; Wang, X.; Zhang, H.; Xia, T.; et al. Surface Defects on Plate-Shaped Silver Nanoparticles Contribute to Its Hazard Potential in a Fish Gill Cell Line and Zebrafish Embryos. ACS Nano 2012, 6, 3745–3759. [Google Scholar] [CrossRef]
- Viswanath, B.; Kim, S. Influence of Nanotoxicity on Human Health and Environment: The Alternative Strategies. Rev. Environ. Contam. Toxicol. Vol. 2016, 242, 61–104. [Google Scholar] [CrossRef]
- Gurr, J.-R.; Wang, A.S.; Chen, C.-H.; Jan, K.-Y. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology 2005, 213, 66–73. [Google Scholar] [CrossRef]
- Fanarraga, M.L.; Suárez, C.L.S.; Barroso, R.V. Nano: Guía de Nanoprevención; Universidad de Cantabria, Instituto de Investigación Valdevilla: Santander, Spain, 2017; ISBN 978-84-697-5191-6. [Google Scholar]
- Elsaesser, A.; Howard, C.V. Toxicology of nanoparticles. Adv. Drug Deliv. Rev. 2012, 64, 129–137. [Google Scholar] [CrossRef]
- Löndahl, J.; Möller, W.; Pagels, J.; Kreyling, W.; Swietlicki, E.; Schmid, O. Measurement Techniques for Respiratory Tract Deposition of Airborne Nanoparticles: A Critical Review. J. Aerosol Med. Pulm. Drug Deliv. 2014, 27, 229–254. [Google Scholar] [CrossRef]
- Bailey, A. The inhalation and deposition of charged particles within the human lung. J. Electrost. 1997, 42, 25–32. [Google Scholar] [CrossRef]
- Melandri, C.; Tarroni, G.; Prodi, V.; De Zaiacomo, T.; Formignani, M.; Lombardi, C. Deposition of charged particles in the human airways. J. Aerosol Sci. 1983, 14, 657–669. [Google Scholar] [CrossRef]
- Garner, K.L.; Keller, A.A. Emerging patterns for engineered nanomaterials in the environment: A review of fate and toxicity studies. J. Nanoparticle Res. 2014, 16, 1–28. [Google Scholar] [CrossRef]
- Yang, W.; Peters, J.I.; Williams III, R.O. Inhaled nanoparticles—A current review. Int. J. Pharm. 2008, 356, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xu, X.-X.; Qiao, Y.; Zhao, X.-G. Experimental Research on the Impact of Alveolar Morphology on Deposition of Inhalable Particles in the Human Pulmonary Acinar Area. J. Med. Biol. Eng. 2019, 39, 470–479. [Google Scholar] [CrossRef]
- Qiao, H.; Liu, W.; Gu, H.; Wang, D.; Wang, Y. The Transport and Deposition of Nanoparticles in Respiratory System by Inhalation. J. Nanomater. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Tsuda, A.; Henry, F.S.; Butler, J.P. Particle Transport and Deposition: Basic Physics of Particle Kinetics. Compr. Physiol. 2013, 3, 1437–1471. [Google Scholar] [CrossRef]
- Prodi, V.; Mularoni, A. Electrostatic lung deposition experiments with humans and animals. Ann. Occup. Hyg. 1985, 29, 229–240. [Google Scholar] [CrossRef]
- Yu, C.P. Theories of electrostatic lung deposition of inhaled aerosols. Ann. Occup. Hyg. 1985, 29, 219–227. [Google Scholar] [CrossRef]
- Jaworek, A. Micro- and nanoparticle production by electrospraying. Powder Technol. 2007, 176, 18–35. [Google Scholar] [CrossRef]
- Fdez-Arroyabe, P.; Kourtidis, K.; Haldoupis, C.; Savoska, S.; Matthews, J.; Mir, L.M.; Kassomenos, P.; Cifra, M.; Barbosa, S.; Chen, X.; et al. Glossary on atmospheric electricity and its effects on biology. Int. J. Biometeorol. 2021, 65, 5–29. [Google Scholar] [CrossRef]
- Bailey, M.R. The new ICRP model for the respiratory tract. Radiat. Prot. Dosim. 1994, 53, 107–114. [Google Scholar] [CrossRef]
- Sonwani, S.; Madaan, S.; Arora, J.; Suryanarayan, S.; Rangra, D.; Mongia, N.; Saxena, P. Inhalation exposure to atmospheric nanoparticles and its associated impacts on human health: A review. Front. Sustain. Cities 2021, 3, 690444. [Google Scholar] [CrossRef]
- Borm, P.J.; Robbins, D.; Haubold, S.; Kuhlbusch, T.; Fissan, H.; Donaldson, K.; Schins, R.; Stone, V.; Kreyling, W.; Lademann, J.; et al. The Potential Risks of Nanomaterials: A Review Carried Out for ECETOC. Part. Fibre Toxicol. 2006, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Baimanov, D.; Zhou, Y.; Chen, C.; Wang, L. Penetration and translocation of functional inorganic nanomaterials into biological barriers. Adv. Drug Deliv. Rev. 2022, 191, 114615. [Google Scholar] [CrossRef]
- Puisney, C.; Baeza-Squiban, A.; Boland, S. Mechanisms of Uptake and Translocation of Nanomaterials in the Lung. Cell. Mol. Toxicol. Nanopart. 2018, 1048, 21–36. [Google Scholar] [CrossRef]
- Bunderson-Schelvan, M.; Holian, A.; Trout, K.L.; Hamilton, R.F. Translocation, Biodistribution, and Fate of Nanomaterials in the Body. In Interaction of Nanomaterials with the Immune System; Springer: Cham, Switzerland, 2020; pp. 99–125. [Google Scholar]
- Kermanizadeh, A.; Balharry, D.; Wallin, H.; Loft, S.; Møller, P. Nanomaterial translocation–the biokinetics, tissue accumulation, toxicity and fate of materials in secondary organs—A review. Crit. Rev. Toxicol. 2015, 45, 837–872. [Google Scholar] [CrossRef]
- Fard, J.K.; Jafari, S.; Eghbal, M.A. A Review of Molecular Mechanisms Involved in Toxicity of Nanoparticles. Adv. Pharm. Bull. 2015, 5, 447–454. [Google Scholar] [CrossRef]
- Hirano, S. A current overview of health effect research on nanoparticles. Environ. Health Prev. Med. 2009, 14, 223–225. [Google Scholar] [CrossRef]
- Schrand, A.M.; Dai, L.; Schlager, J.J.; Hussain, S.M. Toxicity testing of nanomaterials. In New Technologies for Toxicity Testing; Springer: Cham, Switzerland, 2012; pp. 58–75. [Google Scholar]
- Hussain, M.; Madl, P.; Khan, A. Lung deposition predictions of airborne particles and the emergence of contemporary diseases, Part-I. Health 2011, 2, 51–59. [Google Scholar]
- Mann, E.E.; Thompson, L.C.; Shannahan, J.H.; Wingard, C.J. Changes in cardiopulmonary function induced by nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 691–702. [Google Scholar] [CrossRef] [PubMed]
- van Berlo, D.; Hullmann, M.; Schins, R.P.F. Toxicology of Ambient Particulate Matter. Mol. Clin. Environ. Toxicol. 2012, 101, 165–217. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Herrera-Soto, A.; Jury, N.; Maher, B.A.; González-Maciel, A.; Reynoso-Robles, R.; Ruiz-Rudolph, P.; van Zundert, B.; Varela-Nallar, L. Reduced repressive epigenetic marks, increased DNA damage and Alzheimer’s disease hallmarks in the brain of humans and mice exposed to particulate urban air pollution. Environ. Res. 2020, 183, 109226. [Google Scholar] [CrossRef] [PubMed]
- Gonet, T.; Maher, B.A.; Kukutschová, J. Source apportionment of magnetite particles in roadside airborne particulate matter. Sci. Total Environ. 2021, 752, 141828. [Google Scholar] [CrossRef]
- Zhu, S.; Gong, L.; Li, Y.; Xu, H.; Gu, Z.; Zhao, Y. Safety Assessment of Nanomaterials to Eyes: An Important but Neglected Issue. Adv. Sci. 2019, 6, 1802289. [Google Scholar] [CrossRef]
- Wu, T.; Tang, M.; Grigoriadis, N.; Lagoudaki, R.; Tascos, N.; Milonas, I.; Lopez-Campos, J.L.; Calero-Acuña, C.; Lopez-Ramirez, C.; Abad-Arranz, M.; et al. The inflammatory response to silver and titanium dioxide nanoparticles in the central nervous system. Nanomedicine 2018, 13, 233–249. [Google Scholar] [CrossRef]
- Prow, T.W.; Bhutto, I.; Kim, S.Y.; Grebe, R.; Merges, C.; McLeod, D.S.; Uno, K.; Mennon, M.; Rodriguez, L.; Leong, K.; et al. Ocular nanoparticle toxicity and transfection of the retina and retinal pigment epithelium. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 340–349. [Google Scholar] [CrossRef]
- Prow, T.W. Toxicity of nanomaterials to the eye. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 317–333. [Google Scholar] [CrossRef]
- Cosert, K.M.; Kim, S.; Jalilian, I.; Chang, M.; Gates, B.L.; Pinkerton, K.E.; Van Winkle, L.S.; Raghunathan, V.K.; Leonard, B.C.; Thomasy, S.M. Metallic Engineered Nanomaterials and Ocular Toxicity: A Current Perspective. Pharmaceutics 2022, 14, 981. [Google Scholar] [CrossRef]
- Wu, Q.; Guo, D.; Du, Y.; Liu, D.; Wang, D.; Bi, H. UVB Irradiation Enhances TiO2Nanoparticle-induced Disruption of Calcium Homeostasis in Human Lens Epithelial Cells. Photochem. Photobiol. 2014, 90, 1324–1331. [Google Scholar] [CrossRef]
- Patel, J.; Champavat, V. Toxicity of nanomaterials on the gastrointestinal tract. Biointeract. Nanomater. 2014, 3, 259. [Google Scholar]
- Yao, Y.; Zang, Y.; Qu, J.; Tang, M.; Zhang, T. The Toxicity Of Metallic Nanoparticles On Liver: The Subcellular Damages, Mechanisms, And Outcomes. Int. J. Nanomed. 2019, 14, 8787. [Google Scholar] [CrossRef] [PubMed]
- Makhdoumi, P.; Karimi, H.; Khazaei, M. Review on Metal-Based Nanoparticles: Role of Reactive Oxygen Species in Renal Toxicity. Chem. Res. Toxicol. 2020, 33, 2503–2514. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Lou, Y.; He, W.; Song, Z. Aggregation of Nanochemical Microcrystals in Urine Promotes the Formation of Urinary Calculi. J. Chem. 2020, 2020, 8516903. [Google Scholar] [CrossRef]
- Gautam, A.; Singh, D.; Vijayaraghavan, R. Dermal exposure of nanoparticles: An understanding. J. Cell Tissue Res. 2011, 11, 2703–2708. [Google Scholar]
- Babick, F. Dynamic light scattering (DLS). In Characterization of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 137–172. [Google Scholar]
- International Standard 22412–2017; Particle Size Analysis—Dynamic Light Scattering (DLS). ISO: Geneva, Switzerland, 2017.
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. In Characterization of Nanoparticles Intended for Drug Delivery; Humana Press: Totowa, NJ, USA, 2011; pp. 63–70. [Google Scholar]
- Wang, C.P. Laser doppler velocimetry. J. Quant. Spectrosc. Radiat. Transf. 1988, 40, 309–319. [Google Scholar] [CrossRef]
- Uskoković, V.; Castiglione, Z.; Cubas, P.; Zhu, L.; Li, W.; Habelitz, S. Zeta-potential and Particle Size Analysis of Human Amelogenins. J. Dent. Res. 2010, 89, 149–153. [Google Scholar] [CrossRef]
- Salopek, B.; Krasic, D.; Filipovic, S. Measurement and application of zeta-potential. Rudarsko-Geolosko-Naftni Zbornik 1992, 4, 147. [Google Scholar]
- Johnson, D.; Hilal, N.; Bowen, W.R. Basic principles of atomic force microscopy. In Atomic Force Microscopy in Process Engineering; Elsevier Ltd.: Amsterdam, The Netherlands, 2009; pp. 1–30. [Google Scholar]
- Müller, D.J.; Dufrêne, Y.F. Atomic force microscopy: A nanoscopic window on the cell surface. Trends Cell Biol. 2011, 21, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Grobelny, J.; DelRio, F.W.; Pradeep, N.; Kim, D.I.; Hackley, V.A.; Cook, R.F. Size measurement of nanoparticles using atomic force microscopy. In Characterization of Nanoparticles Intended for Drug Delivery; Humana Press: Totowa, NJ, USA, 2011; pp. 71–82. [Google Scholar]
- Carter, C.B.; Williams, D.B. (Eds.) Transmission Electron Microscopy: Diffraction, Imaging, and Spectrometry; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Birtcher, R.; Kirk, M.; Furuya, K.; Lumpkin, G.; Ruault, M.-O. In situ Transmission Electron Microscopy Investigation of Radiation Effects. J. Mater. Res. 2005, 20, 1654–1683. [Google Scholar] [CrossRef]
- Kawasaki, T. Environmental Transmission Electron Microscopy. In Compendium of Surface and Interface Analysis; Springer: Singapore, 2018; pp. 171–175. [Google Scholar]
- Hansen, T.W.; Wagner, J.B. Environmental Transmission Electron Microscopy in an Aberration-Corrected Environment. Microsc. Microanal. 2012, 18, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Modena, M.M.; Rühle, B.; Burg, T.P.; Wuttke, S. Nanoparticle Characterization: What to Measure? Adv. Mater. 2019, 31, e1901556. [Google Scholar] [CrossRef] [PubMed]
- Karatasios, I.; Theoulakis, P.; Kalagri, A.; Sapalidis, A.; Kilikoglou, V. Evaluation of consolidation treatments of marly limestones used in archaeological monuments. Constr. Build. Mater. 2009, 23, 2803–2812. [Google Scholar] [CrossRef]
- Ziegenbalg, G.; Slížková, Z.; Ševčik, R. Inorganic Binders and Consolidants: A Critical Review. In Nanomaterials in Architecture and Art Conservation; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2018; pp. 107–130. [Google Scholar] [CrossRef]
- Chelazzi, D.; Poggi, G.; Jaidar, Y.; Toccafondi, N.; Giorgi, R.; Baglioni, P. Hydroxide nanoparticles for cultural heritage: Consolidation and protection of wall paintings and carbonate materials. J. Colloid Interface Sci. 2013, 392, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Karahan Dağ, F.; Caner-Saltik, E.N.; Tavukçuoğlu, A. Assessing the Usage of Calcium and Magnesium Hydroxide Nanoparticles as Consolidant for Dolostones. In Proceedings of the 10th International Symposium on the Conservation of Monuments in the Mediterranean Basin: Natural and Anthropogenic Hazards and Sustainable Preservation, Athen, Greece, 20-22 September 2017; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 135–142. [Google Scholar]
- Taglieri, G.; Daniele, V.; Quaresima, R. Influence of the Nanolime Suspension Concentration on the Effectiveness of Stone Conservative Treatments. In Special Topics on Materials Science and Technology: The Italian Panorama; Brill Publisher: Leiden, The Netherlands, 2009; pp. 359–366. [Google Scholar] [CrossRef]
- Gomez-Villalba, L.S.; López-Arce, P.; de Buergo, M.A.; Zornoza-Indart, A.; Fort, R. Mineralogical and textural considerations in the assessment of aesthetic changes in dolostones by effect of treatments with Ca(OH)2 nanoparticles. Sci. Technol. Conserv. Cult. Herit. 2013, 235, 329. [Google Scholar]
- Evamy, B.D. Dedolomitization and the Development of Rhombohedral Pores in Limestones. J. Sediment. Res. 1967, 37, 1204–1215. [Google Scholar] [CrossRef]
- Otero, J.; Starinieri, V.; Charola, A.; Taglieri, G. Influence of different types of solvent on the effectiveness of nanolime treatments on highly porous mortar substrates. Constr. Build. Mater. 2020, 230, 117112. [Google Scholar] [CrossRef]
- Camerini, R.; Poggi, G.; Chelazzi, D.; Ridi, F.; Giorgi, R.; Baglioni, P. The carbonation kinetics of calcium hydroxide nanoparticles: A Boundary Nucleation and Growth description. J. Colloid Interface Sci. 2019, 547, 370–381. [Google Scholar] [CrossRef]
- Ševčík, R.; Viani, A.; Machová, D.; Lanzafame, G.; Mancini, L.; Appavou, M.-S. Synthetic calcium carbonate improves the effectiveness of treatments with nanolime to contrast decay in highly porous limestone. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tiano, P.; Cantisani, E.; Sutherland, I.; Paget, J. Biomediated reinforcement of weathered calcareous stones. J. Cult. Heritage 2006, 7, 49–55. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Paolucci, D.; Castelvetro, V.; Bianchi, S.; Mascha, E.; Panariello, L.; Pesce, C.; Weber, J.; Lazzeri, A. Preparation of Water Suspensions of Nanocalcite for Cultural Heritage Applications. Nanomaterials 2018, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Villalba, L.S.; López-Arce, P.; Fort, R. Nucleation of CaCO3 polymorphs from a colloidal alcoholic solution of Ca(OH)2 nanocrystals exposed to low humidity conditions. Appl. Phys. A 2012, 106, 213–217. [Google Scholar] [CrossRef]
- Gomez-Villalba, L.S.; López-Arce, P.; de Buergo, M.A.; Fort, R. Structural stability of a colloidal solution of Ca(OH)2 nanocrystals exposed to high relative humidity conditions. Appl. Phys. A 2011, 104, 1249–1254. [Google Scholar] [CrossRef]
