Reduced Graphene Oxide/Waste-Derived TiO2 Composite Membranes: Preliminary Study of a New Material for Hybrid Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
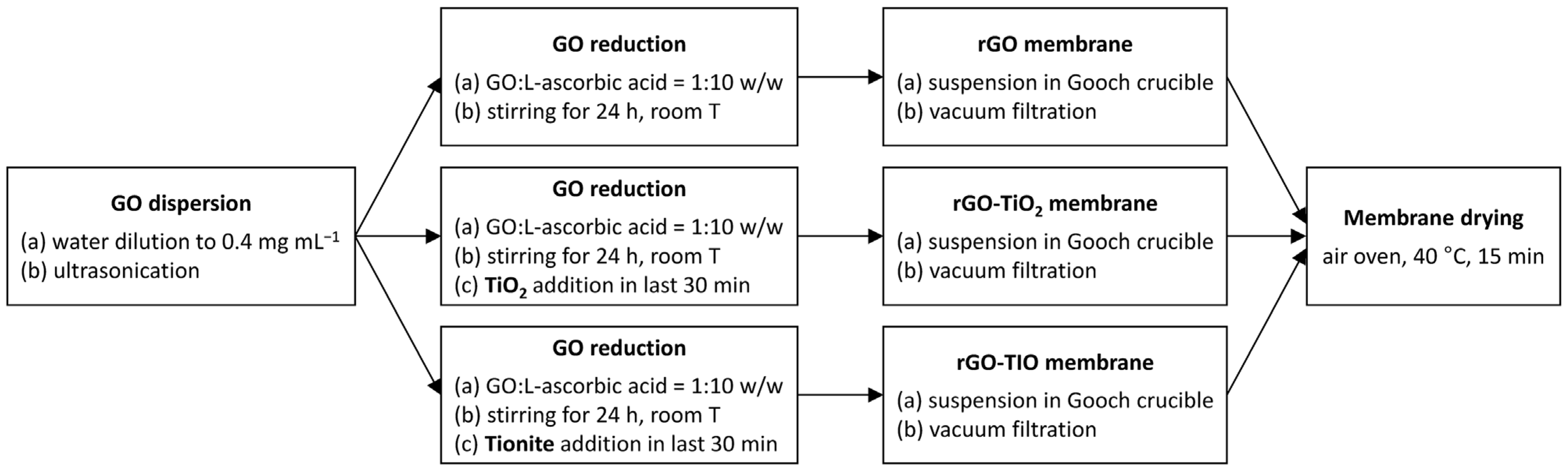
2.2. Membranes Preparation
2.3. Materials and Membranes Characterization
