Variations in Biodistribution and Acute Response of Differently Shaped Titania Nanoparticles in Healthy Rodents
Abstract
1. Introduction
2. Materials and Methods
2.1. NP Synthesis and Characterization
2.2. Animals and Treatments
2.3. ICP-MS Sample Preparation and %ID Calculation
2.4. Blood Analysis and Histopathology
2.5. Statistics
3. Results
3.1. Synthesis and Characterization
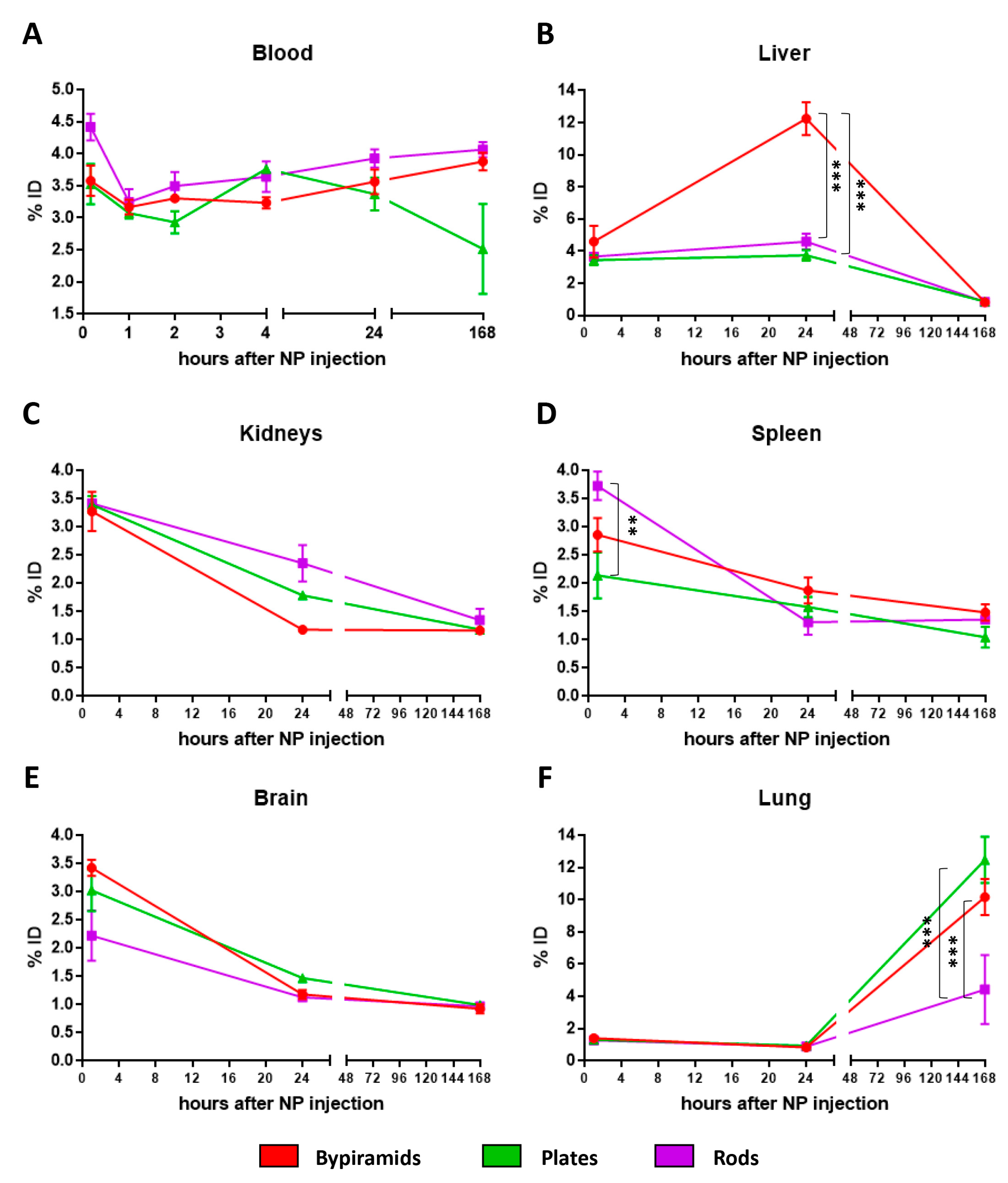
3.2. Influence of the “Shape” on TiO2 NP Biodistribution
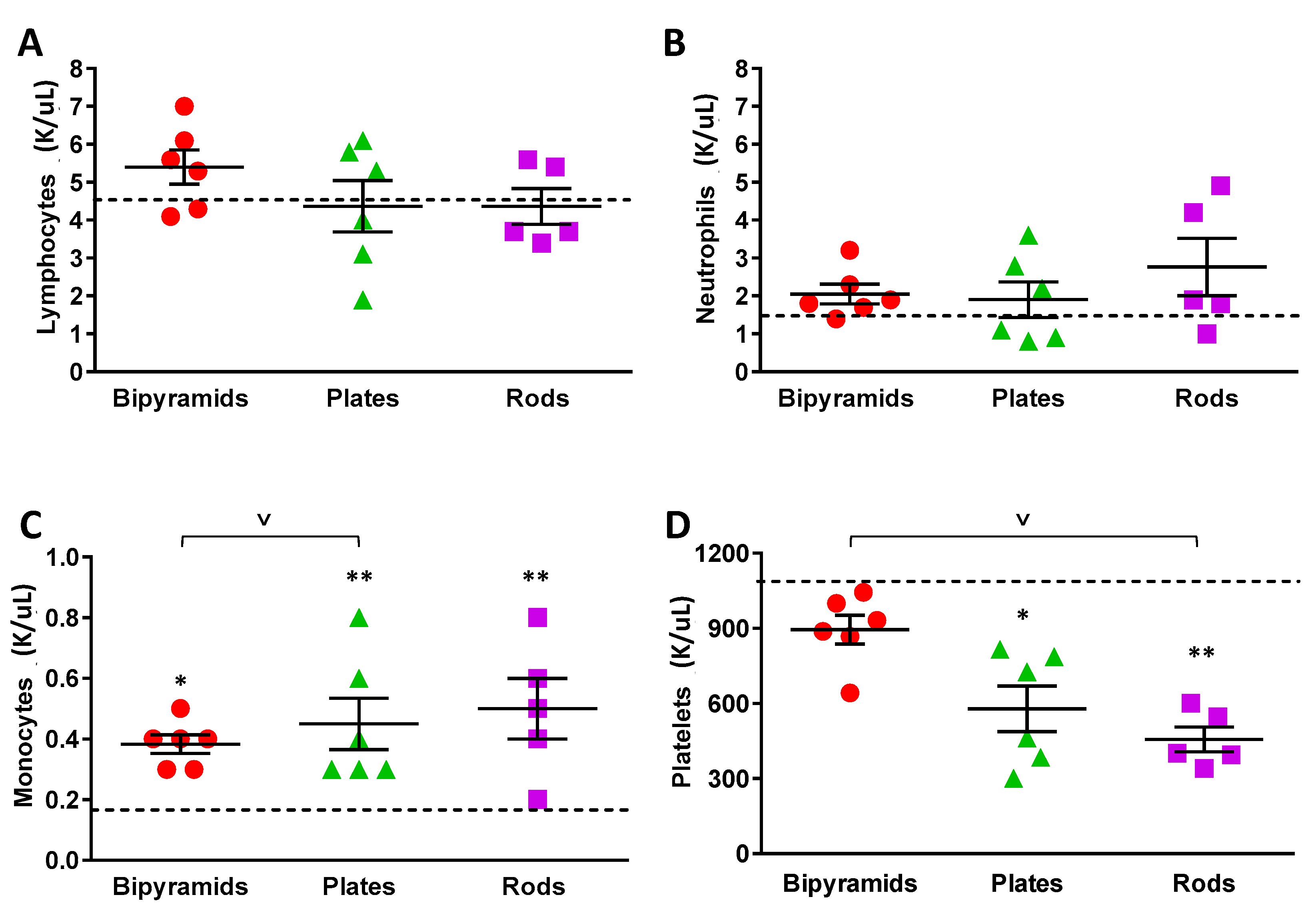
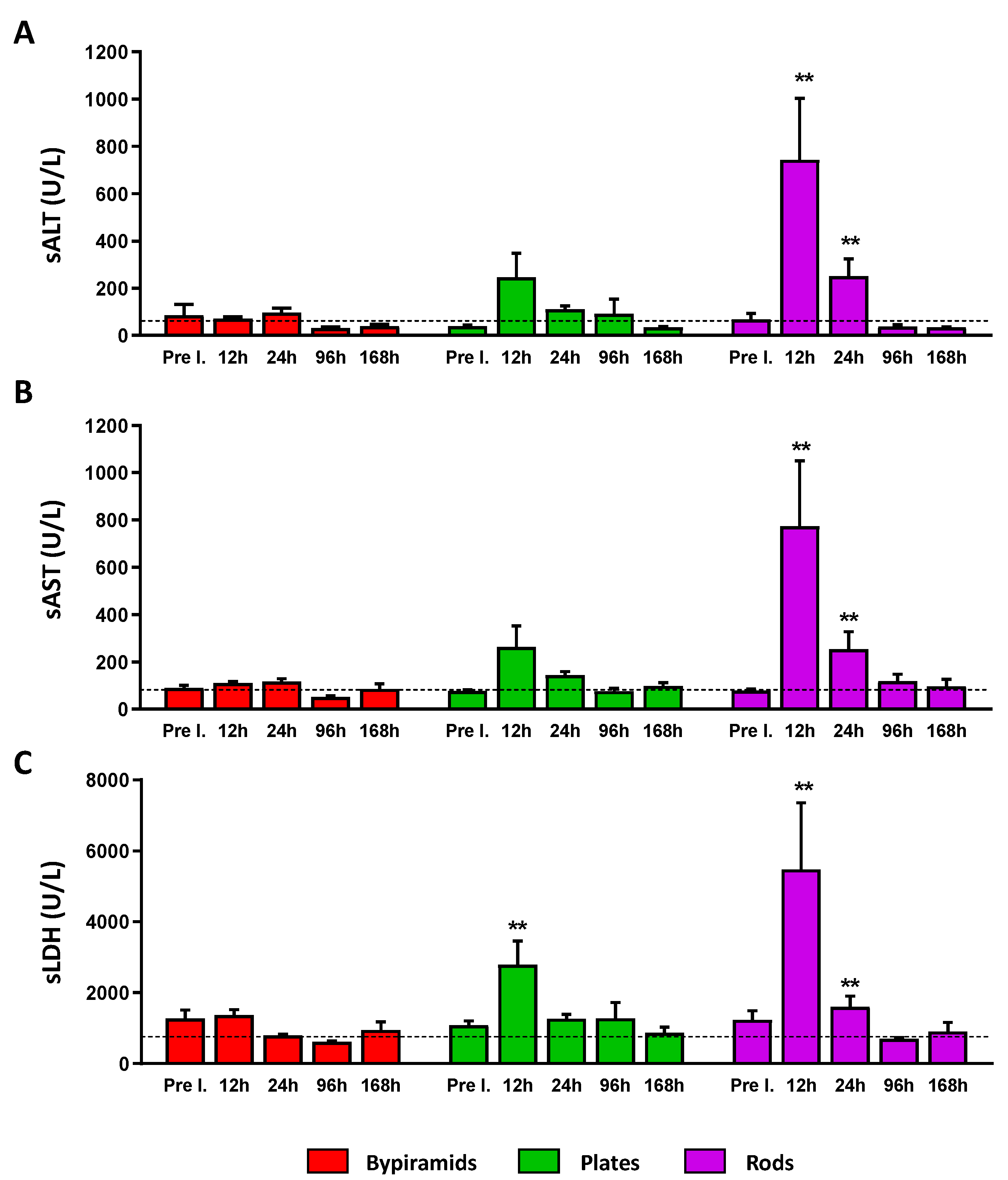
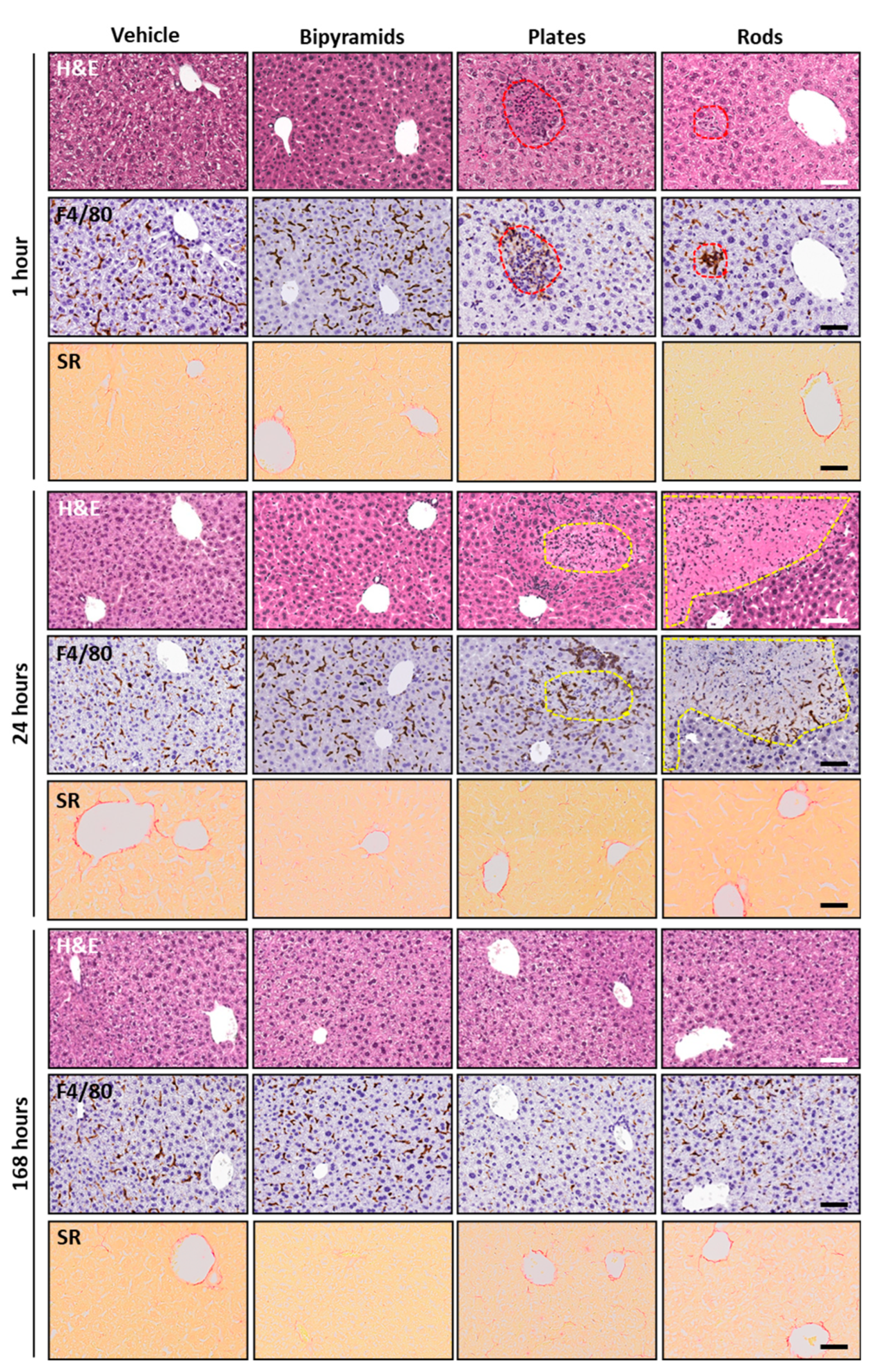
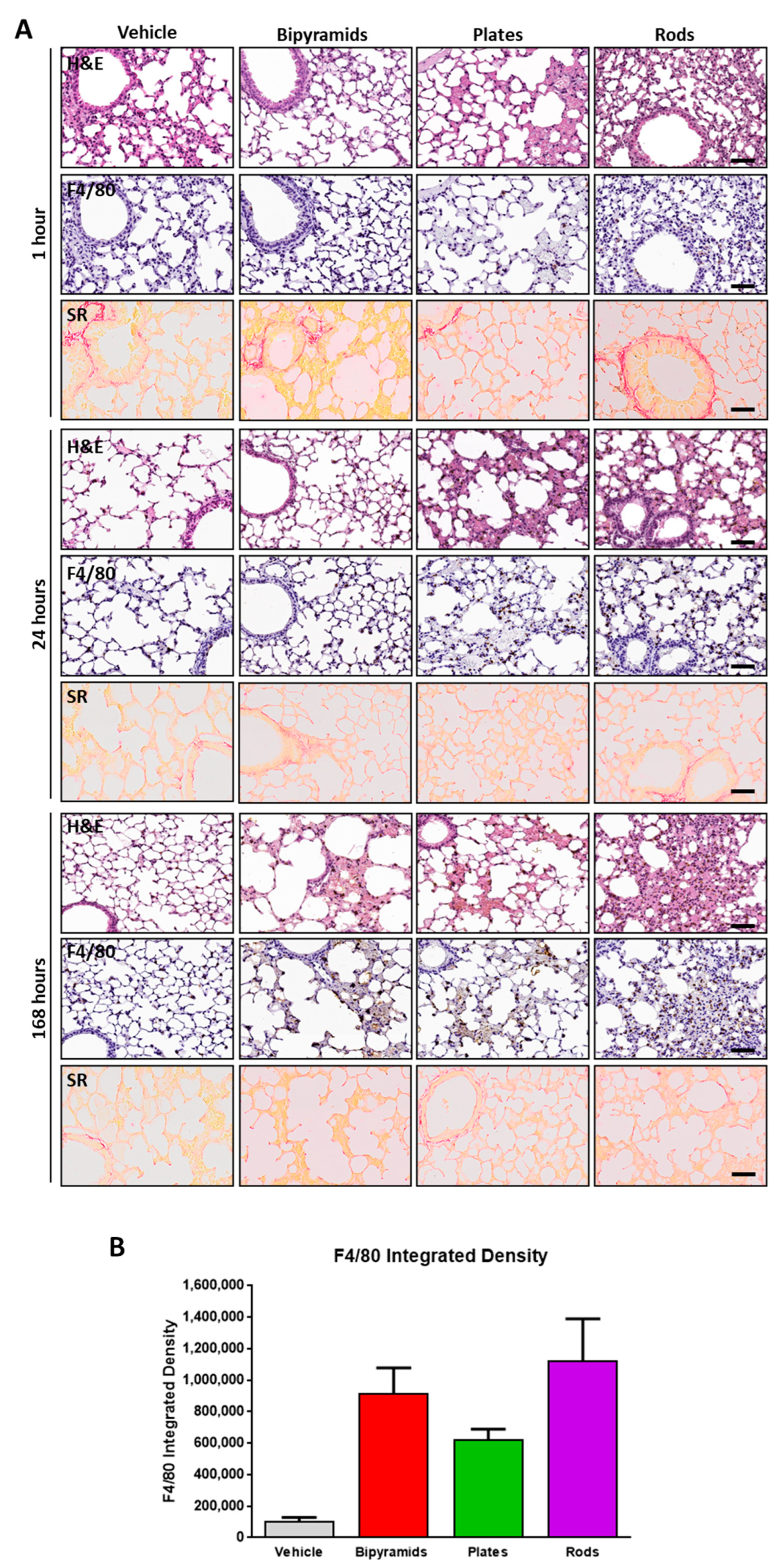
3.3. Influence of the “Shape” on TiO2 NP Biological Effect
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef] [PubMed]
- Rivera Gil, P.; Oberdörster, G.; Elder, A.; Puntes, V.; Parak, W.J. Correlating Physico-Chemical with Toxicological Properties of Nanoparticles: The Present and the Future. ACS Nano 2010, 4, 5527–5531. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Li, N.; Nel, A.E. Potential Health Impact of Nanoparticles. Annu. Rev. Public Health 2009, 30, 137–150. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Is Nano Safe in Foods? Establishing the Factors Impacting the Gastrointestinal Fate and Toxicity of Organic and Inorganic Food-Grade Nanoparticles. Npj Sci. Food 2017, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Nho, R. Pathological Effects of Nano-Sized Particles on the Respiratory System. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102242. [Google Scholar] [CrossRef] [PubMed]