- López-Arce, P.; Gómez-Villalba, L.S.; Martinez-Ramirez, S.; de Buergo, M.; Fort, R. Influence of relative humidity on the carbonation of calcium hydroxide nanoparticles and the formation of calcium carbonate polymorphs. Powder Technol. 2011, 205, 263–269. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Ruiz-Agudo, E. Nanolimes: From synthesis to application. Pure Appl. Chem. 2018, 90, 523–550. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Liu, Q.; Wang, X.; Zhu, J.; Luo, H.; Ji, S. Study on the efficacy of amorphous calcium carbonate as a consolidant for calcareous matrix. Heritage Sci. 2022, 10, 165. [Google Scholar] [CrossRef]
- Otero, J.; Charola, A.E.; Starinieri, V. Preliminary Investigations of Compatible Nanolime Treatments on Indiana Limestone and Weathered Marble Stone. Int. J. Arch. Heritage 2022, 16, 394–404. [Google Scholar] [CrossRef]
- Gherardi, F.; Otero, J.; Blakeley, R.; Colston, B. Application of nanolimes for the consolidation of limestone from the medieval Bishop’s Palace, Lincoln, UK. Stud. Conserv. 2020, 65 (Suppl. 1), P90–P97. [Google Scholar] [CrossRef]
- Iafrate, S.; Sidoti, G.; Capasso, F.E.; Giandomenico, M.; Muca, S.; Daniele, V.; Taglieri, G. New Perspectives for the Consolidation of Mural Paintings in Hypogea with an Innovative Aqueous Nanolime Dispersion, Characterized by Compatible, Sustainable, and Eco-Friendly Features. Nanomaterials 2023, 13, 317. [Google Scholar] [CrossRef] [PubMed]
- Taglieri, G.; Daniele, V.; Del Re, G.; Volpe, R. A New and Original Method to Produce Ca(OH)2 Nanoparticles by Using an Anion Exchange Resin. Adv. Nanopart. 2015, 4, 17–24. [Google Scholar] [CrossRef]
- Ševčík, R.; Mácová, P.; Estébanez, M.P.; Viani, A. Influence of additions of synthetic anhydrous calcium carbonate polymorphs on nanolime carbonation. Constr. Build. Mater. 2019, 228, 116802. [Google Scholar] [CrossRef]
- Ounoughene, G.; Buskens, E.; Santos, R.M.; Cizer, Ö.; Van Gerven, T. Solvochemical carbonation of lime using ethanol: Mechanism and enhancement for direct atmospheric CO2 capture. J. CO2 Util. 2018, 26, 143–151. [Google Scholar] [CrossRef]
- Nouri, S.M.M.; Ebrahim, H.A.; Nejad, B.N. Preparation of a Nano CaO Sorbent for Improvement the Capacity for CO2 Capture Reaction. Synth. React. Inorg. Met. Nano-Met. Chem. 2015, 45, 828–833. [Google Scholar] [CrossRef]
- Giorgi, R.; Dei, L.; Ceccato, M.; Schettino, C.; Baglioni, P. Nanotechnologies for Conservation of Cultural Heritage: Paper and Canvas Deacidification. Langmuir 2002, 18, 8198–8203. [Google Scholar] [CrossRef]
- Burnett, C.A.; Lushniak, B.D.; McCarthy, W.; Kaufman, J. Occupational dermatitis causing days away from work in U.S. private industry, 1993. Am. J. Ind. Med. 1998, 34, 568–573. [Google Scholar] [CrossRef]
- Morris, S.; Lurati, A.; Harbison, R.D.; Bourgeois, M.M.; Johnson, G.T. Alkali Compounds; John Wiley Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 25–31. [Google Scholar]
- Dianat, O.; Azadnia, S.; Mozayeni, M.A. Toxicity of calcium hydroxide nanoparticles on murine fibroblast cell line. Iran. Endod. J. 2015, 10, 49. [Google Scholar]
- Mohamed, H.R.H. Induction of genotoxicity and differential alterations of p53 and inflammatory cytokines expression by acute oral exposure to bulk- or nano-calcium hydroxide particles in mice “Genotoxicity of normal- and nano-calcium hydroxide”. Toxicol. Mech. Methods 2021, 31, 169–181. [Google Scholar] [CrossRef]
- Bartsch, R.; Michaelsen, S.; Hartwig, A.; MAK Commission. Calcium hydroxide [MAK Value Documentations, 2013]. In The MAK-Collection for Occupational Health and Safety: Annual Thresholds and Classifications for the Workplace; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2002; Volume 1, pp. 12–25. [Google Scholar]
- Li, G.; Liang, L.; Yang, J.; Zeng, L.; Xie, Z.; Zhong, Y.; Ruan, X.; Dong, M.; Yang, Z.; Lai, G.; et al. Pulmonary hypofunction due to calcium carbonate nanomaterial exposure in occupational workers: A cross-sectional study. Nanotoxicology 2018, 12, 571–585. [Google Scholar] [CrossRef]
- D’Amora, M.; Liendo, F.; Deorsola, F.A.; Bensaid, S.; Giordani, S. Toxicological profile of calcium carbonate nanoparticles for industrial applications. Colloids Surfaces B Biointerfaces 2020, 190, 110947. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Fernandez, A.; Gómez-Villalba, L.S.; Rabanal, M.E.; Fort, R. New consolidant product based on nanoparticles to preserve the dolomitic stone heritage. In Science, Technology and Cultural Heritage; CRC Press: Boca Raton, FL, USA, 2014; pp. 139–144. [Google Scholar]
- Sierra-Fernandez, A.; Gomez-Villalba, L.S.; Rabanal, M.E.; Fort, R.; Csóka, L. Application of magnesium hydroxide nanocoatings on cellulose fibers with different refining degrees. RSC Adv. 2016, 6, 51583–51590. [Google Scholar] [CrossRef]
- Bessa, M.J.; Brandão, F.; Viana, M.; Gomes, J.F.; Monfort, E.; Cassee, F.R.; Fraga, S.; Teixeira, J.P. Nanoparticle exposure and hazard in the ceramic industry: An overview of potential sources, toxicity and health effects. Environ. Res. 2020, 184, 109297. [Google Scholar] [CrossRef]
- Echeverry-Rendón, M.; Stančič, B.; Muizer, K.; Duque, V.; Calderon, D.J.; Echeverria, F.; Harmsen, M.C. Cytotoxicity Assessment of Surface-Modified Magnesium Hydroxide Nanoparticles. ACS Omega 2022, 7, 17528–17537. [Google Scholar] [CrossRef] [PubMed]
- Ion, R.-M. Analytical Investigations and Advanced Materials for Damage Diagnosis and Conservation of Monument’s Stucco. In Advanced Materials for the Conservation of Stone; Springer: Cham, Switzerland, 2018; pp. 209–221. [Google Scholar]
- Gomez-Villalba, L.S.; Feijoo, J.; Rabanal, M.E.; Fort, R. In-situ electrochemical synthesis of inorganic compounds for materials conservation: Assessment of their effects on the porous structure. Ceram. Int. 2021, 47, 30406–30424. [Google Scholar] [CrossRef]
- Mekmene, O.; Quillard, S.; Rouillon, T.; Bouler, J.-M.; Piot, M.; Gaucheron, F. Effects of pH and Ca/P molar ratio on the quantity and crystalline structure of calcium phosphates obtained from aqueous solutions. Dairy Sci. Technol. 2009, 89, 301–316. [Google Scholar] [CrossRef]
- Recillas, S.; Rodríguez-Lugo, V.; Montero, M.L.; Viquez-Cano, S.; Hernandez, L.; Castano, V.M. Studies on the precipitation behaviour of calcium phosphate solutions. J. Ceram. Process. Res. 2012, 23, 5–10. [Google Scholar]
- Sassoni, E. Hydroxyapatite and Other Calcium Phosphates for the Conservation of Cultural Heritage: A Review. Materials 2018, 11, 557. [Google Scholar] [CrossRef]
- Epple, M. Review of potential health risks associated with nanoscopic calcium phosphate. Acta Biomater. 2018, 77, 1–14. [Google Scholar] [CrossRef]
- Evan, A.P.; Lingeman, J.E.; Coe, F.L.; Shao, Y.; Parks, J.H.; Bledsoe, S.B.; Phillips, C.L.; Bonsib, S.; Worcester, E.M.; Sommer, A.J.; et al. Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int. 2005, 67, 576–591. [Google Scholar] [CrossRef]
- Kono, T.; Sakae, T.; Nakada, H.; Kaneda, T.; Okada, H. Confusion between Carbonate Apatite and Biological Apatite (Carbonated Hydroxyapatite) in Bone and Teeth. Minerals 2022, 12, 170. [Google Scholar] [CrossRef]
- Strutynska, N.; Livitska, O.; Prylutska, S.; Yumyna, Y.; Zelena, P.; Skivka, L.; Malyshenko, A.; Vovchenko, L.; Strelchuk, V.; Prylutskyy, Y.; et al. New nanostructured apatite-type (Na+, Zn2+, CO32−)-doped calcium phosphates: Preparation, mechanical properties and antibacterial activity. J. Mol. Struct. 2020, 1222, 128932. [Google Scholar] [CrossRef]
- Sakae, T.; Nakada, H.; LeGeros, J.P. Historical Review of Biological Apatite Crystallography. J. Hard Tissue Biol. 2015, 24, 111–122. [Google Scholar] [CrossRef]
- Pasero, M.; Kampf, A.R.; Ferraris, C.; Pekov, I.V.; Rakovan, J.; White, T. Nomenclature of the apatite supergroup minerals. Eur. J. Miner. 2010, 22, 163–179. [Google Scholar] [CrossRef]
- Meyer, J.L.; Eanes, E.D. A thermodynamic analysis of the amorphous to crystalline calcium phosphate transformation. Calcif. Tissue Int. 1978, 25, 59–68. [Google Scholar] [CrossRef]
- McConnell, D. Apatite: Its Crystal Chemistry, Mineralogy, Utilization, and Geologic and Biologic Occurrences; Springer Science & Business Media: Berlin, Germany, 2012; Volume 5. [Google Scholar]
- Ptáček, P. Synthetic phase with the structure of apatite. In Chemistry: Apatites and Their Synthetic Analogues-Synthesis, Structure, Properties and Applications; IntechOpen: London, UK, 2016; pp. 177–244. [Google Scholar]
- Ion, R.-M.; Iancu, L.; Vasilievici, G.; Grigore, M.E.; Andrei, R.E.; Radu, G.-I.; Grigorescu, R.M.; Teodorescu, S.; Bucurica, I.A.; Ion, M.-L.; et al. Ion-Substituted Carbonated Hydroxyapatite Coatings for Model Stone Samples. Coatings 2019, 9, 231. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Martel, J.; Cheng, W.-Y.; He, C.-C.; Ojcius, D.; Young, J.D. Membrane Vesicles Nucleate Mineralo-organic Nanoparticles and Induce Carbonate Apatite Precipitation in Human Body Fluids. J. Biol. Chem. 2013, 288, 30571–30584. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Navarrete, D.A. Biological apatites in bone and teeth. In Nanoceramics in Clinical Use: From Materials to Applications, 2nd ed.; Vallet-Regi, M., Arcos Navarrete, D., Eds.; Royal Society of Chemistry: London, UK, 2015; pp. 1–29. [Google Scholar]
- Hesse, A.; Heimbach, D. Causes of phosphate stone formation and the importance of metaphylaxis by urinary acidification: A review. World J. Urol. 1999, 17, 308–315. [Google Scholar] [CrossRef]
- Englert, K.M.; McAteer, J.A.; Lingeman, J.E.; Williams, J.C. High carbonate level of apatite in kidney stones implies infection, but is it predictive? Urolithiasis 2013, 41, 389–394. [Google Scholar] [CrossRef]
- Bazin, D.; Carpentier, X.; Brocheriou, I.; Dorfmuller, P.; Aubert, S.; Chappard, C.; Thiaudière, D.; Reguer, S.; Waychunas, G.; Jungers, P.; et al. Revisiting the localisation of Zn2+ cations sorbed on pathological apatite calcifications made through X-ray absorption spectroscopy. Biochimie 2009, 91, 1294–1300. [Google Scholar] [CrossRef]
- McCarthy, J.; Inkielewicz-Stępniak, I.; Corbalan, J.J.; Radomski, M.W. Mechanisms of Toxicity of Amorphous Silica Nanoparticles on Human Lung Submucosal Cells in Vitro: Protective Effects of Fisetin. Chem. Res. Toxicol. 2012, 25, 2227–2235. [Google Scholar] [CrossRef]
- Napierska, D.; Thomassen, L.C.J.; Lison, D.; Martens, J.A.; Hoet, P.H. The nanosilica hazard: Another variable entity. Part. Fibre Toxicol. 2010, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Rabolli, V.; Thomassen, L.C.; Uwambayinema, F.; Martens, J.A.; Lison, D. The cytotoxic activity of amorphous silica nanoparticles is mainly influenced by surface area and not by aggregation. Toxicol. Lett. 2011, 206, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Franco-Castillo, I.; Hierro, L.; de la Fuente, J.M.; Seral-Ascaso, A.; Mitchell, S.G. Perspectives for antimicrobial nanomaterials in cultural heritage conservation. Chem 2021, 7, 629–669. [Google Scholar] [CrossRef]
- Carrapiço, A.; Martins, M.R.; Caldeira, A.T.; Mirão, J.; Dias, L. Biosynthesis of Metal and Metal Oxide Nanoparticles Using Microbial Cultures: Mechanisms, Antimicrobial Activity and Applications to Cultural Heritage. Microorganisms 2023, 11, 378. [Google Scholar] [CrossRef]
- Castellote, M.; Bengtsson, N. Principles of TiO2 photocatalysis. In Applications of Titanium Dioxide Photocatalysis to Construction Materials; Springer: Dordrecht, The Netherlands, 2011; pp. 5–10. [Google Scholar]
- Munafò, P.; Goffredo, G.B.; Quagliarini, E. TiO2-based nanocoatings for preserving architectural stone surfaces: An overview. Constr. Build. Mater. 2015, 84, 201–218. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X. Titanium dioxide photocatalysis: Present situation and future approaches. Comptes Rendus Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Ashby, M.F.; Ferreira, P.; Schodek, D.L. Nanomaterials, Nanotechnologies and Design: An Introduction for Engineers and Architects; Butterworth-Heinemann: Oxford, UK, 2009. [Google Scholar]
- Shet, V.B.; Navalgund, L.; Joshi, K.; Yumnam, S. Application of Nanoparticles in Construction Industries and Their Toxicity. In Ecological and Health Effects of Building Materials; Springer: Cham, Switzerland, 2022; pp. 147–157. [Google Scholar]
- Zhang, M.; Wu, N.; Yang, J.; Zhang, Z. Photoelectrochemical Antibacterial Platform Based on Rationally Designed Black TiO2–x Nanowires for Efficient Inactivation against Bacteria. ACS Appl. Bio Mater. 2022, 5, 1341–1347. [Google Scholar] [CrossRef]
- Santhosh, G.; Nayaka, G.P. Nanoparticles in Construction Industry and Their Toxicity. In Ecological and Health Effects of Building Materials; Springer: Cham, Switzerland, 2022; pp. 133–146. [Google Scholar]
- Sayes, C.M.; Wahi, R.; Kurian, P.A.; Liu, Y.; West, J.L.; Ausman, K.D.; Warheit, D.B.; Colvin, V.L. Correlating Nanoscale Titania Structure with Toxicity: A Cytotoxicity and Inflammatory Response Study with Human Dermal Fibroblasts and Human Lung Epithelial Cells. Toxicol. Sci. 2006, 92, 174–185. [Google Scholar] [CrossRef]
- Guillard, A.; Gaultier, E.; Cartier, C.; Devoille, L.; Noireaux, J.; Chevalier, L.; Morin, M.; Grandin, F.; Lacroix, M.Z.; Coméra, C.; et al. Basal Ti level in the human placenta and meconium and evidence of a materno-foetal transfer of food-grade TiO2 nanoparticles in an ex vivo placental perfusion model. Part. Fibre Toxicol. 2020, 17, 1–15. [Google Scholar] [CrossRef]
- Saber, A.T.; Jacobsen, N.R.; Mortensen, A.; Szarek, J.; Jackson, P.; Madsen, A.M.; Jensen, K.A.; Koponen, I.K.; Brunborg, G.; Gützkow, K.B.; et al. Nanotitanium dioxide toxicity in mouse lung is reduced in sanding dust from paint. Part. Fibre Toxicol. 2012, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Bacci, M.; Picollo, M.; Trumpy, G.; Tsukada, M.; Kunzelman, D. Non-Invasive Identification of White Pigments on 20Th-Century Oil Paintings by Using Fiber Optic Reflectance Spectroscopy. J. Am. Inst. Conserv. 2007, 46, 27–37. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Hassan, N.F.N.; Middleton, P.S. Anatase—A pigment in ancient artwork or a modern usurper? Anal. Bioanal. Chem. 2006, 384, 1356–1365. [Google Scholar] [CrossRef]
- Aldosari, M.A.; Darwish, S.S.; Adam, M.A.; Elmarzugi, N.A.; Ahmed, S.M. Using ZnO nanoparticles in fungal inhibition and self-protection of exposed marble columns in historic sites. Archaeol. Anthr. Sci. 2019, 11, 3407–3422. [Google Scholar] [CrossRef]
- Sierra-Fernandez, A.; Gomez-Villalba, L.S.; De la Rosa-García, S.C.; Gomez-Cornelio, S.; Quintana, P.; Rabanal, M.; Fort, R. Inorganic Nanomaterials for the Consolidation and Antifungal Protection of Stone Heritage. In Advanced Materials for the Conservation of Stone; Springer: Cham, Switzerland, 2018; pp. 125–149. [Google Scholar]