2.4. Adsorption Tests
2.5. Photocatalytic Activity
3. Results and Discussion
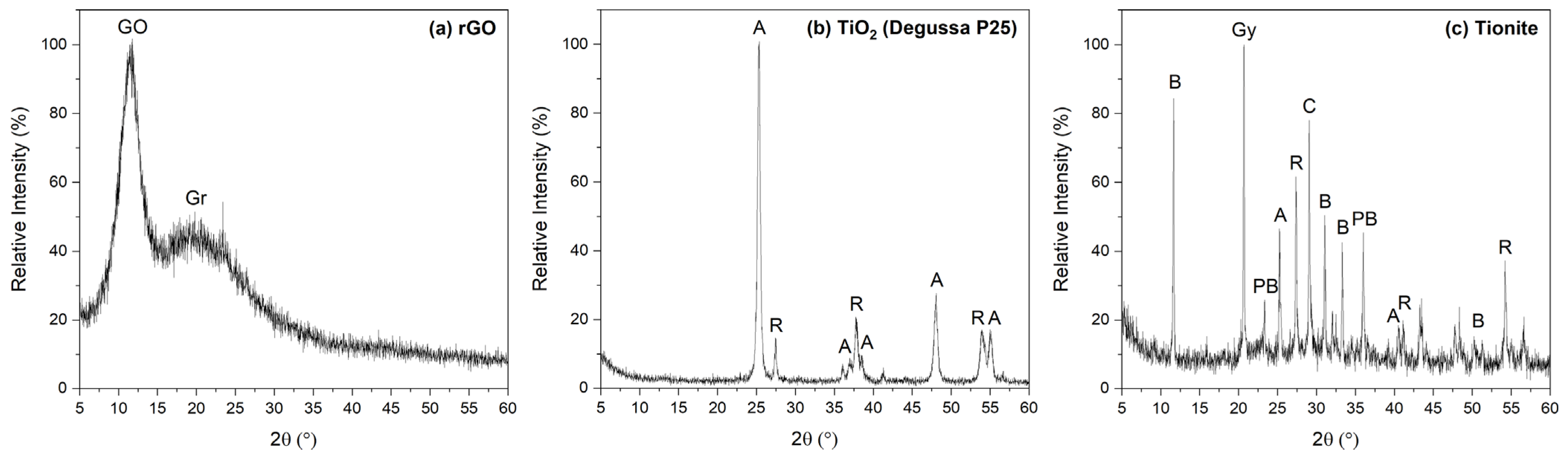
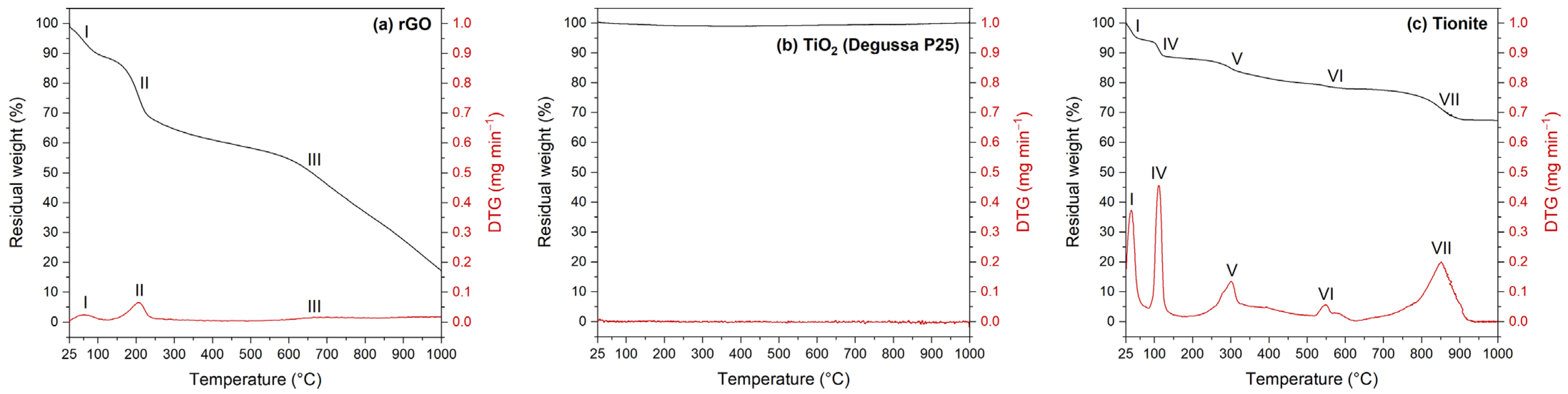
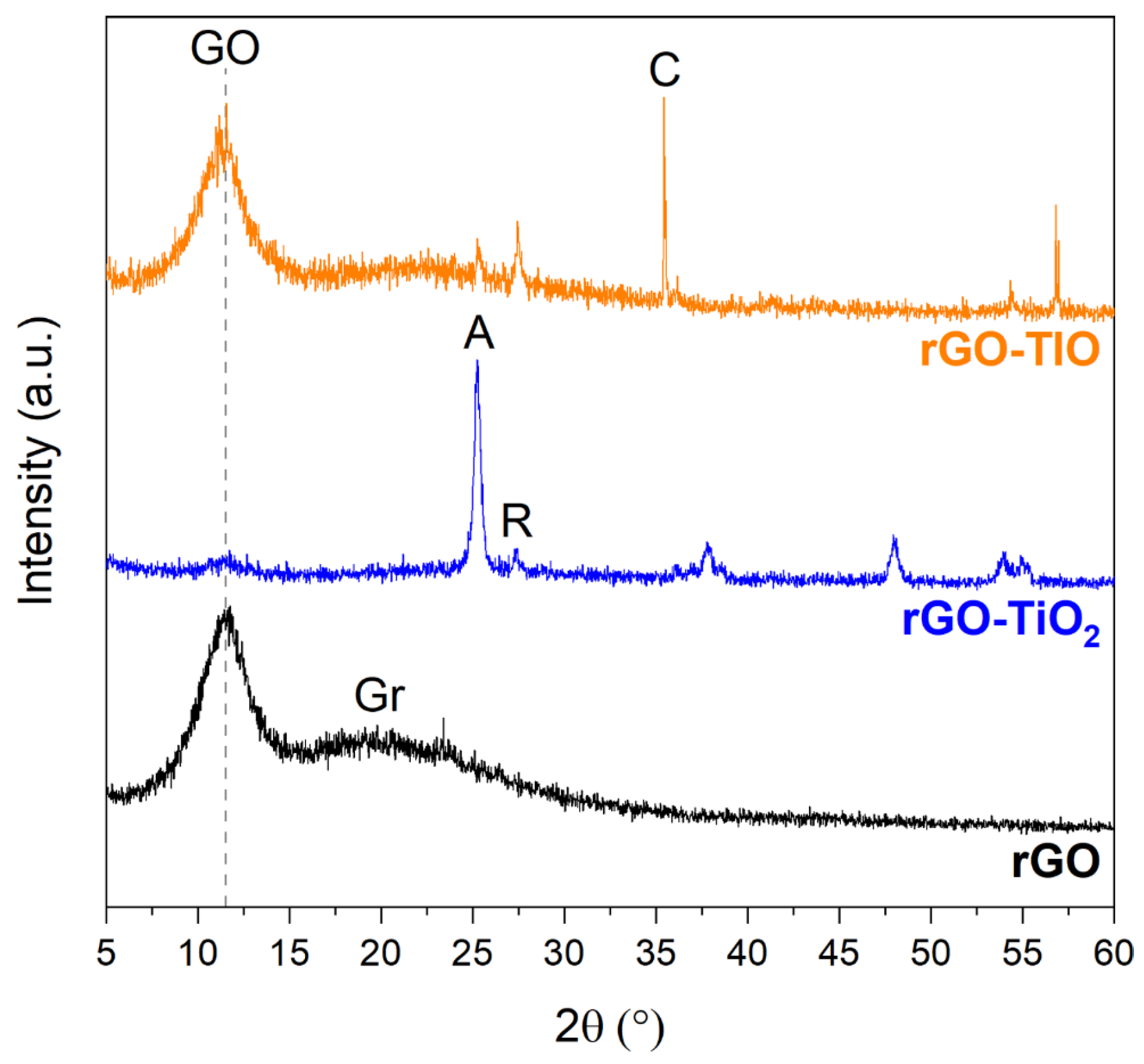
3.1. Starting Materials Characterization
