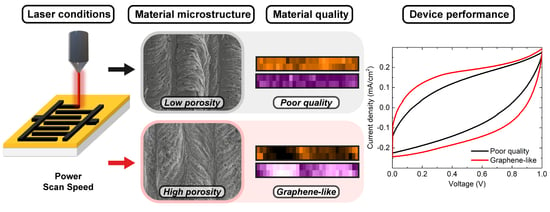
Laser-Induced Graphene Microsupercapacitors: Structure, Quality, and Performance
Abstract
1. Introduction
2. Materials and Methods
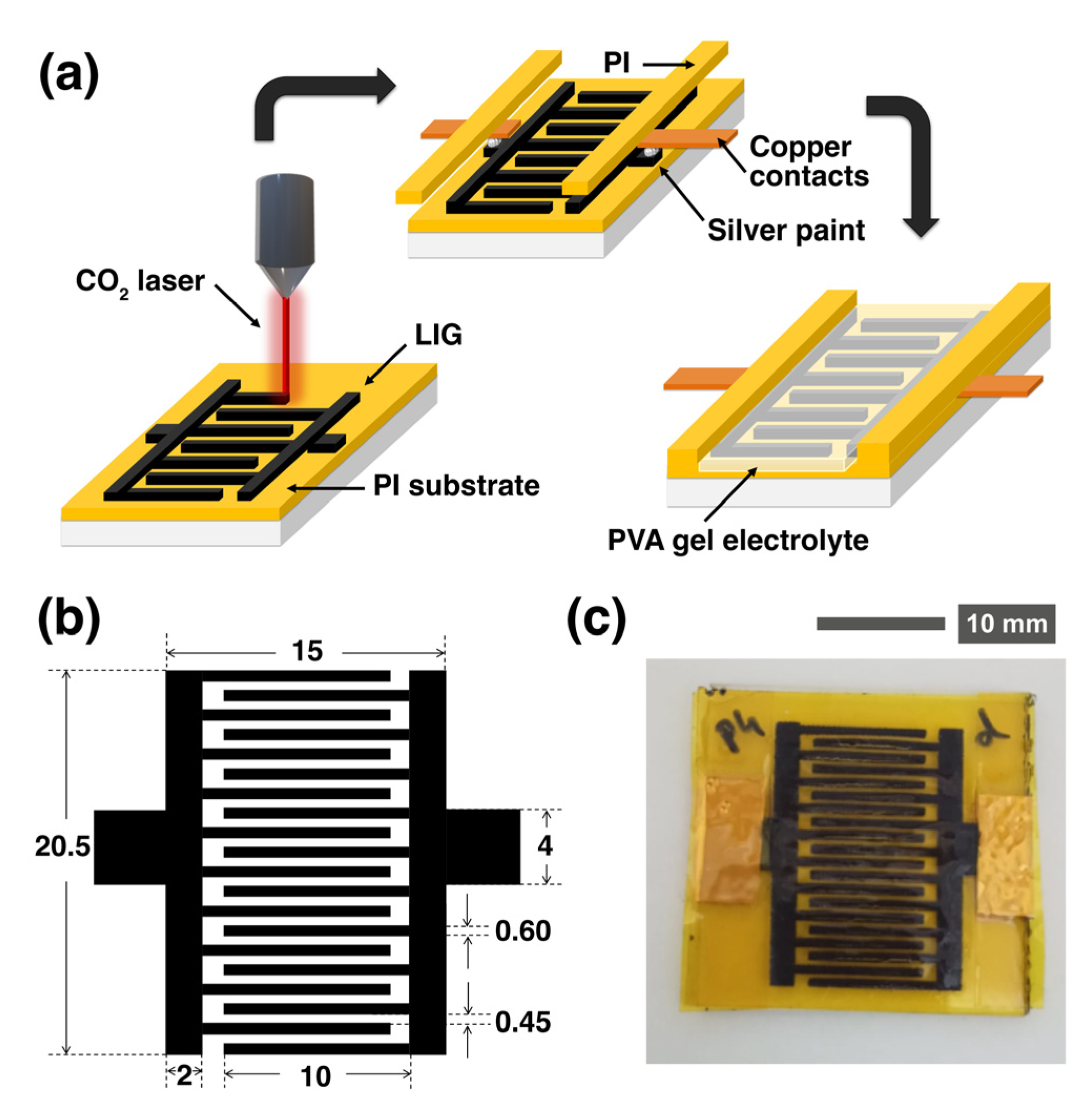
2.1. Laser Pyrolysis
2.2. MSC Fabrication
2.3. Material Characterization
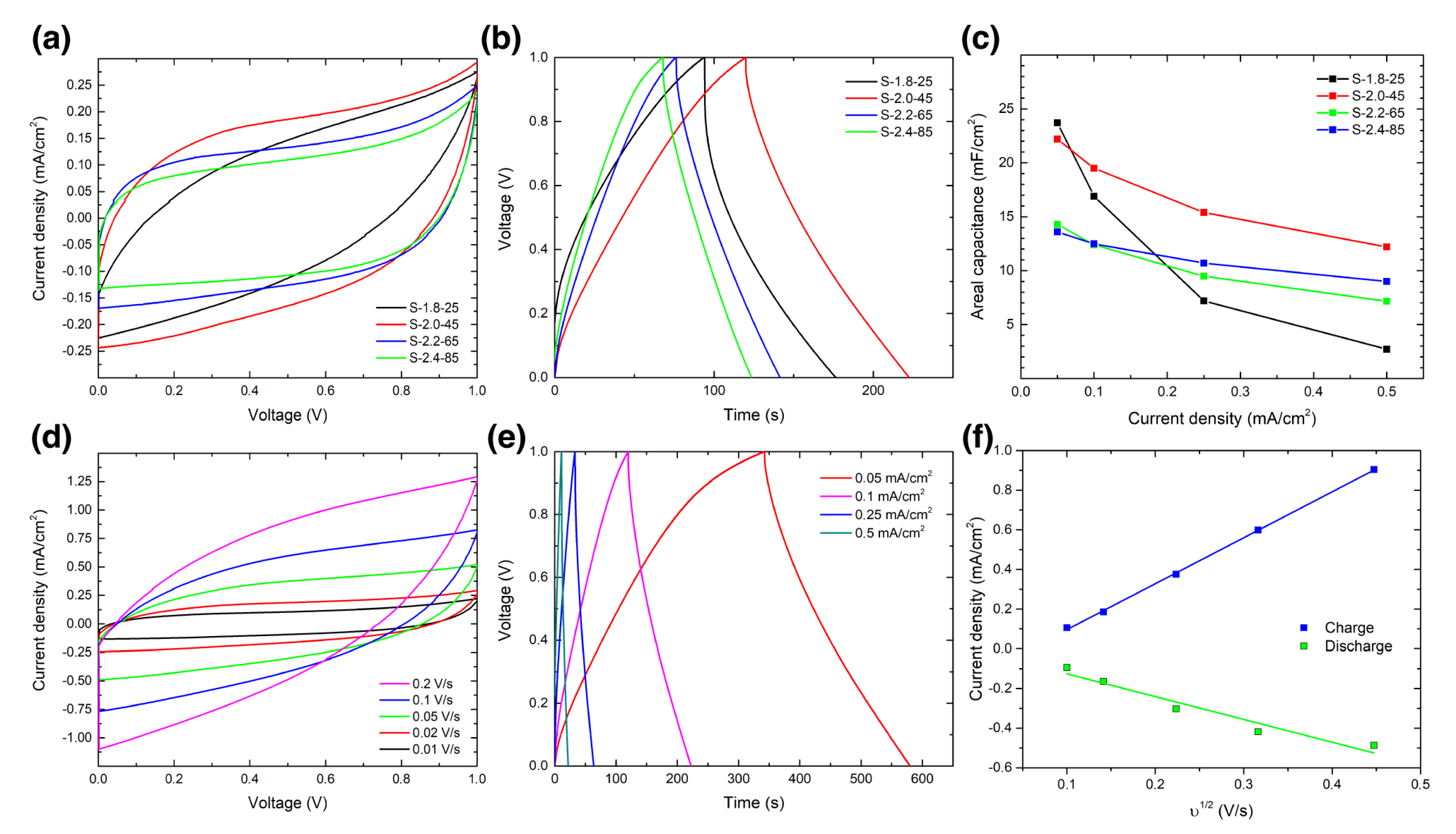
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stoller, M.D.; Park, S.; Yanwu, Z.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of Supercapacitors: Materials and Devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for Electrochemical Capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Kshetri, T.; Tran, D.T.; Nguyen, D.C.; Kim, N.H.; Lau, K.T.; Lee, J.H. Ternary Graphene-Carbon Nanofibers-Carbon Nanotubes Structure for Hybrid Supercapacitor. Chem. Eng. J. 2020, 380, 122543. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, X.C.; Guo, S.; Yu, C.; Zhong, S.; Liu, X. Multi-Functional Graphene/Carbon Nanotube Aerogels for Its Applications in Supercapacitor and Direct Methanol Fuel Cell. Electrochim. Acta 2018, 264, 12–19. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, G.; Hu, H.; Yang, J.; Qian, B.; Ling, Z.; Qiu, J. Ultrasound-Assisted Preparation of Electrospun Carbon Nanofiber/Graphene Composite Electrode for Supercapacitors. J. Power Sources 2013, 243, 350–353. [Google Scholar] [CrossRef]
- Lemine, A.S.; Zagho, M.M.; Altahtamouni, T.M.; Bensalah, N. Graphene a Promising Electrode Material for Supercapacitors—A Review. Int. J. Energy Res. 2018, 42, 4284–4300. [Google Scholar] [CrossRef]
- Xie, P.; Yuan, W.; Liu, X.; Peng, Y.; Yin, Y.; Li, Y.; Wu, Z. Advanced Carbon Nanomaterials for State-of-the-Art Flexible Supercapacitors. Energy Storage Mater. 2021, 36, 56–76. [Google Scholar] [CrossRef]
- Pedrós, J.; Boscá, A.; Martínez, J.; Ruiz-Gómez, S.; Pérez, L.; Barranco, V.; Calle, F. Polyaniline Nanofiber Sponge Filled Graphene Foam as High Gravimetric and Volumetric Capacitance Electrode. J. Power Sources 2016, 317, 35–42. [Google Scholar] [CrossRef]
