Glycerol Electro-Oxidation in Alkaline Medium with Pt-Fe/C Electrocatalysts Synthesized by the Polyol Method: Increased Selectivity and Activity Provided by Less Expensive Catalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrocatalyst Synthesis
2.2. Physical Characterization
2.3. Electrochemical Measurements
2.4. Electrolysis and By-Product Determination
3. Results and Discussion
3.1. Electrocatalyst Physical Characterization
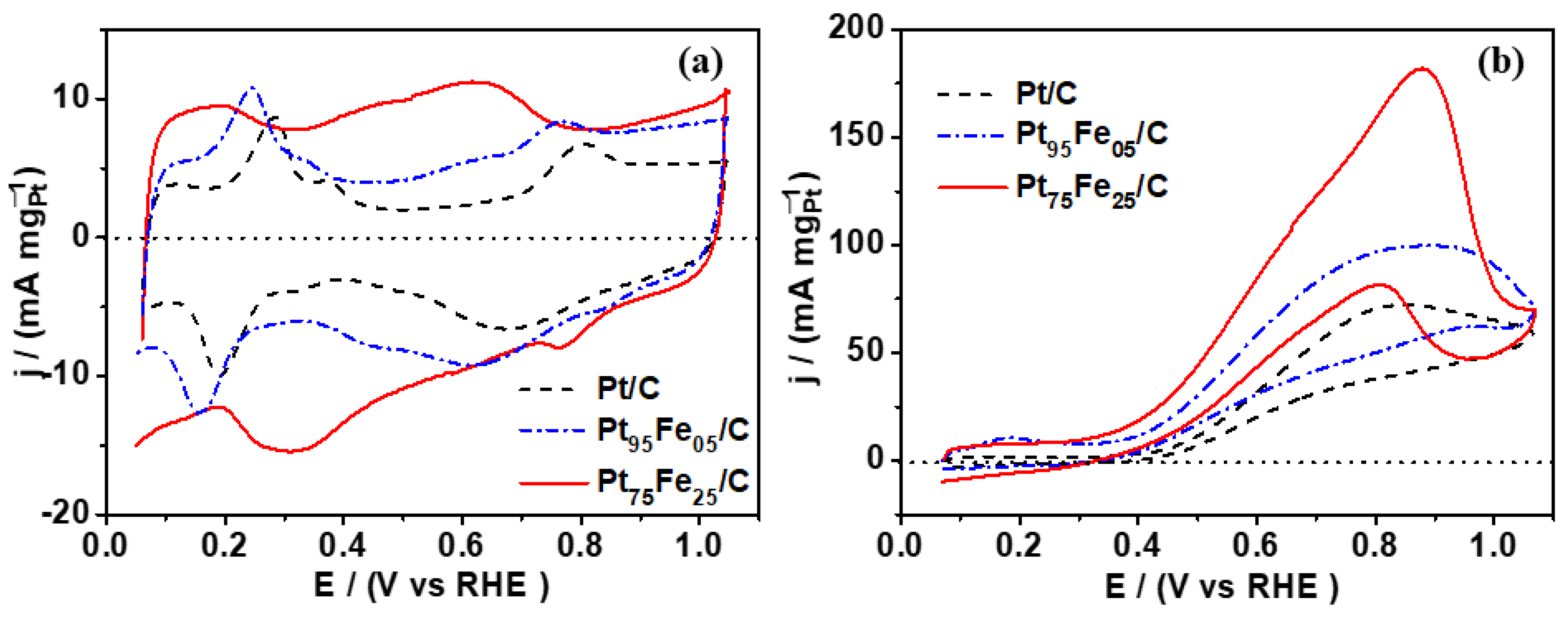
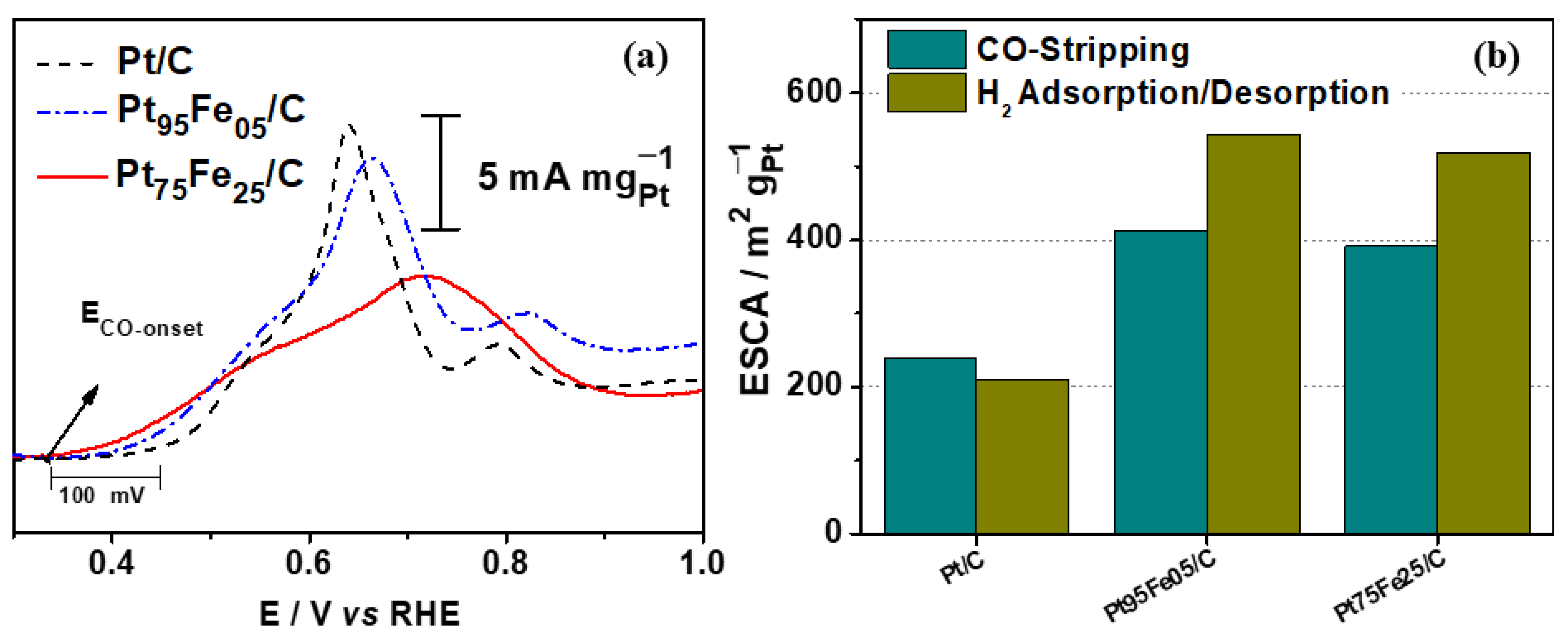
3.2. Electrochemical Characterization
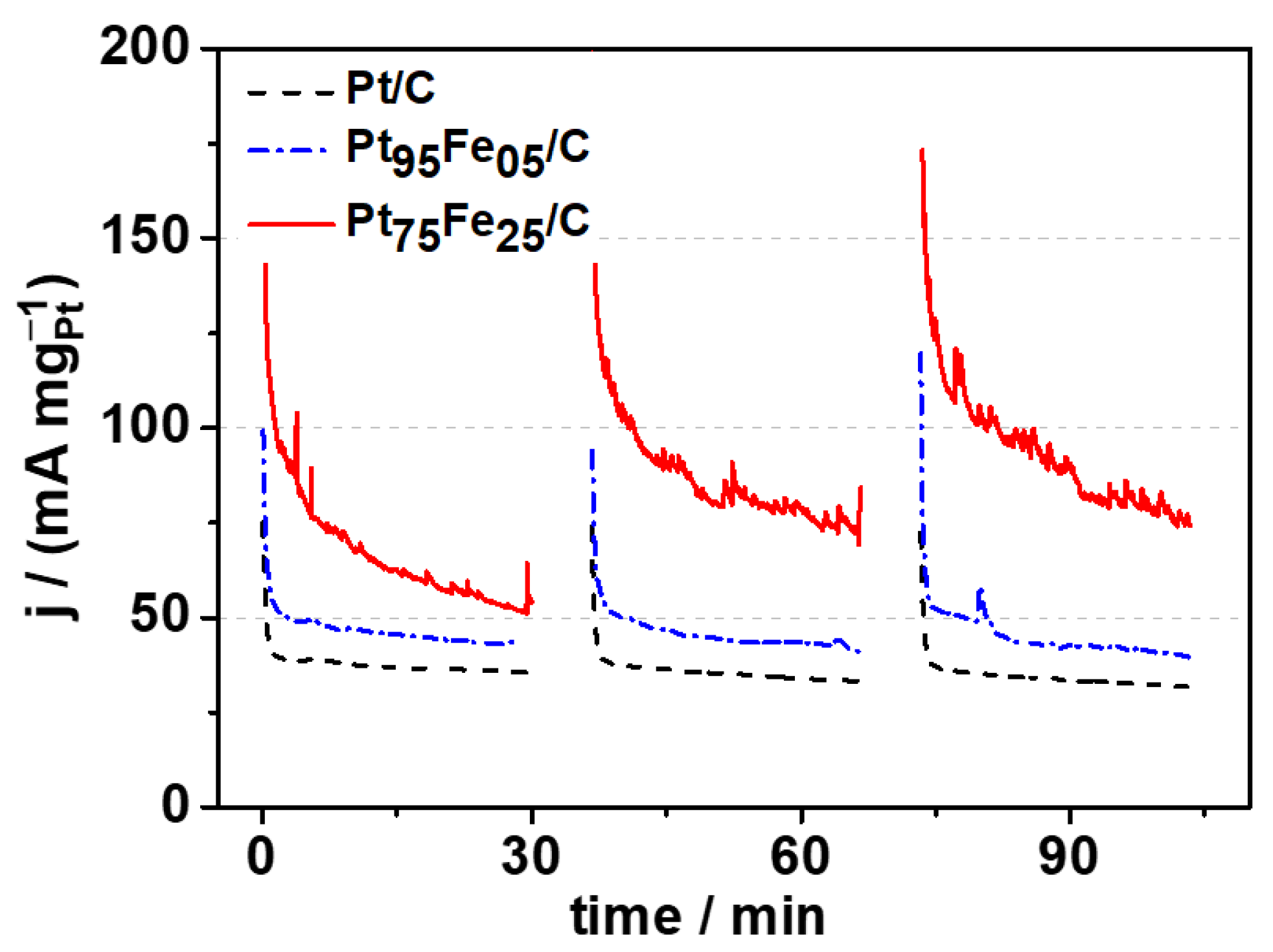
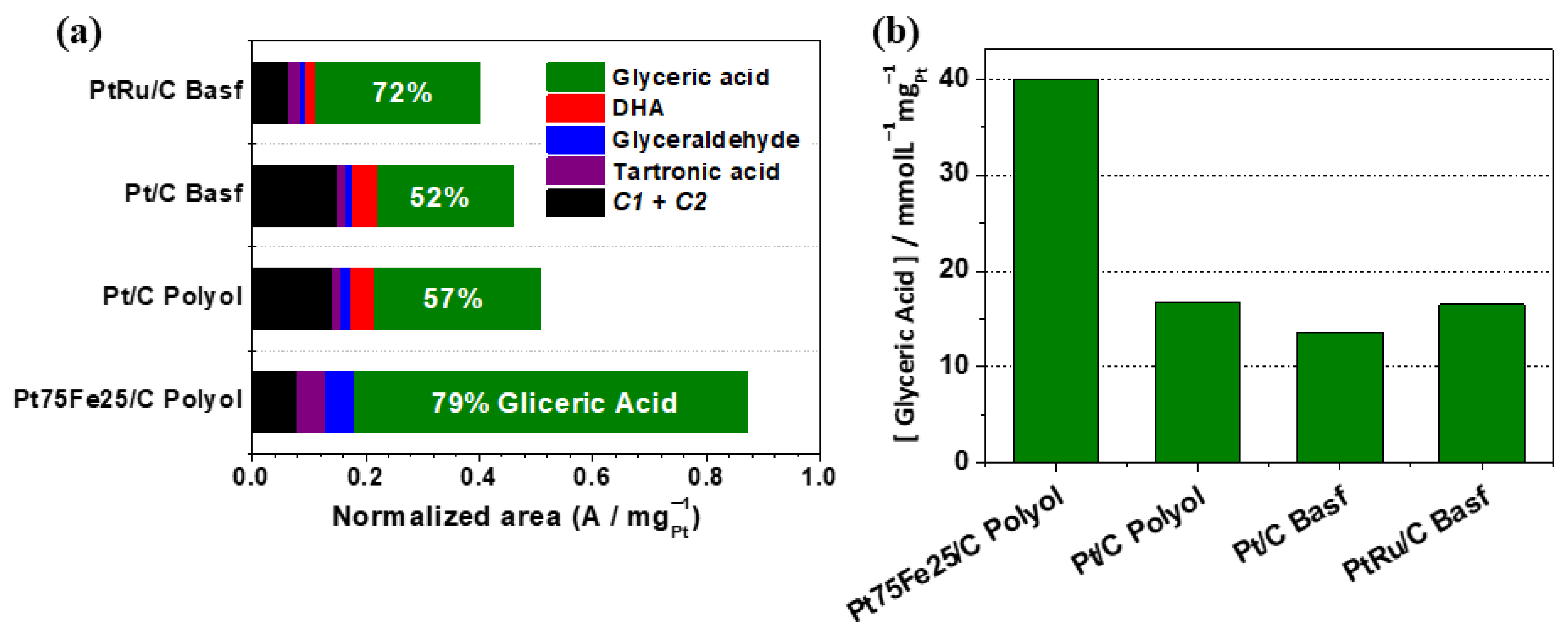
3.3. Electrolysis Study
4. Conclusions
- The polyol method provides good metal distribution and small nanoparticles (<2 nm) in PtFe electrocatalysts.
- Iron addition to platinum increases the active area and the catalytic activity for glycerol and CO electro-oxidation.