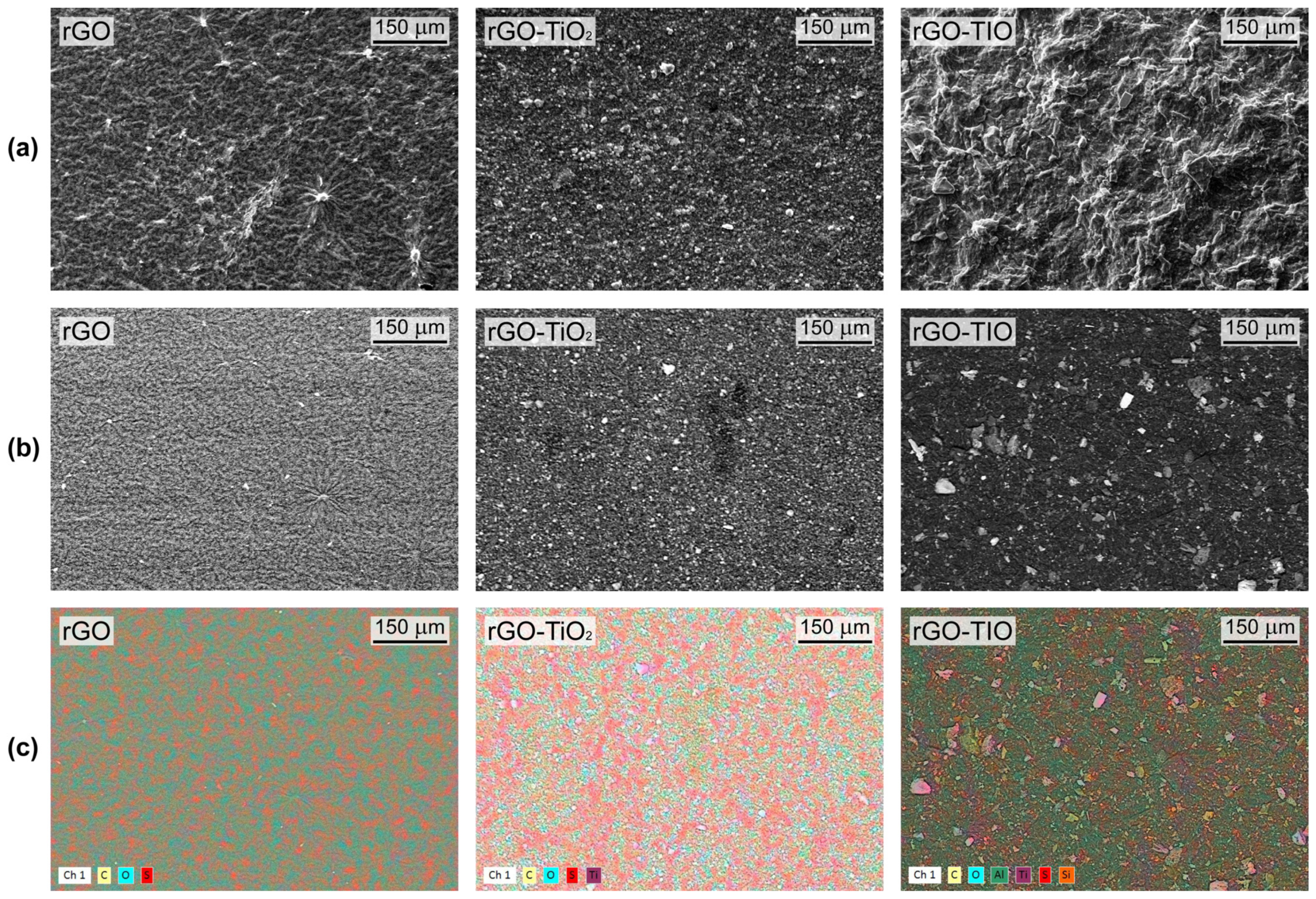
3.2. Membranes Production and Characterization
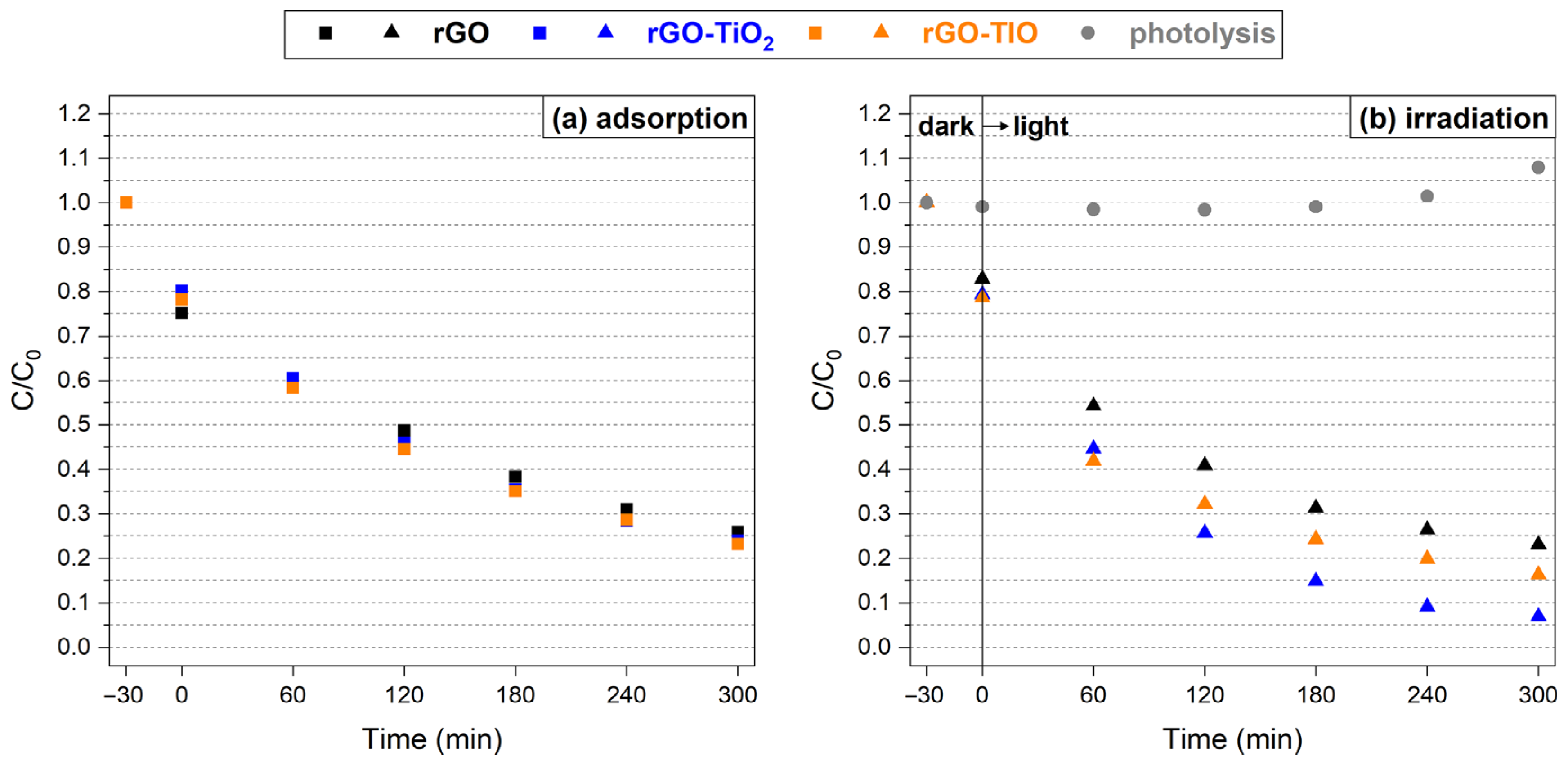
3.3. Metal Capture
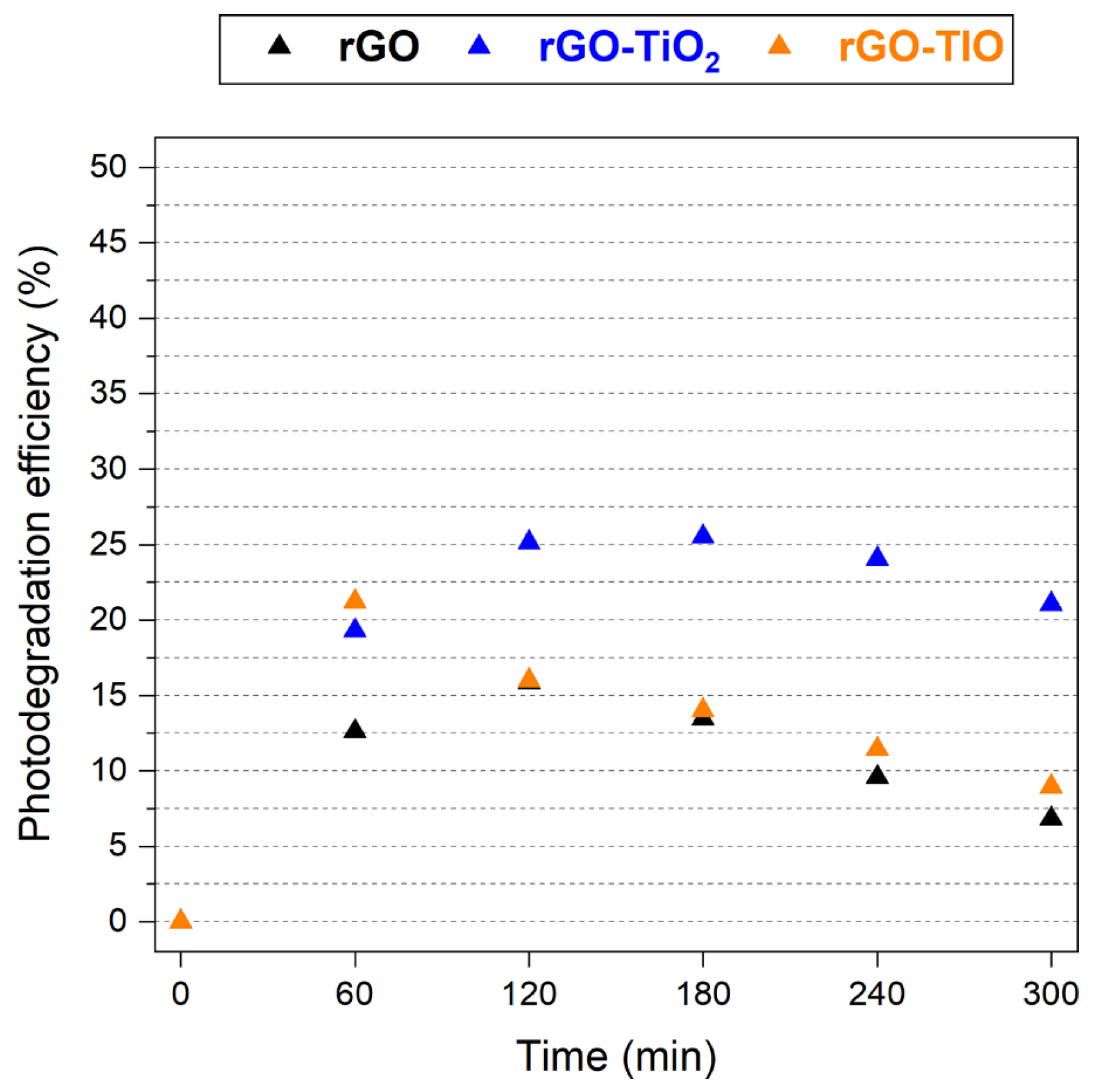
3.4. Photodegradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UN World Water Development Report. 2022. Available online: https://www.unwater.org/publications/un-world-water-development-report-2022 (accessed on 13 February 2023).
- Water Statistics—Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Water_statistics (accessed on 13 February 2023).
