Electrospun Multilayered Films Based on Poly(3-hydroxybutyrate-co-3-hydroxyvalerate), Copolyamide 1010/1014, and Electrosprayed Nanostructured Silica
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Bio-Based Copolyamide
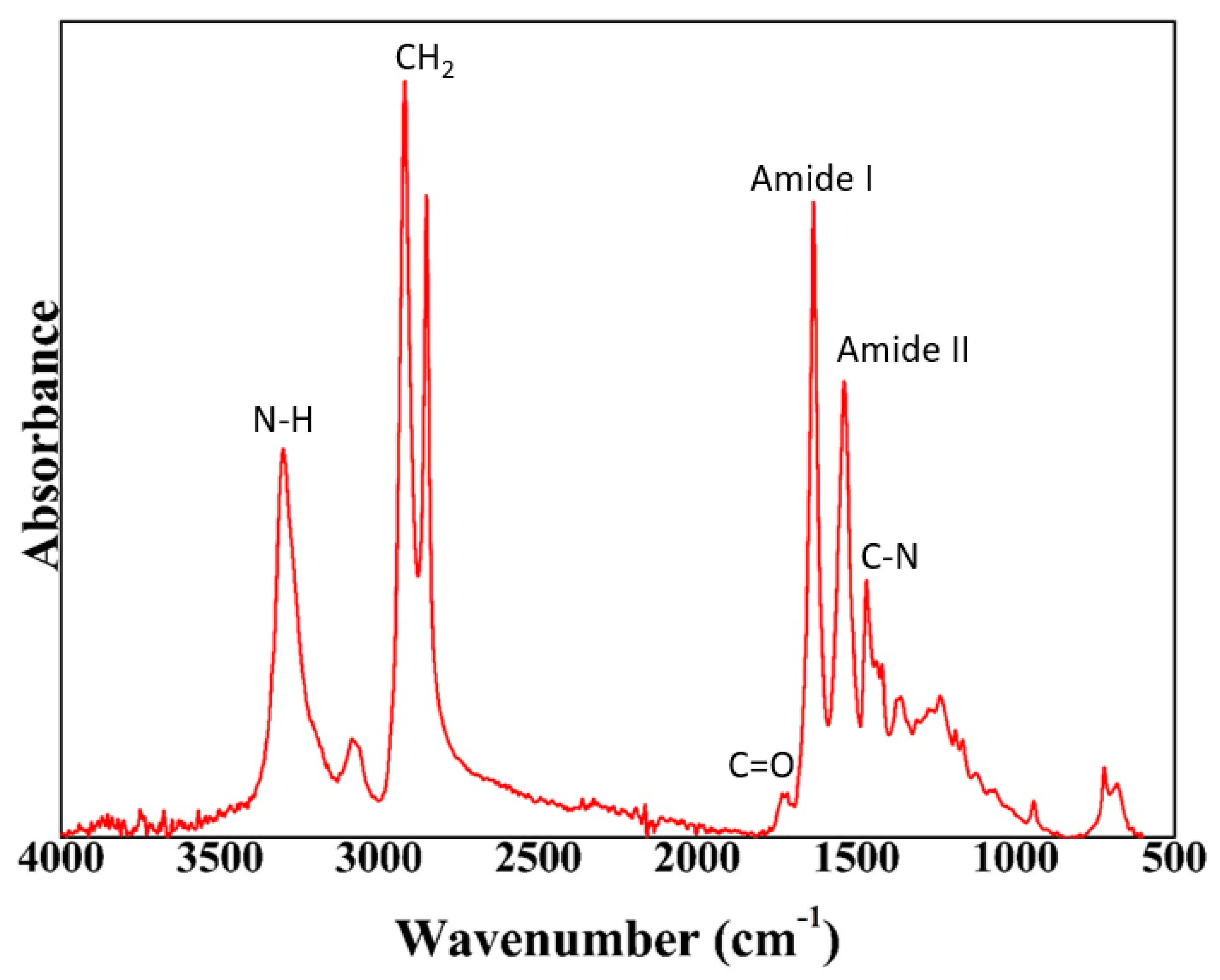
2.3. Polyamide Characterization
2.3.1. ATR–FTIR
2.3.2. DSC
2.3.3. Viscosity Number and Molecular Weight
2.4. Fabrication of the Nanostructured Coatings
2.4.1. Preparation of the Polymer Solutions
2.4.2. Solution Characterization
2.4.3. Electrospinning and Electrospraying Parameters
2.4.4. Thermal Post-Treatment
2.5. Coating Characterization
2.5.1. Thickness
2.5.2. Morphology
2.5.3. Optical Properties
2.5.4. Water Contact Angle
2.5.5. Sliding Angle
2.5.6. Water Vapor Permeance
2.5.7. Oxygen Permeance
2.5.8. Water Uptake
2.5.9. Mechanical Tests
2.6. Statistical Analysis
3. Results and Discussion
3.1. Polyamide Characterization
3.2. Solution Characterization
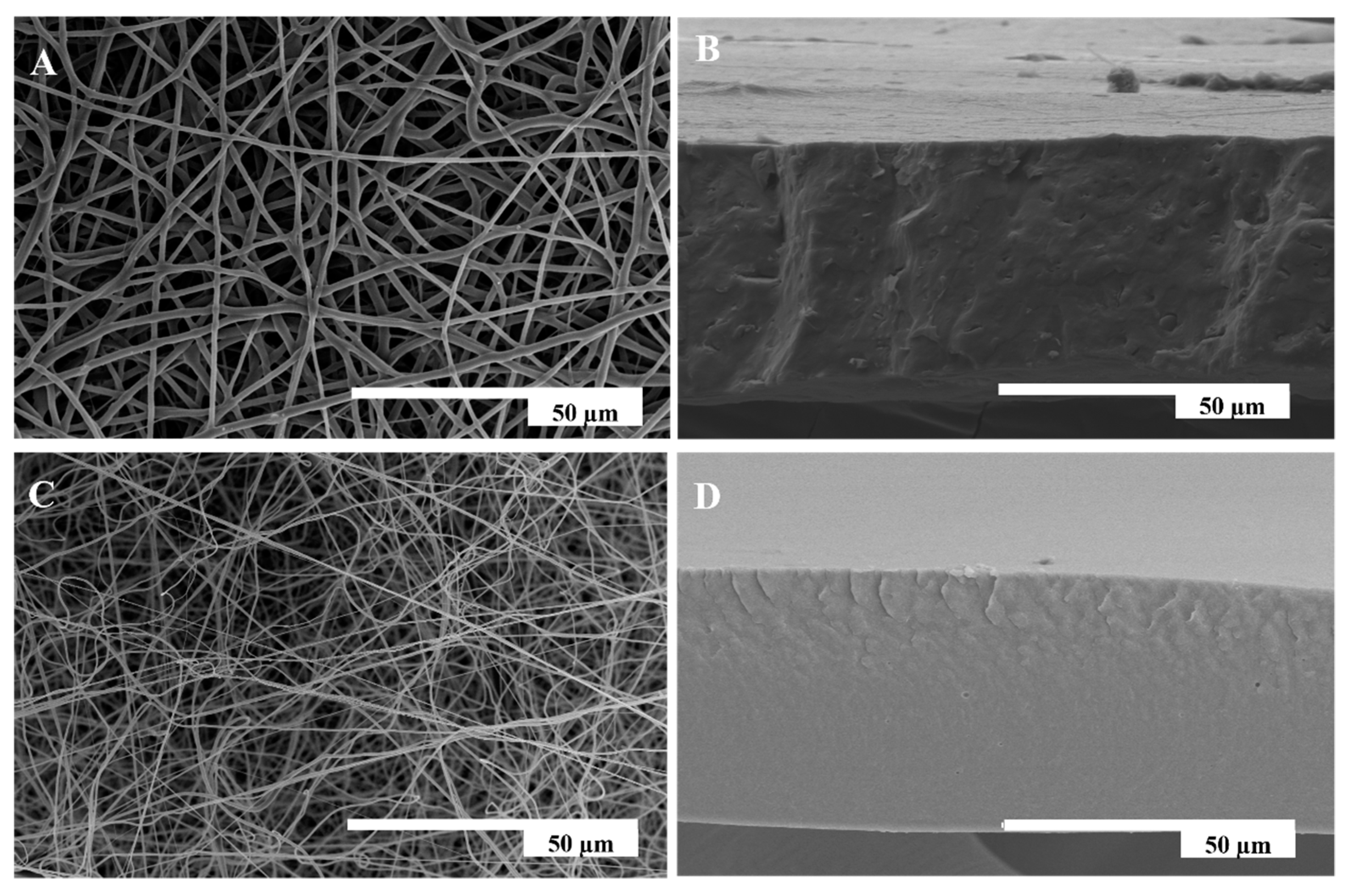
3.3. Morphology of Electrospun Mono- and Multilayer Structures
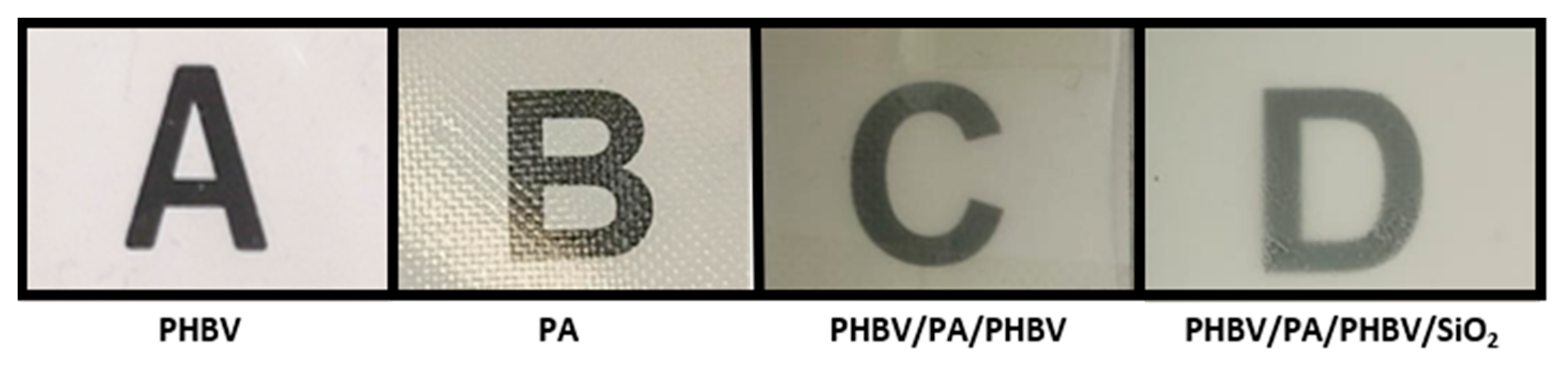
3.4. Optical Properties
3.5. Water Contact Angle and Sliding Angle of Mono- and Multilayer Structures
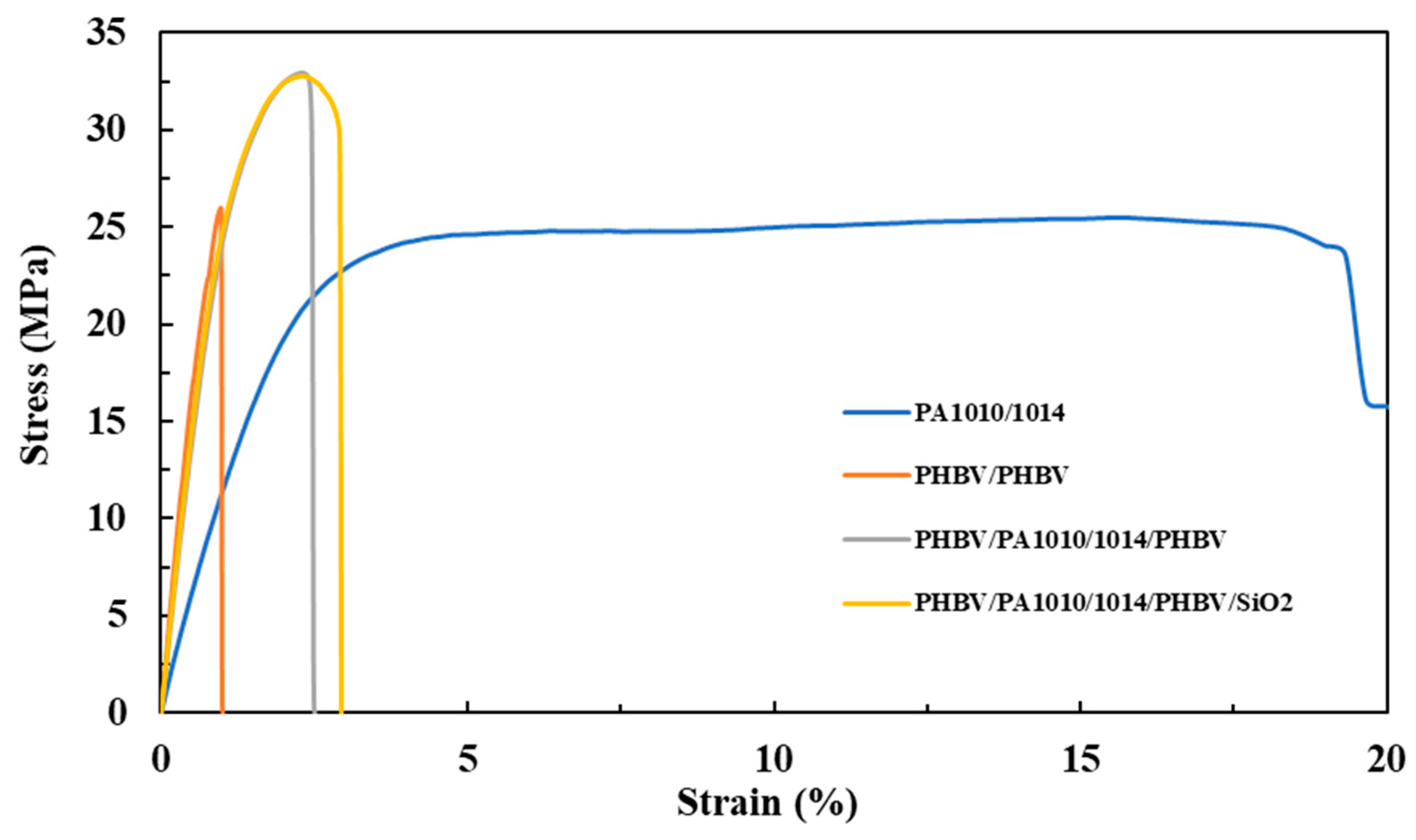
3.6. Mechanical Properties
