Tyrosinase Immobilization Strategies for the Development of Electrochemical Biosensors—A Review
Abstract
1. Introduction
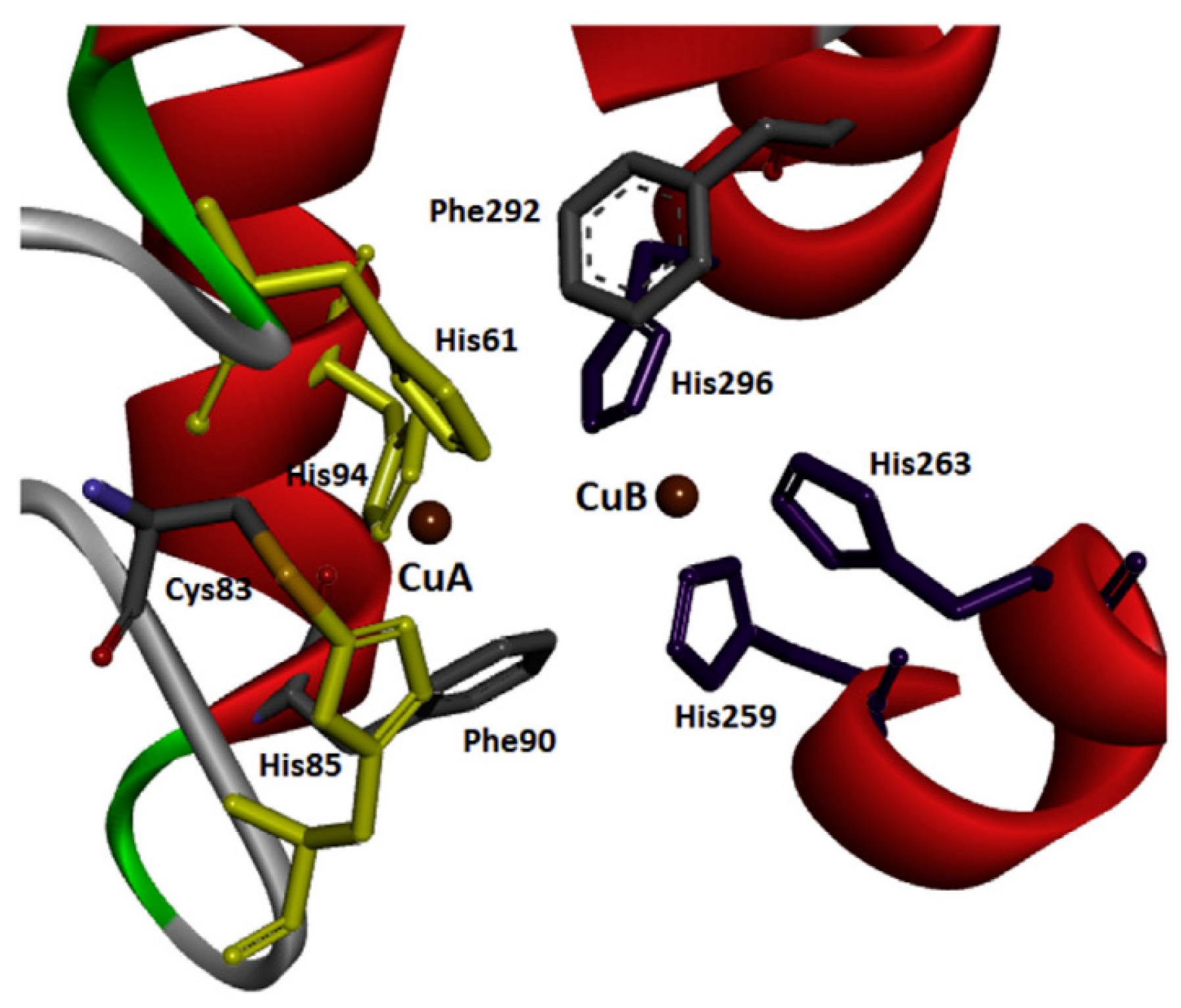
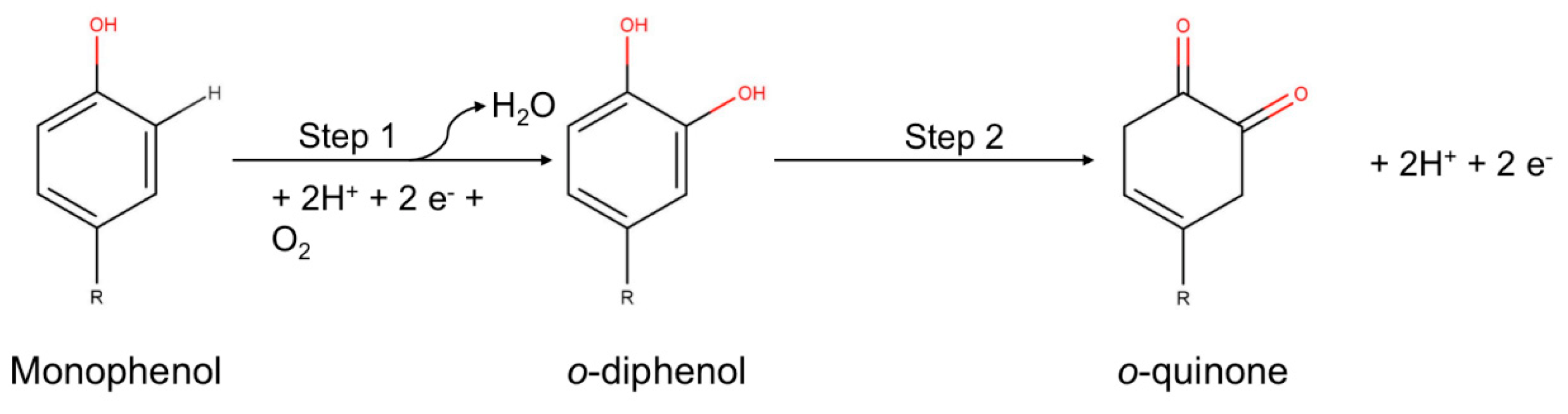
2. Tyrosinase—Sources and General Characteristics
Tyrosinase Inhibition
- -
- Specific tyrosinase inactivators or self-destructive inactivators. This inactivation may be explained by conformational changes, triggered by the substrate and then mediated by solvent molecules, in the tertiary and quaternary structures of tyrosinase [55]. The chemical structure of different substrates is diverse (o-diphenols, ascorbic acid, aminophenols and o-diamine hydroxyhydroquinone), but the process always requires an oxidation-reduction step [56,57,58,59,60,61].
- -
- “true inactivators”, which can have several mechanisms: competitive, non-competitive and mixed. A competitive inhibitor can bind to a free enzyme and thus avoid binding of the substrate to the active site of the enzyme. Such compounds are copper chelators, e.g., aromatic acids (D-tyrosine [60]), phenolic and polyphenolic compounds and some non-aromatic compounds (sulfonamides—such as sulphanilamide, cyanides—such as potassium cyanide, hydroxamic acids—such as benzohydroxamic acid, iodide ions—such as potassium iodide and nitrates—such as nitroglycerin) [50].
3. Tyrosinase-Based Electrochemical Biosensors—Characteristics
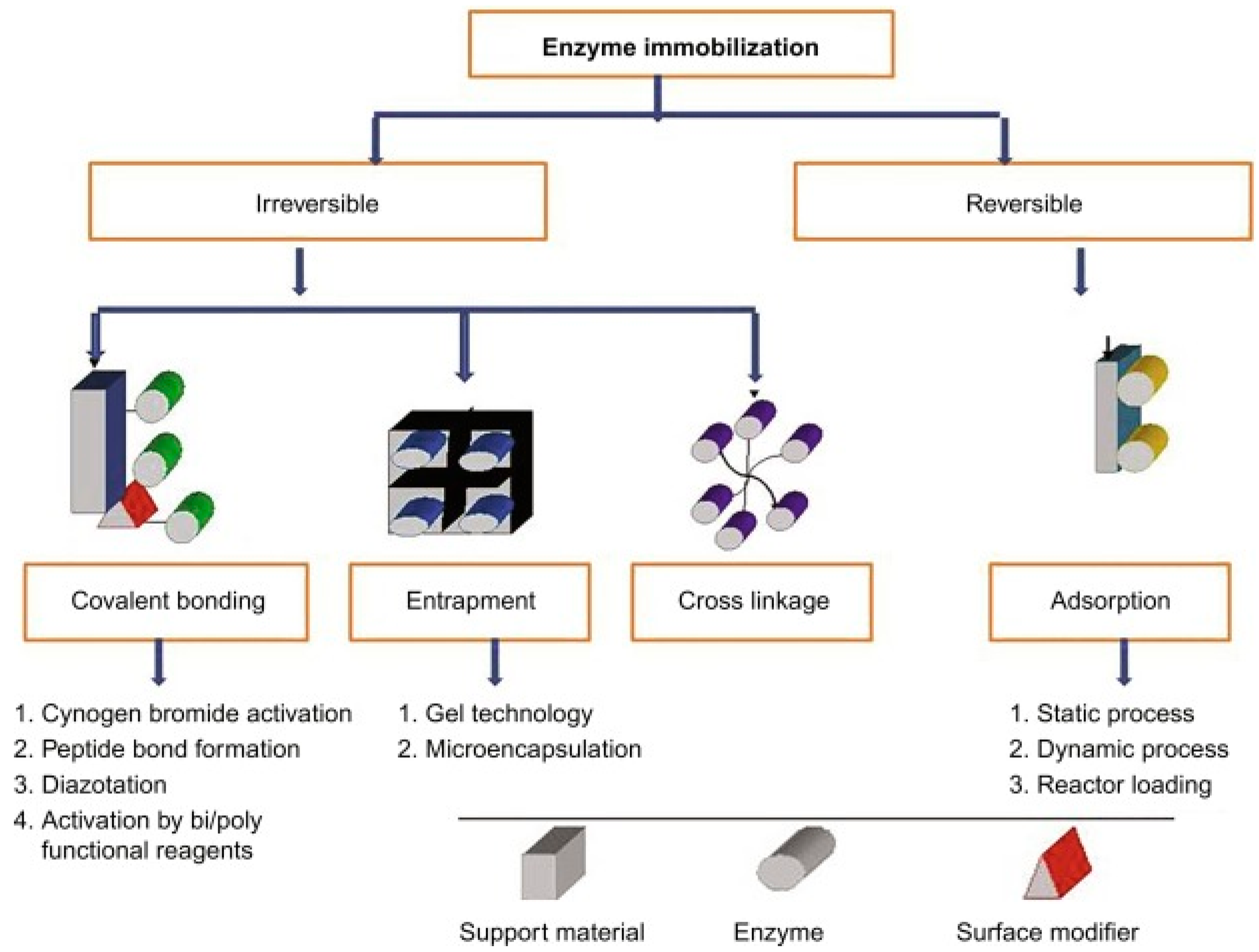
4. Tyrosinase Immobilization Strategies
- (1)
- adsorption
- (2)
- formation of disulphide bonds.
- (1)
- covalent bonding,
- (2)
- reticulation and
- (3)
- capture.