- Ajdary, M.; Moosavi, M.; Rahmati, M.; Falahati, M.; Mahboubi, M.; Mandegary, A.; Jangjoo, S.; Mohammadinejad, R.; Varma, R. Health Concerns of Various Nanoparticles: A Review of Their in Vitro and in Vivo Toxicity. Nanomaterials 2018, 8, 634. [Google Scholar] [CrossRef]
- Horvath, P.; Aulner, N.; Bickle, M.; Davies, A.M.; Nery, E.D.; Ebner, D.; Montoya, M.C.; Östling, P.; Pietiäinen, V.; Price, L.S.; et al. Screening out Irrelevant Cell-Based Models of Disease. Nat. Rev. Drug Discov. 2016, 15, 751–769. [Google Scholar] [CrossRef]
- Talamini, L.; Violatto, M.B.; Cai, Q.; Monopoli, M.P.; Kantner, K.; Krpetić, Ž.; Perez-Potti, A.; Cookman, J.; Garry, D.; Silveira, C.P.; et al. Influence of Size and Shape on the Anatomical Distribution of Endotoxin-Free Gold Nanoparticles. ACS Nano 2017, 11, 5519–5529. [Google Scholar] [CrossRef]
- Wang, J.; Bai, R.; Yang, R.; Liu, J.; Tang, J.; Liu, Y.; Li, J.; Chai, Z.; Chen, C. Size- and Surface Chemistry-Dependent Pharmacokinetics and Tumor Accumulation of Engineered Gold Nanoparticles after Intravenous Administration. Metallomics 2015, 7, 516–524. [Google Scholar] [CrossRef]
- Fiordaliso, F.; Foray, C.; Salio, M.; Salmona, M.; Diomede, L. Realistic Evaluation of Titanium Dioxide Nanoparticle Exposure in Chewing Gum. J. Agric. Food Chem. 2018, 66, 6860–6868. [Google Scholar] [CrossRef]
