Coupling of Mn2O3 with Heteroatom-Doped Reduced Graphene Oxide Aerogels with Improved Electrochemical Performances for Sodium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
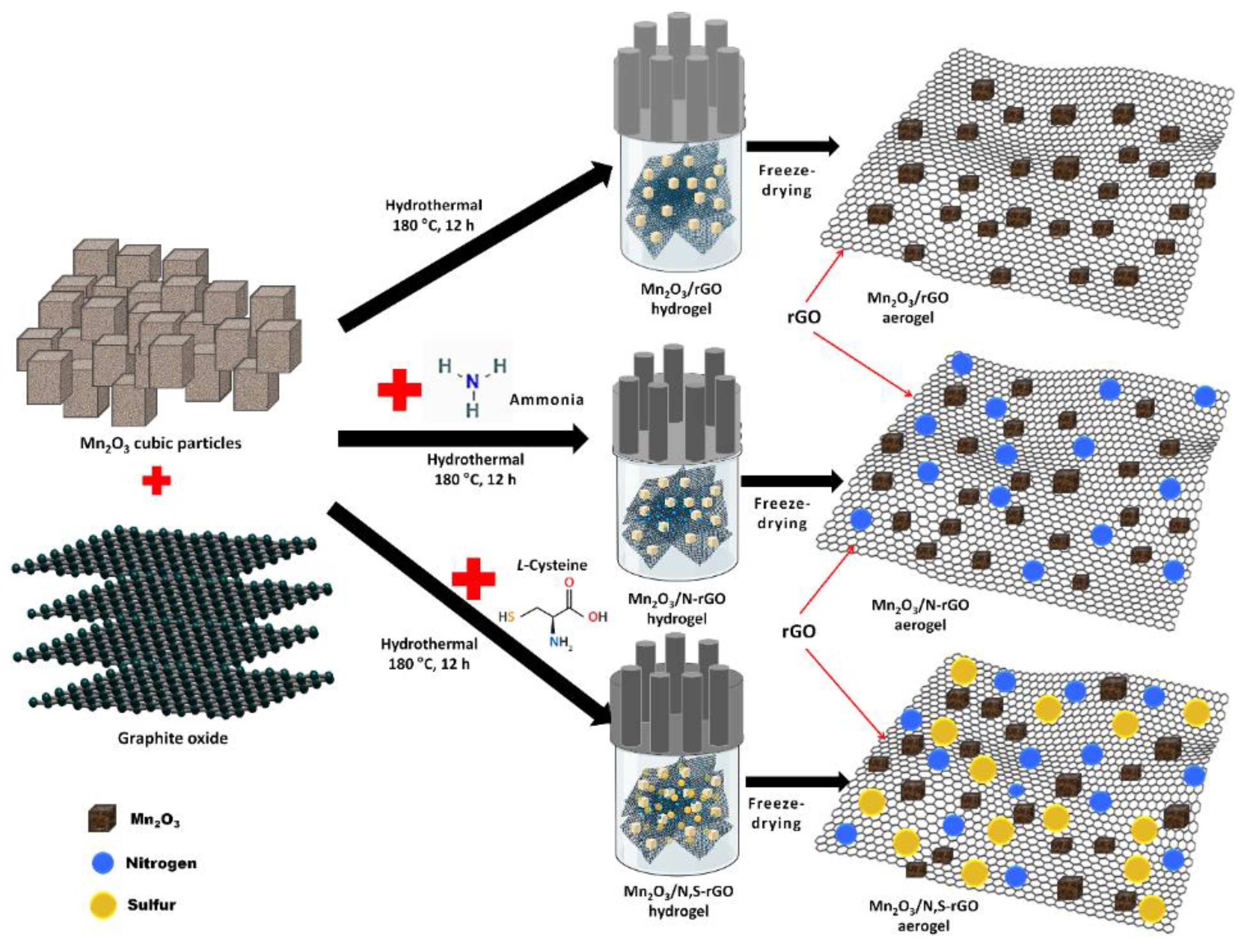
2.2. Synthesis of Mn2O3/rGO and Mn2O3/Heteroatom-Doped rGO Aerogels
2.3. Physical Characterisation
2.4. Electrochemical Characterisation
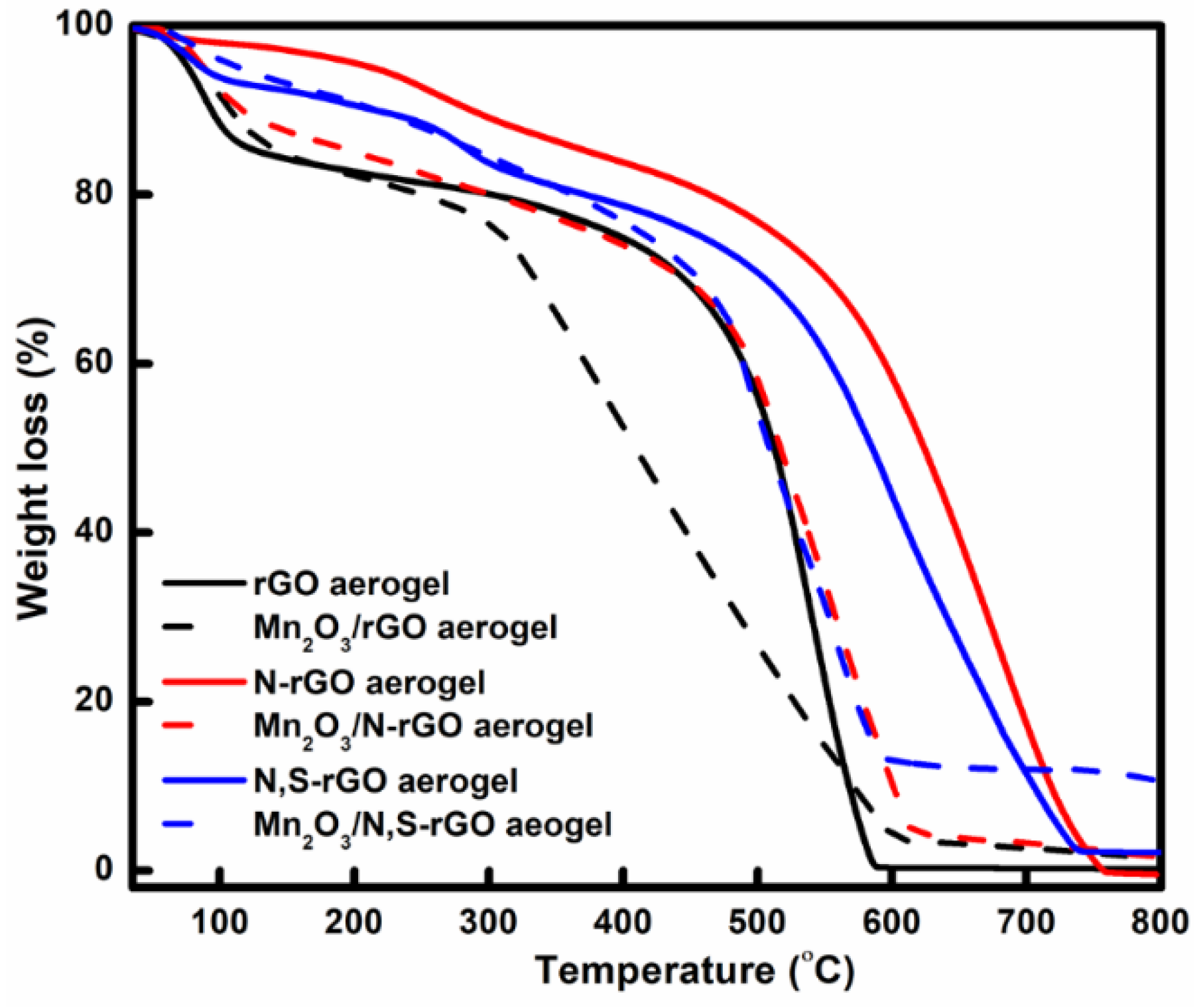
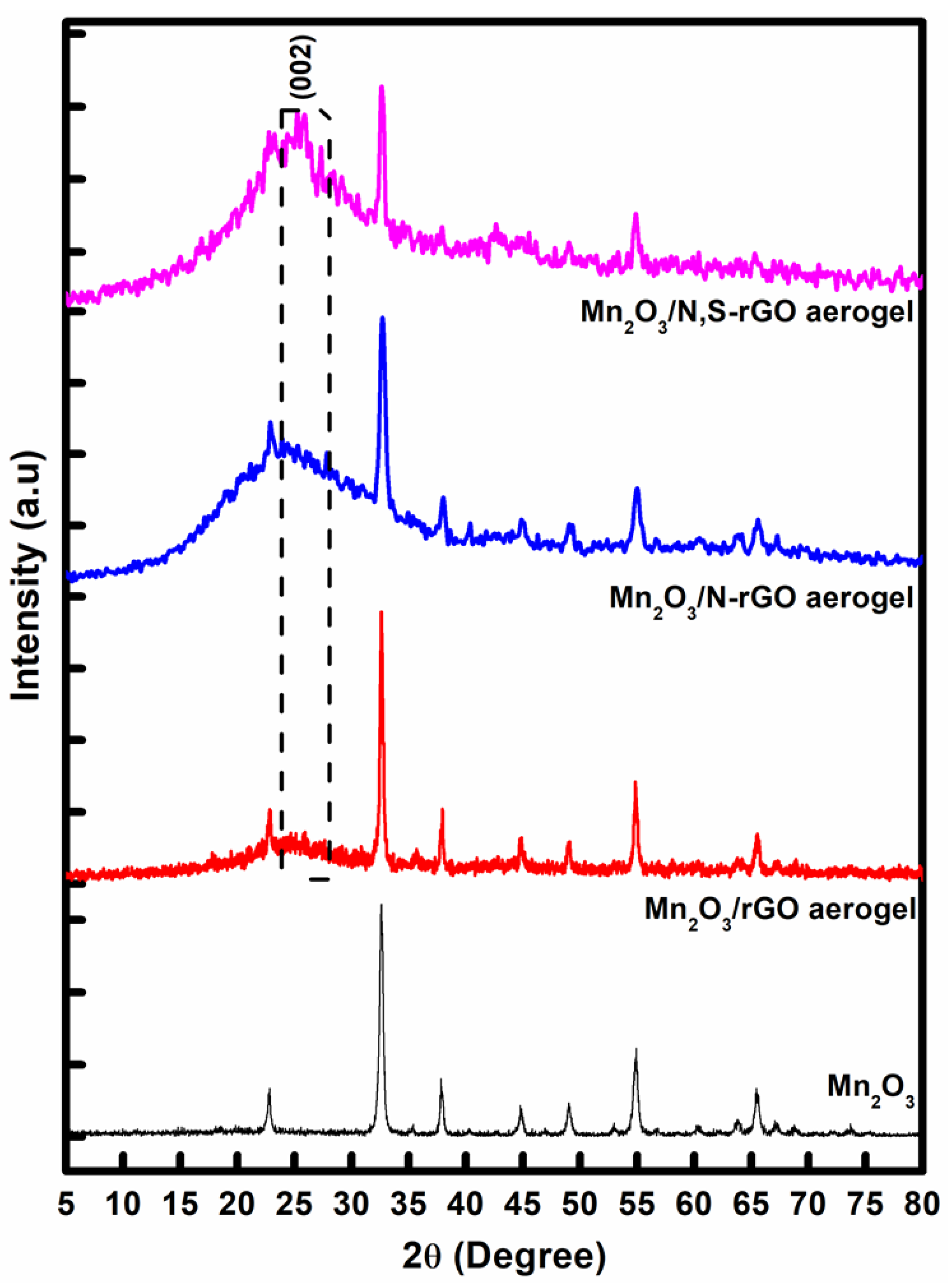
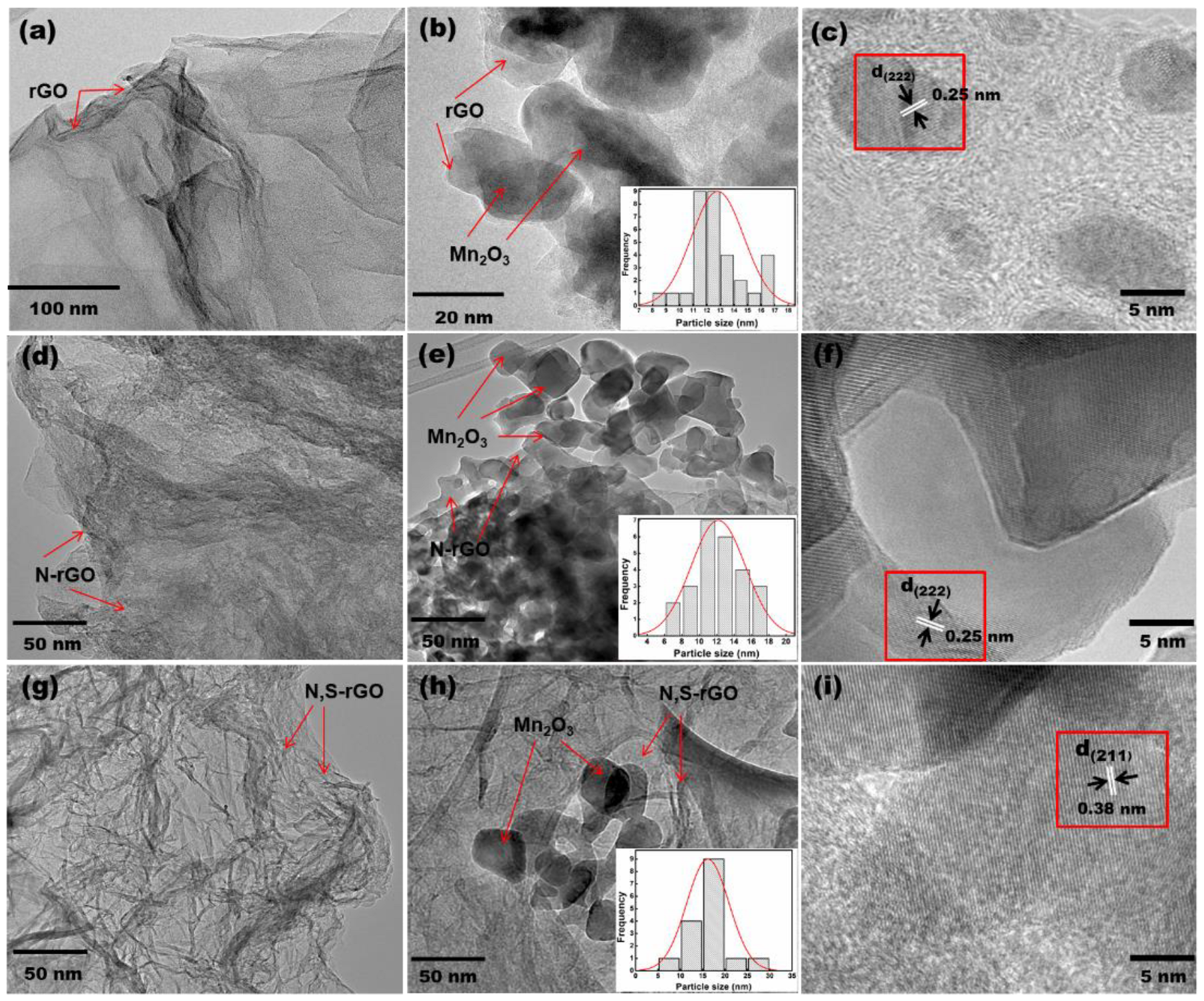
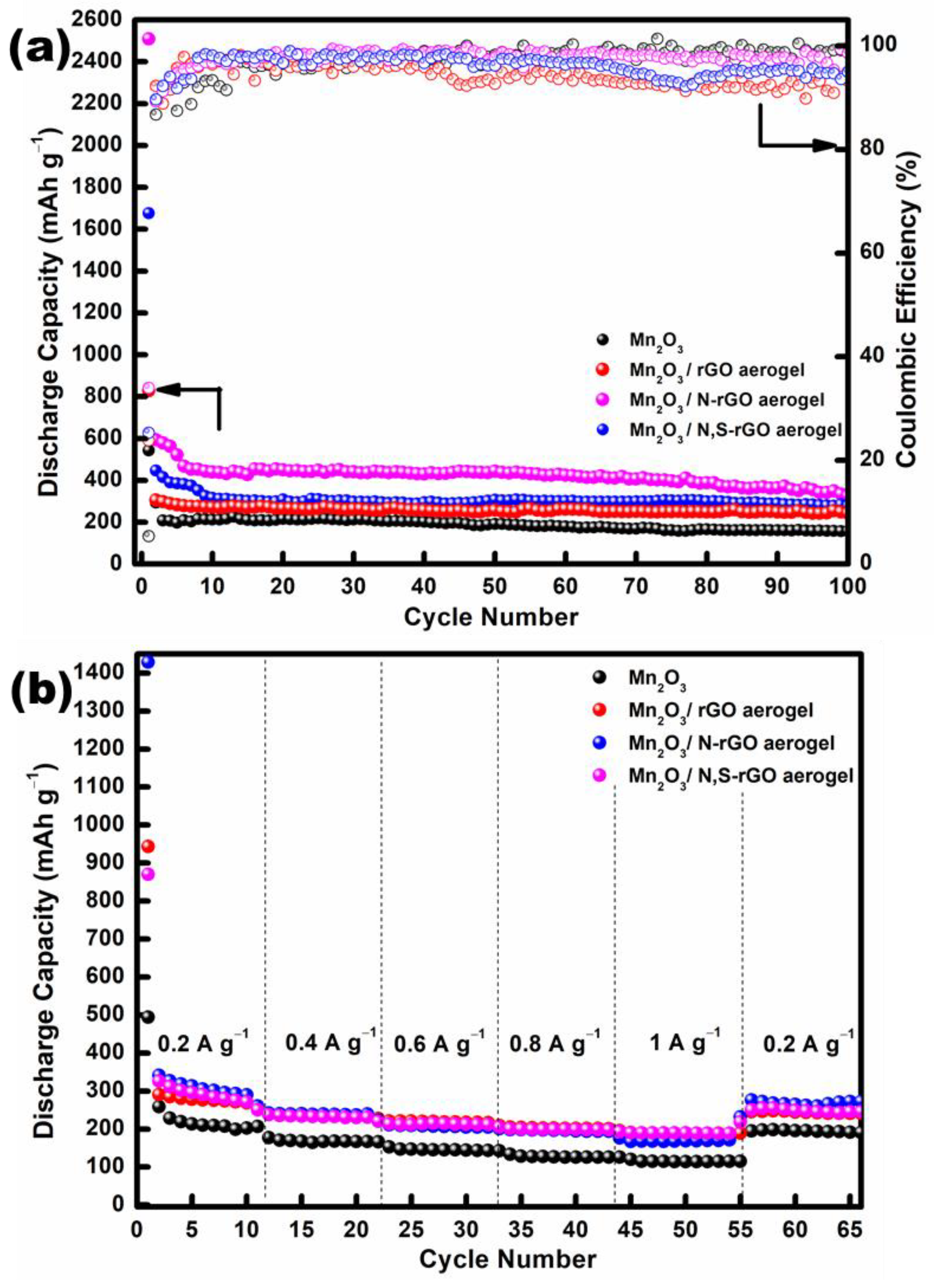
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Wei, Z.; Mao, M.; Wang, H.; Li, Y.; Ma, J. Metal oxide/graphene composite anode materials for sodium-ion batteries. Energy Storage Mater. 2019, 16, 434–454. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.-E.; Yang, Y.; Neale, Z.G.; Massé, R.C.; Cao, J.; Cai, W.; Sui, J.; Cao, G. Reversible and fast Na-ion storage in MoO2/MoSe2 heterostructures for high energy-high power Na-ion capacitors. Energy Storage Mater. 2018, 12, 241–251. [Google Scholar] [CrossRef]
- Cheng, T.; Xu, J.; Tan, Z.; Ye, J.; Tao, Z.; Du, Z.; Wu, Y.; Wu, S.; Ji, H.; Yu, Y. A spray-freezing approach to reduced graphene oxide/MoS2 hybrids for superior energy storage. Energy Storage Mater. 2018, 10, 282–290. [Google Scholar] [CrossRef]
- Hong, S.Y.; Kim, Y.; Park, Y.; Choi, A.; Choi, N.S.; Lee, K.T. Charge carriers in rechargeable batteries: Na ions vs. Li ions. Energy Environ. Sci. 2013, 6, 2067–2081. [Google Scholar] [CrossRef]
- Cao, K.; Jiao, L.; Liu, Y.; Liu, H.; Wang, Y.; Yuan, H. Ultra-high capacity lithium-ion batteries with hierarchical CoO nanowire clusters as binder free electrodes. Adv. Funct. Mater. 2015, 25, 1082–1089. [Google Scholar] [CrossRef]
- Zhang, N.; Han, X.; Liu, Y.; Hu, X.; Zhao, Q.; Chen, J. 3D porous γ-Fe2O3@C nanocomposite as high-performance anode material of Na-ion batteries. Adv. Energy Mater. 2015, 5, 1401123. [Google Scholar] [CrossRef]
- Chen, X.Q.; Lin, H.B.; Zheng, X.W.; Cai, X.; Xia, P.; Zhu, Y.M.; Li, X.P.; Li, W.S. Fabrication of core–shell porous nanocubic Mn2O3@TiO2 as a high-performance anode for lithium ion batteries. J. Mater. Chem. A 2015, 3, 18198–18206. [Google Scholar] [CrossRef]