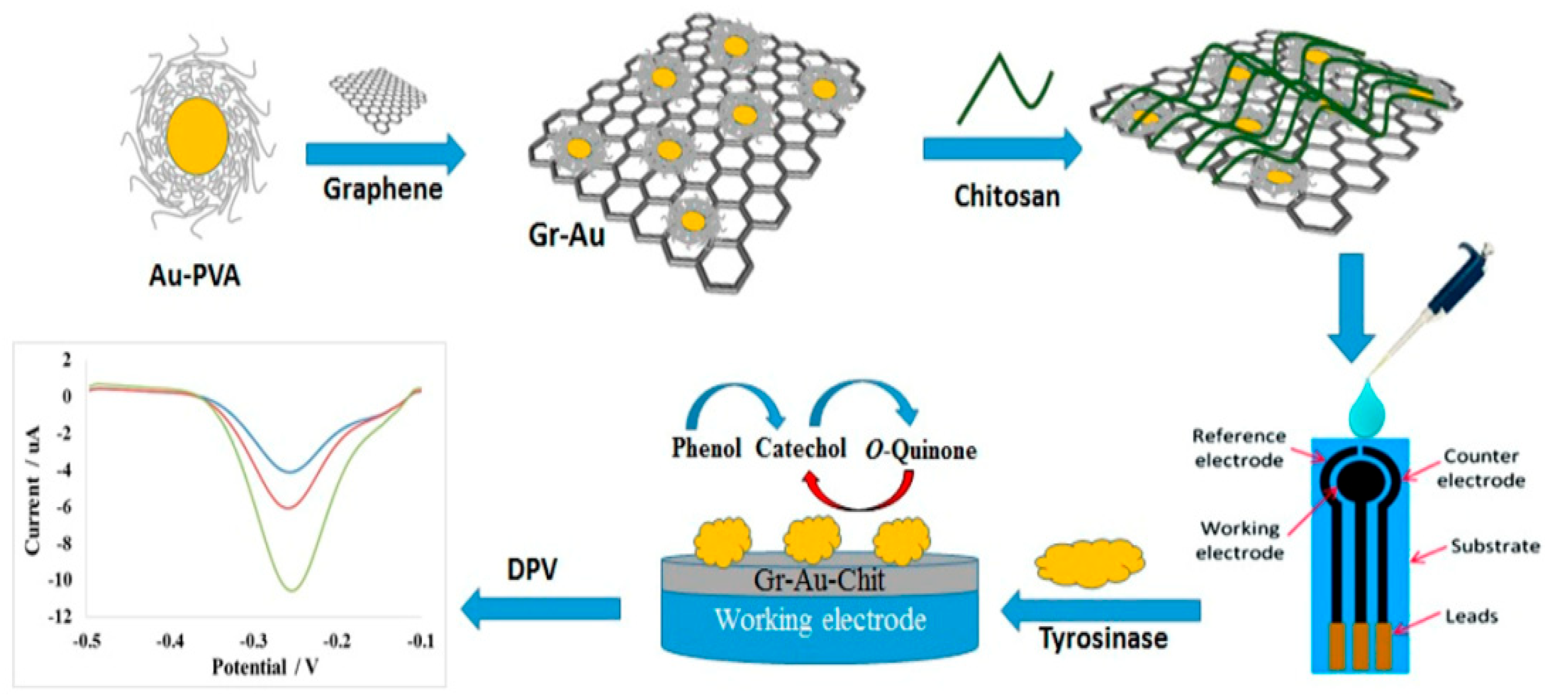
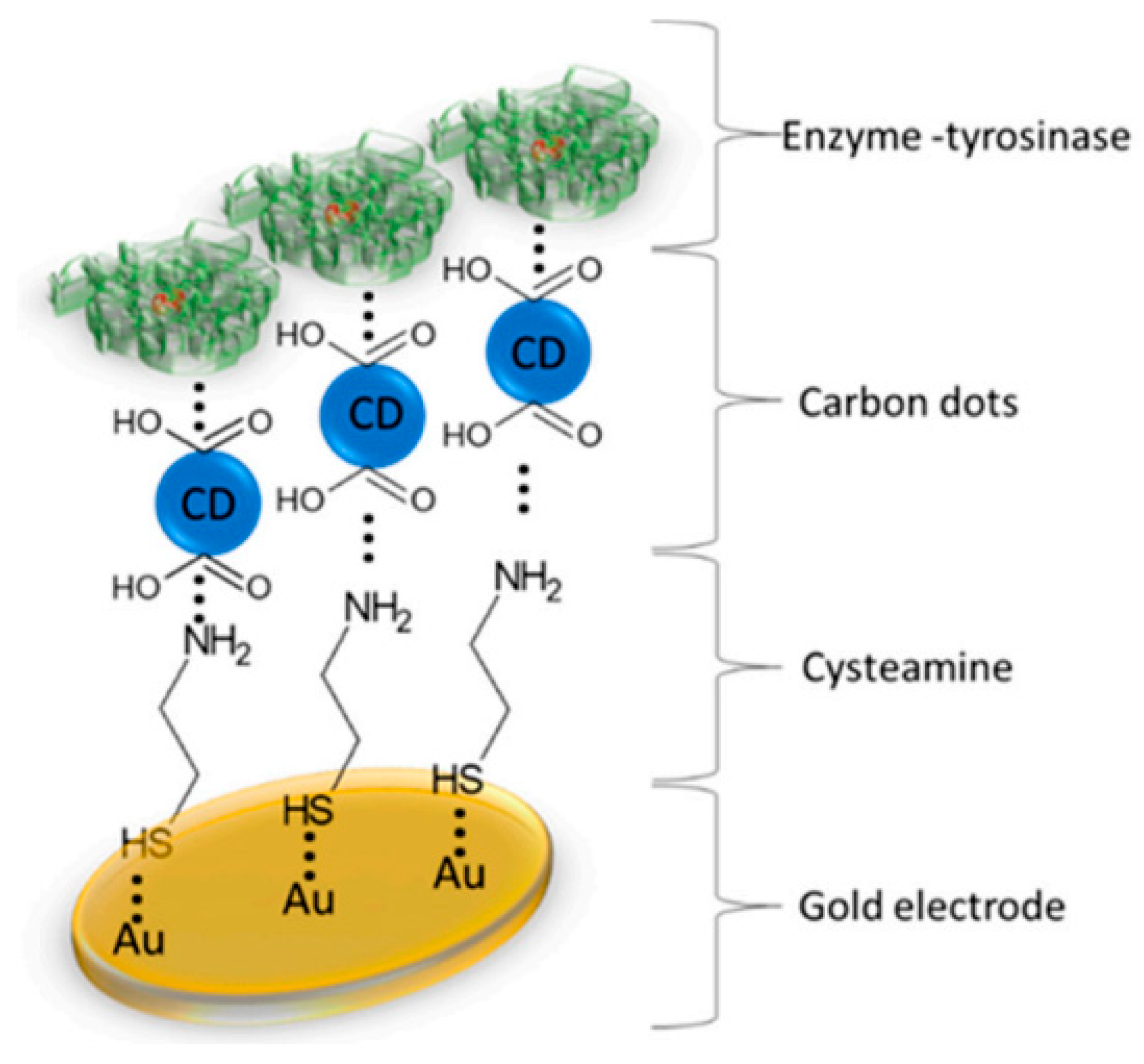
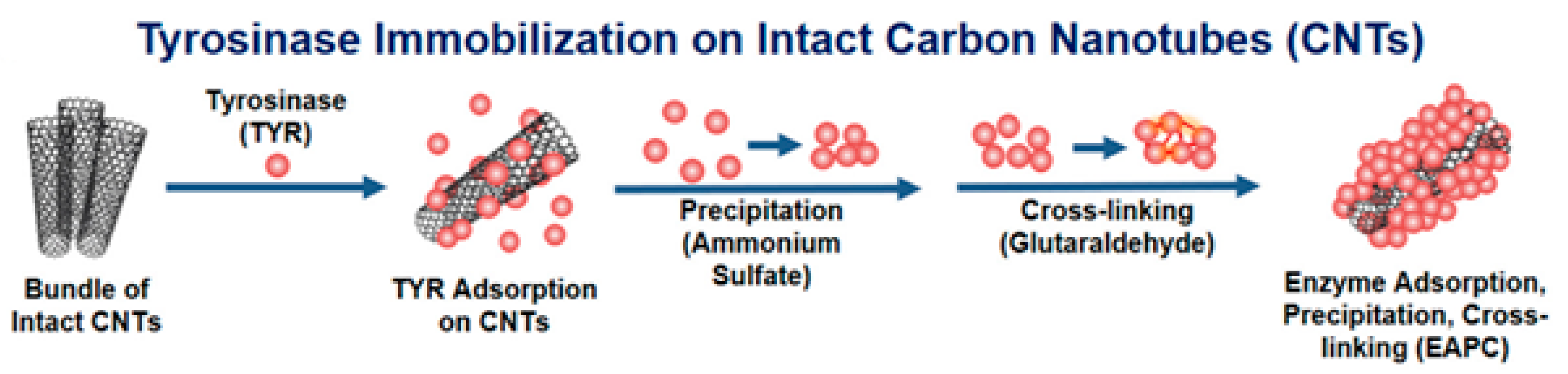
4.1. Physical Adsorption
- (a)
- a static process involving physical contact between the enzyme molecule and the carrier compound, with no dynamics involved.
- (b)
- a dynamic process involving the adherence of the enzyme to the carrier by mechanical agitation, even for commercial production of enzymes in large reactors.
- (c)
- by electrodeposition as follows: the carrier is placed near the electrodes immersed in the enzyme solution, and by passing the current, the adhesion of the enzyme to the carrier compound is initiated [105].