- Tucci, P.; Porta, G.; Agostini, M.; Dinsdale, D.; Iavicoli, I.; Cain, K.; Finazzi-Agró, A.; Melino, G.; Willis, A. Metabolic Effects of TiO2 Nanoparticles, a Common Component of Sunscreens and Cosmetics, on Human Keratinocytes. Cell Death Dis. 2013, 4, e549. [Google Scholar] [CrossRef] [PubMed]
- Rompelberg, C.; Heringa, M.B.; van Donkersgoed, G.; Drijvers, J.; Roos, A.; Westenbrink, S.; Peters, R.; van Bemmel, G.; Brand, W.; Oomen, A.G. Oral Intake of Added Titanium Dioxide and Its Nanofraction from Food Products, Food Supplements and Toothpaste by the Dutch Population. Nanotoxicology 2016, 10, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Iannarelli, L.; Giovannozzi, A.M.; Morelli, F.; Viscotti, F.; Bigini, P.; Maurino, V.; Spoto, G.; Martra, G.; Ortel, E.; Hodoroaba, V.-D.; et al. Shape Engineered TiO2 Nanoparticles in Caenorhabditis Elegans: A Raman Imaging Based Approach to Assist Tissue-Specific Toxicological Studies. RSC Adv. 2016, 6, 70501–70509. [Google Scholar] [CrossRef]
- De Matteis, V. Exposure to Inorganic Nanoparticles: Routes of Entry, Immune Response, Biodistribution and In Vitro/In Vivo Toxicity Evaluation. Toxics 2017, 5, 29. [Google Scholar] [CrossRef]
- Gordon, T.R.; Cargnello, M.; Paik, T.; Mangolini, F.; Weber, R.T.; Fornasiero, P.; Murray, C.B. Nonaqueous Synthesis of TiO 2 Nanocrystals Using TiF 4 to Engineer Morphology, Oxygen Vacancy Concentration, and Photocatalytic Activity. J. Am. Chem. Soc. 2012, 134, 6751–6761. [Google Scholar] [CrossRef]