- Cheng, C.; Huang, Y.; Wang, N.; Jiang, T.; Hu, S.; Zheng, B.; Yuan, H.; Xiao, D. Facile fabrication of Mn2O3 nanoparticle-assembled hierarchical hollow spheres and their sensing for hydrogen peroxide. ACS Appl. Mater. Interfaces 2015, 7, 9526–9533. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Z.; Jin, J.; Cai, Y.; Li, Y.; Tan, H.Y.; Wang, H.E.; Van Tendeloo, G.; Su, B.L. Engineering single crystalline Mn3O4 nano-octahedra with exposed highly active {011} facets for high performance lithium ion batteries. Nanoscale 2014, 6, 6819–6827. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Xu, Y.-F.; Feng, S.-C.; Wu, Z.-G.; Sun, X.-P.; Shen, C.-H.; Wang, J.-Q.; Li, J.-T.; Huang, L.; Sun, S.-G. Hierarchical Mn2O3 hollow microspheres as anode material of lithium ion battery and its conversion reaction mechanism investigated by XANES. ACS Appl. Mater. Interfaces 2015, 7, 8488–8494. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Zhang, Y.; Zhang, Y.; Guo, C.; Tang, B.; Sun, D. MOFs-derived porous Mn2O3 as high-performance anode material for Li-ion battery. J. Mater. Chem. A 2015, 3, 5266–5269. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, X.; Li, J. Facile synthesis of nanostructured MnO2 as anode materials for sodium-ion batteries. ChemNanoMat 2016, 2, 196–200. [Google Scholar] [CrossRef]
- Weng, Y.-T.; Huang, T.Y.; Lim, C.H.; Shao, P.S.; Hy, S.; Kuo, C.Y.; Cheng, J.H.; Hwang, B.J.; Lee, J.F.; Wu, N.L. An unexpected large capacity of ultrafine manganese oxide as a sodium-ion battery anode. Nanoscale 2015, 7, 20075–20081. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hu, M.; Zhang, D.; Yuan, T.; Sun, W.; Xu, B.; Yan, M. Transition metal oxides for high performance sodium ion battery anodes. Nano Energy 2014, 5, 60–66. [Google Scholar] [CrossRef]
- Mahamad, Y.N.F.; Idris, N.H.; Md, D.M.F.; Majid, S.R.; Harun, N.A.; Rahman, M.M. Electrochemical sodiation/desodiation into Mn3O4 nanoparticles. ACS Omega 2020, 5, 29158–29167. [Google Scholar] [CrossRef]
- Alagar, S.; Madhuvilakku, R.; Mariappan, R.; Piraman, S. Nano-architectured porous Mn2O3 spheres/cubes vs rGO for asymmetric supercapacitors applications in novel solid-state electrolyte. J. Power Sources 2019, 441, 227181–227193. [Google Scholar] [CrossRef]
- Yusoff, N.F.M.; Idris, N.H.; Din, M.F.M.; Majid, S.R.; Harun, N.A.; Rahman, M.M. Investigation on the electrochemical performances of Mn2O3 as a potential anode for Na-ion batteries. Sci. Rep. 2020, 10, 9207–9217. [Google Scholar] [CrossRef]