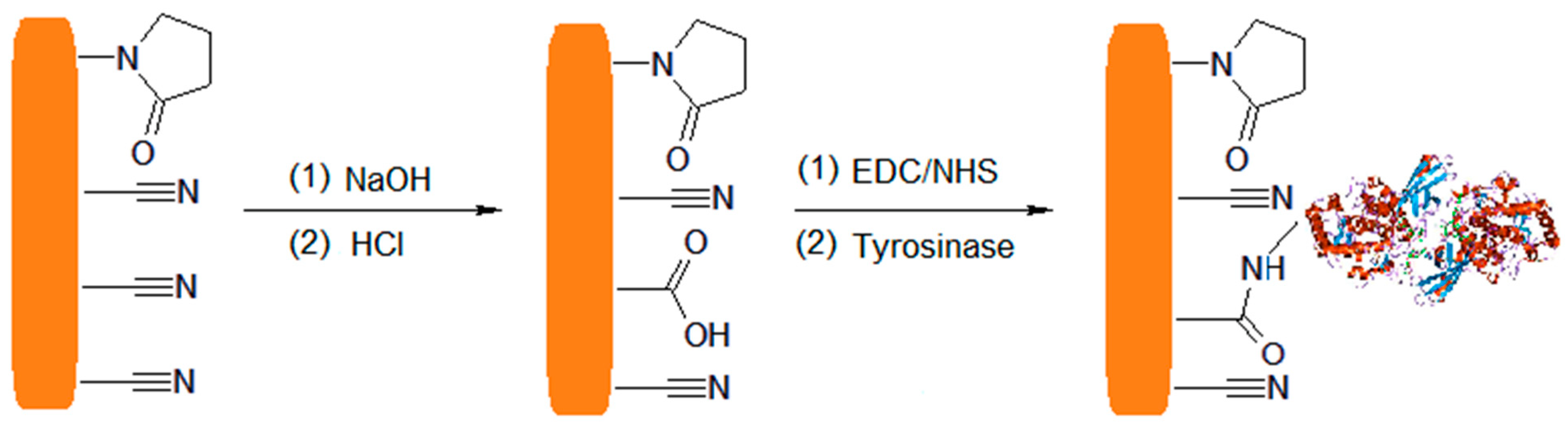
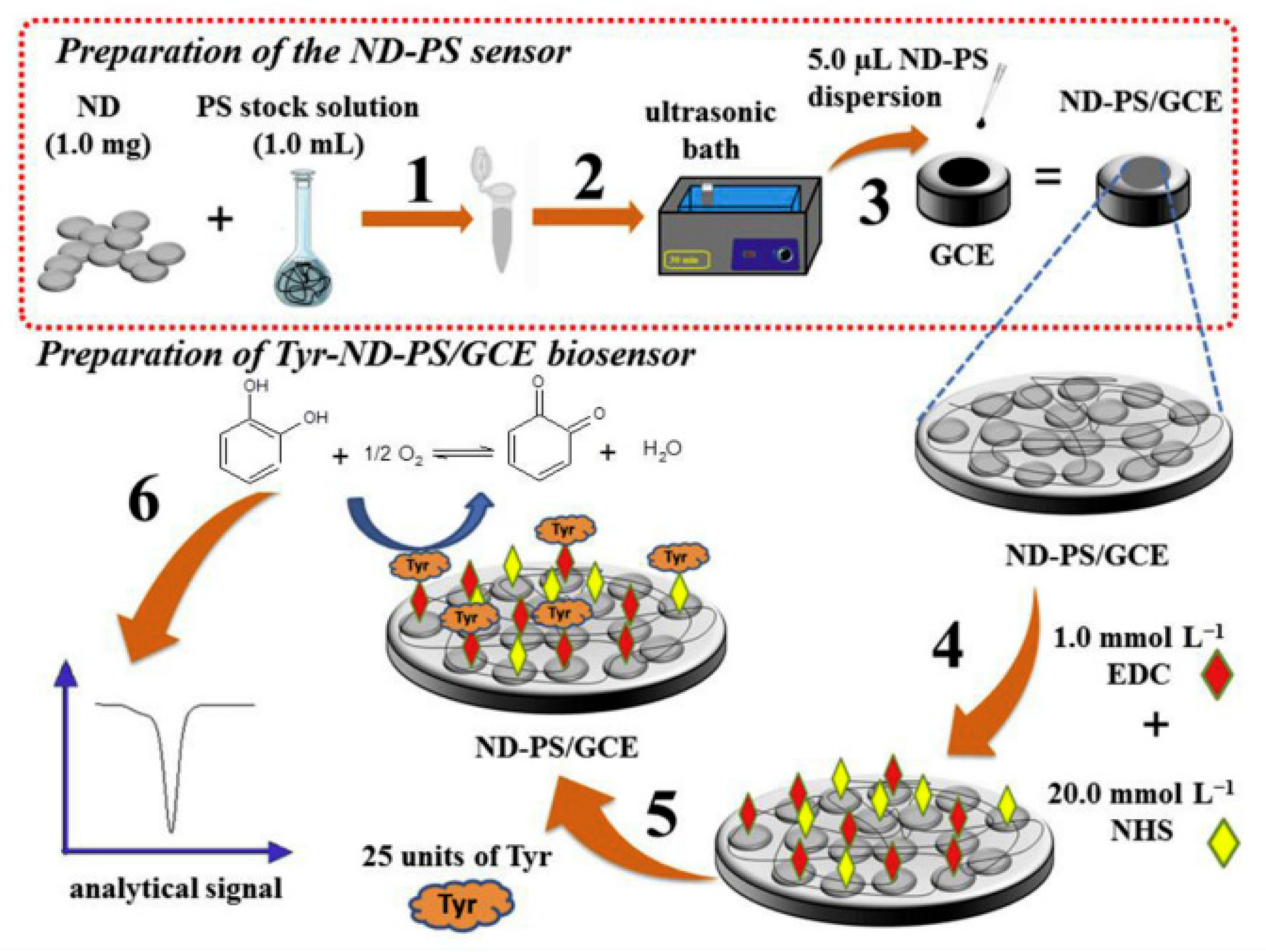
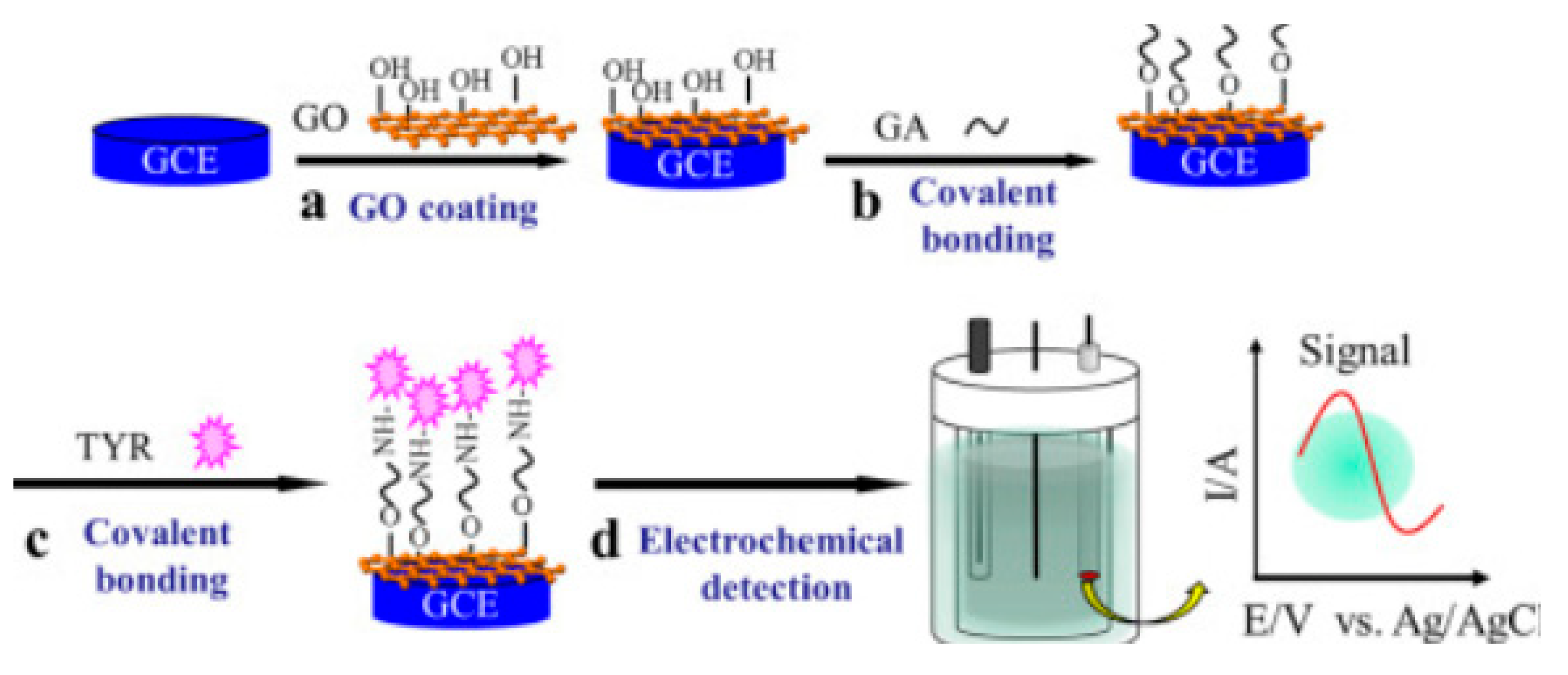
4.2. Covalent Bonding
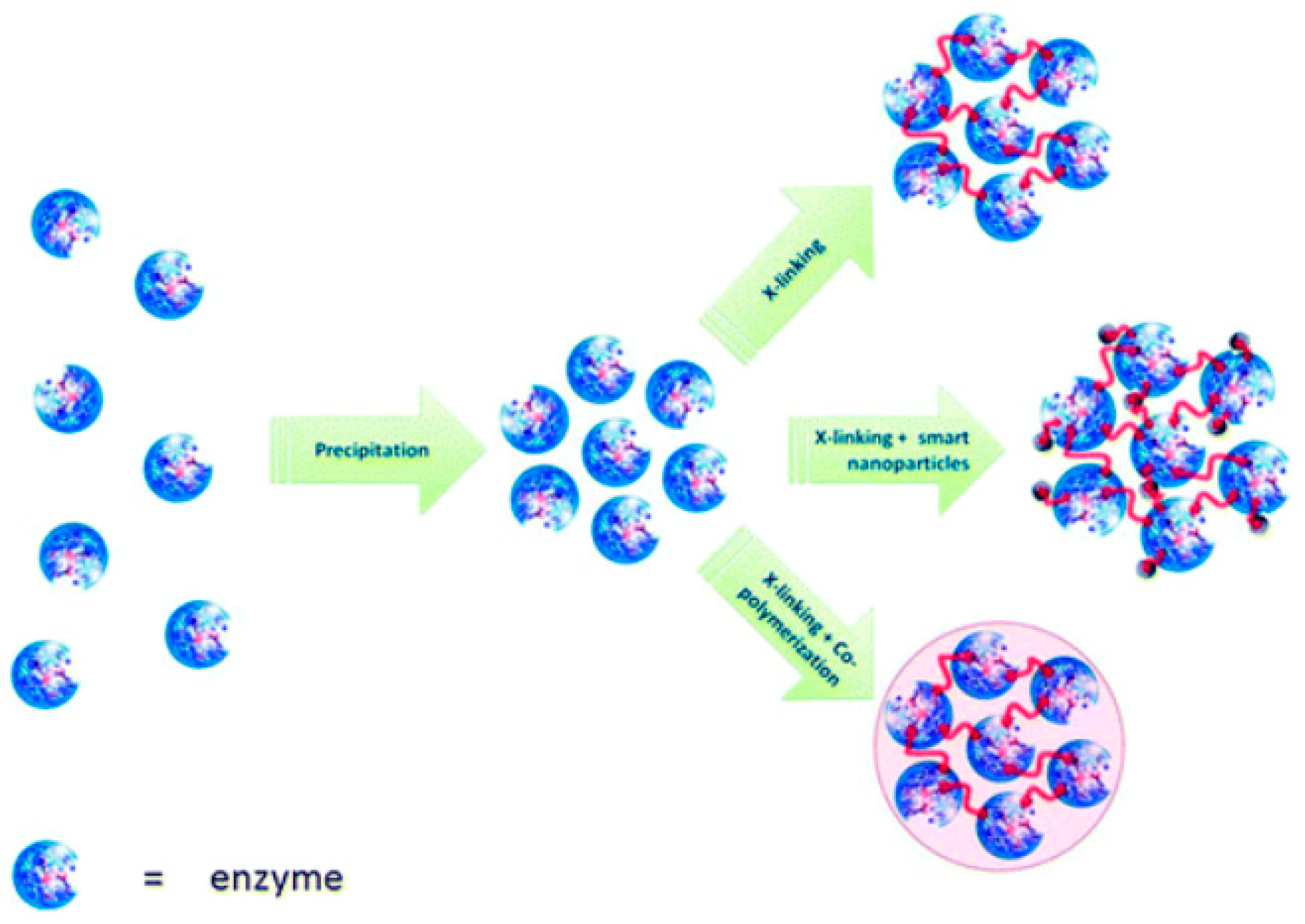
4.3. Cross-Linking
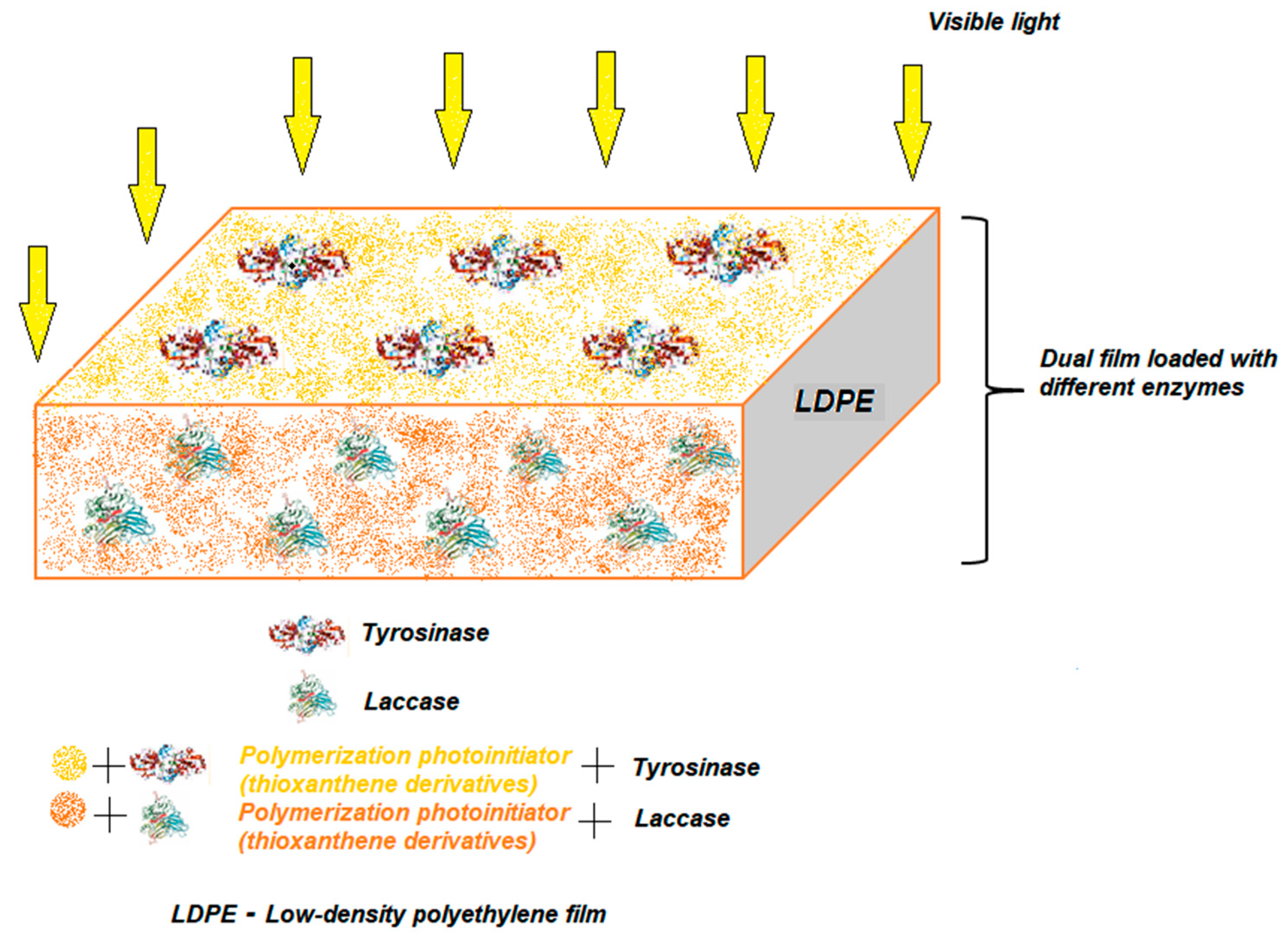
4.4. Entrapment
4.5. Combined Methods of Tyrosinase Immobilization
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eguílaz, M.; Villalonga, R.; Rivas, G. Electrochemical biointerfaces based on carbon nanotubes-mesoporous silica hybrid material: Bioelectrocatalysis of hemoglobin and biosensing applications. Biosens. Bioelectron. 2018, 111, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, Q. The role of nanomaterials in electroanalytical biosensors: A mini review. J. Electroanal. Chem. 2016, 781, 401–409. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Grădinaru, V.R.; Apetrei, C. Sensitive Detection of Rosmarinic Acid Using Peptide-Modified Graphene Oxide Screen-Printed Carbon Electrode. Nanomaterials 2022, 12, 3292. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fang, Y.; Ramasamy, R. Non-Covalent Functionalization of Carbon Nanotubes for Electrochemical Biosensor Development. Sensors 2019, 19, 392. [Google Scholar] [CrossRef]
- Hassan, S.H.; van Ginkel, S.W.; Hussein, M.A.; Abskharon, R.; Oh, S.-E. Toxicity assessment using different bioassays and microbial biosensors. Environ. Int. 2016, 92-93, 106–118. [Google Scholar] [CrossRef]
- Kour, R.; Arya, S.; Young, S.-J.; Gupta, V.; Bandhoria, P.; Khosla, A. Review—Recent Advances in Carbon Nanomaterials as Electrochemical Biosensors. J. Electrochem. Soc. 2020, 167, 037555. [Google Scholar] [CrossRef]
- Sharifi, M.; Karim, A.Y.; Nanakali, N.M.Q.; Salihi, A.; Aziz, F.M.; Hong, J.; Khan, R.H.; Saboury, A.A.; Hasan, A.; Abou-Zied, O.K.; et al. Strategies of enzyme immobilization on nanomatrix supports and their intracellular delivery. J. Biomol. Struct. Dyn. 2019, 38, 2746–2762. [Google Scholar] [CrossRef]
- Sharifi, M.; Sohrabi, M.J.; Hosseinali, S.H.; Hasan, A.; Kani, P.H.; Talaei, A.J.; Karim, A.Y.; Nanakali, N.M.Q.; Salihi, A.; Aziz, F.M.; et al. Enzyme immobilization onto the nanomaterials: Application in enzyme stability and prodrug-activated cancer therapy. Int. J. Biol. Macromol. 2020, 143, 665–676. [Google Scholar] [CrossRef]
- Almulaiky, Y.Q.; El-Shishtawy, R.M.; Aldhahri, M.; Mohamed, S.A.; Afifi, M.; Abdulaal, W.H.; Mahyoub, J.A. Amidrazone modified acrylic fabric activated with cyanuric chloride: A novel and efficient support for horseradish peroxidase immobilization and phenol removal. Int. J. Biol. Macromol. 2019, 140, 949–958. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Ghamdi, S.S.; El-Shishtawy, R.M. Immobilization of horseradish peroxidase on amidoximated acrylic polymer activated by cyanuric chloride. Int. J. Biol. Macromol. 2016, 91, 663–670. [Google Scholar] [CrossRef]
- Leca-Bouvier, B.D.; Blum, L.J. Enzyme for biosensing applications. In Recognition Receptors in Biosensors; Zourob, M., Ed.; Springer: New York, NY, USA, 2010; pp. 177–220. ISBN 978-1-4419-0918-3. [Google Scholar]
- Bonnet, C.; Andreescu, S.; Marty, J.-L. Adsorption: An easy and efficient immobilisation of acetylcholinesterase on screen-printed electrodes. Anal. Chim. Acta 2003, 481, 209–211. [Google Scholar] [CrossRef]
- Wang, P.; Liu, M.; Kan, J. Amperometric phenol biosensor based on polyaniline. Sens. Actuators B Chem. 2009, 140, 577–584. [Google Scholar] [CrossRef]
- Mousty, C.; Lepellec, A.; Cosnier, S.; Novoa, A.; Marks, R. Fabrication of organic phase biosensors based on multilayered polyphenol oxidase protected by an alginate coating. Electrochem. Commun. 2001, 3, 727–732. [Google Scholar] [CrossRef]
- Kim, M.A.; Lee, W.-Y. Amperometric phenol biosensor based on sol-gel silicate/Nafion composite film. Anal. Chim. Acta 2003, 479, 143–150. [Google Scholar] [CrossRef]
- Pedano, M. Amperometric biosensor for the quantification of gentisic acid using polyphenol oxidase modified carbon paste electrode. Talanta 2000, 53, 489–495. [Google Scholar] [CrossRef]
- De Albuquerque, Y.D.T.; Ferreira, L. Amperometric biosensing of carbamate and organophosphate pesticides utilizing screen-printed tyrosinase-modified electrodes. Anal. Chim. Acta 2007, 596, 210–221. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Development of a Novel Electrochemical Biosensor Based on Carbon Nanofibers–Gold Nanoparticles–Tyrosinase for the Detection of Ferulic Acid in Cosmetics. Sensors 2020, 20, 6724. [Google Scholar] [CrossRef]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. New electrochemiluminescent biosensors combining polyluminol and an enzymatic matrix. Anal. Bioanal. Chem. 2009, 394, 971–980. [Google Scholar] [CrossRef]
- Fritzen-Garcia, M.B.; Oliveira, I.R.W.; Zanetti-Ramos, B.G.; Fatibello-Filho, O.; Soldi, V.; Pasa, A.A.; Creczynski-Pasa, T.B. Carbon paste electrode modified with pine kernel peroxidase immobilized on pegylated polyurethane nanoparticles. Sens. Actuators B Chem. 2009, 139, 570–575. [Google Scholar] [CrossRef]
- Bounegru, A.; Apetrei, C. Laccase and Tyrosinase Biosensors Used in the Determination of Hydroxycinnamic Acids. Int. J. Mol. Sci. 2021, 22, 4811. [Google Scholar] [CrossRef]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization strategies to develop enzymatic biosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Murthy, C.; Prabha, C.R. Recent advances in carbon nanotube based electrochemical biosensors. Int. J. Biol. Macromol. 2018, 108, 687–703. [Google Scholar] [CrossRef] [PubMed]
- Theyagarajan, K.; Elancheziyan, M.; Aayushi, P.S.; Thenmozhi, K. Facile strategy for immobilizing horseradish peroxidase on a novel acetate functionalized ionic liquid/MWCNT matrix for electrochemical biosensing. Int. J. Biol. Macromol. 2020, 163, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Zou, Y.; Sun, L.; Xu, F. Direct Electrochemistry and Enhanced Electrocatalysis of Horseradish Peroxidase Based on Flowerlike ZnO-Gold Nanoparticle-Nafion Nanocomposite. Sens. Actuators B Chem. 2009, 136, 158–162. [Google Scholar] [CrossRef]
- Villalonga, R.; Díez, P.; Yáñez-Sedeño, P.; Pingarrón, J.M. Wiring horseradish peroxidase on gold nanoparticles-based nanostructured polymeric network for the construction of mediatorless hydrogen peroxide biosensor. Electrochim. Acta 2011, 56, 4672–4677. [Google Scholar] [CrossRef]
- Kumar, A.; Bisht, M.; Venkatesu, P. Biocompatibility of ionic liquids towards protein stability: A comprehensive overview on the current understanding and their implications. Int. J. Biol. Macromol. 2017, 96, 611–651. [Google Scholar] [CrossRef]
- Liu, G.; Lin, Y.; Ostatná, V.; Wang, J. Enzyme nanoparticles-based electronic biosensor. Chem. Commun. 2005, 3481–3483. [Google Scholar] [CrossRef]
- Bartlett, P.N. Bioelectrochemistry: Fundamentals, Experimental Techniques and Applications; John Wiley & Sons: New York, NY, USA, 2008; ISBN 978-0-470-75383-5. [Google Scholar]
- Porto de Souza Vandenberghe, L.; Karp, S.G.; Binder Pagnoncelli, M.G.; von Linsingen Tavares, M.; Libardi Junior, N.; Valladares Diestra, K.; Viesser, J.A.; Soccol, C.R. Classification of enzymes and catalytic properties. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 11–30. ISBN 978-0-12-819820-9. [Google Scholar]
- Taranto, S. Introduction. Inventing a new politics of family values. In Kitchen Table Politics; University of Pennsylvania Press: Philadelphia, PA, USA, 2017; pp. 1–14. ISBN 978-0-8122-9385-2. [Google Scholar]
- Vanitha, M.; Soundhari, C. Isolation and Characterisation of Mushroom Tyrosinase and Screening of Herbal Extracts for Anti-Tyrosinase Activity. Int. J. ChemTech Res. 2017, 10, 1156–1167. [Google Scholar]
- Strothkamp, K.; Jolley, R.; Mason, H. Quaternary structure of mushroom tyrosinase. Biochem. Biophys. Res. Commun. 1976, 70, 519–524. [Google Scholar] [CrossRef]