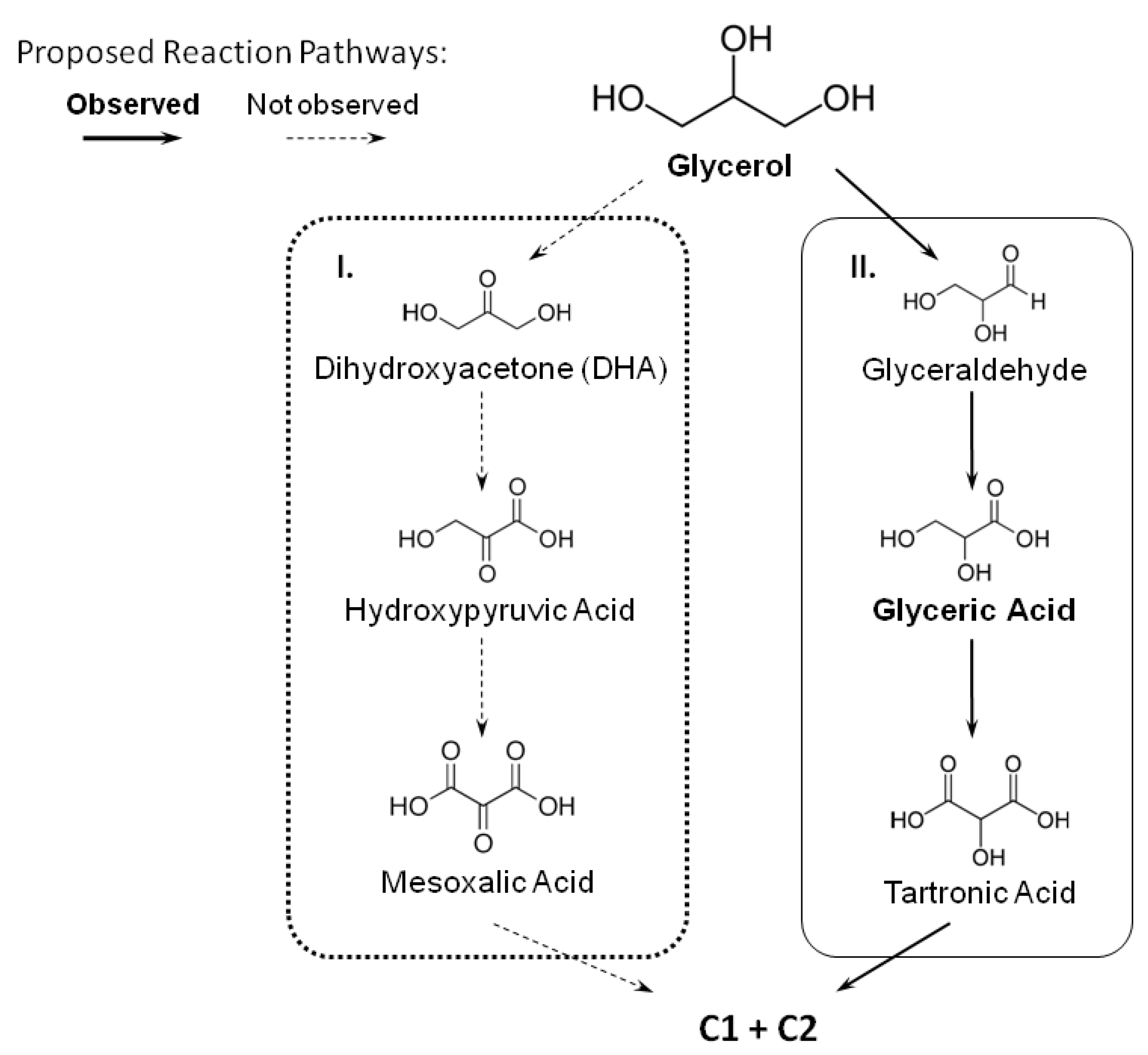
- Initially, glycerol electro-oxidation depends on the type of co-catalyst. PtFe favors the glyceraldehyde oxidation route, and glyceric acid is the main product.
- PtFe presents high selectivity for high-value-added C3-products.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Decarpigny, C.; Aljawish, A.; His, C.; Fertin, B.; Bigan, M.; Dhulster, P.; Millares, M.; Froidevaux, R. Bioprocesses for the Biodiesel Production from Waste Oils and Valorization of Glycerol. Energies 2022, 15, 3381. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, B.; Lawal, A. Recovery and Utilization of Crude Glycerol, a Biodiesel Byproduct. RSC Adv. 2022, 12, 27997–28008. [Google Scholar] [CrossRef]
- Houache, M.S.E.; Hughes, K.; Baranova, E.A. Study on Catalyst Selection for Electrochemical Valorization of Glycerol. Sustain. Energy Fuels 2019, 3, 1892–1915. [Google Scholar] [CrossRef]
- Alaba, P.A.; Lee, C.S.; Abnisa, F.; Aroua, M.K.; Cognet, P.; Peres-Lucchese, Y.; Daud, W.M.A.W. A Review of Recent Progress on Electrocatalysts toward Efficient Glycerol Electrooxidation. Rev. Chem. Eng. 2020, 36, 1. [Google Scholar] [CrossRef]
- Fan, L.; Liu, B.; Liu, X.; Senthilkumar, N.; Wang, G.; Wen, Z. Recent Progress in Electrocatalytic Glycerol Oxidation. Energy Technol. 2021, 9, 2000804. [Google Scholar] [CrossRef]
- Behr, A.; Eilting, J.; Irawadi, K.; Leschinski, J.; Lindner, F. Improved Utilisation of Renewable Resources: New Important Derivatives of Glycerol. Green Chem. 2008, 10, 13–30. [Google Scholar] [CrossRef]
- Coutanceau, C.; Baranton, S.; Kouamé, R.S.B. Selective Electrooxidation of Glycerol Into Value-Added Chemicals: A Short Overview. Front. Chem. 2019, 7, 100. [Google Scholar] [CrossRef]
- Smith, L.R.; Douthwaite, M.; Mugford, K.; Dummer, N.F.; Willock, D.J.; Hutchings, G.J.; Taylor, S.H. Recent Advances on the Valorization of Glycerol into Alcohols. Energies 2022, 15, 6250. [Google Scholar] [CrossRef]
- Li, T.; Harrington, D.A. An Overview of Glycerol Electrooxidation Mechanisms on Pt, Pd and Au. ChemSusChem 2021, 14, 1472–1495. [Google Scholar] [CrossRef]
- Melle, G.; de Souza, M.B.C.; Santiago, P.V.B.; Corradini, P.G.; Mascaro, L.H.; Fernández, P.S.; Sitta, E. Glycerol Electro-Oxidation at Pt in Alkaline Media: Influence of Mass Transport and Cations. Electrochim. Acta 2021, 398, 139318. [Google Scholar] [CrossRef]
- Antolini, E. Glycerol Electro-Oxidation in Alkaline Media and Alkaline Direct Glycerol Fuel Cells. Catalysts 2019, 9, 980. [Google Scholar] [CrossRef]
- Oliveira, V.L.; Morais, C.; Servat, K.; Napporn, T.W.; Tremiliosi-Filho, G.; Kokoh, K.B. Studies of the Reaction Products Resulted from Glycerol Electrooxidation on Ni-Based Materials in Alkaline Medium. Electrochim. Acta 2014, 117, 255–262. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Dong, Z.; Zhang, N.; Zhang, Q.; Xie, C.; Wu, Z.; Xu, G.-R.; Wang, L. One-Step CO Assisted Synthesis of Hierarchical Porous PdRuCu Nanosheets as Advanced Bifunctional Catalysts for Hydrogen Evolution and Glycerol Oxidation. Int. J. Hydrogen Energy 2022, 47, 33319–33328. [Google Scholar] [CrossRef]
- Garcia, A.C.; Morais, C.; Napporn, T.W.; Kokoh, K.B.; Tremiliosi-Filho, G. Unexpected Activity for Glycerol Electro-Oxidation of Nanostructured Pd−Pt and Pd−Pt−Ru Catalysts. ChemElectroChem 2017, 4, 1314–1319. [Google Scholar] [CrossRef]
- Holade, Y.; Morais, C.; Servat, K.; Napporn, T.W.; Kokoh, K.B. Toward the Electrochemical Valorization of Glycerol: Fourier Transform Infrared Spectroscopic and Chromatographic Studies. ACS Catal. 2013, 3, 2403–2411. [Google Scholar] [CrossRef]
- Watanabe, M.; Motoo, S. Electrocatalysis by Ad-Atoms: Part I. Enhancement of the Oxidation of Methanol on Platinum and Palladium by Gold Ad-Atoms. J. Electroanal. Chem. Interfacial Electrochem. 1975, 60, 259–266. [Google Scholar] [CrossRef]