- Hühn, J.; Carrillo-Carrion, C.; Soliman, M.G.; Pfeiffer, C.; Valdeperez, D.; Masood, A.; Chakraborty, I.; Zhu, L.; Gallego, M.; Yue, Z.; et al. Selected Standard Protocols for the Synthesis, Phase Transfer, and Characterization of Inorganic Colloidal Nanoparticles. Chem. Mater. 2017, 29, 399–461. [Google Scholar] [CrossRef]
- Xu, M.; Soliman, M.G.; Sun, X.; Pelaz, B.; Feliu, N.; Parak, W.J.; Liu, S. How Entanglement of Different Physicochemical Properties Complicates the Prediction of in Vitro and in Vivo Interactions of Gold Nanoparticles. ACS Nano 2018, 12, 10104–10113. [Google Scholar] [CrossRef]
- Lin, C.-A.J.; Sperling, R.A.; Li, J.K.; Yang, T.-Y.; Li, P.-Y.; Zanella, M.; Chang, W.H.; Parak, W.J. Design of an Amphiphilic Polymer for Nanoparticle Coating and Functionalization. Small 2008, 4, 334–341. [Google Scholar] [CrossRef]
- Fernández-Argüelles, M.T.; Yakovlev, A.; Sperling, R.A.; Luccardini, C.; Gaillard, S.; Sanz Medel, A.; Mallet, J.-M.; Brochon, J.-C.; Feltz, A.; Oheim, M.; et al. Synthesis and Characterization of Polymer-Coated Quantum Dots with Integrated Acceptor Dyes as FRET-Based Nanoprobes. Nano Lett. 2007, 7, 2613–2617. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Abdelmonem, A.M.; Ali, Z.; Alves, F.; Geiser, M.; Haberl, N.; Hartmann, R.; Hirn, S.; de Aberasturi, D.J.; Kantner, K.; et al. In Vivo Integrity of Polymer-Coated Gold Nanoparticles. Nat. Nanotechnol. 2015, 10, 619–623. [Google Scholar] [CrossRef]