- Bourquelot, E.; Bertrand, G. Le Bleuissement et Le Noircissement Des Champignons. Comp. Rend. Soc. Biol. 1895, 2, 582–584. [Google Scholar]
- Lopez-Tejedor, D.; Palomo, J.M. Efficient purification of a highly active H-subunit of tyrosinase from Agaricus bisporus. Protein Expr. Purif. 2018, 145, 64–70. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Ioniţă, E.; Aprodu, I.; Stănciuc, N.; Râpeanu, G.; Bahrim, G. Advances in structure–function relationships of tyrosinase from Agaricus bisporus—Investigation on heat-induced conformational changes. Food Chem. 2014, 156, 129–136. [Google Scholar] [CrossRef]
- Mayer, A.M. Polyphenol oxidases in plants and fungi: Going places? A review. Phytochemistry 2006, 67, 2318–2331. [Google Scholar] [CrossRef]
- García-Molina, P.; García-Molina, F.; Teruel-Puche, J.A.; Rodríguez-López, J.N.; García-Cánovas, F.; Muñoz-Muñoz, J.L. Considerations about the kinetic mechanism of tyrosinase in its action on monophenols: A review. Mol. Catal. 2022, 518, 112072. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Chemistry of Mixed Melanogenesis—Pivotal Roles of Dopaquinone. Photochem. Photobiol. 2008, 84, 582–592. [Google Scholar] [CrossRef]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure and Function of Human Tyrosinase and Tyrosinase-Related Proteins. Chem.–A Eur. J. 2017, 24, 47–55. [Google Scholar] [CrossRef]
- Garcia-Borron, J.C.; Solano, F. Molecular Anatomy of Tyrosinase and its Related Proteins: Beyond the Histidine-Bound Metal Catalytic Center. Pigment. Cell Res. 2002, 15, 162–173. [Google Scholar] [CrossRef]
- Mostert, A.B. Melanin, the What, the Why and the How: An Introductory Review for Materials Scientists Interested in Flexible and Versatile Polymers. Polymers 2021, 13, 1670. [Google Scholar] [CrossRef]
- Garcia-Jimenez, A.; Puche, J.A.A.T.P.; Garcia-Ruiz, P.A.; Berna, J.; Rodríguez-López, J.N.; Tudela, J.; Garcia-Canovas, F. Action of 2,2′,4,4′-tetrahydroxybenzophenone in the biosynthesis pathway of melanin. Int. J. Biol. Macromol. 2017, 98, 622–629. [Google Scholar] [CrossRef]
- Tembe, S.; D’Souza, S.F. Immobilisation strategies for construction of tyrosinase based biosensors. Mater. Technol. 2014, 30, B190–B195. [Google Scholar] [CrossRef]
- Hammami, A.; Kuliček, J.; Raouafi, N. A naphthoquinone/SAM-mediated biosensor for olive oil polyphenol content. Food Chem. 2016, 209, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Pavinatto, F.J.; Paschoal, C.W.; Arias, A.C. Printed and flexible biosensor for antioxidants using interdigitated ink-jetted electrodes and gravure-deposited active layer. Biosens. Bioelectron. 2015, 67, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Bounegru, A.V.; Apetrei, C. Sensitive Detection of Hydroxytyrosol in Extra Virgin Olive Oils with a Novel Biosensor Based on Single-Walled Carbon Nanotubes and Tyrosinase. Int. J. Mol. Sci. 2022, 23, 9132. [Google Scholar] [CrossRef] [PubMed]
- Capannesi, C.; Palchetti, I.; Mascini, M.; Parenti, A. Electrochemical sensor and biosensor for polyphenols detection in olive oils. Food Chem. 2000, 71, 553–562. [Google Scholar] [CrossRef]
- Fernandes, M.S.; Kerkar, S. Microorganisms as a source of tyrosinase inhibitors: A review. Ann. Microbiol. 2017, 67, 343–358. [Google Scholar] [CrossRef]
- Haudecoeur, R.; Gouron, A.; Dubois, C.; Jamet, H.; Lightbody, M.; Hardré, R.; Milet, A.; Bergantino, E.; Bubacco, L.; Belle, C.; et al. Investigation of Binding-Site Homology between Mushroom and Bacterial Tyrosinases by Using Aurones as Effectors. Chembiochem 2014, 15, 1325–1333. [Google Scholar] [CrossRef]
- Selinheimo, E.; NiEidhin, D.; Steffensen, C.; Nielsen, J.; Lomascolo, A.; Halaouli, S.; Record, E.; O’Beirne, D.; Buchert, J.; Kruus, K. Comparison of the characteristics of fungal and plant tyrosinases. J. Biotechnol. 2007, 130, 471–480. [Google Scholar] [CrossRef]
- Goldfeder, M.; Kanteev, M.; Isaschar-Ovdat, S.; Adir, N.; Fishman, A. Determination of tyrosinase substrate-binding modes reveals mechanistic differences between type-3 copper proteins. Nat. Commun. 2014, 5, 4505. [Google Scholar] [CrossRef]
- Muñoz-Muñoz, J.L.; Garcia-Molina, F.; Varón, R.; Garcia-Ruíz, P.A.; Tudela, J.; García-Cánovas, F.; Rodríguez-López, J.N. Suicide inactivation of the diphenolase and monophenolase activities of tyrosinase. IUBMB Life 2010, 62, 539–547. [Google Scholar] [CrossRef]
- Haghbeen, K.; Saboury, A.A.; Karbassi, F. Substrate share in the suicide inactivation of mushroom tyrosinase. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2004, 1675, 139–146. [Google Scholar] [CrossRef]
- Munoz-Munoz, J.L.; Acosta-Motos, J.R.; Garcia-Molina, F.; Varon, R.; Garcia-Ruíz, P.A.; Tudela, J.; Garcia-Canovas, F.; Rodriguez-Lopez, J.N. Tyrosinase Inactivation in Its Action on Dopa. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2010, 1804, 1467–1475. [Google Scholar] [CrossRef]
- Munoz-Munoz, J.L.; Garcia-Molina, F.; García-Ruiz, P.A.; Varon, R.; Tudela, J.; García-Cánovas, F.; Rodriguez-Lopez, J.N. Stereospecific inactivation of tyrosinase by l- and d-ascorbic acid. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2009, 1794, 244–253. [Google Scholar] [CrossRef]
- Muñoz-Muñoz, J.L.; Garcia-Molina, F.; Berna, J.; Garcia-Ruiz, P.A.; Varon, R.; Tudela, J.; Rodriguez-Lopez, J.N.; Garcia-Canovas, F. Kinetic characterisation of o-aminophenols and aromatic o-diamines as suicide substrates of tyrosinase. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2012, 1824, 647–655. [Google Scholar] [CrossRef]
- Del Mar Garcia-Molina, M.; Muñoz-Muñoz, J.L.; Berna, J.; García-Ruiz, P.A.; Rodriguez-Lopez, J.N.; Garcia-Canovas, F. Catalysis and Inactivation of Tyrosinase in Its Action on Hydroxyhydroquinone. IUBMB life 2014, 66, 122–127. [Google Scholar] [CrossRef]
- Park, J.; Jung, H.; Kim, K.; Lim, K.-M.; Kim, J.-Y.; Jho, E.-H.; Oh, E.-S. D-tyrosine negatively regulates melanin synthesis by competitively inhibiting tyrosinase activity. Pigment. Cell Melanoma Res. 2017, 31, 374–383. [Google Scholar] [CrossRef]
- Mann, T.; Gerwat, W.; Batzer, J.; Eggers, K.; Scherner, C.; Wenck, H.; Stäb, F.; Hearing, V.J.; Röhm, K.H.; Kolbe, L. Inhibition of human tyrosinase requires molecular motifs distinctively different from mushroom tyrosinase. J. Investig. Dermatol. 2018, 138, 1601–1608. [Google Scholar] [CrossRef]
- Yin, S.-J.; Si, Y.-X.; Qian, G.-Y. Inhibitory Effect of Phthalic Acid on Tyrosinase: The Mixed-Type Inhibition and Docking Simulations. Enzym. Res. 2011, 2011, 294724. [Google Scholar] [CrossRef]
- Zhao, D.-Y.; Zhang, M.-X.; Dong, X.-W.; Hu, Y.-Z.; Dai, X.-Y.; Wei, X.; Hider, R.C.; Zhang, J.-C.; Zhou, T. Design and synthesis of novel hydroxypyridinone derivatives as potential tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 3103–3108. [Google Scholar] [CrossRef]
- Jirawattanapong, W.; Saifah, E.; Patarapanich, C. Synthesis of glabridin derivatives as tyrosinase inhibitors. Arch. Pharmacal Res. 2009, 32, 647–654. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Ahmed, A.; Sundramoorthy, A.K.; Furukawa, H.; Arya, S.; Khosla, A. Recent Advances in Electrochemical Biosensors: Applications, Challenges, and Future Scope. Biosensors 2021, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Tucci, M.; Grattieri, M.; Schievano, A.; Cristiani, P.; Minteer, S.D. Microbial amperometric biosensor for online herbicide detection: Photocurrent inhibition of Anabaena variabilis. Electrochim. Acta 2019, 302, 102–108. [Google Scholar] [CrossRef]
- Maalouf, R.; Chebib, H.; Saïkali, Y.; Vittori, O.; Sigaud, M.; Jaffrezic-Renault, N. Amperometric and impedimetric characterization of a glutamate biosensor based on Nafion® and a methyl viologen modified glassy carbon electrode. Biosens. Bioelectron. 2007, 22, 2682–2688. [Google Scholar] [CrossRef] [PubMed]