- Directive 2000/60/EC—Framework for Community Action in the Field of Water Policy. Available online: https://eur-lex.europa.eu/legal-content/EN/LSU/?uri=CELEX%3A32000L0060 (accessed on 13 February 2023).
- American Water Works Association. Water Quality and Treatment: A Handbook of Community Water Supplies, 5th ed.; Letterman, R., Ed.; McGraw-Hill: Denver, CO, USA, 1999; ISBN 9780070016590. [Google Scholar]
- Chen, Y.; Chen, L.; Bai, H.; Li, L. Graphene oxide–chitosan composite hydrogels as broad-spectrum adsorbents for water purification. J. Mater. Chem. A 2013, 1, 1992–2001. [Google Scholar] [CrossRef]
- Prasse, C.; Stalter, D.; Schulte-Oehlmann, U.; Oehlmann, J.; Ternes, T.A. Spoilt for choice: A critical review on the chemical and biological assessment of current wastewater treatment technologies. Water Res. 2015, 87, 237–270. [Google Scholar] [CrossRef] [PubMed]
- Iannicelli-Zubiani, E.M.; Cristiani, C.; Dotelli, G.; Gallo Stampino, P. Recovery of valuable metals from electronic scraps by clays and organo-clays: Study on bi-ionic model solutions. Waste Manag. 2017, 60, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Saleem, H.; Zaidi, S.J. Developments in the Application of Nanomaterials for Water Treatment and Their Impact on the Environment. Nanomaterials 2020, 10, 1764. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, Z.; Jiang, J.; Liu, Y.; Wang, X.; Li, W.; Zheng, G. One-Step Preparation of PVDF/GO Electrospun Nanofibrous Membrane for High-Efficient Adsorption of Cr(VI). Nanomaterials 2022, 12, 3115. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A Review of Removal of Pollutants from Water/Wastewater Using Different Types of Nanomaterials. Adv. Mater. Sci. Eng. 2014, 2014, 825910. [Google Scholar] [CrossRef]
- Cukierman, A.L.; Nunell, G.V.; Bonelli, P.R. Removal of emerging pollutants from water through adsorption onto carbon-based materials. In Emerging and Nanomaterial Contaminants in Wastewater; Elsevier: Amsterdam, The Netherlands, 2019; pp. 159–213. ISBN 9780128146743. [Google Scholar]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Tarcan, R.; Todor-Boer, O.; Petrovai, I.; Leordean, C.; Astilean, S.; Botiz, I. Reduced graphene oxide today. J. Mater. Chem. C 2020, 8, 1198–1224. [Google Scholar] [CrossRef]
- Chenab, K.K.; Sohrabi, B.; Jafari, A.; Ramakrishna, S. Water treatment: Functional nanomaterials and applications from adsorption to photodegradation. Mater. Today Chem. 2020, 16, 100262. [Google Scholar] [CrossRef]
- Latorrata, S.; Cristiani, C.; Basso Peressut, A.; Brambilla, L.; Bellotto, M.; Dotelli, G.; Finocchio, E.; Gallo Stampino, P.; Ramis, G. Reduced Graphene Oxide Membranes as Potential Self-Assembling Filter for Wastewater Treatment. Minerals 2021, 11, 15. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Matafonova, G.; Batoev, V. Recent advances in application of UV light-emitting diodes for degrading organic pollutants in water through advanced oxidation processes: A review. Water Res. 2018, 132, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Abela, S.; Farrugia, C.; Xuereb, R.; Lia, F.; Zammit, E.; Rizzo, A.; Refalo, P.; Grech, M. Photocatalytic Activity of Titanium Dioxide Nanotubes Following Long-Term Aging. Nanomaterials 2021, 11, 2823. [Google Scholar] [CrossRef]
- Avramescu, S.M.; Fierascu, I.; Fierascu, R.C.; Brazdis, R.I.; Nica, A.V.; Butean, C.; Olaru, E.A.; Ulinici, S.; Verziu, M.N.; Dumitru, A. Removal of Paracetamol from Aqueous Solutions by Photocatalytic Ozonation over TiO2-MexOy Thin Films. Nanomaterials 2022, 12, 613. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Xiao, L.; Zhang, J.; Chen, F.; Anpo, M. Preparation and characterization of nitrogen-doped TiO2 photocatalyst in different acid environments. Res. Chem. Intermed. 2006, 32, 717–724. [Google Scholar] [CrossRef]
- Kuvarega, A.T.; Mamba, B.B. Photocatalytic Membranes for Efficient Water Treatment. In Semiconductor Photocatalysis—Materials, Mechanisms and Applications; InTech: Revesby, Australia, 2016; ISBN 978-953-51-2483-2. [Google Scholar]
- Riaz, S.; Park, S.-J. An overview of TiO2-based photocatalytic membrane reactors for water and wastewater treatments. J. Ind. Eng. Chem. 2020, 84, 23–41. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Carbajo, J.; Jiménez, M.; Miralles, S.; Malato, S.; Faraldos, M.; Bahamonde, A. Study of application of titania catalysts on solar photocatalysis: Influence of type of pollutants and water matrices. Chem. Eng. J. 2016, 291, 64–73. [Google Scholar] [CrossRef]
- Ruidíaz-Martínez, M.; Álvarez, M.A.; López-Ramón, M.V.; Cruz-Quesada, G.; Rivera-Utrilla, J.; Sánchez-Polo, M. Hydrothermal Synthesis of rGO-TiO2 Composites as High-Performance UV Photocatalysts for Ethylparaben Degradation. Catalysts 2020, 10, 520. [Google Scholar] [CrossRef]
