Oxygen Vacancy Mediated Band-Gap Engineering via B-Doping for Enhancing Z-Scheme A-TiO2/R-TiO2 Heterojunction Photocatalytic Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of the Catalysts
2.3. Characterizations
2.4. Evaluation of Photodegradation Activity
3. Results and Discussion
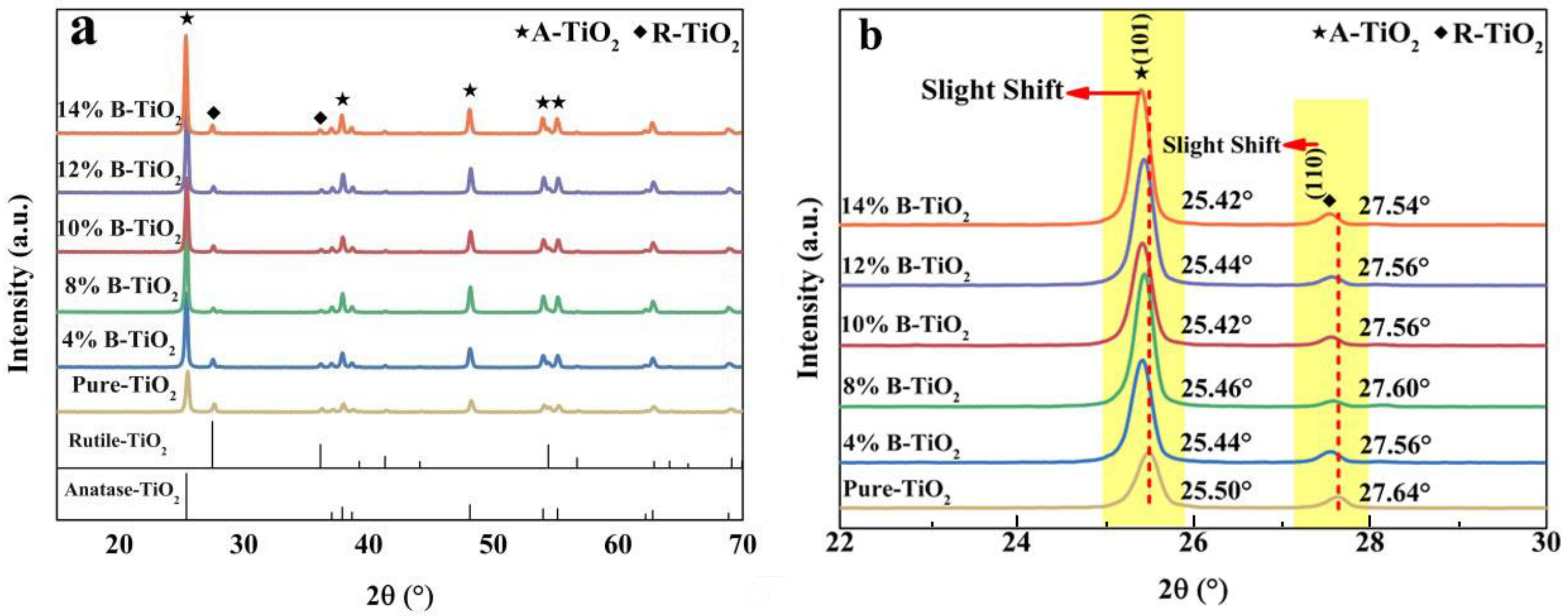
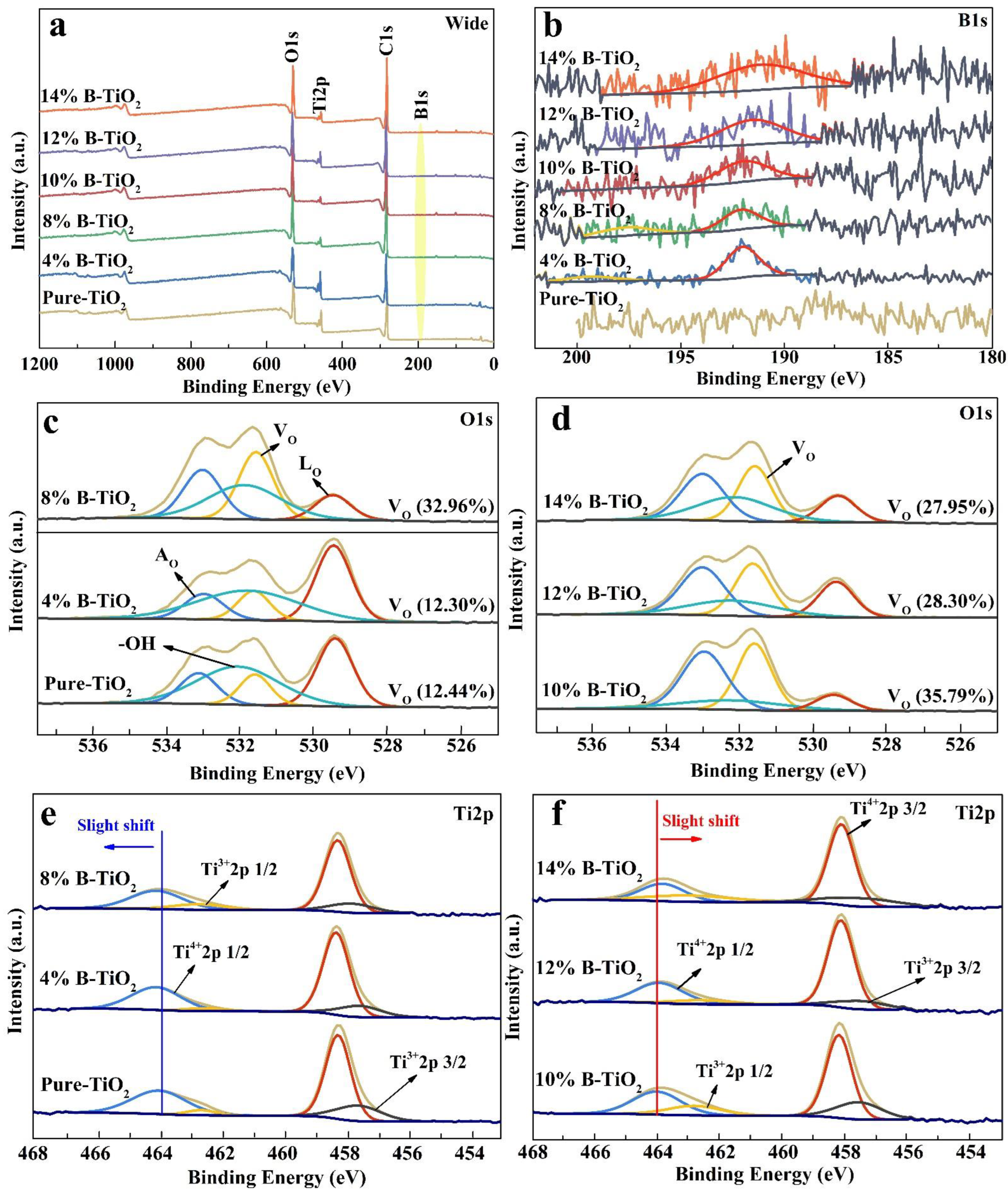
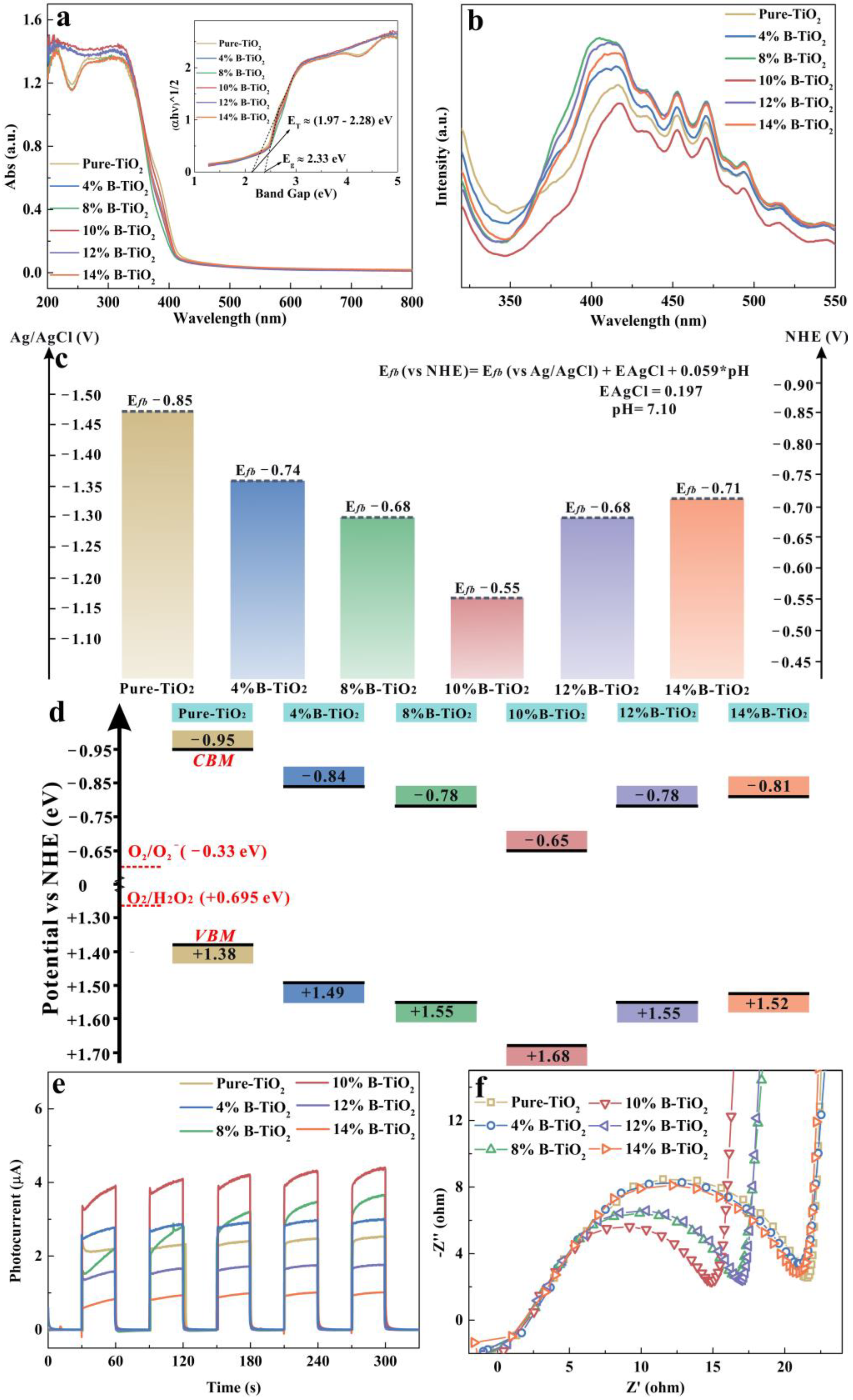
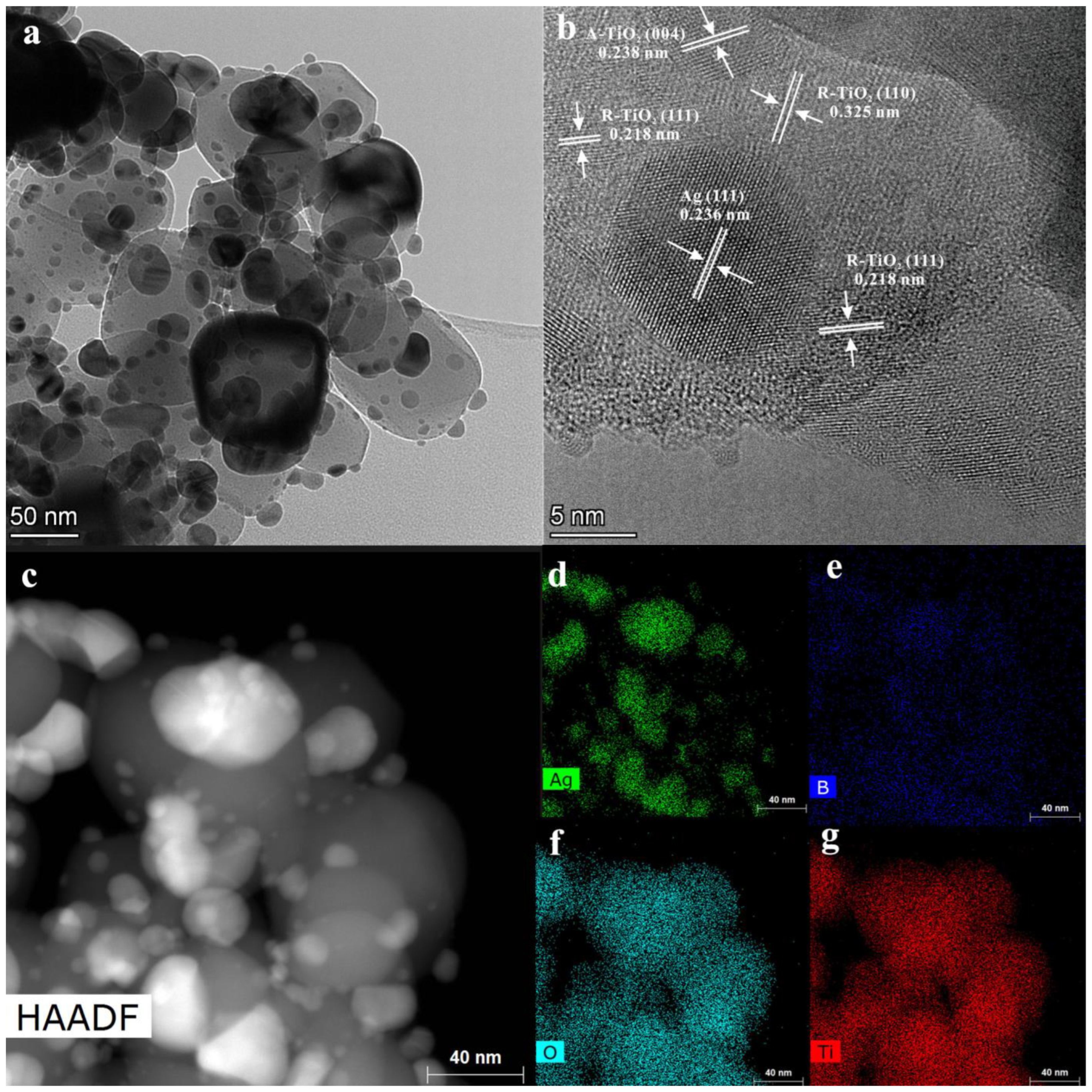
3.1. Microstructure of the Catalysts
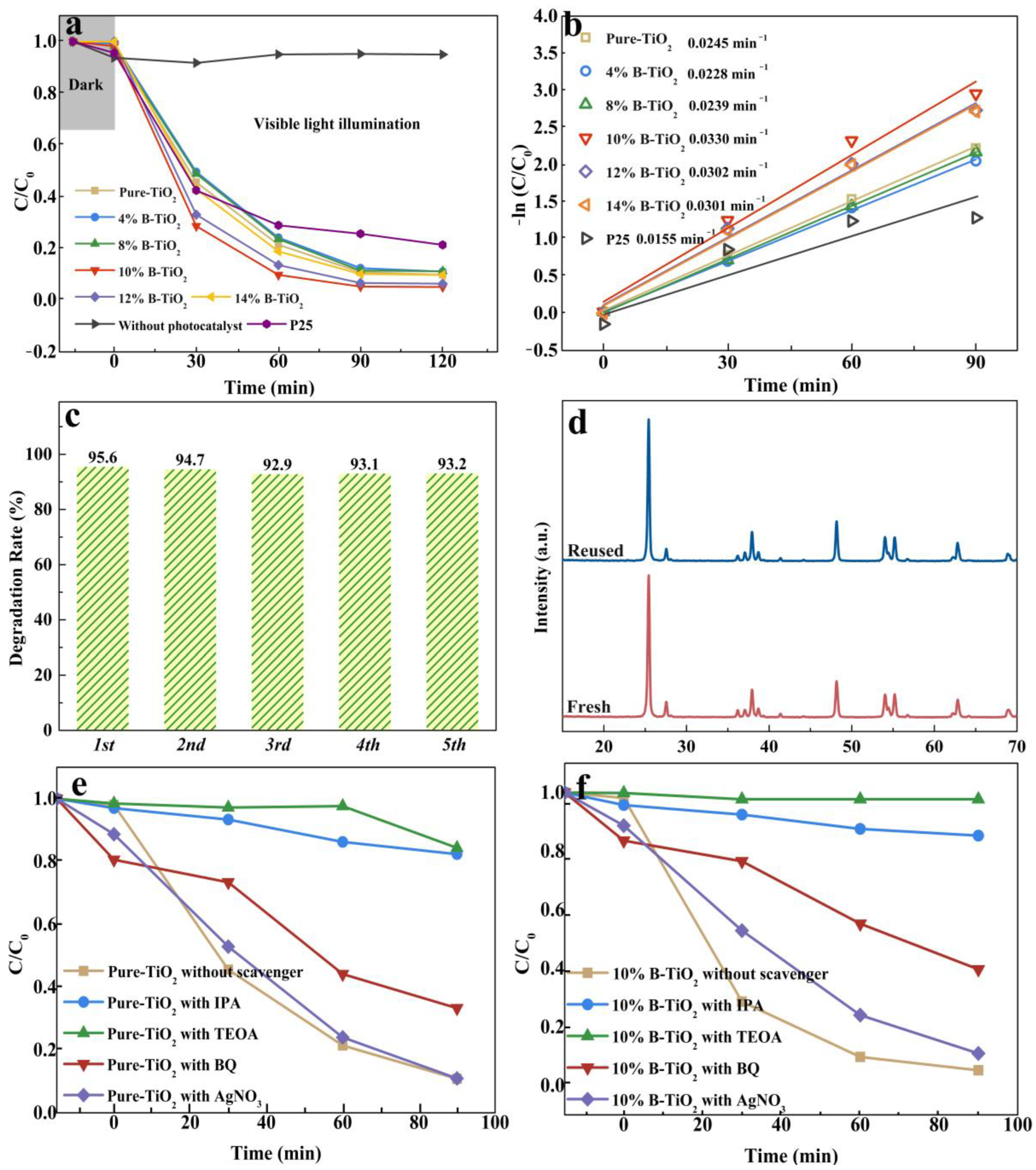
3.2. Photodegradation Activities of the Catalysts
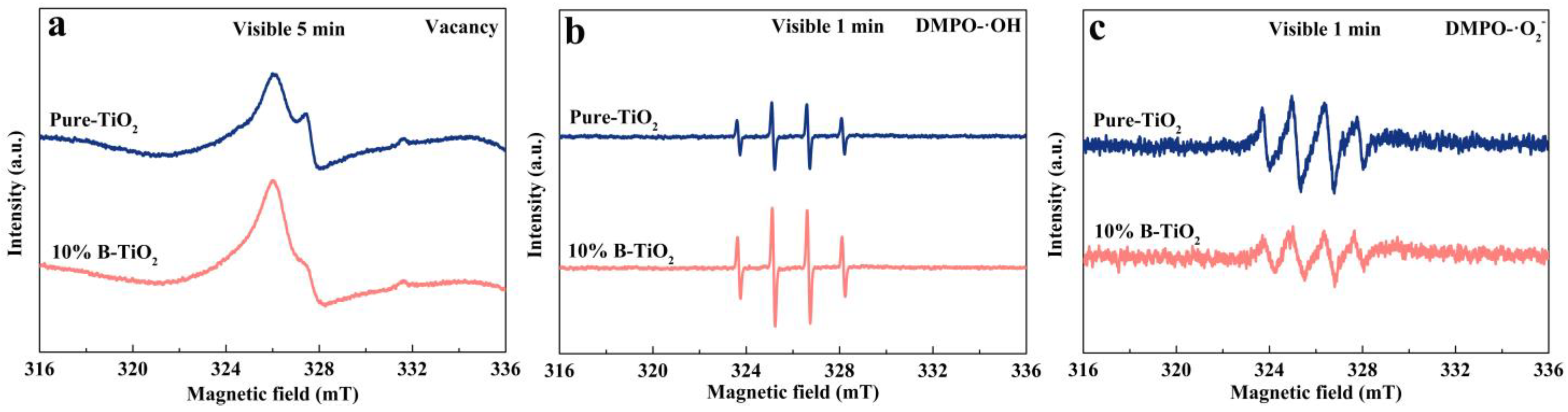
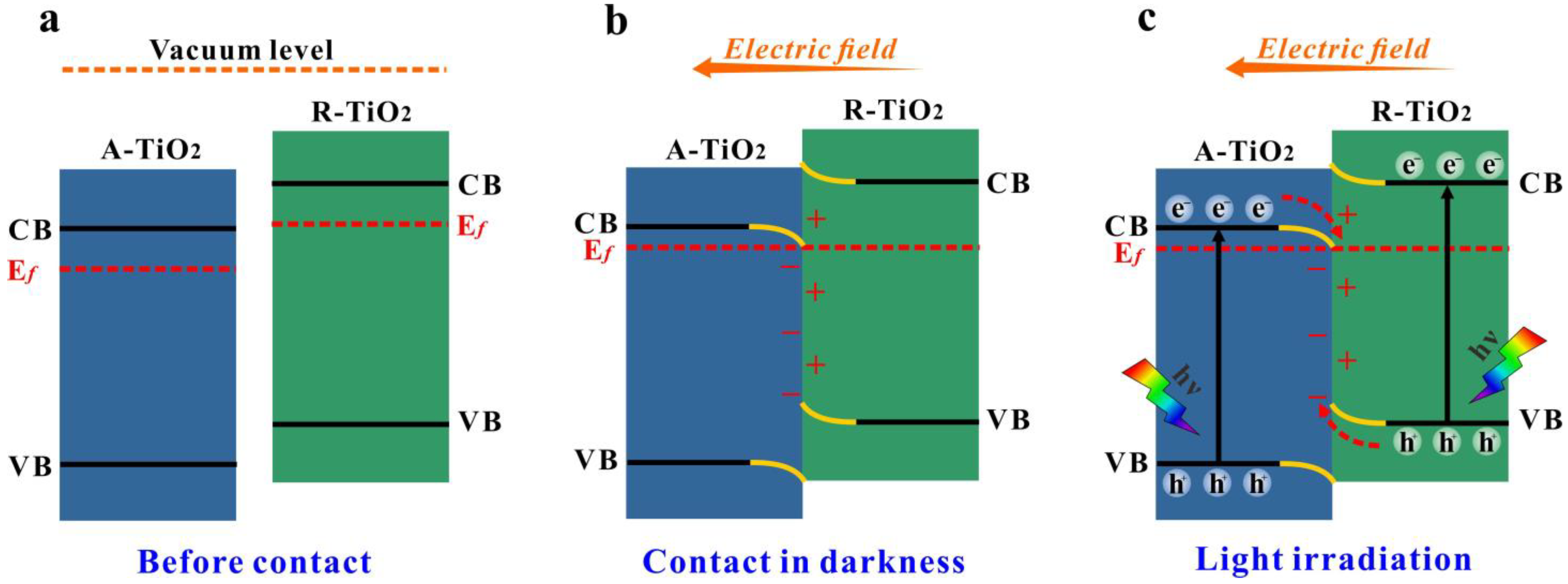
3.3. Photodegradation Mechanism of the Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carey, J.H.; Lawrence, J.; Tosine, H.M. Photodechlorination of PCB’s in the Presence of Titanium Dioxide in Aqueous Suspensions. Bull. Environ. Contam. Toxicol. 1976, 16, 697–701. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Q.; Jiang, W.; Wei, Z.; Zhu, Y. Separation-Free TiO2-Graphene Hydrogel with 3D Network Structure for Efficient Photoelectrocatalytic Mineralization. Appl. Catal. B Environ. 2017, 211, 106–113. [Google Scholar] [CrossRef]
- Deng, Y.; Xing, M.; Zhang, J. An Advanced TiO2/Fe2TiO5/Fe2O3 Triple-Heterojunction with Enhanced and Stable Visible-Light-Driven Fenton Reaction for the Removal of Organic Pollutants. Appl. Catal. B Environ. 2017, 211, 157–166. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, I.S.; Li, Z.; Xia, X.; Lee, W.I.; Dai, S.; Bahnemann, D.W.; Pan, J.H. Nanoporous TiO2 Spheres with Tailored Textural Properties: Controllable Synthesis, Formation Mechanism, and Photochemical Applications. Prog. Mater. Sci. 2020, 109, 100620. [Google Scholar] [CrossRef]
- Friedmann, D.; Hakki, A.; Kim, H.; Choi, W.; Bahnemann, D. ChemInform Abstract: Heterogeneous Photocatalytic Organic Synthesis: State-of-the-Art and Future Perspectives. Chem. Inform. 2016, 47, 5391–5411. [Google Scholar] [CrossRef]
- Fu, X.; Clark, L.A.; Yang, Q.; Anderson, M.A. Enhanced Photocatalytic Performance of Titania-Based Binary Metal Oxides: TiO2/SiO2 and TiO2/ZrO2. Environ. Sci. Technol. 1996, 30, 647–653. [Google Scholar] [CrossRef]
- Espíndola, J.C.; Cristóvão, R.O.; Mendes, A.; Boaventura, R.A.R.; Vilar, V.J.P. Photocatalytic Membrane Reactor Performance towards Oxytetracycline Removal from Synthetic and Real Matrices: Suspended vs Immobilized TiO2-P25. Chem. Eng. J. 2019, 378, 122114. [Google Scholar] [CrossRef]