- Li, X.; Kang, B.; Eom, Y.; Zhong, J.; Lee, H.K.; Kim, H.M.; Song, J.S. Comparison of cytotoxicity effects induced by four different types of nanoparticles in human corneal and conjunctival epithelial cells. Sci. Rep. 2022, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Kermanizadeh, A.; Cassee, F.R.; de Jong, W. The Potential Adverse Effects of Engineered Nanomaterial Exposure to Human Health Following Pulmonary, Oral and Dermal Exposure. In Nanotoxicology in Humans and the Environment; Springer: Cham, Switzerland, 2021; pp. 41–58. [Google Scholar] [CrossRef]
- Gulson, B.; McCall, M.; Korsch, M.; Gomez, L.; Casey, P.; Oytam, Y.; Taylor, A.; McCulloch, M.; Trotter, J.; Kinsley, L.; et al. Small Amounts of Zinc from Zinc Oxide Particles in Sunscreens Applied Outdoors Are Absorbed through Human Skin. Toxicol. Sci. 2010, 118, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Yaqub, A.; Faheem, I.; Anjum, K.M.; Ditta, S.A.; Yousaf, M.Z.; Tanvir, F.; Raza, C. Neurotoxicity of ZnO nanoparticles and associated motor function deficits in mice. Appl. Nanosci. 2020, 10, 177–185. [Google Scholar] [CrossRef]
- Guan, R.; Kang, T.; Lu, F.; Zhang, Z.; Shen, H.; Liu, M. Cytotoxicity, oxidative stress, and genotoxicity in human hepatocyte and embryonic kidney cells exposed to ZnO nanoparticles. Nanoscale Res. Lett. 2012, 7, 602. [Google Scholar] [CrossRef]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.S.; Chen, G. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef]
- Ren, X.; Meng, X.; Chen, D.; Tang, F.; Jiao, J. Using silver nanoparticle to enhance current response of biosensor. Biosens. Bioelectron. 2005, 21, 433–437. [Google Scholar] [CrossRef]
- Mathur, P.; Jha, S.; Ramteke, S.; Jain, N.K. Pharmaceutical aspects of silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S1), 115–126. [Google Scholar] [CrossRef]
- Jouyban, A.; Rahimpour, E. Optical sensors based on silver nanoparticles for determination of pharmaceuticals: An overview of advances in the last decade. Talanta 2020, 217, 121071. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Khan, A.; Marwani, H.M.; Asiri, A.M. Hydrazine sensor based on silver nanoparticle-decorated polyaniline tungstophosphate nanocomposite for use in environmental remediation. Microchim. Acta 2016, 183, 1787–1796. [Google Scholar] [CrossRef]
- Chen, D.; Qiao, X.; Qiu, X.; Chen, J. Synthesis and electrical properties of uniform silver nanoparticles for electronic applications. J. Mater. Sci. 2009, 44, 1076–1081. [Google Scholar] [CrossRef]
- Bapat, M.S.; Singh, H.; Shukla, S.K.; Singh, P.P.; Vo, D.-V.N.; Yadav, A.; Goyal, A.; Sharma, A.; Kumar, D. Evaluating green silver nanoparticles as prospective biopesticides: An environmental standpoint. Chemosphere 2022, 286, 131761. [Google Scholar] [CrossRef]
- Kale, S.K.; Parishwad, G.V.; Husainy, A.S.N.; Patil, A.S.N.H.A.S. Emerging Agriculture Applications of Silver Nanoparticles. ES Food Agrofor. 2021, 3, 17–22. [Google Scholar] [CrossRef]
- Vitali, F.; Raio, A.; Sebastiani, F.; Cherubini, P.; Cavalieri, D.; Cocozza, C. Environmental pollution effects on plant microbiota: The case study of poplar bacterial-fungal response to silver nanoparticles. Appl. Microbiol. Biotechnol. 2019, 103, 8215–8227. [Google Scholar] [CrossRef]
- Carbone, S.; Antisari, L.V.; Gaggia, F.; Baffoni, L.; Di Gioia, D.; Vianello, G.; Nannipieri, P. Bioavailability and biological effect of engineered silver nanoparticles in a forest soil. J. Hazard. Mater. 2014, 280, 89–96. [Google Scholar] [CrossRef]
- Simbine, E.; Rodrigues, L.D.C.; Lapa-Guimarães, J.; Kamimura, E.S.; Corassin, C.H.; De Oliveira, C.A.F. Application of silver nanoparticles in food packages: A review. Food Sci. Technol. 2019, 39, 793–802. [Google Scholar] [CrossRef]
- Carbone, M.; Donia, D.T.; Sabbatella, G.; Antiochia, R. Silver nanoparticles in polymeric matrices for fresh food packaging. J. King Saud Univ.-Sci. 2016, 28, 273–279. [Google Scholar] [CrossRef]
- Lotfizadeh, H.; Rezazadeh, S.; Fathollahi, M.R.; Jokar, J.; Mehrizi, A.A.; Soltannia, B. The effect of silver nanoparticles on the automotive-based paint drying process: An experimental study. Int. J. Adv. Multidiscip. Eng. Sci. 2018, 2, 7–14. [Google Scholar]
- Oliveira, M.J.A.; Otubo, L.; Pires, A.; Brambilla, R.F.; Carvalho, A.C.; Santos, P.S.; Neto, A.O.; Vasquez, P. Silver nanoparticles-based hydrogels synthetized by ionizing radiation for cleaning of tangible cultural heritage surfaces. Radiat. Phys. Chem. 2022, 199, 110345. [Google Scholar] [CrossRef]
- Becerra, J.; Mateo, M.; Ortiz, P.; Nicolás, G.; Zaderenko, A.P. Evaluation of the applicability of nano-biocide treatments on limestones used in cultural heritage. J. Cult. Heritage 2019, 38, 126–135. [Google Scholar] [CrossRef]
- Bellissima, F.; Bonini, M.; Giorgi, R.; Baglioni, P.; Barresi, G.; Mastromei, G.; Perito, B. Antibacterial activity of silver nanoparticles grafted on stone surface. Environ. Sci. Pollut. Res. 2014, 21, 13278–13286. [Google Scholar] [CrossRef]
- Baglioni, P.; Carretti, E.; Chelazzi, D. Nanomaterials in art conservation. Nat. Nanotechnol. 2015, 10, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Ilieș, A.; Hodor, N.; Pantea, E.; Ilieș, D.C.; Indrie, L.; Zdrîncă, M.; Iancu, S.; Caciora, T.; Chiriac, A.; Ghergheles, C.; et al. Antibacterial Effect of Eco-Friendly Silver Nanoparticles and Traditional Techniques on Aged Heritage Textile, Investigated by Dark-Field Microscopy. Coatings 2022, 12, 1688. [Google Scholar]
- Tortella, G.; Rubilar, O.; Durán, N.; Diez, M.; Martínez, M.; Parada, J.; Seabra, A. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef]
- Budama-Kilinc, Y.; Cakir-Koc, R.; Zorlu, T.; Ozdemir, B.; Karavelioglu, Z.; Egil, A.C.; Kecel-Gunduz, S. Assessment of Nano-Toxicity and Safety Profiles of Silver Nanoparticles; IntechOpen: London, UK, 2018; p. 75645. [Google Scholar]
- Brule, S.V.D.; Ambroise, J.; Lecloux, H.; Levard, C.; Soulas, R.; De Temmerman, P.-J.; Palmai-Pallag, M.; Marbaix, E.; Lison, D. Dietary silver nanoparticles can disturb the gut microbiota in mice. Part. Fibre Toxicol. 2015, 13, 38. [Google Scholar] [CrossRef]
- Rodriguez-Garraus, A.; Azqueta, A.; Vettorazzi, A.; de Cerain, A.L. Genotoxicity of Silver Nanoparticles. Nanomaterials 2020, 10, 251. [Google Scholar] [CrossRef]
- Kim, S.; Ryu, D.-Y. Silver nanoparticle-induced oxidative stress, genotoxicity and apoptosis in cultured cells and animal tissues. J. Appl. Toxicol. 2013, 33, 78–89. [Google Scholar] [CrossRef]
- Ramalingam, V. Multifunctionality of gold nanoparticles: Plausible and convincing properties. Adv. Colloid Interface Sci. 2019, 271, 101989. [Google Scholar] [CrossRef] [PubMed]
- Senior, K.; Müller, S.; Schacht, V.J.; Bunge, M. Antimicrobial Precious-Metal Nanoparticles and their Use in Novel Materials. Recent Pat. Food Nutr. Agric. 2012, 4, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, P.; Chelazzi, D. How science can contribute to the remedial conservation of cultural heritage. Chem. Eur. J. 2021, 27, 10798–10806. [Google Scholar] [CrossRef]
- Ion, R.-M.; Nyokong, T.; Nwahara, N.; Suica-Bunghez, R.; Iancu, L.; Teodorescu, S.; Dulama, I.D.; Stirbescu, R.M.; Gheboianu, A.; Grigorescu, R.M. Wood preservation with gold hydroxyapatite system. Heritage Sci. 2018, 6, 37. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. How toxic are gold nanoparticles? The state-of-the-art. Nano Res. 2015, 8, 1771–1799. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef]
- Khan, M.A.R.; Al Mamun, M.S.; Ara, M.H. Review on platinum nanoparticles: Synthesis, characterization, and applications. Microchem. J. 2021, 171, 106840. [Google Scholar] [CrossRef]
- Ortega-Morales, B.O.; Reyes-Estebanez, M.M.; Gaylarde, C.C.; Camacho-Chab, J.C.; Sanmartín, P.; Chan-Bacab, M.J.; Granados-Echegoyen, C.A.; Pereañez-Sacarias, J.E. Antimicrobial properties of nanomaterials used to control microbial colonization of stone substrata. In Advanced Materials for the Conservation of Stone; Springer: Berlin/Heidelberg, Germany, 2018; pp. 277–298. [Google Scholar]
- Lin, X.; Wu, M.; Wu, D.; Kuga, S.; Endo, D.; Huang, Y. Platinum nanoparticles using wood nanomaterials: Eco-friendly synthesis, shape control and catalytic activity for p-nitrophenol reduction. Green Chem. 2011, 13, 283–287. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Ryu, H.-S.; Bae, I.-H.; Lee, K.-G.; Hwang, H.-S.; Lee, K.-H.; Koh, J.-T.; Cho, J.-H. Antibacterial effect of silver-platinum coating for orthodontic appliances. Angle Orthod. 2011, 82, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Czubacka, E.; Czerczak, S. Are platinum nanoparticles safe to human health? Med. Pr. 2019, 70, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Bayade, G.; Wu, M.R.; Massicotte, R.; Deryabin, D.G.E.; Yahia, L.H. Biocidal properties of copper nanoparticles. Eng. Biomater. 2021, 24, 159. [Google Scholar]
- Pinna, D.; Galeotti, M.; Perito, B.; Daly, G.; Salvadori, B. In situ long-term monitoring of recolonization by fungi and lichens after innovative and traditional conservative treatments of archaeological stones in Fiesole (Italy). Int. Biodeterior. Biodegrad. 2018, 132, 49–58. [Google Scholar] [CrossRef]
- Ditaranto, N.; Loperfido, S.; van der Werf, I.; Mangone, A.; Cioffi, N.; Sabbatini, L. Synthesis and analytical characterisation of copper-based nanocoatings for bioactive stone artworks treatment. Anal. Bioanal. Chem. 2011, 399, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Akhtari, M.; Nicholas, D. Evaluation of particulate zinc and copper as wood preservatives for termite control. Eur. J. Wood Wood Prod. 2013, 71, 395–396. [Google Scholar] [CrossRef]
- Pohanka, M. Copper and copper nanoparticles toxicity and their impact on basic functions in the body. Bratisl. Med. J. 2019, 120, 397–409. [Google Scholar] [CrossRef]
- Prajapati, V.; Sharma, P.K.; Banik, A. Carbon nanotubes and its applications. Int. J. Pharm. Sci. Res. 2011, 2, 1099. [Google Scholar]
- Iijima, S. Carbon nanotubes: Past, present, and future. Phys. B Condens. Matter 2002, 323, 1–5. [Google Scholar] [CrossRef]
- Jiménez-Rodríguez, J.A.; Arana-Sosa, V.; Franco, A. Síntesis de nanotubos de carbono multicapa sobre sustratos metálicos por el método de depósito químico de vapores: No todos los nanotubos son iguales. Mundo Nano Rev. Interdiscip. Nanoci. Nanotecnol. 2017, 10, 93–108. [Google Scholar] [CrossRef]
- Lehman, J.H.; Terrones, M.; Mansfield, E.; Hurst, K.E.; Meunier, V. Evaluating the characteristics of multiwall carbon nanotubes. Carbon 2011, 49, 2581–2602. [Google Scholar] [CrossRef]
- Sianipar, M.; Kim, S.H.; Khoiruddin, K.; Iskandar, F.; Wenten, I.G. Functionalized carbon nanotube (CNT) membrane: Progress and challenges. RSC Adv. 2017, 7, 51175–51198. [Google Scholar] [CrossRef]
- Kausar, H.; Khan, M.S.; Islam, A.; Ahmad, A.; Kant, R.; Nami, S.A. Polycarbazole-multiwalled carbon nanotubes based nanocomposite: Synthesis, spectral, biocidal and Acetaldehyde sensing studies. J. Mol. Struct. 2021, 1229, 129704. [Google Scholar] [CrossRef]
- Aschberger, K.; Campia, I.; Pesudo, L.Q.; Radovnikovic, A.; Reina, V. Chemical alternatives assessment of different flame retardants—A case study including multi-walled carbon nanotubes as synergist. Environ. Int. 2017, 101, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Pitroda, J.; Jethwa, B.; Dave, S.K. A critical review on carbon nanotubes. Int. J. Constr. Res. Civ. Eng. 2016, 2, 36–42. [Google Scholar]
- Bussy, C.; Methven, L.; Kostarelos, K. Hemotoxicity of carbon nanotubes. Adv. Drug Deliv. Rev. 2013, 65, 2127–2134. [Google Scholar] [CrossRef]
- Park, Y.-H.; Jeong, S.H.; Lee, E.Y.; Lee, S.-H.; Choi, B.H.; Kim, M.-K.; Son, S.W. Assessment of dermal irritation potential of MWCNT. Toxicol. Environ. Health Sci. 2010, 2, 115–118. [Google Scholar] [CrossRef]
- Foldvari, M.; Bagonluri, M. Carbon nanotubes as functional excipients for nanomedicines: II. Drug delivery and biocompatibility issues. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 183–200. [Google Scholar] [CrossRef]
- Mohanta, D.; Patnaik, S.; Sood, S.; Das, N. Carbon nanotubes: Evaluation of toxicity at biointerfaces. J. Pharm. Anal. 2019, 9, 293–300. [Google Scholar] [CrossRef]
- Wang, L.; Mercer, R.R.; Rojanasakul, Y.; Qiu, A.; Lu, Y.; Scabilloni, J.F.; Wu, N.; Castranova, V. Direct Fibrogenic Effects of Dispersed Single-Walled Carbon Nanotubes on Human Lung Fibroblasts. J. Toxicol. Environ. Health Part A 2010, 73, 410–422. [Google Scholar] [CrossRef]
- Kakoti, B.B.; Kataki, M.S.; Pathak, Y. Nanotoxicity of Nanobiomaterials in Ocular System and Its Evaluation. In Nano-Biomaterials For Ophthalmic Drug Delivery; Springer: Cham, Switzerland, 2016; pp. 495–533. [Google Scholar]
- Bardi, G.; Nunes, A.; Gherardini, L.; Bates, K.; Al-Jamal, K.T.; Gaillard, C.; Prato, M.; Bianco, A.; Pizzorusso, T.; Kostarelos, K. Functionalized Carbon Nanotubes in the Brain: Cellular Internalization and Neuroinflammatory Responses. PLoS ONE 2013, 8, e80964. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Y.; Sun, B.; Chen, C. Understanding the Toxicity of Carbon Nanotubes. Accounts Chem. Res. 2013, 46, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, H.; Wang, H. Flame retardant mechanism and surface modification of magnesium hydroxide flame retardant. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 170, p. 032028. [Google Scholar]
- Zhang, Z.; Feng, L.; Qiu, F.; Lan, B. Advances in fire-retardant inorganic nanomaterials. Prog. Chem. 2004, 16, 508. [Google Scholar]
- Walter, M.D.; Wajer, M.T. Overview of Flame Retardants including Magnesium Hydroxide; Martin Marietta Magnesia Specialties: Baltimore, MD, USA, 2015. [Google Scholar]
- Petrova, S.; Soudek, P.; Vanek, T. Flame retardants, their use and environmental impact. Chem. Listy 2015, 109, 679–686. [Google Scholar]
- Purser, D. Toxicity of fire retardants in relation to life safety and environmental hazards. In Fire Retardant Materials; Woodhead Publishing: Sawston, UK, 2001; pp. 69–127. [Google Scholar]
- Janas, D.; Rdest, M.; Koziol, K.K. Flame-retardant carbon nanotube films. Appl. Surf. Sci. 2017, 411, 177–181. [Google Scholar] [CrossRef]
- Zhang, L.; Bian, Y.; Kuai, D. Preparation and flame retardant property of nano-aluminum hydroxide foam for preventing spontaneous coal combustion. Fuel 2021, 304, 121494. [Google Scholar] [CrossRef]
- Domingo, J.L. Reproductive and developmental toxicity of aluminum: A review. Neurotoxicol. Teratol. 1995, 17, 515–521. [Google Scholar] [CrossRef]
- He, P.; Zou, Y.; Hu, Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum. Vaccines Immunother. 2015, 11, 477–488. [Google Scholar] [CrossRef]
- Warheit, D.B.; Sayes, C.M.; Frame, S.R.; Reed, K.L. Pulmonary exposures to Sepiolite nanoclay particulates in rats: Resolution following multinucleate giant cell formation. Toxicol. Lett. 2010, 192, 286–293. [Google Scholar] [CrossRef]