- Usharani, B.; Manivannan, V. Enhanced photocatalytic activity of reduced graphene oxide-TiO2 nanocomposite for picric acid degradation. Inorg. Chem. Commun. 2022, 142, 109660. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Likodimos, V.; Figueiredo, J.L.; Faria, J.L.; Falaras, P.; Silva, A.M.T. Advanced nanostructured photocatalysts based on reduced graphene oxide–TiO2 composites for degradation of diphenhydramine pharmaceutical and methyl orange dye. Appl. Catal. B Environ. 2012, 123–124, 241–256. [Google Scholar] [CrossRef]
- Luna-Sanguino, G.; Ruíz-Delgado, A.; Duran-Valle, C.J.; Malato, S.; Faraldos, M.; Bahamonde, A. Impact of water matrix and oxidant agent on the solar assisted photodegradation of a complex mix of pesticides over titania-reduced graphene oxide nanocomposites. Catal. Today 2021, 380, 114–124. [Google Scholar] [CrossRef]
- Kocijan, M.; Ćurković, L.; Bdikin, I.; Otero-Irurueta, G.; Hortigüela, M.J.; Gonçalves, G.; Radošević, T.; Vengust, D.; Podlogar, M. Immobilised rGO/TiO2 Nanocomposite for Multi-Cycle Removal of Methylene Blue Dye from an Aqueous Medium. Appl. Sci. 2021, 12, 385. [Google Scholar] [CrossRef]
- Sundaran, S.P.; Reshmi, C.R.; Sagitha, P.; Sujith, A. Polyurethane nanofibrous membranes decorated with reduced graphene oxide–TiO2 for photocatalytic templates in water purification. J. Mater. Sci. 2020, 55, 5892–5907. [Google Scholar] [CrossRef]
- Zouzelka, R.; Remzova, M.; Plsek, J.; Brabec, L.; Rathousky, J. Immobilized rGO/TiO2 Photocatalyst for Decontamination of Water. Catalysts 2019, 9, 708. [Google Scholar] [CrossRef]
- Wang, H.; Wang, G.; Zhang, Y.; Ma, Y.; Zhang, Q.; Pu, H.; Xu, W.; Gao, D.; Wang, B.; Qi, X. Preparation of RGO/TiO2 photocatalyst and the mechanism of its hydrothermal process. J. Chin. Chem. Soc. 2019, 66, 734–739. [Google Scholar] [CrossRef]
- Leshuk, T.; Everett, P.; Krishnakumar, H.; Wong, K.; Linley, S.; Gu, F. Mesoporous Magnetically Recyclable Photocatalysts for Water Treatment. J. Nanosci. Nanotechnol. 2013, 13, 3127–3132. [Google Scholar] [CrossRef]
- Lee, J.S.; You, K.H.; Park, C.B. Highly Photoactive, Low Bandgap TiO2 Nanoparticles Wrapped by Graphene. Adv. Mater. 2012, 24, 1084–1088. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, K. Reduced graphene oxide–TiO2 nanocomposite with high photocatalystic activity for the degradation of rhodamine B. J. Mol. Catal. A Chem. 2011, 345, 101–107. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; Wang, X.; Yu, H.; Yu, J.; Lei, M.; Wang, Y. One-step synthesis of easy-recycling TiO2-rGO nanocomposite photocatalysts with enhanced photocatalytic activity. Appl. Catal. B Environ. 2013, 132–133, 452–459. [Google Scholar] [CrossRef]
- Olowoyo, J.O.; Kumar, M.; Singh, B.; Oninla, V.O.; Babalola, J.O.; Valdés, H.; Vorontsov, A.V.; Kumar, U. Self-assembled reduced graphene oxide-TiO2 nanocomposites: Synthesis, DFTB+ calculations, and enhanced photocatalytic reduction of CO2 to methanol. Carbon N. Y. 2019, 147, 385–397. [Google Scholar] [CrossRef]
- Kanta, U.; Thongpool, V.; Sangkhun, W.; Wongyao, N.; Wootthikanokkhan, J. Preparations, Characterizations, and a Comparative Study on Photovoltaic Performance of Two Different Types of Graphene/TiO2 Nanocomposites Photoelectrodes. J. Nanomater. 2017, 2017, 2758294. [Google Scholar] [CrossRef]
- Yahya, N.; Aziz, F.; Jamaludin, N.; Mutalib, M.A.; Ismail, A.; Salleh, W.W.; Jaafar, J.; Yusof, N.; Ludin, N.A. A review of integrated photocatalyst adsorbents for wastewater treatment. J. Environ. Chem. Eng. 2018, 6, 7411–7425. [Google Scholar] [CrossRef]
- Jia, F.; Xiao, X.; Nashalian, A.; Shen, S.; Yang, L.; Han, Z.; Qu, H.; Wang, T.; Ye, Z.; Zhu, Z.; et al. Advances in graphene oxide membranes for water treatment. Nano Res. 2022, 15, 6636–6654. [Google Scholar] [CrossRef]
- Luna-Sanguino, G.; Ruíz-Delgado, A.; Tolosana-Moranchel, A.; Pascual, L.; Malato, S.; Bahamonde, A.; Faraldos, M. Solar photocatalytic degradation of pesticides over TiO2-rGO nanocomposites at pilot plant scale. Sci. Total Environ. 2020, 737, 140286. [Google Scholar] [CrossRef]
- Graphenea Graphene Oxide, Product Datasheet. Available online: https://cdn.shopify.com/s/files/1/0191/2296/files/Graphenea_GO_4mgmL_Datasheet_202109.pdf?v=1632927913 (accessed on 13 February 2023).
- Sriwong, C.; Choojun, K.; Tejangkura, W.; Prasanseang, W. Preparation and Photocatalytic Activities of TiO2-rGO Nanocomposite Catalysts for MB Dye Degradation over Sunlight Irradiation. Mater. Sci. Forum 2018, 936, 47–52. [Google Scholar] [CrossRef]
- Dietmar, K.; Goverde, T.; Carsten, B. Particle World—Technical Papers of QUANTACHROME. Available online: https://www.3p-instruments.com/wp-content/uploads/PDF/particleworld/PW-02/Particle_World_2.pdf (accessed on 13 February 2023).
