Facile Synthesis of ZnO/WO3 Nanocomposite Porous Films for High-Performance Gas Sensing of Multiple VOCs
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. In Situ Fabrication of ZnO/WO3 Ordered Porous Films
2.3. Characterization
2.4. Gas-Sensing Tests
3. Results and Discussion
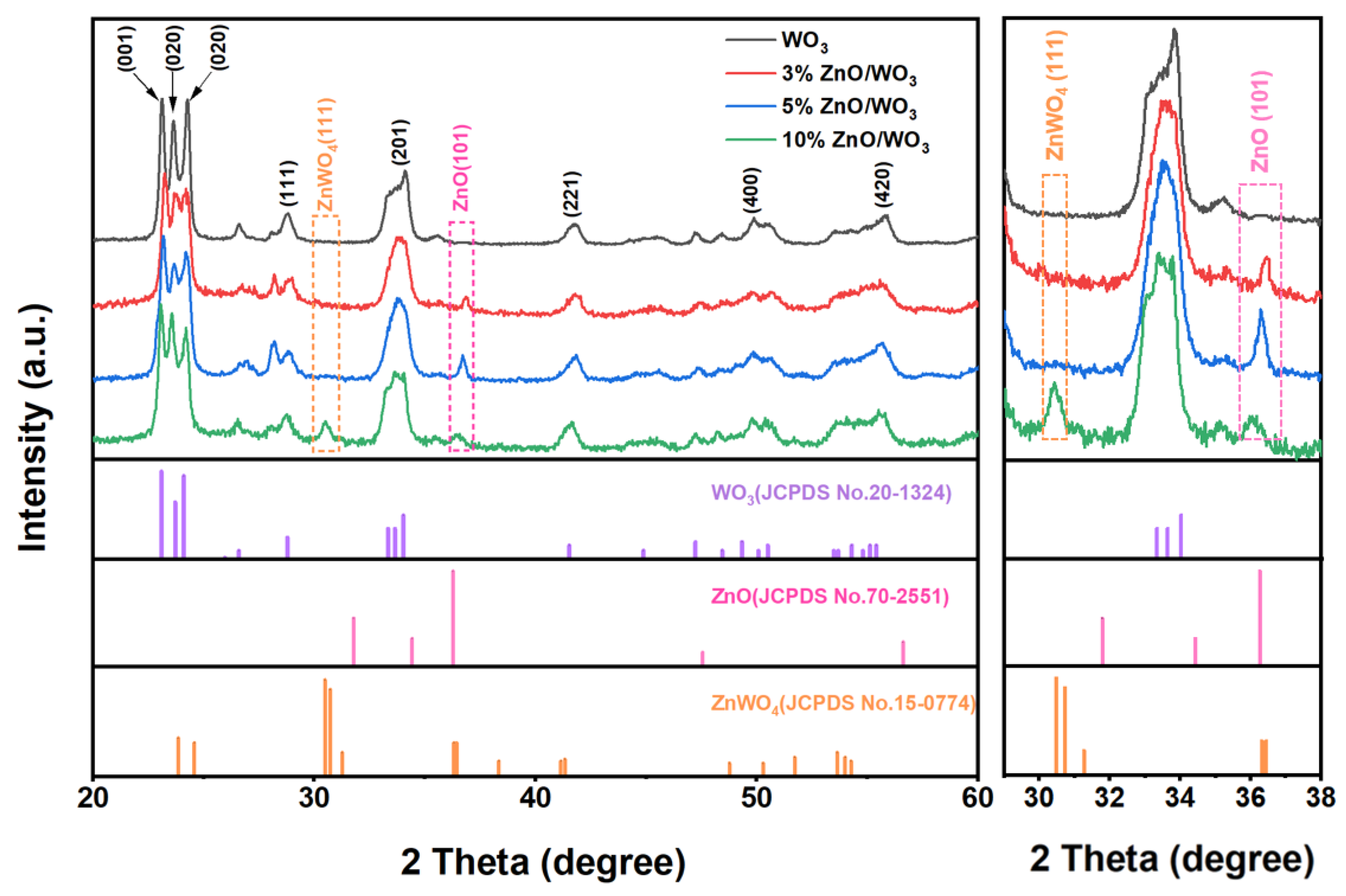
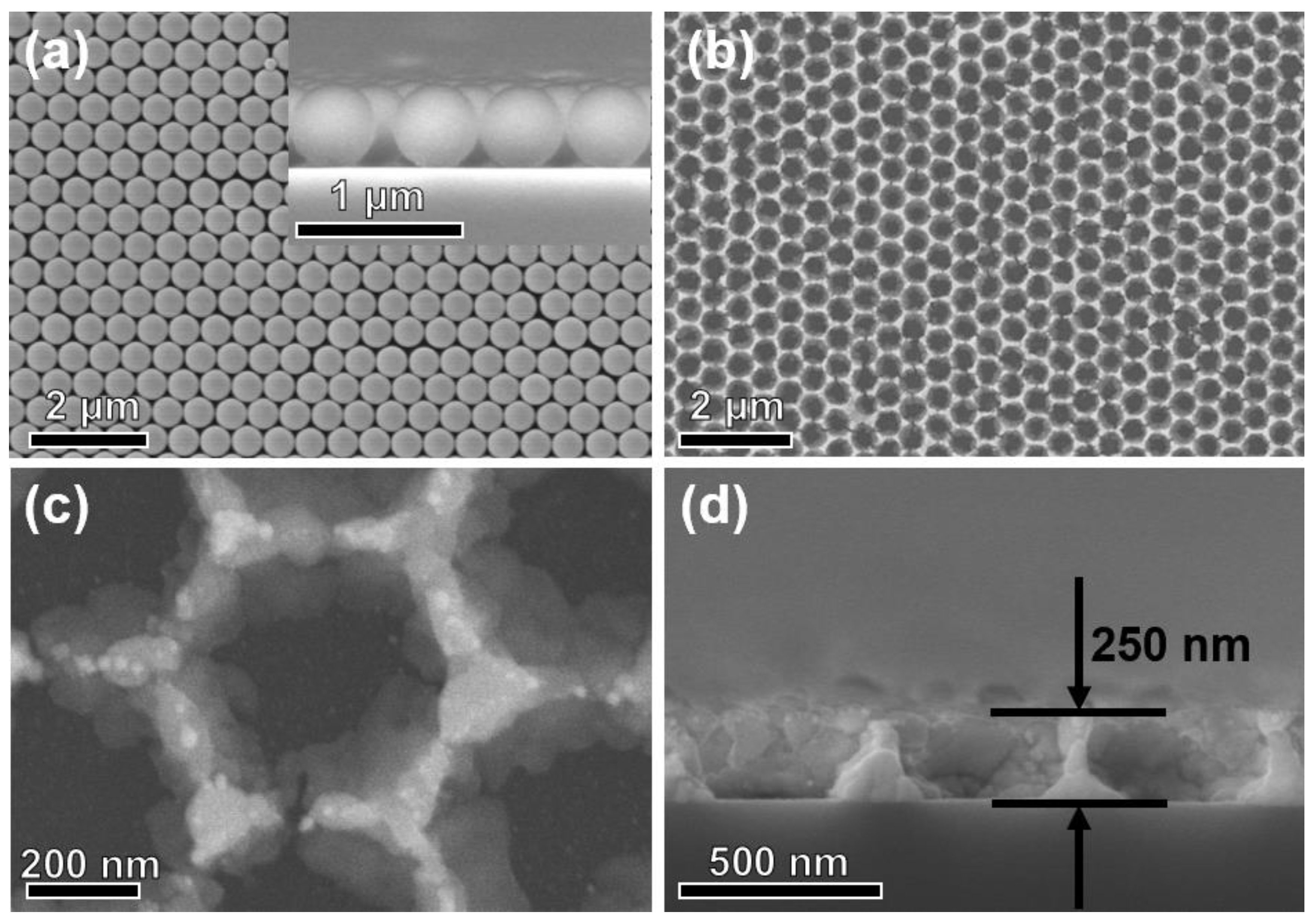
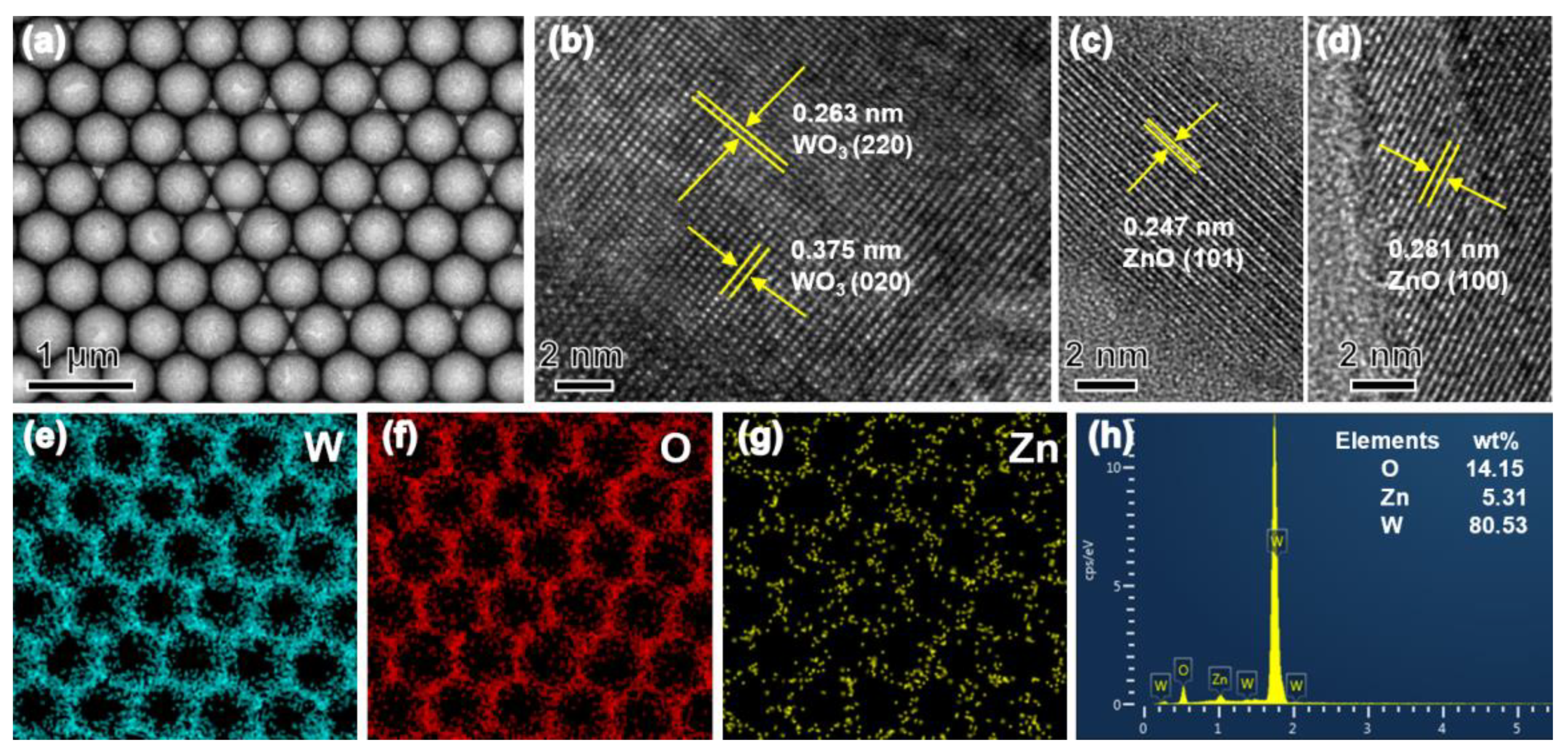
3.1. Structural and Morphological Characteristics
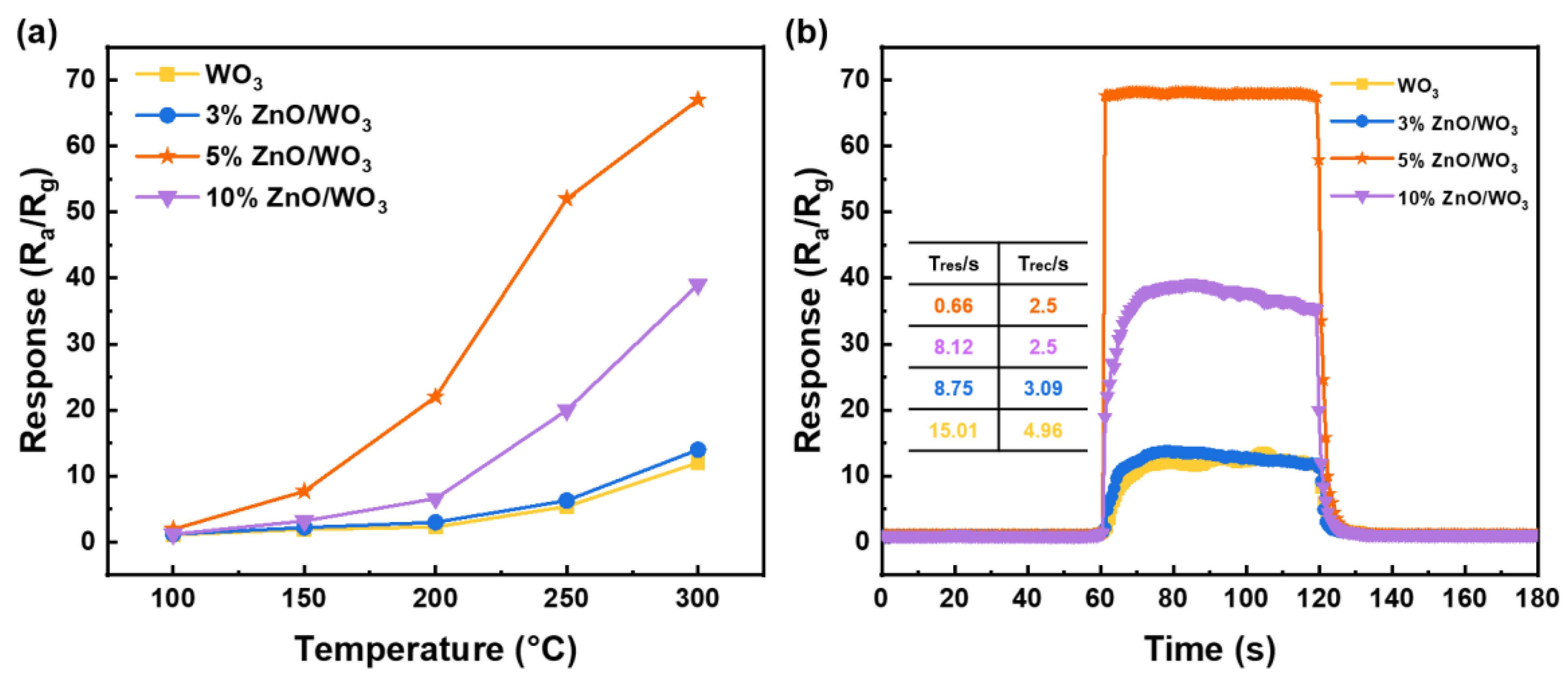
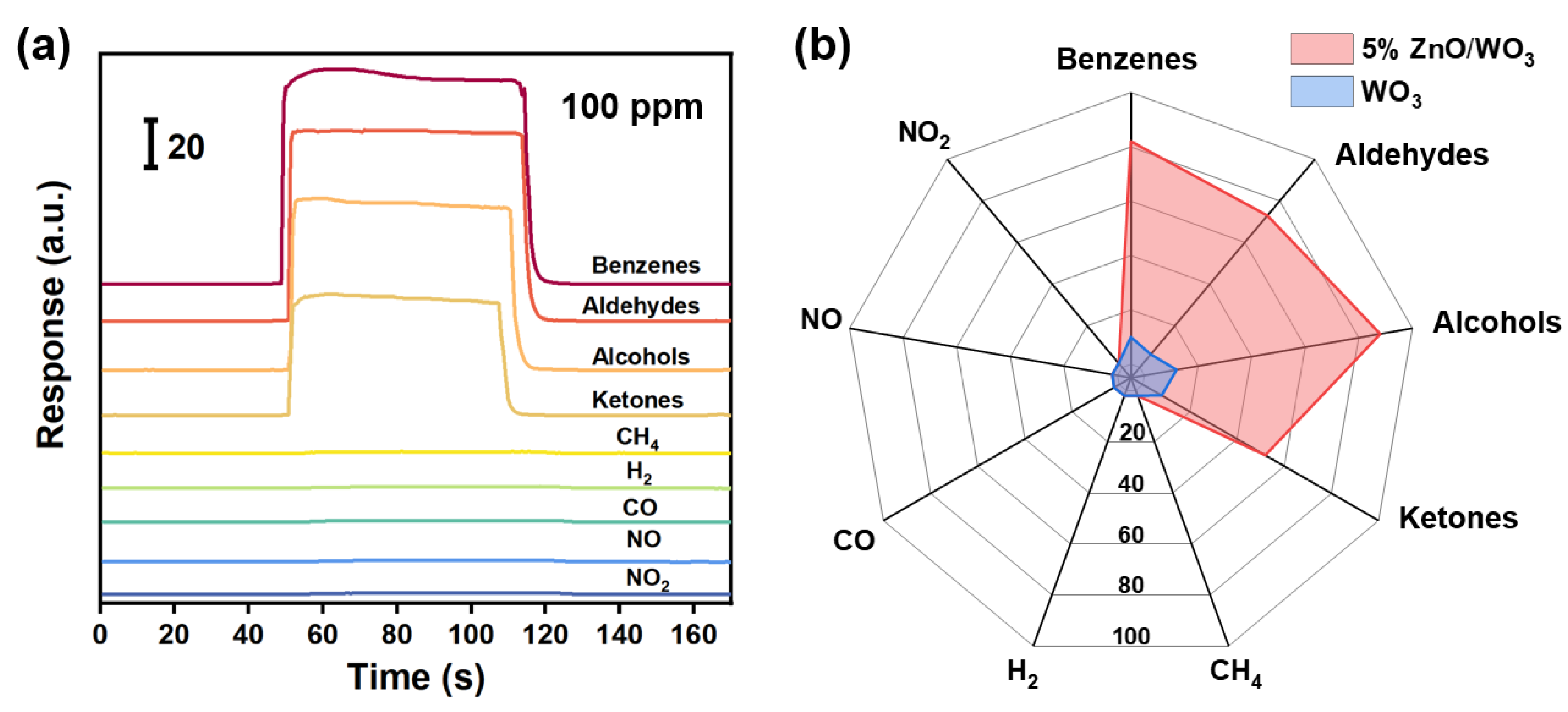
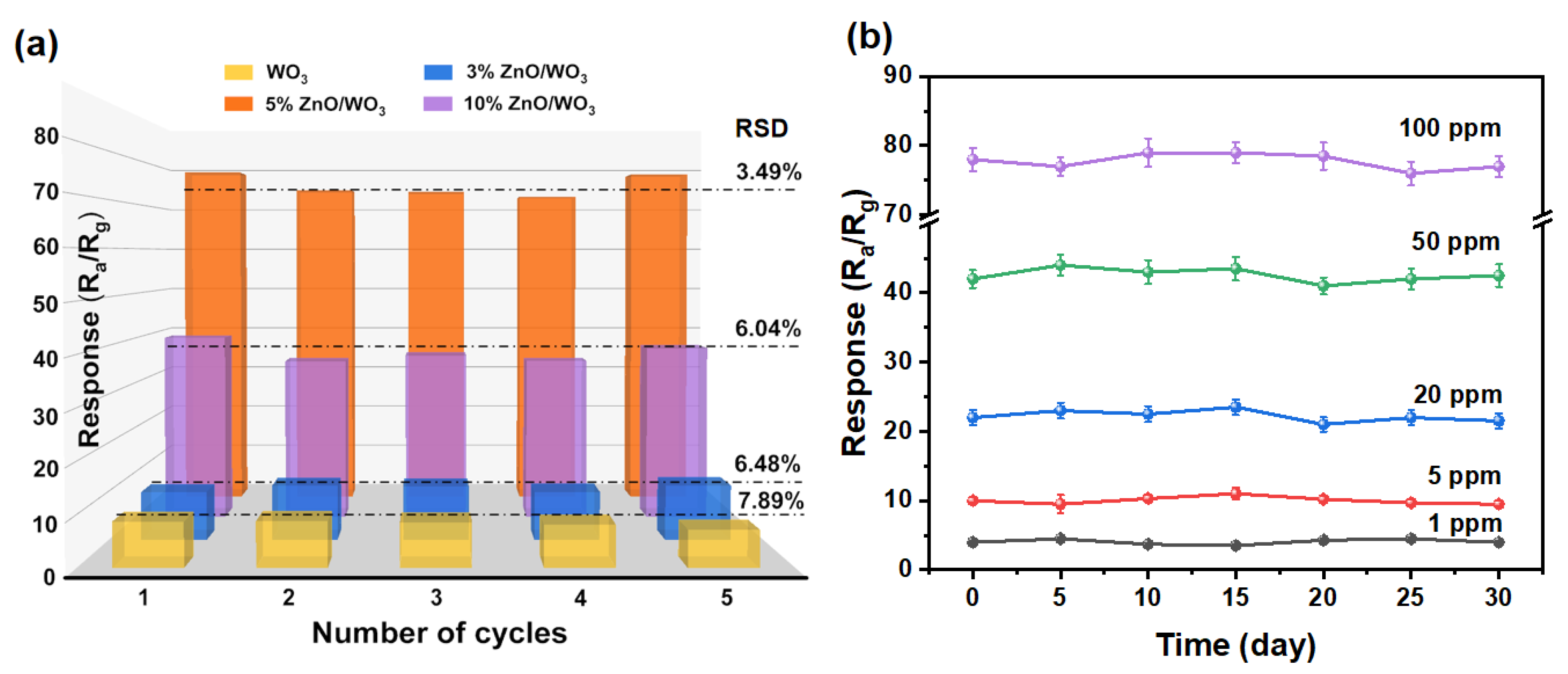
3.2. Gas-Sensing Performances
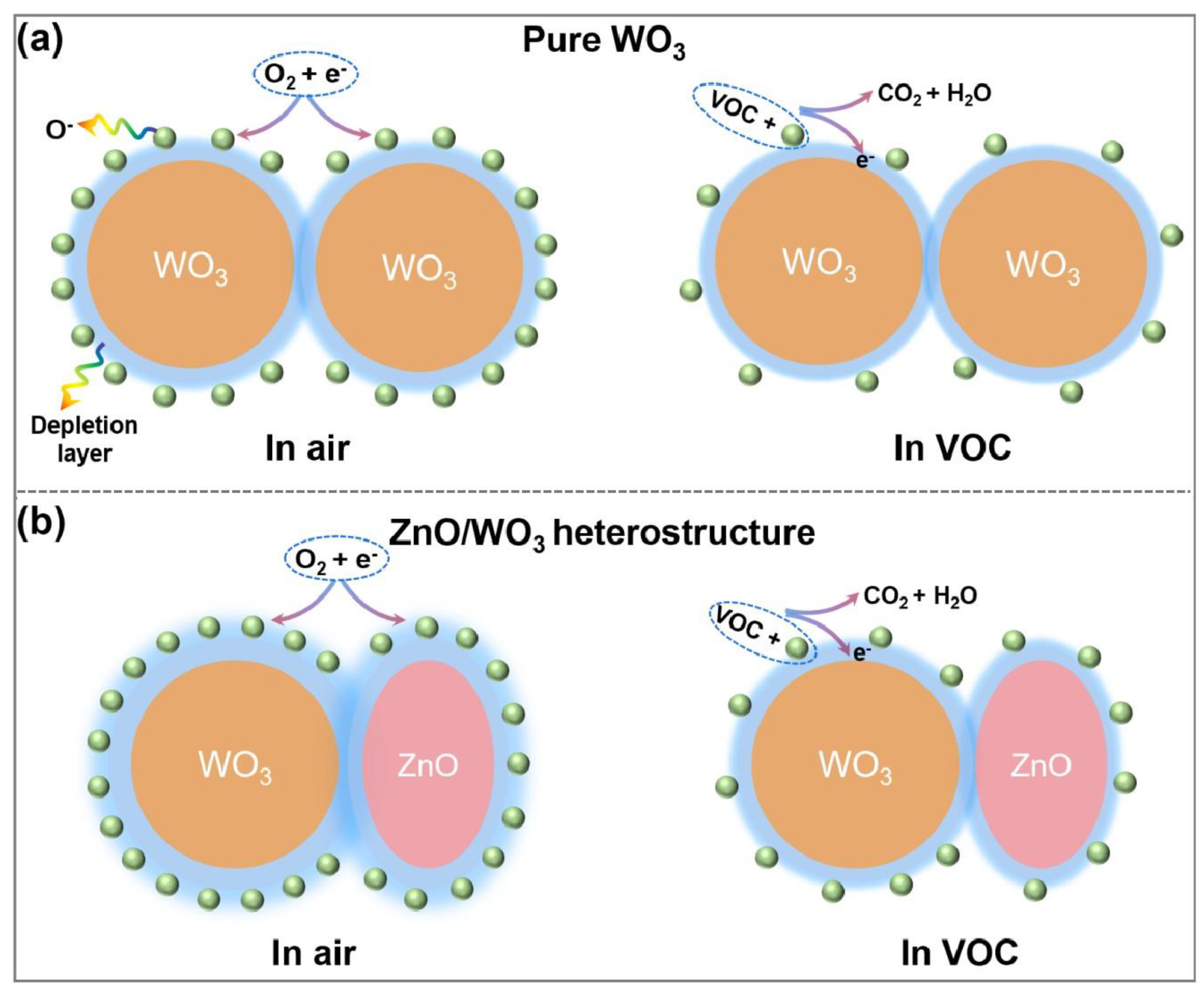
3.3. Gas-Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ehn, M.; Thornton, J.A.; Kleist, E.; Sipila, M.; Junninen, H.; Pullinen, I.; Springer, M.; Rubach, F.; Tillmann, R.; Lee, B.; et al. A large source of low-volatility secondary organic aerosol. Nature 2014, 506, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Moufawad, T.; Gomes, M.C.; Fourmentin, S. Deep eutectic solvents as absorbents for VOC and VOC mixtures in static and dynamic processes. Chem. Eng. J. 2022, 448, 137619. [Google Scholar] [CrossRef]
- Sha, Q.; Zhu, M.; Huang, H.; Wang, Y.; Huang, Z.; Zhang, X.; Tang, M.; Lu, M.; Chen, C.; Shiet, B.; et al. A newly integrated dataset of volatile organic compounds (VOCs) source profiles and implications for the future development of VOCs profiles in China. Sci. Total Environ. 2021, 793, 148348. [Google Scholar] [CrossRef]
- Yang, C.; Miao, G.; Pi, Y.; Xia, Q.; Wu, J.; Li, Z.; Xiao, J. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review. Chem. Eng. J. 2019, 370, 1128–1153. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Dankwort, T.; Mishra, Y.K.; Kienle, L. Cubic mesoporous Pd-WO3 loaded graphitic carbon nitride (g-CN) nanohybrids: Highly sensitive and temperature dependent VOC sensors. J. Mater. Chem. A 2018, 6, 10718–10730. [Google Scholar] [CrossRef]