- Watanabe, M.; Motoo, S. Electrocatalysis by Ad-Atoms: Part II. Enhancement of the Oxidation of Methanol on Platinum by Ruthenium Ad-Atoms. J. Electroanal. Chem. Interfacial Electrochem. 1975, 60, 267–273. [Google Scholar] [CrossRef]
- Watanabe, M.; Motoo, S. Electrocatalysis by Ad-Atoms: Part III. Enhancement of the Oxidation of Carbon Monoxide on Platinum by Ruthenium Ad-Atoms. J. Electroanal. Chem. Interfacial Electrochem. 1975, 60, 275–283. [Google Scholar] [CrossRef]
- González-Cobos, J.; Baranton, S.; Coutanceau, C. Development of Bismuth-Modified PtPd Nanocatalysts for the Electrochemical Reforming of Polyols into Hydrogen and Value-Added Chemicals. ChemElectroChem 2016, 3, 1694–1704. [Google Scholar] [CrossRef]
- de Souza, M.B.C.; Vicente, R.A.; Yukuhiro, V.Y.; Pires, C.T.G.V.M.T.; Cheuquepán, W.; Bott-Neto, J.L.; Solla-Gullón, J.; Fernández, P.S. Bi-Modified Pt Electrodes toward Glycerol Electrooxidation in Alkaline Solution: Effects on Activity and Selectivity. ACS Catal. 2019, 9, 5104–5110. [Google Scholar] [CrossRef]
- Ren, F.; Zhang, Z.; Liang, Z.; Shen, Q.; Luan, Y.; Xing, R.; Fei, Z.; Du, Y. Synthesis of PtRu Alloy Nanofireworks as Effective Catalysts toward Glycerol Electro-Oxidation in Alkaline Media. J. Colloid Interface Sci. 2022, 608, 800–808. [Google Scholar] [CrossRef]
- Yan, H.; Yao, S.; Yin, B.; Liang, W.; Jin, X.; Feng, X.; Liu, Y.; Chen, X.; Yang, C. Synergistic Effects of Bimetallic PtRu/MCM-41 Nanocatalysts for Glycerol Oxidation in Base-Free Medium: Structure and Electronic Coupling Dependent Activity. Appl. Catal. B Environ. 2019, 259, 118070. [Google Scholar] [CrossRef]
- Garcia, A.C.; Ferreira, E.B.; Silva de Barros, V.V.; Linares, J.J.; Tremiliosi-Filho, G. PtAg/MnOx/C as a Promising Electrocatalyst for Glycerol Electro-Oxidation in Alkaline Medium. J. Electroanal. Chem. 2017, 793, 188–196. [Google Scholar] [CrossRef]
- Weng, X.; Liu, Q.; Wang, A.-J.; Yuan, J.; Feng, J.-J. Simple One-Pot Synthesis of Solid-Core@porous-Shell Alloyed PtAg Nanocrystals for the Superior Catalytic Activity toward Hydrogen Evolution and Glycerol Oxidation. J. Colloid Interface Sci. 2017, 494, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shen, Y.; Xi, J.; Luo, X. Selective Electro-Oxidation of Glycerol to Dihydroxyacetone by PtAg Skeletons. ACS Appl. Mater. Interfaces 2019, 11, 28953–28959. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shen, Y.; Piao, J. Sustainable Conversion of Glycerol into Value-Added Chemicals by Selective Electro-Oxidation on Pt-Based Catalysts. ChemElectroChem 2018, 5, 1636–1643. [Google Scholar] [CrossRef]
- Bhunia, K.; Khilari, S.; Pradhan, D. Monodispersed PtPdNi Trimetallic Nanoparticles-Integrated Reduced Graphene Oxide Hybrid Platform for Direct Alcohol Fuel Cell. ACS Sustain. Chem. Eng. 2018, 6, 7769–7778. [Google Scholar] [CrossRef]
- Antolini, E. Iron-Containing Platinum-Based Catalysts as Cathode and Anode Materials for Low-Temperature Acidic Fuel Cells: A Review. RSC Adv. 2016, 6, 3307–3325. [Google Scholar] [CrossRef]
- Vinayan, B.P.; Sahoo, M.; Ramaprabhu, S. Platinum-Iron Alloy Nanoparticles Dispersed Multiwalled Carbon Nanotubes As Cathode Electrocatalyst for PEMFC. In Proceedings of the 2011 International Conference on Nanoscience, Technology and Societal Implications, Bhubaneswar, India, 8–10 December 2011; pp. 1–5. [Google Scholar] [CrossRef]
- Yu, J.; Gao, W.; Liu, F.; Ju, Y.; Zhao, F.; Yang, Z.; Chu, X.; Che, S.; Hou, Y. Tuning Crystal Structure and Magnetic Property of Dispersible FePt Intermetallic Nanoparticles. Sci. China Mater. 2018, 61, 961–968. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, M.; Liu, J.; Liu, R.; Zhao, J. Glycerol-Stabilized NaBH4 Reduction at Room-Temperature for the Synthesis of a Carbon-Supported PtxFe Alloy with Superior Oxygen Reduction Activity for a Microbial Fuel Cell. Electrochim. Acta 2014, 141, 331–339. [Google Scholar] [CrossRef]
