Synthesis and Functional Characterization of CoxFe3−xO4-BaTiO3 Magnetoelectric Nanocomposites for Biomedical Applications
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Synthesis of the Samples
2.2.1. Synthesis of CoxFe3−xO4 Phase
2.2.2. Synthesis of the Barium Titanate Phase
2.3. Characterization
2.4. Cell Line
2.5. MTS-Assay
3. Results and Discussions
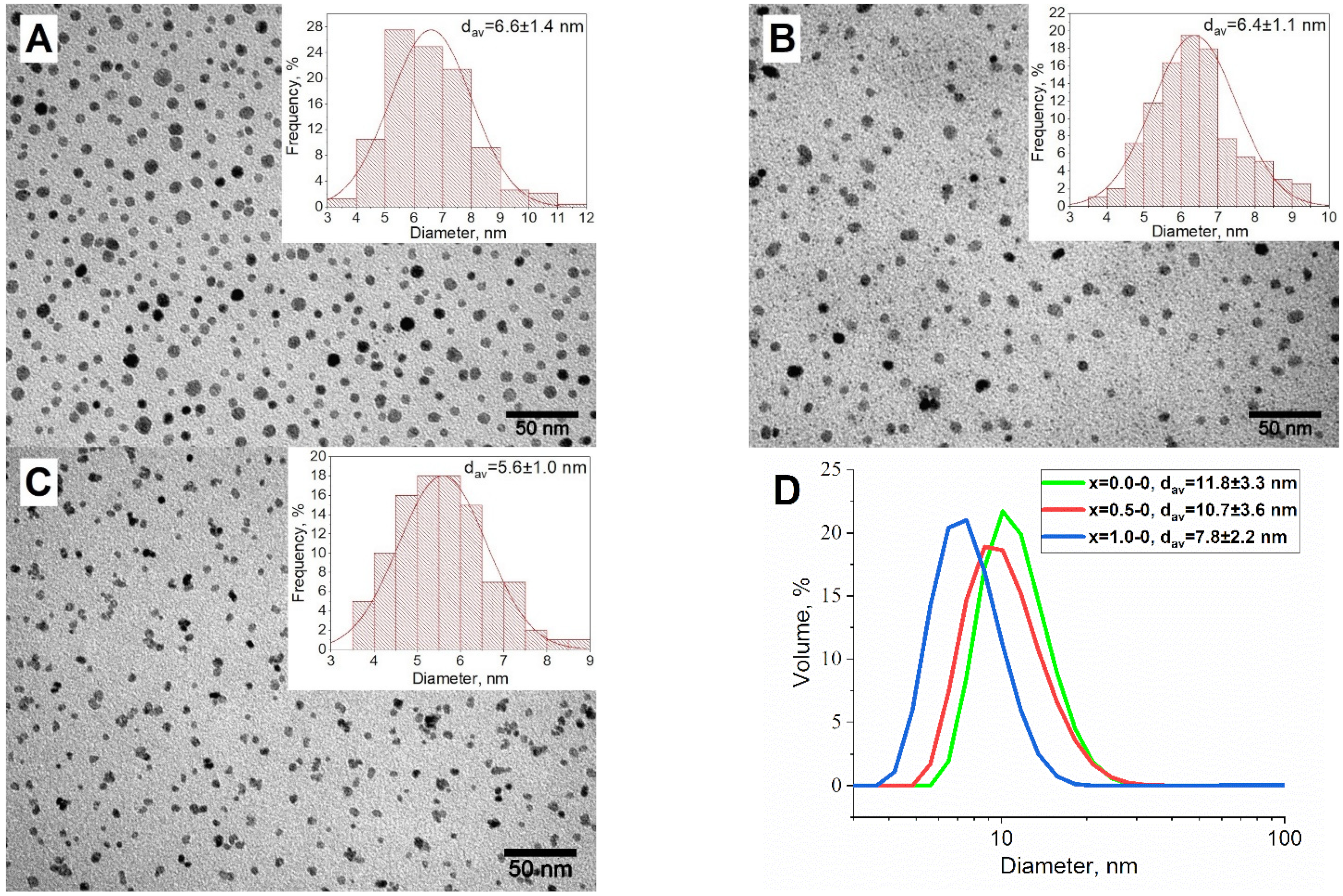
3.1. Magnetic Phase Synthesis
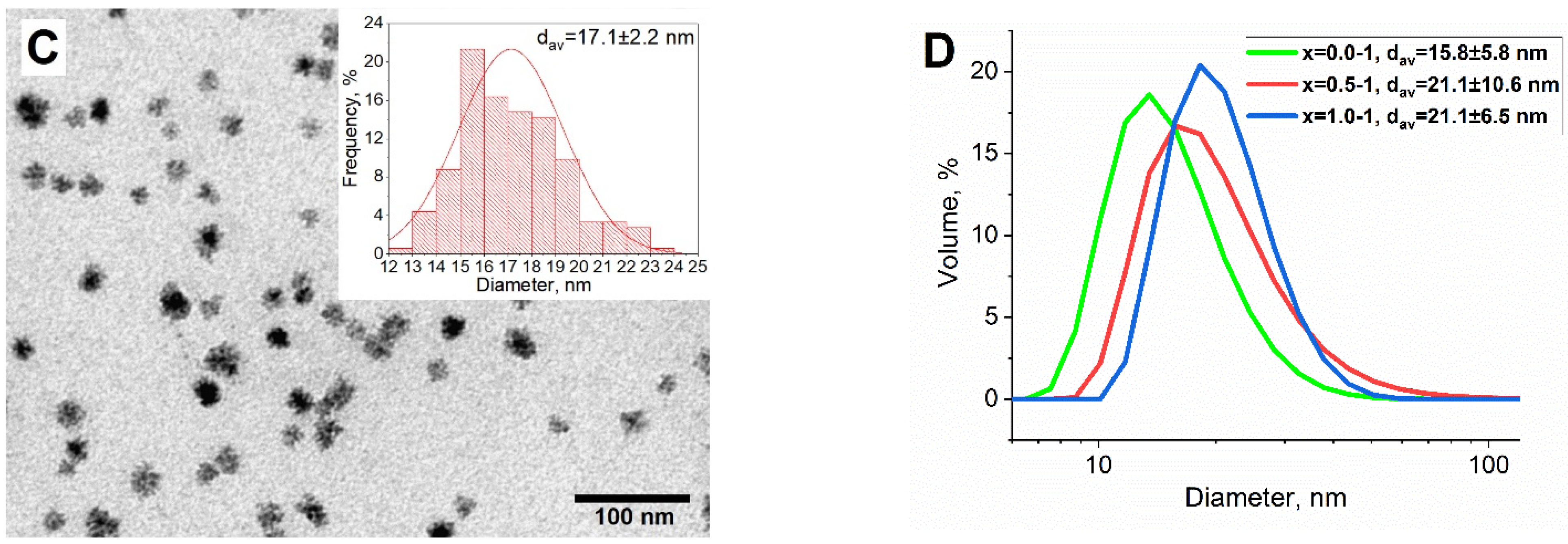
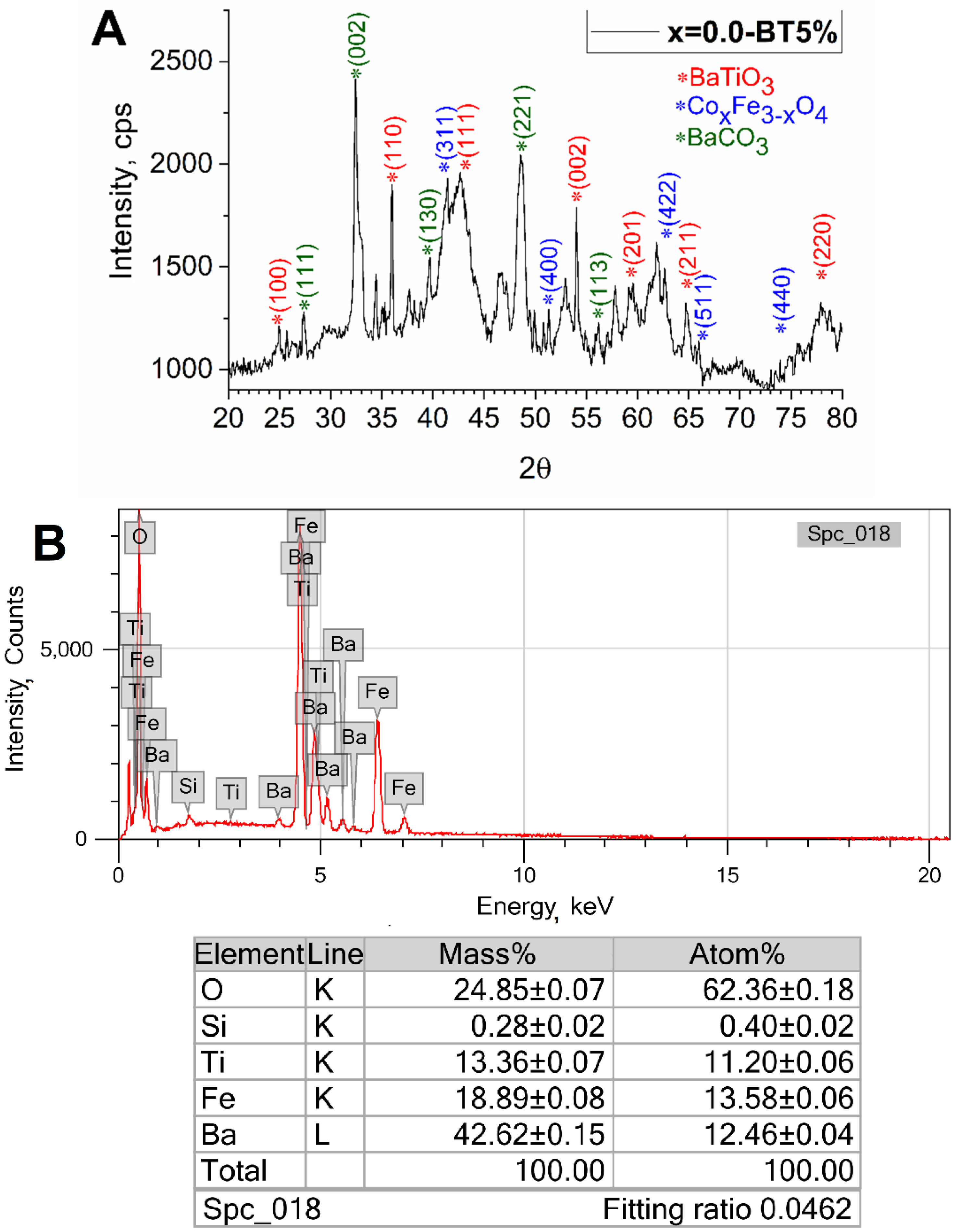
3.2. Investigation of Shell Formation Conditions
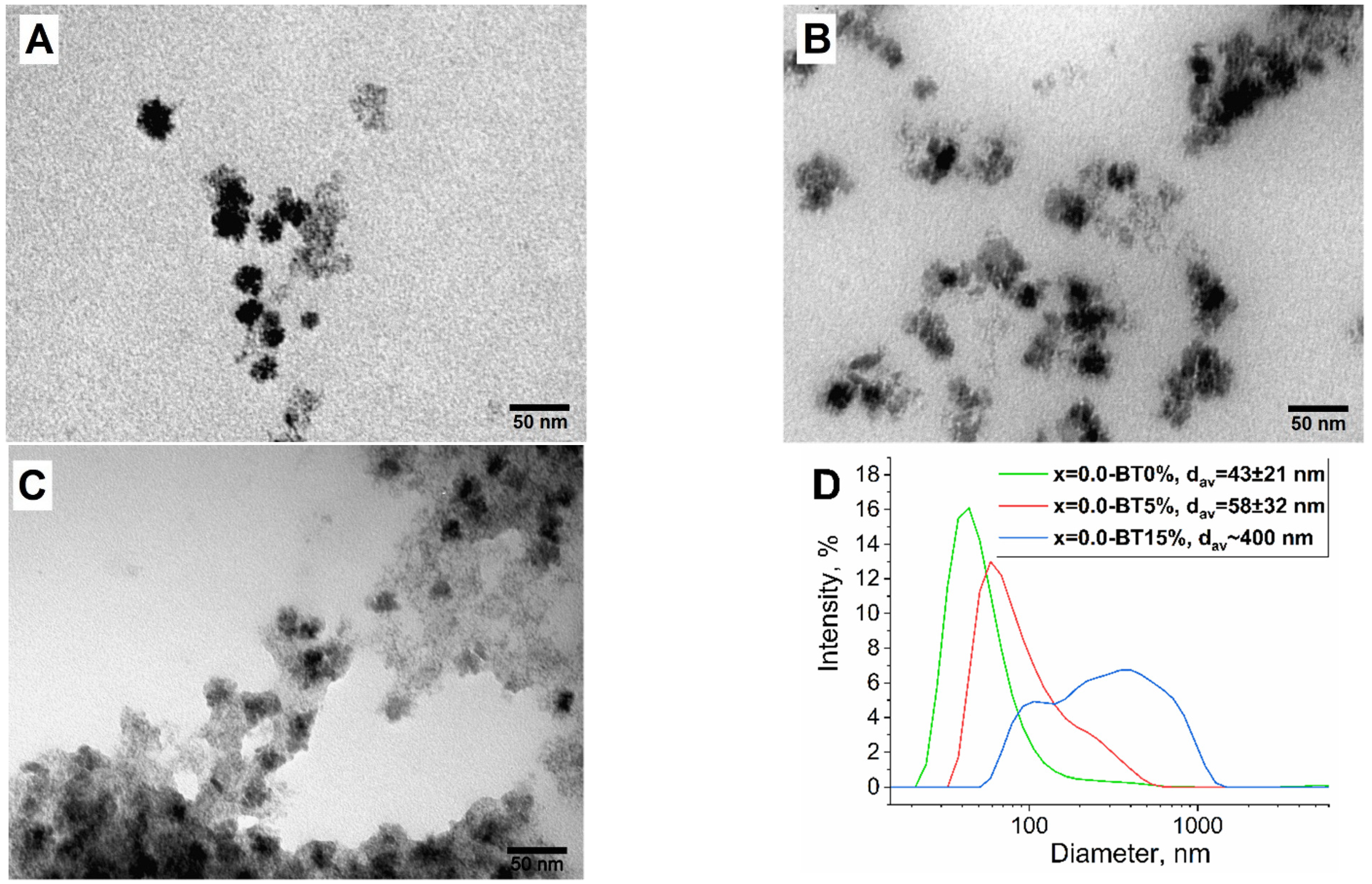
3.3. Synthesis of MENC Series
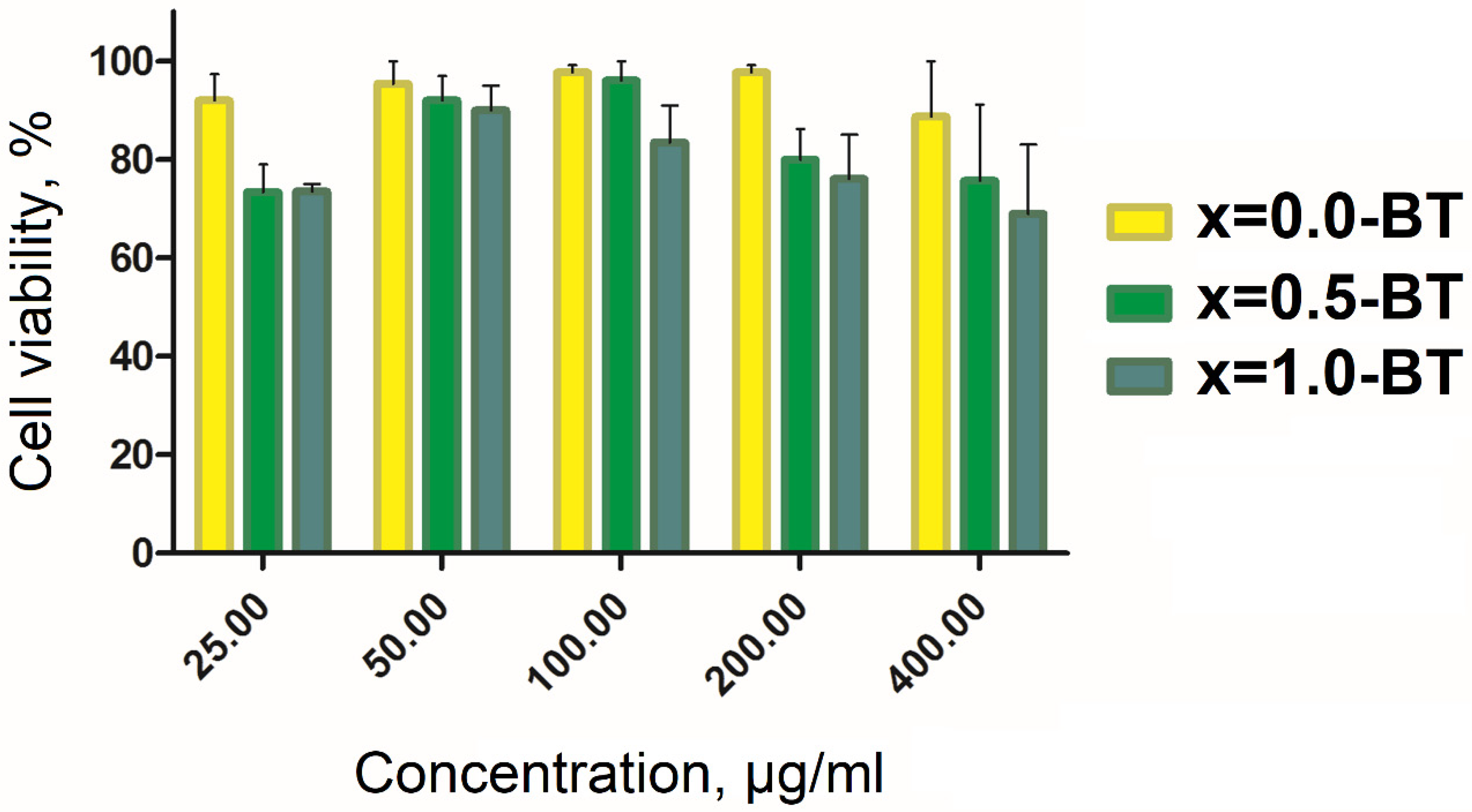
3.4. Study of the MENC Cytotoxicity on CT-26 Cell Line
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vopson, M.M. Fundamentals of Multiferroic Materials and Their Possible Applications. Crit. Rev. Solid State Mater. Sci. 2015, 40, 223–250. [Google Scholar] [CrossRef]
- Bichurin, M.; Petrov, R.; Tatarenko, A. Magnetoelectric Composites: Modeling and Application. Adv. Mater. 2020, 9, 15. [Google Scholar] [CrossRef]
- Kargol, A.; Malkinski, L.; Caruntu, G. Biomedical Applications of Multiferroic Nanoparticles. In Advanced Magnetic Materials; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Kopyl, S.; Surmenev, R.; Surmeneva, M.; Fetisov, Y.; Kholkin, A. Magnetoelectric Effect: Principles and Applications in Biology and Medicine—A Review. Mater. Today Bio 2021, 12, 100149. [Google Scholar] [CrossRef] [PubMed]
- Amirov, A. Multiferroic, Magnetic, and Magnetoelectric Nanomaterials for Medical Applications. In Magnetic Materials and Technologies for Medical Applications; Woodhead Publishing: Sawston, UK, 2022; pp. 469–484. [Google Scholar] [CrossRef]
- Park, T.-J.; Papaefthymiou, G.; Viescas, A.; Moodenbaugh, A.; Wong, S. Size-Dependent Magnetic Properties of Single-Crystalline Multiferroic BiFeO3 Nanoparticles. Nano Lett. 2007, 7, 766–772. [Google Scholar] [CrossRef]
- Rosales-González, O.; Sánchez-De Jesús, F.; Cortés-Escobedo, C.A.; Bolarín-Miró, A.M. Crystal Structure and Multiferroic Behavior of Perovskite YFeO3. Ceram. Int. 2018, 44, 15298–15303. [Google Scholar] [CrossRef]
- Schileo, G. Recent Developments in Ceramic Multiferroic Composites Based on Core/Shell and Other Heterostructures Obtained by Sol-Gel Routes. Prog. Solid State Chem. 2013, 41, 87–98. [Google Scholar] [CrossRef]
- Rao, B.N.; Kaviraj, P.; Vaibavi, S.R.; Kumar, A.; Bajpai, S.K.; Arockiarajan, A. Investigation of Magnetoelectric Properties and Biocompatibility of CoFe2O4-BaTiO3 Core-Shell Nanoparticles for Biomedical Applications. J. Appl. Phys. 2017, 122, 164102. [Google Scholar] [CrossRef]
