Comparative Study on Gas-Sensing Properties of 2D (MoS2, WS2)/PANI Nanocomposites-Based Sensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Liquid-Exfoliated MoS2 and WS2 Sheets
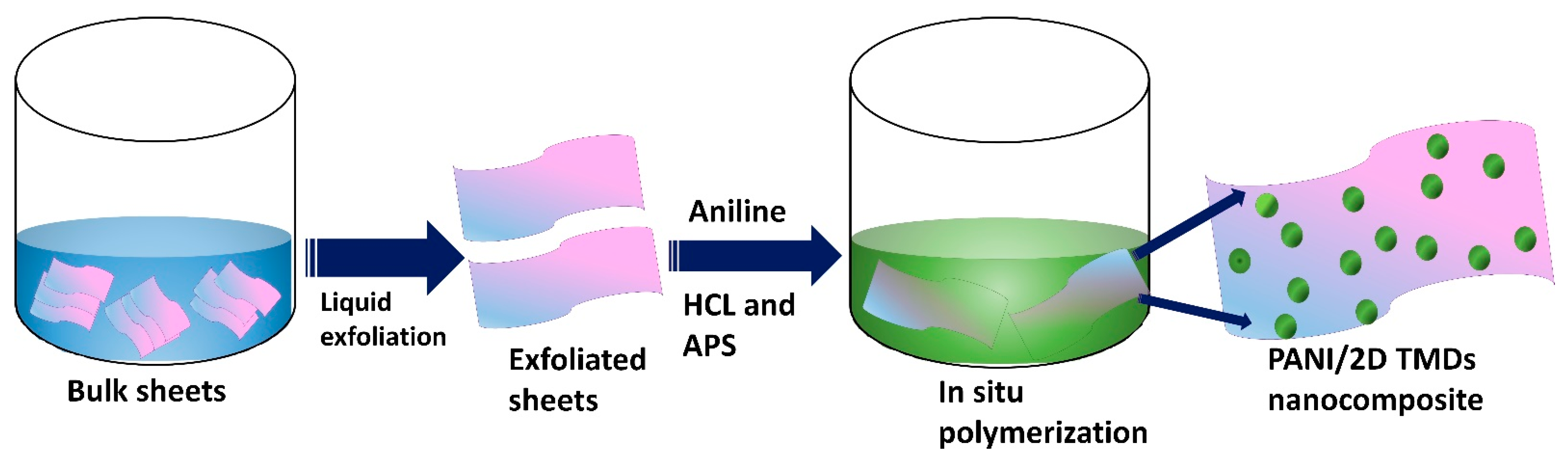
2.2. Synthesis of Liquid-Exfoliated Sheet/PANI Composites
2.3. Materials’ Characterization
3. Results and Discussion
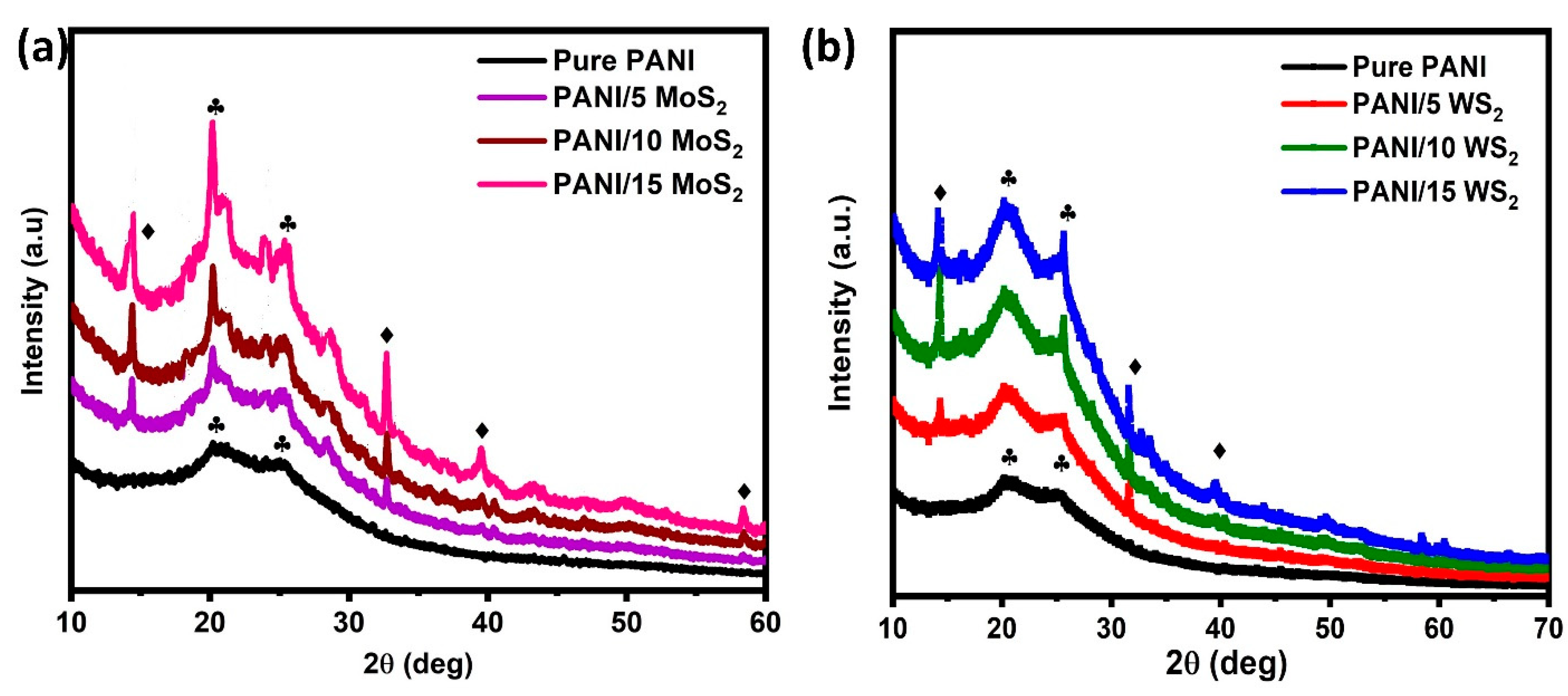
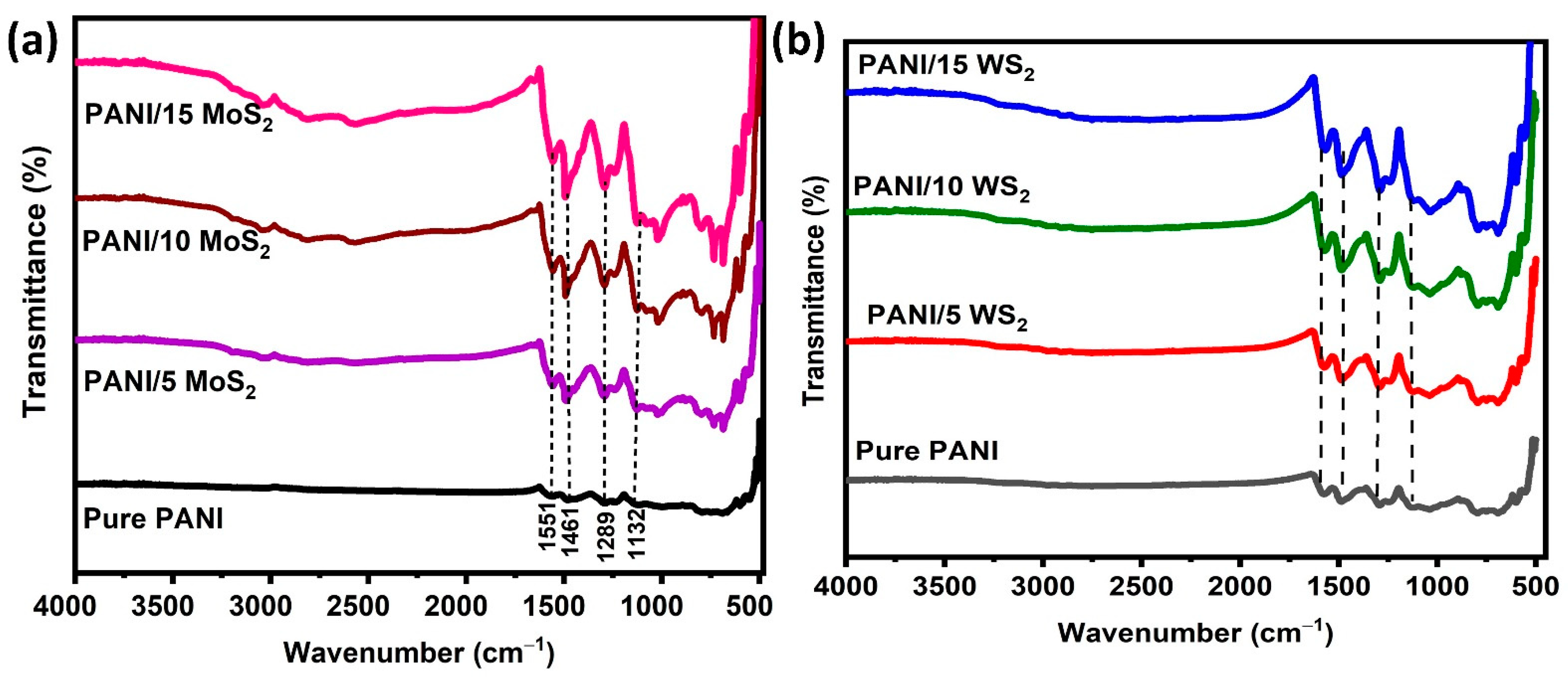
3.1. Structural Analysis
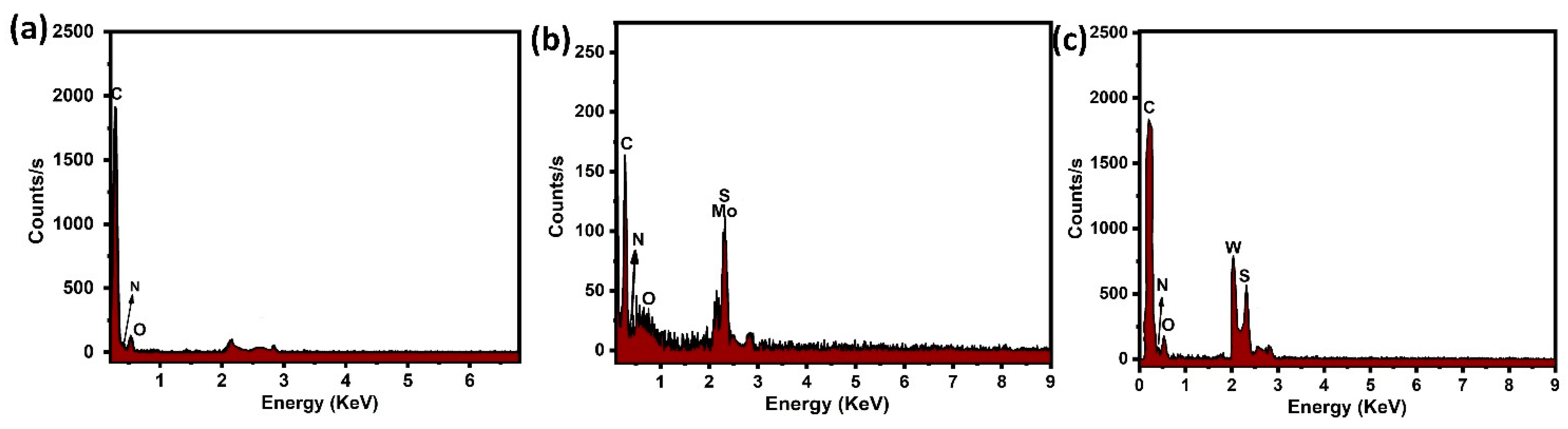
3.2. Morphological Analysis
3.3. Optical Analysis