- Velasco, A.; Ryu, Y.K.; Boscá, A.; Ladrón-De-Guevara, A.; Hunt, E.; Zuo, J.; Pedrós, J.; Calle, F.; Martinez, J. Recent Trends in Graphene Supercapacitors: From Large Area to Microsupercapacitors. Sustain. Energy Fuels 2021, 5, 1235–1254. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-Induced Porous Graphene Films from Commercial Polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, M.F.; Kaner, R.B. Direct Laser Writing of Graphene Electronics. ACS Nano 2014, 8, 8725–8729. [Google Scholar] [CrossRef]
- Tiliakos, A.; Ceaus, C.; Iordache, S.M.; Vasile, E.; Stamatin, I. Morphic Transitions of Nanocarbons via Laser Pyrolysis of Polyimide Films. J. Anal. Appl. Pyrolysis 2016, 121, 275–286. [Google Scholar] [CrossRef]
- Wang, F.; Wang, K.; Zheng, B.; Dong, X.; Mei, X.; Lv, J.; Duan, W.; Wang, W. Laser-Induced Graphene: Preparation, Functionalization and Applications. Mater. Technol. 2018, 33, 340–356. [Google Scholar] [CrossRef]
- Ladrón-de-Guevara, A.; Boscá, A.; Pedrós, J.; Climent-Pascual, E.; de Andrés, A.; Calle, F.; Martínez, J. Reduced Graphene Oxide/Polyaniline Electrochemical Supercapacitors Fabricated by Laser. Appl. Surf. Sci. 2019, 467–468, 691–697. [Google Scholar] [CrossRef]
- Cao, L.; Zhu, S.; Pan, B.; Dai, X.; Zhao, W.; Liu, Y.; Xie, W.; Kuang, Y.; Liu, X. Stable and Durable Laser-Induced Graphene Patterns Embedded in Polymer Substrates. Carbon 2020, 163, 85–94. [Google Scholar] [CrossRef]
- Ye, R.; James, D.K.; Tour, J.M. Laser-Induced Graphene: From Discovery to Translation. Adv. Mater. 2019, 31, e1803621. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, M.; Hao, J.; Wu, K.; Li, C.; Hu, C. Visible Light Laser-Induced Graphene from Phenolic Resin: A New Approach for Directly Writing Graphene-Based Electrochemical Devices on Various Substrates. Carbon 2018, 127, 287–296. [Google Scholar] [CrossRef]
- Chyan, Y.; Ye, R.; Li, Y.; Singh, S.P.; Arnusch, C.J.; Tour, J.M. Laser-Induced Graphene by Multiple Lasing: Toward Electronics on Cloth, Paper, and Food. ACS Nano 2018, 12, 2176–2183. [Google Scholar] [CrossRef]
- Lamberti, A.; Perrucci, F.; Caprioli, M.; Serrapede, M.; Fontana, M.; Bianco, S.; Ferrero, S.; Tresso, E. New Insights on Laser-Induced Graphene Electrodes for Flexible Supercapacitors: Tunable Morphology and Physical Properties. Nanotechnology 2017, 28, 174002. [Google Scholar] [CrossRef] [PubMed]
- Abdulhafez, M.; Tomaraei, G.N.; Bedewy, M. Fluence-Dependent Morphological Transitions in Laser-Induced Graphene Electrodes on Polyimide Substrates for Flexible Devices. ACS Appl. Nano Mater. 2021, 4, 2973–2986. [Google Scholar] [CrossRef]
