Single-Walled vs. Multi-Walled Carbon Nanotubes: Influence of Physico-Chemical Properties on Toxicogenomics Responses in Mouse Lungs
Abstract
1. Introduction
2. Materials and Methods
2.1. Physico-Chemical Properties of CNTs
2.2. Material Dispersion
2.3. Animal Exposure and Sample Collection
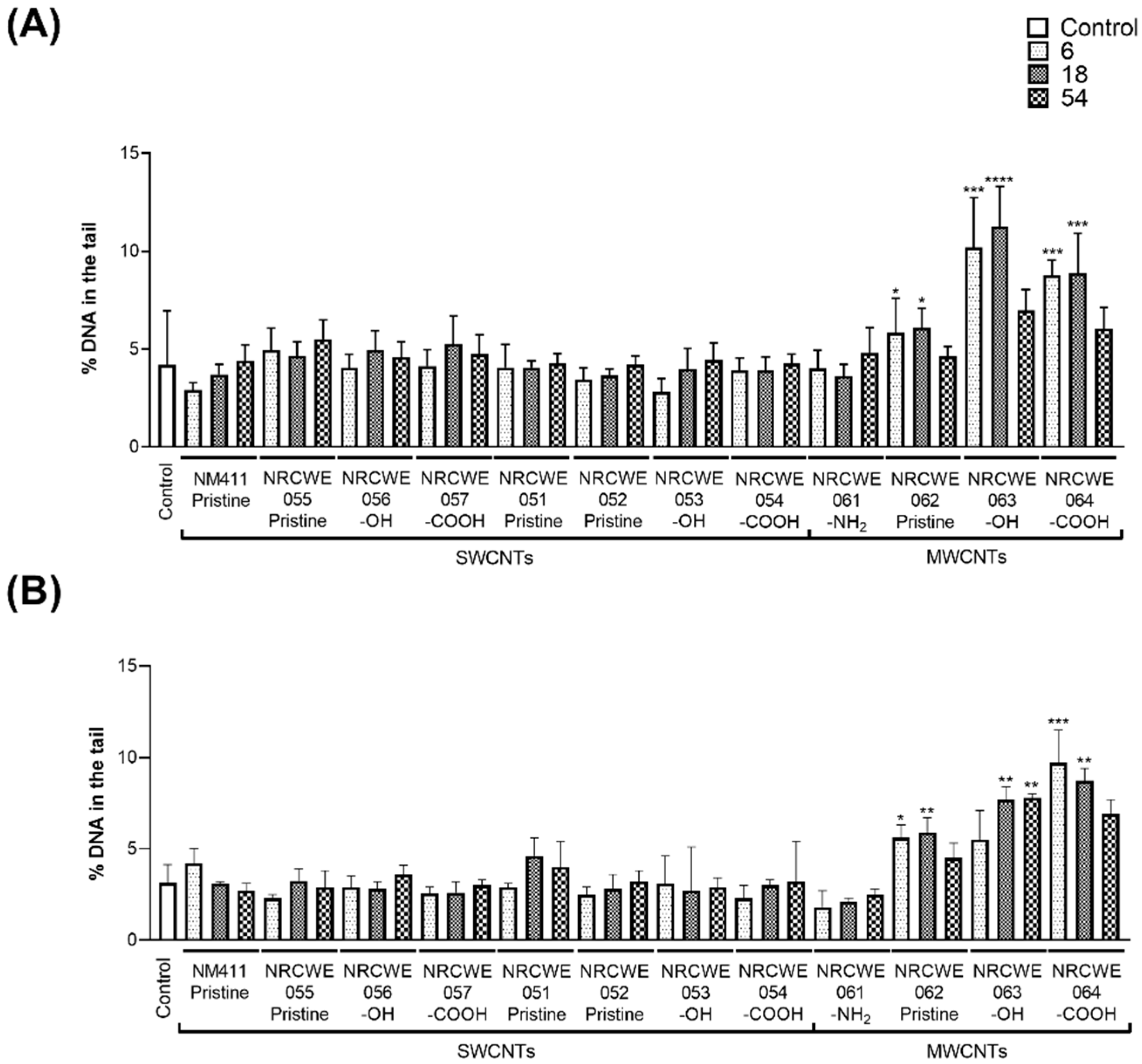
2.4. Comet Assay
2.5. BALF Neutrophil Influx
2.6. Total RNA Extraction
2.7. Microarrays
2.8. Statistical Analysis of Microarray Data
2.9. Gene Ontology (GO) and Pathway Analysis of Differentially Expressed Genes (DEGs)
2.10. WGCNA and GSEA
2.10.1. WGCNA
2.10.2. GSEA
2.11. Pro-Fibrotic Transcriptomic Signature
2.12. Potency Ranking of CNTs
3. Results
3.1. DNA Strand Breaks by Comet Assay
3.2. BALF Neutrophil Influx
3.3. Gene Expression Analysis
3.4. Canonical Pathways Analysis
3.5. WGCNA Dynamic Modules and GSEA
3.6. Pro-Fibrotic Transcriptomic Signature
3.7. Potency Ranking of CNTs
4. Discussion
4.1. Genotoxic Effects of CNTs
4.2. Pro-Inflammatory Effects of CNTs
4.3. Pro-Fibrotic Effects of CNTs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Connell, M.J. Carbon Nanotubes Properties and Applications; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Saleemi, M.A.; Hosseini Fouladi, M.; Yong, P.V.C.; Chinna, K.; Palanisamy, N.K.; Wong, E.H. Toxicity of Carbon Nanotubes: Molecular Mechanisms, Signaling Cascades, and Remedies in Biomedical Applications. Chem. Res. Toxicol. 2021, 34, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Pitroda, D.J.; Jethwa, B.; Dave, D. A critical review on carbon nanotubes. Int. J. Constr. Res. Civ. Eng. 2016, 2, 36–42. [Google Scholar] [CrossRef]
- National Institute for Occupational Safety & Health. Current Intelligence Bulletin 65: Occupational Exposure to Carbon Nanotubes and Nanofibers; National Institute for Occupational Safety & Health: Washington, DC, USA, 2013.