- Priya, S.; Halder, J.; Mandal, D.; Chowdhury, A.; Singh, T.; Chandra, A. Hierarchical SnO2 nanostructures for potential VOC sensor. J. Mater. Sci 2021, 56, 9883–9893. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, W.; Li, Q. SnO2 as a gas sensor in detection of volatile organic compounds: A review. Sens. Actuator A Phys. 2022, 346, 113845. [Google Scholar] [CrossRef]
- Chaudhary, V.; Nehra, S.P. Pt-sensitized MoO3/mpg-CN mesoporous nanohybrid: A highly sensitive VOC sensor. Microporous Mesoporous Mater. 2021, 315, 110906. [Google Scholar] [CrossRef]
- Joos, P.E.; Godoi, A.F.L.; De Jong, R.; de Zeeuw, J.; Van Grieken, R. Trace analysis of benzene, toluene, ethylbenzene and xylene isomers in environmental samples by low-pressure gas chromatography-ion trap mass spectrometry. J. Chromatogr. A 2003, 985, 191–196. [Google Scholar] [CrossRef]
- Rezende, G.C.; Le Calve, S.; Brandner, J.J.; Newport, D. Characterization of a modular microfluidic photoionization detector. Sens. Actuators B Chem. 2020, 324, 128667. [Google Scholar] [CrossRef]
- Silva, L.I.B.; Ferreira, F.D.P.; Rocha-Santos, T.A.P.; Duarte, A.C. Carbon nanotube field-effect transistor detector associated to gas chromatography for speciation of benzene, toluene, ethylbenzene, (o-, m- and p-)xylene. J. Chromatogr. A 2009, 1216, 6517–6521. [Google Scholar] [CrossRef] [PubMed]
- Young, C.R.; Menegazzo, N.; Riley, A.E.; Brons, C.H.; DiSanzo, F.P.; Givens, J.L.; Martin, J.L.; Disko, M.M.; Mizaikoff, B. Infrared hollow waveguide sensors for simultaneous gas phase detection of benzene, toluene, and xylenes in field environments. Anal. Chem. 2011, 83, 6141–6147. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Duan, L.; Xia, K.; Chen, Y.; Li, Y.; Deng, S.; Xu, J.; Hou, Z. Microwave synthesized 2D WO3 nanosheets for VOCs gas sensors. Nanomaterials 2022, 12, 3211. [Google Scholar] [CrossRef] [PubMed]