- Guduru, R.; Liang, P.; Runowicz, C.; Nair, M.; Atluri, V.; Khizroev, S. Magneto-Electric Nanoparticles to Enable Field-Controlled High-Specificity Drug Delivery to Eradicate Ovarian Cancer Cells. Sci. Rep. 2013, 3, 2953. [Google Scholar] [CrossRef]
- Rodzinski, A.; Guduru, R.; Liang, P.; Hadjikhani, A.; Stewart, T.; Stimphil, E.; Runowicz, C.; Cote, R.; Altman, N.; Datar, R.; et al. Targeted and Controlled Anticancer Drug Delivery and Release with Magnetoelectric Nanoparticles. Sci. Rep. 2016, 6, 20867. [Google Scholar] [CrossRef]
- Nair, M.; Guduru, R.; Liang, P.; Hong, J.; Sagar, V.; Khizroev, S. Externally Controlled On-Demand Release of Anti-HIV Drug Using Magneto-Electric Nanoparticles as Carriers. Nat. Commun. 2013, 4, 1707. [Google Scholar] [CrossRef]
- Varvaro, G.; Omelyanchik, A.; Peddis, D. Ferroic Transition Metal Oxide Nano-heterostructures: From Fundamentals to Applications. In Tailored Functional Oxide Nanomaterials; Wiley: Hoboken, NJ, USA, 2022; pp. 405–437. [Google Scholar] [CrossRef]
- Golovin, Y.I.; Klyachko, N.L.; Majouga, A.G.; Sokolsky, M.; Kabanov, A. Theranostic Multimodal Potential of Magnetic Nanoparticles Actuated by Non-Heating Low Frequency Magnetic Field in the New-Generation Nanomedicine. J. Nanopart. Res. 2017, 19, 63. [Google Scholar] [CrossRef]
- Stimphil, E.; Nagesetti, A.; Guduru, R.; Stewart, T.; Rodzinski, A.; Liang, P.; Khizroev, S. Physics Considerations in Targeted Anticancer Drug Delivery by Magnetoelectric Nanoparticles. Appl. Phys. Rev. 2017, 4, 021101. [Google Scholar] [CrossRef]
- Song, H.; Listyawan, M.A.; Ryu, J. Core–Shell Magnetoelectric Nanoparticles: Materials, Synthesis, Magnetoelectricity, and Applications. Actuators 2022, 11, 380. [Google Scholar] [CrossRef]
- Guduru, R.; Khizroev, S. Magnetic Field-Controlled Release of Paclitaxel Drug from Functionalized Magnetoelectric Nanoparticles. Part. Part. Syst. Charact. 2014, 31, 605–611. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Mandal, K. Large Magnetoelectric Properties in CoFe2O4:BaTiO3core-Shell Nanocomposites. J. Magn. Magn. Mater. 2015, 377, 441–445. [Google Scholar] [CrossRef]
- Raidongia, K.; Nag, A.; Sundaresan, A.; Rao, C.N.R. Multiferroic and Magnetoelectric Properties of Core-Shell CoFe2 O4 @ BaTiO3 Nanocomposites. Appl. Phys. Lett. 2010, 97, 2008–2011. [Google Scholar] [CrossRef]