3.7. Barrier Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tibbetts, J.H. Managing Marine Plastic Pollution: Policy Initiatives to Address Wayward Waste. Environ. Health Perspect. 2015, 123, A90–A93. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Park, H.-M.; Ha, C.-S. Bio-Nanocomposites for Food Packaging Applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Debeaufort, F.; Quezada-Gallo, J.-A.; Voilley, A. Edible Films and Coatings: Tomorrow’s Packagings: A Review. Crit. Rev. Food Sci. Nutr. 1998, 38, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, W.; Zhu, W.; McClements, D.J.; Liu, X.; Liu, F. A Review of Multilayer and Composite Films and Coatings for Active Biodegradable Packaging. NPJ Sci. Food 2022, 6, 18. [Google Scholar] [CrossRef]
- Jain, R.; Shetty, S.; Yadav, K.S. Unfolding the electrospinning potential of biopolymers for preparation of nanofibers. J. Drug Deliv. Sci. Technol. 2020, 57, 101604. [Google Scholar] [CrossRef]
- Sun, F.; Jiang, H.; Wang, H.; Zhong, Y.; Xu, Y.; Xing, Y.; Yu, M.; Feng, L.-W.; Tang, Z.; Liu, J. Soft Fiber Electronics Based on Semiconducting Polymer. Chem. Rev. 2023. [Google Scholar] [CrossRef]
- Liang, F.-C.; Kuo, C.-C.; Chen, B.-Y.; Cho, C.-J.; Hung, C.-C.; Chen, W.-C.; Borsali, R. RGB-Switchable Porous Electrospun Nanofiber Chemoprobe-Filter Prepared from Multifunctional Copolymers for Versatile Sensing of PH and Heavy Metals. ACS Appl. Mater. Interfaces 2017, 9, 16381–16396. [Google Scholar] [CrossRef]