- Larin, A.; Womble, P.C.; Dobrokhotov, V. Novel highly-integrated mems based solid state detectors for analytical gas chromatography. Sens. Actuators B Chem. 2018, 256, 1057–1068. [Google Scholar] [CrossRef]
- Oliver, K.D.; Adams, J.R.; Daughtrey, E.H.; McClenny, W.A.; Yoong, M.J.; Pardee, M.A.; Almasi, E.B.; Kirshen, N.A. Technique for monitoring toxic VOCs in air: Sorbent preconcentration, closed-cycle cooler cryofocusing and GC/MS analysis. Environ. Sci. Technol. 1996, 30, 1939–1945. [Google Scholar] [CrossRef]
- Silva, L.I.B.; Rocha-Santos, T.A.R.; Duarte, A.C. Remote optical fibre microsensor for monitoring BTEX in confined industrial atmospheres. Talanta 2009, 78, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.; Yang, C.; Grundmann, M.; Liu, X.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horizons 2019, 6, 470–506. [Google Scholar] [CrossRef]
- Adilakshmi, G.; Reddy, A.S.; Reddy, P.S.; Reddy, C.S. Electron beam evaporated nanostructure WO3 films for gas sensor application. Mat. Sci. Eng. B Adv. 2021, 273, 115421. [Google Scholar] [CrossRef]
- Rabeel, M.; Javed, S.; Khan, R.; Akram, M.A.; Rehman, S.; Kim, D.-k.; Khan, M.F. Controlling the wettability of ZnO thin films by spray pyrolysis for photocatalytic applications. Materials 2022, 15, 3364. [Google Scholar] [CrossRef]
- Dediu, V.; Busila, M.; Tucureanu, V.; Bucur, F.I.; Iliescu, F.S.; Brincoveanu, O.; Iliescu, C. Synthesis of ZnO/Au nanocomposite for antibacterial applications. Nanomaterials 2022, 12, 3832. [Google Scholar] [CrossRef]