- Gupta, S.S.; Singh, K.P.; Gupta, S.; Dusinska, M.; Rahman, Q. Do Carbon Nanotubes and Asbestos Fibers Exhibit Common Toxicity Mechanisms? Nanomaterials 2022, 12, 1708. [Google Scholar] [CrossRef] [PubMed]
- Pauluhn, J. Subchronic 13-week inhalation exposure of rats to multiwalled carbon nanotubes: Toxic effects are determined by density of agglomerate structures, not fibrillar structures. Toxicol. Sci. 2010, 113, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Kasai, T.; Umeda, Y.; Ohnishi, M.; Mine, T.; Kondo, H.; Takeuchi, T.; Matsumoto, M.; Fukushima, S. Lung carcinogenicity of inhaled multi-walled carbon nanotube in rats. Part. Fibre Toxicol. 2016, 13, 53. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the evaluation of carcinogenic risks to humans. In Some Nanomaterials and Some Fibres; International Agency for Research on Cancer: Lyon, France, 2017; ISBN 978-92-832-0177-9. [Google Scholar]
- Rahman, L.; Jacobsen, N.R.; Aziz, S.A.; Wu, D.; Williams, A.; Yauk, C.L.; White, P.; Wallin, H.; Vogel, U.; Halappanavar, S. Multi-walled carbon nanotube-induced genotoxic, inflammatory and pro-fibrotic responses in mice: Investigating the mechanisms of pulmonary carcinogenesis. Mutat. Res. Genet. Toxicol. Environ. Mutagen 2017, 823, 28–44. [Google Scholar] [CrossRef]
- Poulsen, S.S.; Saber, A.T.; Williams, A.; Andersen, O.; Købler, C.; Atluri, R.; Pozzebon, M.E.; Mucelli, S.P.; Simion, M.; Rickerby, D.; et al. MWCNTs of different physicochemical properties cause similar inflammatory responses, but differences in transcriptional and histological markers of fibrosis in mouse lungs. Toxicol. Appl. Pharmacol. 2015, 284, 16–32. [Google Scholar] [CrossRef]
- Fujita, K.; Fukuda, M.; Endoh, S.; Maru, J.; Kato, H.; Nakamura, A.; Shinohara, N.; Uchino, K.; Honda, K. Pulmonary and pleural inflammation after intratracheal instillation of short single-walled and multi-walled carbon nanotubes. Toxicol. Lett. 2016, 257, 23–37. [Google Scholar] [CrossRef]
- Poulsen, S.S.; Jackson, P.; Kling, K.; Knudsen, K.B.; Skaug, V.; Kyjovska, Z.O.; Thomsen, B.L.; Clausen, P.A.; Atluri, R.; Berthing, T.; et al. Multi-walled carbon nanotube physicochemical properties predict pulmonary inflammation and genotoxicity. Nanotoxicology 2016, 10, 1263–1275. [Google Scholar] [CrossRef]
- Sager, T.M.; Wolfarth, M.W.; Andrew, M.; Hubbs, A.; Friend, S.; Chen, T.H.; Porter, D.W.; Wu, N.; Yang, F.; Hamilton, R.F.; et al. Effect of multi-walled carbon nanotube surface modification on bioactivity in the C57BL/6 mouse model. Nanotoxicology 2014, 8, 317–327. [Google Scholar] [CrossRef]
- Rahman, L.; Williams, A.; Gelda, K.; Nikota, J.; Wu, D.; Vogel, U.; Halappanavar, S. 21st Century Tools for Nanotoxicology: Transcriptomic Biomarker Panel and Precision-Cut Lung Slice Organ Mimic System for the Assessment of Nanomaterial-Induced Lung Fibrosis. Small 2020, 16, e2000272. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, X.; Ji, Z.; Sun, B.; Zhang, H.; Chang, C.H.; Lin, S.; Meng, H.; Liao, Y.P.; Wang, M.; et al. Surface charge and cellular processing of covalently functionalized multiwall carbon nanotubes determine pulmonary toxicity. ACS Nano 2013, 7, 2352–2368. [Google Scholar] [CrossRef] [PubMed]
- Wils, R.S.; Jacobsen, N.R.; Di Ianni, E.; Roursgaard, M.; Møller, P. Reactive oxygen species production, genotoxicity and telomere length in FE1-Muta™Mouse lung epithelial cells exposed to carbon nanotubes. Nanotoxicology 2021, 15, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Bengtson, S.; Jacobsen, N.R.; Jackson, P.; Nocun, M.; Saber, A.T.; Jensen, K.A.; Wallin, H.; Vogel, U. Influence of dispersion medium on nanomaterial-induced pulmonary inflammation and DNA strand breaks: Investigation of carbon black, carbon nanotubes and three titanium dioxide nanoparticles. Mutagenesis 2017, 32, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.; Pedersen, L.M.; Kyjovska, Z.O.; Jacobsen, N.R.; Saber, A.T.; Hougaard, K.S.; Vogel, U.; Wallin, H. Validation of freezing tissues and cells for analysis of DNA strand break levels by comet assay. Mutagenesis 2013, 28, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Kerr, M.K.; Churchill, G.A. Statistical design and the analysis of gene expression microarray data. Genet. Res. 2001, 77, 123–128. [Google Scholar] [CrossRef]
- Wu, H.; Kerr, M.K.; Cui, X.; Churchill, G.A. MAANOVA: A Software Package for the Analysis of Spotted cDNA Microarray Experiments. In The Analysis of Gene Expression Data: Methods and Software; Parmigiani, G., Garrett, E.S., Irizarry, R.A., Zeger, S.L., Eds.; Springer: New York, NY, USA, 2003; pp. 313–341. [Google Scholar]
- Cui, X.; Hwang, J.T.; Qiu, J.; Blades, N.J.; Churchill, G.A. Improved statistical tests for differential gene expression by shrinking variance components estimates. Biostatistics 2005, 6, 59–75. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Searle, S.R.; Speed, F.M.; Milliken, G.A. Population Marginal Means in the Linear Model: An Alternative to Least Squares Means. Am. Stat. 1980, 34, 216–221. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Labib, S.; Williams, A.; Kuo, B.; Yauk, C.L.; White, P.A.; Halappanavar, S. A framework for the use of single-chemical transcriptomics data in predicting the hazards associated with complex mixtures of polycyclic aromatic hydrocarbons. Arch. Toxicol. 2017, 91, 2599–2616. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, T.; Misra, V.; Zhen, L.; Tankersley, C.G.; Tuder, R.M.; Biswal, S. Cigarette smoke-induced emphysema in A/J mice is associated with pulmonary oxidative stress, apoptosis of lung cells, and global alterations in gene expression. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 296, L888–L900. [Google Scholar] [CrossRef] [PubMed]