- Koncki, R. Recent developments in potentiometric biosensors for biomedical analysis. Anal. Chim. Acta 2007, 599, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Rafeeq, H.; Qasim, M.; Jabeen, Z.; Bilal, M.; Franco, M.; Iqbal, H.M.N. Engineered tyrosinases with broadened bio-catalysis scope: Immobilization using nanocarriers and applications. 3 Biotech 2021, 11, 1–18. [Google Scholar] [CrossRef]
- Forzato, C.; Vida, V.; Berti, F. Biosensors and Sensing Systems for Rapid Analysis of Phenolic Compounds from Plants: A Comprehensive Review. Biosensors 2020, 10, 105. [Google Scholar] [CrossRef]
- Li, Y.-F.; Liu, Z.-M.; Liu, Y.-L.; Yang, Y.-H.; Shen, G.-L.; Yu, R.-Q. A mediator-free phenol biosensor based on immobilizing tyrosinase to ZnO nanoparticles. Anal. Biochem. 2006, 349, 33–40. [Google Scholar] [CrossRef]
- Kristensen, S.B.; van Mourik, T.; Pedersen, T.B.; Sørensen, J.L.; Muff, J. Simulation of electrochemical properties of naturally occurring quinones. Sci. Rep. 2020, 10, 13571. [Google Scholar] [CrossRef]
- Takeda, K.; Kusuoka, R.; Birrell, J.A.; Yoshida, M.; Igarashi, K.; Nakamura, N. Bioelectrocatalysis based on direct electron transfer of fungal pyrroloquinoline quinone-dependent dehydrogenase lacking the cytochrome domain. Electrochim. Acta 2020, 359, 136982. [Google Scholar] [CrossRef]
- Hashim, H.S.; Fen, Y.W.; Omar, N.A.S.; Fauzi, N.I.M. Sensing Methods for Hazardous Phenolic Compounds Based on Graphene and Conducting Polymers-Based Materials. Chemosensors 2021, 9, 291. [Google Scholar] [CrossRef]
- Cerrato-Alvarez, M.; Bernalte, E.; Bernalte-García, M.J.; Pinilla-Gil, E. Fast and direct amperometric analysis of polyphenols in beers using tyrosinase-modified screen-printed gold nanoparticles biosensors. Talanta 2019, 193, 93–99. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. Development of a Novel Biosensor Based on Tyrosinase/Platinum Nanoparticles/Chitosan/Graphene Nanostructured Layer with Applicability in Bioanalysis. Materials 2019, 12, 1009. [Google Scholar] [CrossRef]
- Zdarta, J.; Staszak, M.; Jankowska, K.; Kaźmierczak, K.; Degórska, O.; Nguyen, L.N.; Kijeńska-Gawrońska, E.; Pinelo, M.; Jesionowski, T. The response surface methodology for optimization of tyrosinase immobilization onto electrospun polycaprolactone–chitosan fibers for use in bisphenol A removal. Int. J. Biol. Macromol. 2020, 165, 2049–2059. [Google Scholar] [CrossRef]
- Wee, Y.; Park, S.; Kwon, Y.H.; Ju, Y.; Yeon, K.-M.; Kim, J. Tyrosinase-immobilized CNT based biosensor for highly-sensitive detection of phenolic compounds. Biosens. Bioelectron. 2019, 132, 279–285. [Google Scholar] [CrossRef]
- Inroga, F.A.D.; Rocha, M.O.; Lavayen, V.; Arguello, J. Development of a tyrosinase-based biosensor for bisphenol A detection using gold leaf–like microstructures. J. Solid State Electrochem. 2019, 23, 1659–1666. [Google Scholar] [CrossRef]
- Wang, Y.; Zhai, F.; Hasebe, Y.; Jia, H.; Zhang, Z. A highly sensitive electrochemical biosensor for phenol derivatives using a graphene oxide-modified tyrosinase electrode. Bioelectrochemistry 2018, 122, 174–182. [Google Scholar] [CrossRef]
- Manan, F.A.A.; Hong, W.W.; Abdullah, J.; Yusof, N.A.; Ahmad, I. Nanocrystalline cellulose decorated quantum dots based tyrosinase biosensor for phenol determination. Mater. Sci. Eng. C 2019, 99, 37–46. [Google Scholar] [CrossRef]
- Ibáñez-Redín, G.; Silva, T.A.; Vicentini, F.C.; Fatibello-Filho, O. Effect of carbon black functionalization on the analytical performance of a tyrosinase biosensor based on glassy carbon electrode modified with dihexadecylphosphate film. Enzym. Microb. Technol. 2018, 116, 41–47. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Chou, Y.-C.; Tsai, J.-H.; Huang, T.-M.; Chen, J.-Z.; Yeh, Y.-C. Tyrosinase/Chitosan/Reduced Graphene Oxide Modified Screen-Printed Carbon Electrode for Sensitive and Interference-Free Detection of Dopamine. Appl. Sci. 2019, 9, 622. [Google Scholar] [CrossRef]
- Wong, A.; dos Santos, A.M.; Fatibello-Filho, O.; Sotomayor, M. Amperometric Tyrosinase Biosensor Based on Carbon Black Paste Electrode for Sensitive Detection of Catechol in Environmental Samples. Electroanalysis 2020, 33, 431–437. [Google Scholar] [CrossRef]
- Alpat, Ş.; Özdemir, K.; Alpat, S.K. Voltammetric Determination of Epinephrine in Pharmaceutical Sample with a Tyrosinase Nanobiosensor. J. Sens. 2016, 2016, e5653975. [Google Scholar] [CrossRef]
- Švancara, I.; Walcarius, A.; Kalcher, K.; Vytřas, K. Carbon paste electrodes in the new millennium. Open Chem. 2009, 7, 598–656. [Google Scholar] [CrossRef]
- Mascarenhas, R.J.; Satpati, A.K.; Yellappa, S.; Sherigara, B.S.; Bopiah, A.K. Wax-Impregnated Carbon Paste Electrode Modified with Mercuric Oxalate for the Simultaneous Determination of Heavy Metal Ions in Medicinal Plants and Ayurvedic Tablets. Anal. Sci. 2006, 22, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Malongo, T.K.; Patris, S.; Macours, P.; Cotton, F.; Nsangu, J.; Kauffmann, J.-M. Highly sensitive determination of iodide by ion chromatography with amperometric detection at a silver-based carbon paste electrode. Talanta 2008, 76, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Gopal, P.; Narasimha, G.; Reddy, T.M. Development, validation and enzyme kinetic evaluation of multi walled carbon nano tubes mediated tyrosinase based electrochemical biosensing platform for the voltammetric monitoring of epinephrine. Process. Biochem. 2020, 92, 476–485. [Google Scholar] [CrossRef]
- Qu, Z.; Na, W.; Liu, X.; Liu, H.; Su, X. A novel fluorescence biosensor for sensitivity detection of tyrosinase and acid phosphatase based on nitrogen-doped graphene quantum dots. Anal. Chim. Acta 2018, 997, 52–59. [Google Scholar] [CrossRef]
- Vicentini, F.C.; Garcia, L.L.; Figueiredo-Filho, L.C.; Janegitz, B.C.; Fatibello-Filho, O. A biosensor based on gold nanoparticles, dihexadecylphosphate, and tyrosinase for the determination of catechol in natural water. Enzym. Microb. Technol. 2016, 84, 17–23. [Google Scholar] [CrossRef]
- Cho, I.-H.; Kim, D.H.; Park, S. Electrochemical biosensors: Perspective on functional nanomaterials for on-site analysis. Biomater. Res. 2020, 24, 1–12. [Google Scholar] [CrossRef]
- Daraee, H.; Eatemadi, A.; Abbasi, E.; Aval, S.F.; Kouhi, M.; Akbarzadeh, A. Application of gold nanoparticles in biomedical and drug delivery. Artif. Cells Nanomed. Biotechnol. 2014, 44, 410–422. [Google Scholar] [CrossRef]
- Saifuddin, N.; Raziah, A.Z.; Junizah, A.R. Carbon Nanotubes: A Review on Structure and Their Interaction with Proteins. J. Chem. 2012, 2013, 676815. [Google Scholar] [CrossRef]
- Verma, R.K.; Slotboom, J.; Heldner, M.R.; Kellner-Weldon, F.; Kottke, R.; Ozdoba, C.; Weisstanner, C.; Kamm, C.P.; Wiest, R. Correction: Characterization of Microcirculation in Multiple Sclerosis Lesions by Dynamic Texture Parameter Analysis (DTPA). PLoS ONE 2013, 8, e67610. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Iqbal, H.M.N.; Hu, H.; Zhang, X. Bio-based degradation of emerging endocrine-disrupting and dye-based pollutants using cross-linked enzyme aggregates. Environ. Sci. Pollut. Res. 2017, 24, 7035–7041. [Google Scholar] [CrossRef]
- Dinu, A.; Apetrei, C. Quantification of Tyrosine in Pharmaceuticals with the New Biosensor Based on Laccase-Modified Polypyrrole Polymeric Thin Film. Polymers 2022, 14, 441. [Google Scholar] [CrossRef]
- Jun, L.Y.; Yon, L.S.; Mubarak, N.; Bing, C.H.; Pan, S.; Danquah, M.K.; Abdullah, E.; Khalid, M. An overview of immobilized enzyme technologies for dye and phenolic removal from wastewater. J. Environ. Chem. Eng. 2019, 7, 102961. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Che Marzuki, N.H.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Tischer, W.; Wedekind, F. Immobilized enzymes: Methods and applications. In Biocatalysis—From Discovery to Application; Fessner, W.-D., Archelas, A., Demirjian, D.C., Furstoss, R., Griengl, H., Jaeger, K.-E., Morís-Varas, E., Öhrlein, R., Reetz, M.T., Reymond, J.-L., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 95–126. ISBN 978-3-540-68116-8. [Google Scholar]