- Baek, M.; Lee, J.-A.; Choi, S.-J. Toxicological effects of a cationic clay, montmorillonite in vitro and in vivo. Mol. Cell. Toxicol. 2012, 8, 95–101. [Google Scholar] [CrossRef]
- Maisanaba, S.; Gutiérrez-Praena, D.; Pichardo, S.; Moreno, F.J.; Jordá, M.; Cameán, A.M.; Aucejo, S.; Jos, Á. Toxic effects of a modified montmorillonite clay on the human intestinal cell line Caco-2. J. Appl. Toxicol. 2014, 34, 714–725. [Google Scholar] [CrossRef]
- Fistos, T.; Fierascu, I.; Doni, M.; Chican, I.E.; Fierascu, R.C. A Short Overview of Recent Developments in the Application of Polymeric Materials for the Conservation of Stone Cultural Heritage Elements. Materials 2022, 15, 6294. [Google Scholar] [CrossRef] [PubMed]
- Azadi, N.; Parsimehr, H.; Ershad-Langroudi, A. Cultural heritage protection via hybrid nanocomposite coating. Plast. Rubber Compos. 2020, 49, 414–424. [Google Scholar] [CrossRef]
- Mosquera, M.J.; Carrascosa, L.A.; Badreldin, N. Producing superhydrophobic/oleophobic coatings on Cultural Heritage building materials. Pure Appl. Chem. 2018, 90, 551–561. [Google Scholar] [CrossRef]
- Colangiuli, D.; Lettieri, M.; Masieri, M.; Calia, A. Field study in an urban environment of simultaneous self-cleaning and hydrophobic nanosized TiO2-based coatings on stone for the protection of building surface. Sci. Total. Environ. 2019, 650, 2919–2930. [Google Scholar] [CrossRef] [PubMed]
- Pinho, L.; Rojas, M.; Mosquera, M.J. Ag–SiO2–TiO2 nanocomposite coatings with enhanced photoactivity for self-cleaning application on building materials. Appl. Catal. B Environ. 2015, 178, 144–154. [Google Scholar] [CrossRef]
- Kapridaki, C.; Pinho, L.; Mosquera, M.J.; Maravelaki-Kalaitzaki, P. Producing photoactive, transparent and hydrophobic SiO2-crystalline TiO2 nanocomposites at ambient conditions with application as self-cleaning coatings. Appl. Catal. B Environ. 2014, 156–157, 416–427. [Google Scholar] [CrossRef]
- Petersen, E.J.; Lam, T.; Gorham, J.M.; Scott, K.C.; Long, C.J.; Stanley, D.; Sharma, R.; Liddle, J.A.; Pellegrin, B.; Nguyen, T. Methods to assess the impact of UV irradiation on the surface chemistry and structure of multiwall carbon nanotube epoxy nanocomposites. Carbon 2014, 69, 194–205. [Google Scholar] [CrossRef]
- Nowack, B.; David, R.M.; Fissan, H.; Morris, H.; Shatkin, J.A.; Stintz, M.; Zepp, R.; Brouwer, D. Potential release scenarios for carbon nanotubes used in composites. Environ. Int. 2013, 59, 1–11. [Google Scholar] [CrossRef]
- Nguyen, T.; Pellegrin, B.; Bernard, C.; Rabb, S.; Stuztman, P.; Gorham, J.; Gu, X.; Yu, L.L.; Chin, J.W. Characterization of Surface Accumulation and Release of Nanosilica During Irradiation of Polymer Nanocomposites by Ultraviolet Light. J. Nanosci. Nanotechnol. 2012, 12, 6202–6215. [Google Scholar] [CrossRef]
- Pacheco, I.; Buzea, C. Nanomaterials and Nanocomposites: Classification and Toxicity. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–38. [Google Scholar]
- Parandhaman, T.; Dey, M.D.; Das, S.K. Biofabrication of supported metal nanoparticles: Exploring the bioinspiration strategy to mitigate the environmental challenges. Green Chem. 2019, 21, 5469–5500. [Google Scholar] [CrossRef]
- Hussain, I.; Singh, N.B.; Singh, A.; Singh, H.; Singh, S.C. Green synthesis of nanoparticles and its potential application. Biotechnol. Lett. 2016, 38, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Chouke, P.B.; Shrirame, T.; Potbhare, A.K.; Mondal, A.; Chaudhary, A.R.; Mondal, S.; Thakare, S.R.; Nepovimova, E.; Valis, M.; Kuca, K.; et al. Bioinspired metal/metal oxide nanoparticles: A road map to potential applications. Mater. Today Adv. 2022, 16, 100314. [Google Scholar] [CrossRef]
- Nangare, S.N.; Patil, P.O. Green Synthesis of Silver Nanoparticles: An Eco-Friendly Approach. Nano Biomed. Eng. 2020, 12, 281–296. [Google Scholar] [CrossRef]
- Prasad, A.R.; Garvasis, J.; Oruvil, S.K.; Joseph, A. Bio-inspired green synthesis of zinc oxide nanoparticles using Abelmoschus esculentus mucilage and selective degradation of cationic dye pollutants. J. Phys. Chem. Solids 2019, 127, 265–274. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lin, J.; Chen, Z.; Megharaj, M.; Naidu, R. Green synthesized iron nanoparticles by green tea and eucalyptus leaves extracts used for removal of nitrate in aqueous solution. J. Clean. Prod. 2014, 83, 413–419. [Google Scholar] [CrossRef]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of gold nanotriangles and silver nanoparticles using Aloevera plant extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef]
- Davar, F.; Majedi, A.; Mirzaei, A. Green Synthesis of ZnO Nanoparticles and Its Application in the Degradation of Some Dyes. J. Am. Ceram. Soc. 2015, 98, 1739–1746. [Google Scholar] [CrossRef]
- Nabi, G.; Aain, Q.U.; Khalid, N.R.; Tahir, M.B.; Rafique, M.; Rizwan, M.; Hussain, S.; Iqbal, T.; Majid, A. A Review on Novel Eco-Friendly Green Approach to Synthesis TiO2 Nanoparticles Using Different Extracts. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1552–1564. [Google Scholar] [CrossRef]
- Eisenberger, I.; Nentwich, M.; Fiedeler, U.; Gazsó, A.; Simkó, M. Nano Regulation in Austria (I): Chemical and Product Safety (NanoTrust Dossier No. 018en–January 2011); Institut für Technikfolgen-Abschätzung: Wien, Austria, 2011. [Google Scholar]
- Gazs, D.F.A. Nano risk governance: The Austrian case. Int. J. Perform. Eng. 2015, 11, 569. [Google Scholar]
- Hansen, S.F.; Jensen, K.A.; Baun, A. NanoRiskCat: A conceptual tool for categorization and communication of exposure potentials and hazards of nanomaterials in consumer products. J. Nanopart. Res. 2014, 16, 2195. [Google Scholar] [CrossRef]
- Gaffet, E.; Bloch, D.; Gouget, B.; Herlin-Boime, N.; Honnert, B.; Lombard, A.; Michael, R.; Tardif, F. Nanomatériaux et Sécurité au Travail: Rapport du Groupe de l’Afsset, Agents Physiques, Nouvelles Technologies et Grands Aménagements; Afsset (Agence Française de Sécurité Sanitaire de l’environnement et du Travail): Maisons-Alfort, France, 2008. [Google Scholar]
- BAuA/VCI. Empfehlung für die Gefährdungsbeurteilung bei Tätigkeiten mit Nanomaterialien am Arbeitsplatz. Bundesanstalt für Arbeitsschutz und Arbeitsmedizin (BAuA)/Verband der Chemischen Industrie e.V. (VCI), Germany. 2012. Available online: https://www.baua.de/DE/Angebote/Publikationen/Kooperation/Gd4.html (accessed on 26 February 2023).
- Ispesl-INAIL. White Book Exposure To Engineered Nanomaterials and Occupational Health and Safety Effects. 2011. Available online: https://www.inail.it/cs/internet/docs/alg-libro_bianco-esposizione-a-nanomateriali-eng.pdf (accessed on 26 February 2023).
- Schmidt-Ott, A.; Butselaar-Orthlieb, V.; van Winsen, J.; Bosma, D. Nanosafety Guidelines: Preventing Exposure to Nanomaterials at the Faculty of Applied Sciences; Delft University of Technology, Workgroup Nanosafety of the Faculty of Applied Sciences: Delft, The Netherlands, 2010. [Google Scholar]
- Van Est, R.; Walhout, B.; Rerimassie, V.; Stemerding, D.; Hanssen, L. Governance of nanotechnology in the Netherlands: Informing and engaging in different social spheres. Int. J. Emerg. Technol. Soc. 2012, 10, 6–26. [Google Scholar]
- Swiss Federal Government’s Central Information Hub for Nanotechnology. Available online: https://www.bag.admin.ch/bag/en/home/gesund-leben/umwelt-und-gesundheit/chemikalien/nanotechnologie.html (accessed on 26 February 2023).
- Royo, S. Instituto Nacional de Seguridad e Higiene en el Trabajo. Arch. Prevención Riesgos Labor. 2016, 19, 124–126. [Google Scholar]
- Veiga-Álvarez, Á.; Sánchez-De-Alcázar, D.; Martínez-Negro, M.; Barbu, A.; González-Díaz, J.B.; Maquea-Blasco, J. Riesgos para la salud y recomendaciones en el manejo de nanopartículas en entornos laborales. Med. Segur. Trab. 2015, 61, 143–161. [Google Scholar] [CrossRef]
- British Standard Institute (BSI). Nanotechnologies—Part 2: Guide to Safe Handling and Disposal of Manufactured Nanomaterials vol PD 6699-2:2007 UK. 2007. Available online: https://www.bsigroup.com/en-GB/industries-and-sectors/nanotechnology/ (accessed on 26 February 2023).
- Benko, H. ISO technical committee 229 nanotechnologies. In Metrology and Standardization of Nanotechnology: Protocols and Industrial Innovations; Wiley Online Library: Hoboken, NJ, USA, 2017; pp. 259–268. [Google Scholar]
- Bochon, A. Evolution of the European commission recommendation for a code of conduct for responsible nanosciences and nanotechnology research. Nanotech. L. Bus. 2011, 8, 117. [Google Scholar]
- Hullmann, A. European Activities in the Field of Ethical, Legal and Social Aspects (ELSA) and Governance of Nanotechnology; DG Research; European Commission: Brussels, Belgium, 2008; Available online: https://www.nanowerk.com/nanotechnology/reports/reportpdf/report122.pdf (accessed on 25 February 2023).
- European Commission. A Code of Conduct for Responsible Nanosciences and Nanotechnologies Research. 2008. Available online: https://ec.europa.eu/commission/presscorner/detail/en/IP_08_193 (accessed on 26 February 2023).
- Haslinger, J.; Hocke, P.; Hauser, C. Nanotechnology in the Media–On the Reporting in Representative Daily Newspapers in Austria, Germany and Switzerland (NanoTrust Dossier No. 037en–October 2012). 2020. Available online: https://epub.oeaw.ac.at/0xc1aa5576_0x002d5aed.pdf (accessed on 11 April 2023).
- Hodson, L.; Eastlake, A. Occupational Exposure Sampling for Engineered Nanomaterials; Technical Report, DHHS (NIOSH) Publication No. 2022-153; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health: Cincinnati, OH, USA, 2022. [CrossRef]
- NIOSH. Current Strategies for Engineering Controls in Nanomaterial Production and Downstream Handling Processes (NIOSH Pub. 2014-102); National Institute of Occupational Safety Health: Cincinnati, OH, USA, 2014.
- Harmon, S.H. Book Review: International Handbook on Regulating Nanotechnologies. Scripted 2012, 9, 271–273. [Google Scholar] [CrossRef]
- Ostiguy, C.; Roberge, B.; Woods, C.; Soucy, B. Les Nanoparticules de Synthèse-Connaissances Actuelles sur les Risques et les Mesures de Prévention en SST, 2nd ed.; IRSST: Montréal, QC, Canada, 2010. [Google Scholar]
- NanoSafe Australia 2007. Current OHS Best Practices for the Australian Nanotechnology Industry—A Position Paper by the NanoSafe Australia Network. RMIT University, Melbourne, Australia. Available online: https://nanotech.law.asu.edu/Documents/2011/06/72nuxiavskpg_532_3444.pdf (accessed on 25 February 2023).
- Jarvis, D.S.; Richmond, N. Regulation and governance of nanotechnology in China: Regulatory challenges and effectiveness. Eur. J. Law Technol. 2011, 2, 3. Available online: https://ejlt.org/index.php/ejlt/article/view/94/155 (accessed on 11 April 2023).
- Besserman, J.; Mentzer, R.A. Review of global process safety regulations: United States, European Union, United Kingdom, China, India. J. Loss Prev. Process. Ind. 2017, 50, 165–183. [Google Scholar] [CrossRef]
- Savolainen, K. Nanosafety in Europe 2015–2025: Towards Safe and Sustainable Nanomaterials and Nanotechnology Innovations; Finnish Institute of Occupational Health: Helsinki, Finland, 2013. [Google Scholar]
- Rasmussen, K.; Rauscher, H.; Mech, A.; Sintes, J.R.; Gilliland, D.; González, M.; Kearns, P.; Moss, K.; Visser, M.; Groenewold, M.; et al. Physico-chemical properties of manufactured nanomaterials—Characterisation and relevant methods. An outlook based on the OECD Testing Programme. Regul. Toxicol. Pharmacol. 2017, 92, 8–28. [Google Scholar] [CrossRef]
- OECD. OECD Working Party on Nanotechnology (WPN): Vision Statement; OECD: Paris, France, 2007; Available online: http://www.oecd.org/sti/nano (accessed on 11 April 2023).
- Murashov, V.; Howard, J. (Eds.) Nanotechnology Standards; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Organization for Economic Cooperation and Development (OECD). Publications in the Series on the Safety of Manufactured Nanomaterials. Available online: https://www.oecd.org/chemicalsafety/nanosafety/publications-series-safety-manufactured-nanomaterials.htm (accessed on 27 March 2023).
- Organization for Economic Cooperation and Development (OECD). Report of the OECD Workshop on the Safety of Manufactured Nanomaterials Building Co-Operation, Co-Ordination and Communication. Series on the Safety of Manufactured Nanomaterials, Report No.1 ENV/JM/MONO(2006)19. Available online: https://one.oecd.org/document/ENV/JM/MONO(2006)19/en/pdf (accessed on 27 March 2023).
- Organization for Economic Cooperation and Development (OECD). Considerations for Using Dissolution as a Function of Surface Chemistry to Evaluate Environmental Behaviour of Nanomaterials in Risk Assessments: A Preliminary Case Study Using Silver Nanoparticles. Series on the Safety of Manufactured Nanomaterials, Report No. 62 ENV/JM/MONO(2015)44. Available online: https://one.oecd.org/document/env/jm/mono(2015)44/en/pdf (accessed on 27 March 2023).
- Rasmussen, K.; González, M.; Kearns, P.; Sintes, J.R.; Rossi, F.; Sayre, P. Review of achievements of the OECD Working Party on Manufactured Nanomaterials’ Testing and Assessment Programme. From exploratory testing to test guidelines. Regul. Toxicol. Pharmacol. 2016, 74, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Organization for Economic Cooperation and Development (OECD). Strategies, Techniques and Sampling Protocols for Determining the Concentrations of Manufactured Nanomaterials in Air at the Workplace. Series on the Safety of Manufactured Nanomaterials, Report No. 82. ENV/JM/MONO(2017)30. Available online: https://one.oecd.org/document/env/jm/mono(2017)30/en/pdf (accessed on 27 March 2023).
- Organization for Economic Cooperation and Development (OECD). Consumer and Environmental Exposure to Manufactured Nanomaterials Information Used to Characterize Exposures: Analysis of a Survey. Series on the Safety of Manufactured Nanomaterials, Report No. 84 ENV/JM/MONO(2017)32. Available online: https://one.oecd.org/document/env/jm/mono(2017)32/en/pdf (accessed on 27 March 2023).
- Rasmussen, K.; Rauscher, H.; Kearns, P.; González, M.; Sintes, J.R. Developing OECD test guidelines for regulatory testing of nanomaterials to ensure mutual acceptance of test data. Regul. Toxicol. Pharmacol. 2019, 104, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Organization for Economic Cooperation and Development (OECD). Ability of Biopersistent/Biodurable Manufactured Nanomaterials (MNs) to Induce Lysosomal Membrane Permeabilization (LMP) as a Prediction of Their Long-Term Toxic Effects, Report 92. Available online: https://www.natlawreview.com/article/oecd-publishes-report-ability-biopersistentbiodurable-manufactured-nanomaterials-to (accessed on 27 March 2023).
- Organization for Economic Cooperation and Development (OECD). Important Issues on Risk Assessment of Manufactured Nanomaterials. Series on the Safety of Manufactured Nanomaterials, Report No. 103, ENV/CBC/MONO(2022)3. Available online: https://one.oecd.org/document/ENV/CBC/MONO(2022)3/en/pdf (accessed on 27 March 2023).