- Eshete, M.; Li, X.; Yang, L.; Wang, X.; Zhang, J.; Xie, L.; Deng, L.; Zhang, G.; Jiang, J. Charge Steering in Heterojunction Photocatalysis: General Principles, Design, Construction, and Challenges. Small Sci. 2023, 1, 2200041. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Dhodamani, A.G.; Suzuki, N.; Terashima, C.; Fujishima, A.; Mathe, V.L. Enhanced Photocatalytic Performance of Ultrasound Treated GO/TiO2 Composite for Photocatalytic Degradation of Salicylic Acid under Sunlight Illumination. Ultrason. Sonochem. 2020, 61, 104849. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Wu, Y.; Sun, L.; Zhang, L.; Chang, X.; Zhang, X.; Wang, X. Enhanced Photocatalytic Activity by Tailoring the Interface in TiO2–ZrTiO4 Heterostructure in TiO2–ZrTiO4–SiO2 Ternary System. Ceram. Int. 2019, 45, 17163–17172. [Google Scholar] [CrossRef]
- Qiang, W.; Wei, L.; Shaodan, W.; Yu, B. Superior Environment Resistance of Quartz Crystal Microbalance with Anatase TiO2/ZnO Nanorod Composite Films. Appl. Surf. Sci. 2015, 347, 755–762. [Google Scholar] [CrossRef]
- He, X.; Wu, M.; Ao, Z.; Lai, B.; Zhou, Y.; An, T.; Wang, S. Metal–Organic Frameworks Derived C/TiO2 for Visible Light Photocatalysis: Simple Synthesis and Contribution of Carbon Species. J. Hazard. Mater. 2021, 403, 124048. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Jaroniec, M. Direct Z-Scheme Photocatalysts: Principles, Synthesis, and Applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. A Review on TiO2-Based Z-Scheme Photocatalysts. Chin. J. Catal. 2017, 38, 1936–1955. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Liang, J.; Wu, Z.; Wang, H. Construction of an All-Solid-State Z-Scheme Photocatalyst Based on Graphite Carbon Nitride and Its Enhancement to Catalytic Activity. Environ. Sci. Nano 2018, 5, 599–615. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-Scheme Heterojunction Photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Zeng, Z.; Jing, D.; Guo, L. Efficient Hydrogen Production in a Spotlight Reactor with Plate Photocatalyst of TiO2/NiO Heterojunction Supported on Nickel Foam. Energy 2021, 228, 120578. [Google Scholar] [CrossRef]
- Drmosh, Q.A.; Hezam, A.; Hendi, A.H.Y.; Qamar, M.; Yamani, Z.H.; Byrappa, K. Ternary Bi2S3/MoS2/TiO2 with Double Z-Scheme Configuration as High Performance Photocatalyst. Appl. Surf. Sci. 2020, 499, 143938. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, C.; Zuo, G.; Guo, Y.; Xiao, W.; Dai, Y.; Kong, J.; Xu, X.; Zhou, Y.; Xie, A.; et al. 0D/2D Co3O4/TiO2 Z-Scheme Heterojunction for Boosted Photocatalytic Degradation and Mechanism Investigation. Appl. Catal. B Environ. 2020, 278, 119298. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, X.; Easton, C.D.; Li, Q.; Xia, Y.; Yin, Y.; Hu, X.; Hu, J.; Xia, D.; McCarthy, D.T.; et al. An in Situ Assembled WO3–TiO2 Vertical Heterojunction for Enhanced Z-Scheme Photocatalytic Activity. Nanoscale 2020, 12, 8775–8784. [Google Scholar] [CrossRef]