- Li, Y.; Luong, D.X.; Zhang, J.; Tarkunde, Y.R.; Kittrell, C.; Sargunaraj, F.; Ji, Y.; Arnusch, C.J.; Tour, J.M. Laser-Induced Graphene in Controlled Atmospheres: From Superhydrophilic to Superhydrophobic Surfaces. Adv. Mater. 2017, 29, 1700496. [Google Scholar] [CrossRef]
- Ma, W.; Zhu, J.; Wang, Z.; Song, W.; Cao, G. Recent Advances in Preparation and Application of Laser-Induced Graphene in Energy Storage Devices. Mater. Today Energy 2020, 18, 100569. [Google Scholar] [CrossRef]
- Le, T.S.D.; Phan, H.P.; Kwon, S.; Park, S.; Jung, Y.; Min, J.; Chun, B.J.; Yoon, H.; Ko, S.H.; Kim, S.W.; et al. Recent Advances in Laser-Induced Graphene: Mechanism, Fabrication, Properties, and Applications in Flexible Electronics. Adv. Funct. Mater. 2022, 32, 2205158. [Google Scholar] [CrossRef]
- Vivaldi, F.M.; Dallinger, A.; Bonini, A.; Poma, N.; Sembranti, L.; Biagini, D.; Salvo, P.; Greco, F.; di Francesco, F. Three-Dimensional (3D) Laser-Induced Graphene: Structure, Properties, and Application to Chemical Sensing. ACS Appl. Mater. Interfaces 2021, 13, 30245–30260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, R.; Feng, J.; Xie, Y.; Zhou, T. Laser Direct Writing of Flexible Heaters on Polymer Substrates. Ind. Eng. Chem. Res. 2021, 60, 11161–11170. [Google Scholar] [CrossRef]
- Mamleyev, E.R.; Heissler, S.; Nefedov, A.; Weidler, P.G.; Nordin, N.; Kudryashov, V.V.; Länge, K.; MacKinnon, N.; Sharma, S. Laser-Induced Hierarchical Carbon Patterns on Polyimide Substrates for Flexible Urea Sensors. NPJ Flexible Electron. 2019, 3, 2. [Google Scholar] [CrossRef]
- Bobinger, M.R.; Romero, F.J.; Salinas-Castillo, A.; Becherer, M.; Lugli, P.; Morales, D.P.; Rodríguez, N.; Rivadeneyra, A. Flexible and Robust Laser-Induced Graphene Heaters Photothermally Scribed on Bare Polyimide Substrates. Carbon N Y 2019, 144, 116–126. [Google Scholar] [CrossRef]
- Shangguan, Q.; Zhao, Y.; Song, Z.; Wang, J.; Yang, H.; Chen, J.; Liu, C.; Cheng, S.; Yang, W.; Yi, Z. High Sensitivity Active Adjustable Graphene Absorber for Refractive Index Sensing Applications. Diam. Relat. Mater. 2022, 128, 109273. [Google Scholar] [CrossRef]