- Xiarchos, I.; Morozinis, A.K.; Kavouras, P.; Charitidis, C. A Nanocharacterization, materials modeling, and research integrity as enablers of sound risk assessment: Designing responsible nanotechnology. Small 2020, 16, 2001590. [Google Scholar] [CrossRef] [PubMed]
- Vinković Vrček, I.; Apartsin, E.; Ottaviani, M.F.; Riveiro, M.E.; Catalano, E. Consensus Protocols for Full Physico Chemical Characterization of New/Existing Chemical Entities and/or Nanomaterials. Cost Action CA17140. Cancer Nanomedicine, from the Bench to the Bedside (Nano2Clinic) Deliverable D2.1: Physico-Chemical Characterization. 2020. Available online: https://www.nano2clinic.eu/sites/default/files/downloads/CA17140_Deliverable2.1.pdf (accessed on 11 April 2023).
- Pedersen, E.; Fant, K. Guidance Document on Good In Vitro Method Practices (GIVIMP): Series on Testing and Assessment No. 286; OECD: Paris, France, 2018. [Google Scholar] [CrossRef]
- Ulrey, A.; Kolle, S.; Landsiedel, R.; Hill, E. How a GIVIMP certification program can increase confidence in in vitro methods. Altex 2021, 38, 316–318. [Google Scholar] [CrossRef]
- Ulrey, A.; Kolle, S.; Landsiedel, R.; Hill, E. P17-14 Good in vitro method practices (GIVIMP) certification: A roadmap for implementation and harmonization. Toxicol. Lett. 2022, 368, S232. [Google Scholar] [CrossRef]
- Arts, J.H.E.; Hadi, M.; Irfan, M.-A.; Keene, A.M.; Kreiling, R.; Lyon, D.; Maier, M.; Michel, K.; Petry, T.; Sauer, U.G.; et al. A decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping). Regul. Toxicol. Pharmacol. 2015, 71, S1–S27. [Google Scholar] [CrossRef]
- Kaur, J.; Sohal, I.S.; Singh, H.; Gupta, N.K.; Sehrawat, S.; Puri, S.; Bello, D.; Khatri, M. Toxicity screening and ranking of diverse engineered nanomaterials using established hierarchical testing approaches with a complementary in vivo zebrafish model. Environ. Sci. Nano 2022, 9, 2726–2749. [Google Scholar] [CrossRef]
- Fadeel, B. Understanding the immunological interactions of engineered nanomaterials: Role of the bio-corona. WIREs Nanomed. Nanobiotechnol. 2022, 14, e1798. [Google Scholar] [CrossRef]
- Park, H.-G.; Yeo, M.-K. Nanomaterial regulatory policy for human health and environment. Mol. Cell. Toxicol. 2016, 12, 223–236. [Google Scholar] [CrossRef]
- Locascio, L.E.; Reipa, V.; Zook, J.M.; Pleus, R.C. Nanomaterial Toxicity: Emerging Standards and Efforts to Support Standards Development. In Nanotechnology Standards. Nanostructure Science and Technology; Murashov, V., Howard, J., Eds.; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Doa, M.J. Categorization of Engineered Nanomaterials For Regulatory Decision-Making. In Metrology and Standardization of Nanotechnology: Protocols and Industrial Innovations; Wiley Online Library: Hoboken, NJ, USA, 2017; pp. 327–342. [Google Scholar]
- ISO 29701:2010; Nanotechnologies Endotoxin Test on Nanomaterial Samples for In Vitro Systems—Limulus Amebocyte Lysate (LAL) Test 2010, Upgraded in 2021. ISO: Geneva, Switzerland, 2010. Available online: https://www.iso.org/standard/45640.html (accessed on 11 April 2023).
- Smulders, S.; Kaiser, J.-P.; Zuin, S.; Van Landuyt, K.L.; Golanski, L.; Vanoirbeek, J.; Wick, P.; Hoet, P.H.M. Contamination of nanoparticles by endotoxin: Evaluation of different test methods. Part. Fibre Toxicol. 2012, 9, 41. [Google Scholar] [CrossRef]
- Scimeca, J.; Verron, E. Nano-engineered biomaterials: Safety matters and toxicity evaluation. Mater. Today Adv. 2022, 15, 100260. [Google Scholar] [CrossRef]
- Zalk, D.M.; Nelson, D.I. History and Evolution of Control Banding: A Review. J. Occup. Environ. Hyg. 2008, 5, 330–346. [Google Scholar] [CrossRef]
- Hodson, L.; Methner, M.; Zumwalde, R.D. Approaches to Safe Nanotechnology; Managing the Health and Safety Concerns Associated with Engineered Nanomaterials; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2009. [Google Scholar]
- Schulte, P.A.; Geraci, C.L.; Murashov, V.; Kuempel, E.D.; Zumwalde, R.D.; Castranova, V.; Hoover, M.D.; Hodson, L.; Martinez, K.F. Occupational safety and health criteria for responsible development of nanotechnology. J. Nanopart. Res. 2014, 16, 1–17. [Google Scholar] [CrossRef]
- Eastlake, A.; Zumwalde, R.; Geraci, C. Can Control Banding be Useful for the Safe Handling of Nanomaterials? A Systematic Review. J. Nanopart. Res. 2016, 18, 169. [Google Scholar] [CrossRef] [PubMed]
- Zalk, D.M.; Paik, S.Y.; Swuste, P. Evaluating the Control Banding Nanotool: A qualitative risk assessment method for controlling nanoparticle exposures. J. Nanopart. Res. 2009, 11, 1685–1704. [Google Scholar] [CrossRef]
- Väänänen, V.; Rydman, E.; Ilves, M.; Hannukainen, K.; Norppa, H.; Von Wright, H.; Honkalampi, U.; Tsitko, I.; Rouhiainen, J. Evaluation of the suitability of the developed methodology for nanoparticle health and safety studies. In Proceedings of the Scale-Up Nanoparticles in Modern Papermaking (SUNPAP 2012), Milan, Italy, 19–20 June 2012. [Google Scholar]
- Dimou, K.; Emond, C. Nanomaterials, and Occupational Health and Safety—A Literature Review About Control Banding and a Semi-Quantitative Method Proposed for Hazard Assessment. J. Phys. Conf. Ser. 2017, 838, 12020. [Google Scholar] [CrossRef]
- Ramos, D.; Almeida, L.; Gomes, M. Application of Control Banding to Workplace Exposure to Nanomaterials in the Textile Industry. In Occupational and Environmental Safety and Health; Springer: Cham, Switzerland, 2019; pp. 105–113. [Google Scholar]
- Dugheri, S.; Cappelli, G.; Trevisani, L.; Kemble, S.; Paone, F.; Rigacci, M.; Bucaletti, E.; Squillaci, D.; Mucci, N.; Arcangeli, G. A Qualitative and Quantitative Occupational Exposure Risk Assessment to Hazardous Substances during Powder-Bed Fusion Processes in Metal-Additive Manufacturing. Safety 2022, 8, 32. [Google Scholar] [CrossRef]
- Silva, F.; Arezes, P.; Swuste, P. Risk assessment and control in engineered nanoparticles occupational exposure. In Occupational Safety and Hygiene; CRC Press: Boca Raton, FL, USA, 2013; pp. 197–202. [Google Scholar] [CrossRef]
- Groso, A.; Magrez, A.; Meyer, T. Safety Management of Nanomaterials. Procedia Eng. 2012, 42, 1587–1596. [Google Scholar] [CrossRef]
- Silva, F.; Arezes, P.; Swuste, P. Risk management: Controlling occupational exposure to nanoparticles in construction. In Nanotechnology in Eco-Efficient Construction; Woodhead Publishing: Sawston, UK, 2019; pp. 755–784. [Google Scholar]
- López-Alonso, M.; Díaz-Soler, B.; Martínez-Rojas, M.; Fito-López, C.; Martínez-Aires, M.D. Management of Occupational Risk Prevention of Nanomaterials Manufactured in Construction Sites in the EU. Int. J. Environ. Res. Public Health 2020, 17, 9211. [Google Scholar] [CrossRef]
- Yarahmadi, R.; Dizaji, R.A.; Farshad, A.A.; Teimuri, F.; Soleimani, M. Occupational risk assessment of engineered nanomaterials by control banding method in chemistry laboratories. J. Am. Sci. 2013, 9, 42–47. [Google Scholar]
- Tielemans, E.; Noy, D.; Schinkel, J.; Heussen, H.; Van Der Schaaf, D.; West, J.; Fransman, W. Stoffenmanager Exposure Model: Development of a Quantitative Algorithm. Ann. Work. Expo Health 2008, 52, 443–454. [Google Scholar] [CrossRef]
- Marquart, H.; Heussen, H.; Le Feber, M.; Noy, D.; Tielemans, E.; Schinkel, J.; West, J.; Van Der Schaaf, D. ‘Stoffenmanager’, a Web-Based Control Banding Tool Using an Exposure Process Model. Ann. Occup. Hyg. 2008, 52, 429–441. [Google Scholar] [CrossRef]
- Landberg, H.E.; Axmon, A.; Westberg, H.; Tinnerberg, H. A Study of the Validity of Two Exposure Assessment Tools: Stoffenmanager and the Advanced REACH Tool. Ann. Work. Expo. Health 2017, 61, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Jiménez, A.; Varet, J.; Poland, C.; Fern, G.J.; Hankin, S.M.; van Tongeren, M. A comparison of control banding tools for nanomaterials. J. Occup. Environ. Hyg. 2016, 13, 936–949. [Google Scholar] [CrossRef]
- Barberio, G.; Scalbi, S.; Buttol, P.; Masoni, P.; Righi, S. Combining life cycle assessment and qualitative risk assessment: The case study of alumina nanofluid production. Sci. Total. Environ. 2014, 496, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, R.; Zhou, X.; Jin, S.; Zhang, Y.; Xu, Z.; Guo, L.; Tang, S. Risk Assessment of Nanoparticle Exposure in a Calcium Carbonate Manufacturing Workshop with Six Control Banding Tools. J. Nanosci. Nanotechnol. 2020, 20, 3610–3619. [Google Scholar] [CrossRef]
- Silva, F.; Sousa, S.P.B.; Arezes, P.; Swuste, P.; Ribeiro, M.C.S.; Baptista, J.S. Qualitative risk assessment during polymer mortar test specimens preparation—Methods comparison. J. Phys. Conf. Ser. 2015, 617, 012037. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, X.; Zou, H.; Wang, Q.; Zhou, Z.; Chen, R.; Yuan, W.; Luan, Y.; Quan, C.; Zhang, M. Exposure characterization and risk assessment of ultrafine particles from the blast furnace process in a steelmaking plant. J. Occup. Health 2021, 63, e12257. [Google Scholar] [CrossRef]
- Freeland, J.; Hulme, J.; Kinnison, D.; Mitchell, A.; Veitch, P.; Aitken, R.; Hankin, S.; Poland, C.; Bard, D.; Gibson, R.; et al. Working Safely with Nanomaterials in Research Development, Edinburgh, GB. The UK NanoSafety Partnership Group 44pp 2012. Available online: http://eprints.soton.ac.uk/id/eprint/363448 (accessed on 11 April 2023).
- Methner, M.; Beaucham, C.; Crawford, C.; Hodson, L.; Geraci, C. Field Application of the Nanoparticle Emission Assessment Technique (NEAT): Task-Based Air Monitoring During the Processing of Engineered Nanomaterials (ENM) at Four Facilities. J. Occup. Environ. Hyg. 2012, 9, 543–555. [Google Scholar] [CrossRef]
- Eastlake, A.C.; Beaucham, C.; Martinez, K.F.; Dahm, M.; Sparks, C.; Hodson, L.L.; Geraci, C.L. Refinement of the Nanoparticle Emission Assessment Technique into the Nanomaterial Exposure Assessment Technique (NEAT 2.0). J. Occup. Environ. Hyg. 2016, 13, 708–717. [Google Scholar] [CrossRef]
- Tsai, C.-J.; Huang, C.-Y.; Chen, S.-C.; Ho, C.-E.; Chen, C.-W.; Chang, C.-P.; Tsai, S.-J.; Ellenbecker, M.J. Exposure assessment of nano-sized and respirable particles at different workplaces. J. Nanopart. Res. 2011, 13, 4161–4172. [Google Scholar] [CrossRef]
- Garcia, A.; Eastlake, A.; Topmiller, J.L.; Sparks, C.; Martinez, K.; Geraci, C.L. Nano-metal oxides: Exposure and engineering control assessment. J. Occup. Environ. Hyg. 2017, 14, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Starost, K.; Frijns, E.; Van Laer, J.; Faisal, N.; Egizabal, A.; Elizextea, C.; Blazquez, M.; Nelissen, I.; Njuguna, J. Assessment of nanoparticles release into the environment during drilling of carbon nanotubes/epoxy and carbon nanofibres/epoxy nanocomposites. J. Hazard. Mater. 2017, 340, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Strickland, J.A.; Foureman, G.L. US EPA’s acute reference exposure methodology for acute inhalation exposures. Sci. Total Environ. 2002, 288, 51–63. [Google Scholar] [CrossRef]
- Setzer, R.W.; Kimmel, C.A. Use of NOAEL, benchmark dose, and other models for human risk assessment of hormonally active substances. Pure Appl. Chem. 2003, 75, 2151–2158. [Google Scholar] [CrossRef]
- Davis, J.A.; Gift, J.S.; Zhao, Q.J. Introduction to benchmark dose methods and U.S. EPA’s benchmark dose software (BMDS) version 2.1.1. Toxicol. Appl. Pharmacol. 2011, 254, 181–191. [Google Scholar] [CrossRef]
- Pink, M.; Verma, N.; Schmitz-Spanke, S. Benchmark dose analyses of toxic endpoints in lung cells provide sensitivity and toxicity ranking across metal oxide nanoparticles and give insights into the mode of action. Toxicol. Lett. 2020, 331, 218–226. [Google Scholar] [CrossRef]
- Baralić, K.; Javorac, D.; Antonijević, E.; Buha-Đorđević, A.; Ćurčić, M.; Đukić-Ćosić, D.; Antonijević, B. Relevance and evaluation of the benchmark dose in toxicology. Arh. Za Farm. 2020, 70, 130–141. [Google Scholar] [CrossRef]
- Haber, L.T.; Dourson, M.L.; Allen, B.C.; Hertzberg, R.C.; Parker, A.; Vincent, M.; Maier, A.; Boobis, A.R. Benchmark dose (BMD) modeling: Current practice, issues, and challenges. Crit. Rev. Toxicol. 2018, 48, 387–415. [Google Scholar] [CrossRef]
- Shao, K.; Shapiro, A.J. A Web-Based System for Bayesian Benchmark Dose Estimation. Environ. Health Perspect. 2018, 126, 017002. [Google Scholar] [CrossRef]
- Piegorsch, W.W.; An, L.; Wickens, A.A.; West, R.W.; Peña, E.A.; Wu, W. Information-theoretic model-averaged benchmark dose analysis in environmental risk assessment. Environmetrics 2013, 24, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Lewinski, N.; Zhu, H.; Drezek, R. Evaluating strategies for risk assessment of nanomaterials. In Nanotoxicity: From In Vivo and In Vitro Models to Health Risks; John Wiley & Sons: Chichester, UK, 2009; pp. 459–498. [Google Scholar]
- Ramchandran, V.; Gernand, J.M. Evaluation of Risk and Uncertainty for Model-Predicted NOAELs of Engineered Nanomaterials Based on Dose-Response-Recovery Clusters. ASCE-ASME J. Risk Uncertain. Eng. Syst. Part B Mech. Eng. 2023, 9, 011205. [Google Scholar] [CrossRef]
- Bemis, J.C.; Wills, J.W.; Bryce, S.M.; Torous, D.K.; Dertinger, S.D.; Slob, W. Comparison of in vitro and in vivo clastogenic potency based on benchmark dose analysis of flow cytometric micronucleus data. Mutagenesis 2016, 31, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.M.; Kluxen, F.M.; Ritz, C. A Review of Recent Advances in Benchmark Dose Methodology. Risk Anal. 2019, 39, 2295–2315. [Google Scholar] [CrossRef] [PubMed]
- EFSA Scientific Committee; Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, K.H.; More, S.; Mortensen, A.; Naegeli, H.; Noteborn, H.; et al. Update: Use of the benchmark dose approach in risk assessment. EFSA J. 2017, 15, e04658. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Cheng, H.; Zheng, Y.; Zhao, J.; Qu, K. A universal automated method for determining the bacteriostatic activity of nanomaterials. J. Hazard. Mater. 2021, 413, 125320. [Google Scholar] [CrossRef]
- Weldon, B.A.; Griffith, W.C.; Workman, T.; Scoville, D.K.; Kavanagh, T.J.; Faustman, E.M. In vitro to in vivo benchmark dose comparisons to inform risk assessment of quantum dot nanomaterials. WIREs Nanomed. Nanobiotechnol. 2018, 10, e1507. [Google Scholar] [CrossRef]
- Halappanavar, S.; Rahman, L.; Nikota, J.; Poulsen, S.S.; Ding, Y.; Jackson, P.; Wallin, H.; Schmid, O.; Vogel, U.; Williams, A. Ranking of nanomaterial potency to induce pathway perturbations associated with lung responses. Nanoimpact 2019, 14, 100158. [Google Scholar] [CrossRef]
- Gosens, I.; Kermanizadeh, A.; Jacobsen, N.R.; Lenz, A.-G.; Bokkers, B.; De Jong, W.H.; Krystek, P.; Tran, L.; Stone, V.; Wallin, H.; et al. Comparative Hazard Identification by a Single Dose Lung Exposure of Zinc Oxide and Silver Nanomaterials in Mice. PLoS ONE 2015, 10, e0126934. [Google Scholar] [CrossRef]
- Pini, M.; Salieri, B.; Ferrari, A.M.; Nowack, B.; Hischier, R. Human health characterization factors of nano-TiO2 for indoor and outdoor environments. Int. J. Life Cycle Assess. 2016, 21, 1452–1462. [Google Scholar] [CrossRef]
- Jagiello, K.; Halappanavar, S.; Rybińska-Fryca, A.; Willliams, A.; Vogel, U.; Puzyn, T. Transcriptomics-Based and AOP-Informed Structure–Activity Relationships to Predict Pulmonary Pathology Induced by Multiwalled Carbon Nanotubes. Small 2021, 17, e2003465. [Google Scholar] [CrossRef] [PubMed]
- Jamuna, B.A.; Ravishankar, R.V. Environmental risk, human health, and toxic effects of nanoparticles. In Nanomaterials for Environmental Protection; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 523–535. [Google Scholar]
- Schulte, P.A.; Kuempel, E.D.; Geraci, C.L. Risk Assessment and Risk Management of Nanomaterials in the Workplace: Translating Research to Practice. Ann. Occup. Hyg. 2012, 56, 491–505. [Google Scholar] [CrossRef]
- European Agency for Health and Hygiene at Work 2013 E-fact 72: Tools for the Management of Nanomaterials in the Workplace and Prevention Measures. Available online: https://osha.europa.eu/en/emerging-risks/nanomaterials (accessed on 11 April 2023).