- Almeida, T.S.; Van Wassen, A.R.; VanDover, R.B.; de Andrade, A.R.; Abruña, H.D. Combinatorial PtSnM (M = Fe, Ni, Ru and Pd) Nanoparticle Catalyst Library toward Ethanol Electrooxidation. J. Power Sources 2015, 284, 623–630. [Google Scholar] [CrossRef]
- Fashedemi, O.O.; Ozoemena, K.I. Comparative Electrocatalytic Oxidation of Ethanol, Ethylene Glycol and Glycerol in Alkaline Medium at Pd-Decorated FeCo@Fe/C Core-Shell Nanocatalysts. Electrochim. Acta 2014, 128, 279–286. [Google Scholar] [CrossRef]
- Fashedemi, O.O.; Miller, H.A.; Marchionni, A.; Vizza, F.; Ozoemena, K.I. Electro-Oxidation of Ethylene Glycol and Glycerol at Palladium-Decorated FeCo@Fe Core–Shell Nanocatalysts for Alkaline Direct Alcohol Fuel Cells: Functionalized MWCNT Supports and Impact on Product Selectivity. J. Mater. Chem. A 2015, 3, 7145–7156. [Google Scholar] [CrossRef]
- Palma, L.M.; Almeida, T.S.; Oliveira, V.L.; Tremiliosi-Filho, G.; Gonzalez, E.R.; de Andrade, A.R.; Servat, K.; Morais, C.; Napporn, T.W.; Kokoh, K.B. Identification of Chemicals Resulted in Selective Glycerol Conversion as Sustainable Fuel on Pd-Based Anode Nanocatalysts. RSC Adv. 2014, 4, 64476–64483. [Google Scholar] [CrossRef]
- Almeida, T.S.; Garbim, C.; Silva, R.G.; De Andrade, A.R. Addition of Iron Oxide to Pt-Based Catalyst to Enhance the Catalytic Activity of Ethanol Electrooxidation. J. Electroanal. Chem. 2017, 796, 49–56. [Google Scholar] [CrossRef]
- Wang, F.; Fang, B.; Yu, X.; Feng, L. Coupling Ultrafine Pt Nanocrystals over the Fe2P Surface as a Robust Catalyst for Alcohol Fuel Electro-Oxidation. ACS Appl. Mater. Interfaces 2019, 11, 9496–9503. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; An, Y.; Zhou, S.; Wang, Z.; Long, J.; Liao, W.; Chen, M.; Wang, Q. Precisely Tuning the Electronic Structure of Ordered PtFe Alloy Supported on Multi-Walled Carbon Nanotubes for Enhanced Methanol Oxidation. J. Alloy. Compd. 2023, 937, 168347. [Google Scholar] [CrossRef]
- Xu, H.; Song, P.; Wang, J.; Gao, F.; Zhang, Y.; Shiraishi, Y.; Du, Y. High-Quality Platinum–Iron Nanodendrites with a Multibranched Architecture as Efficient Electrocatalysts for the Ethanol Oxidation Reaction. ChemCatChem 2018, 10, 2195–2199. [Google Scholar] [CrossRef]
- Mari, E.; Tsai, P.-C.; Eswaran, M.; Ponnusamy, V.K. Efficient Electro-Catalytic Oxidation of Ethylene Glycol Using Flower-like Graphitic Carbon Nitride/Iron Oxide/Palladium Nanocomposite for Fuel Cell Application. Fuel 2020, 280, 118646. [Google Scholar] [CrossRef]
- Jin, X.; Yan, H.; Zeng, C.; Thapa, P.S.; Subramaniam, B.; Chaudhari, R.V. Phase Transformed PtFe Nanocomposites Show Enhanced Catalytic Performances in Oxidation of Glycerol to Tartronic Acid. Ind. Eng. Chem. Res. 2017, 56, 13157–13164. [Google Scholar] [CrossRef]
- Jin, X.; Zhao, M.; Yan, W.; Zeng, C.; Bobba, P.; Thapa, P.S.; Subramaniam, B.; Chaudhari, R.V. Anisotropic Growth of PtFe Nanoclusters Induced by Lattice-Mismatch: Efficient Catalysts for Oxidation of Biopolyols to Carboxylic Acid Derivatives. J. Catal. 2016, 337, 272–283. [Google Scholar] [CrossRef]
- Wan, H.; Dai, C.; Jin, L.; Luo, S.; Meng, F.; Chen, G.; Duan, Y.; Liu, C.; Xu, Q.; Lu, J.; et al. Electro-Oxidation of Glycerol to High-Value-Added C1–C3 Products by Iron-Substituted Spinel Zinc Cobalt Oxides. ACS Appl. Mater. Interfaces 2022, 14, 14293–14301. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.-G.; Tran, G.-S.; Chiang, C.-L.; Lin, Y.-G.; Chang, H.-E.; Kuo, H.-H.; Chiang, C.-Y.; Hsu, Y.-J. Au@NiSx Yolk@Shell Nanostructures as Dual-Functional Electrocatalysts for Concomitant Production of Value-Added Tartronic Acid and Hydrogen Fuel (Adv. Funct. Mater. 4/2023). Adv. Funct. Mater. 2023, 33, 2370021. [Google Scholar] [CrossRef]
- Gharibi, H.; Mirzaie, R.A.; Shams, E.; Zhiani, M.; Khairmand, M. Preparation of Platinum Electrocatalysts Using Carbon Supports for Oxygen Reduction at a Gas-Diffusion Electrode. J. Power Sources 2005, 139, 61–66. [Google Scholar] [CrossRef]