- Stewart, T.S.; Nagesetti, A.; Guduru, R.; Liang, P.; Stimphil, E.; Hadjikhani, A.; Salgueiro, L.; Horstmyer, J.; Cai, R.; Schally, A.; et al. Magnetoelectric Nanoparticles for Delivery of Antitumor Peptides into Glioblastoma Cells by Magnetic Fields. Nanomedicine 2018, 13, 423–438. [Google Scholar] [CrossRef]
- Balbaa, A.O.; El-fattah, A.A.; Awad, N.M.; Abdellatif, A. Effects of Nanoscale Electric Fields on the Histology of Liver Cell Dysplasia. Nanomedicine 2019, 14, 515–528. [Google Scholar] [CrossRef]
- Guduru, R.; Liang, P.; Yousef, M.; Horstmyer, J.; Khizroev, S. Mapping the Brain’s Electric Fields with Magnetoelectric Nanoparticles. Bioelectron. Med. 2018, 4, 10. [Google Scholar] [CrossRef]
- Friedrich, R.M.; Zabel, S.; Galka, A.; Lukat, N.; Wagner, J.M.; Kirchhof, C.; Quandt, E.; McCord, J.; Selhuber-Unkel, C.; Siniatchkin, M.; et al. Magnetic Particle Mapping Using Magnetoelectric Sensors as an Imaging Modality. Sci. Rep. 2019, 9, 2086. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.; Novoselova, I.P.; Schupletsova, V.; Andrade, R.; Dunec, N.A.; Litvinova, L.S.; Safronov, A.P.; Yurova, K.A.; Kulesh, N.A.; Dzyuman, A.N.; et al. Nanoparticles for Magnetic Biosensing Systems. J. Magn. Magn. Mater. 2017, 431, 249–254. [Google Scholar] [CrossRef]
- Khlusov, I.A.; Zagrebin, L.; Shestov, S.S.; Itin, V.I.; Sedoi, V.S.; Feduschak, T.A.; Terekhova, O.G.; Magaeva, A.A.; Naiden, E.P.; Antipov, S.A.; et al. Colony-Forming Activity of Unipotent Hemopoietic Precursors under the Effect of Nanosized Ferrites in a Constant Magnetic Field in Vitro. Bull. Exp. Biol. Med. 2008, 145, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Andujar, C.; Walter, A.; Cotin, G.; Bordeianu, C.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Design of Iron Oxide-Based Nanoparticles for MRI and Magnetic Hyperthermia. Nanomedicine 2016, 11, 1889–1910. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Tsai, M.H.; Li, K.Y.; Hou, C.H.; Lin, F.H. Carbon-Doped TiO2 Activated by X-Ray Irradiation for the Generation of Reactive Oxygen Species to Enhance Photodynamic Therapy in Tumor Treatment. Int. J. Mol. Sci. 2019, 20, 2072. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Ortiz-García, I.; Jiménez-Guerra, A.; Monsalve-Guil, L.; Muñoz-Guzón, F.; Perez, R.A.; Gil, F.J. Comparison between Sandblasted Acid-Etched and Oxidized Titanium Dental Implants: In Vivo Study. Int. J. Mol. Sci. 2019, 20, 3267. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Khan, M.A.M.; Alhadlaq, H.A.; Alshamsan, A. Cobalt Iron Oxide Nanoparticles Induce Cytotoxicity and Regulate the Apoptotic Genes through ROS in Human Liver Cells (HepG2). Colloids Surf. B Biointerfaces 2016, 148, 665–673. [Google Scholar] [CrossRef]
