Nanobionics: A Sustainable Agricultural Approach towards Understanding Plant Response to Heavy Metals, Drought, and Salt Stress
Abstract
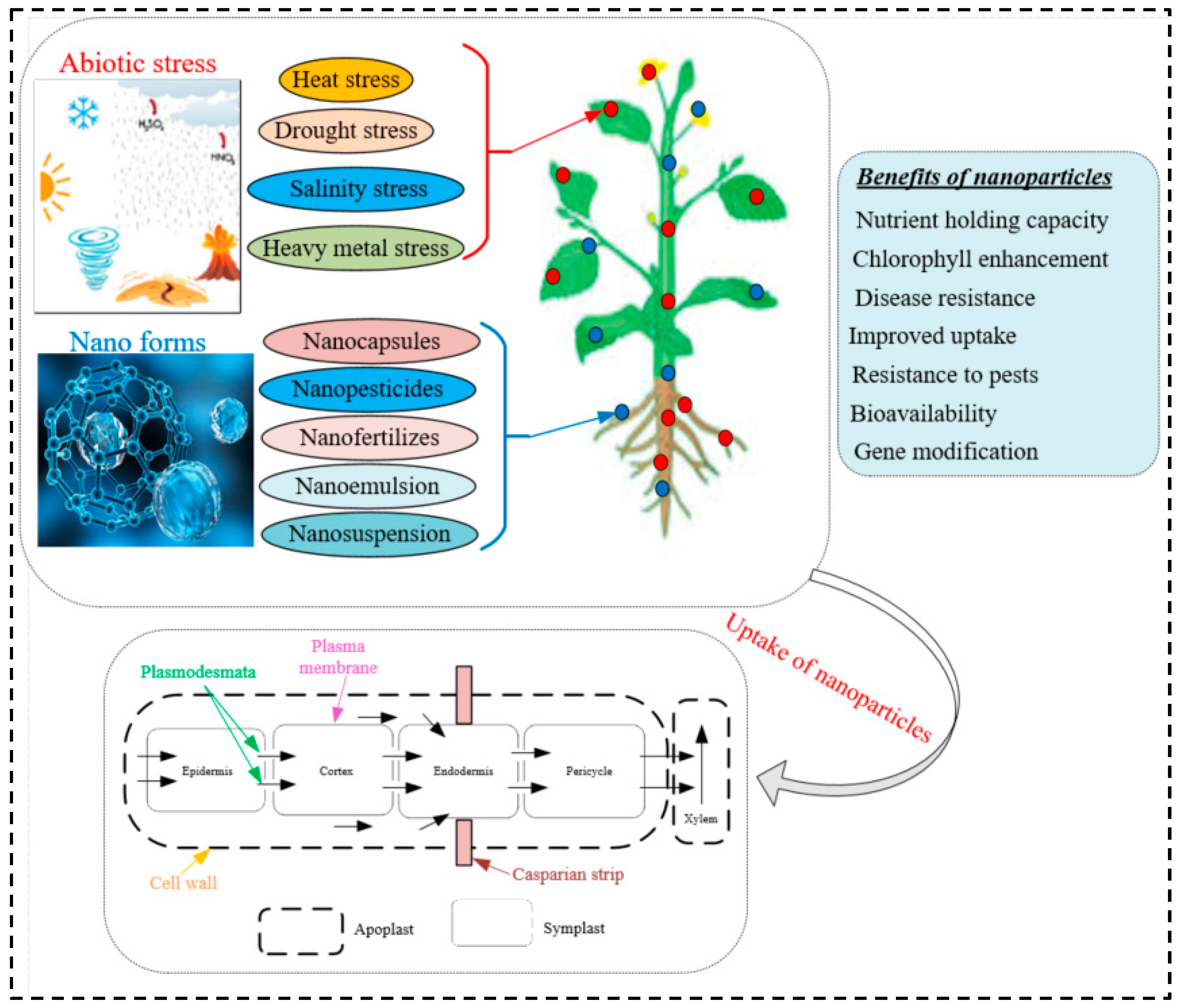
1. Introduction
2. Nanobionics and Physiological Traits
3. Nanobionics and Growth Indices
4. Nanobionics and Root Architecture
5. Nanobionics and Stress Tolerance
6. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amritha, M.S.; Sridharan, K.; Puthur, J.T.; Dhankher, O.P. Priming with Nanoscale Materials for Boosting Abiotic Stress Tolerance in Crop Plants. J. Agric. Food Chem. 2021, 69, 10017–10035. [Google Scholar]
- Anwar, K.; Joshi, R.; Dhankher, O.P.; Singla-Pareek, S.L.; Pareek, A. Elucidating the Response of Crop Plants towards Individual, Combined and Sequentially Occurring Abiotic Stresses. Int. J. Mol. Sci. 2021, 22, 6119. [Google Scholar] [CrossRef]
- Turgut-Kara, N.; Arikan, B.; Celik, H. Epigenetic Memory and Priming in Plants. Genetica 2020, 148, 47–54. [Google Scholar] [CrossRef]
- Balestrini, R.; Chitarra, W.; Antoniou, C.; Ruocco, M.; Fotopoulos, V. Improvement of Plant Performance under Water Deficit with the Employment of Biological and Chemical Priming Agents. J. Agric. Sci. 2018, 156, 680–688. [Google Scholar] [CrossRef]
- Sharma, A.; Vishwakarma, K.; Singh, N.K.; Prakash, V.; Ramawat, N.; Prasad, R.; Sahi, S.; Singh, V.P.; Tripathi, D.K.; Sharma, S. Synergistic Action of Silicon Nanoparticles and Indole Acetic Acid in Alleviation of Chromium (CrVI) Toxicity in Oryza Sativa Seedlings. J. Biotechnol. 2022, 343, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Latif, S.; Saeed, F.; Ali, I.; Ullah, S.; Alsahli, A.A.; Jan, S.; Ahmad, P. Seed Priming with Titanium Dioxide Nanoparticles Enhances Seed Vigor, Leaf Water Status, and Antioxidant Enzyme Activities in Maize (Zea Mays L.) under Salinity Stress. J. King Saud Univ. 2021, 33, 101207. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Yusuf, M.; Khan, S.T.; Hayat, S. Zinc Oxide Nanoparticle-Mediated Changes in Photosynthetic Efficiency and Antioxidant System of Tomato Plants. Photosynthetica 2018, 56, 678–686. [Google Scholar] [CrossRef]
- Pereira, A.E.S.; Grillo, R.; Mello, N.F.S.; Rosa, A.H.; Fraceto, L.F. Application of Poly (Epsilon-Caprolactone) Nanoparticles Containing Atrazine Herbicide as an Alternative Technique to Control Weeds and Reduce Damage to the Environment. J. Hazard. Mater. 2014, 268, 207–215. [Google Scholar] [CrossRef]
- Hatami, M.; Kariman, K.; Ghorbanpour, M. Engineered Nanomaterial-Mediated Changes in the Metabolism of Terrestrial Plants. Sci. Total Environ. 2016, 571, 275–291. [Google Scholar] [CrossRef]
- Castillo-Henríquez, L.; Alfaro-Aguilar, K.; Ugalde-Álvarez, J.; Vega-Fernández, L.; Montes de Oca-Vásquez, G.; Vega-Baudrit, J.R. Green Synthesis of Gold and Silver Nanoparticles from Plant Extracts and Their Possible Applications as Antimicrobial Agents in the Agricultural Area. Nanomaterials 2020, 10, 1763. [Google Scholar] [CrossRef]
- Jat, S.K.; Bhattacharya, J.; Sharma, M.K. Nanomaterial Based Gene Delivery: A Promising Method for Plant Genome Engineering. J. Mater. Chem. B 2020, 8, 4165–4175. [Google Scholar] [CrossRef] [PubMed]