- John-Schuster, G.; Hager, K.; Conlon, T.M.; Irmler, M.; Beckers, J.; Eickelberg, O.; Yildirim, A. Cigarette smoke-induced iBALT mediates macrophage activation in a B cell-dependent manner in COPD. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 307, L692–L706. [Google Scholar] [CrossRef] [PubMed]
- Zeidler-Erdely, P.C.; Kashon, M.L.; Li, S.; Antonini, J.M. Response of the mouse lung transcriptome to welding fume: Effects of stainless and mild steel fumes on lung gene expression in A/J and C57BL/6J mice. Respir. Res. 2010, 11, 70. [Google Scholar] [CrossRef]
- Miller, M.A.; Danhorn, T.; Cruickshank-Quinn, C.I.; Leach, S.M.; Jacobson, S.; Strand, M.J.; Reisdorph, N.A.; Bowler, R.P.; Petrache, I.; Kechris, K. Gene and metabolite time-course response to cigarette smoking in mouse lung and plasma. PLoS ONE 2017, 12, e0178281. [Google Scholar] [CrossRef]
- Gebel, S.; Diehl, S.; Pype, J.; Friedrichs, B.; Weiler, H.; Schüller, J.; Xu, H.; Taguchi, K.; Yamamoto, M.; Müller, T. The transcriptome of Nrf2-/- mice provides evidence for impaired cell cycle progression in the development of cigarette smoke-induced emphysematous changes. Toxicol. Sci. 2010, 115, 238–252. [Google Scholar] [CrossRef]
- Morissette, M.C.; Lamontagne, M.; Bérubé, J.C.; Gaschler, G.; Williams, A.; Yauk, C.; Couture, C.; Laviolette, M.; Hogg, J.C.; Timens, W.; et al. Impact of cigarette smoke on the human and mouse lungs: A gene-expression comparison study. PLoS ONE 2014, 9, e92498. [Google Scholar] [CrossRef]
- Ansari, S.; Baumer, K.; Boué, S.; Dijon, S.; Dulize, R.; Ekroos, K.; Elamin, A.; Foong, C.; Guedj, E.; Hoeng, J.; et al. Comprehensive systems biology analysis of a 7-month cigarette smoke inhalation study in C57BL/6 mice. Sci. Data 2016, 3, 150077. [Google Scholar] [CrossRef]
- Stevens, T.; Hester, S.; Gilmour, M.I. Differential Transcriptional Changes in Mice Exposed to Chemically Distinct Diesel Samples. Biomed Inf. Insights 2010, 3, 29–52. [Google Scholar] [CrossRef]
- Stevens, T.; Krantz, Q.T.; Linak, W.P.; Hester, S.; Gilmour, M.I. Increased transcription of immune and metabolic pathways in naive and allergic mice exposed to diesel exhaust. Toxicol. Sci. 2008, 102, 359–370. [Google Scholar] [CrossRef]
- Ciencewicki, J.M.; Verhein, K.C.; Gerrish, K.; McCaw, Z.R.; Li, J.; Bushel, P.R.; Kleeberger, S.R. Effects of mannose-binding lectin on pulmonary gene expression and innate immune inflammatory response to ozone. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, L280–L291. [Google Scholar] [CrossRef] [PubMed]
- Backus, G.S.; Howden, R.; Fostel, J.; Bauer, A.K.; Cho, H.Y.; Marzec, J.; Peden, D.B.; Kleeberger, S.R. Protective role of interleukin-10 in ozone-induced pulmonary inflammation. Environ. Health Perspect. 2010, 118, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.K.; Rondini, E.A.; Hummel, K.A.; Degraff, L.M.; Walker, C.; Jedlicka, A.E.; Kleeberger, S.R. Identification of candidate genes downstream of TLR4 signaling after ozone exposure in mice: A role for heat-shock protein 70. Environ. Health Perspect. 2011, 119, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Jedlicka, A.E.; Chang, F.H.; Marzec, J.; Bauer, A.K.; Kleeberger, S.R. Transcriptomics Underlying Pulmonary Ozone Pathogenesis Regulated by Inflammatory Mediators in Mice. Antioxidants 2021, 10, 1489. [Google Scholar] [CrossRef]
- Rowan-Carroll, A.; Halappanavar, S.; Williams, A.; Somers, C.M.; Yauk, C.L. Mice exposed in situ to urban air pollution exhibit pulmonary alterations in gene expression in the lipid droplet synthesis pathways. Environ. Mol. Mutagen 2013, 54, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Moreno-Vinasco, L.; Huang, Y.; Lang, G.D.; Linares, J.D.; Goonewardena, S.N.; Grabavoy, A.; Samet, J.M.; Geyh, A.S.; Breysse, P.N.; et al. Murine lung responses to ambient particulate matter: Genomic analysis and influence on airway hyperresponsiveness. Environ. Health Perspect. 2008, 116, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lang, G.D.; Moreno-Vinasco, L.; Huang, Y.; Goonewardena, S.N.; Peng, Y.J.; Svensson, E.C.; Natarajan, V.; Lang, R.M.; Linares, J.D.; et al. Particulate matter induces cardiac arrhythmias via dysregulation of carotid body sensitivity and cardiac sodium channels. Am. J. Respir. Cell Mol. Biol. 2012, 46, 524–531. [Google Scholar] [CrossRef]
- Peng, R.; Sridhar, S.; Tyagi, G.; Phillips, J.E.; Garrido, R.; Harris, P.; Burns, L.; Renteria, L.; Woods, J.; Chen, L.; et al. Bleomycin induces molecular changes directly relevant to idiopathic pulmonary fibrosis: A model for “active” disease. PLoS ONE 2013, 8, e59348. [Google Scholar] [CrossRef]
- Brass, D.M.; Yang, I.V.; Kennedy, M.P.; Whitehead, G.S.; Rutledge, H.; Burch, L.H.; Schwartz, D.A. Fibroproliferation in LPS-induced airway remodeling and bleomycin-induced fibrosis share common patterns of gene expression. Immunogenetics 2008, 60, 353–369. [Google Scholar] [CrossRef]