- Huang, S.-Z.; Jin, J.; Cai, Y.; Li, Y.; Deng, Z.; Zeng, J.-Y.; Liu, J.; Wang, C.; Hasan, T.; Su, B.-L. Three-dimensional (3D) bicontinuous hierarchically porous Mn2O3 single crystals for high performance lithium-ion batteries. Sci. Rep. 2015, 5, 14686–14698. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Yan, B.; Li, D.; Lawes, S.; Sun, X. Significant impact of 2D graphene nanosheets on large volume change tin-based anodes in lithium-ion batteries: A review. J. Power Sources 2015, 274, 869–884. [Google Scholar] [CrossRef]
- Jiang, X.; Yu, W.; Wang, H.; Xu, H.; Liu, X.; Ding, Y. Enhancing the performance of MnO by double carbon modification for advanced lithium-ion battery anodes. J. Mater. Chem. A 2016, 4, 920–925. [Google Scholar] [CrossRef]
- Bai, Z.; Zhang, Y.; Zhang, Y.; Guo, C.; Tang, B. A large-scale, green route to synthesize of leaf-like mesoporous CuO as high-performance anode materials for lithium ion batteries. Electrochim. Acta 2015, 159, 29–34. [Google Scholar] [CrossRef]
- Li, Y.; Ye, D.; Liu, W.; Shi, B.; Guo, R.; Pei, H.; Xie, J. A three-dimensional core-shell nanostructured composite of polypyrrole wrapped MnO2/reduced graphene oxide/carbon nanotube for high performance lithium ion batteries. J. Colloid Interface Sci. 2017, 493, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Liu, B.; Ye, J.; Xu, C. Well encapsulated Mn3O4 octahedra in graphene nanosheets with much enhanced Li-storage performances. J. Colloid Interface Sci. 2017, 504, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.S.; Zhou, G.; Yin, L.C.; Ren, W.; Li, F.; Cheng, H.M. Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 2012, 1, 107–131. [Google Scholar] [CrossRef]
- Buqa, H.; Goers, D.; Holzapfel, M.; Spahr, M.E.; Novák, P. High rate capability of graphite negative electrodes for lithium-ion batteries. J. Electrochem. Soc. 2005, 152, A474–A481. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y. The main problems and solutions in practical application of anode materials for sodium ion batteries and the latest research progress. Int. J. Energy Res. 2021, 45, 9753–9779. [Google Scholar] [CrossRef]
- Wang, Z.; Qie, L.; Yuan, L.; Zhang, W.; Hu, X.; Huang, Y. Functionalized N-doped interconnected carbon nanofibers as an anode material for sodium-ion storage with excellent performance. Carbon 2013, 55, 328–334. [Google Scholar] [CrossRef]