- Sitia, G.; Fiordaliso, F.; Violatto, M.B.; Alarcon, J.F.; Talamini, L.; Corbelli, A.; Ferreira, L.M.; Tran, N.L.; Chakraborty, I.; Salmona, M.; et al. Food-Grade Titanium Dioxide Induces Toxicity in the Nematode Caenorhabditis Elegans and Acute Hepatic and Pulmonary Responses in Mice. Nanomaterials 2022, 12, 1669. [Google Scholar] [CrossRef]
- Sitia, G.; Iannacone, M.; Aiolfi, R.; Isogawa, M.; van Rooijen, N.; Scozzesi, C.; Bianchi, M.E.; von Andrian, U.H.; Chisari, F.V.; Guidotti, L.G. Kupffer Cells Hasten Resolution of Liver Immunopathology in Mouse Models of Viral Hepatitis. PLoS Pathog. 2011, 7, e1002061. [Google Scholar] [CrossRef] [PubMed]
- Brain, J.D.; Molina, R.M.; DeCamp, M.M.; Warner, A.E. Pulmonary Intravascular Macrophages: Their Contribution to the Mononuclear Phagocyte System in 13 Species. Am. J. Physiol. Lung Cell. Mol. Physiol. 1999, 276, L146–L154. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The Effect of Nanoparticle Size on in Vivo Pharmacokinetics and Cellular Interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Black, K.C.L.; Wang, Y.; Luehmann, H.P.; Cai, X.; Xing, W.; Pang, B.; Zhao, Y.; Cutler, C.S.; Wang, L.V.; Liu, Y.; et al. Radioactive 198 Au-Doped Nanostructures with Different Shapes for In Vivo Analyses of Their Biodistribution, Tumor Uptake, and Intratumoral Distribution. ACS Nano 2014, 8, 4385–4394. [Google Scholar] [CrossRef] [PubMed]
- Kaga, S.; Truong, N.P.; Esser, L.; Senyschyn, D.; Sanyal, A.; Sanyal, R.; Quinn, J.F.; Davis, T.P.; Kaminskas, L.M.; Whittaker, M.R. Influence of Size and Shape on the Biodistribution of Nanoparticles Prepared by Polymerization-Induced Self-Assembly. Biomacromolecules 2017, 18, 3963–3970. [Google Scholar] [CrossRef]