- Wei, T.; Zhu, Y.-N.; An, X.; Liu, L.-M.; Cao, X.; Liu, H.; Qu, J. Defect Modulation of Z-Scheme TiO2/Cu2O Photocatalysts for Durable Water Splitting. ACS Catal. 2019, 9, 8346–8354. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, W.; Meng, Y.; Xia, S. A Direct Z-Scheme Heterojunction with Boosted Transportation of Photogenerated Charge Carriers for Highly Efficient Photodegradation of PFOA: Reaction Kinetics and Mechanism. Appl. Catal. B Environ. 2021, 285, 119851. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, W.; Wang, R.; Zhang, Z.; Qiu, S. Interface Engineering of Hierarchical Photocatalyst for Enhancing Photoinduced Charge Transfers. Appl. Catal. B Environ. 2021, 283, 119632. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Z.; Zhang, J.; Zhang, Z. Fabrication of Vertical Orthorhombic/Hexagonal Tungsten Oxide Phase Junction with High Photocatalytic Performance. Appl. Catal. B Environ. 2017, 207, 207–217. [Google Scholar] [CrossRef]
- Qiu, Y.; Ouyang, F. Fabrication of TiO2 Hierarchical Architecture Assembled by Nanowires with Anatase/TiO2(B) Phase-Junctions for Efficient Photocatalytic Hydrogen Production. Appl. Surf. Sci. 2017, 403, 691–698. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Wang, Z.; Cao, Y.; Feng, J.; Wang, Z.; Ma, Y. Fabrication of TiO2 (B)/Anatase Heterophase Junctions in Nanowires via a Surface-Preferred Phase Transformation Process for Enhanced Photocatalytic Activity. Chin. J. Catal. 2018, 39, 1500–1510. [Google Scholar] [CrossRef]
- Niu, P.; Wu, G.; Chen, P.; Zheng, H.; Cao, Q.; Jiang, H. Optimization of Boron Doped TiO2 as an Efficient Visible Light-Driven Photocatalyst for Organic Dye Degradation With High Reusability. Front. Chem. 2020, 8, 172. [Google Scholar] [CrossRef]
- Ng, B.; Putri, L.K.; Kong, X.Y.; Teh, Y.W.; Pasbakhsh, P.; Chai, S. Z-Scheme Photocatalytic Systems for Solar Water Splitting. Adv. Sci. 2020, 7, 1903171. [Google Scholar] [CrossRef]
- Chen, F.; Ma, Z.; Ye, L.; Ma, T.; Zhang, T.; Zhang, Y.; Huang, H. Macroscopic Spontaneous Polarization and Surface Oxygen Vacancies Collaboratively Boosting CO2 Photoreduction on BiOIO3 Single Crystals. Adv. Mater. 2020, 32, 1908350. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Shi, W.; Ling, P.; Sun, Y.; Jiao, X.; Gao, S.; Liang, L.; Xu, J.; Yan, W.; et al. Efficient Visible-Light-Driven CO2 Reduction Mediated by Defect-Engineered BiOBr Atomic Layers. Angew. Chem. 2018, 130, 8855–8859. [Google Scholar] [CrossRef]