- Hallock, M.F.; Greenley, P.; DiBerardinis, L.; Kallin, D. Potential risks of nanomaterials and how to safely handle materials of uncertain toxicity. ACS Chem. Health Saf. 2009, 16, 16–23. [Google Scholar] [CrossRef]
- Health and Safety Executive (HSE) Using Nanomaterials at Work. Including Carbon Nanotubes (CNTs) and Other Bioper-sistent High Aspect Ratio Nanomaterials HARN. HSG 272; 2013. Available online: https://www.hse.gov.uk/pubns/books/hsg272.pdf (accessed on 11 April 2023).
- Ruiz Pérez, J.; Salcines Suárez, C.L.; Valiente, R. Criterios de selección de equipos de protección respiratoria frente a nanomateriales. In Actas II Congreso Prevencionar 2019: Desde la Teoría a la Práctica; Seguridad, Salud, Bienestar: Madrid, Spain, 2020; ISBN 978-84-09-16021-1. [Google Scholar]
- Goede, H.; Vries, Y.C.-D.; Kuijpers, E.; Fransman, W. A Review of Workplace Risk Management Measures for Nanomaterials to Mitigate Inhalation and Dermal Exposure. Ann. Work Expo. Health 2018, 62, 907–922. [Google Scholar] [CrossRef] [PubMed]
- Oksel, C.; Subramanian, V.; Semenzin, E.; Ma, C.Y.; Hristozov, D.; Wang, X.Z.; Hunt, N.; Costa, A.; Fransman, W.; Marcomini, A.; et al. Evaluation of existing control measures in reducing health and safety risks of engineered nanomaterials. Environ. Sci. Nano 2016, 3, 869–882. [Google Scholar] [CrossRef]
- Ding, Y.; Kuhlbusch, T.A.; van Tongeren, M.; Jiménez, A.S.; Tuinman, I.; Chen, R.; Alvarez, I.L.; Mikołajczyk, U.; Nickel, C.; Meyer, J.; et al. Airborne engineered nanomaterials in the workplace—A review of release and worker exposure during nanomaterial production and handling processes. J. Hazard. Mater. 2017, 322, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Conti, J.A.; Killpack, K.; Gerritzen, G.; Huang, L.; Mircheva, M.; Delmas, M.; Harthorn, B.H.; Appelbaum, R.P.; Holden, P.A. Health and Safety Practices in the Nanomaterials Workplace: Results from an International Survey. Environ. Sci. Technol. 2008, 42, 3155–3162. [Google Scholar] [CrossRef] [PubMed]
- Oksel, C.; Ma, C.Y.; Wang, X.Z. Structure-activity relationship models for hazard assessment and risk management of engineered nanomaterials. Procedia Eng. 2015, 102, 1500–1510. [Google Scholar] [CrossRef]
- Myojo, T.; Nagata, T.; Verbeek, J. The Effectiveness of Specific Risk Mitigation Techniques Used in the Production and Handling of Manufactured Nanomaterials: A Systematic Review. J. UOEH 2017, 39, 187–199. [Google Scholar] [CrossRef]
- Sundarrajan, S.; Chandrasekaran, A.R.; Ramakrishna, S. An Update on Nanomaterials-Based Textiles for Protection and Decontamination. J. Am. Ceram. Soc. 2010, 93, 3955–3975. [Google Scholar] [CrossRef]
- Damokhi, A.; Yousefinejad, S.; Fakherpour, A.; Jahangiri, M. Improvement of performance and function in respiratory protection equipment using nanomaterials. J. Nanopart. Res. 2022, 24, 76. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska, A.; Bayan, M.A.H.; Nakielski, P.; Petronella, F.; De Sio, L.; Pierini, F. Nanotechnology Transition Roadmap toward Multifunctional Stimuli-Responsive Face Masks. ACS Appl. Mater. Interfaces 2022, 14, 46123–46144. [Google Scholar] [CrossRef] [PubMed]
- EN 149:2001+A1:2009; Respiratory Protective Devices—Filtering Half Masks to Protect against Particles. Requirements Marking, Testing. European Commission: Brussels, Belgium, 2009. Available online: https://static.austrian-standards.at/covid-normen/OENORM-EN149e.pdf (accessed on 11 April 2023).
- AS/NZS 1716-2012; Respiratory Protective Devices. Standards Australia: Sydney, Australia, 2012. Available online: https://www.standards.org.au/standards-catalogue/sa-snz/publicsafety/sf-010/as-slash-nzs--1716-2012 (accessed on 25 February 2023)Australian/New Zealand Standard.
- Bollinger, N.J. DHHS (NIOSH) (2004) NIOSH Respirator Selection logic publication 2005-100. Available online: https://www.cdc.gov/niosh/docs/2005-100/default.html (accessed on 25 February 2023).
- Abbasinia, M.; Karimie, S.; Haghighat, M.; Mohammadfam, I. Application of nanomaterials in personal respiratory protection equipment: A literature review. Safety 2018, 4, 47. [Google Scholar] [CrossRef]
- Cherrie, J.W.; Apsley, A.; Cowie, H.; Steinle, S.; Mueller, W.; Lin, C.; Horwell, C.J.; Sleeuwenhoek, A.; Loh, M. Effectiveness of face masks used to protect Beijing residents against particulate air pollution. Occup. Environ. Med. 2018, 75, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Woskie, S. Workplace practices for engineered nanomaterial manufacturers. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 685–692. [Google Scholar] [CrossRef]
- Dolez, P.; Vinches, L.; Perron, G.; Vu-Khanh, T.; Plamondon, P.; L’Espérance, G.; Wilkinson, K.; Cloutier, Y.; Dion, C.; Truchon, G. Development of a Method of Measuring Nanoparticle Penetration through Protective Glove Materials under Conditions Simulating Workplace Use; Report R-785 IRSST; IRSST: Montréal, QC, Canada, 2013. [Google Scholar]
- Vinches, L.; Hallé, S.; Peyrot, C.; Wilkinson, K.J. Which Gloves are Efficient to Protect Against Titanium Dioxide Nanoparticles in Work Conditions? Int. J. Theor. Appl. Nanotechnol. 2014, 2, 24–29. [Google Scholar] [CrossRef]
- Buitrago, E.; Novello, A.M.; Fink, A.; Riediker, M.; Rothen-Rutishauser, B.; Meyer, T. NanoSafe III: A User Friendly Safety Management System for Nanomaterials in Laboratories and Small Facilities. Nanomaterials 2021, 11, 2768. [Google Scholar] [CrossRef]
- Stephens, B.; Maynard, A.; Hopke, P.K. Control of Airborne Particles: Filtration. In Handbook of Indoor Air Quality; Zhang, Y., Hopke, P.K., Mandin, C., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Fdez-Arroyabe, P.; Suárez, C.L.S.; Nita, I.-A.; Kassomenos, P.; Petrou, E.; Santurtún, A. Electrical characterization of circulation weather types in Northern Spain based on atmospheric nanoparticles measurements: A pilot study. Sci. Total. Environ. 2020, 704, 135320. [Google Scholar] [CrossRef]
- Fernández de Arróyabe Hernáez, P.; Salcines Suárez, C.L.; Kassomenos, P.; Santurtún Zarrabeitia, A.; Petäjä, T. Electric charge of atmospheric nanoparticles and its potential implications with human health. Sci. Total Environ. 2022, 808, 152106. [Google Scholar]
- Schweinheim, C. Setting standards for HEPA filter efficiency. Filtr. Sep. 2015, 52, 13–15. [Google Scholar] [CrossRef]
- Vijayan, V.; Paramesh, H.; Salvi, S.; Dalal, A.K. Enhancing indoor air quality—The air filter advantage. Lung India 2015, 32, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Komaladewi, A.; Khoiruddin, K.; Surata, I.; Subagia, I.A.; Wenten, I. Recent advances in antimicrobial air filter. E3S Web Conf. 2018, 67, 03016. [Google Scholar] [CrossRef]
- European Communities Council (1989) Directive 89/654/EEC—Workplace Requirements. Latest Update: 19/03/2021. Available online: https://osha.europa.eu/en/legislation/directives/2) (accessed on 11 April 2023).
- Coelho, D.A.; de Oliveira Matias, J.C. The Benefits of Occupational Health and Safety Standards. In Handbook of Standards and Guidelines in Ergonomics and Human Factors, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 413–440. [Google Scholar]
- Coelho, D.A.; Matias, J.C.; Filipe, J.N. The Benefits of Occupational Health and Safety Standards. In Handbook of Standards and Guidelines in Ergonomics and Human Factors, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2021; Chapter 32; pp. 541–568. Available online: https://lccn.loc.gov/2020056180 (accessed on 11 April 2023).
- Document 6699-2 (2007); Nanotechnologies—Part 2: Guide to Safe Handling and Disposal of Manufactured Nanomaterials; BSI: London, UK, 2007; Available online: http://www.safenano.org/research/bsi-nanotechnologies-guide-to-safe-handling-disposal-of-manufactured-nanomaterials/ (accessed on 25 February 2023).
- Leung, M.K.H.; Liu, C.; Chan, A. Industrial Ventilation: A Manual of Recommended Practice Industrial Ventilation: A Manual of Recommended Practice, 2004. J. Occup. Health 2005, 47, 540–547. [Google Scholar] [CrossRef]
- Denev, J.A.; Durst, F.; Mohr, B. Room Ventilation and Its Influence on the Performance of Fume Cupboards: A Parametric Numerical Study. Ind. Eng. Chem. Res. 1997, 36, 458–466. [Google Scholar] [CrossRef]
- Dittrich, E. Fume Cupboards and Ventilated Units. In The Sustainable Laboratory Handbook: Design, Equipment, Operation; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 225–240. [Google Scholar]
- Dockx, P. Lab ventilation and energy consumption. In The Sustainable Laboratory Handbook: Design, Equipment, Operation; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 363–378. [Google Scholar]
- Rebar, R.; Halley, M. Biosafety Compliance: A Global Perspective. In Biological Safety: Principles and Practices; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 417–436. [Google Scholar]
- ISO TR 12885:2018; Nanotechnologies—Health and Safety Practices in Occupational Settings Relevant to Nanotechnologies. International Organization for Standardization (ISO): Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/67446.html (accessed on 11 April 2023).
- BS 1822-1:2009; High Efficiency Air Filters (EPA, HEPA and ULPA). BSI British Standard: London, UK, 2009. Available online: https://img.antpedia.com/standard/pdf/Q76/1703/BS%20EN%201822-4-2009_3125.pdf (accessed on 11 April 2023).
- EN 1822-1:2019; High Efficiency Air Filters (EPA, HEPA and ULPA)—Part 1: Classification, Performance Testing, Marking. Last Update 8 June 2022. European Committee For Standardization (CEN): Brussels, Belgium, 2019. Available online: https://www.airum.com/frontend/immagini/files/EN%201822-1.PDF (accessed on 11 April 2023).
- Sandle, T. Cleanrooms and Associated Controlled Environments-Biocontamination Control: Dissecting the Standard. Inst. Valid. Technol. IVT Netw. 2021, 25, 1. Available online: https://bpi.bioprocessintl.com/hubfs/IVT%20-%20GXP%20Archive/1-21-Cleanrooms%20and%20associated%20controlled%20environments-1.pdf (accessed on 11 April 2023).
- Handscomb, J.R.; Eng, P. Use of ASHRAE Standards, ACGIH Industrial Ventilation Guidelines and Client Standards for Industrial Buildings. In Proceedings of the ASHRAE Topical Conference, Toronto, ON, Canada, 22–24 June 2022. [Google Scholar]
- Amoabediny, G.H.; Naderi, A.; Malakootikhah, J.; Koohi, M.K.; Mortazavi, S.A.; Naderi, M.; Rashedi, H. Guidelines for safe handling, use and disposal of nanoparticles. J. Phys. Conf. Ser. 2009, 170, 012037. [Google Scholar] [CrossRef]
- Suman, T.Y.; Pei, D.S. Nanomaterial waste management. In Nanomaterials Recycling; Elsevier: Amsterdam, The Netherlands, 2022; pp. 21–36. [Google Scholar]
- Brunelli, A.; Calgaro, L.; Semenzin, E.; Cazzagon, V.; Giubilato, E.; Marcomini, A.; Badetti, E. Leaching of nanoparticles from nano-enabled products for the protection of cultural heritage surfaces: A review. Environ. Sci. Eur. 2021, 33, 48. [Google Scholar] [CrossRef]
- Johnston, L.J.; Gonzalez-Rojano, N.; Wilkinson, K.J.; Xing, B. Key challenges for evaluation of the safety of engineered nanomaterials. NanoImpact 2020, 18, 100219. [Google Scholar] [CrossRef]
- Nielsen, M.B.; Baun, A.; Mackevica, A.; Thit, A.; Wallinder, I.O.; Gallego, J.A.; Clausen, L.P.W.; Rissler, J.; Skjolding, L.M.; Nilsson, A.C.; et al. Nanomaterials in the Euro- pean chemicals legislation—Methodological challenges for registration and environmental safety assessment. Environ. Sci. Nano 2021, 8, 731–747. [Google Scholar] [CrossRef]
- Clausen, L.P.W.; Hansen, S.F. The ten decrees of nanomaterials regulations. Nat. Nanotechnol. 2018, 13, 766–768. [Google Scholar] [CrossRef]
- UNE EN ISO 2812-1:2018; Paints and Varnishes—Determination of Resistance to Liquids—Part 1: Immersion in Liquids Other than Water (ISO 2812-1:2017). ISO: Geneva, Switzerland, 2017. Available online: https://www.en-standard.eu/une-en-iso-2812-1-2018-paints-and-varnishes-determination-of-resistance-to-liquids-part-1-immersion-in-liquids-other-than-water-iso-2812-1-2017/ (accessed on 11 April 2023).
- AWPA E11, 16th Edition, 2022—Standard Method for Accelerated Evaluation of Preservative Leaching 2022. Available online: https://global.ihs.com/doc_detail.cfm?document_name=AWPA%20E11&item_s_key=002702500 (accessed on 11 April 2023).
- CEN/TS 16637-1; Construction Products-Assessment of Release of Dangerous Sub- Stances—Part 1: Guidance for the Determination of Leaching Tests and Additional Testing Step (2018). CEN (European Committee for Standardization): Brussels, Belgium. Available online: https://standards.iteh.ai/catalog/standards/cen/28608948-5d69-4d45-a9e7-474a13d4d283/pren-16637-1/ (accessed on 11 April 2023).