- Ansari, S.M.; Bhor, R.D.; Pai, K.R.; Sen, D.; Mazumder, S.; Ghosh, K.; Kolekar, Y.D.; Ramana, C. Cobalt Nanoparticles for Biomedical Applications: Facile Synthesis, Physiochemical Characterization, Cytotoxicity Behavior and Biocompatibility. Appl. Surf. Sci. 2017, 414, 171–187. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Khan, M.A.M.; Alhadlaq, H.A.; Alshamsan, A. Barium Titanate (BaTiO3) Nanoparticles Exert Cytotoxicity through Oxidative Stress in Human Lung Carcinoma (A549) Cells. Nanomaterials 2020, 10, 2309. [Google Scholar] [CrossRef]
- Lu, L.T.; Dung, N.T.; Tung, L.D.; Thanh, C.T.; Quy, O.K.; Chuc, N.V.; Maenosono, S.; Thanh, N.T.K. Synthesis of Magnetic Cobalt Ferrite Nanoparticles with Controlled Morphology, Monodispersity and Composition: The Influence of Solvent, Surfactant, Reductant and Synthetic Conditions. Nanoscale 2015, 7, 19596–19610. [Google Scholar] [CrossRef]
- Chiba, K.; Chikazumi, S. Magnetocrystalline Anisotropy of Magnetite. J. Magn. Magn. Mater. 1983, 31–34, 813–814. [Google Scholar] [CrossRef]
- Li, Y.; Lu, X.; Yang, S.; Duan, W.; Zhang, Y.; Zhou, C.; Li, K.; Zhang, Y.; Shi, Q. Synthesis of Monodisperse Ferromagnetic CoxFe3−xO4 Colloidal Particles with Magnetically Tunable Optical Properties. CrystEngComm 2019, 21, 2310–2319. [Google Scholar] [CrossRef]
- Abakumov, M.; Nizamov, T.; Yanchen, L.; Shchetinin, I.; Savchenko, A.; Zhukov, D.; Majouga, A. Versatile Seed-Mediated Method of CoxFe3-XO4 Nanoparticles Synthesis in Glycol Media via Thermal Decomposition. Mater. Lett. 2020, 276, 128210. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, Y.; Huang, R.; Zhao, Y.; Zhou, H. Multiferroic Ferrite/Perovskite Oxide Core/Shell Nanostructures. J. Mater. Chem. 2010, 20, 10665. [Google Scholar] [CrossRef]
- Maity, D.; Chandrasekharan, P.; Pradhan, P.; Chuang, K.-H.; Xue, J.-M.; Feng, S.-S.; Ding, J. Novel Synthesis of Superparamagnetic Magnetite Nanoclusters for Biomedical Applications. J. Mater. Chem. 2011, 21, 14717. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, X.; Zhang, H.; Guo, L.; Li, L. Sol-Gel Based Synthesis of Ultrafine Tetragonal BaTiO3. J. Sol-Gel Sci. Technol. 2013, 67, 182–187. [Google Scholar] [CrossRef]
- Zhan, H.; Yang, X.; Wang, C.; Chen, J.; Wen, Y.; Liang, C.; Greer, H.F.; Wu, M.; Zhou, W. Multiple Nucleation and Crystal Growth of Barium Titanate. Cryst. Growth Des. 2012, 12, 1247–1253. [Google Scholar] [CrossRef]