- Cheng, Z.; Liao, J.; He, B.; Zhang, F.; Zhang, F.; Huang, X.; Zhou, L. One-Step Fabrication of Graphene Oxide Enhanced Magnetic Composite Gel for Highly Efficient Dye Adsorption and Catalysis. ACS Sustain. Chem. Eng. 2015, 3, 1677–1685. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, R.; Long, F.; Wang, J. Development and Application of Tetrabromobisphenol A Imprinted Electrochemical Sensor Based on Graphene/Carbon Nanotubes Three-Dimensional Nanocomposites Modified Carbon Electrode. Talanta 2015, 134, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, Q.; Chen, Z.; Yang, H.; Cheng, S.; Yang, W.; Yi, Z.; Wu, X.; Wang, S.; Yi, Y.; Wu, P. Design of Ultra-Narrow Band Graphene Refractive Index Sensor. Sensors 2022, 22, 6483. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Lin, J.; Ye, R.; Samuel, E.L.G.; Tour, J.M. Flexible and Stackable Laser-Induced Graphene Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 3414–3419. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.J.; Salinas-Castillo, A.; Rivadeneyra, A.; Albrecht, A.; Godoy, A.; Morales, D.P.; Rodriguez, N. In-Depth Study of Laser Diode Ablation of Kapton Polyimide for Flexible Conductive Substrates. Nanomaterials 2018, 8, 517. [Google Scholar] [CrossRef]
- Feurer, T.; Sauerbrey, R.; Smayling, M.C.; Story, B.J. Ultraviolet-Laser-Induced Permanent Electrical Conductivity in Polyimide. Appl. Phys. A Solids Surf. 1993, 56, 275–281. [Google Scholar] [CrossRef]
- Wang, H.; Xiang, Z.; Wan, J.; Song, Y.; Zhang, H. Double-Sided Laser-Induced Graphene Based Smart Bracelet for Sensing and Energy. In Proceedings of the 2021 IEEE 34th International Conference on Micro Electro Mechanical Systems (MEMS), Gainesville, FL, USA, 25–29 January 2021; pp. 34–37. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, L.; Xia, Y.; Qiu, R.; Liu, W.; Wu, M.; Zhu, Y.; Zhu, S.; Jia, C.; Zhu, M.; et al. Flexible High-Resolution Triboelectric Sensor Array Based on Patterned Laser-Induced Graphene for Self-Powered Real-Time Tactile Sensing. Adv. Funct. Mater. 2021, 31, 1–9. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, D.; Chen, Z.; Yu, H.; Chen, Q.; Sun, D. Laser Induced Graphene Patterns on a Thin Polyimide Film via a Cooling Plate. In Proceedings of the 2021 IEEE 16th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Xiamen, China, 25–29 April 2021; pp. 1650–1654. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Fernandes, A.J.S.; Leitão, C.; Deuermeier, J.; Marques, A.C.; Martins, R.; Fortunato, E.; Costa, F.M. Laser-Induced Graphene Strain Sensors Produced by Ultraviolet Irradiation of Polyimide. Adv. Funct. Mater. 2018, 28, 1805271. [Google Scholar] [CrossRef]