- Klug, H.; Alexander, L. X-ray Diffraction Procedures: For Polycrystalline and Amorphous Materials, 2nd ed.; Wiley: New York, NY, USA, 1974; ISBN 978-0-471-49369-3. [Google Scholar]
- Chemical Equilibrium Diagrams|KTH. Available online: https://www.kth.se/che/medusa (accessed on 13 February 2023).
- Gázquez, M.J.; Bolívar, J.P.; García-Tenorio, R.; Vaca, F. Physicochemical characterization of raw materials and co-products from the titanium dioxide industry. J. Hazard. Mater. 2009, 166, 1429–1440. [Google Scholar] [CrossRef]
- Pérez-Moreno, S.M.; Gázquez, M.J.; Barneto, A.G.; Bolívar, J.P. Thermal characterization of new fire-insulating materials from industrial inorganic TiO2 wastes. Thermochim. Acta 2013, 552, 114–122. [Google Scholar] [CrossRef]
- Masset, P.; Poinso, J.Y.; Poignet, J.C. TG/DTA/MS Study of the thermal decomposition of FeSO4·6H2O. J. Therm. Anal. Calorim. 2006, 83, 457–462. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Poston, J.A., Jr.; Fisher, E.P.; Shen, M.-S.; Miltz, A.L. Decomposition of the sulfates of copper, iron (II), iron (III), nickel, and zinc: XPS, SEM, DRIFTS, XRD, and TGA study. Appl. Surf. Sci. 1999, 152, 219–236. [Google Scholar] [CrossRef]
- Thomas, P.S.; Hirschausen, D.; White, R.E.; Guerbois, J.P.; Ray, A.S. Characterisation of the oxidation products of pyrite by thermogravimetric and evolved gas analysis. J. Therm. Anal. Calorim. 2003, 72, 769–776. [Google Scholar] [CrossRef]
- Villagrán-Zaccardi, Y.A.; Egüez-Alava, H.; De Buysser, K.; Gruyaert, E.; De Belie, N. Calibrated quantitative thermogravimetric analysis for the determination of portlandite and calcite content in hydrated cementitious systems. Mater. Struct. 2017, 50, 179. [Google Scholar] [CrossRef]
- Kłosek-Wawrzyn, E.; Małolepszy, J.; Murzyn, P. Sintering Behavior of Kaolin with Calcite. Procedia Eng. 2013, 57, 572–582. [Google Scholar] [CrossRef]
- Yang, S.; Yue, W.; Huang, D.; Chen, C.; Lin, H.; Yang, X. A facile green strategy for rapid reduction of graphene oxide by metallic zinc. RSC Adv. 2012, 2, 8827. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.-W.; Jung, W.-G. Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Shnitov, V.V.; Dideikin, A.T.; Aleksenskii, A.E.; Vul’, S.P.; Baidakova, M.V.; Pronin, I.I.; Kirilenko, D.A.; Brunkov, P.N.; Weise, J.; et al. Nanoscale Perforation of Graphene Oxide during Photoreduction Process in the Argon Atmosphere. J. Phys. Chem. C 2016, 120, 28261–28269. [Google Scholar] [CrossRef]
- Zedek, R.; Djedjiga, H.; Megherbi, M.; Belkaid, M.S.; Ntsoenzok, E. Effects of slight Fe (III)-doping on structural and optical properties of TiO2 nanoparticles. J. Sol-Gel Sci. Technol. 2021, 100, 44–54. [Google Scholar] [CrossRef]
- Yu, J.; Xiang, Q.; Zhou, M. Preparation, characterization and visible-light-driven photocatalytic activity of Fe-doped titania nanorods and first-principles study for electronic structures. Appl. Catal. B Environ. 2009, 90, 595–602. [Google Scholar] [CrossRef]
- Ismael, M. Enhanced photocatalytic hydrogen production and degradation of organic pollutants from Fe (III) doped TiO2 nanoparticles. J. Environ. Chem. Eng. 2020, 8, 103676. [Google Scholar] [CrossRef]
- Agüera, A.; Almansa, E.; Malato, S.; Maldonado, M.I.; Fernández-Alba, A.R. Evaluation of photocatalytic degradation of imidacloprid in industrial water by GC-MS and LC-MS. Analusis 1998, 26, 245–250. [Google Scholar] [CrossRef]
- Kitsiou, V.; Filippidis, N.; Mantzavinos, D.; Poulios, I. Heterogeneous and homogeneous photocatalytic degradation of the insecticide imidacloprid in aqueous solutions. Appl. Catal. B Environ. 2009, 86, 27–35. [Google Scholar] [CrossRef]
- Lacson, C.F.Z.; de Luna, M.D.G.; Dong, C.; Garcia-Segura, S.; Lu, M.-C. Fluidized-bed Fenton treatment of imidacloprid: Optimization and degradation pathway. Sustain. Environ. Res. 2018, 28, 309–314. [Google Scholar] [CrossRef]
- Yari, K.; Seidmohammadi, A.; Khazaei, M.; Bhatnagar, A.; Leili, M. A comparative study for the removal of imidacloprid insecticide from water by chemical-less UVC, UVC/TiO2 and UVC/ZnO processes. J. Environ. Health Sci. Eng. 2019, 17, 337–351. [Google Scholar] [CrossRef] [PubMed]