- Gholami, P.; Khataee, A.; Bhatnagar, A.; Vahid, B. Synthesis of N-doped magnetic WO3-x@Mesoporous carbon using a diatom template and plasma modification: Visible-light-driven photocatalytic activities. ACS Appl. Mater. Interfaces 2021, 13, 13072–13086. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, T.; Wang, Y.; Huang, D.; He, G.; Han, Y.; Hu, N.; Su, Y.; Zhou, Z.; Zhang, Y.; et al. Enhanced formaldehyde detection based on Ni doping of SnO2 nanoparticles by one-step synthesis. Sens. Actuators B Chem. 2018, 263, 120–128. [Google Scholar] [CrossRef]
- Kang, J.-g.; Park, J.-S.; Lee, H.-J. Pt-doped SnO2 thin film based micro gas sensors with high selectivity to toluene and HCHO. Sens. Actuators B Chem. 2017, 248, 1011–1016. [Google Scholar] [CrossRef]
- Sanze, S.; Gurlo, A.; Hess, C. Monitoring gas sensors at work: Operando Raman-FTIR study of ethanol detection by indium oxide. Angew. Chem. Int. Ed. 2013, 52, 3607–3610. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, T.; Zhang, R.; Lou, Z.; Deng, J.; Zhang, T. Comparison of toluene sensing performances of zinc stannate with different morphology-based gas sensors. Sens. Actuators B Chem. 2016, 227, 448–455. [Google Scholar] [CrossRef]
- Yao, M.S.; Tang, W.X.; Wang, G.E.; Nath, B.; Xu, G. MOF thin film-coated metal oxide nanowire array: Significantly improved chemiresistor sensor performance. Adv. Mater. 2016, 28, 5229–5234. [Google Scholar] [CrossRef]
- Luo, S.; Fu, G.; Chen, H.; Liu, Z.; Hong, Q. Gas-sensing properties and complex impedance analysis of Ce-added WO3 nanoparticles to VOC gases. Solid State Electron. 2007, 51, 913–919. [Google Scholar] [CrossRef]
- Hanh, N.H.; Van Duy, L.; Hung, C.M.; Van Duy, N.; Heo, Y.W.; Van Hieu, N.; Hoa, N.D. VOC gas sensor based on hollow cubic assembled nanocrystal Zn2SnO4 for breath analysis. Sens. Actuator A Phys. 2020, 302, 111834. [Google Scholar] [CrossRef]