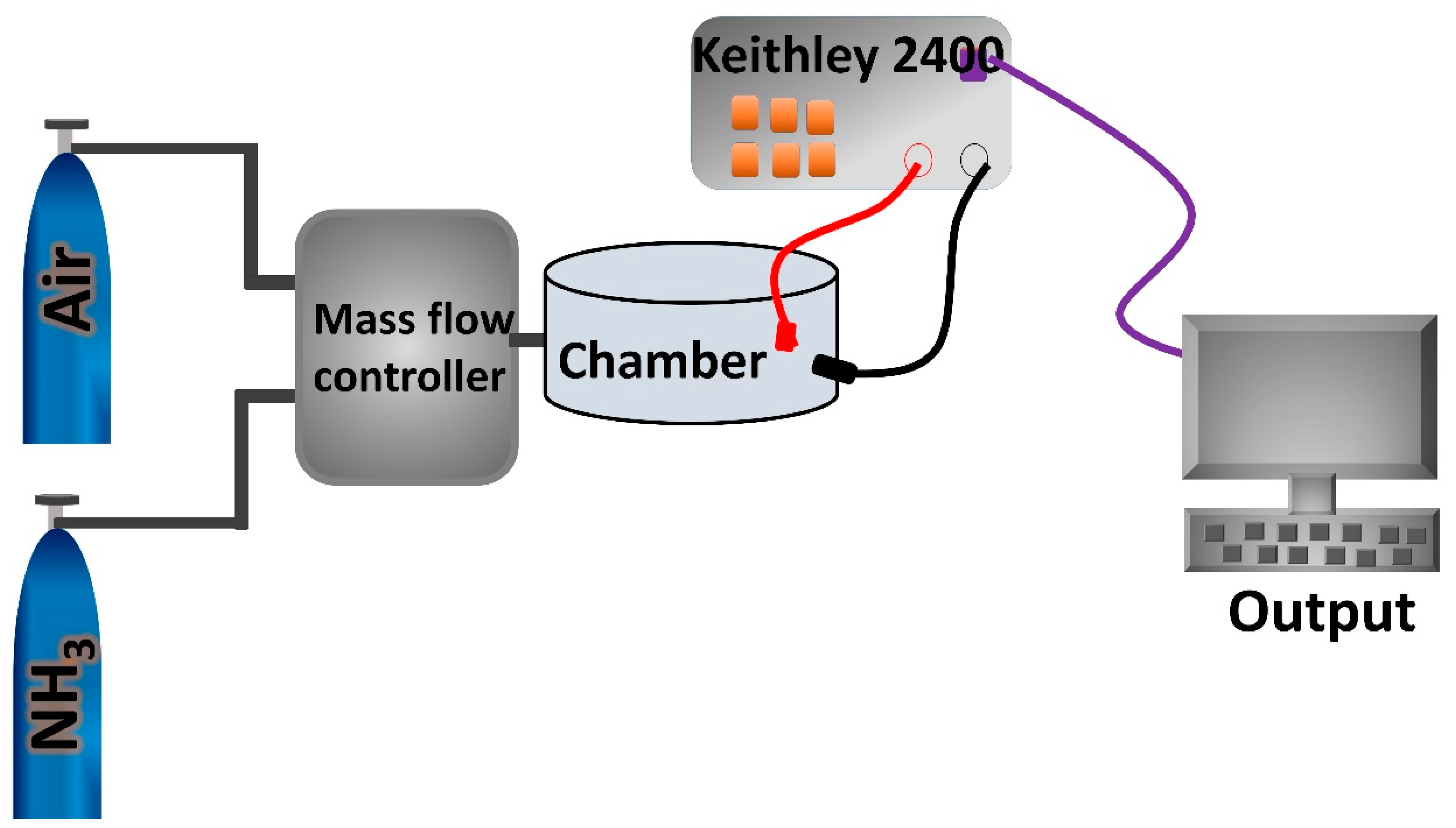
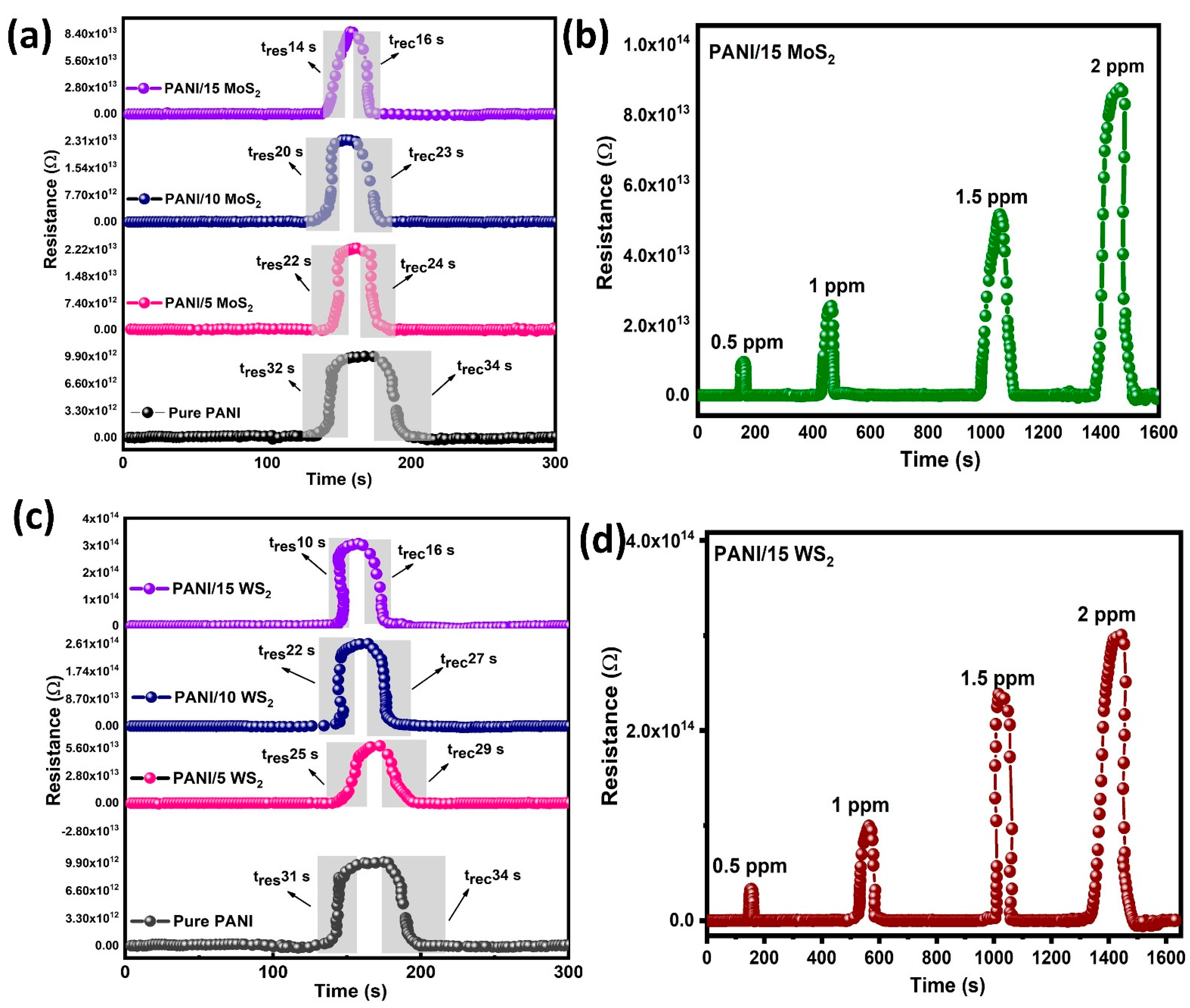
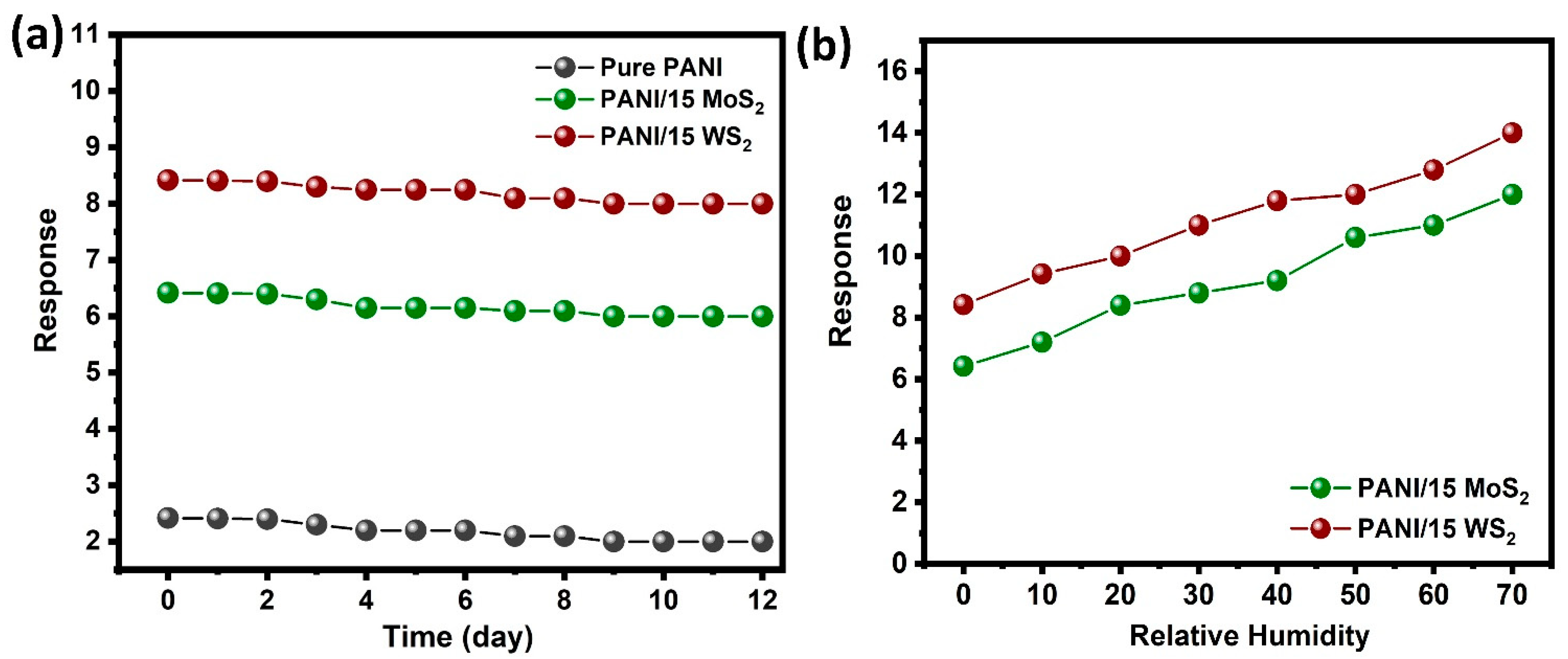
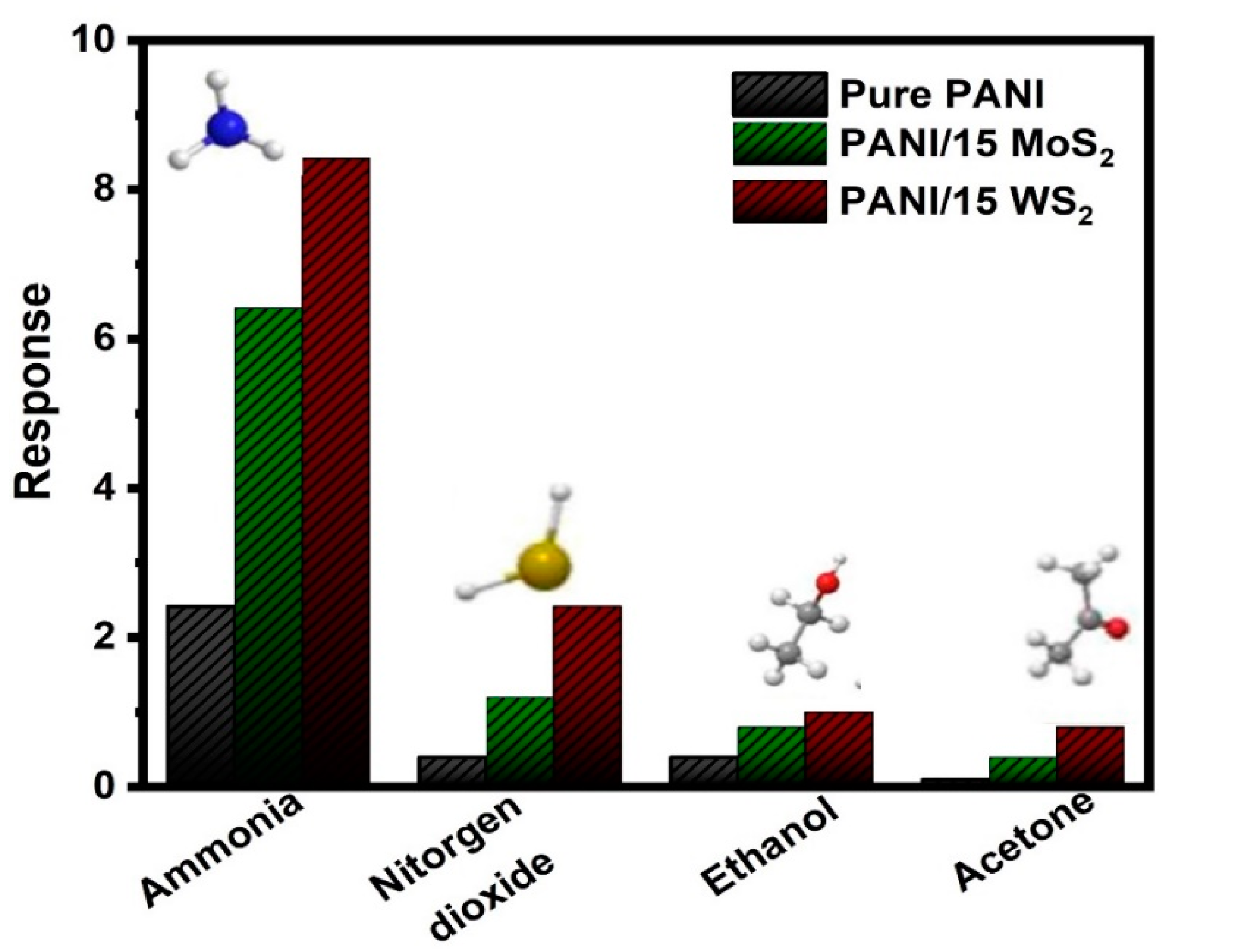
3.4. Gas Sensing Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Shen, W.; Chen, X. A room temperature operated ammonia gas sensor based on Ag-decorated TiO2 quantum dot clusters. RSC Adv. 2019, 9, 24519–24526. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.J.; Španěl, P.; Smith, D. Breath analysis of ammonia, volatile organic compounds and deuterated water vapor in chronic kidney disease and during