- Jing, M.; Chen, Z.; Li, Z.; Li, F.; Chen, M.; Zhou, M.; He, B.; Chen, L.; Hou, Z.; Chen, X. Facile synthesis of ZnS/N,S co-doped carbon composite from zinc metal complex for high-performance sodium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 704–712. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Z.; Du, K.; Zhao, X.; Chen, W.; Lai, Y.; Li, J. Dopamine derived nitrogen-doped carbon sheets as anode materials for high-performance sodium ion batteries. Carbon 2015, 91, 88–95. [Google Scholar] [CrossRef]
- Yue, X.; Huang, N.; Jiang, Z.; Tian, X.; Wang, Z.; Hao, X.; Jiang, Z.J. Nitrogen-rich graphene hollow microspheres as anode materials for sodium-ion batteries with super-high cycling and rate performance. Carbon 2018, 130, 574–583. [Google Scholar] [CrossRef]
- Deng, X.; Xie, K.; Li, L.; Zhou, W.; Sunarso, J.; Shao, Z. Scalable synthesis of self-standing sulfur-doped flexible graphene films as recyclable anode materials for low-cost sodium-ion batteries. Carbon 2016, 107, 67–73. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Hassan, F.M.; Li, J.; Fan, X.; Batmaz, R.; Xiao, X.; Chen, Z. Sulfur covalently bonded graphene with large capacity and high rate for high-performance sodium-ion batteries anodes. Nano Energy 2015, 15, 746–754. [Google Scholar] [CrossRef]
- Quan, B.; Jin, A.; Yu, S.H.; Kang, S.M.; Jeong, J.; Abruña, H.D.; Jin, L.; Piao, Y.; Sung, Y.E. Solvothermal-derived S-doped graphene as an anode material for sodium-ion batteries. Adv. Sci. 2018, 5, 1700880–1700913. [Google Scholar] [CrossRef] [PubMed]
- Mahamad Yusoff, N.F.; Idris, N.H.; Md Din, M.F.; Majid, S.R.; Harun, N.A. Enhanced electrochemical performances of Mn3O4/heteroatom-doped reduced graphene oxide aerogels as an anode for sodium-ion batteries. Nanomaterials 2022, 12, 3569. [Google Scholar] [CrossRef]
- Hummers, J.W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Hu, N.; Gao, R.; Wang, Y.; Wang, Y.; Chai, J.; Yang, Z.; Kong, E.S.W.; Zhang, Y. The preparation and characterization of non-covalently functionalized graphene. J. Nanosci. Nanotechnol. 2012, 12, 99–104. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, G. Preparation of highly conductive graphene hydrogels for fabricating supercapacitors with high rate capability. J. Phys. Chem. C 2011, 115, 17206–17212. [Google Scholar] [CrossRef]