- Kandhol, N.; Bansal, R.; Parveen, N.; Singh, V.P.; Chauhan, D.K.; Sonah, H.; Sahi, S.; Grillo, R.; Peralta-Videa, J.; Deshmukh, R. Nanoparticles as a Potential Protective Agent for Arsenic Toxicity Alleviation in Plants. Environ. Pollut. 2022, 300, 118887. [Google Scholar] [CrossRef]
- De Oliveira, J.L.; Campos, E.V.R.; Pereira, A.E.S.; Pasquoto, T.; Lima, R.; Grillo, R.; Andrade, D.J.D.; Santos, F.A.D.; Fraceto, L.F. Zein Nanoparticles as Eco-Friendly Carrier Systems for Botanical Repellents Aiming Sustainable Agriculture. J. Agric. Food Chem. 2018, 66, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.P.; Mitter, N. How Nanocarriers Delivering Cargos in Plants Can Change the GMO Landscape. Nat. Nanotechnol. 2019, 14, 512–514. [Google Scholar] [CrossRef]
- Guo, J.; Mattos, B.D.; Tardy, B.L.; Moody, V.M.; Xiao, G.; Ejima, H.; Cui, J.; Liang, K.; Richardson, J.J. Porous Inorganic and Hybrid Systems for Drug Delivery: Future Promise in Combatting Drug Resistance and Translation to Botanical Applications. Curr. Med. Chem. 2019, 26, 6107–6131. [Google Scholar] [CrossRef]
- El-Badri, A.M.A.; Batool, M.; Mohamed, I.A.A.; Khatab, A.; Sherif, A.; Wang, Z.; Salah, A.; Nishawy, E.; Ayaad, M.; Kuai, J. Modulation of Salinity Impact on Early Seedling Stage via Nano-Priming Application of Zinc Oxide on Rapeseed (Brassica Napus L.). Plant Physiol. Biochem. 2021, 166, 376–392. [Google Scholar] [CrossRef]
- Abbasi Khalaki, M.; Ghorbani, A.; Dadjou, F. Influence of Nano-Priming on Festuca ovina Seed Germination and Early Seedling Traits under Drought Stress, in Laboratory Condition. Ecopersia 2019, 7, 133–139. [Google Scholar]
- Khan, M.N.; Li, Y.; Khan, Z.; Chen, L.; Liu, J.; Hu, J.; Wu, H.; Li, Z. Nanoceria Seed Priming Enhanced Salt Tolerance in Rapeseed through Modulating ROS Homeostasis and α-Amylase Activities. J. Nanobiotechnol. 2021, 19, 276. [Google Scholar] [CrossRef]
- Abou-Zeid, H.; Ismail, G. The Role of Priming with Biosynthesized Silver Nanoparticles in the Response of Triticum aestivum L to Salt Stress. Egypt. J. Bot. 2018, 58, 73–85. [Google Scholar]
- Yousefi, S.; Kartoolinejad, D.; Naghdi, R. Effects of Priming with Multi-Walled Carbon Nanotubes on Seed Physiological Characteristics of Hopbush (Dodonaea viscosa L.) under Drought Stress. Int. J. Environ. Stud. 2017, 74, 528–539. [Google Scholar] [CrossRef]
- Rai-Kalal, P.; Tomar, R.S.; Jajoo, A. Seed Nanopriming by Silicon Oxide Improves Drought Stress Alleviation Potential in Wheat Plants. Funct. Plant Biol. 2021, 48, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Elkhatib, E.; Attia, M.G.; Mahdy, A.M.; Mostafa, R.A. Priming with Mango Peels Nanoparticles Enhances Seed Germination of Maize (Zea Mays l.) under Salt Stress. Alexandria Sci. Exch. J. 2019, 40, 767–780. [Google Scholar] [CrossRef]