- Tao, L.Q.; Tian, H.; Liu, Y.; Ju, Z.Y.; Pang, Y.; Chen, Y.Q.; Wang, D.Y.; Tian, X.G.; Yan, J.C.; Deng, N.Q.; et al. An Intelligent Artificial Throat with Sound-Sensing Ability Based on Laser Induced Graphene. Nat. Commun. 2017, 8, 14579. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Penner, R.M. Energy Storage in Nanomaterials—Capacitive, Pseudocapacitive, or Battery-Like? ACS Nano 2018, 12, 2081–2083. [Google Scholar] [CrossRef]
- Voiry, D.; Chhowalla, M.; Gogotsi, Y.; Kotov, N.A.; Li, Y.; Penner, R.M.; Schaak, R.E.; Weiss, P.S. Best Practices for Reporting Electrocatalytic Performance of Nanomaterials. ACS Nano 2018, 12, 9635–9638. [Google Scholar] [CrossRef]
- Sun, S.; Wang, S.; Li, S.; Li, Y.; Zhang, Y.; Chen, J.; Zhang, Z.; Fang, S.; Wang, P. Asymmetric Supercapacitors Based on a NiCo2O4/Three Dimensional Graphene Composite and Three Dimensional Graphene with High Energy Density. J. Mater. Chem. A Mater. 2016, 4, 18646–18653. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999; ISBN 0-306-45736-9. [Google Scholar]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Díez-Betriu, X.; Álvarez-García, S.; Botas, C.; Álvarez, P.; Sánchez-Marcos, J.; Prieto, C.; Menéndez, R.; de Andrés, A. Raman Spectroscopy for the Study of Reduction Mechanisms and Optimization of Conductivity in Graphene Oxide Thin Films. J. Mater. Chem. C Mater. 2013, 1, 6905–6912. [Google Scholar] [CrossRef]
- Ni, Z.; Wang, Y.; Yu, T.; Shen, Z. Raman Spectroscopy and Imaging of Graphene. Nano Res. 2008, 1, 273–291. [Google Scholar] [CrossRef]
- Silva, D.L.; Campos, J.L.E.; Fernandes, T.F.D.; Rocha, J.N.; Machado, L.R.P.; Soares, E.M.; Miquita, D.R.; Miranda, H.; Rabelo, C.; Vilela Neto, O.P.; et al. Raman Spectroscopy Analysis of Number of Layers in Mass-Produced Graphene Flakes. Carbon 2020, 161, 181–189. [Google Scholar] [CrossRef]