- Ghosh, A.; Ghosh, S.; Seshadhri, G.M.; Ramaprabhu, S. Green synthesis of nitrogen-doped self-assembled porous carbon-metal oxide composite towards energy and environmental applications. Sci. Rep. 2019, 9, 5187. [Google Scholar] [CrossRef]
- Kang, J.-H.; Chen, J.-S. Using ethylenediamine to prepare three dimensional nitrogen-doped graphene aerogel/sulfur composite for lithium-sulfur batteries. Diam. Relat. Mater. 2018, 88, 222–229. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Ma, C.; Shi, J.; Zhao, Y. Highly monodispersed graphene/SnO2 hybrid nanosheets as bifunctional anode materials of Li-ion and Na-ion batteries. J. Alloys Compd. 2020, 821, 153506–153516. [Google Scholar] [CrossRef]
- Zhang, L.; Ge, D.; Geng, H.; Zheng, J.; Cao, X.; Gu, H. Synthesis of porous Mn2O3 embedded in reduced graphene oxide as advanced anode materials for lithium storage. New J. Chem. 2017, 41, 7102–7107. [Google Scholar] [CrossRef]
- Ouyang, Z.; Lei, Y.; Chen, Y.; Zhang, Z.; Jiang, Z.; Hu, J.; Lin, Y. Preparation and specific capacitance properties of sulfur, nitrogen co-doped graphene quantum dots. Nanoscale Res. Lett. 2019, 14, 1–9. [Google Scholar] [CrossRef]
- Meng, J.; Cao, Y.; Suo, Y.; Liu, Y.; Zhang, J.; Zheng, X. Facile fabrication of 3D SiO2@graphene aerogel composites as anode material for lithium ion batteries. Electrochim. Acta 2015, 176, 1001–1009. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Winter, A.; Chen, L.; Sun, Y.; Turchanin, A.; Feng, X.; Müllen, K. Three-dimensional nitrogen and boron co-doped graphene for high-performance all-solid-state supercapacitors. Adv. Mater. 2012, 24, 5130–5135. [Google Scholar] [CrossRef]
- Rochman, R.A.; Wahyuningsih, S.; Ramelan, A.H.; Hanif, Q.A. Preparation of nitrogen and sulphur Co-doped reduced graphene oxide (rGO-NS) using N and S heteroatom of thiourea. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012119–012127. [Google Scholar] [CrossRef]
- Ding, Y.; Hou, C.; Li, B.; Lei, Y. Sensitive hydrazine detection using a porous Mn2O3 nanofibers-based sensor. Electroanalysis 2011, 23, 1245–1251. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, Y.; Han, C.; He, Z.; Ma, W.; Meng, W.; Jiang, Y.; Dai, L.; Wang, L. Superior lithium storage performance of hierarchical N-doped carbon encapsulated NaTi2(PO4)3 microflower. Ceram. Int. 2020, 46, 1954–1961. [Google Scholar] [CrossRef]
- Wall, M. The Raman spectroscopy of graphene and the determination of layer thickness. Thermo Sci 2011, 5, 1–5. [Google Scholar]