- Degler, D.; Weimar, U.; Barsan, N. Current understanding of the fundamental mechanisms of doped and loaded semiconducting metal-oxide-based gas sensing materials. ACS Sens. 2019, 4, 2228–2249. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Zhu, X.; Li, Y.; Cai, W. Bilayer Au nanoparticle-decorated WO3 porous thin films: On-chip fabrication and enhanced NO2 gas sensing performances with high selectivity. Sens. Actuators B Chem. 2019, 280, 192–200. [Google Scholar] [CrossRef]
- Yang, S.; Sun, J.; Xu, L.; Zhou, Q.; Chen, X.; Zhu, S.; Dong, B.; Lu, G.; Song, H. Au@ZnO functionalized three-dimensional macroporous WO3: A application of selective H2S gas sensor for exhaled breath biomarker detection. Sens. Actuators B Chem. 2020, 324, 128725. [Google Scholar] [CrossRef]
- Hu, J.; Xiong, X.; Guan, W.; Long, H.; Zhang, L.; Wang, H. Self-templated flower-like WO3-In2O3 hollow microspheres for conductometric acetone sensors. Sens. Actuators B Chem. 2022, 361, 131705. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Guo, J.; Wang, X.; Wang, Y.; Ma, Q. In-situ growth of hierarchical SnO2/In2O3 heterostructures with multiple effective n-n heterojunctions for superior triethylamine-sensing performances. Sens. Actuators B Chem. 2022, 369, 132377. [Google Scholar] [CrossRef]
- Qin, S.; Tang, P.; Feng, Y.; Li, D. Novel ultrathin mesoporous ZnO-SnO2 n-n heterojunction nanosheets with high sensitivity to ethanol. Sens. Actuators B Chem. 2020, 309, 127801. [Google Scholar] [CrossRef]
- Suematsu, K.; Watanabe, K.; Tou, A.; Sun, Y.; Shimanoe, K. Ultraselective toluene-gas sensor: Nanosized gold loaded on zinc oxide nanoparticles. Anal. Chem. 2018, 90, 1959–1966. [Google Scholar] [CrossRef]