- Norouzian, D. Enzyme Immobilization: The State of Art in Biotechnology. Iran. J. Biotechnol. 2003, 1, 197–206. [Google Scholar]
- Sheldon, R.A. Cross-linked enzyme aggregates (CLEA®s): Stable and recyclable biocatalysts. Biochem. Soc. Trans. 2007, 35, 1583–1587. [Google Scholar] [CrossRef]
- Li, S.; Hu, J.; Liu, B. Use of chemically modified PMMA microspheres for enzyme immobilization. Biosystems 2004, 77, 25–32. [Google Scholar] [CrossRef]
- Foresti, M.; Ferreira, M. Chitosan-immobilized lipases for the catalysis of fatty acid esterifications. Enzym. Microb. Technol. 2007, 40, 769–777. [Google Scholar] [CrossRef]
- Sneha, H.P.; Beulah, K.C.; Murthy, P.S. Enzyme immobilization methods and applications in the food industry. In Enzymes in Food Biotechnology, 1st ed.; Kuddus, M., Ed.; Academic Press: London, UK, 2019; pp. 645–658. [Google Scholar] [CrossRef]
- An, N.; Zhou, C.H.; Zhuang, X.Y.; Tong, D.S.; Yu, W.H. Immobilization of enzymes on clay minerals for biocatalysts and biosensors. Appl. Clay Sci. 2015, 114, 283–296. [Google Scholar] [CrossRef]
- Song, Y.; Chen, S.; Li, L.; Zeng, Y.; Hu, X. The Hypopigmentation Mechanism of Tyrosinase Inhibitory Peptides Derived from Food Proteins: An Overview. Molecules 2022, 27, 2710. [Google Scholar] [CrossRef] [PubMed]
- Brady, D.; Jordaan, J. Advances in enzyme immobilisation. Biotechnol. Lett. 2009, 31, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, B.; Solomon, P.R. Immobilization of Lipases—A Review. Part I: Enzyme Immobilization. ChemBioEng Rev. 2019, 6, 157–166. [Google Scholar] [CrossRef]
- Mateo, C.; Pessela, B.C.C.; Fuentes, M.; Torres, R.; Betancor, L.; Hidalgo, A.; Fernandez-Lorente, G.; Fernandez-Lafuente, R.; Guisán, J.M. Stabilization of Multimeric Enzymes Via Immobilization and Further Cross-Linking with Aldehyde-Dextran. Phytoremediation 2006, 22, 129–141. [Google Scholar] [CrossRef]
- Pavel, I.-A.; Prazeres, S.F.; Montalvo, G.; Ruiz, C.G.; Nicolas, V.; Celzard, A.; Dehez, F.; Canabady-Rochelle, L.; Canilho, N.; Pasc, A. Effect of Meso vs Macro Size of Hierarchical Porous Silica on the Adsorption and Activity of Immobilized β-Galactosidase. Langmuir 2017, 33, 3333–3340. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Fartas, F.M.; Abdullah, J.; Yusof, N.A.; Sulaiman, Y.; Saiman, M.I. Biosensor Based on Tyrosinase Immobilized on Graphene-Decorated Gold Nanoparticle/Chitosan for Phenolic Detection in Aqueous. Sensors 2017, 17, 1132. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Basu, S.; Jha, S.K. Dopamine biosensor based on surface functionalized nanostructured nickel oxide platform. Biosens. Bioelectron. 2016, 84, 72–81. [Google Scholar] [CrossRef]
- Wu, S.; Wang, H.; Tao, S.; Wang, C.; Zhang, L.; Liu, Z.; Meng, C. Magnetic loading of tyrosinase-Fe3O4/mesoporous silica core/shell microspheres for high sensitive electrochemical biosensing. Anal. Chim. Acta 2011, 686, 81–86. [Google Scholar] [CrossRef]
- Wang, Y.; Hasebe, Y. Highly sensitive flow-biosensor for toxic phenolic compounds using tyrosinase and acridine orange-adsorbed carbon felt. J. Environ. Sci. 2009, 21, S100–S104. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhang, Z.; Sobhy, A.; Sato, S.; Uchida, M.; Hasebe, Y. Natural Molybdenite- and Tyrosinase-Based Amperometric Catechol Biosensor Using Acridine Orange as a Glue, Anchor, and Stabilizer for the Adsorbed Tyrosinase. ACS Omega 2021, 6, 13719–13727. [Google Scholar] [CrossRef]
- Abdollahi, K.; Yazdani, F.; Panahi, R. Fabrication of the robust and recyclable tyrosinase-harboring biocatalyst using ethylenediamine functionalized superparamagnetic nanoparticles: Nanocarrier characterization and immobilized enzyme properties. J. Biol. Inorg. Chem. 2019, 24, 943–959. [Google Scholar] [CrossRef]
- Júnior, A.; Ladeira, Y.; França, A.; Souza, R.; Moraes, A.; Wojcieszak, R.; Itabaiana, I.; Miranda, A. Multicatalytic Hybrid Materials for Biocatalytic and Chemoenzymatic Cascades—Strategies for Multicatalyst (Enzyme) Co-Immobilization. Catalysts 2021, 11, 936. [Google Scholar] [CrossRef]
- Wu, L.; Lu, X.; Niu, K.; Dhanjai; Chen, J. Tyrosinase nanocapsule based nano-biosensor for ultrasensitive and rapid detection of bisphenol A with excellent stability in different application scenarios. Biosens. Bioelectron. 2020, 165, 112407. [Google Scholar] [CrossRef]
- Thangaraj, B.; Muniyandi, B.; Ranganathan, S.; Xin, H. Functionalized Magnetic Nanoparticles for Catalytic Application—A Review. Rev. Adv. Sci. Eng. 2015, 4, 106–119. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. Developments in support materials for immobilization of oxidoreductases: A comprehensive review. Adv. Colloid Interface Sci. 2018, 258, 1–20. [Google Scholar] [CrossRef]
- Oktay, B.; Demir, S.; Kayaman-Apohan, N. Immobilization of α-amylase onto poly(glycidyl methacrylate) grafted electrospun fibers by ATRP. Mater. Sci. Eng. C 2015, 50, 386–393. [Google Scholar] [CrossRef]
- Smith, S.; Goodge, K.; Delaney, M.; Struzyk, A.; Tansey, N.; Frey, M. A Comprehensive Review of the Covalent Immobilization of Biomolecules onto Electrospun Nanofibers. Nanomaterials 2020, 10, 2142. [Google Scholar] [CrossRef]
- Nataraj, S.; Yang, K.; Aminabhavi, T. Polyacrylonitrile-based nanofibers—A state-of-the-art review. Prog. Polym. Sci. 2011, 37, 487–513. [Google Scholar] [CrossRef]
- Zheng, H.; Yan, Z.; Wang, M.; Chen, J.; Zhang, X. Biosensor based on polyaniline-polyacrylonitrile-graphene hybrid assemblies for the determination of phenolic compounds in water samples. J. Hazard. Mater. 2019, 378, 120714. [Google Scholar] [CrossRef]
- Nicolucci, C.; Rossi, S.; Menale, C.; Godjevargova, T.; Ivanov, Y.; Bianco, M.; Mita, L.; Bencivenga, U.; Mita, D.G.; Diano, N. Biodegradation of bisphenols with immobilized laccase or tyrosinase on polyacrylonitrile beads. Biodegradation 2010, 22, 673–683. [Google Scholar] [CrossRef] [PubMed]
- A simple and efficient method for removal of phenolic contaminants in wastewater using polyacrylamide entrapped mushroom tyrosinase. J. Appl. Biol. Biotechnol. 2020, 8, 22–27. [CrossRef]
- Guisan, J.M. Immobilization of enzymes and cells. In Methods in Biotechnology; Bolivar, J.M., López-Gallego, F., Rocha-Martín, J., Eds.; Humana Press: Totowa, NJ, USA, 2006; Volume 22, ISBN 978-1-58829-290-2. [Google Scholar]
- Camargo, J.R.; Baccarin, M.; Raymundo-Pereira, P.A.; Campos, A.M.; Oliveira, G.G.; Fatibello-Filho, O.; Oliveira, O.N., Jr.; Janegitz, B.C. Electrochemical Biosensor Made with Tyrosinase Immobilized in a Matrix of Nanodiamonds and Potato Starch for Detecting Phenolic Compounds. Anal. Chim. Acta 2018, 1034, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Shepherd, J.H.; Shepherd, D.V.; Ghose, S.; Kew, S.J.; Cameron, R.E.; Best, S.M.; Brooks, R.A.; Wardale, J.; Rushton, N. Effect of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide and N-hydroxysuccinimide concentrations on the mechanical and biological characteristics of cross-linked collagen fibres for tendon repair. Regen. Biomater. 2015, 2, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Avvakumova, S.; Colombo, M.; Galbiati, E.; Mazzucchelli, S.; Rotem, R.; Prosperi, D. Bioengineered approaches for site orientation of peptide-based ligands of nanomaterials. In Biomedical Applications of Functionalized Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 139–169. ISBN 978-0-323-50878-0. [Google Scholar]
- Chen, F.M.F.; Benoiton, N.L. A General Method for Formylating Sensitive Amino Acid Esters. Synthesis 1979, 1979, 709–710. [Google Scholar] [CrossRef]
- Bu, J.; Peng, Z.; Zhao, F.; McLuckey, S.A. Enhanced Reactivity in Nucleophilic Acyl Substitution Ion/Ion Reactions Using Triazole-Ester Reagents. J. Am. Soc. Mass Spectrom. 2017, 28, 1254–1261. [Google Scholar] [CrossRef]