- Inada, M.; Enomoto, N.; Hayashi, K.; Hojo, J.; Komarneni, S. Facile Synthesis of Nanorods of Tetragonal Barium Titanate Using Ethylene Glycol. Ceram. Int. 2015, 41, 5581–5587. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Nizamov, T.R.; Abakumov, M.A.; Shchetinin, I.V.; Savchenko, A.G.; Majouga, A.G. Effect of Magnetite Nanoparticle Morphology on the Parameters of MRI Relaxivity. Bull. Russ. Acad. Sci. Phys. 2018, 82, 1214–1221. [Google Scholar] [CrossRef]
- Vopson, M.M.; Fetisov, Y.K.; Caruntu, G.; Srinivasan, G. Measurement Techniques of the Magneto-Electric Coupling in Multiferroics. Materials 2017, 10, 963. [Google Scholar] [CrossRef]
- Omelyanchik, A.; Antipova, V.; Gritsenko, C.; Kolesnikova, V.; Murzin, D.; Han, Y.; Turutin, A.; Kubasov, I.; Kislyuk, A.M.; Ilina, T.S.; et al. Boosting Magnetoelectric Effect in Polymer-Based Nanocomposites. Nanomaterials 2021, 11, 1154. [Google Scholar] [CrossRef]
- Spizzo, F.; Sgarbossa, P.; Sieni, E.; Semenzato, A.; Dughiero, F.; Forzan, M.; Bertani, R.; del Bianco, L. Synthesis of Ferrofluids Made of Iron Oxide Nanoflowers: Interplay between Carrier Fluid and Magnetic Properties. Nanomaterials 2017, 7, 373. [Google Scholar] [CrossRef] [PubMed]
- Albarqi, H.A.; Wong, L.H.; Schumann, C.; Sabei, F.Y.; Korzun, T.; Li, X.; Hansen, M.N.; Dhagat, P.; Moses, A.S.; Taratula, O.; et al. Biocompatible Nanoclusters with High Heating Efficiency for Systemically Delivered Magnetic Hyperthermia. ACS Nano 2019, 13, 6383–6395. [Google Scholar] [CrossRef] [PubMed]
- Karaagac, O.; Yildiz, B.B.; Köçkar, H. The Influence of Synthesis Parameters on One-Step Synthesized Superparamagnetic Cobalt Ferrite Nanoparticles with High Saturation Magnetization. J. Magn. Magn. Mater. 2019, 473, 262–267. [Google Scholar] [CrossRef]
- López-Ortega, A.; Lottini, E.; Fernández, C.D.J.; Sangregorio, C. Exploring the Magnetic Properties of Cobalt-Ferrite Nanoparticles for the Development of a Rare-Earth-Free Permanent Magnet. Chem. Mater. 2015, 27, 4048–4056. [Google Scholar] [CrossRef]
- Galizia, P.; Baldisserri, C.; Capiani, C.; Galassi, C. Multiple Parallel Twinning Overgrowth in Nanostructured Dense Cobalt Ferrite. Mater. Des. 2016, 109, 19–26. [Google Scholar] [CrossRef]
- Vega-Chacón, J.; Picasso, G.; Avilés-Félix, L.; Jafelicci, M. Influence of Synthesis Experimental Parameters on the Formation of Magnetite Nanoparticles Prepared by Polyol Method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 015014. [Google Scholar] [CrossRef]
- Lak, A.; Disch, S.; Bender, P. Embracing Defects and Disorder in Magnetic Nanoparticles. Adv. Sci. 2021, 8, 2002682. [Google Scholar] [CrossRef]