- Dong, C.; Lian, C.; Hu, S.; Deng, Z.; Gong, J.; Li, M.; Liu, H.; Xing, M.; Zhang, J. Size-Dependent Activity and Selectivity of Carbon Dioxide Photocatalytic Reduction over Platinum Nanoparticles. Nat. Commun. 2018, 9, 1252. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, Y.; Yu, Y.; Zhang, B. Oxygen Vacancy Engineering in Photocatalysis. Sol. RRL 2020, 4, 2000037. [Google Scholar] [CrossRef]
- Sarkar, A.; Khan, G.G. The Formation and Detection Techniques of Oxygen Vacancies in Titanium Oxide-Based Nanostructures. Nanoscale 2019, 11, 3414–3444. [Google Scholar] [CrossRef]
- You, M.; Kim, T.G.; Sung, Y.-M. Synthesis of Cu-Doped TiO2 Nanorods with Various Aspect Ratios and Dopant Concentrations. Cryst. Growth Des. 2010, 10, 983–987. [Google Scholar] [CrossRef]
- Bilgin Simsek, E. Solvothermal Synthesized Boron Doped TiO2 Catalysts: Photocatalytic Degradation of Endocrine Disrupting Compounds and Pharmaceuticals under Visible Light Irradiation. Appl. Catal. B Environ. 2017, 200, 309–322. [Google Scholar] [CrossRef]
- Li, L.; Yang, Y.; Liu, X.; Fan, R.; Shi, Y.; Li, S.; Zhang, L.; Fan, X.; Tang, P.; Xu, R.; et al. A Direct Synthesis of B-Doped TiO2 and Its Photocatalytic Performance on Degradation of RhB. Appl. Surf. Sci. 2013, 265, 36–40. [Google Scholar] [CrossRef]
- Wang, W.-K.; Chen, J.-J.; Gao, M.; Huang, Y.-X.; Zhang, X.; Yu, H.-Q. Photocatalytic Degradation of Atrazine by Boron-Doped TiO2 with a Tunable Rutile/Anatase Ratio. Appl. Catal. B Environ. 2016, 195, 69–76. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with Oxygen Vacancies: Synthesis, Properties and Photocatalytic Applications. Nanoscale 2013, 5, 3601. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, M.; Pastrana-Martínez, L.M.; Pereira, M.F.R.; Faria, J.L.; Figueiredo, J.L.; Silva, A.M.T. N/S-Doped Graphene Derivatives and TiO2 for Catalytic Ozonation and Photocatalysis of Water Pollutants. Chem. Eng. J. 2018, 348, 888–897. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Xu, C.; Wu, Y.; Hu, X.; Hou, X. Boron-Doped Rutile TiO2/Anatase TiO2/ZrTiO4 Ternary Heterojunction Photocatalyst with Optimized Phase Interface and Band Structure. Ceram. Int. 2020, 46, 20943–20953. [Google Scholar] [CrossRef]
- Arif, M.; Zhang, M.; Mao, Y.; Bu, Q.; Ali, A.; Qin, Z.; Muhmood, T.; Liu, X.; Zhou, B.; Chen, S.M. Oxygen Vacancy Mediated Single Unit Cell Bi2WO6 by Ti Doping for Ameliorated Photocatalytic Performance. J. Colloid Interface Sci. 2021, 581, 276–291. [Google Scholar] [CrossRef] [PubMed]