- Campiglio, C.E.; Contessi Negrini, N.; Farè, S.; Draghi, L.J.M. Cross-Linking Strategies for Electrospun Gelatin Scaffolds. Materials 2019, 12, 2476. [Google Scholar] [CrossRef]
- Karim, N.; Lee, H.J. Amperometric phenol biosensor based on covalent immobilization of tyrosinase on Au nanoparticle modified screen printed carbon electrodes. Talanta 2013, 116, 991–996. [Google Scholar] [CrossRef]
- Soares, I.P.; da Silva, A.G.; Alves, R.D.F.; Corrêa, R.A.M.D.S.; Ferreira, L.F.; Franco, D.L. Electrochemical enzymatic biosensor for tyramine based on polymeric matrix derived from 4-mercaptophenylacetic acid. J. Solid State Electrochem. 2019, 23, 985–995. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef]
- Xiao, Z.; Xie, Y.; Militz, H.; Mai, C. Effect of glutaraldehyde on water related properties of solid wood. Holzforschung 2010, 64, 483–488. [Google Scholar] [CrossRef]
- El-Moghazy, A.Y.; Soliman, E.A.; Ibrahim, H.Z.; Marty, J.-L.; Istamboulie, G.; Noguer, T. Biosensor based on electrospun blended chitosan-poly (vinyl alcohol) nanofibrous enzymatically sensitized membranes for pirimiphos-methyl detection in olive oil. Talanta 2016, 155, 258–264. [Google Scholar] [CrossRef]
- Park, J.-M.; Kim, M.; Park, H.-S.; Jang, A.; Min, J.; Kim, Y.-H. Immobilization of lysozyme-CLEA onto electrospun chitosan nanofiber for effective antibacterial applications. Int. J. Biol. Macromol. 2013, 54, 37–43. [Google Scholar] [CrossRef]
- Huang, X.-J.; Ge, D.; Xu, Z.-K. Preparation and characterization of stable chitosan nanofibrous membrane for lipase immobilization. Eur. Polym. J. 2007, 43, 3710–3718. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, Y.; Zhang, Z.; Shen, Y.; Li, M. Tyrosinase Modified Poly(thionine) Electrodeposited Glassy Carbon Electrode for Amperometric Determination of Catechol. Electrochemistry 2017, 85, 17–22. [Google Scholar] [CrossRef]
- Polatoğlu, I.; Aydin, L. A new design strategy with stochastic optimization on the preparation of magnetite cross-linked tyrosinase aggregates (MCLTA). Process. Biochem. 2020, 99, 131–138. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Kim, M. An Overview of Techniques in Enzyme Immobilization. Appl. Sci. Converg. Technol. 2017, 26, 157–163. [Google Scholar] [CrossRef]
- Xu, J.; Sun, J.; Wang, Y.; Sheng, J.; Wang, F.; Sun, M. Application of Iron Magnetic Nanoparticles in Protein Immobilization. Molecules 2014, 19, 11465–11486. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, J.; Liu, Z.; Chu, Z.; Jin, W. Nanomaterial-based electrochemical enzymatic biosensors for recognizing phenolic compounds in aqueous effluents. Environ. Res. 2022, 214, 113858. [Google Scholar] [CrossRef]
- Talekar, S.; Ghodake, V.; Ghotage, T.; Rathod, P.; Deshmukh, P.; Nadar, S.; Mulla, M.; Ladole, M. Novel magnetic cross-linked enzyme aggregates (magnetic CLEAs) of alpha amylase. Bioresour. Technol. 2012, 123, 542–547. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Cui, J.D.; Jia, S.R. Optimization protocols and improved strategies of cross-linked enzyme aggregates technology: Current development and future challenges. Crit. Rev. Biotechnol. 2013, 35, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Samoylova, Y.V.; Sorokina, K.N.; Piligaev, A.V.; Parmon, V.N. Preparation of Stable Cross-Linked Enzyme Aggregates (CLEAs) of a Ureibacillus thermosphaericus Esterase for Application in Malathion Removal from Wastewater. Catalysts 2018, 8, 154. [Google Scholar] [CrossRef]
- Wahab, M.K.H.A.; El-Enshasy, H.A.; Abu Bakar, F.D.; Murad, A.M.A.; Jahim, J.M.; Illias, R.M. Improvement of cross-linking and stability on cross-linked enzyme aggregate (CLEA)-xylanase by protein surface engineering. Process. Biochem. 2019, 86, 40–49. [Google Scholar] [CrossRef]
- Moehlenbrock, M.J.; Minteer, S.D. Enzyme stabilization and immobilization: Methods and protocols. In Methods in Molecular Biology; Moehlenbrock, M.J., Minteer, S.D., Eds.; Springer: New York, NY, USA, 2017; Volume 1504, ISBN 978-1-4939-6497-0. [Google Scholar]
- Tapdigov, S.Z. The bonding nature of the chemical interaction between trypsin and chitosan based carriers in immobilization process depend on entrapped method: A review. Int. J. Biol. Macromol. 2021, 183, 1676–1696. [Google Scholar] [CrossRef]
- Sabbah, M.; Giosafatto, C.V.L.; Esposito, M.; di Pierro, P.; Mariniello, L.; Porta, R. Transglutaminase cross-linked edible films and coatings for food applications. In Enzymes in Food Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 369–388. ISBN 978-0-12-813280-7. [Google Scholar]
- Elias, N.; Chandren, S.; Razak, F.I.A.; Jamalis, J.; Widodo, N.; Wahab, R.A. Characterization, optimization and stability studies on Candida rugosa lipase supported on nanocellulose reinforced chitosan prepared from oil palm biomass. Int. J. Biol. Macromol. 2018, 114, 306–316. [Google Scholar] [CrossRef]
- Polatoğlu, I. Electrochemical Sensing Platform Based on Tyrosinase Immobilized Magnetite Chitosan Nanobiocomposite Film and Its Application as Catechol Biosensor. J. Electrochem. Soc. 2019, 166, B1620–B1629. [Google Scholar] [CrossRef]
- Siar, E.-H.; Arana-Peña, S.; Barbosa, O.; Zidoune, M.N.; Fernandez-Lafuente, R. Immobilization/Stabilization of Ficin Extract on Glutaraldehyde-Activated Agarose Beads. Variables That Control the Final Stability and Activity in Protein Hydrolyses. Catalysts 2018, 8, 149. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Studies on the Detection of Oleuropein from Extra Virgin Olive Oils Using Enzymatic Biosensors. Int. J. Mol. Sci. 2022, 23, 12569. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. Biosensor based on tyrosinase immobilized on a single-walled carbon nanotube-modified glassy carbon electrode for detection of epinephrine. Int. J. Nanomed. 2013, 8, 4391. [Google Scholar] [CrossRef]
- Florescu, M.; David, M. Tyrosinase-Based Biosensors for Selective Dopamine Detection. Sensors 2017, 17, 1314. [Google Scholar] [CrossRef]
- Singh, R.K.; Tiwari, M.K.; Singh, R.; Lee, J.-K. From Protein Engineering to Immobilization: Promising Strategies for the Upgrade of Industrial Enzymes. Int. J. Mol. Sci. 2013, 14, 1232–1277. [Google Scholar] [CrossRef]
- Sheldon, R.A. Characteristic features and biotechnological applications of cross-linked enzyme aggregates (CLEAs). Appl. Microbiol. Biotechnol. 2011, 92, 467–477. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Wahab, R.A.; Elias, N.; Abdullah, F.; Ghoshal, S.K. On the taught new tricks of enzymes immobilization: An all-inclusive overview. React. Funct. Polym. 2020, 152, 104613. [Google Scholar] [CrossRef]
- Reis, C.; Sousa, E.; Serpa, J.; Oliveira, R.; Santos, J. Design of immobilized enzyme biocatalysts: Drawbacks and opportunities. Química Nova 2019, 42, 768–783. [Google Scholar] [CrossRef]
- Pinto, S.C.; Rodrigues, A.R.; Saraiva, J.A.; Lopes-Da-Silva, J.A. Catalytic activity of trypsin entrapped in electrospun poly(ϵ-caprolactone) nanofibers. Enzym. Microb. Technol. 2015, 79-80, 8–18. [Google Scholar] [CrossRef]
- Ndlovu, T.; Ba, S.; Malinga, S.P. Overview of Recent Advances in Immobilisation Techniques for Phenol Oxidases in Solution. Catalysts 2020, 10, 467. [Google Scholar] [CrossRef]
- Kochana, J.; Wapiennik, K.; Kozak, J.; Knihnicki, P.; Pollap, A.; Woźniakiewicz, M.; Nowak, J.; Kościelniak, P. Tyrosinase-based biosensor for determination of bisphenol A in a flow-batch system. Talanta 2015, 144, 163–170. [Google Scholar] [CrossRef]
- López, M.S.-P.; López-Ruiz, B. Electrochemical biosensor based on ionic liquid polymeric microparticles. An analytical platform for catechol. Microchem. J. 2018, 138, 173–179. [Google Scholar] [CrossRef]