- Quintero-Ruiz, J.; Ruiz-Rosas, R.; Quílez-Bermejo, J.; Salinas-Torres, D.; Cazorla-Amorós, D.; Morallón, E. Preparation of Pt/CNT Thin-Film Electrodes by Electrochemical Potential Pulse Deposition for Methanol Oxidation. C 2021, 7, 32. [Google Scholar] [CrossRef]
- Xiong, Y.; Xia, Y. Shape-Controlled Synthesis of Metal Nanostructures: The Case of Palladium. Adv. Mater. 2007, 19, 3385–3391. [Google Scholar] [CrossRef]
- Wu, S.-H.; Chen, D.-H. Synthesis and Characterization of Nickel Nanoparticles by Hydrazine Reduction in Ethylene Glycol. J. Colloid Interface Sci. 2003, 259, 282–286. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, J.; Wiley, B.; Xia, Y.; Aloni, S.; Yin, Y. Understanding the Role of Oxidative Etching in the Polyol Synthesis of Pd Nanoparticles with Uniform Shape and Size. J. Am. Chem. Soc. 2005, 127, 7332–7333. [Google Scholar] [CrossRef]
- Antolini, E. Carbon Supports for Low-Temperature Fuel Cell Catalysts. Appl. Catal. B Environ. 2009, 88, 1–24. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction, 2nd ed.; Addison-Wesley series in metallurgy and materials; Addison-Wesley Publishing Company, Inc.: Reading, MA, USA, 1978. [Google Scholar]
- Taylor, S.; Fabbri, E.; Levecque, P.; Schmidt, T.J.; Conrad, O. The Effect of Platinum Loading and Surface Morphology on Oxygen Reduction Activity. Electrocatalysis 2016, 7, 287–296. [Google Scholar] [CrossRef]
- Vidaković, T.; Christov, M.; Sundmacher, K. The Use of CO Stripping for in Situ Fuel Cell Catalyst Characterization. Electrochim. Acta 2007, 52, 5606–5613. [Google Scholar] [CrossRef]
- Bishnoi, A.; Kumar, S.; Joshi, N. Chapter 9—Wide-Angle X-Ray Diffraction (WXRD): Technique for Characterization of Nanomaterials and Polymer Nanocomposites. In Microscopy Methods in Nanomaterials Characterization; Thomas, S., Thomas, R., Zachariah, A.K., Mishra, R.K., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 313–337. [Google Scholar] [CrossRef]
- Jiang, L.; Hsu, A.; Chu, D.; Chen, R. Ethanol Electro-Oxidation on Pt/C and PtSn/C Catalysts in Alkaline and Acid Solutions. Int. J. Hydrogen Energy 2010, 35, 365–372. [Google Scholar] [CrossRef]
- Warwick, M.E.A.; Kaunisto, K.; Carraro, G.; Gasparotto, A.; Maccato, C.; Barreca, D. A Study of Pt/α-Fe2O3 Nanocomposites by XPS. Surf. Sci. Spectra 2015, 22, 47–57. [Google Scholar] [CrossRef]
- Song, G.; Yang, H.; Sun, Y.; Wang, J.; Qu, W.; Zhang, Q.; Ma, L.; Feng, Y. Promoting Effects of Fe2O3 to Pt Electrocatalysts toward Methanol Oxidation Reaction in Alkaline Electrolyte. Chin. J. Catal. 2017, 38, 554–562. [Google Scholar] [CrossRef]
- Yaqoob, L.; Noor, T.; Iqbal, N. Recent Progress in Development of Efficient Electrocatalyst for Methanol Oxidation Reaction in Direct Methanol Fuel Cell. Int. J. Energy Res. 2021, 45, 6550–6583. [Google Scholar] [CrossRef]
- Yu, E.H.; Wang, X.; Krewer, U.; Li, L.; Scott, K. Direct Oxidation Alkaline Fuel Cells: From Materials to Systems. Energy Environ. Sci. 2012, 5, 5668–5680. [Google Scholar] [CrossRef]
- Fujiwara, N.; Siroma, Z.; Yamazaki, S.; Ioroi, T.; Senoh, H.; Yasuda, K. Direct Ethanol Fuel Cells Using an Anion Exchange Membrane. J. Power Sources 2008, 185, 621–626. [Google Scholar] [CrossRef]
- Caneppele, G.L.; Almeida, T.S.; Zanata, C.R.; Teixeira-Neto, É.; Fernández, P.S.; Camara, G.A.; Martins, C.A. Exponential Improving in the Activity of Pt/C Nanoparticles towards Glycerol Electrooxidation by Sb Ad-Atoms Deposition. Appl. Catal. B Environ. 2017, 200, 114–120. [Google Scholar] [CrossRef]
- Da Silva, R.G.; Aquino Neto, S.; Kokoh, K.B.; De Andrade, A.R. Electroconversion of Glycerol in Alkaline Medium: From Generation of Energy to Formation of Value-Added Products. J. Power Sources 2017, 351, 174–182. [Google Scholar] [CrossRef]
- Almeida, T.S.; Yu, Y.; de Andrade, A.R.; Abruña, H.D. Employing Iron and Nickel to Enhance Ethanol Oxidation of Pd-Based Anodes in Alkaline Medium. Electrochim. Acta 2019, 295, 751–758. [Google Scholar] [CrossRef]