- Li, Z.; Li, S.; Song, Z.; Yang, X.; Wang, Z.; Zhang, H.; Guo, L.; Sun, C.; Liu, H.; Shao, J.; et al. Influence of nickel doping on ultrahigh toluene sensing performance of core-shell zno microsphere gas sensor. Chemosensors 2022, 10, 327. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, J.; Li, M.; Li, Z.; Yuan, Y.; Yang, X.; Guo, L. Highly efficient toluene gas sensor based on spinel structured hollow urchin-like core-shell ZnFe2O4 spheres. Sens. Actuators B Chem. 2021, 349, 130734. [Google Scholar] [CrossRef]
- Li, F.; Li, C.; Zhu, L.; Guo, W.; Shen, L.; Wen, S.; Ruan, S. Enhanced toluene sensing performance of gold-functionalized WO3 center dot H2O nanosheets. Sens. Actuators B Chem. 2016, 223, 761–767. [Google Scholar] [CrossRef]
- Kim, N.-H.; Choi, S.-J.; Yang, D.-J.; Bae, J.; Park, J.; Kim, I.-D. Highly sensitive and selective hydrogen sulfide and toluene sensors using Pd functionalized WO3 nanofibers for potential diagnosis of halitosis and lung cancer. Sens. Actuators B Chem. 2014, 193, 574–581. [Google Scholar] [CrossRef]
- Huang, J.; Xu, X.; Gu, C.; Yang, M.; Yang, M.; Liu, J. Large-scale synthesis of hydrated tungsten oxide 3D architectures by a simple chemical solution route and their gas-sensing properties. J. Mater. Chem. 2011, 21, 13283–13289. [Google Scholar] [CrossRef]
- Lv, Y.-K.; Yao, B.-H.; Liu, Z.-Q.; Liang, S.; Liu, Q.-C.; Zhai, K.; Li, Z.J.; Yao, H.C. Hierarchical Au-Loaded WO3 hollow microspheres with high sensitive and selective properties to toluene and xylene. IEEE Sens. J. 2019, 19, 5413–5420. [Google Scholar] [CrossRef]
- Ma, J.; Ren, Y.; Zhou, X.; Liu, L.; Zhu, Y.; Cheng, X.; Xu, P.; Li, X.; Deng, Y.; Zhao, D. Pt nanoparticles sensitized ordered mesoporous WO3 semiconductor: Gas sensing performance and mechanism study. Adv. Funct. Mater. 2018, 28, 1705268. [Google Scholar] [CrossRef]