- Rahaman, H.; Laha, R.M.; Maiti, D.K.; Ghosh, S.K. Fabrication of Mn2O3 nanorods: An efficient catalyst for selective transformation of alcohols to aldehydes. RSC Adv. 2015, 5, 33923–33929. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Wu, D.; Xia, W.; Zhao, H.; Jia, D. Hydrothermal synthesis of nitrogen-doped graphene hydrogels using amino acids with different acidities as doping agents. J. Mater. Chem. A 2014, 2, 8352–8361. [Google Scholar] [CrossRef]
- Li, D.; Chen, H.; Liu, G.; Wei, M.; Ding, L.-X.; Wang, S.; Wang, H. Porous nitrogen doped carbon sphere as high performance anode of sodium-ion battery. Carbon 2015, 94, 888–894. [Google Scholar] [CrossRef]
- Zou, L.; Lai, Y.; Hu, H.; Wang, M.; Zhang, K.; Zhang, P.; Fang, J.; Li, J. N/S co-doped 3D porous carbon nanosheet networks enhancing anode performance of sodium-ion batteries. Chem. A Eur. J. 2017, 23, 14261–14266. [Google Scholar] [CrossRef]
- Gopi, C.V.V.M.; Vinodh, R.; Sambasivam, S.; Obaidat, I.M.; Singh, S.; Kim, H.J. Co9S8-Ni3S2/CuMn2O4-NiMn2O4 and MnFe2O4-ZnFe2O4/graphene as binder-free cathode and anode materials for high energy density supercapacitors. Chem. Eng. J. 2020, 381, 122640. [Google Scholar] [CrossRef]
- Li, W.; Zhou, M.; Li, H.; Wang, K.; Cheng, S.; Jiang, K. A high performance sulfur-doped disordered carbon anode for sodium ion batteries. Energy Environ. Sci. 2015, 8, 2916–2921. [Google Scholar] [CrossRef]
- Wang, B.; Li, F.; Wang, X.; Wang, G.; Wang, H.; Bai, J. Mn3O4 nanotubes encapsulated by porous graphene sheets with enhanced electrochemical properties for lithium/sodium-ion batteries. Chem. Eng. J. 2019, 364, 57–69. [Google Scholar] [CrossRef]
- Valvo, M.; Philippe, B.; Lindgren, F.; Tai, C.-W.; Edström, K. Insight into the processes controlling the electrochemical reactions of nanostructured iron oxide electrodes in Li-and Na-half cells. Electrochim. Acta 2016, 194, 74–83. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Wang, G.; Xia, Y.; Wang, H. One-dimensional hybrid nanocomposite of high-density monodispersed Fe3O4 nanoparticles and carbon nanotubes for high-capacity storage of lithium and sodium. J. Mater. Chem. A 2016, 4, 18532–18542. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Bakenov, Z.; Tuiyebayeva, M.; Konarov, A.; Chen, P. Synthesis of hierarchical porous sulfur/polypyrrole/multiwalled carbon nanotube composite cathode for lithium batteries. Electrochim. Acta 2014, 143, 49–55. [Google Scholar] [CrossRef]