- Anemone, G.; Climent-Pascual, E.; Yu, H.K.; al Taleb, A.; Jiménez-Villacorta, F.; Prieto, C.; Wodtke, A.M.; de Andrés, A.; Farías, D. Quality of Graphene on Sapphire: Long-Range Order from Helium Diffraction versus Lattice Defects from Raman Spectroscopy. RSC Adv. 2016, 6, 21235–21245. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive Contributions to Electrochemical Energy Storage in TiO2 (Anatase) Nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Mustarelli, P.; Quartarone, E.; Tomasi, C.; Magistris, A. New Materials for Polymer Electrolytes. Solid State Ion. 2000, 135, 81–86. [Google Scholar] [CrossRef]
- Khandelwal, M.; van Tran, C.; In, J. Nitrogen and Phosphorous Co-Doped Laser-Induced Graphene: A High-Performance Electrode Material for Supercapacitor Applications. Appl. Surf. Sci. 2022, 576, 151714. [Google Scholar] [CrossRef]
- Zaccagnini, P.; Lamberti, A. A Perspective on Laser-Induced Graphene for Micro-Supercapacitor Application. Appl. Phys. Lett. 2022, 120, 100501. [Google Scholar] [CrossRef]
- Kim, K.Y.; Choi, H.; van Tran, C.; In, J. Simultaneous Densification and Nitrogen Doping of Laser-Induced Graphene by Duplicated Pyrolysis for Supercapacitor Applications. J. Power Sources 2019, 441, 227199. [Google Scholar] [CrossRef]
- Li, L.; Zhang, J.; Peng, Z.; Li, Y.; Gao, C.; Ji, Y.; Ye, R.; Kim, N.D.; Zhong, Q.; Yang, Y.; et al. High-Performance Pseudocapacitive Microsupercapacitors from Laser-Induced Graphene. Adv. Mater. 2016, 28, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, M.; van Tran, C.; Lee, J.; In, J. Nitrogen and Boron Co-Doped Densified Laser-Induced Graphene for Supercapacitor Applications. Chem. Eng. J. 2021, 428, 131119. [Google Scholar] [CrossRef]
- Yagati, A.K.; Behrent, A.; Beck, S.; Rink, S.; Goepferich, A.M.; Min, J.; Lee, M.H.; Baeumner, A.J. Laser-Induced Graphene Interdigitated Electrodes for Label-Free or Nanolabel-Enhanced Highly Sensitive Capacitive Aptamer-Based Biosensors. Biosens. Bioelectron. 2020, 164, 112272. [Google Scholar] [CrossRef] [PubMed]
- Barber, R.; Cameron, S.; Devine, A.; McCombe, A.; Kirsty Pourshahidi, L.; Cundell, J.; Roy, S.; Mathur, A.; Casimero, C.; Papakonstantinou, P.; et al. Laser Induced Graphene Sensors for Assessing PH: Application to Wound Management. Electrochem. Commun. 2021, 123, 106914. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; Teh, K.S.; Qi, M.; Zang, X.; Ding, X.; Cui, Y.; Xie, Y.; Wu, Y.; Ma, H.; et al. High-Voltage Flexible Microsupercapacitors Based on Laser-Induced Graphene. ACS Appl. Mater. Interfaces 2018, 10, 26357–26364. [Google Scholar] [CrossRef]