- Lasprilla-Botero, J.; Torres-Giner, S.; Pardo-Figuerez, M.; Álvarez-Láinez, M.; Lagaron, J.M. Superhydrophobic Bilayer Coating Based on Annealed Electrospun Ultrathin Poly (ε-Caprolactone) Fibers and Electrosprayed Nanostructured Silica Microparticles for Easy Emptying Packaging Applications. Coatings 2018, 8, 173. [Google Scholar] [CrossRef]
- Cherpinski, A.; Szewczyk, P.K.; Gruszczyński, A.; Stachewicz, U.; Lagaron, J.M. Oxygen-Scavenging Multilayered Biopapers Containing Palladium Nanoparticles Obtained by the Electrospinning Coating Technique. Nanomaterials 2019, 9, 262. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Torres-Giner, S.; Angulo, I.; Pardo-Figuerez, M.; Hilliou, L.; Escuin, J.M.; Cabedo, L.; Nevo, Y.; Prieto, C.; Lagaron, J.M. High-Oxygen-Barrier Multilayer Films Based on Polyhydroxyalkanoates and Cellulose Nanocrystals. Nanomaterials 2021, 11, 1443. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Lopez, K.J.; Torres-Giner, S.; Angulo, I.; Pardo-Figuerez, M.; Escuin, J.M.; Bourbon, A.I.; Cabedo, L.; Nevo, Y.; Cerqueira, M.A.; Lagaron, J.M. Development of Active Barrier Multilayer Films Based on Electrospun Antimicrobial Hot-Tack Food Waste Derived Poly (3-Hydroxybutyrate-Co-3-Hydroxyvalerate) and Cellulose Nanocrystal Interlayers. Nanomaterials 2020, 10, 2356. [Google Scholar] [CrossRef]
- Lafraya, A.; Prieto, C.; Pardo-Figuerez, M.; Chiva, A.; Lagaron, J.M. Super-Repellent Paper Coated with Electrospun Biopolymers and Electrosprayed Silica of Interest in Food Packaging Applications. Nanomaterials 2021, 11, 3354. [Google Scholar] [CrossRef]