- Ye, J.; Zhao, H.; Song, W.; Wang, N.; Kang, M.; Li, Z. Enhanced electronic conductivity and sodium-ion adsorption in N/S co-doped ordered mesoporous carbon for high-performance sodium-ion battery anode. J. Power Sources 2019, 412, 606–614. [Google Scholar] [CrossRef]
- Kandula, S.; Bae, J.; Cho, J.; Son, J.G. Gram-scale synthesis of rGO wrapped porous α-Fe2O3 as an advanced anode material for Na-ion batteries with superior cyclic stability. Compos. Part B Eng. 2021, 220, 108995–109005. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Z.; Zou, C.; Jin, H.; Wang, S.; Li, L. Structural engineering of electrode materials to boost high-performance sodium-ion batteries. Cell Rep. Phys. Sci. 2021, 2, 100551–100579. [Google Scholar] [CrossRef]
- Li, M.; Zhu, K.; Zhao, H.; Meng, Z. Recent progress on graphene-based nanocomposites for electrochemical sodium-ion storage. Nanomaterials 2022, 12, 2837. [Google Scholar] [CrossRef]
- Xie, X.; Chen, S.; Sun, B.; Wang, C.; Wang, G. 3D networked tin oxide/graphene aerogel with a hierarchically porous architecture for high-rate performance sodium-ion batteries. ChemSusChem 2015, 8, 2948–2955. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Lee, H.-W.; Pasta, M.; Yuan, H.; Zheng, G.; Sun, Y.; Li, Y.; Cui, Y. A phosphorene–graphene hybrid material as a high-capacity anode for sodium-ion batteries. Nat. Nanotechnol. 2015, 10, 980–985. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Ren, W.; Xu, L.; Li, F.; Cheng, H.-M. Doped graphene sheets as anode materials with superhigh rate and large capacity for lithium ion batteries. ACS Nano 2011, 5, 5463–5471. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.M.; Lee, J.W.; Shin, W.H.; Choi, Y.J.; Shin, H.J.; Kang, J.K.; Choi, J.W. Nitrogen-doped graphene for high-performance ultracapacitors and the importance of nitrogen-doped sites at basal planes. Nano Lett. 2011, 11, 2472–2477. [Google Scholar] [CrossRef]
- Li, F.; Ma, J.; Ren, H.; Wang, H.; Wang, G. Fabrication of MnO nanowires implanted in graphene as an advanced anode material for sodium-ion batteries. Mater. Lett. 2017, 206, 132–135. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, G.; Yan, D.; Lu, T.; Pan, L. MnO@C nanorods derived from metal-organic frameworks as anode for superiorly stable and long-life sodium-ion batteries. J. Alloys Compd. 2017, 710, 575–580. [Google Scholar] [CrossRef]