- Park, S.; Ahn, Y.-J. Multi-Walled Carbon Nanotubes and Silver Nanoparticles Differentially Affect Seed Germination, Chlorophyll Content, and Hydrogen Peroxide Accumulation in Carrot (Daucus carota L.). Biocatal. Agric. Biotechnol. 2016, 8, 257–262. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Ahmad, Z.; Xie, Y. The Effect of Silicon Nanoparticles on the Seed Germination and Seedling Growth of Moso Bamboo (Phyllostachys edulis) under Cadmium Stress. Polish J. Environ. Stud. 2021, 30, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Liang, L.; Li, W.; Ashraf, U.; Ma, L.; Tang, X.; Pan, S.; Tian, H.; Mo, Z. ZnO Nanoparticle-Based Seed Priming Modulates Early Growth and Enhances Physio-Biochemical and Metabolic Profiles of Fragrant Rice against Cadmium Toxicity. J. Nanobiotechnol. 2021, 19, 75. [Google Scholar] [CrossRef]
- Van Nguyen, D.; Nguyen, H.M.; Le, N.T.; Nguyen, K.H.; Nguyen, H.T.; Le, H.M.; Nguyen, A.T.; Dinh, N.T.T.; Hoang, S.A.; Van Ha, C. Copper Nanoparticle Application Enhances Plant Growth and Grain Yield in Maize under Drought Stress Conditions. J. Plant Growth Regul. 2022, 41, 364–375. [Google Scholar] [CrossRef]
- Faraji, J.; Sepehri, A. Exogenous Nitric Oxide Improves the Protective Effects of TiO2 Nanoparticles on Growth, Antioxidant System, and Photosynthetic Performance of Wheat Seedlings Under Drought Stress. J. Soil Sci. Plant Nutr. 2020, 20, 703–714. [Google Scholar] [CrossRef]
- Alabdallah, N.M.; Alzahrani, H.S. The Potential Mitigation Effect of ZnO Nanoparticles on [Abelmoschus esculentus L. Moench] Metabolism under Salt Stress Conditions. Saudi J. Biol. Sci. 2020, 27, 3132–3137. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Dutt, M.; Shalan, A.M.; El-Kady, M.E.; El-Boray, M.S.; Shabana, Y.M.; Grosser, J.W. Silicon Nanoparticles Mitigate Oxidative Stress of in Vitro-Derived Banana (Musa acuminata ‘Grand Nain’) under Simulated Water Deficit or Salinity Stress. S. Afr. J. Bot. 2020, 132, 155–163. [Google Scholar] [CrossRef]
- Xin, X.; Zhao, F.; Rho, J.Y.; Goodrich, S.L.; Sumerlin, B.S.; He, Z. Use of Polymeric Nanoparticles to Improve Seed Germination and Plant Growth under Copper Stress. Sci. Total Environ. 2020, 745, 141055. [Google Scholar] [CrossRef]
- An, J.; Hu, P.; Li, F.; Wu, H.; Shen, Y.; White, J.C.; Tian, X.; Li, Z.; Giraldo, J.P. Emerging Investigator Series: Molecular Mechanisms of Plant Salinity Stress Tolerance Improvement by Seed Priming with Cerium Oxide Nanoparticles. Environ. Sci. Nano 2020, 7, 2214–2228. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Mansoori, G.A. Reliability for Drug Targeting in Cancer Treatment through Nanotechnology (a Psychometric Approach). Molecules 2014, 1, 8. [Google Scholar] [CrossRef]
- Giraldo, J.P.; Landry, M.P.; Faltermeier, S.M.; McNicholas, T.P.; Iverson, N.M.; Boghossian, A.A.; Reuel, N.F.; Hilmer, A.J.; Sen, F.; Brew, J.A. Plant Nanobionics Approach to Augment Photosynthesis and Biochemical Sensing. Nat. Mater. 2014, 13, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xianyu, Y. When Nano Meets Plants: A Review on the Interplay between Nanoparticles and Plants. Nano Today 2021, 38, 101143. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, B.; Wang, Q.; Shi, X.; Xiao, Z.; Lin, J.; Fang, X. Carbon Nanotubes as Molecular Transporters for Walled Plant Cells. Nano Lett. 2009, 9, 1007–1010. [Google Scholar] [CrossRef]
- Zhu, X.-G.; Long, S.P.; Ort, D.R. Improving Photosynthetic Efficiency for Greater Yield. Annu. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef]