dialysis. Bioanalysis 2014, 6, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.; Sheikhi, M.H. Highly sensitive wireless H2S gas sensors at room temperature based on CuO-SWCNT hybrid nanomaterials. Sens. Actuators B Chem. 2016, 231, 474–483. [Google Scholar] [CrossRef]
- Shao, F.; Hernandez-Ramirez, F.; Prades, J.D.; Morante, J.R.; Lopez, N. Assessment and modeling of NH3-SnO2 interactions using individual nanowires. Procedia Eng. 2012, 47, 293–297. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Chen, N.; Han, B.; Xiao, X.; Chen, G.; Djerdj, I.; Wang, Y. Nanoparticle cluster gas sensor: Pt activated SnO2 nanoparticles for NH 3 detection with ultrahigh sensitivity. Nanoscale 2015, 7, 14872–14880. [Google Scholar] [CrossRef]
- Little, J.L.; Howard, A.S. Qualitative Gas Chromatography–Mass Spectrometry Analyses Using Amines as Chemical Ionization Reagent Gases. J. Am. Soc. Mass Spectrom. 2013, 24, 1913–1918. [Google Scholar] [CrossRef]
- Passaro, V.M.; Dell’Olio, F.; De Leonardis, F. Ammonia optical sensing by microring resonators. Sensors 2007, 7, 2741–2749. [Google Scholar] [CrossRef]