- Quesada-González, M.; Boscher, N.D.; Carmalt, C.J.; Parkin, I.P. Interstitial Boron-Doped TiO2 Thin Films: The Significant Effect of Boron on TiO2 Coatings Grown by Atmospheric Pressure Chemical Vapor Deposition. ACS Appl. Mater. Interfaces 2016, 8, 25024–25029. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Peng, F.; Wang, H.; Yu, H.; Zhang, S.; Zhao, H. A New Insight into Regulating High Energy Facets of Rutile TiO2. J. Mater. Chem. A 2013, 1, 4182. [Google Scholar] [CrossRef]
- Abdullah, S.A.; Sahdan, M.Z.; Nafarizal, N.; Saim, H.; Embong, Z.; Rohaida, C.H.C.; Adriyanto, F. Influence of Substrate Annealing on Inducing Ti3+ and Oxygen Vacancy in TiO2 Thin Films Deposited via RF Magnetron Sputtering. Appl. Surf. Sci. 2018, 462(DEC.31), 575–582. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Hua, L.; Li, S.; Zhang, X.; Sheng, W.; Cao, S. High Photocatalytic Activity of Hierarchical SiO2@C-Doped TiO2 Hollow Spheres in UV and Visible Light towards Degradation of Rhodamine B. J. Hazard. Mater. 2017, 340, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Ząbek, P.; Eberl, J.; Kisch, H. On the Origin of Visible Light Activity in Carbon-Modified Titania. Photochem. Photobiol. Sci. 2009, 8, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Maarisetty, D.; Baral, S.S. Defect Engineering in Photocatalysis: Formation, Chemistry, Optoelectronics, and Interface Studies. J. Mater. Chem. A 2020, 8, 18560–18604. [Google Scholar] [CrossRef]
- Zhang, L.; Tse, M.S.; Tan, O.K.; Wang, Y.X.; Han, M. Facile Fabrication and Characterization of Multi-Type Carbon-Doped TiO2 for Visible Light-Activated Photocatalytic Mineralization of Gaseous Toluene. J. Mater. Chem. A 2013, 1, 4497. [Google Scholar] [CrossRef]
- Lin, J.; Heo, Y.-U.; Nattestad, A.; Sun, Z.; Wang, L.; Kim, J.H.; Dou, S.X. 3D Hierarchical Rutile TiO2 and Metal-Free Organic Sensitizer Producing Dye-Sensitized Solar Cells 8.6% Conversion Efficiency. Sci. Rep. 2014, 4, 5769. [Google Scholar] [CrossRef]
- Yadav, A.A.; Kang, S.-W.; Hunge, Y.M. Photocatalytic Degradation of Rhodamine B Using Graphitic Carbon Nitride Photocatalyst. J. Mater. Sci. Mater. Electron. 2021, 32, 15577–15585. [Google Scholar] [CrossRef]
- Khalid, N.R.; Mazia, U.; Tahir, M.B.; Niaz, N.A.; Javid, M.A. Photocatalytic Degradation of RhB from an Aqueous Solution Using Ag3PO4/N-TiO2 Heterostructure. J. Mol. Liq. 2020, 313, 113522. [Google Scholar] [CrossRef]