- Blankenship, R.E.; Tiede, D.M.; Barber, J.; Brudvig, G.W.; Fleming, G.; Ghirardi, M.; Gunner, M.R.; Junge, W.; Kramer, D.M.; Melis, A. Comparing Photosynthetic and Photovoltaic Efficiencies and Recognizing the Potential for Improvement. Science 2011, 332, 805–809. [Google Scholar] [CrossRef]
- Dufil, G.; Bernacka-Wojcik, I.; Armada-Moreira, A.; Stavrinidou, E. Plant Bioelectronics and Biohybrids: The Growing Contribution of Organic Electronic and Carbon-Based Materials. Chem. Rev. 2021, 122, 4847–4883. [Google Scholar] [CrossRef]
- Zhang, J.; Boghossian, A.A.; Barone, P.W.; Rwei, A.; Kim, J.-H.; Lin, D.; Heller, D.A.; Hilmer, A.J.; Nair, N.; Reuel, N.F. Single Molecule Detection of Nitric Oxide Enabled by d (AT) 15 DNA Adsorbed to near Infrared Fluorescent Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2011, 133, 567–581. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Firoz, M.; Al-Khaishany, M.Y. Role of Nanoparticles in Plants. In Nanotechnology and Plant Sciences; Springer: Cham, Switzerland, 2015; pp. 19–35. [Google Scholar]
- Tullii, G.; Gobbo, F.; Costa, A.; Antognazza, M.R. A Prototypical Conjugated Polymer Regulating Signaling in Plants. Adv. Sustain. Syst. 2022, 6, 2100048. [Google Scholar] [CrossRef]
- Prasad, A.; Astete, C.E.; Bodoki, A.E.; Windham, M.; Bodoki, E.; Sabliov, C.M. Zein Nanoparticles Uptake and Translocation in Hydroponically Grown Sugar Cane Plants. J. Agric. Food Chem. 2017, 66, 6544–6551. [Google Scholar] [CrossRef]
- Pradhan, S.; Mailapalli, D.R. Interaction of Engineered Nanoparticles with the Agri-Environment. J. Agric. Food Chem. 2017, 65, 8279–8294. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.H.; Misra, R.P.; Giraldo, J.P.; Kwak, S.-Y.; Son, Y.; Landry, M.P.; Swan, J.W.; Blankschtein, D.; Strano, M.S. Lipid Exchange Envelope Penetration (LEEP) of Nanoparticles for Plant Engineering: A Universal Localization Mechanism. Nano Lett. 2016, 16, 1161–1172. [Google Scholar] [CrossRef]
- Kwak, S.-Y.; Lew, T.T.S.; Sweeney, C.J.; Koman, V.B.; Wong, M.H.; Bohmert-Tatarev, K.; Snell, K.D.; Seo, J.S.; Chua, N.-H.; Strano, M.S. Chloroplast-Selective Gene Delivery and Expression in Planta Using Chitosan-Complexed Single-Walled Carbon Nanotube Carriers. Nat. Nanotechnol. 2019, 14, 447–455. [Google Scholar] [CrossRef]
- Wu, H.; Sun, Q.; Chen, J.; Wang, G.-Y.; Wang, D.; Zeng, X.-F.; Wang, J.-X. Citric Acid-Assisted Ultrasmall CeO2 Nanoparticles for Efficient Photocatalytic Degradation of Glyphosate. Chem. Eng. J. 2021, 425, 130640. [Google Scholar] [CrossRef]
- Thagun, C.; Chuah, J.; Numata, K. Targeted Gene Delivery into Various Plastids Mediated by Clustered Cell-penetrating and Chloroplast-targeting Peptides. Adv. Sci. 2019, 6, 1902064. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.-Y.; Wong, M.H.; Lew, T.T.S.; Bisker, G.; Lee, M.A.; Kaplan, A.; Dong, J.; Liu, A.T.; Koman, V.B.; Sinclair, R. Nanosensor Technology Applied to Living Plant Systems. Annu. Rev. Anal. Chem. 2017, 10, 113–140. [Google Scholar] [CrossRef]