- Singh, V.; Yadav, P.; Mishra, V. Recent advances on classification 2018, properties, synthesis, and characterization of nanomaterials. In Green Synthesis of Nanomaterials for Bioenergy Applications; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 83–97. [Google Scholar]
- Franzoni, E.; Sassoni, E.; Graziani, G. Brushing, poultice or immersion? The role of the application technique on the performance of a novel hydroxyapatite-based consolidating treatment for limestone. J. Cult. Heritage 2015, 16, 173–184. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Wang, Y.; Wang, M.; Singh, V.; Men, X.; Zhang, Z. Rapid fabrication of a transparent superhydrophobic coating: Potential application with pollution-free under construction. Appl. Phys. A 2020, 126, 1–10. [Google Scholar] [CrossRef]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bonazza, A.; De Nuntiis, P.; Mandrioli, P.; Sabbioni, C. Aerosol Impact on Cultural Heritage: Deterioration Processes and Strategies for Preventive Conservation. In Atmospheric Aerosols: Life Cycles and Effects on Air Quality and Climate; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 645–670. [Google Scholar] [CrossRef]
- Fdez-Arroyabe, P.; Salcines, C.; Santurtún, A.; Setién, I.; Kassomenos, P.; Petäjä, T. Methodology to measure atmospheric nanoparticles charge. MethodsX 2023, 10, 102148. [Google Scholar] [CrossRef] [PubMed]
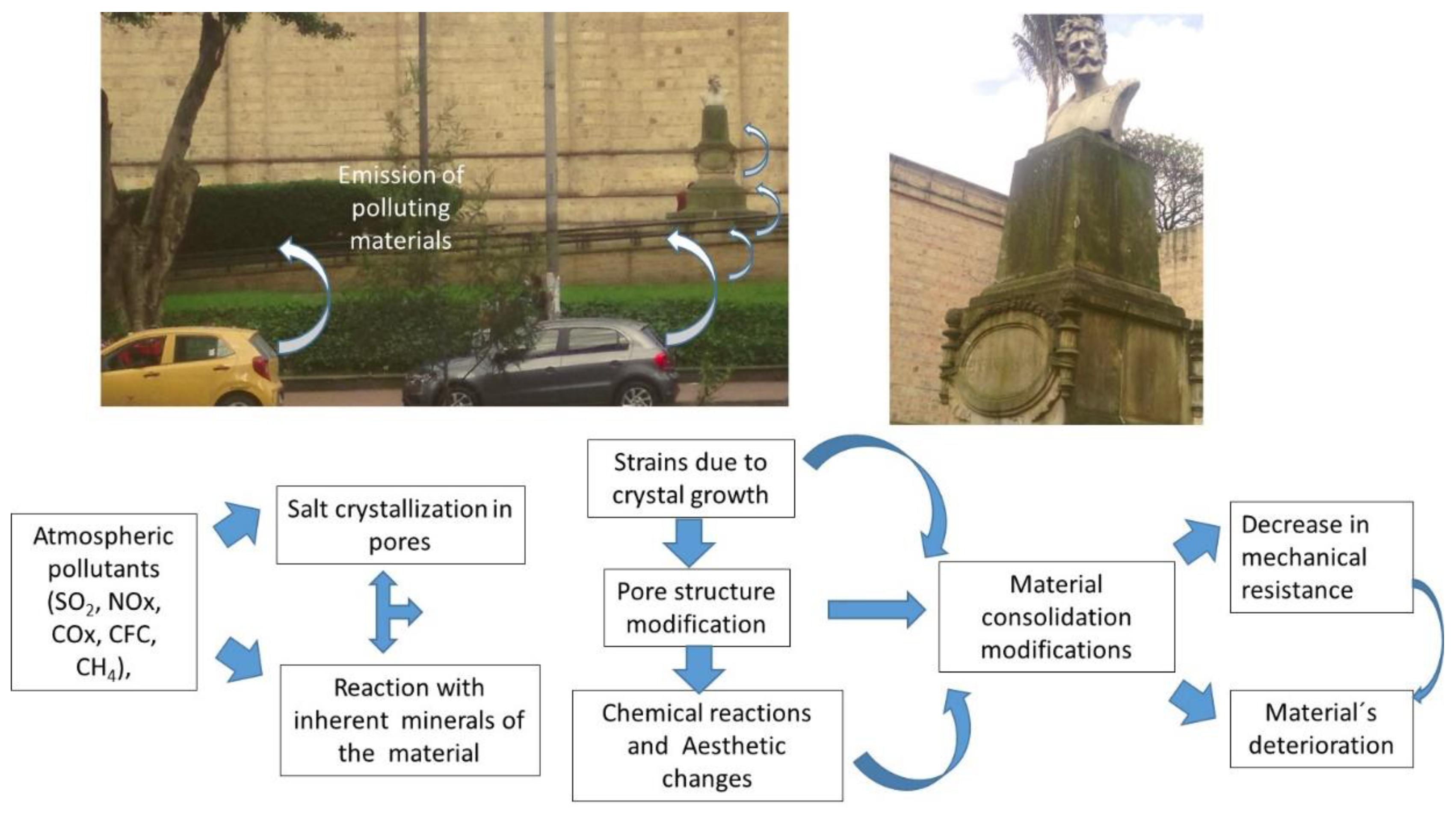
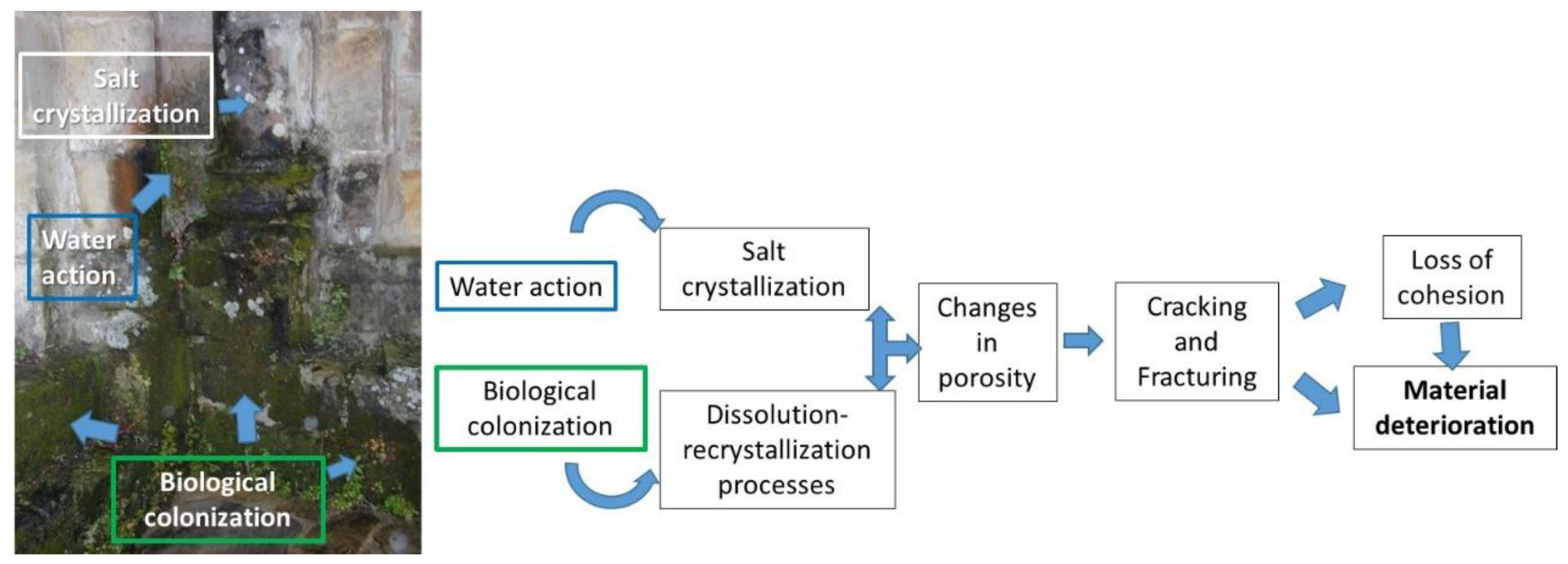
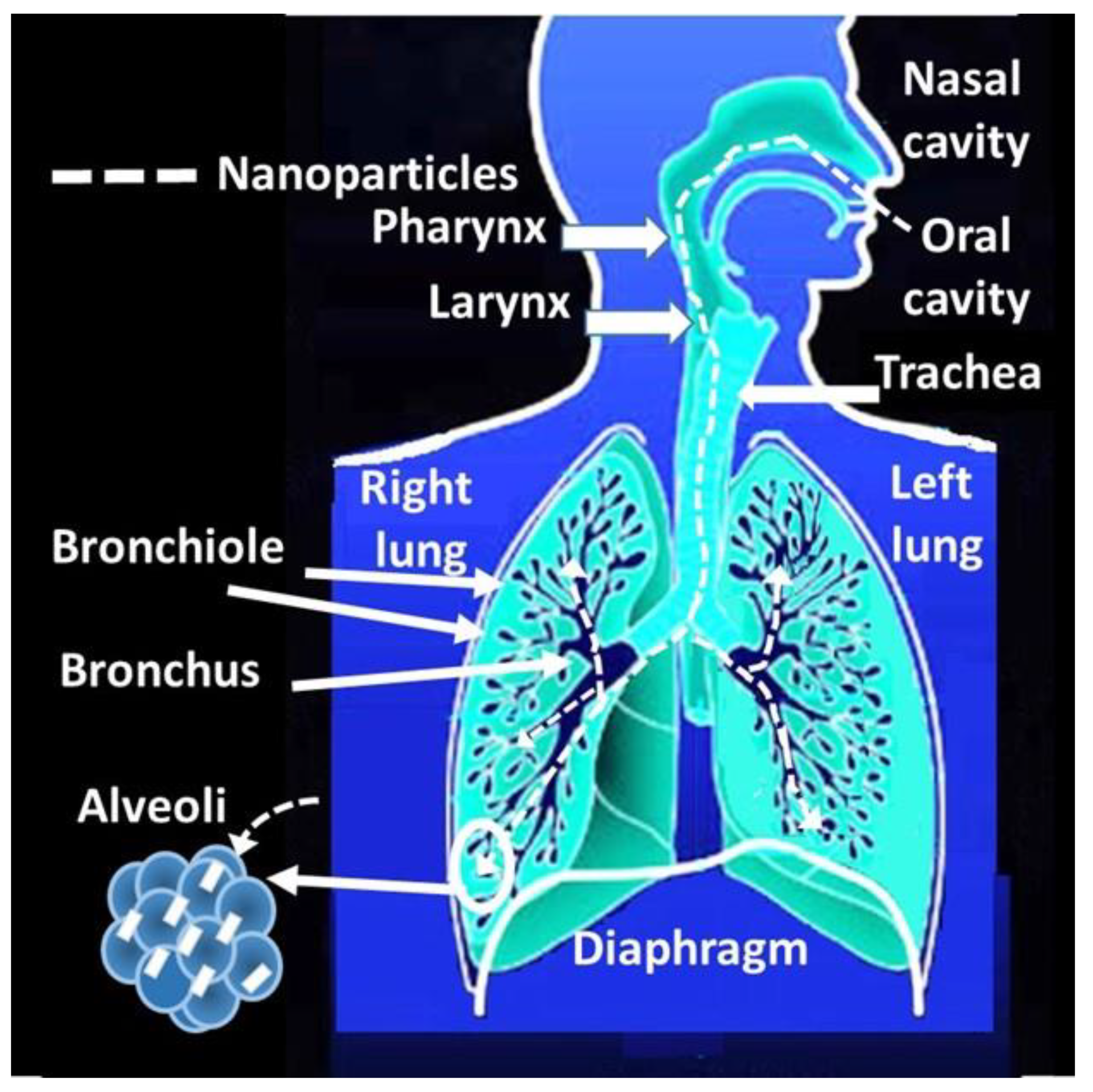
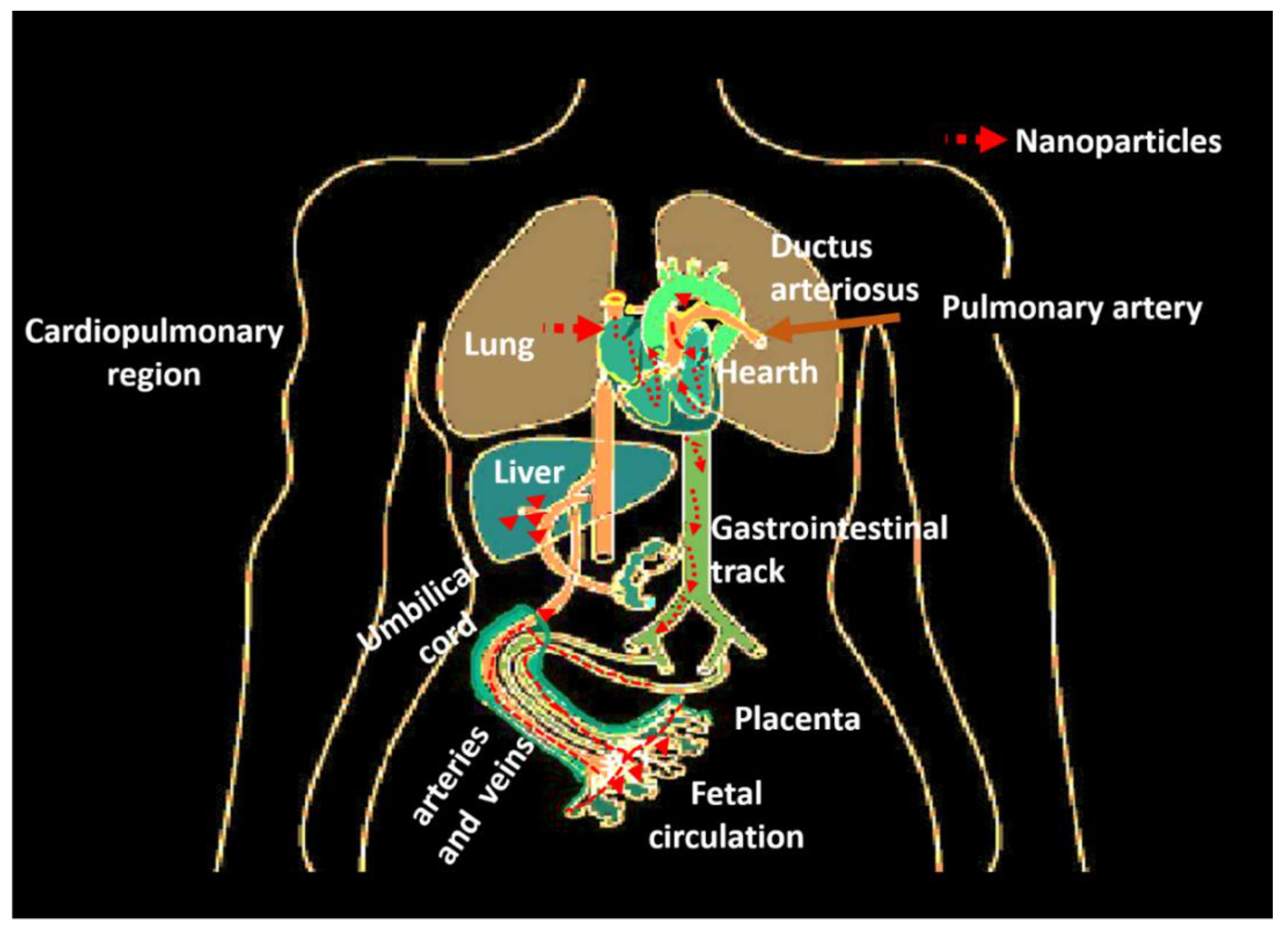
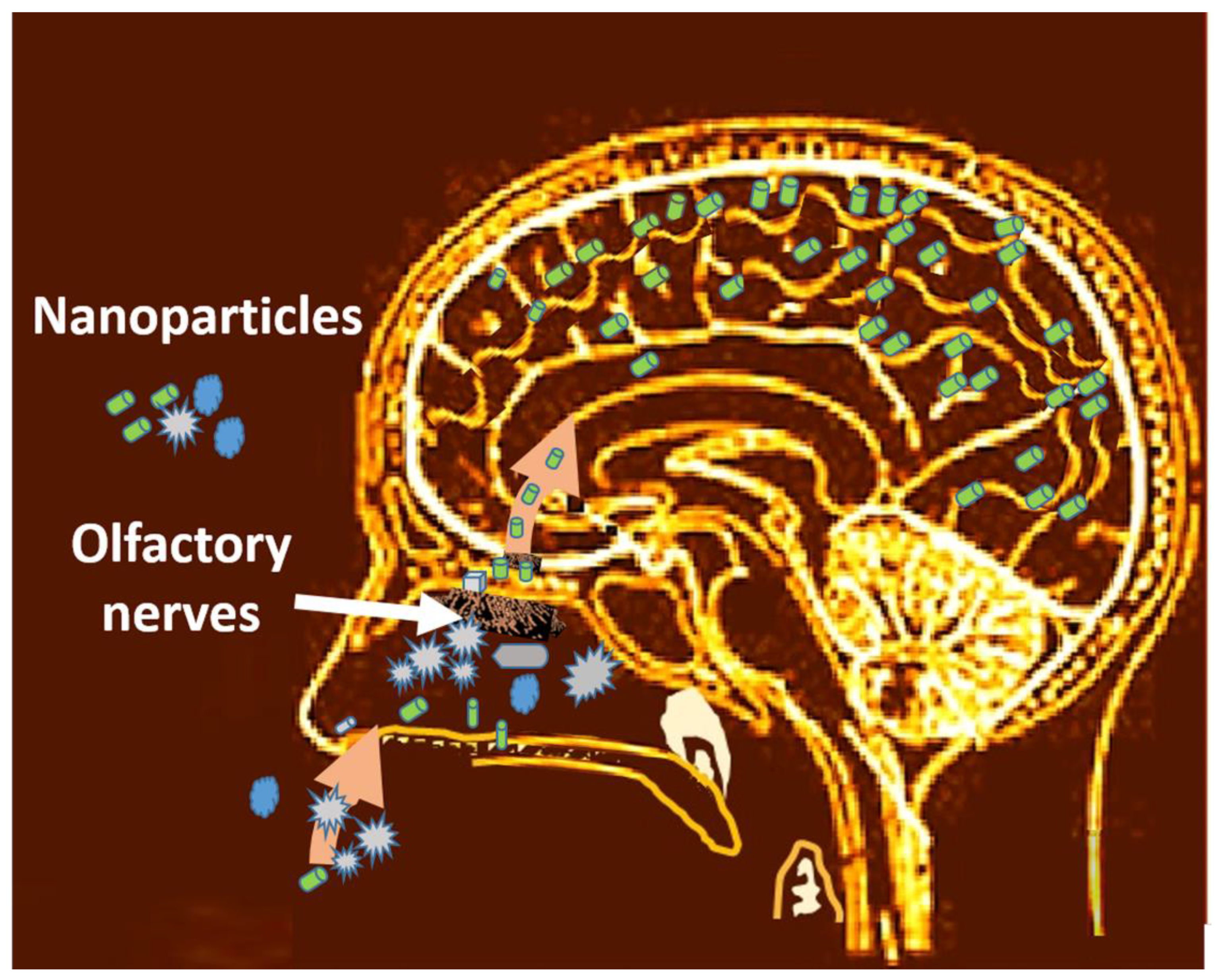
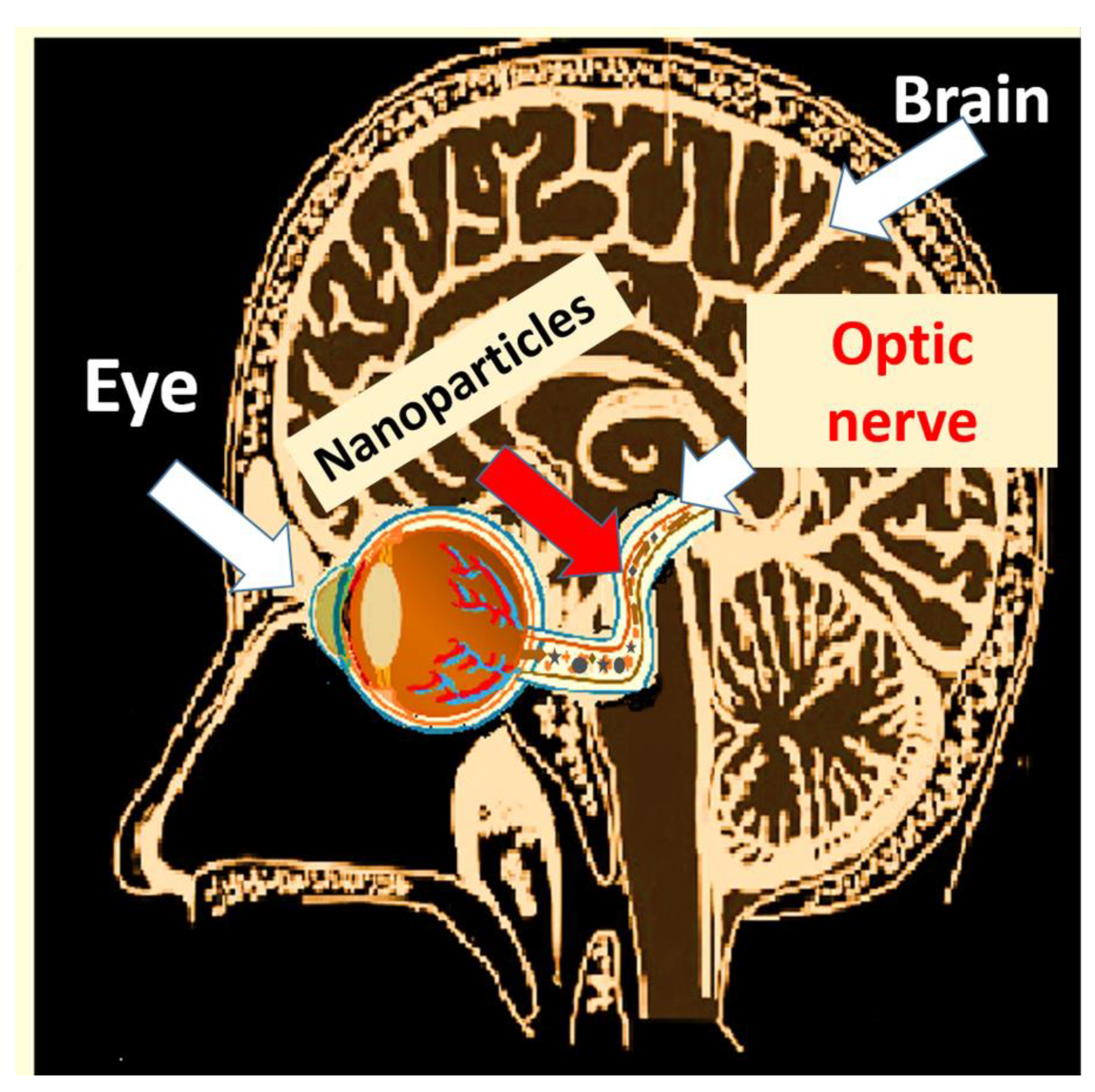

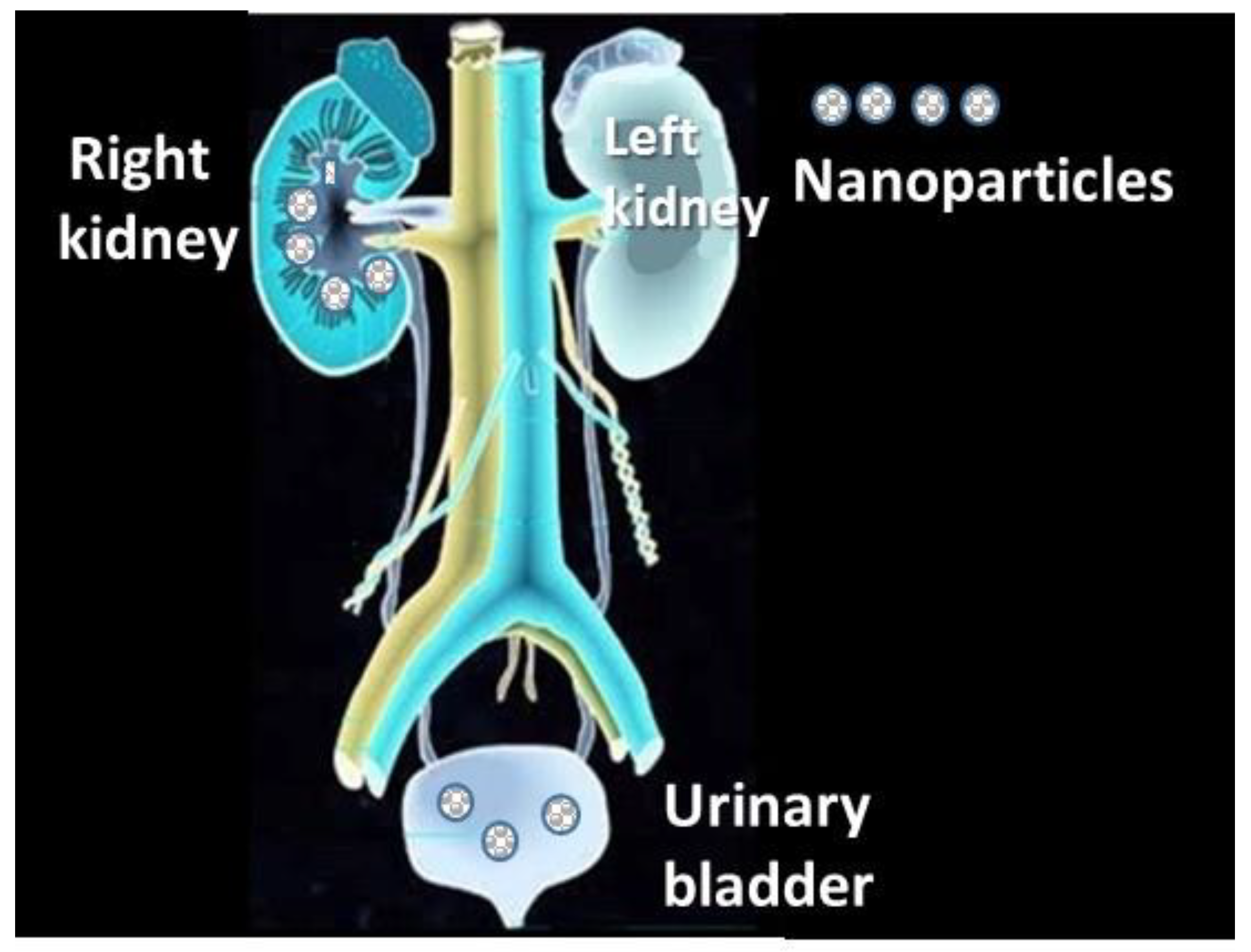
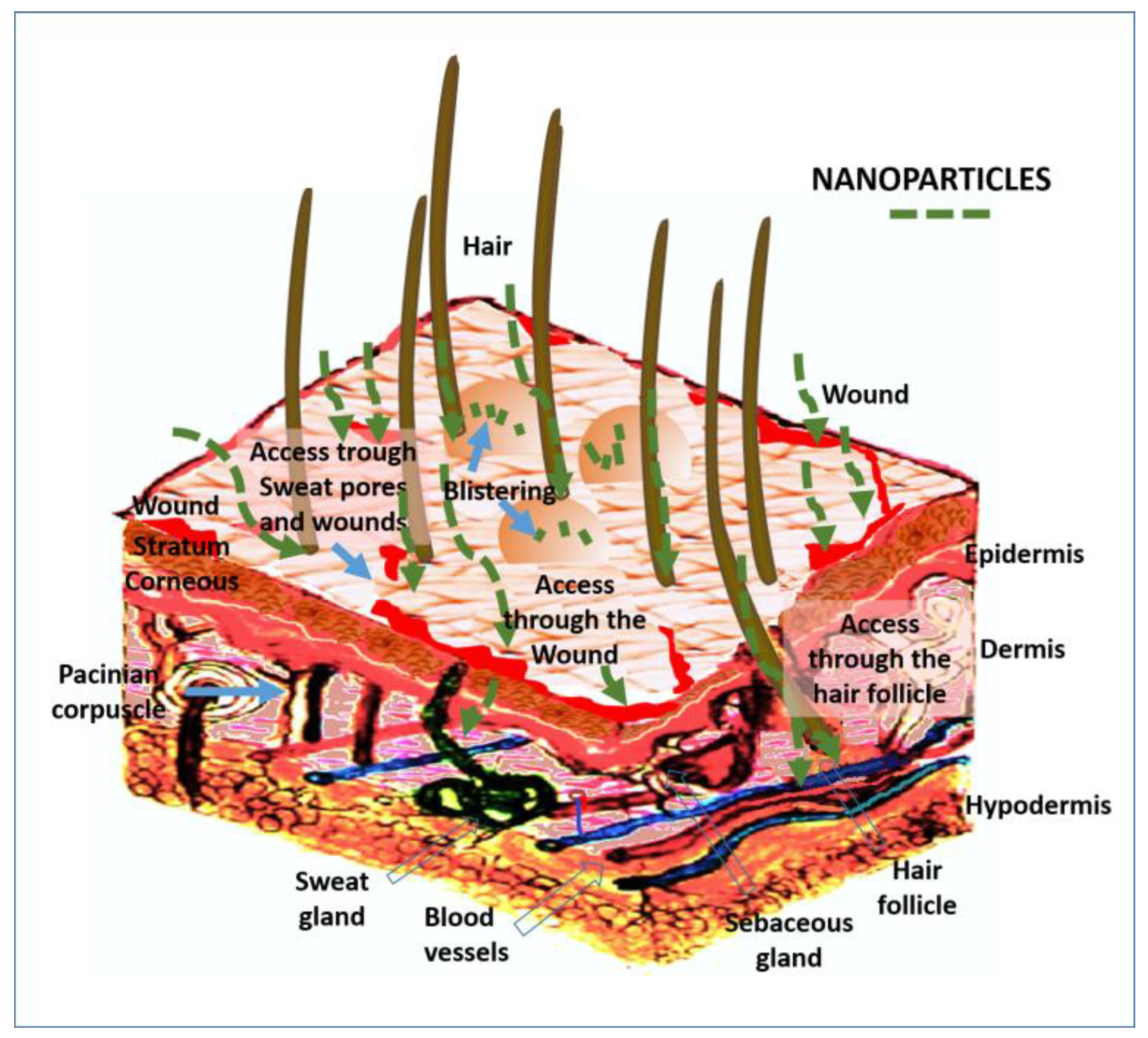
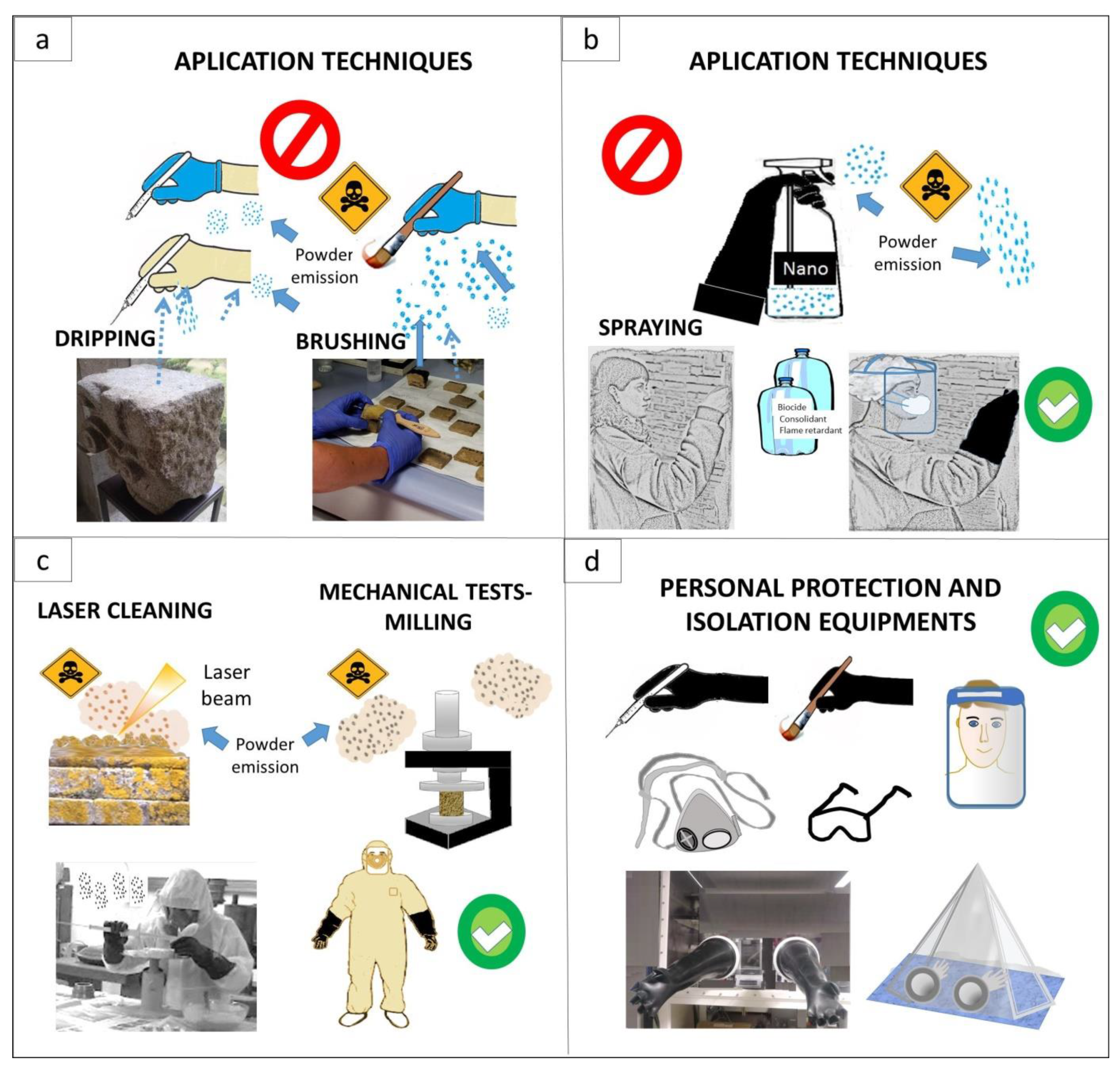
| Year/ Period | Strategies, Activities | Report No. |
|---|---|---|
| 2006–2015 | Definition of criteria for the safety of manufactured Nms Design of testing guidelines on: The ecotoxicology and environmental fate of manufactured Nms Inhalation toxicity tests Genotoxicity Preliminary guidance about advances in the safety of manufactured Nms | 1 [505] 62 [506] |
| 2016 | Categorization of nanostructured materials, physical-chemical parameters, methods for regulating nanomaterials SWCNT/MWCNT fullerenes, silver, gold, titanium oxide, silicon oxide, and metallic compounds in CNT | 63–79 [504] |