- Stoehr, L.C.; Gonzalez, E.; Stampfl, A.; Casals, E.; Duschl, A.; Puntes, V.; Oostingh, G.J. Shape Matters: Effects of Silver Nanospheres and Wires on Human Alveolar Epithelial Cells. Part. Fibre Toxicol. 2011, 8, 36. [Google Scholar] [CrossRef]
- Shakeel, M.; Jabeen, F.; Shabbir, S.; Asghar, M.S.; Khan, M.S.; Chaudhry, A.S. Toxicity of Nano-Titanium Dioxide (TiO2-NP) Through Various Routes of Exposure: A Review. Biol. Trace Elem. Res. 2016, 172, 5. [Google Scholar] [CrossRef]
- Iavicoli, I.; Leso, V.; Bergamaschi, A. Toxicological Effects of Titanium Dioxide Nanoparticles: A Review of In Vivo Studies. J. Nanomater. 2012, 2012, 964381. [Google Scholar] [CrossRef]
- Tsoi, K.M.; MacParland, S.A.; Ma, X.-Z.; Spetzler, V.N.; Echeverri, J.; Ouyang, B.; Fadel, S.M.; Sykes, E.A.; Goldaracena, N.; Kaths, J.M.; et al. Mechanism of Hard-Nanomaterial Clearance by the Liver. Nat. Mater 2016, 15, 1212–1221. [Google Scholar] [CrossRef]
- Bertrand, N.; Leroux, J.-C. The Journey of a Drug-Carrier in the Body: An Anatomo-Physiological Perspective. J. Control. Release 2012, 161, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Violatto, M.B.; Santangelo, C.; Capelli, C.; Frapolli, R.; Ferrari, R.; Sitia, L.; Tortarolo, M.; Talamini, L.; Previdi, S.; Moscatelli, D.; et al. Longitudinal Tracking of Triple Labeled Umbilical Cord Derived Mesenchymal Stromal Cells in a Mouse Model of Amyotrophic Lateral