- Wang, W.; Lu, L.; Li, Z.; Xie, Y. Laser Induced 3D Porous Graphene Dots: Bottom-up Growth Mechanism, Multi-Physics Coupling Effect and Surface Wettability. Appl. Surf. Sci 2022, 592, 153242. [Google Scholar] [CrossRef]
- Wang, W.; Lu, L.; Xie, Y.; Mei, X.; Tang, Y.; Wu, W.; Liang, R. Tailoring the Surface Morphology and Nanoparticle Distribution of Laser-Induced Graphene/Co3O4 for High-Performance Flexible Microsupercapacitors. Appl. Surf. Sci 2020, 504, 144487. [Google Scholar] [CrossRef]
- Wang, W.; Lu, L.; Lu, X.; Liang, Z.; Lin, H.; Li, Z.; Wu, X.; Lin, L.; Xie, Y. Scorpion-Inspired Dual-Bionic, Microcrack-Assisted Wrinkle Based Laser Induced Graphene-Silver Strain Sensor with High Sensitivity and Broad Working Range for Wireless Health Monitoring System. Nano Res. 2022, 16, 1228–1241. [Google Scholar] [CrossRef]
- Liao, J.; Zhang, X.; Sun, Z.; Chen, H.; Fu, J.; Si, H.; Ge, C.; Lin, S. Laser-Induced Graphene-Based Wearable Epidermal Ion-Selective Sensors for Noninvasive Multiplexed Sweat Analysis. Biosensors 2022, 12, 397. [Google Scholar] [CrossRef]
- Karimi, G.; Lau, I.; Fowler, M.; Pope, M. Parametric Study of Laser-Induced Graphene Conductive Traces and Their Application as Flexible Heaters. Int J. Energy Res. 2021, 45, 13712–13725. [Google Scholar] [CrossRef]
- Wang, W.; Lu, L.; Li, Z.; Lin, L.; Liang, Z.; Lu, X.; Xie, Y. Fingerprint-Inspired Strain Sensor with Balanced Sensitivity and Strain Range Using Laser-Induced Graphene. ACS Appl Mater. Interfaces 2022, 14, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ji, H.; Lv, X.; Zeng, C.; Li, H.; Li, F.; Qu, B.; Cui, F.; Zhou, Q. Laser-Induced Graphene (LIG)-Driven Medical Sensors for Health Monitoring and Diseases Diagnosis. Microchim. Acta 2022, 189, 54. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, L.; Wang, Z.; Wang, S.; Xu, R. Boosting the Performance of Flexible In-Plane Micro-Supercapacitors by Engineering MoS2 Nanoparticles Embedded in Laser-Induced Graphene. J. Alloys Compd. 2021, 887, 161514. [Google Scholar] [CrossRef]