- Ruíz-Santoyo, V.; Marañon-Ruiz, V.F.; Romero-Toledo, R.; González Vargas, O.A.; Pérez-Larios, A. Photocatalytic Degradation of Rhodamine B and Methylene Orange Using TiO2-ZrO2 as Nanocomposite. Catalysts 2021, 11, 1035. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Wei, X.; Liu, G.; Li, J. ZnO Nanoparticles Implanted in TiO2 Macrochannels as an Effective Direct Z-Scheme Heterojunction Photocatalyst for Degradation of RhB. Appl. Surf. Sci. 2018, 456, 666–675. [Google Scholar] [CrossRef]
- Bian, H.; Zhang, Z.; Xu, X.; Gao, Y.; Wang, T. Photocatalytic Activity of Ag/ZnO/AgO/TiO2 Composite. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 124, 114236. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.; Bu, Y.; Wang, X.; Zhang, J.; Huang, J.; Ma, R.; Zhao, J. Photocatalytic Enhancement of Floating Photocatalyst: Layer-by-Layer Hybrid Carbonized Chitosan and Fe-N-Codoped TiO2 on Fly Ash Cenospheres. Appl. Surf. Sci. 2017, 391, 236–250. [Google Scholar] [CrossRef]
- Ali, M.H.H.; Al-Afify, A.D.; Goher, M.E. Preparation and Characterization of Graphene–TiO2 Nanocomposite for Enhanced Photodegradation of Rhodamine-B Dye. Egypt. J. Aquat. Res. 2018, 44, 263–270. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Y.; He, D.; Zhang, J.; Fan, Z.; Xie, T. Interface Junction at Anatase/Rutile in Mixed-Phase TiO2: Formation and Photo-Generated Charge Carriers Properties. Chem. Phys. Lett. 2011, 504, 71–75. [Google Scholar] [CrossRef]
- Yu, Y.; Wen, W.; Qian, X.-Y.; Liu, J.-B.; Wu, J.-M. UV and Visible Light Photocatalytic Activity of Au/TiO2 Nanoforests with Anatase/Rutile Phase Junctions and Controlled Au Locations. Sci. Rep. 2017, 7, 41253. [Google Scholar] [CrossRef]
- Kasinathan, K.; Kennedy, J.; Elayaperumal, M.; Henini, M.; Malik, M. Photodegradation of Organic Pollutants RhB Dye Using UV Simulated Sunlight on Ceria Based TiO2 Nanomaterials for Antibacterial Applications. Sci. Rep. 2016, 6, 38064. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Q.; Shi, X.; Song, Q.; Zhou, C.; Jiang, D. Photocatalytic Reduction of CO2 into CH4 over Ru-Doped TiO2: Synergy of Ru and Oxygen Vacancies. J. Colloid Interface Sci. 2022, 608, 2809–2819. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Dvoranová, D.; Lajaunie, L.; Rozman, N.; Figueiredo, B.; Seabra, M.P.; Škapin, A.S.; Calvino, J.J.; Brezová, V.; Labrincha, J.A. Graphene-TiO2 Hybrids for Photocatalytic Aided Removal of VOCs and Nitrogen Oxides from Outdoor Environment. Chem. Eng. J. 2021, 405, 126651. [Google Scholar] [CrossRef]
- Fónagy, O.; Szabó-Bárdos, E.; Horváth, O. 1,4-Benzoquinone and 1,4-Hydroquinone Based Determination of Electron and Superoxide Radical Formed in Heterogeneous Photocatalytic Systems. J. Photochem. Photobiol. Chem. 2021, 407, 113057. [Google Scholar] [CrossRef]