- Sriramprabha, R.; Divagar, M.; Mangalaraj, D.; Ponpandian, N.; Viswanathan, C. Formulation of SnO2/graphene nanocomposite modified electrode for synergitic electrochemcial detection of dopamine. Adv. Mater. Lett. 2015, 6, 973–977. [Google Scholar] [CrossRef]
- Maziarz, W.; Kusior, A.; Trenczek-Zajac, A. Nanostructured TiO2-based gas sensors with enhanced sensitivity to reducing gases. Beilstein J. Nanotechnol. 2016, 7, 1718–1726. [Google Scholar] [CrossRef]
- Hang, N.T.; Zhang, S.; Noh, J.-S.; Yang, W. Study on the optimization of graphene sensors using Ag-nanostructures decoration. Thin Solid Film. 2018, 660, 631–636. [Google Scholar] [CrossRef]
- Sharma, H.J.; Jamkar, D.V.; Kondawar, S.B. Electrospun nanofibers of conducting polyaniline/Al-SnO2 composites for hydrogen sensing applications. Procedia Mater. Sci. 2015, 10, 186–194. [Google Scholar] [CrossRef]
- Ko, K.Y.; Park, K.; Lee, S.; Kim, Y.; Woo, W.J.; Kim, D.; Song, J.-G.; Park, J.; Kim, H. Recovery improvement for large-area tungsten diselenide gas sensors. ACS Appl. Mater. Interfaces 2018, 10, 23910–23917. [Google Scholar] [CrossRef] [PubMed]