- Clerici, F.; Fontana, M.; Bianco, S.; Serrapede, M.; Perrucci, F.; Ferrero, S.; Tresso, E.; Lamberti, A. In Situ MoS2 Decoration of Laser-Induced Graphene as Flexible Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2016, 8, 10459–10465. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, Z.; Huang, C.; Zhang, B.; Xing, C.; Chen, H.; Cheng, H.; Wang, J.; Tang, S. High-Energy All-in-One Stretchable Micro-Supercapacitor Arrays Based on 3D Laser-Induced Graphene Foams Decorated with Mesoporous ZnP Nanosheets for Self-Powered Stretchable Systems. Nano Energy 2021, 81, 105609. [Google Scholar] [CrossRef]
- Xu, R.; Liu, P.; Ji, G.; Gao, L.; Zhao, J. Versatile Strategy to Design Flexible Planar-Integrated Microsupercapacitors Based on Co3O4-Decorated Laser-Induced Graphene. ACS Appl. Energy Mater. 2020, 3, 10676–10684. [Google Scholar] [CrossRef]
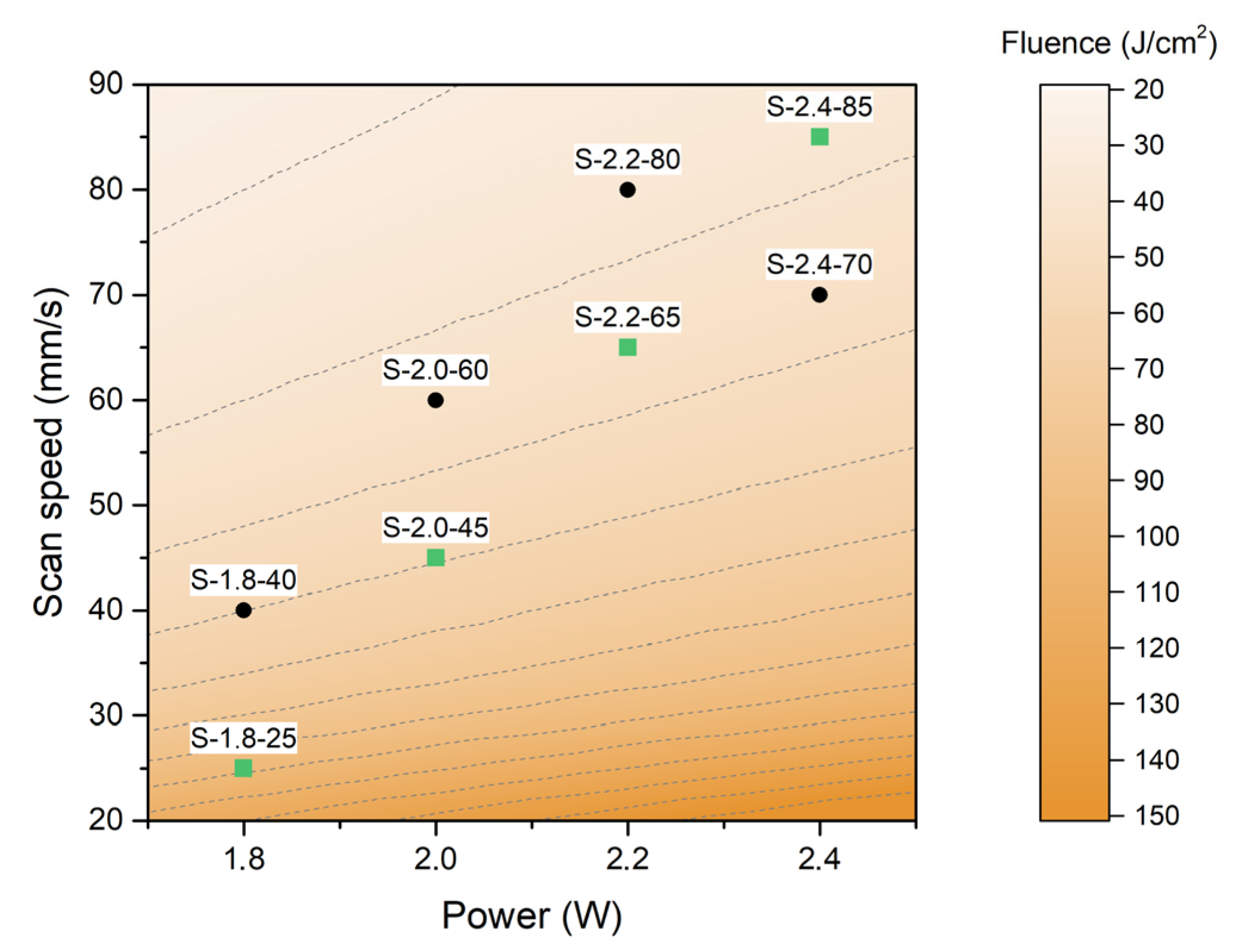
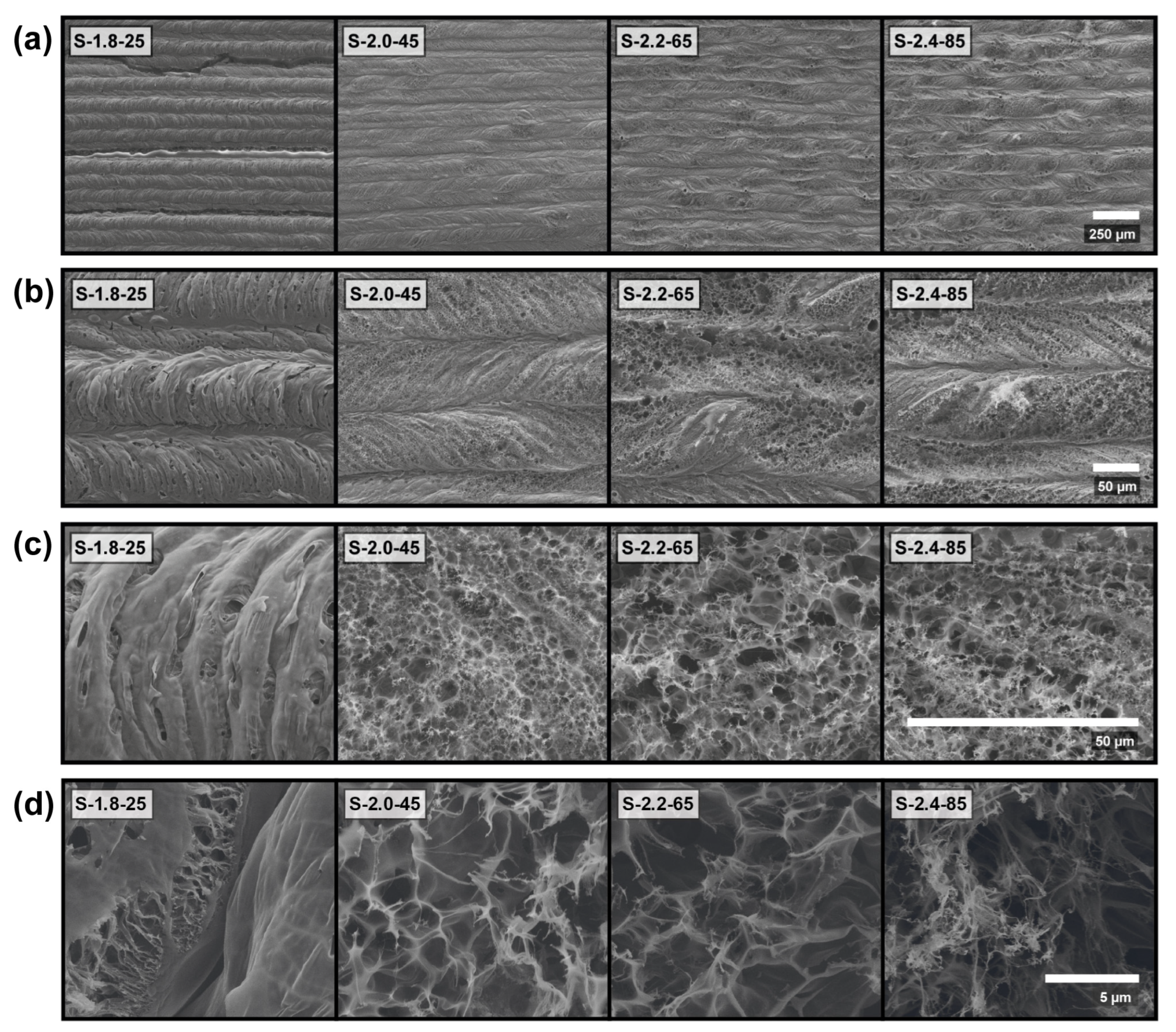


| Sample Name | Power (W) | Scan Speed (mm/s) | Fluence (J/cm2) |
|---|---|---|---|
| S-1.8-25 (*) | 1.8 | 25 | 96 |
| S-1.8-40 | 1.8 | 40 | 60 |
| S-2.0-45 (*) | 2.0 | 45 | 59.3 |
| S-2.0-60 | 2.0 | 60 | 44.4 |
| S-2.2-65 (*) | 2.2 | 65 | 45.1 |
| S-2.2-80 | 2.2 | 80 | 36.7 |
| S-2.4-70 | 2.4 | 70 | 45.7 |
| S-2.4-85 (*) | 2.4 | 85 | 37.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velasco, A.; Ryu, Y.K.; Hamada, A.; de Andrés, A.; Calle, F.; Martinez, J. Laser-Induced Graphene Microsupercapacitors: Structure, Quality, and Performance. Nanomaterials 2023, 13, 788. https://doi.org/10.3390/nano13050788
Velasco A, Ryu YK, Hamada A, de Andrés A, Calle F, Martinez J. Laser-Induced Graphene Microsupercapacitors: Structure, Quality, and Performance. Nanomaterials. 2023; 13(5):788. https://doi.org/10.3390/nano13050788
Chicago/Turabian StyleVelasco, Andres, Yu Kyoung Ryu, Assia Hamada, Alicia de Andrés, Fernando Calle, and Javier Martinez. 2023. "Laser-Induced Graphene Microsupercapacitors: Structure, Quality, and Performance" Nanomaterials 13, no. 5: 788. https://doi.org/10.3390/nano13050788
APA StyleVelasco, A., Ryu, Y. K., Hamada, A., de Andrés, A., Calle, F., & Martinez, J. (2023). Laser-Induced Graphene Microsupercapacitors: Structure, Quality, and Performance. Nanomaterials, 13(5), 788. https://doi.org/10.3390/nano13050788