- Li, X.; Hu, Z.; Liu, J.; Li, D.; Zhang, X.; Chen, J.; Fang, J. Ga Doped ZnO Photonic Crystals with Enhanced Photocatalytic Activity and Its Reaction Mechanism. Appl. Catal. B Environ. 2016, 195, 29–38. [Google Scholar] [CrossRef]
- Zou, Y.; Hu, Y.; Uhrich, A.; Shen, Z.; Peng, B.; Ji, Z.; Muhler, M.; Zhao, G.; Wang, X.; Xu, X. Steering Accessible Oxygen Vacancies for Alcohol Oxidation over Defective Nb2O5 under Visible Light Illumination. Appl. Catal. B Environ. 2021, 298, 120584. [Google Scholar] [CrossRef]
- Li, N.; Li, R.; Zhao, J.; Liang, L.; Yu, Y.; Kong, L.; Chen, G.; Yan, B. Multi-Interface Mn3O4@ZnO/TiO2 with Controllable Charge Transfer Routes for Highly Selective Denitrification under Ultrasonic-Assisted Visible Light Photocatalysis. Chem. Eng. J. 2020, 394, 124997. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Z.; Zhao, K.; Lin, S.; Li, H.; Gao, X. Photocatalytic Nitrogen Fixation: Oxygen Vacancy Modified Novel Micro-Nanosheet Structure Bi2O2CO3 with Band Gap Engineering. J. Colloid Interface Sci. 2021, 583, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Bhandary, N.; Singh, A.P.; Kumar, S.; Ingole, P.P.; Thakur, G.S.; Ganguli, A.K.; Basu, S. In Situ Solid-State Synthesis of a AgNi/g-C3N4 Nanocomposite for Enhanced Photoelectrochemical and Photocatalytic Activity. ChemSusChem 2016, 9, 2816–2823. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Li, K.; Tang, J. Cu2O/Reduced Graphene Oxide Composites for the Photocatalytic Conversion of CO2. ChemSusChem 2014, 7, 1086–1093. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, W.; Feng, C.; Yang, Z.; Yang, Y. Replacement/Etching Route to ZnSe Nanotube Arrays and Their Enhanced Photocatalytic Activities. Ind. Eng. Chem. Res. 2012, 51, 4208–4214. [Google Scholar] [CrossRef]
- Ângelo, J.; Magalhães, P.; Andrade, L.; Mendes, A. Characterization of TiO2-Based Semiconductors for Photocatalysis by Electrochemical Impedance Spectroscopy. Appl. Surf. Sci. 2016, 387, 183–189. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, Z.; Zhu, C.; Hu, X.; Zhang, X.; Zhu, H.; Fan, J.; Lin, S.H. C/B Codoping Effect on Band Gap Narrowing and Optical Performance of TiO2 Photocatalyst: A Spin-Polarized DFT Study. J. Mater. Chem. A 2013, 1, 4516. [Google Scholar] [CrossRef]
- Wei, S.; Wang, F.; Yan, P.; Dan, M.; Cen, W.; Yu, S.; Zhou, Y. Interfacial Coupling Promoting Hydrogen Sulfide Splitting on the Staggered Type II g-C3N4/R-TiO2 Heterojunction. J. Catal. 2019, 377, 122–132. [Google Scholar] [CrossRef]
- Du, L.; Jin, C.; Cheng, Y.; Xu, L.; An, X.; Shang, W.; Zhang, Y.; Rao, X. Improvement of Antibacterial Activity of Hydrothermal Treated TC4 Substrate through an In-Situ Grown TiO2/g-C3N4 Z-Scheme Heterojunction Film. J. Alloys Compd. 2020, 842, 155612. [Google Scholar] [CrossRef]