- Khatri, K.; Rathore, M.S. Plant Nanobionics and Its Applications for Developing Plants with Improved Photosynthetic Capacity. Photosynthesis—From Its Evolution to Future Improvements in Photosynthetic Efficiency Using Nanomaterials; BoD–Books on Demand: London, UK, 2018. [Google Scholar] [CrossRef]
- Mingyu, S.; Chao, L.; Chunxiang, Q.; Lei, Z.; Liang, C.; Hao, H.; Xiaoqing, L.; Xiao, W.; Fashui, H. Nano-Anatase Relieves the Inhibition of Electron Transport Caused by Linolenic Acid in Chloroplasts of Spinach. Biol. Trace Elem. Res. 2008, 122, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Fan, X.; Li, X.; Zhang, Z.; Sun, L.; Fu, Z.; Lavoie, M.; Pan, X.; Qian, H. Distinct Physiological and Molecular Responses in Arabidopsis Thaliana Exposed to Aluminum Oxide Nanoparticles and Ionic Aluminum. Environ. Pollut. 2017, 228, 517–527. [Google Scholar] [CrossRef]
- Anand, K.V.; Anugraga, A.R.; Kannan, M.; Singaravelu, G.; Govindaraju, K. Bio-Engineered Magnesium Oxide Nanoparticles as Nano-Priming Agent for Enhancing Seed Germination and Seedling Vigour of Green Gram (Vigna radiata L.). Mater. Lett. 2020, 271, 127792. [Google Scholar] [CrossRef]
- Lahiani, M.H.; Dervishi, E.; Chen, J.; Nima, Z.; Gaume, A.; Biris, A.S.; Khodakovskaya, M.V. Impact of Carbon Nanotube Exposure to Seeds of Valuable Crops. ACS Appl. Mater. Interfaces 2013, 5, 7965–7973. [Google Scholar] [CrossRef]
- Rahman, M.S.; Chakraborty, A.; Mazumdar, S.; Nandi, N.C.; Bhuiyan, M.N.I.; Alauddin, S.M.; Khan, I.A.; Hossain, M.J. Effects of Poly (Vinylpyrrolidone) Protected Platinum Nanoparticles on Seed Germination and Growth Performance of Pisum sativum. Nano-Struct. Nano-Objects 2020, 21, 100408. [Google Scholar] [CrossRef]
- Sun, D.; Hussain, H.I.; Yi, Z.; Rookes, J.E.; Kong, L.; Cahill, D.M. Mesoporous Silica Nanoparticles Enhance Seedling Growth and Photosynthesis in Wheat and Lupin. Chemosphere 2016, 152, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodzadeh, H.; Nabavi, M.; Kashefi, H. Effect of Nanoscale Titanium Dioxide Particles on the Germination and Growth of Canola (Brassica napus). J. Ornam. Hortic. Plants 2013, 3, 25–32. [Google Scholar]
- Larue, C.; Laurette, J.; Herlin-Boime, N.; Khodja, H.; Fayard, B.; Flank, A.-M.; Brisset, F.; Carriere, M. Accumulation, Translocation and Impact of TiO2 Nanoparticles in Wheat (Triticum aestivum spp.): Influence of Diameter and Crystal Phase. Sci. Total Environ. 2012, 431, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Boykov, I.N.; Shuford, E.; Zhang, B. Nanoparticle Titanium Dioxide Affects the Growth and MicroRNA Expression of Switchgrass (Panicum virgatum). Genomics 2019, 111, 450–456. [Google Scholar] [CrossRef]
- Raja, K.; Sowmya, R.; Sudhagar, R.; Moorthy, P.S.; Govindaraju, K.; Subramanian, K.S. Biogenic ZnO and Cu Nanoparticles to Improve Seed Germination Quality in Blackgram (Vigna mungo). Mater. Lett. 2019, 235, 164–167. [Google Scholar] [CrossRef]