- Nie, Y.; Qi, X.; Wu, R.; Yang, R.; Wang, H.; Deng, M.; Zhang, S.; Lu, S.; Gu, Z.; Liu, X. Structurally Ordered PtFe Intermetallic Nanocatalysts toward Efficient Electrocatalysis of Methanol Oxidation. Appl. Surf. Sci. 2021, 569, 151004. [Google Scholar] [CrossRef]
- Cai, Z.; Lu, Z.; Bi, Y.; Li, Y.; Kuang, Y.; Sun, X. Superior Anti-CO Poisoning Capability: Au-Decorated PtFe Nanocatalysts for High-Performance Methanol Oxidation. Chem. Commun. 2016, 52, 3903–3906. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, S.; Li, P.; Ibraheem, S.; Li, J.; Deng, J.; Wei, Z. Surface Ru Enriched Structurally Ordered Intermetallic PtFe@PtRuFe Core-Shell Nanostructure Boosts Methanol Oxidation Reaction Catalysis. Appl. Catal. B Environ. 2019, 252, 120–127. [Google Scholar] [CrossRef]
- Cassani, A.; Tuleushova, N.; Wang, Q.; Guesmi, H.; Bonniol, V.; Cambedouzou, J.; Tingry, S.; Bechelany, M.; Cornu, D.; Holade, Y. Fe-Modified Pd as an Effective Multifunctional Electrocatalyst for Catalytic Oxygen Reduction and Glycerol Oxidation Reactions in Alkaline Media. ACS Appl. Energy Mater. 2021, 4, 9944–9960. [Google Scholar] [CrossRef]
- Palma, L.M.; Almeida, T.S.; Morais, C.; Napporn, T.W.; Kokoh, K.B.; de Andrade, A.R. Effect of Co-Catalyst on the Selective Electrooxidation of Glycerol over Ruthenium-Based Nanomaterials. ChemElectroChem 2017, 4, 39–45. [Google Scholar] [CrossRef]
- Luo, H.; Yukuhiro, V.Y.; Fernández, P.S.; Feng, J.; Thompson, P.; Rao, R.R.; Cai, R.; Favero, S.; Haigh, S.J.; Durrant, J.R.; et al. Role of Ni in PtNi Bimetallic Electrocatalysts for Hydrogen and Value-Added Chemicals Coproduction via Glycerol Electrooxidation. ACS Catal. 2022, 12, 14492–14506. [Google Scholar] [CrossRef]
- Zhou, Y.; Shen, Y.; Luo, X.; Liu, G.; Cao, Y. Boosting Activity and Selectivity of Glycerol Oxidation over Platinum–Palladium–Silver Electrocatalysts via Surface Engineering. Nanoscale Adv. 2020, 2, 3423–3430. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, B.; Xu, X.; Jing, P.; Zhang, J. Heterogeneous Pt-Bi Hybrid Nanoparticle Decorated Self-Standing Hollow TiN Nanowire as an Efficient Catalyst for Glycerol Electrooxidation. J. Power Sources 2022, 543, 231836. [Google Scholar] [CrossRef]
- Zhou, Y.; Shen, Y.; Xi, J. Seed-Mediated Synthesis of PtxAuy@Ag Electrocatalysts for the Selective Oxidation of Glycerol. Appl. Catal. B Environ. 2019, 245, 604–612. [Google Scholar] [CrossRef]
- García, G.; Tsiouvaras, N.; Pastor, E.; Peña, M.A.; Fierro, J.L.G.; Martínez-Huerta, M.V. Ethanol Oxidation on PtRuMo/C Catalysts: In Situ FTIR Spectroscopy and DEMS Studies. Int. J. Hydrogen Energy 2012, 37, 7131–7140. [Google Scholar] [CrossRef]
- Şener, T.; Demirci, U.B.; Gül, Ö.F.; Ata, A. Pd–MnO2–Fe2O3/C as Electrocatalyst for the Formic Acid Electrooxidation. Int. J. Hydrogen Energy 2015, 40, 6920–6926. [Google Scholar] [CrossRef]
- Jiang, L.; Sun, G.; Sun, S.; Liu, J.; Tang, S.; Li, H.; Zhou, B.; Xin, Q. Structure and Chemical Composition of Supported Pt–Sn Electrocatalysts for Ethanol Oxidation. Electrochim. Acta 2005, 50, 5384–5389. [Google Scholar] [CrossRef]
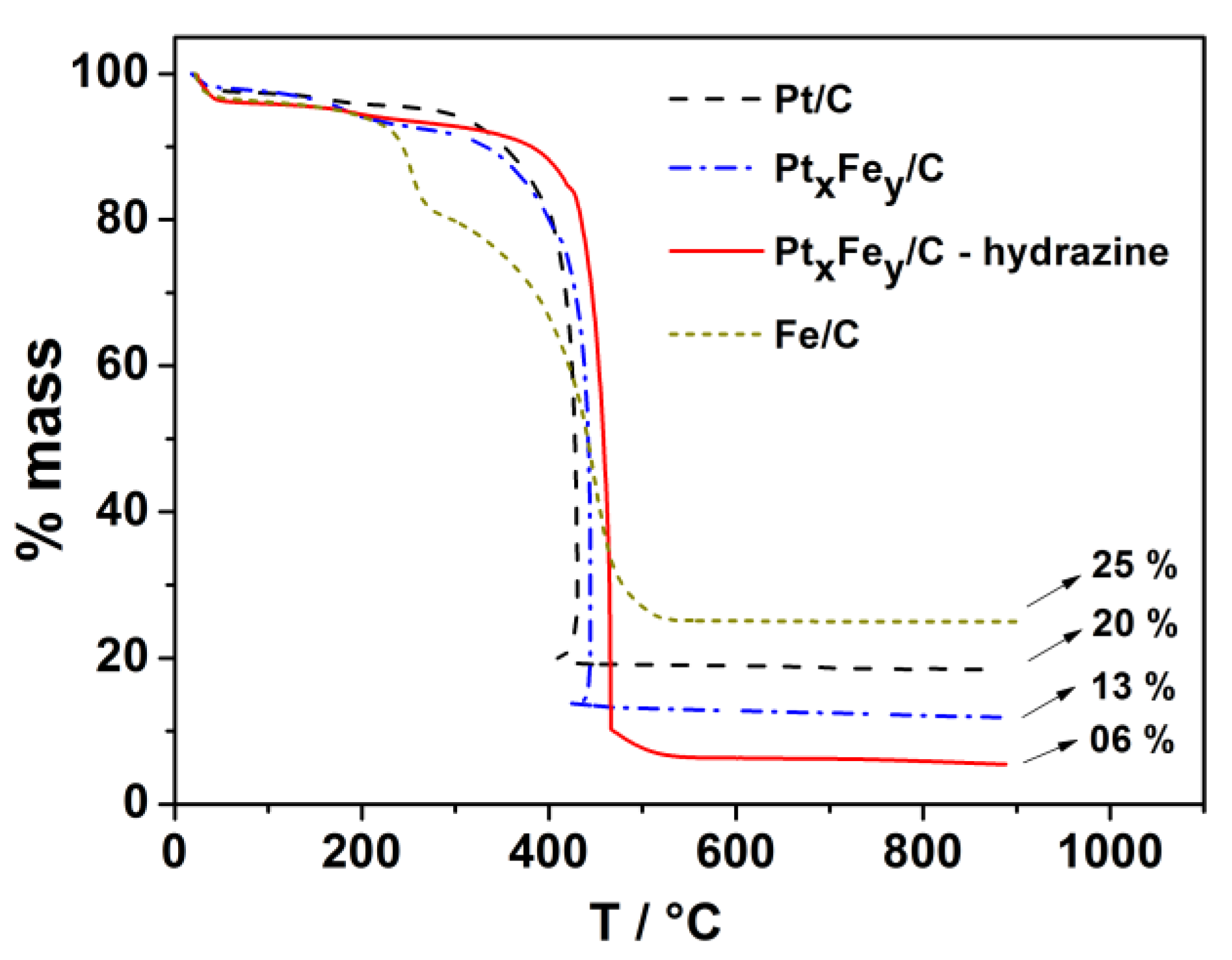
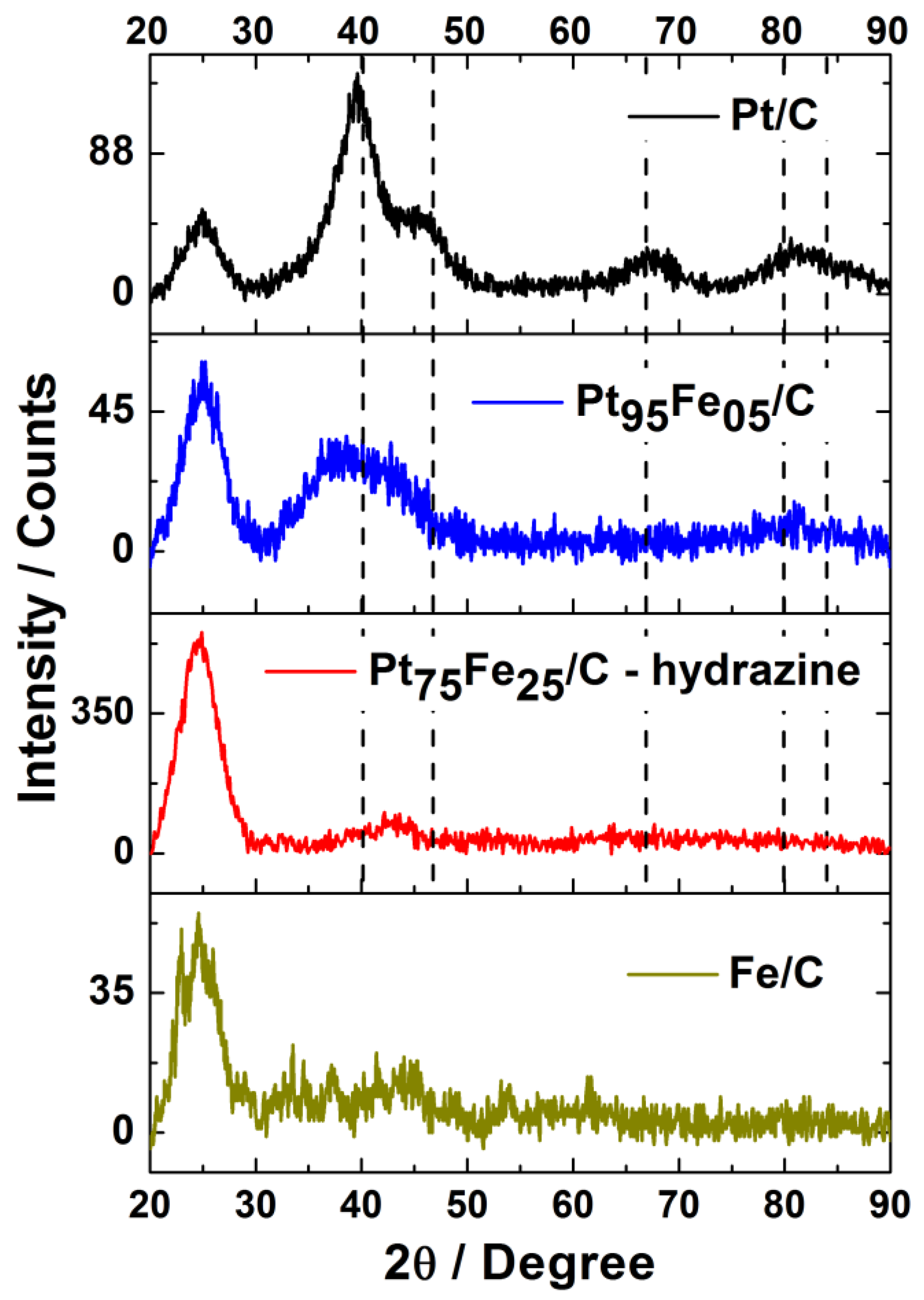
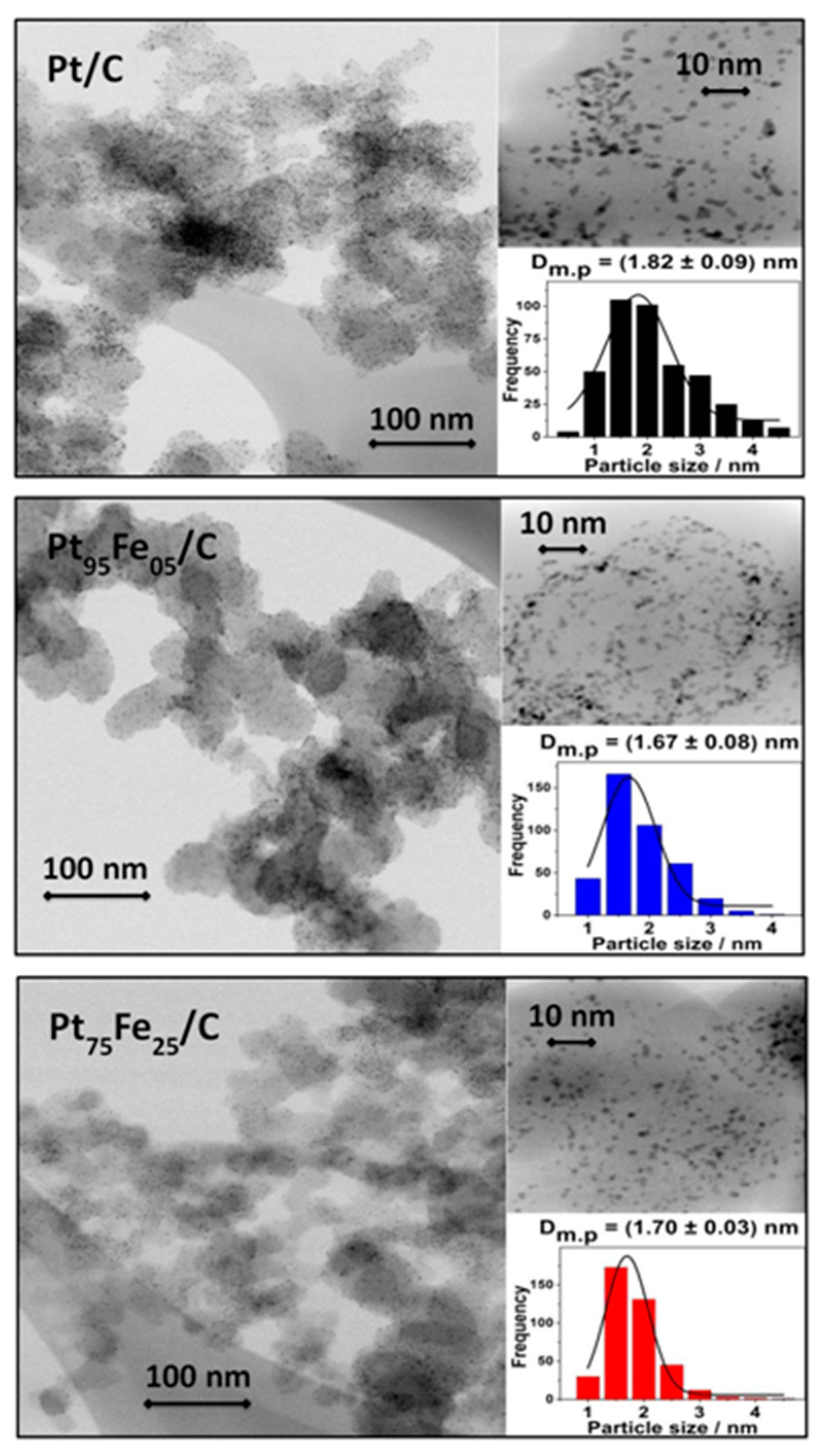
| Nominal Composition | Experimental Composition (EDX) | TG (%M) | d XRD [nm] | TEM [nm] |
|---|---|---|---|---|
| Fe/C | Fe/C | 25 | - | - |
| Pt/C | Pt/C | 20 | 1.4 | 1.82 |
| Pt50Fe50/C | Pt95Fe05/C | 13 | - | 1.67 |
| Pt50Fe50/C-hydrazine | Pt75Fe25/C | 6 | - | 1.70 |
| Composition | Eonset V vs. RHE | Anodic Peak (mA mgPt−1) @ +0.87 V vs. RHE | Catalytic Activity (mA mgPt−1) @ +0.70 V vs. RHE | ECSA (m2 gPt−1) |
|---|---|---|---|---|
| Pt/C | 0.40 | 65 | 30 | 240 |
| Pt95Fe05/C | 0.35 | 88 | 40 | 415 |
| Pt75Fe25/C | 0.35 | 192 | 75 | 390 |
| Catalyst Composition Synthesis Method | Electrolyte | Peak Current (If) Peak Current Potential (Ef) | Condition Electrolysis | Selectivity (%) Gly Conversion (%) | [REF] Year |