- El-Shafai, N.M.; El-Shaer, A.; Eraky, M.R.; Ibrahim, M.M.; Ramadan, M.S.; El-Mehasseb, I.M. Enhancing electron density, electrochemical, and dielectric properties of nanohybrid materials for advanced photocatalytic antifouling and energy storage. Diam. Relat. Mater. 2021, 119, 108543. [Google Scholar] [CrossRef]
- Behera, L.; Barik, B.; Mohapatra, S. Improved photodegradation and antimicrobial activity of hydrothermally synthesized 0.2Ce-TiO2/RGO under visible light. Colloids Surf. A Physicochem. Eng. Asp. 2021, 620, 126553. [Google Scholar] [CrossRef]
- Tismanar, I.; Obreja, A.C.; Buiu, O.; Duta, A. VIS-active TiO2—Graphene oxide composite thin films for photocatalytic applications. Appl. Surf. Sci. 2021, 538, 147833. [Google Scholar] [CrossRef]



| Membrane | Expected Weight (mg) | Actual Weight (mg) | Thickness (µm) |
|---|---|---|---|
| rGO | 8 | 7.53 ± 0.31 | 11.80 ± 2.57 |
| rGO–TiO2 | 16 | 15.00 ± 0.92 | 35.82 ± 9.43 |
| rGO–TIO | 16 | 12.33 ± 0.64 | 53.11 ± 8.01 |
| Sample | Crystallite Dimensions (nm) | |
|---|---|---|
| Anatase | Rutile | |
| TiO2 | 19 | 76 |
| rGO–TiO2 | 19 | 36 |
| TIO | 48 | 101 |
| rGO–TIO | 31 | 60 |
| Membrane | Qm,Fe (mmol gmembrane−1) | Qm,Cu (mmol gmembrane−1) |
|---|---|---|
| rGO | 0.19 | 0.05 |
| rGO–TiO2 | 0.11 | 0.10 |
| rGO–TIO | 0.18 | 0.09 |
| Membrane | %wtFe a (ICP-OES) | %wtFe (SEM-EDX) | %wtCu a (ICP-OES) | %wtCu (SEM-EDX) |
|---|---|---|---|---|
| rGO | 1.08 | 0.11 | 0.31 | 0.31 |
| rGO–TiO2 | 0.62 | 1.31 | 0.63 | 0.72 |
| rGO–TIO | 1.02 | 1.22 | 0.56 | 1.18 |
| Test | Membrane | Time (min) | pH | TOC (mg L−1) | Anions a (mg L−1) | Residual nitrogen (mg L−1) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CH3COO− | HCO2− | NO2− | NO3− | NH4+ | TOT | |||||
| photolysis | - | −30 | 6.9 | 2.55 | - | 0.11 | - | - | 0.01 | 0.01 |
| 300 | 7.0 | 6.29 | - | 0.70 | 0.03 | - | 0.11 | 0.14 | ||
| adsorption | rGO | −30 | 6.1 | 2.25 | - | - | - | - | - | - |
| 300 | 2.9 | 9.67 | >2 (2.85) | 0.16 | - | - | 0.05 | 0.05 | ||
| rGO–TiO2 | −30 | 5.5 | 2.75 | - | 0.07 | - | - | - | - | |
| 300 | 2.6 | 12.80 | - | 0.35 | - | - | 0.05 | 0.05 | ||
| rGO–TIO | −30 | 6.4 | 2.57 | - | 0.08 | - | - | - | - | |
| 300 | 4.1 | 11.74 | >2 (2.16) | 0.20 | - | - | 0.01 | - | ||
| photocatalysis | rGO | −30 | 6.9 | 2.61 | - | 0.11 | 0.02 | - | 0.01 | 0.03 |
| 300 | 3.5 | 15.31 | >2 (2.44) | 0.77 | - | 0.05 | 0.13 | 0.18 | ||
| rGO–TiO2 | −30 | 6.5 | 2.52 | - | 0.11 | - | - | 0.02 | 0.02 | |
| 300 | 3.5 | 12.47 | 1.18 | 1.18 | 0.01 | 0.06 | 0.09 | 0.16 | ||
| rGO–TIO | −30 | 6.6 | 2.30 | - | 0.11 | - | - | 0.01 | 0.01 | |
| 300 | 4.5 | 4.76 | - | >2 (2.58) | - | 0.06 | 0.07 | 0.13 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basso Peressut, A.; Cristiani, C.; Dotelli, G.; Dotti, A.; Latorrata, S.; Bahamonde, A.; Gascó, A.; Hermosilla, D.; Balzarotti, R. Reduced Graphene Oxide/Waste-Derived TiO2 Composite Membranes: Preliminary Study of a New Material for Hybrid Wastewater Treatment. Nanomaterials 2023, 13, 1043. https://doi.org/10.3390/nano13061043
Basso Peressut A, Cristiani C, Dotelli G, Dotti A, Latorrata S, Bahamonde A, Gascó A, Hermosilla D, Balzarotti R. Reduced Graphene Oxide/Waste-Derived TiO2 Composite Membranes: Preliminary Study of a New Material for Hybrid Wastewater Treatment. Nanomaterials. 2023; 13(6):1043. https://doi.org/10.3390/nano13061043
Chicago/Turabian StyleBasso Peressut, Andrea, Cinzia Cristiani, Giovanni Dotelli, Anna Dotti, Saverio Latorrata, Ana Bahamonde, Antonio Gascó, Daphne Hermosilla, and Riccardo Balzarotti. 2023. "Reduced Graphene Oxide/Waste-Derived TiO2 Composite Membranes: Preliminary Study of a New Material for Hybrid Wastewater Treatment" Nanomaterials 13, no. 6: 1043. https://doi.org/10.3390/nano13061043
APA StyleBasso Peressut, A., Cristiani, C., Dotelli, G., Dotti, A., Latorrata, S., Bahamonde, A., Gascó, A., Hermosilla, D., & Balzarotti, R. (2023). Reduced Graphene Oxide/Waste-Derived TiO2 Composite Membranes: Preliminary Study of a New Material for Hybrid Wastewater Treatment. Nanomaterials, 13(6), 1043. https://doi.org/10.3390/nano13061043