- Júnior, L.M.; de Oliveira, L.M.; Bócoli, P.F.J.; Cristianini, M.; Padula, M.; Anjos, C.A.R. Morphological, Thermal and Mechanical Properties of Polyamide and Ethylene Vinyl Alcohol Multilayer Flexible Packaging after High-Pressure Processing. J. Food Eng. 2020, 276, 109913. [Google Scholar] [CrossRef]
- Baniasadi, H.; Trifol, J.; Lipponen, S.; Seppälä, J. Sustainable Composites of Surface-Modified Cellulose with Low–Melting Point Polyamide. Mater. Today Chem. 2021, 22, 100590. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Spoljaric, S.; Seppälä, J. Redefining Polyamide Property Profiles via Renewable Long-Chain Aliphatic Segments: Towards Impact Resistance and Low Water Absorption. Eur. Polym. J. 2018, 109, 16–25. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Vicente, A.A.; Reis, M.A.M.; Torres-Giner, S.; Lagaron, J.M. Antimicrobial and Antioxidant Performance of Various Essential Oils and Natural Extracts and Their Incorporation into Biowaste Derived Poly (3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Layers Made from Electrospun Ultrathin Fibers. Nanomaterials 2019, 9, 144. [Google Scholar] [CrossRef]
- Yan, M.; Yang, H. Improvement of Polyamide 1010 with Silica Nanospheres via in Situ Melt Polycondensation. Polym. Compos. 2012, 33, 1770–1776. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.; Yan, D. Synthesis and Crystallization Behavior of Nylon 12, 14. I. Preparation and Melting Behavior. J. Appl. Polym. Sci. 2003, 88, 1581–1589. [Google Scholar] [CrossRef]