- Guan, Y.; Liu, L.; Chen, C.; Kang, X.; Xie, Q. Effective immobilization of tyrosinase via enzyme catalytic polymerization of l -DOPA for highly sensitive phenol and atrazine sensing. Talanta 2016, 160, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Vaghela, C.; Kulkarni, M.; Karve, M.; Zinjarde, S. Selective electrochemical sensing of bisphenol derivatives using novel bioelectrode of agarose-guar gum-graphene oxide immobilized with tyrosinase. J. Environ. Chem. Eng. 2022, 10, 107360. [Google Scholar] [CrossRef]
- Wang, J.; Myung, N.V.; Yun, M.; Monbouquette, H.G. Glucose oxidase entrapped in polypyrrole on high-surface-area Pt electrodes: A model platform for sensitive electroenzymatic biosensors. J. Electroanal. Chem. 2005, 575, 139–146. [Google Scholar] [CrossRef]
- Palomera, N.; Vera, J.L.; Meléndez, E.; Ramirez-Vick, J.E.; Tomar, M.S.; Arya, S.K.; Singh, S.P. Redox active poly(pyrrole-N-ferrocene-pyrrole) copolymer-based mediator-less biosensors. J. Electroanal. Chem. 2011, 658, 33–37. [Google Scholar] [CrossRef]
- Lete, C.; Berger, D.; Matei, C.; Lupu, S. Electrochemical and microgravimetric studies of poly[3,4-ethylenedioxythiophene]-tyrosinase biocomposite material electrodeposited onto gold electrodes by a sinusoidal voltages method. J. Solid State Electrochem. 2016, 20, 3043–3051. [Google Scholar] [CrossRef]
- Li, Y.-M.; Yuan, J.; Ren, H.; Ji, C.-Y.; Tao, Y.; Wu, Y.; Chou, L.-Y.; Zhang, Y.-B.; Cheng, L. Fine-Tuning the Micro-Environment to Optimize the Catalytic Activity of Enzymes Immobilized in Multivariate Metal–Organic Frameworks. J. Am. Chem. Soc. 2021, 143, 15378–15390. [Google Scholar] [CrossRef]
- Liu, T.; Li, P.; Yao, N.; Kong, T.; Cheng, G.; Chen, S.; Luo, W. Self-Sacrificial Template-Directed Vapor-Phase Growth of MOF Assemblies and Surface Vulcanization for Efficient Water Splitting. Adv. Mater. 2019, 31, e1806672. [Google Scholar] [CrossRef]
- Liu, C.-S.; Li, J.; Pang, H. Metal-organic framework-based materials as an emerging platform for advanced electrochemical sensing. Coord. Chem. Rev. 2020, 410, 213222. [Google Scholar] [CrossRef]
- Bai, W.; Li, S.; Ma, J.; Cao, W.; Zheng, J. Ultrathin 2D metal–organic framework (nanosheets and nanofilms)-based xD–2D hybrid nanostructures as biomimetic enzymes and supercapacitors. J. Mater. Chem. A 2019, 7, 9086–9098. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, Y.; Peng, Y.; Huang, Z.; Ma, Q.; Zhang, H. Two-dimensional metal–organic framework nanosheets: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 6267–6295. [Google Scholar] [CrossRef]
- Ma, J.; Yuan, J.; Xu, Y.; Jiang, Y.; Bai, W.; Zheng, J. Ultrasensitive electrochemical determination of bisphenol A in food samples based on a strategy for activity enhancement of enzyme: Layer-by-layer self-assembly of tyrosinase between two-dimensional porphyrin metal–organic framework nanofilms. Chem. Eng. J. 2022, 446, 137001. [Google Scholar] [CrossRef]
- Liu, D.-M.; Chen, J.; Shi, Y.-P. Tyrosinase immobilization on aminated magnetic nanoparticles by physical adsorption combined with covalent crosslinking with improved catalytic activity, reusability and storage stability. Anal. Chim. Acta 2018, 1006, 90–98. [Google Scholar] [CrossRef]
- Baluta, S.; Lesiak, A.; Cabaj, J. Simple and Cost-Effective Electrochemical Method for Norepinephrine Determination Based on Carbon Dots and Tyrosinase. Sensors 2020, 20, 4567. [Google Scholar] [CrossRef]
- Baluta, S.; Meloni, F.; Halicka, K.; Szyszka, A.; Zucca, A.; Pilo, M.I.; Cabaj, J. Differential pulse voltammetry and chronoamperometry as analytical tools for epinephrine detection using a tyrosinase-based electrochemical biosensor. RSC Adv. 2022, 12, 25342–25353. [Google Scholar] [CrossRef]
- Meloni, F.; Spychalska, K.; Zając, D.; Pilo, M.I.; Zucca, A.; Cabaj, J. Application of a Thiadiazole-derivative in a Tyrosinase-based Amperometric Biosensor for Epinephrine Detection. Electroanalysis 2021, 33, 1639–1645. [Google Scholar] [CrossRef]

| Immobilization Strategy | Advantages | Drawbacks |
|---|---|---|
| Physical adsorption |
|
|
| Affinity |
|
|
| Cross-linking |
|
|
| Entrapment |
|
|
| Covalent coupling |
|
|
| Biosensor | Immobilization Technique | Analyte | LOD | Sensitivity | Ref. |
|---|---|---|---|---|---|
| Biosensor based on tyrosinase onto graphene-decorated gold nanoparticle/chitosan (Gr-Au-Chit/Tyr) nanocomposite-modified screen-printed carbon electrode (SPCE) | Physical adsorption | Phenol | 1.6 × 10−2 µM | 0.624 µA/µM | [113] |
| Tyrosinase/NiO/ITO biosensor based tyrosinase, nickel oxide nanoparticles deposited on indium tin oxide | Dopamine | 1.038 μM | 0.0602 µA/µM | [114] | |
| Magnetic loading of tyrosinase-Fe3O4/mesoporous silica core/shell microspheres | Phenol | 1 × 10−3 μM | 78µA/mM | [115] | |
| Flow-biosensor based tyrosinase and acridine orange-adsorbed carbon felt (TYR/AO-CF) | p-Chlorophenol | 2.13 × 10−2 μM | 1.41 µA/µM | [116] | |
| Biosensor based tyrosinase immobilized in a matrix of nanodiamonds and potato starch (Tyr-ND-PS/GCE) | Covalent bond | Catechol | 0.39 μM | - | [130] |
| Tyrosinase modified Au nanoparticles on a screen-printed electrode (Tyr–AuNP–SPE) biosensor | Phenol | 0.4994 μM | 166.830 μA/µM | [136] | |
| Electrochemical enzymatic biosensor based on polymeric matrix derived from 4-mercaptophenylacetic acid (Poly (MPAA)/CGL) | Tyramine | 3.16 μM | - | [137] | |
| Tyrosinase Modified Poly(thionine) Electrodeposited Glassy Carbon Electrode (TYR/GA/pTN/GCE) | Catechol | 6.0 µM | 5.04 × 10−3 µA/μM | [143] | |
| Graphene oxide-modified tyrosinase electrode (TYR/GA/GO/GCE) | Catechol | 3 × 10−2 μM | - | [80] | |
| Gold electrodes modified with cobalt (II)-porphyrin and tyrosinase(CoP) (CoP-Tyr Biosensor) | Cross-linking | Dopamine | 0.43 µM | 1.22 µA·cm−2/µM | [162] |
| Screen-printed electrode modified with single-layer carbon nanotubes and tyrosinase (SPE-SWCNT-Ty) | Hydroxytyrosol | 3.49 × 10−2 μM | - | [48] | |
| Screen printed electrode modified with single-layer carbon-nanotube and tyrosinase (SPE/SWCNT/Tyr) | Oleuropein | 9.53 × 10−2 μM | 0.0718 µA·cm−2/µM | [160] | |
| Tyrosinase immobilized on a single-walled carbon nanotube-modified glassy carbon electrode | Epinephrine | 2.54 µM | - | [161] | |
| Sol–gel TiO2 matrix modified with multi-walled carbon nanotubes (MWCNTs), polycationic polymer poly(diallyldimethylammonium chloride), (PDDA) and Nafion (TYR/TiO2/MWCNTs/PDDA/Nafion) | Entrapment | Bisphenol A | 6.6 μM | 3.263 µA·cm−2/µM | [170] |
| Electrochemical biosensor based on tyrosinase and ionic liquid polymeric microparticles | Catechol | 20 µM | 1.795 × 10−4 µA·cm−2/µM | [171] | |
| Bioelectrode of agarose-guar gum-graphene oxide immobilized with tyrosinase | Bisphenol A | 5 µM | 2.904 µA·cm−2/µM | [173] | |
| Gold electrode based poly(3,4-ethylenedioxythiophene)-tyrosinase (PEDOT-Ty) biocomposite material | Dopamine Hydroquinone | 3.9 µM 5.6 µM | - | [176] | |
| Au electrode modified with poly(L-DOPA)-tyrosinase (PDM-Tyr) composite | Phenol p-Chlorophenol | 2 × 10−3 µM 3 × 10−2 µM | 5.122 μA/µM 1.293 μA/µM | [172] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bounegru, A.V.; Apetrei, C. Tyrosinase Immobilization Strategies for the Development of Electrochemical Biosensors—A Review. Nanomaterials 2023, 13, 760. https://doi.org/10.3390/nano13040760
Bounegru AV, Apetrei C. Tyrosinase Immobilization Strategies for the Development of Electrochemical Biosensors—A Review. Nanomaterials. 2023; 13(4):760. https://doi.org/10.3390/nano13040760
Chicago/Turabian StyleBounegru, Alexandra Virginia, and Constantin Apetrei. 2023. "Tyrosinase Immobilization Strategies for the Development of Electrochemical Biosensors—A Review" Nanomaterials 13, no. 4: 760. https://doi.org/10.3390/nano13040760
APA StyleBounegru, A. V., & Apetrei, C. (2023). Tyrosinase Immobilization Strategies for the Development of Electrochemical Biosensors—A Review. Nanomaterials, 13(4), 760. https://doi.org/10.3390/nano13040760