- Decato, B.E.; Ammar, R.; Reinke-Breen, L.; Thompson, J.R.; Azzara, A.V. Transcriptome analysis reveals key genes modulated by ALK5 inhibition in a bleomycin model of systemic sclerosis. Rheumatology 2022, 61, 1717–1727. [Google Scholar] [CrossRef]
- Lino Cardenas, C.L.; Henaoui, I.S.; Courcot, E.; Roderburg, C.; Cauffiez, C.; Aubert, S.; Copin, M.C.; Wallaert, B.; Glowacki, F.; Dewaeles, E.; et al. miR-199a-5p Is upregulated during fibrogenic response to tissue injury and mediates TGFbeta-induced lung fibroblast activation by targeting caveolin-1. PLoS Genet. 2013, 9, e1003291. [Google Scholar] [CrossRef] [PubMed]
- Citrin, D.E.; Shankavaram, U.; Horton, J.A.; Shield, W., 3rd; Zhao, S.; Asano, H.; White, A.; Sowers, A.; Thetford, A.; Chung, E.J. Role of type II pneumocyte senescence in radiation-induced lung fibrosis. J. Natl. Cancer Inst. 2013, 105, 1474–1484. [Google Scholar] [CrossRef]
- Moritake, T.; Fujita, H.; Yanagisawa, M.; Nakawatari, M.; Imadome, K.; Nakamura, E.; Iwakawa, M.; Imai, T. Strain-dependent damage in mouse lung after carbon ion irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e95–e102. [Google Scholar] [CrossRef] [PubMed]
- Beach, T.A.; Johnston, C.J.; Groves, A.M.; Williams, J.P.; Finkelstein, J.N. Radiation induced pulmonary fibrosis as a model of progressive fibrosis: Contributions of DNA damage, inflammatory response and cellular senescence genes. Exp. Lung Res. 2017, 43, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Jackson, I.L.; Baye, F.; Goswami, C.P.; Katz, B.P.; Zodda, A.; Pavlovic, R.; Gurung, G.; Winans, D.; Vujaskovic, Z. Gene expression profiles among murine strains segregate with distinct differences in the progression of radiation-induced lung disease. Dis. Model. Mech. 2017, 10, 425–437. [Google Scholar] [CrossRef]
- Britto, C.J.; Niu, N.; Khanal, S.; Huleihel, L.; Herazo-Maya, J.D.; Thompson, A.; Sauler, M.; Slade, M.D.; Sharma, L.; Dela Cruz, C.S.; et al. BPIFA1 regulates lung neutrophil recruitment and interferon signaling during acute inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L321–L333. [Google Scholar] [CrossRef]
- Yang, I.V.; Alper, S.; Lackford, B.; Rutledge, H.; Warg, L.A.; Burch, L.H.; Schwartz, D.A. Novel regulators of the systemic response to lipopolysaccharide. Am. J. Respir. Cell Mol. Biol. 2011, 45, 393–402. [Google Scholar] [CrossRef]
- Walters, K.A.; Olsufka, R.; Kuestner, R.E.; Wu, X.; Wang, K.; Skerrett, S.J.; Ozinsky, A. Prior infection with Type A Francisella tularensis antagonizes the pulmonary transcriptional response to an aerosolized Toll-like receptor 4 agonist. BMC Genom. 2015, 16, 874. [Google Scholar] [CrossRef]
- Reece, J.J.; Siracusa, M.C.; Scott, A.L. Innate immune responses to lung-stage helminth infection induce alternatively activated alveolar macrophages. Infect Immun. 2006, 74, 4970–4981. [Google Scholar] [CrossRef]
- Reece, J.J.; Siracusa, M.C.; Southard, T.L.; Brayton, C.F.; Urban, J.F., Jr.; Scott, A.L. Hookworm-induced persistent changes to the immunological environment of the lung. Infect. Immun. 2008, 76, 3511–3524. [Google Scholar] [CrossRef]
- Savin, I.A.; Markov, A.V.; Zenkova, M.A.; Sen’kova, A.V. Asthma and Post-Asthmatic Fibrosis: A Search for New Promising Molecular Markers of Transition from Acute Inflammation to Pulmonary Fibrosis. Biomedicines 2022, 10, 1017. [Google Scholar] [CrossRef] [PubMed]
- Ilves, M.; Kinaret, P.A.S.; Ndika, J.; Karisola, P.; Marwah, V.; Fortino, V.; Fedutik, Y.; Correia, M.; Ehrlich, N.; Loeschner, K.; et al. Surface PEGylation suppresses pulmonary effects of CuO in allergen-induced lung inflammation. Part. Fibre Toxicol. 2019, 16, 28. [Google Scholar] [CrossRef]
- Rahman, L.; Wu, D.; Johnston, M.; William, A.; Halappanavar, S. Toxicogenomics analysis of mouse lung responses following exposure to titanium dioxide nanomaterials reveal their disease potential at high doses. Mutagenesis 2017, 32, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Halappanavar, S.; Rahman, L.; Nikota, J.; Poulsen, S.S.; Ding, Y.; Jackson, P.; Wallin, H.; Schmid, O.; Vogel, U.; Williams, A. Ranking of nanomaterial potency to induce pathway perturbations associated with lung responses. NanoImpact 2019, 14, 100158. [Google Scholar] [CrossRef]
- Søs Poulsen, S.; Jacobsen, N.R.; Labib, S.; Wu, D.; Husain, M.; Williams, A.; Bøgelund, J.P.; Andersen, O.; Købler, C.; Mølhave, K.; et al. Transcriptomic analysis reveals novel mechanistic insight into murine biological responses to multi-walled carbon nanotubes in lungs and cultured lung epithelial cells. PLoS ONE 2013, 8, e80452. [Google Scholar] [CrossRef] [PubMed]
- Rydman, E.M.; Ilves, M.; Koivisto, A.J.; Kinaret, P.A.; Fortino, V.; Savinko, T.S.; Lehto, M.T.; Pulkkinen, V.; Vippola, M.; Hämeri, K.J.; et al. Inhalation of rod-like carbon nanotubes causes unconventional allergic airway inflammation. Part. Fibre Toxicol. 2014, 11, 48. [Google Scholar] [CrossRef]
- Guo, N.L.; Wan, Y.W.; Denvir, J.; Porter, D.W.; Pacurari, M.; Wolfarth, M.G.; Castranova, V.; Qian, Y. Multiwalled carbon nanotube-induced gene signatures in the mouse lung: Potential predictive value for human lung cancer risk and prognosis. J. Toxicol. Environ. Health A 2012, 75, 1129–1153. [Google Scholar] [CrossRef]