- Wudy, K.; Drummer, D. Aging Effects of Polyamide 12 in Selective Laser Sintering: Molecular Weight Distribution and Thermal Properties. Addit. Manuf. 2019, 25, 1–9. [Google Scholar] [CrossRef]
- Nirmala, R.; Panth, H.R.; Yi, C.; Nam, K.T.; Park, S.-J.; Kim, H.Y.; Navamathavan, R. Effect of Solvents on High Aspect Ratio Polyamide-6 Nanofibers via Electrospinning. Macromol. Res. 2010, 18, 759–765. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Liu, C. Film Transparency and Opacity Measurements. Food Anal. Methods 2022, 15, 2840–2846. [Google Scholar] [CrossRef]
- Han, J.H.; Floros, J.D. Casting Antimicrobial Packaging Films and Measuring Their Physical Properties and Antimicrobial Activity. J. Plast. Film Sheeting 1997, 13, 287–298. [Google Scholar] [CrossRef]
- Lee, W.-K.; Jung, W.-B.; Nagel, S.R.; Odom, T.W. Stretchable Superhydrophobicity from Monolithic, Three-Dimensional Hierarchical Wrinkles. Nano Lett. 2016, 16, 3774–3779. [Google Scholar] [CrossRef]
- Ma, M.; Hill, R.M. Superhydrophobic Surfaces. Curr. Opin. Colloid Interface Sci. 2006, 11, 193–202. [Google Scholar] [CrossRef]
- ASTM E 96/E96M; Standard Test Methods for Water Vapor Transmission of Materials. ASTM: West Conshohocken, PA, USA, 2015.
- ASTM D570; On Standard Test Method for Water Absorption of Plastics. ASTM International: West Conshohocken, PA, USA, 2005.