- Galizia, P.; Cernea, M.; Mihalache, V.; Diamandescu, L.; Maizza, G.; Galassi, C. Easy Batch-Scale Production of Cobalt Ferrite Nanopowders by Two-Step Milling: Structural and Magnetic Characterization. Mater. Des. 2017, 130, 327–335. [Google Scholar] [CrossRef]
- Duong, G.; Roland, G.; Turtelli, R.S. Magnetoelectric Properties of CoFe2O4-BaTiO3Core-Shell Structure Composite. IEEE Trans. Magn. 2006, 42, 3611–3613. [Google Scholar] [CrossRef]
- Bibani, M.; Breitwieser, R.; Aubert, A.; Loyau, V.; Mercone, S.; Ammar, S.; Mammeri, F. Tailoring the Magnetic Properties of Cobalt Ferrite Nanoparticles Using the Polyol Process. Beilstein J. Nanotechnol. 2019, 10, 1166–1176. [Google Scholar] [CrossRef]
- Bozorth, R.M.; Tilden, E.F.; Williams, A.J. Anisotropy and Magnetostriction of Some Ferrites. Phys. Rev. 1955, 99, 1788–1798. [Google Scholar]
- Gao, J.; Xue, D.; Liu, W.; Zhou, C.; Ren, X. Recent Progress on BaTiO3-Based Piezoelectric Ceramics for Actuator Applications. Actuators 2017, 6, 24. [Google Scholar] [CrossRef]
- Trivedi, H.; Shvartsman, V.; Lupascu, D.C.; Medeiros, M.S.A.; Pullar, R.C.; Kholkin, A.L.; Zelenovskiy, P.; Sosnovskikh, A.; Shur, V.Y. Local Manifestations of a Static Magnetoelectric Effect in Nanostructured BaTiO3-BaFe12O9 Composite Multiferroics. Nanoscale 2015, 7, 4489–4496. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, E.; Toledo, D.; Smith, I.T.; Navarrete, B.; Furman, N.; Hernandez, A.F.; Telusma, M.; McDaniel, D.; Liang, P.; et al. Colossal Magnetoelectric Effect in Core-Shell Magnetoelectric Nanoparticles. Nano Lett. 2020, 20, 5765–5772. [Google Scholar] [CrossRef]
- Soetaert, F.; Korangath, P.; Serantes, D.; Fiering, S.; Ivkov, R. Cancer Therapy with Iron Oxide Nanoparticles: Agents of Thermal and Immune Therapies. Adv. Drug Deliv. Rev. 2020, 163–164, 65–83. [Google Scholar] [CrossRef]
- Shakil, M.S.; Hasan, M.A.; Uddin, M.F.; Islam, A.; Nahar, A.; Das, H.; Khan, M.N.I.; Dey, B.P.; Rokeya, B.; Hoque, S.M. In Vivo Toxicity Studies of Chitosan-Coated Cobalt Ferrite Nanocomplex for Its Application as MRI Contrast Dye. ACS Appl. Bio Mater. 2020, 3, 7952–7964. [Google Scholar] [CrossRef]
- Petrarca, C.; Poma, A.M.; Vecchiotti, G.; Bernardini, G.; Niu, Q.; Cattaneo, A.G.; di Gioacchino, M.; Sabbioni, E. Cobalt Magnetic Nanoparticles as Theranostics: Conceivable or Forgettable? Nanotechnol. Rev. 2020, 9, 1522–1538. [Google Scholar] [CrossRef]
- Gonzales, M.; Krishnan, K.M. Phase Transfer of Highly Monodisperse Iron Oxide Nanocrystals with Pluronic F127 for Biomedical Applications. J. Magn. Magn. Mater. 2007, 311, 59–62. [Google Scholar] [CrossRef]