- Bourdon, J.A.; Saber, A.T.; Jacobsen, N.R.; Williams, A.; Vogel, U.; Wallin, H.; Halappanavar, S.; Yauk, C.L. Carbon black nanoparticle intratracheal instillation does not alter cardiac gene expression. Cardiovasc. Toxicol. 2013, 13, 406–412. [Google Scholar] [CrossRef]
- Husain, M.; Kyjovska, Z.O.; Bourdon-Lacombe, J.; Saber, A.T.; Jensen, K.A.; Jacobsen, N.R.; Williams, A.; Wallin, H.; Halappanavar, S.; Vogel, U.; et al. Carbon black nanoparticles induce biphasic gene expression changes associated with inflammatory responses in the lungs of C57BL/6 mice following a single intratracheal instillation. Toxicol. Appl. Pharmacol. 2015, 289, 573–588. [Google Scholar] [CrossRef]
- Jackson, P.; Hougaard, K.S.; Vogel, U.; Wu, D.; Casavant, L.; Williams, A.; Wade, M.; Yauk, C.L.; Wallin, H.; Halappanavar, S. Exposure of pregnant mice to carbon black by intratracheal instillation: Toxicogenomic effects in dams and offspring. Mutat. Res. 2012, 745, 73–83. [Google Scholar] [CrossRef]
- Yousefi, S.; Sharma, S.K.; Stojkov, D.; Germic, N.; Aeschlimann, S.; Ge, M.Q.; Flayer, C.H.; Larson, E.D.; Redai, I.G.; Zhang, S.; et al. Oxidative damage of SP-D abolishes control of eosinophil extracellular DNA trap formation. J. Leukoc. Biol. 2018, 104, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, J.J.; Jolly, R.A.; Goldstein, K.M.; Stevens, J.L. Assessing Concordance of Drug-Induced Transcriptional Response in Rodent Liver and Cultured Hepatocytes. PLoS Comput. Biol. 2016, 12, e1004847. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Halappanavar, S. Application of biclustering of gene expression data and gene set enrichment analysis methods to identify potentially disease causing nanomaterials. Beilstein J. Nanotechnol. 2015, 6, 2438–2448. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Halappanavar, S. Application of bi-clustering of gene expression data and gene set enrichment analysis methods to identify potentially disease causing nanomaterials. Data Brief 2017, 15, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Korotkevich, G.; Sukhov, V.; Sergushichev, A.A. Fast gene set enrichment analysis. bioRxiv 2019. [Google Scholar] [CrossRef]
- Lewis, C.C.; Yang, J.Y.; Huang, X.; Banerjee, S.K.; Blackburn, M.R.; Baluk, P.; McDonald, D.M.; Blackwell, T.S.; Nagabhushanam, V.; Peters, W.; et al. Disease-specific gene expression profiling in multiple models of lung disease. Am. J. Respir. Crit. Care Med. 2008, 177, 376–387. [Google Scholar] [CrossRef]
- Thomson, E.M.; Williams, A.; Yauk, C.L.; Vincent, R. Overexpression of tumor necrosis factor-α in the lungs alters immune response, matrix remodeling, and repair and maintenance pathways. Am. J. Pathol. 2012, 180, 1413–1430. [Google Scholar] [CrossRef]
- Schaffer, B.E.; Park, K.S.; Yiu, G.; Conklin, J.F.; Lin, C.; Burkhart, D.L.; Karnezis, A.N.; Sweet-Cordero, E.A.; Sage, J. Loss of p130 accelerates tumor development in a mouse model for human small-cell lung carcinoma. Cancer Res. 2010, 70, 3877–3883. [Google Scholar] [CrossRef]
- Ochoa, C.E.; Mirabolfathinejad, S.G.; Ruiz, V.A.; Evans, S.E.; Gagea, M.; Evans, C.M.; Dickey, B.F.; Moghaddam, S.J. Interleukin 6, but not T helper 2 cytokines, promotes lung carcinogenesis. Cancer Prev. Res. 2011, 4, 51–64. [Google Scholar] [CrossRef]
- Lu, X.; Jain, V.V.; Finn, P.W.; Perkins, D.L. Hubs in biological interaction networks exhibit low changes in expression in experimental asthma. Mol. Syst. Biol. 2007, 3, 98. [Google Scholar] [CrossRef]
- Pandiri, A.R.; Sills, R.C.; Ziglioli, V.; Ton, T.V.; Hong, H.H.; Lahousse, S.A.; Gerrish, K.E.; Auerbach, S.S.; Shockley, K.R.; Bushel, P.R.; et al. Differential transcriptomic analysis of spontaneous lung tumors in B6C3F1 mice: Comparison to human non-small cell lung cancer. Toxicol. Pathol. 2012, 40, 1141–1159. [Google Scholar] [CrossRef] [PubMed]
- Delgado, O.; Batten, K.G.; Richardson, J.A.; Xie, X.J.; Gazdar, A.F.; Kaisani, A.A.; Girard, L.; Behrens, C.; Suraokar, M.; Fasciani, G.; et al. Radiation-enhanced lung cancer progression in a transgenic mouse model of lung cancer is predictive of outcomes in human lung and breast cancer. Clin. Cancer Res. 2014, 20, 1610–1622. [Google Scholar] [CrossRef]
- Thomas, R.S.; Pluta, L.; Yang, L.; Halsey, T.A. Application of genomic biomarkers to predict increased lung tumor incidence in 2-year rodent cancer bioassays. Toxicol. Sci. 2007, 97, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Baek, H.A.; Yu, H.; Lee, H.J.; Park, B.H.; Ullenbruch, M.; Liu, J.; Nakashima, T.; Choi, Y.Y.; Wu, G.D.; et al. FIZZ2/RELM-β induction and role in pulmonary fibrosis. J. Immunol. 2011, 187, 450–461. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. NTP Research Reports. In NTP Research Report on National Toxicology Program Approach to Genomic Dose-Response Modeling: Research Report 5; National Toxicology Program: Durham, NC, USA, 2018. [Google Scholar]