- Belfer, S.; Purinson, Y.; Kedem, O. Surface Modification of Commercial Polyamide Reverse Osmosis Membranes by Radical Grafting: An ATR-FTIR Study. Acta Polym. 1998, 49, 574–582. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Boronat, T.; Balart, R.; Torres-Giner, S. Evaluation of the Engineering Performance of Different Bio-Based Aliphatic Homopolyamide Tubes Prepared by Profile Extrusion. Polym. Test. 2017, 61, 421–429. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, X.; Berglund, L.A. An Unusual Crystallization Behavior in Polyamide 6/Montmorillonite Nanocomposites. Macromol. Rapid Commun. 2001, 22, 1438–1440. [Google Scholar] [CrossRef]
- Baniasadi, H.; Lipponen, S.; Asplund, M.; Seppälä, J. High-Concentration Lignin Biocomposites with Low-Melting Point Biopolyamide. Chem. Eng. J. 2023, 451, 138564. [Google Scholar] [CrossRef]
- Wang, Z.; Song, M.; Li, X.; Chen, J.; Liang, T.; Chen, X.; Yan, Y. Copolymerization-Regulated Hydrogen Bonds: A New Routine for High-Strength Copolyamide 6/66 Fibers. Polymer 2022, 14, 3517. [Google Scholar] [CrossRef] [PubMed]
- Feijoo, P.; Samaniego-Aguilar, K.; Sánchez-Safont, E.; Torres-Giner, S.; Lagaron, J.M.; Gamez-Perez, J.; Cabedo, L. Development and Characterization of Fully Renewable and Biodegradable Polyhydroxyalkanoate Blends with Improved Thermoformability. Polymer 2022, 14, 2527. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Castro-Mayorga, J.L.; Reis, M.A.M.; Sammon, C.; Cabedo, L.; Torres-Giner, S.; Lagaron, J.M. Preparation and Characterization of Electrospun Food Biopackaging Films of Poly (3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Derived from Fruit Pulp Biowaste. Front. Sustain. Food Syst. 2018, 2, 38. [Google Scholar] [CrossRef]
- Palangetic, L.; Reddy, N.K.; Srinivasan, S.; Cohen, R.E.; McKinley, G.H.; Clasen, C. Dispersity and Spinnability: Why Highly Polydisperse Polymer Solutions Are Desirable for Electrospinning. Polymer 2014, 55, 4920–4931. [Google Scholar] [CrossRef]
- Amariei, N.; Manea, L.R.; Bertea, A.P.; Bertea, A.; Popa, A. The Influence of Polymer Solution on the Properties of Electrospun 3D Nanostructures. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 209, p. 012092. [Google Scholar]
- Figueroa-Lopez, K.J.; Cabedo, L.; Lagaron, J.M.; Torres-Giner, S. Development of Electrospun Poly (3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Monolayers Containing Eugenol and Their Application in Multilayer Antimicrobial Food Packaging. Front. Nutr. 2020, 7, 140. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Z.; Lǚ, Y. Electrospinning of Nylon-6, 66, 1010 Terpolymer. Eur. Polym. J. 2006, 42, 1696–1704. [Google Scholar] [CrossRef]
- Cherpinski, A.; Torres-Giner, S.; Cabedo, L.; Méndez, J.A.; Lagaron, J.M. Multilayer Structures Based on Annealed Electrospun Biopolymer Coatings of Interest in Water and Aroma Barrier Fiber-based Food Packaging Applications. J. Appl. Polym. Sci. 2018, 135, 45501. [Google Scholar] [CrossRef]
- Cherpinski, A.; Torres-Giner, S.; Cabedo, L.; Lagaron, J.M. Post-Processing Optimization of Electrospun Submicron Poly (3-Hydroxybutyrate) Fibers to Obtain Continuous Films of Interest in Food Packaging Applications. Food Addit. Contam. Part A 2017, 34, 1817–1830. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Lagaron, J.M. Zein-based Ultrathin Fibers Containing Ceramic Nanofillers Obtained by Electrospinning. I. Morphology and Thermal Properties. J. Appl. Polym. Sci. 2010, 118, 778–789. [Google Scholar] [CrossRef]