Sclerosis. Stem Cell Res. 2015, 15, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Nold, P.; Hartmann, R.; Feliu, N.; Kantner, K.; Gamal, M.; Pelaz, B.; Hühn, J.; Sun, X.; Jungebluth, P.; del Pino, P.; et al. Optimizing Conditions for Labeling of Mesenchymal Stromal Cells (MSCs) with Gold Nanoparticles: A Prerequisite for in Vivo Tracking of MSCs. J. Nanobiotechnol. 2017, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. Action of Nanoparticles on Platelet Activation and Plasmatic Coagulation. CMC 2016, 23, 408–430. [Google Scholar] [CrossRef]
- Renga, B.; Scavizzi, F. Platelets and Cardiovascular Risk. Acta Cardiol. 2017, 72, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Parakhonskiy, B.; Zyuzin, M.V.; Yashchenok, A.; Carregal-Romero, S.; Rejman, J.; Möhwald, H.; Parak, W.J.; Skirtach, A.G. The Influence of the Size and Aspect Ratio of Anisotropic, Porous CaCO3 Particles on Their Uptake by Cells. J. Nanobiotechnol. 2015, 13, 53. [Google Scholar] [CrossRef]
- Feliu, N.; Hühn, J.; Zyuzin, M.V.; Ashraf, S.; Valdeperez, D.; Masood, A.; Said, A.H.; Escudero, A.; Pelaz, B.; Gonzalez, E.; et al. Quantitative Uptake of Colloidal Particles by Cell Cultures. Sci. Total Environ. 2016, 568, 819–828. [Google Scholar] [CrossRef]
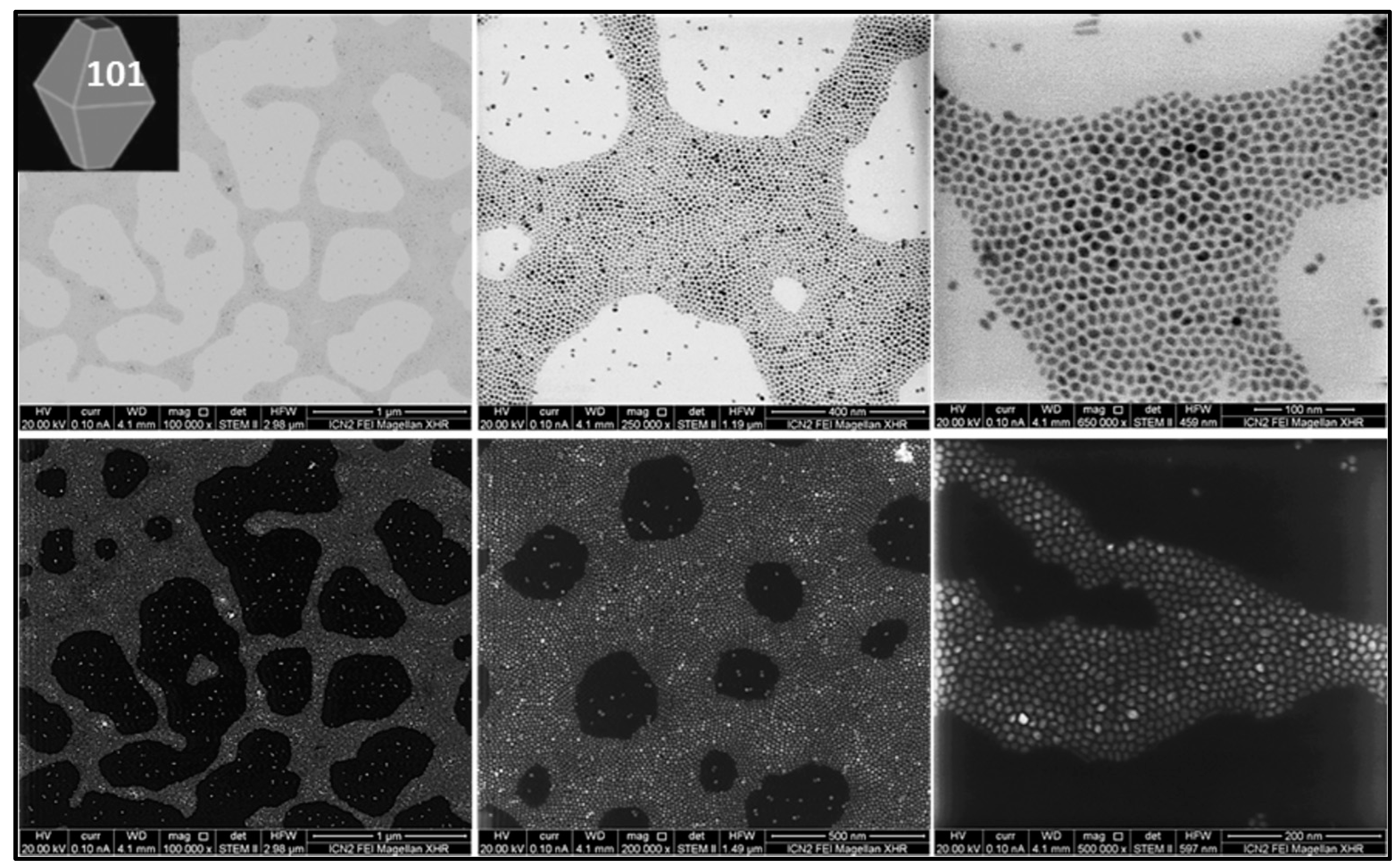
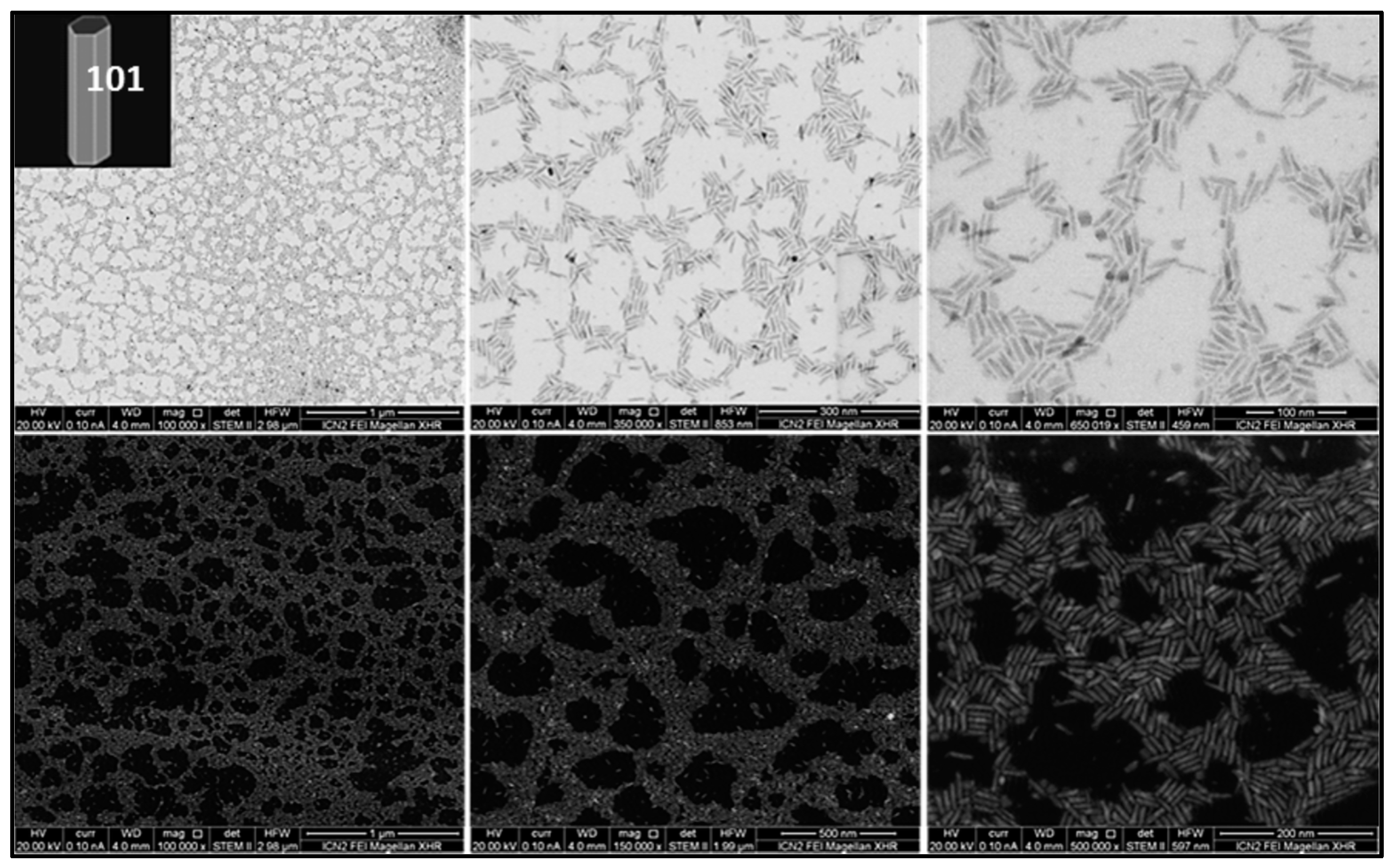
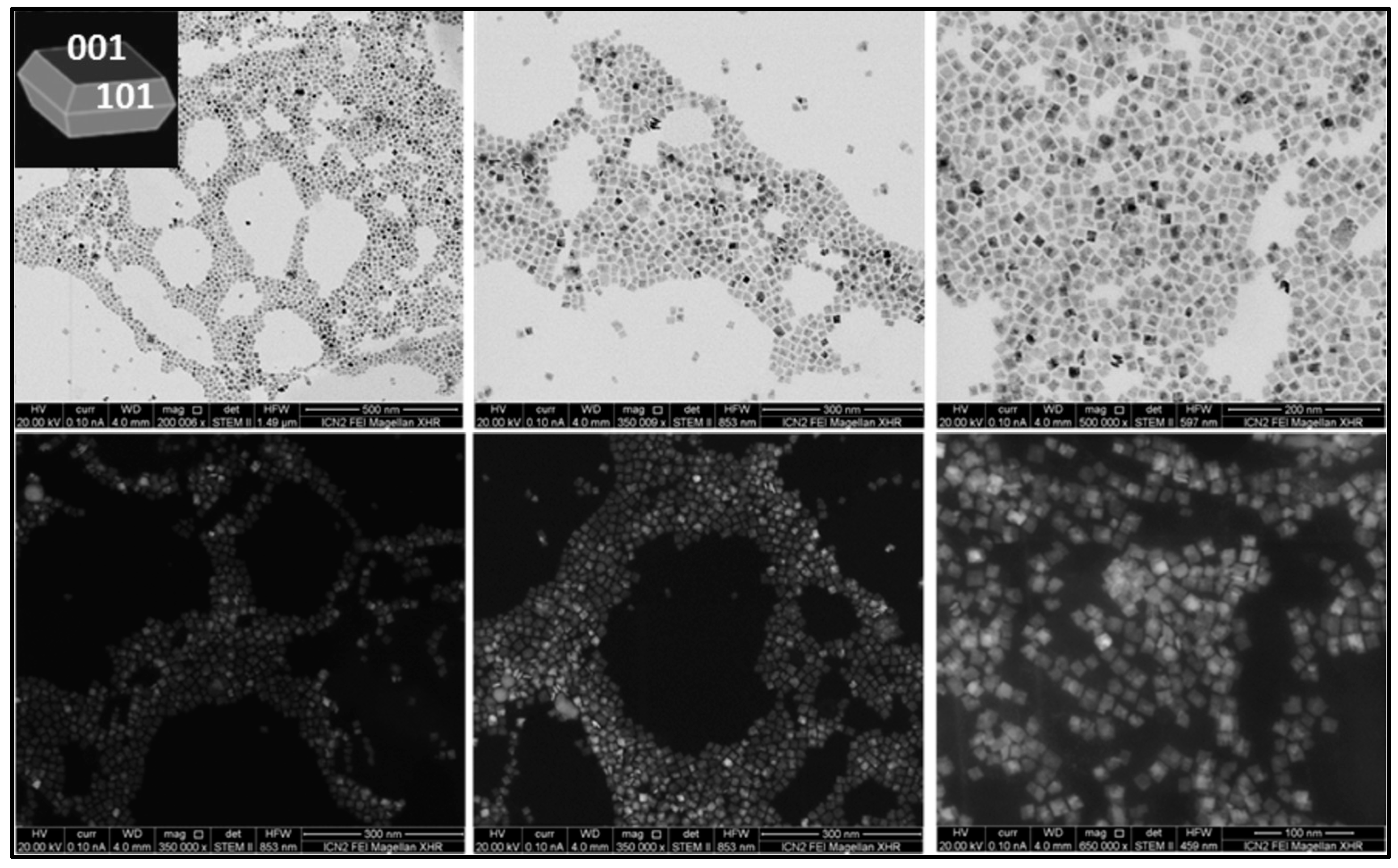
| Sample | Size Distribution |
|---|---|
| TiO2 bipyramids | 7.5 ± 0.7 nm side |
| TiO2 rods | 27.8 ± 2.1 nm length 6.3 ± 1.0 nm width |