- Walker, T.S.; Bais, H.P.; Grotewold, E.; Vivanco, J.M. Root Exudation and Rhizosphere Biology. Plant Physiol. 2003, 132, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Guo, H.; Ma, C.; Li, C.; Chefetz, B.; Polubesova, T.; Xing, B. Maize (Zea mays L.) Root Exudates Modify the Surface Chemistry of CuO Nanoparticles: Altered Aggregation, Dissolution and Toxicity. Sci. Total Environ. 2019, 690, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Duan, H.; Wang, X.; Zhang, H.; Zhang, Z. Effects of rice root exudates on aggregation, dissolution and bioaccumulation of differently-charged Ag nanoparticles. RSC Adv. 2022, 12, 9435–9444. [Google Scholar] [CrossRef]
- Butnariu, M.; Butu, A. Plant Nanobionics: Application of Nanobiosensors in Plant Biology. Plant Nanobionics 2019, 1, 337–376. [Google Scholar]
- Cervantes-Avilés, P.; Huang, X.; Keller, A.A. Dissolution and Aggregation of Metal Oxide Nanoparticles in Root Exudates and Soil Leachate: Implications for Nanoagrochemical Application. Environ. Sci. Technol. 2021, 55, 13443–13451. [Google Scholar] [CrossRef]
- McManus, P.; Hortin, J.; Anderson, A.J.; Jacobson, A.R.; Britt, D.W.; Stewart, J.; McLean, J.E. Rhizosphere Interactions between Copper Oxide Nanoparticles and Wheat Root Exudates in a Sand Matrix: Influences on Copper Bioavailability and Uptake. Environ. Toxicol. Chem. 2018, 37, 2619–2632. [Google Scholar] [CrossRef]
- Geldner, N. The Endodermis. Annu. Rev. Plant Biol. 2013, 64, 531–558. [Google Scholar] [CrossRef]
- Bao, Y.; Ma, J.; Pan, C.; Guo, A.; Li, Y.; Xing, B. Citric acid enhances Ce uptake and accumulation in rice seedlings exposed to CeO2 nanoparticles and iron plaque attenuates the enhancement. Chemosphere 2020, 240, 124897. [Google Scholar] [CrossRef] [PubMed]
- Bañuelos, G.S.; Stushnoff, C.; Walse, S.S.; Zuber, T.; Yang, S.I.; Pickering, I.J.; Freeman, J.L. Biofortified, Selenium Enriched, Fruit and Cladode from Three Opuntia Cactus Pear Cultivars Grown on Agricultural Drainage Sediment for Use in Nutraceutical Foods. Food Chem. 2012, 135, 9–16. [Google Scholar] [CrossRef]
- Su, Y.; Ashworth, V.; Kim, C.; Adeleye, A.S.; Rolshausen, P.; Roper, C.; White, J.; Jassby, D. Delivery, Uptake, Fate, and Transport of Engineered Nanoparticles in Plants: A Critical Review and Data Analysis. Environ. Sci. Nano 2019, 6, 2311–2331. [Google Scholar] [CrossRef]
- Ranjan, A.; Rajput, V.D.; Kumari, A.; Mandzhieva, S.S.; Sushkova, S.; Prazdnova, E.V.; Zargar, S.M.; Raza, A.; Minkina, T.; Chung, G. Nanobionics in Crop Production: An Emerging Approach to Modulate Plant Functionalities. Plants 2022, 11, 692. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Zhang, W.; Ma, X. Cerium Oxide Nanoparticles Alter the Salt Stress Tolerance of Brassica napus L. by Modifying the Formation of Root Apoplastic Barriers. Environ. Pollut. 2017, 229, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Chen, F.; Yu, K.; Xiao, Z.; Yu, X.; Wang, Z.; Xing, B. Early Development of Apoplastic Barriers and Molecular Mechanisms in Juvenile Maize Roots in Response to La2O3 Nanoparticles. Sci. Total Environ. 2019, 653, 675–683. [Google Scholar] [CrossRef]
- Chen, T.; Cai, X.; Wu, X.; Karahara, I.; Schreiber, L.; Lin, J. Casparian Strip Development and Its Potential Function in Salt Tolerance. Plant Signal. Behav. 2011, 6, 1499–1502. [Google Scholar] [CrossRef]
- Li, L.; Luo, Y.; Li, R.; Zhou, Q.; Peijnenburg, W.J.G.M.; Yin, N.; Yang, J.; Tu, C.; Zhang, Y. Effective Uptake of Submicrometre Plastics by Crop Plants via a Crack-Entry Mode. Nat. Sustain. 2020, 3, 929–937. [Google Scholar] [CrossRef]