- Tang, X.; Alavi, S. Recent Advances in Starch, Polyvinyl Alcohol Based Polymer Blends, Nanocomposites and Their Biodegradability. Carbohydr. Polym. 2011, 85, 7–16. [Google Scholar] [CrossRef]
- Chen, W.; Fadeev, A.Y.; Hsieh, M.C.; Öner, D.; Youngblood, J.; McCarthy, T.J. Ultrahydrophobic and Ultralyophobic Surfaces: Some Comments and Examples. Langmuir 1999, 15, 3395–3399. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; M’Bengue, M.-S.; Torres-Giner, S.; Cabedo, L.; Prieto, C.; Lagaron, J.M. Barrier Biopaper Multilayers Obtained by Impregnation of Electrospun Poly (3-Hydroxybutyrate-Co-3-Hydroxyvalerate) with Protein and Polysaccharide Hydrocolloids. Carbohydr. Polym. Technol. Appl. 2021, 2, 100150. [Google Scholar] [CrossRef]
- Feng, W.; Wang, P.; Zou, G.; Ren, Z.; Ji, J. Synthesis and Characterization of Semiaromatic Copolyamide 10T/1014 with High Performance and Flexibility. Des. Monomers Polym. 2018, 21, 33–42. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Torres-Giner, S.; Aldureid, A.; Cabedo, L.; Lagaron, J.M. Reactive Melt Mixing of Poly (3-Hydroxybutyrate)/Rice Husk Flour Composites with Purified Biosustainably Produced Poly (3-Hydroxybutyrate-Co-3-Hydroxyvalerate). Materials 2019, 12, 2152. [Google Scholar] [CrossRef]
- Lagarón, J.-M. Multifunctional and Nanoreinforced Polymers for Food Packaging. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–28. [Google Scholar]
- Wang, L.; Guo, Y.; Chen, Y.; Chen, T.; Zhu, S.; Zhang, T.; Liu, S. Enhanced Mechanical and Water Absorption Properties of Rice Husk-Derived Nano-SiO2 Reinforced PHBV Composites. Polymer 2018, 10, 1022. [Google Scholar] [CrossRef]
- Jördens, C.; Wietzke, S.; Scheller, M.; Koch, M. Investigation of the Water Absorption in Polyamide and Wood Plastic Composite by Terahertz Time-Domain Spectroscopy. Polym. Test. 2010, 29, 209–215. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Hilliou, L.; Melendez-Rodriguez, B.; Figueroa-Lopez, K.J.; Madalena, D.; Cabedo, L.; Covas, J.A.; Vicente, A.A.; Lagaron, J.M. Melt Processability, Characterization, and Antibacterial Activity of Compression-Molded Green Composite Sheets Made of Poly (3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Reinforced with Coconut Fibers Impregnated with Oregano Essential Oil. Food Packag. Shelf Life 2018, 17, 39–49. [Google Scholar] [CrossRef]
- Fasihi, M.; Abolghasemi, M.R. Oxygen Barrier and Mechanical Properties of Masterbatch-based PA6/Nanoclay Composite Films. J. Appl. Polym. Sci. 2012, 125, E2–E8. [Google Scholar] [CrossRef]
- Sun, L.; Li, H.; Huang, Y.; Wu, J.; Runt, J.; Kuo, M.; Huang, K.; Yeh, J. Oxygen Barrier, Free Volume and Miscibility Properties of Fully Bio-Based Polyamide 1010/Poly (Vinyl Alcohol) Blends. J. Polym. Res. 2019, 26, 1–10. [Google Scholar] [CrossRef]



| Sample | Temperature (°C) | Roll Speed (m/min) |
|---|---|---|
| PA1010/1014 | 120 | 0.52 |
| PHBV | 125 | 0.52 |
| PHBV/PA1010/1014/PHBV | 130 | 0.52 |
| PHBV/PA1010/1014/PHBV/SiO2 | 130 | 0.52 |