- Reardon, A.J.F.; Rowan-Carroll, A.; Ferguson, S.S.; Leingartner, K.; Gagne, R.; Kuo, B.; Williams, A.; Lorusso, L.; Bourdon-Lacombe, J.A.; Carrier, R.; et al. Potency Ranking of Per- and Polyfluoroalkyl Substances Using High-Throughput Transcriptomic Analysis of Human Liver Spheroids. Toxicol. Sci. 2021, 184, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Crizer, D.M.; Ramaiahgari, S.C.; Ferguson, S.S.; Rice, J.R.; Dunlap, P.E.; Sipes, N.S.; Auerbach, S.S.; Merrick, B.A.; DeVito, M.J. Benchmark Concentrations for Untargeted Metabolomics versus Transcriptomics for Liver Injury Compounds in In Vitro Liver Models. Toxicol. Sci. 2021, 181, 175–186. [Google Scholar] [CrossRef]
- Shvedova, A.A.; Yanamala, N.; Kisin, E.R.; Khailullin, T.O.; Birch, M.E.; Fatkhutdinova, L.M. Integrated Analysis of Dysregulated ncRNA and mRNA Expression Profiles in Humans Exposed to Carbon Nanotubes. PLoS ONE 2016, 11, e0150628. [Google Scholar] [CrossRef]
- Di Ianni, E.; Erdem, J.S.; Møller, P.; Sahlgren, N.M.; Poulsen, S.S.; Knudsen, K.B.; Zienolddiny, S.; Saber, A.T.; Wallin, H.; Vogel, U.; et al. In vitro-in vivo correlations of pulmonary inflammogenicity and genotoxicity of MWCNT. Part. Fibre Toxicol. 2021, 18, 25. [Google Scholar] [CrossRef]
- Saleh, D.M.; Luo, S.; Ahmed, O.H.M.; Alexander, D.B.; Alexander, W.T.; Gunasekaran, S.; El-Gazzar, A.M.; Abdelgied, M.; Numano, T.; Takase, H.; et al. Assessment of the toxicity and carcinogenicity of double-walled carbon nanotubes in the rat lung after intratracheal instillation: A two-year study. Part. Fibre Toxicol. 2022, 19, 30. [Google Scholar] [CrossRef]
- Saleh, D.M.; Alexander, W.T.; Numano, T.; Ahmed, O.H.M.; Gunasekaran, S.; Alexander, D.B.; Abdelgied, M.; El-Gazzar, A.M.; Takase, H.; Xu, J.; et al. Comparative carcinogenicity study of a thick, straight-type and a thin, tangled-type multi-walled carbon nanotube administered by intra-tracheal instillation in the rat. Part. Fibre Toxicol. 2020, 17, 48. [Google Scholar] [CrossRef]
- Numano, T.; Higuchi, H.; Alexander, D.B.; Alexander, W.T.; Abdelgied, M.; El-Gazzar, A.M.; Saleh, D.; Takase, H.; Hirose, A.; Naiki-Ito, A.; et al. MWCNT-7 administered to the lung by intratracheal instillation induces development of pleural mesothelioma in F344 rats. Cancer Sci. 2019, 110, 2485–2492. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Naya, M.; Takehara, H.; Kataura, H.; Fujita, K.; Ema, M. A 104-week pulmonary toxicity assessment of long and short single-wall carbon nanotubes after a single intratracheal instillation in rats. Inhal. Toxicol. 2017, 29, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Naya, M.; Kobayashi, N.; Endoh, S.; Maru, J.; Honda, K.; Ema, M.; Tanaka, J.; Fukumuro, M.; Hasegawa, K.; Nakajima, M.; et al. In vivo genotoxicity study of single-wall carbon nanotubes using comet assay following intratracheal instillation in rats. Regul. Toxicol. Pharmacol. 2012, 64, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, N.R.; Møller, P.; Jensen, K.A.; Vogel, U.; Ladefoged, O.; Loft, S.; Wallin, H. Lung inflammation and genotoxicity following pulmonary exposure to nanoparticles in ApoE-/- mice. Part. Fibre Toxicol. 2009, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Tabei, Y.; Sugino, S.; Fukui, H.; Nishioka, A.; Hagiwara, Y.; Sato, K.; Yoneda, T.; Tada, A.; Koyama, T. Comparison of the effects of multiwall carbon nanotubes on the epithelial cells and macrophages. Nanotoxicology 2019, 13, 861–878. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Spellberg, B.; Edwards, J.E., Jr. Type 1/Type 2 immunity in infectious diseases. Clin. Infect. Dis. 2001, 32, 76–102. [Google Scholar] [CrossRef]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, S. T Cells in Fibrosis and Fibrotic Diseases. Front. Immunol. 2020, 11, 1142. [Google Scholar] [CrossRef]
- Dong, J.; Ma, Q. Type 2 Immune Mechanisms in Carbon Nanotube-Induced Lung Fibrosis. Front. Immunol. 2018, 9, 1120. [Google Scholar] [CrossRef]
- Dong, J.; Ma, Q. In vivo activation of a T helper 2-driven innate immune response in lung fibrosis induced by multi-walled carbon nanotubes. Arch. Toxicol. 2016, 90, 2231–2248. [Google Scholar] [CrossRef] [PubMed]
- Nikota, J.; Banville, A.; Goodwin, L.R.; Wu, D.; Williams, A.; Yauk, C.L.; Wallin, H.; Vogel, U.; Halappanavar, S. Stat-6 signaling pathway and not Interleukin-1 mediates multi-walled carbon nanotube-induced lung fibrosis in mice: Insights from an adverse outcome pathway framework. Part. Fibre Toxicol. 2017, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Labib, S.; Williams, A.; Yauk, C.L.; Nikota, J.K.; Wallin, H.; Vogel, U.; Halappanavar, S. Nano-risk Science: Application of toxicogenomics in an adverse outcome pathway framework for risk assessment of multi-walled carbon nanotubes. Part. Fibre Toxicol. 2016, 13, 15. [Google Scholar] [CrossRef]
- Hadrup, N.; Zhernovkov, V.; Jacobsen, N.R.; Voss, C.; Strunz, M.; Ansari, M.; Schiller, H.B.; Halappanavar, S.; Poulsen, S.S.; Kholodenko, B.; et al. Acute Phase Response as a Biological Mechanism-of-Action of (Nano)particle-Induced Cardiovascular Disease. Small 2020, 16, e1907476. [Google Scholar] [CrossRef]
- Gruys, E.; Toussaint, M.J.; Niewold, T.A.; Koopmans, S.J. Acute phase reaction and acute phase proteins. J. Zhejiang Univ. Sci. B 2005, 6, 1045–1056. [Google Scholar] [CrossRef]