- Spielman-Sun, E.; Avellan, A.; Bland, G.D.; Tappero, R.V.; Acerbo, A.S.; Unrine, J.M.; Giraldo, J.P.; Lowry, G.V. Nanoparticle Surface Charge Influences Translocation and Leaf Distribution in Vascular Plants with Contrasting Anatomy. Environ. Sci. Nano 2019, 6, 2508–2519. [Google Scholar] [CrossRef]
- Lv, Z.; Sun, H.; Du, W.; Li, R.; Mao, H.; Kopittke, P.M. Interaction of Different-Sized ZnO Nanoparticles with Maize (Zea mays): Accumulation, Biotransformation and Phytotoxicity. Sci. Total Environ. 2021, 796, 148927. [Google Scholar] [CrossRef]
- Berhin, A.; de Bellis, D.; Franke, R.B.; Buono, R.A.; Nowack, M.K.; Nawrath, C. The Root Cap Cuticle: A Cell Wall Structure for Seedling Establishment and Lateral Root Formation. Cell 2019, 176, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Bhat, J.A.; Chen, C.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P.; Yu, F. Zinc Oxide Nanoparticles (ZnO-NPs) Induce Salt Tolerance by Improving the Antioxidant System and Photosynthetic Machinery in Tomato. Plant Physiol. Biochem. 2021, 161, 122–130. [Google Scholar] [CrossRef]
- Bidi, H.; Fallah, H.; Niknejad, Y.; Tari, D.B. Iron Oxide Nanoparticles Alleviate Arsenic Phytotoxicity in Rice by Improving Iron Uptake, Oxidative Stress Tolerance and Diminishing Arsenic Accumulation. Plant Physiol. Biochem. 2021, 163, 348–357. [Google Scholar] [CrossRef]
- Balážová, Ľ.; Baláž, M.; Babula, P. Zinc Oxide Nanoparticles Damage Tobacco BY-2 Cells by Oxidative Stress Followed by Processes of Autophagy and Programmed Cell Death. Nanomaterials 2020, 10, 1066. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, L.; Gu, J.; Ma, H.; Wu, H. Carbon-Based Nanomaterials for Sustainable Agriculture: Their Application as Light Converters, Nanosensors, and Delivery Tools. Plants 2022, 11, 511. [Google Scholar] [CrossRef]
- López-Vargas, E.R.; González-García, Y.; Pérez-Álvarez, M.; Cadenas-Pliego, G.; González-Morales, S.; Benavides-Mendoza, A.; Cabrera, R.I.; Juárez-Maldonado, A. Seed Priming with Carbon Nanomaterials to Modify the Germination, Growth, and Antioxidant Status of Tomato Seedlings. Agronomy 2020, 10, 639. [Google Scholar] [CrossRef]
- Das, A.; Das, B. Nanotechnology a Potential Tool to Mitigate Abiotic Stress in Crop Plants. In Abiotic and Biotic Stress in Plants; IntechOpen: London, UK, 2019. [Google Scholar]
- Tripathi, D.K.; Singh, S.; Singh, S.; Dubey, N.K.; Chauhan, D.K. Impact of Nanoparticles on Photosynthesis: Challenges and Opportunities. Mater. Focus 2016, 5, 405–411. [Google Scholar]
- Fan, X.; Xu, J.; Lavoie, M.; Peijnenburg, W.; Zhu, Y.; Lu, T.; Fu, Z.; Zhu, T.; Qian, H. Multiwall Carbon Nanotubes Modulate Paraquat Toxicity in Arabidopsis thaliana. Environ. Pollut. 2018, 233, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, Z.H.; Akram, M.W.; Begum, R.; Wu, W.; Irfan, A. Inorganic nanoparticles for reduction of hexavalent chromium: Physicochemical aspects. J. Hazard. Mater. 2021, 402, 123535. [Google Scholar] [CrossRef] [PubMed]
| Plant Species | Applied NPs | Abiotic Stress | Concentration and Mode of Application | The Result of the Experiment | References |