- Nizamov, T.R.; Garanina, A.S.; Uvarova, V.I.; Naumenko, V.A.; Schetinin, I.V.; Savchenko, A.G. The Use of Iron Oxide Magnetic Nanospheres and Nanocubes for Targeted Doxorubicin Delivery into 4T1 Mouse Breast Carcinoma Cells. Bull. Russ. State Med. Univ. 2018, 7, 125–133. [Google Scholar] [CrossRef]
- Betal, S.; Dutta, M.; Shrestha, B.; Cotica, L.; Tang, L.; Bhalla, A.; Guo, R. Cell Permeation Using Core-Shell Magnetoelectric Nanoparticles. Integr. Ferroelectr. 2016, 174, 186–194. [Google Scholar] [CrossRef]
- Kaushik, A.; Nikkhah-Moshaie, R.; Sinha, R.; Bhardwaj, V.; Atluri, V.; Jayant, R.D.; Yndart, A.; Kateb, B.; Pala, N.; Nair, M. Investigation of Ac-Magnetic Field Stimulated Nanoelectroporation of Magneto-Electric Nano-Drug-Carrier inside CNS Cells. Sci. Rep. 2017, 7, srep45663. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Jayant, R.D.; Nikkhah-Moshaie, R.; Bhardwaj, V.; Roy, U.; Huang, Z.; Ruiz, A.; Yndart, A.; Atluri, V.; El-Hage, N.; et al. Magnetically Guided Central Nervous System Delivery and Toxicity Evaluation of Magneto-Electric Nanocarriers. Sci. Rep. 2016, 6, 25309. [Google Scholar] [CrossRef] [PubMed]


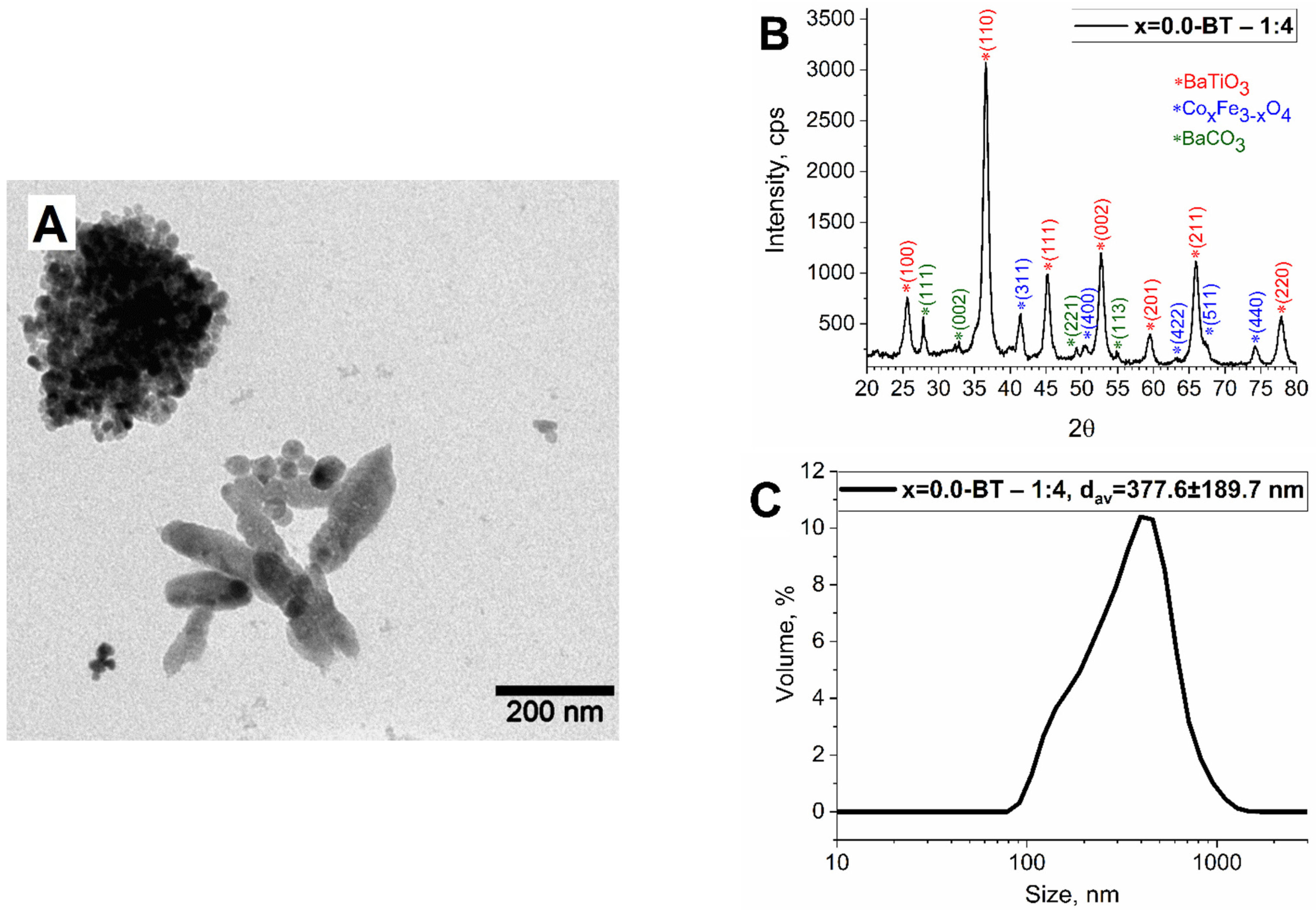
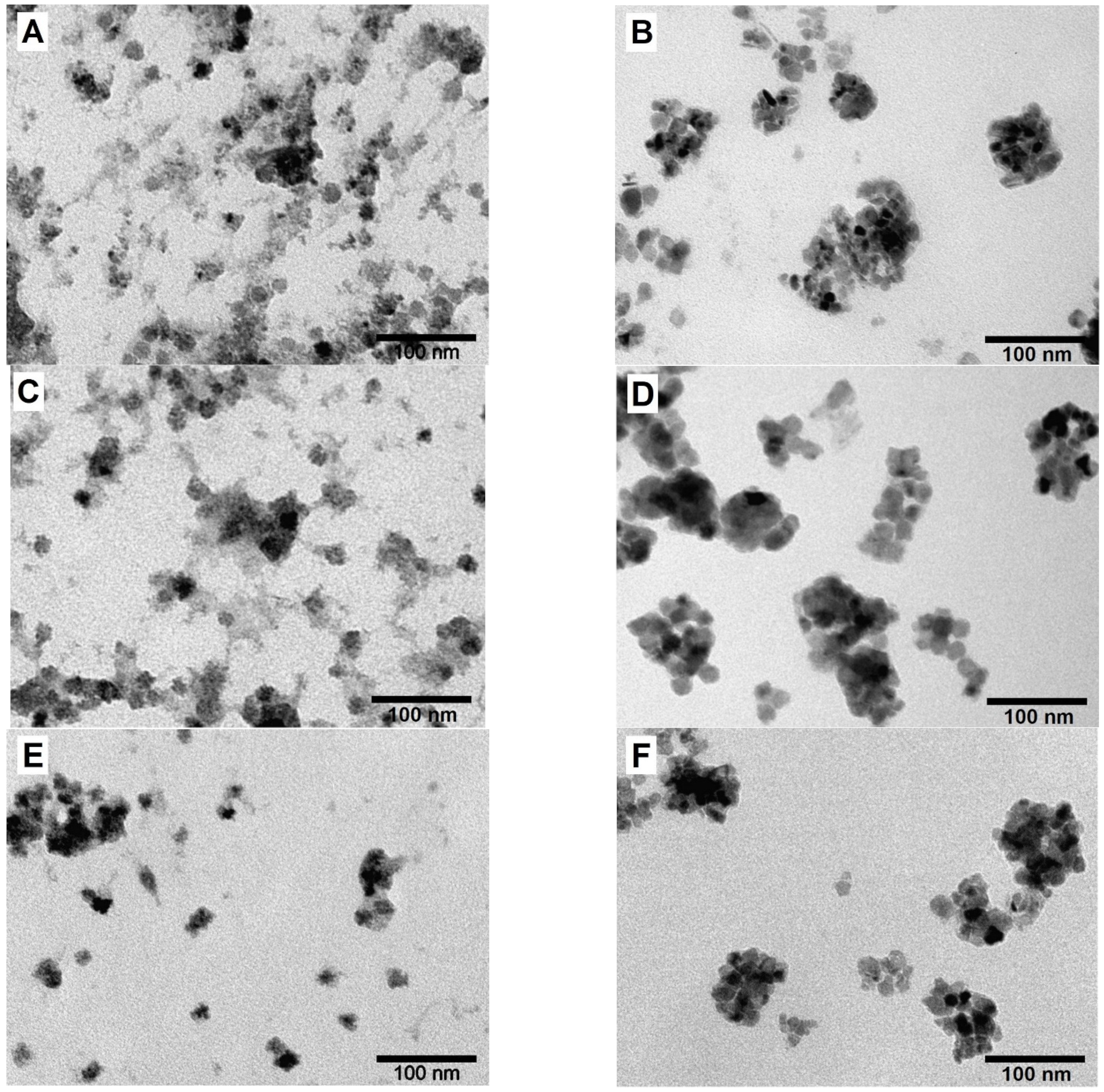
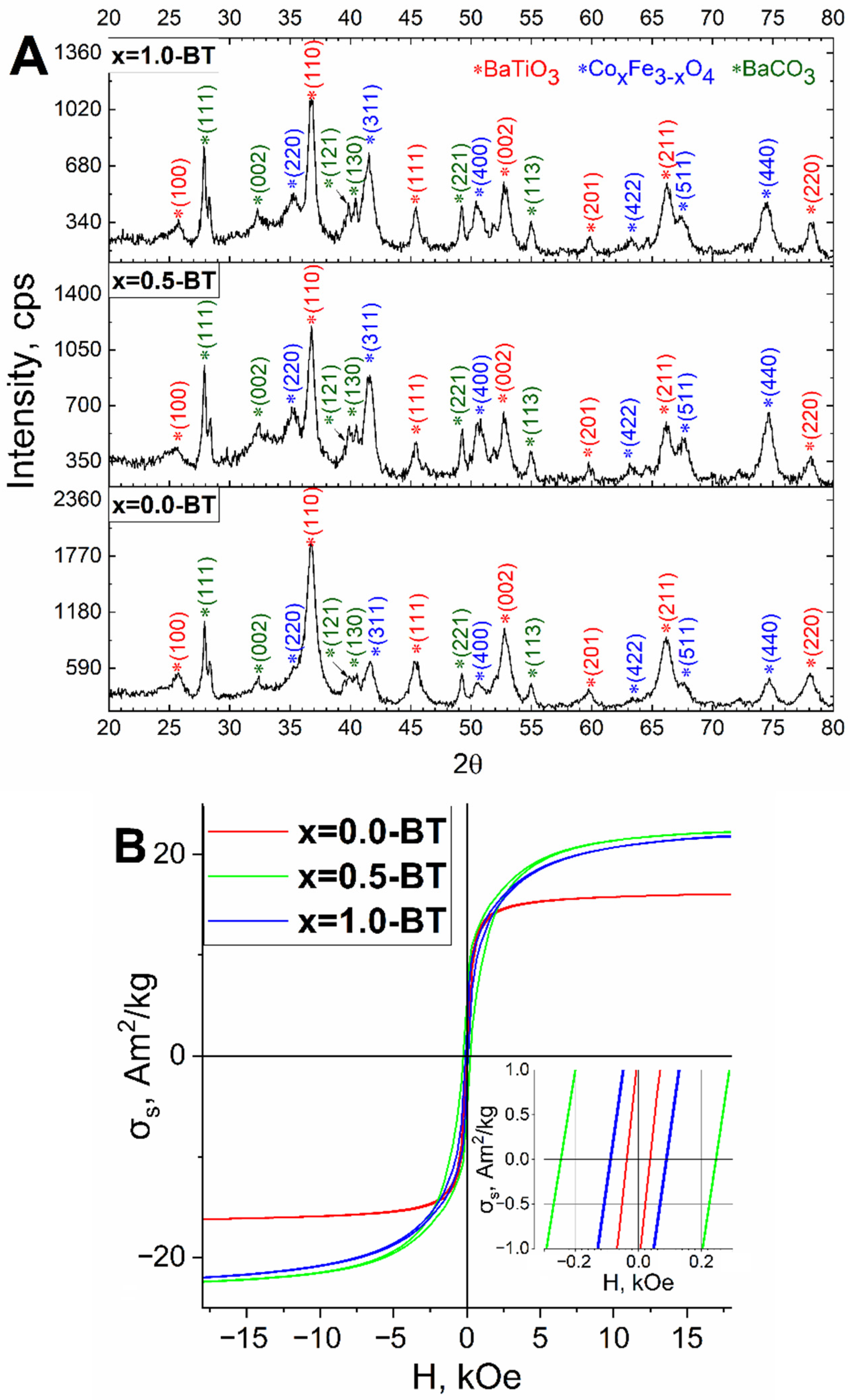
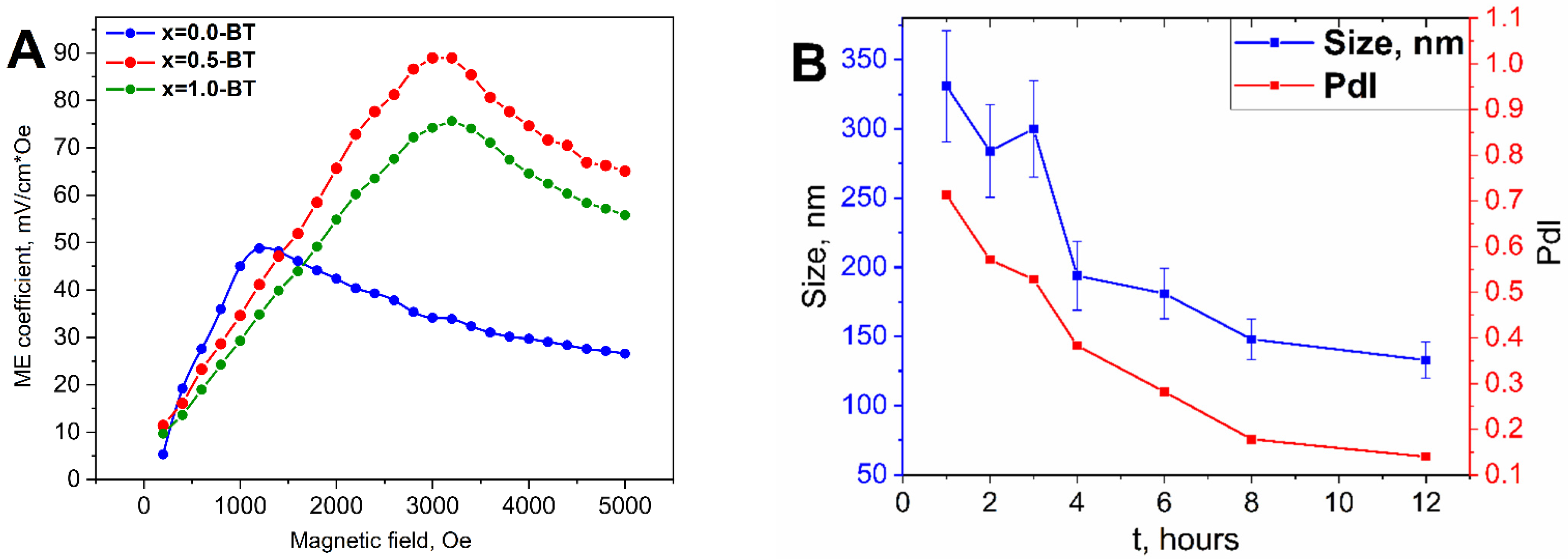

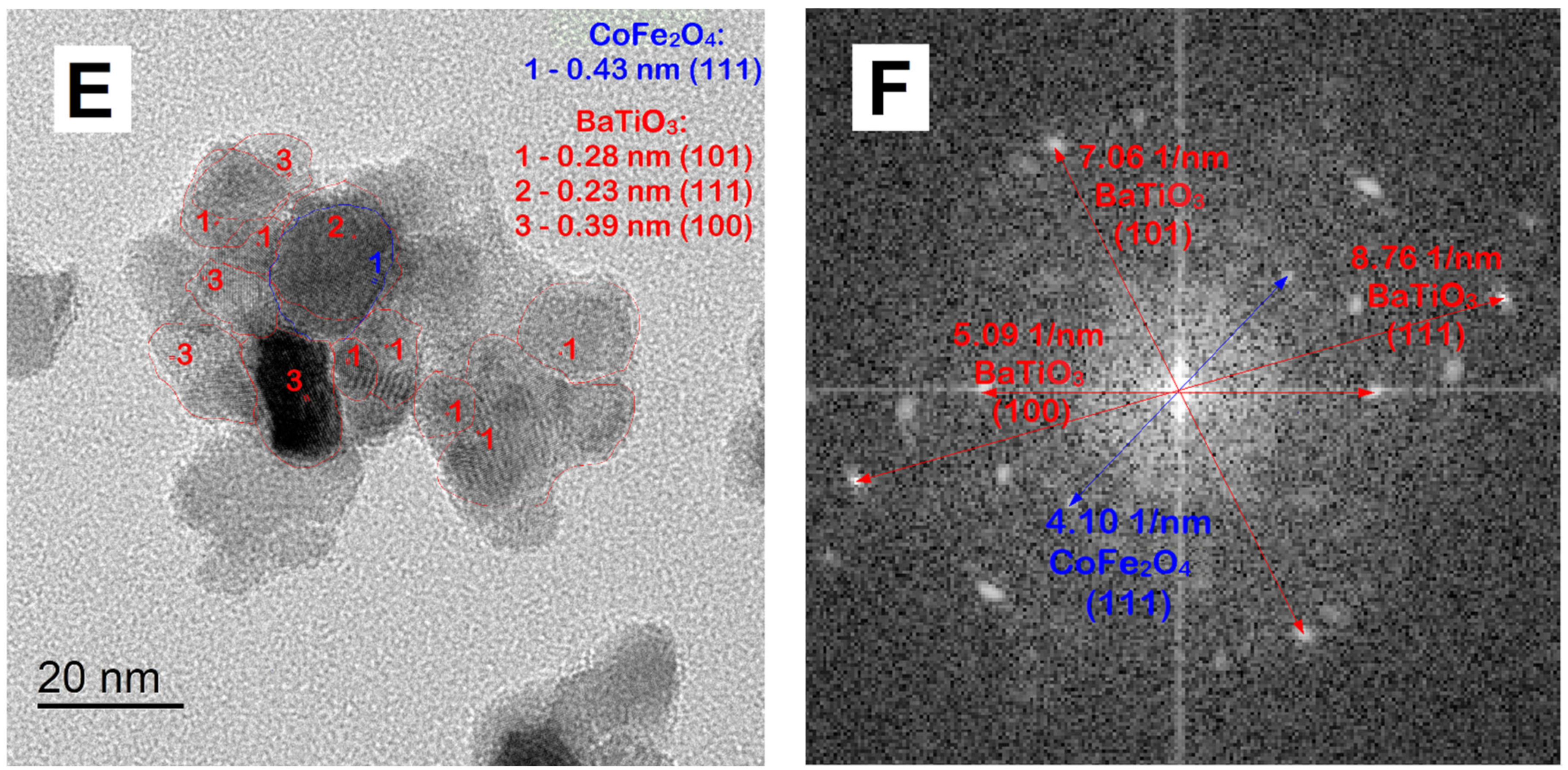
| Sample | Lattice Parameter, Å | Space Group | Phase Composition, % | Crystallite Size, nm | |
|---|---|---|---|---|---|
| x = 0.0-1 | a | 8.385 ± 0.001 | Fd3m | 100% CoxFe3−xO4 | 12.4 ± 1.2 |
| x = 0.5-1 | a | 8.395 ± 0.001 | Fd3m | 100% CoxFe3−xO4 | 9.1 ± 0.9 |
| x = 1.0-1 | a | 8.412 ± 0.001 | Fd3m | 100% CoxFe3−xO4 | 7.4 ± 0.7 |
| x = 0.0-BT | a | 4.019 ± 0.001 | 99: P4mm | 61 ± 3% BaTiO3 | 10.1 ± 1.0 |
| c | 4.018 ± 0.001 | ||||
| a | 8.376 ± 0.001 | 227: Fd-3m | 20 ± 2% CoxFe3−xO4 | 12.8 ± 1.3 | |
| a | 6.440 ± 0.001 | 62: Pnma | 18 ± 2% BaCO3 | 41.3 ± 4.1 | |
| b | 5.309 ± 0.001 | ||||
| c | 8.905 ± 0.001 | ||||
| x = 0.5-BT | a | 4.018 ± 0.001 | 99: P4mm | 29 ± 2% BaTiO3 | 13.9 ± 1.4 |
| c | 4.019 ± 0.001 | ||||
| a | 8.338 ± 0.001 | 227: Fd-3m | 49 ± 3% CoxFe3−xO4 | 13.7 ± 1.4 | |
| a | 6.439 ± 0.001 | 62: Pnma | 21 ± 2% BaCO3 | 50.4 ± 5.0 | |
| b | 5.309 ± 0.001 | ||||
| c | 8.904 ± 0.001 | ||||
| x = 1.0-BT | a | 4.019 ± 0.001 | 99: P4mm | 43 ± 2% BaTiO3 | 14.5 ± 1.5 |
| c | 4.020 ± 0.001 | ||||
| a | 8.376 ± 0.001 | 227: Fd-3m | 44 ± 2% CoxFe3−xO4 | 9.0 ± 0.9 | |
| a | 5.580 ± 0.001 | 62: Pnma | 13 ± 1% BaCO3 | 39.3 ± 3.9 | |
| b | 8.902 ± 0.001 | ||||
| c | 6.431 ± 0.001 | ||||