- Meng, Z.; Aykanat, A.; Mirica, K.A. Welding metallophthalocyanines into bimetallic molecular meshes for ultrasensitive, low-power chemiresistive detection of gases. J. Am. Chem. Soc. 2019, 141, 2046–2053. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.A.; Xiao, D.J.; Gonzalez, M.I.; Darago, L.E.; Herm, Z.R.; Grandjean, F.; Long, J.R. Reversible CO scavenging via adsorbate-dependent spin state transitions in an Iron(II)-Triazolate metal-organic framework. J. Am. Chem. Soc. 2016, 138, 5594–5602. [Google Scholar] [CrossRef]
- Chi, X.; Liu, C.; Liu, L.; Li, Y.; Wang, Z.; Bo, X.; Liu, L.; Su, C. Tungsten trioxide nanotubes with high sensitive and selective properties to acetone. Sens. Actuators B Chem. 2014, 194, 33–37. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Han, L.; Li, X.; Xu, Y. Highly sensitive and selective triethylamine gas sensor based on hierarchical radial CeO2/ZnO n-n heterojunction. Sens. Actuators B Chem. 2022, 367, 132031. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, Y.; Ma, P.; Li, S.; Fan, F.; Cui, K.; Ge, S.; Zhang, Y.; Yu, J. Low-power and high-performance trimethylamine gas sensor based on n-n heterojunction microbelts of perylene Diimide/CdS. Anal. Chem. 2019, 91, 5591–5598. [Google Scholar] [CrossRef]
- Yuan, H.; Aljneibi, S.A.A.A.; Yuan, J.; Wang, Y.; Liu, H.; Fang, J.; Tang, C.; Yan, X.; Cai, H.; Gu, Y.; et al. ZnO nanosheets abundant in oxygen vacancies derived from metal-organic frameworks for ppb-level gas sensing. Adv. Mater. 2019, 31, 1807161. [Google Scholar] [CrossRef]

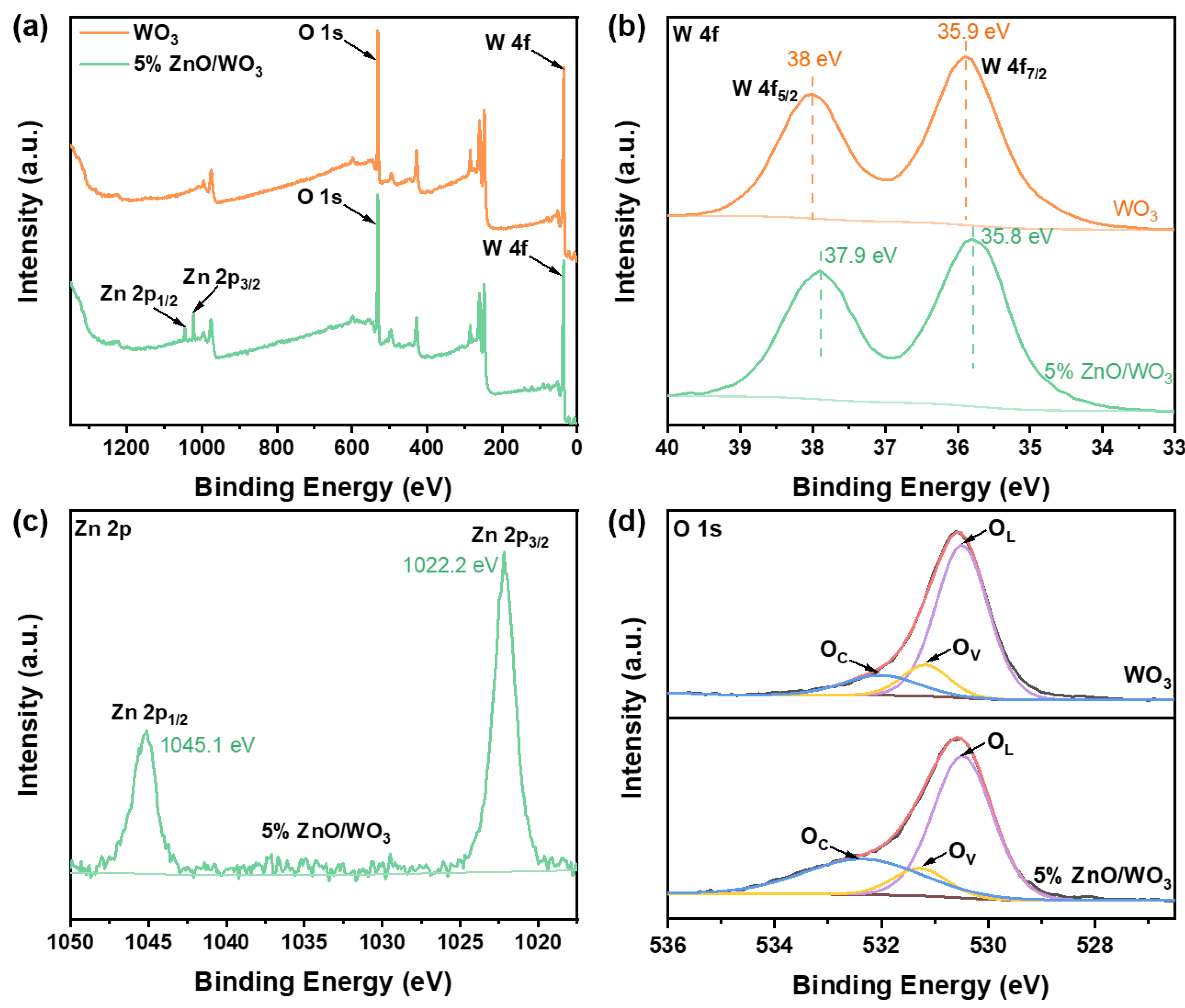

| Samples | OL (eV) | OV (eV) | OC (eV) | OL (%) | OV (%) | OC (%) | OV + OC (%) |
|---|---|---|---|---|---|---|---|
| WO3 | 530.3 | 531.2 | 532.1 | 73 | 13 | 14 | 27 |
| 5% ZnO/WO3 | 530.3 | 531.3 | 532.4 | 57 | 14 | 29 | 43 |
| Sensing Materials | Con. (ppm) | Tem. (°C) | Response (Ra/Rg) | Res./Rec. Time (s) | Detection Limit (ppm) | Ref. |