- Qin, Y.; Jiang, Z.; Guo, L.; Huang, J.; Jiang, Z.J. Controlled thermal oxidation derived Mn3O4 encapsulated in nitrogen doped carbon as an anode for lithium/sodium ion batteries with enhanced performance. Chem. Eng. J. 2021, 406, 126894–126906. [Google Scholar] [CrossRef]

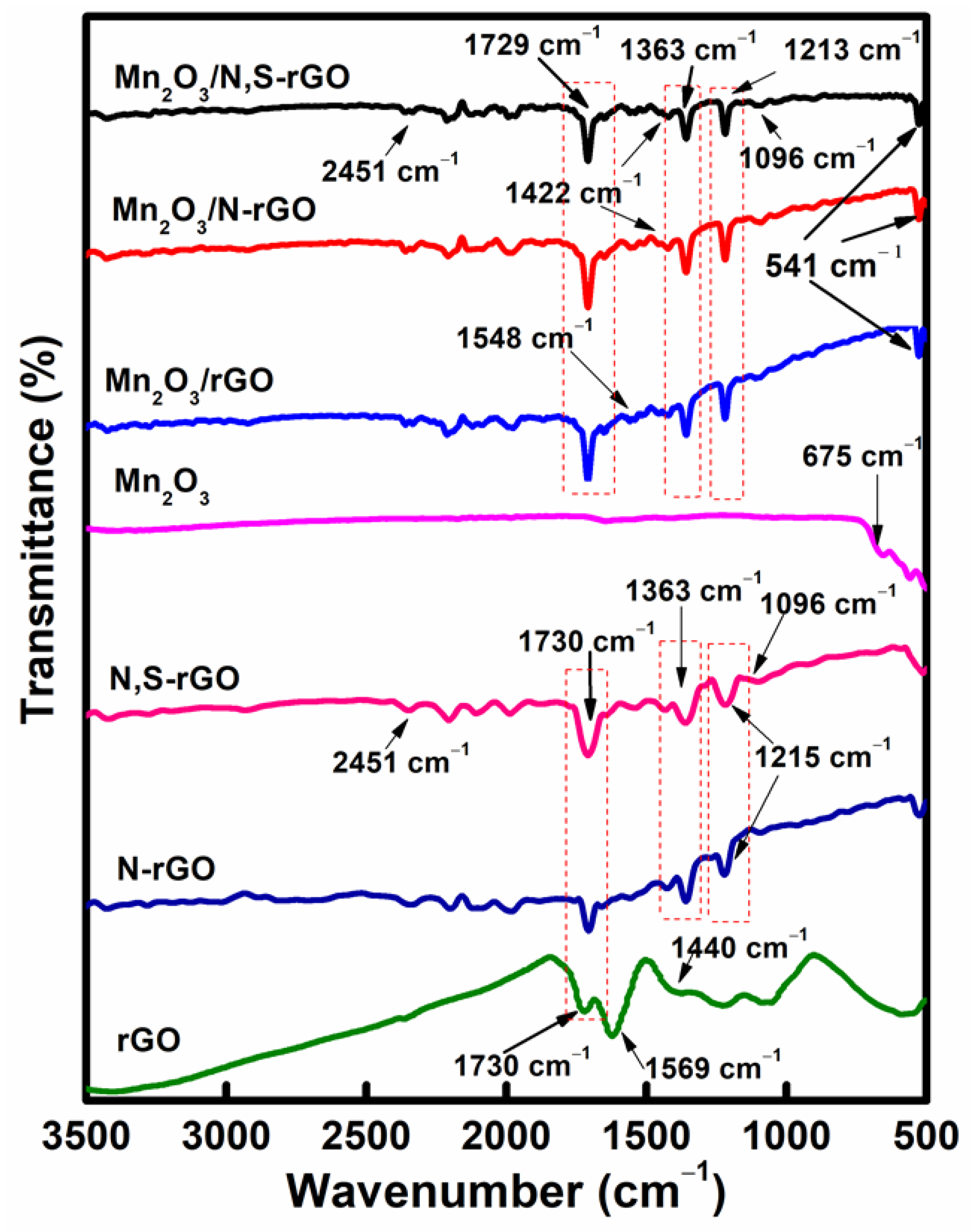

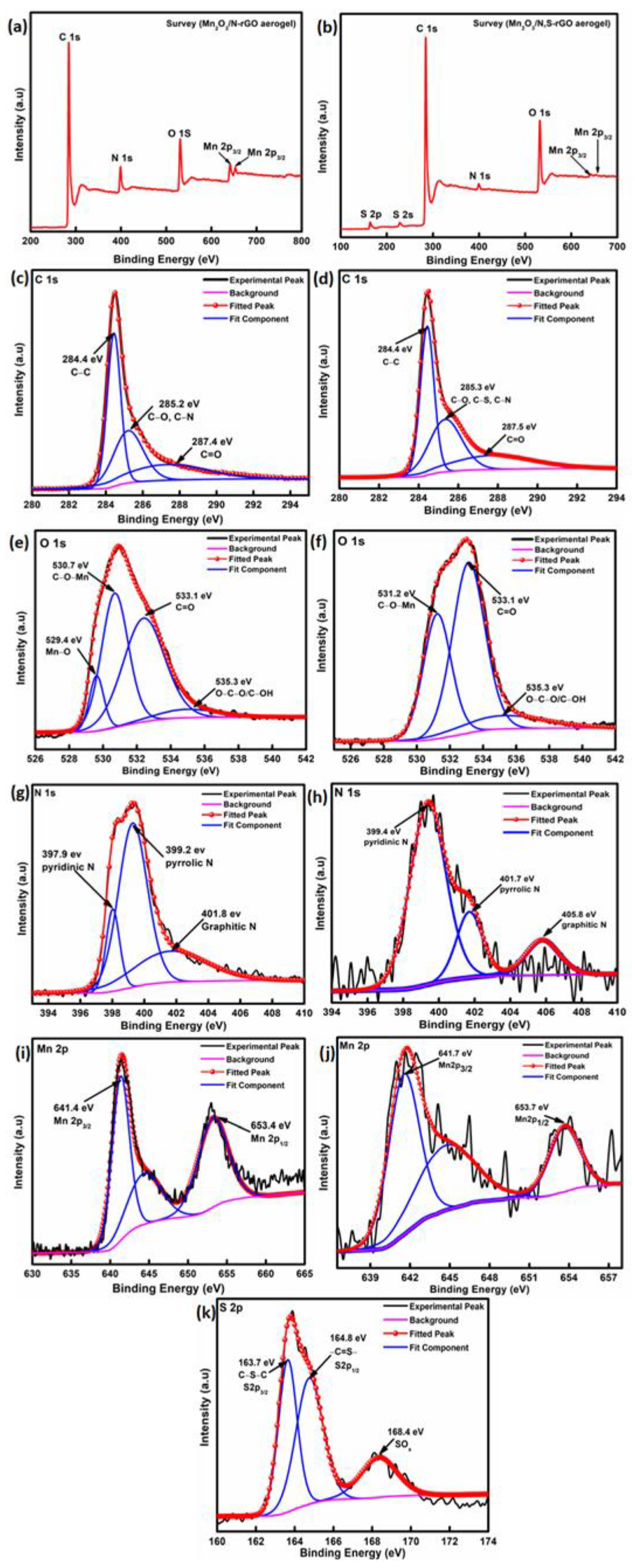
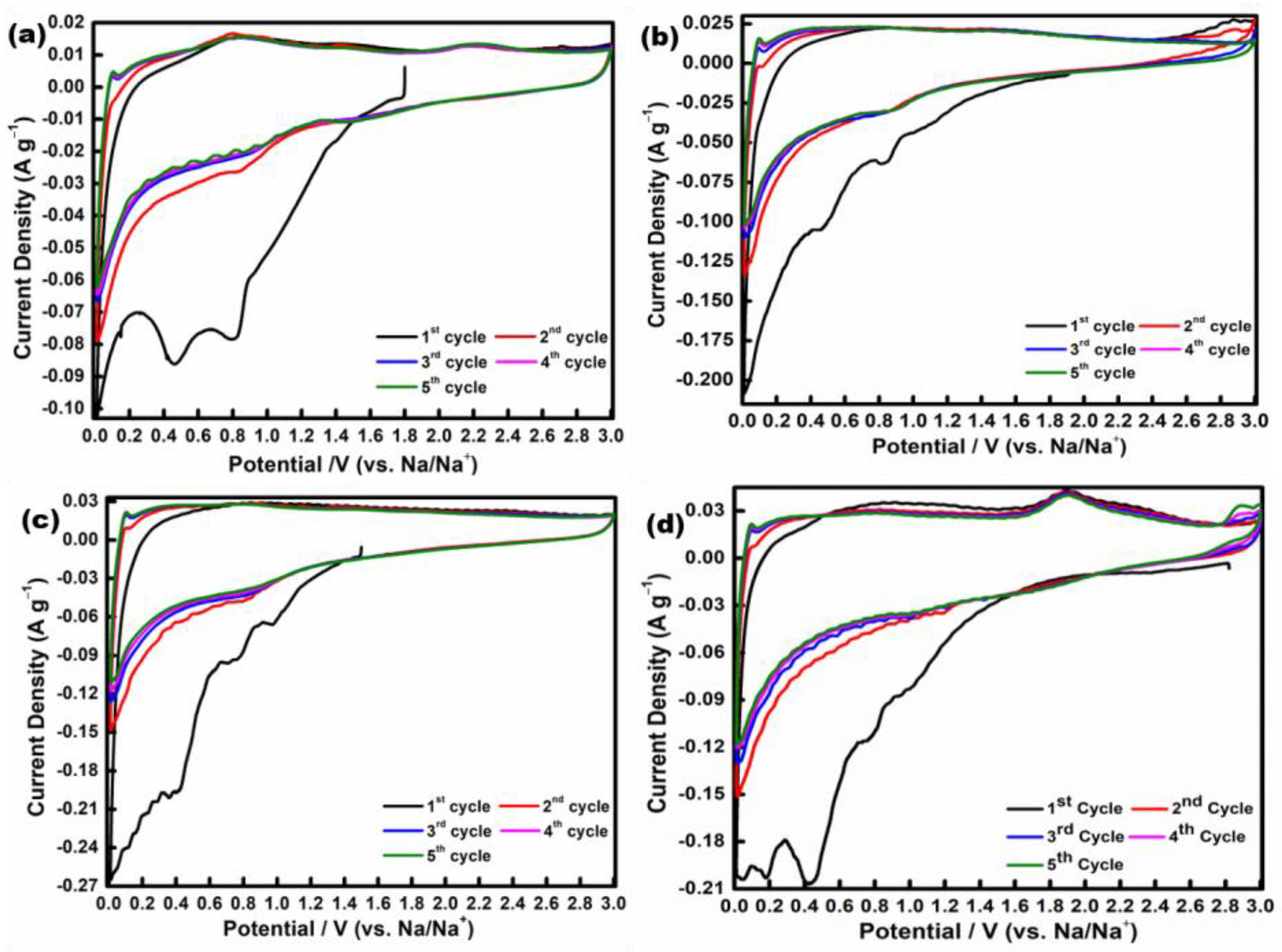
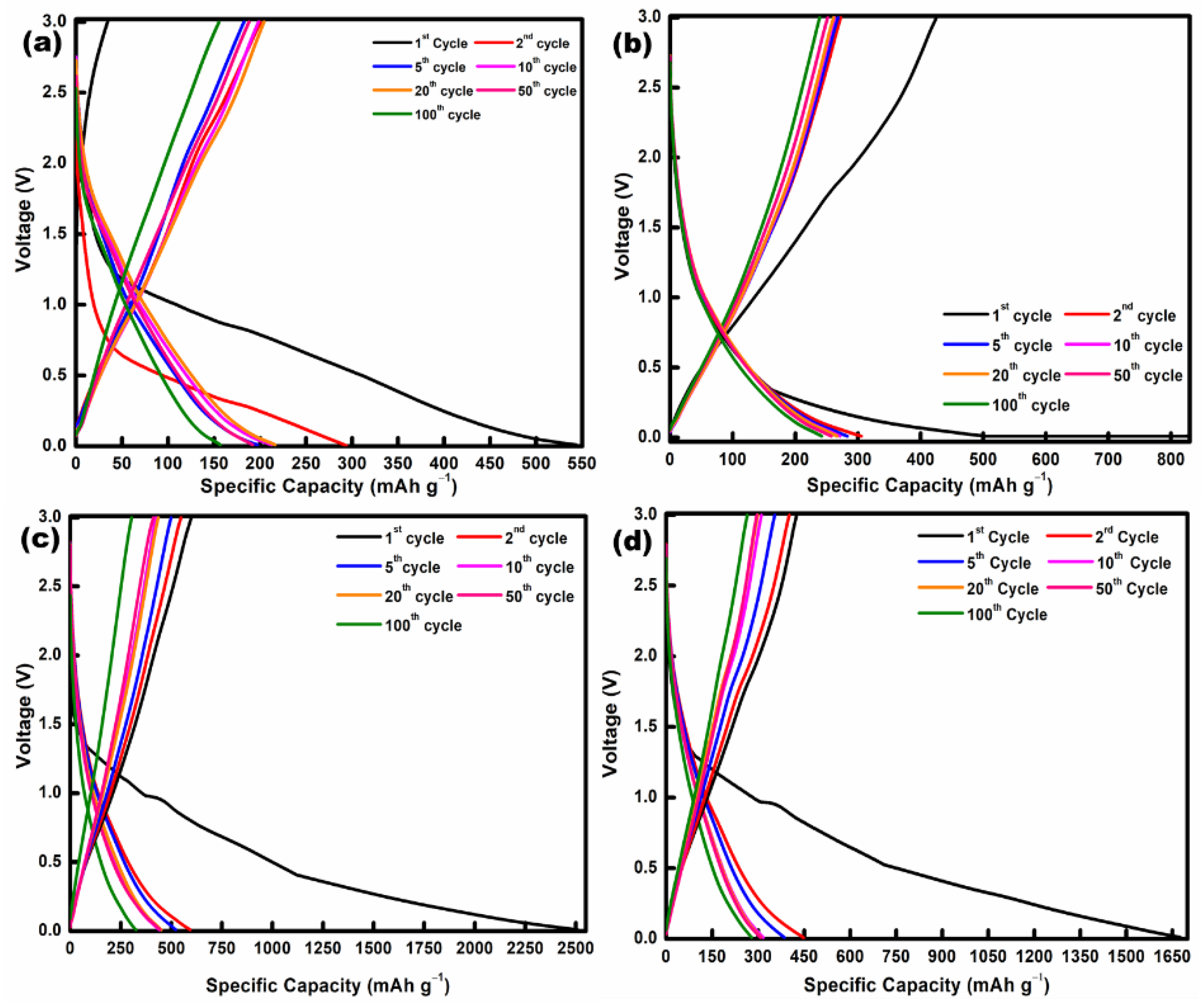
| Mn-Based Anode Materials | Initial Discharge Capacity (mAh g−1) | Discharge Capacity at 2nd Cycle (mAh g−1) | Discharge Capacity after 100 Cycles (mAh g−1) | Reference |
|---|---|---|---|---|
| MnO/graphene | 553 | 316 | 191 | [68] |
| MnO/C | 560 | - | 260 | [69] |
| Mn3O4/pGS | 700 | - | 233 | [56] |
| Mn3O4/NC | 710.8 | - | 249.5 | [70] |
| Mn3O4/N-rGO aerogel | 1950 | 470 | 374 | [35] |
| Mn3O4/N,S-rGO aerogel | 884 | 425 | 281 | [35] |
| Mn2O3/N-rGO aerogel | 2507 | 594 | 325 | This work |
| Mn2O3/N,S-rGO aerogel | 1677 | 448 | 279 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahamad Yusoff, N.F.; Idris, N.H.; Md Din, M.F.; Majid, S.R.; Harun, N.A.; Noerochim, L. Coupling of Mn2O3 with Heteroatom-Doped Reduced Graphene Oxide Aerogels with Improved Electrochemical Performances for Sodium-Ion Batteries. Nanomaterials 2023, 13, 732. https://doi.org/10.3390/nano13040732
Mahamad Yusoff NF, Idris NH, Md Din MF, Majid SR, Harun NA, Noerochim L. Coupling of Mn2O3 with Heteroatom-Doped Reduced Graphene Oxide Aerogels with Improved Electrochemical Performances for Sodium-Ion Batteries. Nanomaterials. 2023; 13(4):732. https://doi.org/10.3390/nano13040732
Chicago/Turabian StyleMahamad Yusoff, Nor Fazila, Nurul Hayati Idris, Muhamad Faiz Md Din, Siti Rohana Majid, Noor Aniza Harun, and Lukman Noerochim. 2023. "Coupling of Mn2O3 with Heteroatom-Doped Reduced Graphene Oxide Aerogels with Improved Electrochemical Performances for Sodium-Ion Batteries" Nanomaterials 13, no. 4: 732. https://doi.org/10.3390/nano13040732
APA StyleMahamad Yusoff, N. F., Idris, N. H., Md Din, M. F., Majid, S. R., Harun, N. A., & Noerochim, L. (2023). Coupling of Mn2O3 with Heteroatom-Doped Reduced Graphene Oxide Aerogels with Improved Electrochemical Performances for Sodium-Ion Batteries. Nanomaterials, 13(4), 732. https://doi.org/10.3390/nano13040732