| TiO2 plates | 13.6 ± 2.1 nm side 4.5 ± 0.3 nm thickness |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Violatto, M.B.; Sitia, G.; Talamini, L.; Morelli, A.; Tran, N.L.; Zhang, Q.; Masood, A.; Pelaz, B.; Chakraborty, I.; Cui, D.; et al. Variations in Biodistribution and Acute Response of Differently Shaped Titania Nanoparticles in Healthy Rodents. Nanomaterials 2023, 13, 1174. https://doi.org/10.3390/nano13071174
Violatto MB, Sitia G, Talamini L, Morelli A, Tran NL, Zhang Q, Masood A, Pelaz B, Chakraborty I, Cui D, et al. Variations in Biodistribution and Acute Response of Differently Shaped Titania Nanoparticles in Healthy Rodents. Nanomaterials. 2023; 13(7):1174. https://doi.org/10.3390/nano13071174
Chicago/Turabian StyleViolatto, Martina B., Giovanni Sitia, Laura Talamini, Annalisa Morelli, Ngoc Lan Tran, Qian Zhang, Atif Masood, Beatriz Pelaz, Indranath Chakraborty, Daxiang Cui, and et al. 2023. "Variations in Biodistribution and Acute Response of Differently Shaped Titania Nanoparticles in Healthy Rodents" Nanomaterials 13, no. 7: 1174. https://doi.org/10.3390/nano13071174
APA StyleViolatto, M. B., Sitia, G., Talamini, L., Morelli, A., Tran, N. L., Zhang, Q., Masood, A., Pelaz, B., Chakraborty, I., Cui, D., Parak, W. J., Salmona, M., Bastús, N. G., Puntes, V., & Bigini, P. (2023). Variations in Biodistribution and Acute Response of Differently Shaped Titania Nanoparticles in Healthy Rodents. Nanomaterials, 13(7), 1174. https://doi.org/10.3390/nano13071174