|---|---|---|---|---|---|---|
| Zn2SnO4 sheet | 100 | 280 | 25.2 | 1/3.5 | 5 | [26] |
| Au-ZnO-NP | 100 | 377 | 97 | 65/360 | / | [37] |
| Ni–ZnO core–shell spheres | 100 | 325 | 210 | 2/77 | 0.5 | [38] |
| Au-functionalized WO3·H2O | 100 | 300 | 50 | 2/9 | / | [40] |
| Pd-NPs/Pd-embedded WO3 NFs | 5 | 350 | 10 | 10.9/16.1 | 0.2 | [41] |
| Core–shell ZnFe2O4 spheres | 100 | 275 | 55.26 | 3/105 | 0.2 | [39] |
| WO3 microflowers | 100 | 320 | 16.7 | 2/12 | 1 | [42] |
| Hierarchical Au-loaded WO3 hollow microspheres | 100 | 340 | 24 | 8/5 | 5 | [43] |
| ZnO/WO3 composite ordered porous films | 50 | 300 | 68 | 0.7/2.5 | 0.1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, B.; Zhang, H.; Zhao, Q.; Liu, W.; Wei, Y.; Lu, Y.; Xiao, T.; Kong, J.; Cai, W. Facile Synthesis of ZnO/WO3 Nanocomposite Porous Films for High-Performance Gas Sensing of Multiple VOCs. Nanomaterials 2023, 13, 733. https://doi.org/10.3390/nano13040733
Lei B, Zhang H, Zhao Q, Liu W, Wei Y, Lu Y, Xiao T, Kong J, Cai W. Facile Synthesis of ZnO/WO3 Nanocomposite Porous Films for High-Performance Gas Sensing of Multiple VOCs. Nanomaterials. 2023; 13(4):733. https://doi.org/10.3390/nano13040733
Chicago/Turabian StyleLei, Biao, Hongwen Zhang, Qian Zhao, Weiwei Liu, Yi Wei, Yanyan Lu, Tingting Xiao, Jinglin Kong, and Weiping Cai. 2023. "Facile Synthesis of ZnO/WO3 Nanocomposite Porous Films for High-Performance Gas Sensing of Multiple VOCs" Nanomaterials 13, no. 4: 733. https://doi.org/10.3390/nano13040733
APA StyleLei, B., Zhang, H., Zhao, Q., Liu, W., Wei, Y., Lu, Y., Xiao, T., Kong, J., & Cai, W. (2023). Facile Synthesis of ZnO/WO3 Nanocomposite Porous Films for High-Performance Gas Sensing of Multiple VOCs. Nanomaterials, 13(4), 733. https://doi.org/10.3390/nano13040733