| Sample | Phase Composition | Hc, (Oe) | σr, A•m2/kg | σs, A•m2/kg | σs (per Magnetic Phase), A•m2/kg |
|---|---|---|---|---|---|
| x = 0.0-1 | CoxFe3−xO4 (100%) | 49 ± 2.5 | 5.14 ± 0.05 | 70.9 ± 0.7 | 70.9 ± 0.7 |
| x = 0.5-1 | CoxFe3−xO4 (100%) | 200 ± 10 | 7.66 ± 0.08 | 70.5 ± 0.7 | 70.5 ± 0.7 |
| x = 1.0-1 | CoxFe3−xO4 (100%) | 49 ± 2.5 | 6.65 ± 0.08 | 66.2 ± 0.7 | 66.2 ± 0.7 |
| x = 0.0-BT | BaTiO3 (61 ± 3%), CoxFe3−xO4 (20 ± 2%), BaCO3 (18 ± 2%) | 36 ± 2.5 | 1.01 ± 0.01 | 13.2 ± 0.1 | 66.0 ± 0.7 |
| x = 0.5-BT | BaTiO3 (29 ± 2%), CoxFe3−xO4 (49 ± 3%), BaCO3 (21 ± 2%) | 240 ± 11 | 4.32 ± 0.04 | 20.2 ± 0.2 | 40.8 ± 0.5 |
| x = 1.0-BT | BaTiO3 (43 ± 2%) CoxFe3−xO4 (44 ± 2%) BaCO3 (13 ± 1%) | 89 ± 5 | 2.24 ± 0.02 | 21.9 ± 0.2 | 49.8 ± 0.6 |
| Sample | C(Co), mg/mL | C(Fe), mg/ml | Empirical Formula |
|---|---|---|---|
| 0.0-1 | 0.00 | 2.65 ± 0.05 | Fe3O4 |
| 0.5-1 | 0.52 ± 0.05 | 3.04 ± 0.05 | Co0.42Fe2.58O4 |
| 1.0-1 | 3.05 ± 0.05 | 6.42 ± 0.05 | Co0.93Fe2.07O4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nizamov, T.R.; Amirov, A.A.; Kuznetsova, T.O.; Dorofievich, I.V.; Bordyuzhin, I.G.; Zhukov, D.G.; Ivanova, A.V.; Gabashvili, A.N.; Tabachkova, N.Y.; Tepanov, A.A.; et al. Synthesis and Functional Characterization of CoxFe3−xO4-BaTiO3 Magnetoelectric Nanocomposites for Biomedical Applications. Nanomaterials 2023, 13, 811. https://doi.org/10.3390/nano13050811
Nizamov TR, Amirov AA, Kuznetsova TO, Dorofievich IV, Bordyuzhin IG, Zhukov DG, Ivanova AV, Gabashvili AN, Tabachkova NY, Tepanov AA, et al. Synthesis and Functional Characterization of CoxFe3−xO4-BaTiO3 Magnetoelectric Nanocomposites for Biomedical Applications. Nanomaterials. 2023; 13(5):811. https://doi.org/10.3390/nano13050811
Chicago/Turabian StyleNizamov, Timur R., Abdulkarim A. Amirov, Tatiana O. Kuznetsova, Irina V. Dorofievich, Igor G. Bordyuzhin, Dmitry G. Zhukov, Anna V. Ivanova, Anna N. Gabashvili, Nataliya Yu. Tabachkova, Alexander A. Tepanov, and et al. 2023. "Synthesis and Functional Characterization of CoxFe3−xO4-BaTiO3 Magnetoelectric Nanocomposites for Biomedical Applications" Nanomaterials 13, no. 5: 811. https://doi.org/10.3390/nano13050811
APA StyleNizamov, T. R., Amirov, A. A., Kuznetsova, T. O., Dorofievich, I. V., Bordyuzhin, I. G., Zhukov, D. G., Ivanova, A. V., Gabashvili, A. N., Tabachkova, N. Y., Tepanov, A. A., Shchetinin, I. V., Abakumov, M. A., Savchenko, A. G., & Majouga, A. G. (2023). Synthesis and Functional Characterization of CoxFe3−xO4-BaTiO3 Magnetoelectric Nanocomposites for Biomedical Applications. Nanomaterials, 13(5), 811. https://doi.org/10.3390/nano13050811