| 2017 | Sampling strategies, techniques, and protocols for determining the concentrations of manufactured nanomaterials in the workplace air | 80–84 [504] |
| 2018 | Inhalation toxicity of submicron particles, in vitro methods for human hazard assessment, biodurability of nanomaterials, and different types of risk assessments of manufactured nanomaterials | 85–88 Test Guidelines 318, 412–413 [504] |
| 2019 | Physical-chemical parameters measurement | 89–91 [504] |
| 2020 | Biopersistence/biodurability of manufactured nanomaterials, categorization of Nms risks | 92–97 [511] |
| 2021 | Evaluating tools and models used to assess the environmental exposure to manufactured Nms Functional evaluation and statistical analysis | 98–102 [504] |
| 2022 | Sustainability and safe design | 103–105 [512] |
| Procedure | Risk | Reccomendations |
|---|---|---|
| Spraying | Dispersion through the air of nanoparticles: contact with the skin, inhalation. Evaporation of harmful organic solvents and release of nanoparticles [629] | |
| Brushing | Skin exposure to nanoparticles | |
| Inmersion | Splash and dispersion though the air, soils, and rivers, contact with the skin, inhalation [257,630] | |
| Cleaning, milling | Dispersion through the air of nanoparticles: dermal and ocular contact, inhalation [631] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez-Villalba, L.S.; Salcines, C.; Fort, R. Application of Inorganic Nanomaterials in Cultural Heritage Conservation, Risk of Toxicity, and Preventive Measures. Nanomaterials 2023, 13, 1454. https://doi.org/10.3390/nano13091454
Gomez-Villalba LS, Salcines C, Fort R. Application of Inorganic Nanomaterials in Cultural Heritage Conservation, Risk of Toxicity, and Preventive Measures. Nanomaterials. 2023; 13(9):1454. https://doi.org/10.3390/nano13091454
Chicago/Turabian StyleGomez-Villalba, Luz Stella, Ciro Salcines, and Rafael Fort. 2023. "Application of Inorganic Nanomaterials in Cultural Heritage Conservation, Risk of Toxicity, and Preventive Measures" Nanomaterials 13, no. 9: 1454. https://doi.org/10.3390/nano13091454
APA StyleGomez-Villalba, L. S., Salcines, C., & Fort, R. (2023). Application of Inorganic Nanomaterials in Cultural Heritage Conservation, Risk of Toxicity, and Preventive Measures. Nanomaterials, 13(9), 1454. https://doi.org/10.3390/nano13091454