- Jiang, W.; Qu, D.; An, L.; Gao, X.; Wen, Y.; Wang, X.; Sun, Z. Purposely Constructing Direct Z-scheme Photocatalyst by Photo-deposition Technique. J. Mater. Chem. A. 2019, 7, 31. [Google Scholar] [CrossRef]
- Kashiwaya, S.; Morasch, J.; Streibel, V.; Toupance, T.; Jaegermann, W.; Klein, A. The Work Function of TiO2. Surfaces 2018, 1, 73–89. [Google Scholar] [CrossRef]


| Photocatalyst | C0 (mg/L) | Dosage (mg) | Light Source | Degradation | Time (min) | Kinetic Rate (min−1) | Ref. |
|---|---|---|---|---|---|---|---|
| Ag3PO4/N-TiO2 | 10 | 20 | 150 W Xe lamp | 94.0% | 120 | 0.0194 | [51] |
| TiO2-ZrO2 | 30 | 200 | UV-light | 96.5% | 150 | 0.0218 | [52] |
| TiO2/ZnO | 4.79 | 40 | UV-light | 95.0% | 120 | 0.0107 | [53] |
| Ag/ZnO/AgO/TiO2 | 10 | 30 | 350 W Xe lamp | 99.3% | 100 | 0.0230 | [54] |
| Fe-N-codoped TiO2 | 8 | 200 | 500 W Xe lamp | 93.13% | 250 | 0.0102 | [55] |
| graphene–TiO2 | 30 | 375 | UV-light | 96% | 60 | 0.0280 | [56] |
| B-doped TiO2 | 5 | - | 500 W Xe lamp | >95% | 90 | - | [27] |
| B-doped TiO2 | 4.79 | 5 | Xe lamp | >95% | 120 | 0.038 | [36] |
| A/R-TiO2 | 10 | 25 | UV-light | about 100% | 50 | - | [57] |
| Au/A/R-TiO2 | - | - | UV-light | 97% | 60 | 0.0470 | [58] |
| Ce-doped TiO2 | 15 | 15 | UV-Vis light. | 99.89% | 480 | - | [59] |
| A/R-TiO2 | 10 | 30 | 300 W Xe lamp | 94.8% | 90 | 0.0370 | Thiswork |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Xu, C.; Wang, W.; Chen, L.; Li, X.; Wu, Y. Oxygen Vacancy Mediated Band-Gap Engineering via B-Doping for Enhancing Z-Scheme A-TiO2/R-TiO2 Heterojunction Photocatalytic Performance. Nanomaterials 2023, 13, 794. https://doi.org/10.3390/nano13050794
Liu C, Xu C, Wang W, Chen L, Li X, Wu Y. Oxygen Vacancy Mediated Band-Gap Engineering via B-Doping for Enhancing Z-Scheme A-TiO2/R-TiO2 Heterojunction Photocatalytic Performance. Nanomaterials. 2023; 13(5):794. https://doi.org/10.3390/nano13050794
Chicago/Turabian StyleLiu, Changqing, Chenggang Xu, Wanting Wang, Long Chen, Xu Li, and Yuanting Wu. 2023. "Oxygen Vacancy Mediated Band-Gap Engineering via B-Doping for Enhancing Z-Scheme A-TiO2/R-TiO2 Heterojunction Photocatalytic Performance" Nanomaterials 13, no. 5: 794. https://doi.org/10.3390/nano13050794
APA StyleLiu, C., Xu, C., Wang, W., Chen, L., Li, X., & Wu, Y. (2023). Oxygen Vacancy Mediated Band-Gap Engineering via B-Doping for Enhancing Z-Scheme A-TiO2/R-TiO2 Heterojunction Photocatalytic Performance. Nanomaterials, 13(5), 794. https://doi.org/10.3390/nano13050794