|---|---|---|---|---|---|
| Pt75Fe25/C Polyol | 0.5 M Gly + 0.1 M NaOH | If = 192 mA mgPt−1 (10 mV s−1) Ef = +0.87 V vs. RHE | E = 0.7 V vs. RHE (4 h) | Glycerate (79%) Glycerol (5–10%) | This Work |
| PdFe/C Pechini/microwave | 0.1 M Gly + 0.1 M NaOH | If = 28 mA mgPd−1 (50 mVs−1) Ef = 1.2 V vs. RHE | E = 0.8 V vs. RHE | Glycerate (2 mM) | [35] 2014 |
| PdFe/rGO Reduction with NaBH4 | 0.1 M Gly + 1 M KOH | If = 1.1 A mgPd−1 (50 mV s−1) Ef = 0.9 V vs. RHE | E = 0.8 V vs. RHE (2 h) | Glycerate (45%) | [67] 2021 |
| NiFe/C H2 flow at 300 °C | 0.1 M Gly + 0.1 M NaOH | I = 60 mA gM−1 (50 mV s−1) E = 1.6 V vs. RHE | E = 1.6 V vs. RHE (8 h) | Formate (4%) Glycerol (14%) | [12] 2014 |
| NiFeCo/C H2 flow at 300 °C | 0.1 M Gly + 0.1 M NaOH | I = 60 mA gM−1 (50 mV s−1) E = 1.6 V vs. RHE | E = 1.6 V vs. RHE (8 h) | Formate (34%) Glycerol (13%) | [12] 2014 |
| Pt86Ru/C Pechini/microwave | 0.5 M Gly + 1.0 M NaOH | If = 525 mA mgPt−1 (10 mVs−1) Ef = 1.2 V vs. RHE | E = 0.7 V vs. RHE (4 h) | DHA (8 mM) | [68] 2017 |
| Ru@Pt/CNTs Reduction with NaBH4 | 0.5 M Gly + 1 m NaOH | If = 215 mA mgPt−1(10 mV s−1) Ef= −0.46 V vs. Hg/HgO | E = −0.2 V vs. Hg/HgO (12 h) | Glycerate Glycerol (40%) | [62] 2017 |
| Ni@Pt/CNTs Reduction with NaBH4 | 0.5 M Gly + 1 m NaOH | If = 275 mA mgPt−1 (10 mV s−1) Ef = −0.49 V vs. Hg/HgO | E = −0.2 V vs. Hg/HgO (6 h) | Glycerate Glycerol (60%) | [62] 2017 |
| PtAg Galvanic replacement | 1 M Gly + 0.1 M KOH | If = 7.6 mA cm−2 (50 mV s−1) Ef = 1.0 V vs. RHE | E = 0.7 V vs. RHE | DHA (83%) | [25] 2019 |
| PtRh/GNS Polyol | 0.5 ML Gly + 0.5 M KOH | If = 4.5 mA cm−2 (50 mV s−1) Ef = −0.25 V vs. SCE | E = 0.2 V vs. SCE | Glycerate (50%) | [26] 2018 |
| PtNi2 | 0.1 M Gly + 1 M KOH | If = 2 AmgPt−1 (50 mV s−1) Ef = 0.85 V vs. RHE | E = 0.8 V vs. RHE (2h) | Tartronate (60%) | [69] 2022 |
| Pt@Ag-NaCl Hydrothermal | 1 M Gly + 0.1 M KO (If) 0.5 M Gly + 0.5 M KOH (Elect.) | If = 1.2 mA cm−2 (50 mV s−1) Ef = 1.0 V vs. RHE | E = 0.5V vs. RHE | DHA (75%) | [70] 2020 |
| Pt 95Bi05/TiN-HNWs | 0.05 M Gly + 1.0 M KOH (If) 1 M Gly + 1.0 M KOH (Elect.) | If = 307 mA mgPt−1 (20 mV s−1) Ef = 1.0 V vs. RHE | E = 1.0 V vs. RHE (8h) | Glycerate (37%) Glycerol (87%) | [71] 2022 |
| PtPd@Ag-NH3 Hydrothermal | 1 M Gly + 0.1 M KO (If) 0.5 M Gly + 0.5 M KOH (Elect.) | If = 9.2 mA cm−2 (50 mV s−1) Ef = 1.0 V vs. RHE | E = 0.5 V vs. RHE | DHA (70%) | [70] 2020 |
| Pt4Au6@Ag Hydrothermal | 1 M Gly + 0.1 M KOH (If) 0.5 M Gly + 0.5 M KOH (Elect.) | If = 2.8 mA cm−2 (50 mV s−1) Ef = 1.0 V vs. RHE | E = 1.1 V vs. RHE | DHA (77%) | [72] 2019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, V.S.; Almeida, T.S.; De Andrade, A.R. Glycerol Electro-Oxidation in Alkaline Medium with Pt-Fe/C Electrocatalysts Synthesized by the Polyol Method: Increased Selectivity and Activity Provided by Less Expensive Catalysts. Nanomaterials 2023, 13, 1173. https://doi.org/10.3390/nano13071173
Lima VS, Almeida TS, De Andrade AR. Glycerol Electro-Oxidation in Alkaline Medium with Pt-Fe/C Electrocatalysts Synthesized by the Polyol Method: Increased Selectivity and Activity Provided by Less Expensive Catalysts. Nanomaterials. 2023; 13(7):1173. https://doi.org/10.3390/nano13071173
Chicago/Turabian StyleLima, Vanderlei S., Thiago S. Almeida, and Adalgisa R. De Andrade. 2023. "Glycerol Electro-Oxidation in Alkaline Medium with Pt-Fe/C Electrocatalysts Synthesized by the Polyol Method: Increased Selectivity and Activity Provided by Less Expensive Catalysts" Nanomaterials 13, no. 7: 1173. https://doi.org/10.3390/nano13071173
APA StyleLima, V. S., Almeida, T. S., & De Andrade, A. R. (2023). Glycerol Electro-Oxidation in Alkaline Medium with Pt-Fe/C Electrocatalysts Synthesized by the Polyol Method: Increased Selectivity and Activity Provided by Less Expensive Catalysts. Nanomaterials, 13(7), 1173. https://doi.org/10.3390/nano13071173