|---|---|---|---|---|---|
| Phyllostachys edulis | SiO2 NPs | Cd | 100 and 200 μM; seed germination | Increased seed germination rate and percentage and mean germination time | [24] |
| Yuxiangyouzhan and Xiangyaxiangzhan | ZnO NPs | Cd | 0, 25, 50, and 100 mg/L−1 through seedlings | Increased plant length and fresh weight | [25] |
| Zea mays L. | CuNPs | Drought stress | 3.33, 4.44, and 5.55 mg/L plant priming | An increase in the biomass, anthocyanin, chlorophyll, and carotenoid contents along with number of seeds the grain yielded | [26] |
| T. aestivum | TiO2 | Drought | 500, 1000, and 2000 mg/kg amended soil | Improved stomatal conductance, transpiration rate, antioxidative enzymes | [27] |
| Abelmoschus esculentus L. | ZnO | Salinity | 10 mg/L | A rise in photosynthetic pigments, an increase in SOD and CAT activity, a decrease in proline, and a total increase in the amount of soluble sugars | [28] |
| Musa acuminata | SiO2 | Salinity and water deficit | 0, 200, 400, and 600 mg/L in vitro | Improved photosynthesis, preserved K+ and Na+ balance, decreased cell wall damage, and increased shoot growth and chlorophyll content | [29] |
| Zea mays L. | Polysuc-cinimide nanoparticles (PSI-NPs) | Cu | 200 mg/L−1 through seeds | Increased the effectiveness of the water supply, which in turn raised the germination rate and percentage. Longer shoots, roots, and seedling fresh biomass all indicated PSI-NPs’ ability to reduce Cu stress | [30] |
| Gossypium hirsutum L. | Poly (acrylic acid)-coated cerium oxide nanoparticles (PNC) | Salinity | 500 mg/L−1 through seedlings | In response to salinity stress, it was found that the length, vitality, fresh and dry weight, and root anatomical structure of seedlings all increased | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faizan, M.; Karabulut, F.; Alam, P.; Yusuf, M.; Tonny, S.H.; Adil, M.F.; Sehar, S.; Ahmed, S.M.; Hayat, S. Nanobionics: A Sustainable Agricultural Approach towards Understanding Plant Response to Heavy Metals, Drought, and Salt Stress. Nanomaterials 2023, 13, 974. https://doi.org/10.3390/nano13060974
Faizan M, Karabulut F, Alam P, Yusuf M, Tonny SH, Adil MF, Sehar S, Ahmed SM, Hayat S. Nanobionics: A Sustainable Agricultural Approach towards Understanding Plant Response to Heavy Metals, Drought, and Salt Stress. Nanomaterials. 2023; 13(6):974. https://doi.org/10.3390/nano13060974
Chicago/Turabian StyleFaizan, Mohammad, Fadime Karabulut, Pravej Alam, Mohammad Yusuf, Sadia Haque Tonny, Muhammad Faheem Adil, Shafaque Sehar, S. Maqbool Ahmed, and Shamsul Hayat. 2023. "Nanobionics: A Sustainable Agricultural Approach towards Understanding Plant Response to Heavy Metals, Drought, and Salt Stress" Nanomaterials 13, no. 6: 974. https://doi.org/10.3390/nano13060974
APA StyleFaizan, M., Karabulut, F., Alam, P., Yusuf, M., Tonny, S. H., Adil, M. F., Sehar, S., Ahmed, S. M., & Hayat, S. (2023). Nanobionics: A Sustainable Agricultural Approach towards Understanding Plant Response to Heavy Metals, Drought, and Salt Stress. Nanomaterials, 13(6), 974. https://doi.org/10.3390/nano13060974