| Sample | Surface Tension (mN/m) | Conductivity (µS/cm) | Viscosity (cP) |
|---|---|---|---|
| PHBV | 31.0 ± 3.8 a | 1.79 ± 0.03 a | 2799.8 ± 45.7 a |
| PA1010/1014 | 19.4 ± 0.6 b | 1.29 ± 0.07 a | 640.3 ± 4.2 b |
| SiO2 | 26.3 ± 1.3 a | 1.33 ± 0.99 a | 339.2 ± 8.3 c |
| Processing Parameters | PHBV Solution | BioPA Solution | SiO2 Dispersion |
|---|---|---|---|
| Flowrate per needle (mL/h) | 4.0 | 2.4 | 1.2 |
| Voltage emitter (kV) | 10.0 | 15.0 | 5.0 |
| Voltage collector (kV) | 22.5 | 20.0 | 18.0 |
| Distance collector (cm) | 20.0 | 23.5 | 22.5 |
| Drum speed (rpm) | 200 | 200 | 200 |
| Temperature (°C) | 30 | 30 | 30 |
| Relative humidity (%) | 30 | 30 | 30 |
| Sample | T (%) (Equation (5)) | T (mm−1) (Equation (4)) |
|---|---|---|
| PHBV | 23.7 ± 1.4 a | 9.9 ± 2.3 a |
| PA1010/1014 | 52.1 ± 0.3 b | 8.1 ± 0.9 a |
| PHBV/PA1010/1014/PHBV PHBV/ PA1010/1014/PHBV/SiO2 | 6.5 ± 1.9 c 2.6 ± 0.1 d | 10.8 ± 0.3 a,b 14.0 ± 0.1 b |
| Sample | WCA (°) |
|---|---|
| PHBV | 82.5 ± 1.0 a |
| PA1010/1014 | 86.3 ± 0.2 b |
| PHBV/PA1010/1014/PHBV/SiO2 | 146.2 ± 0.2 c |
| Samples | E (MPa) | σy (MPa) | εb (%) |
|---|---|---|---|
| PHBV/PHBV | 3347 ± 261 a | 22.0 ± 6.7 a | 1.0 ± 0.1 a |
| PA1010/1014 | 1202 ± 246 b | 25.2 ± 3.1 a,b | 21.9 ± 4.4 b |
| PHBV/ PA1010/1014/PHBV | 2925 ± 86 a | 33.3 ± 2.4 b | 2.4 ± 0.4 a |
| PHBV/ PA1010/1014/PHBV/SiO2 | 2898 ± 162 a | 32.3 ± 1.1 b | 2.8 ± 0.3 a |
| Sample | Thickness (µm) | WVP × 1011 (kg m−2s−1Pa−1) | OP × 1015 (m3 m−2s−1Pa−1) | Water Uptake (%wt) |
|---|---|---|---|---|
| PHBV/PHBV | 97 ± 5 a | 1.04 ± 0.30 a | 7.00 ± 0.07 a | 0.20 ± 0.20 a |
| PA1010/1014 | 72 ± 8 b | 161 ± 68 b | 31.3 ± 7.0 b,* | 1.10 ± 0.53 b |
| PHBV/PA1010/1014/PHBV | 184 ± 13 c | 0.30 ± 0.03 a | 3.95 ± 0.40 a,c | 0.57 ± 0.21 a,b |
| PHBV/ PA1010/1014/PHBV/SiO2 | 160 ± 8 d | 1.02 ± 0.06 a | 5.16 ± 0.02 a,d | 0.38 ± 0.17 a,b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcoaldi, C.; Pardo-Figuerez, M.; Prieto, C.; Arnal, C.; Torres-Giner, S.; Cabedo, L.; Lagaron, J.M. Electrospun Multilayered Films Based on Poly(3-hydroxybutyrate-co-3-hydroxyvalerate), Copolyamide 1010/1014, and Electrosprayed Nanostructured Silica. Nanomaterials 2023, 13, 972. https://doi.org/10.3390/nano13060972
Marcoaldi C, Pardo-Figuerez M, Prieto C, Arnal C, Torres-Giner S, Cabedo L, Lagaron JM. Electrospun Multilayered Films Based on Poly(3-hydroxybutyrate-co-3-hydroxyvalerate), Copolyamide 1010/1014, and Electrosprayed Nanostructured Silica. Nanomaterials. 2023; 13(6):972. https://doi.org/10.3390/nano13060972
Chicago/Turabian StyleMarcoaldi, Chiara, Maria Pardo-Figuerez, Cristina Prieto, Carmen Arnal, Sergio Torres-Giner, Luis Cabedo, and Jose M. Lagaron. 2023. "Electrospun Multilayered Films Based on Poly(3-hydroxybutyrate-co-3-hydroxyvalerate), Copolyamide 1010/1014, and Electrosprayed Nanostructured Silica" Nanomaterials 13, no. 6: 972. https://doi.org/10.3390/nano13060972
APA StyleMarcoaldi, C., Pardo-Figuerez, M., Prieto, C., Arnal, C., Torres-Giner, S., Cabedo, L., & Lagaron, J. M. (2023). Electrospun Multilayered Films Based on Poly(3-hydroxybutyrate-co-3-hydroxyvalerate), Copolyamide 1010/1014, and Electrosprayed Nanostructured Silica. Nanomaterials, 13(6), 972. https://doi.org/10.3390/nano13060972