- Vietri, L.; Fui, A.; Bergantini, L.; d’Alessandro, M.; Cameli, P.; Sestini, P.; Rottoli, P.; Bargagli, E. Serum amyloid A: A potential biomarker of lung disorders. Respir. Investig. 2020, 58, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Saber, A.T.; Jacobsen, N.R.; Jackson, P.; Poulsen, S.S.; Kyjovska, Z.O.; Halappanavar, S.; Yauk, C.L.; Wallin, H.; Vogel, U. Particle-induced pulmonary acute phase response may be the causal link between particle inhalation and cardiovascular disease. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 517–531. [Google Scholar] [CrossRef]
- Poulsen, S.S.; Knudsen, K.B.; Jackson, P.; Weydahl, I.E.; Saber, A.T.; Wallin, H.; Vogel, U. Multi-walled carbon nanotube-physicochemical properties predict the systemic acute phase response following pulmonary exposure in mice. PLoS ONE 2017, 12, e0174167. [Google Scholar] [CrossRef]
- Saber, A.T.; Lamson, J.S.; Jacobsen, N.R.; Ravn-Haren, G.; Hougaard, K.S.; Nyendi, A.N.; Wahlberg, P.; Madsen, A.M.; Jackson, P.; Wallin, H.; et al. Particle-induced pulmonary acute phase response correlates with neutrophil influx linking inhaled particles and cardiovascular risk. PLoS ONE 2013, 8, e69020. [Google Scholar] [CrossRef]
- Husain, M.; Saber, A.T.; Guo, C.; Jacobsen, N.R.; Jensen, K.A.; Yauk, C.L.; Williams, A.; Vogel, U.; Wallin, H.; Halappanavar, S. Pulmonary instillation of low doses of titanium dioxide nanoparticles in mice leads to particle retention and gene expression changes in the absence of inflammation. Toxicol. Appl. Pharmacol. 2013, 269, 250–262. [Google Scholar] [CrossRef]
- Halappanavar, S.; Saber, A.T.; Decan, N.; Jensen, K.A.; Wu, D.; Jacobsen, N.R.; Guo, C.; Rogowski, J.; Koponen, I.K.; Levin, M.; et al. Transcriptional profiling identifies physicochemical properties of nanomaterials that are determinants of the in vivo pulmonary response. Environ. Mol. Mutagen. 2015, 56, 245–264. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Rahmani, F.; Jacobsen, N.R.; Saber, A.T.; Jackson, P.; Bengtson, S.; Williams, A.; Wallin, H.; Halappanavar, S.; Vogel, U. Acute phase response and inflammation following pulmonary exposure to low doses of zinc oxide nanoparticles in mice. Nanotoxicology 2019, 13, 1275–1292. [Google Scholar] [CrossRef] [PubMed]
- Halappanavar, S.; Jackson, P.; Williams, A.; Jensen, K.A.; Hougaard, K.S.; Vogel, U.; Yauk, C.L.; Wallin, H. Pulmonary response to surface-coated nanotitanium dioxide particles includes induction of acute phase response genes, inflammatory cascades, and changes in microRNAs: A toxicogenomic study. Environ. Mol. Mutagen. 2011, 52, 425–439. [Google Scholar] [CrossRef]
- Madison, M.C.; Landers, C.T.; Gu, B.H.; Chang, C.Y.; Tung, H.Y.; You, R.; Hong, M.J.; Baghaei, N.; Song, L.Z.; Porter, P.; et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J. Clin. Investig. 2019, 129, 4290–4304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, T.; Pan, M.; Wang, W.; Huang, W.; Yuan, Y.; Xie, Z.; Chen, Y.; Peng, J.; Li, X.; et al. SIRT1 prevents cigarette smoking-induced lung fibroblasts activation by regulating mitochondrial oxidative stress and lipid metabolism. J. Transl. Med. 2022, 20, 222. [Google Scholar] [CrossRef]
- Kononenko, V.; Erman, A.; Petan, T.; Križaj, I.; Kralj, S.; Makovec, D.; Drobne, D. Harmful at non-cytotoxic concentrations: SiO(2)-SPIONs affect surfactant metabolism and lamellar body biogenesis in A549 human alveolar epithelial cells. Nanotoxicology 2017, 11, 419–429. [Google Scholar] [CrossRef]
- Solorio-Rodríguez, A.; Escamilla-Rivera, V.; Uribe-Ramírez, M.; González-Pozos, S.; Hernández-Soto, J.; Rafael-Vázquez, L.; De Vizcaya-Ruiz, A. In vitro cytotoxicity study of superparamagnetic iron oxide and silica nanoparticles on pneumocyte organelles. Toxicol. In Vitro 2021, 72, 105071. [Google Scholar] [CrossRef]
- Lin, J.; Jiang, Y.; Luo, Y.; Guo, H.; Huang, C.; Peng, J.; Cao, Y. Multi-walled carbon nanotubes (MWCNTs) transformed THP-1 macrophages into foam cells: Impact of pulmonary surfactant component dipalmitoylphosphatidylcholine. J. Hazard. Mater. 2020, 392, 122286. [Google Scholar] [CrossRef]
- Agudelo, C.W.; Samaha, G.; Garcia-Arcos, I. Alveolar lipids in pulmonary disease. A review. Lipids Health Dis. 2020, 19, 122. [Google Scholar] [CrossRef]
- Sherratt, M.J.; Hopkinson, L.; Naven, M.; Hibbert, S.A.; Ozols, M.; Eckersley, A.; Newton, V.L.; Bell, M.; Meng, Q.J. Circadian rhythms in skin and other elastic tissues. Matrix. Biol. 2019, 84, 97–110. [Google Scholar] [CrossRef]
- Pekovic-Vaughan, V.; Gibbs, J.; Yoshitane, H.; Yang, N.; Pathiranage, D.; Guo, B.; Sagami, A.; Taguchi, K.; Bechtold, D.; Loudon, A.; et al. The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev. 2014, 28, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.E.; Beesley, S.; Plumb, J.; Singh, D.; Farrow, S.; Ray, D.W.; Loudon, A.S.I. Circadian timing in the lung; a specific role for bronchiolar epithelial cells. Endocrinology 2009, 150, 268–276. [Google Scholar] [CrossRef]
- Sundar, I.K.; Yao, H.; Sellix, M.T.; Rahman, I. Circadian clock-coupled lung cellular and molecular functions in chronic airway diseases. Am. J. Respir. Cell Mol. Biol. 2015, 53, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Halappanavar, S.; Sharma, M.; Wallin, H.; Vogel, U.; Sullivan, K.; Clippinger, A.J. AOP173: Substance Interaction with the Lung Resident Cell Membrane Components Leading to Lung Fibrosis. Available online: https://aopwiki.org/aops/173 (accessed on 31 July 2022).
- Marchioni, A.; Tonelli, R.; Cerri, S.; Castaniere, I.; Andrisani, D.; Gozzi, F.; Bruzzi, G.; Manicardi, L.; Moretti, A.; Demurtas, J.; et al. Pulmonary Stretch and Lung Mechanotransduction: Implications for Progression in the Fibrotic Lung. Int. J. Mol. Sci. 2021, 22, 6443. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Niu, Y.; Liang, K.; Du, Y. Mechanical communication in fibrosis progression. Trends Cell Biol. 2022, 32, 70–90. [Google Scholar] [CrossRef] [PubMed]
- Manke, A.; Luanpitpong, S.; Dong, C.; Wang, L.; He, X.; Battelli, L.; Derk, R.; Stueckle, T.A.; Porter, D.W.; Sager, T.; et al. Effect of fiber length on carbon nanotube-induced fibrogenesis. Int. J. Mol. Sci. 2014, 15, 7444–7461. [Google Scholar] [CrossRef]











| Group | Name | Length | Diameter | CNT Type | Carbon | BET | OH | Fe2O3 | CoO | NiO | MgO | MnO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (nm) | (nm) | (%) | (m2/g) | (mmol/g) | Content * | Content * | Content * | Content * | Content * | |||
| SWCNTs | NM-411 | 2 a | Pristine a | 93.1 | 861 | 0.89 | ||||||
| NRCWE-055 | 1–2 | Pristine | 91.9 | 453.1 | 1.55 | 4.39 | 1.33 | 0.04 | 0.029 | 0.027 | ||
| NRCWE-056 | 1–2 | -OH | 89.6 | 356.7 | 2.76 | 1.26 | 3.65 | 0.1 | 0.04 | 0.026 | ||
| NRCWE-057 | 1–2 | -COOH | 83.1 | 281.6 | 6.02 | 2.2 | 2.74 | 0.14 | 0.177 | 0.044 | ||
| NRCWE-051 | 1–2 | Pristine | 90.2 | 442.6 | 1.13 | 1.63 | 1.08 | 0.06 | 0.041 | - | ||
| NRCWE-052 | 1–2 | Pristine | 92.9 | 405.7 | 0.83 | 1.05 | 1.23 | 0.12 | 0.028 | 0.009 | ||
| NRCWE-053 | 1–2 | -OH | 88.2 | 367.8 | 1.85 | 0.85 | 3.82 | 0.1 | 0.021 | 0.023 | ||
| NRCWE-054 | 1–2 | -COOH | 87.9 | 370.8 | 3.03 | 1.59 | 3.81 | 0.13 | 0.027 | - | ||
| MWCNTs | NRCWE-061 | 730.85 | 16.42 | -NH2 | 96.5 | 170.4 | 0.42 | 0.59 | 0.001 | 1.97 | 0.01 | 0.005 |
| NRCWE-062 | 468 | 8.82 | Pristine | 89.1 | 443.2 | 2.59 | 0.58 | 4.6 | 0.22 | 0.074 | - | |
| NRCWE-063 | 345.35 | 14.18 | -OH | 88.2 | 426.4 | 2.66 | 1.95 | 5.87 | 0.49 | 0.072 | 0.023 | |
| NRCWE-064 | 213.6 | 7.46 | -COOH | 88.4 | 445.2 | 3.21 | 1.8 | 5.51 | 0.3 | 0.027 | 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solorio-Rodriguez, S.A.; Williams, A.; Poulsen, S.S.; Knudsen, K.B.; Jensen, K.A.; Clausen, P.A.; Danielsen, P.H.; Wallin, H.; Vogel, U.; Halappanavar, S. Single-Walled vs. Multi-Walled Carbon Nanotubes: Influence of Physico-Chemical Properties on Toxicogenomics Responses in Mouse Lungs. Nanomaterials 2023, 13, 1059. https://doi.org/10.3390/nano13061059
Solorio-Rodriguez SA, Williams A, Poulsen SS, Knudsen KB, Jensen KA, Clausen PA, Danielsen PH, Wallin H, Vogel U, Halappanavar S. Single-Walled vs. Multi-Walled Carbon Nanotubes: Influence of Physico-Chemical Properties on Toxicogenomics Responses in Mouse Lungs. Nanomaterials. 2023; 13(6):1059. https://doi.org/10.3390/nano13061059
Chicago/Turabian StyleSolorio-Rodriguez, Silvia Aidee, Andrew Williams, Sarah Søs Poulsen, Kristina Bram Knudsen, Keld Alstrup Jensen, Per Axel Clausen, Pernille Høgh Danielsen, Håkan Wallin, Ulla Vogel, and Sabina Halappanavar. 2023. "Single-Walled vs. Multi-Walled Carbon Nanotubes: Influence of Physico-Chemical Properties on Toxicogenomics Responses in Mouse Lungs" Nanomaterials 13, no. 6: 1059. https://doi.org/10.3390/nano13061059
APA StyleSolorio-Rodriguez, S. A., Williams, A., Poulsen, S. S., Knudsen, K. B., Jensen, K. A., Clausen, P. A., Danielsen, P. H., Wallin, H., Vogel, U., & Halappanavar, S. (2023). Single-Walled vs. Multi-Walled Carbon Nanotubes: Influence of Physico-Chemical Properties on Toxicogenomics Responses in Mouse Lungs. Nanomaterials, 13(6), 1059. https://doi.org/10.3390/nano13061059