- Ai, W.; Kou, L.; Hu, X.; Wang, Y.; Krasheninnikov, A.V.; Sun, L.; Shen, X. Enhanced sensitivity of MoSe2 monolayer for gas adsorption induced by electric field. J. Phys. Condens. Matter 2019, 31, 445301. [Google Scholar] [CrossRef] [PubMed]
- Ravindra, N.M.; Tang, W.; Rassay, S. Transition Metal Dichalcogenides Properties and Applications. In Semiconductors; Springer: Berlin/Heidelberg, Germany, 2019; pp. 333–396. [Google Scholar]
- Xu, J.; Zhang, J.; Zhang, W.; Lee, C.S. Interlayer nanoarchitectonics of two-dimensional transition-metal dichalcogenides nanosheets for energy storage and conversion applications. Adv. Energy Mater. 2017, 7, 1700571. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Peng, L.; Wu, C.; Sun, X.; Hu, S.; Lin, C.; Dai, J.; Yang, J.; Xie, Y. Giant moisture responsiveness of VS2 ultrathin nanosheets for novel touchless positioning interface. Adv. Mater. 2012, 24, 1969–1974. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, Y.; Zhang, H.; Zhang, D.W.; Zhou, P.; Huang, J. Suspended SnS2 layers by light assistance for ultrasensitive ammonia detection at room temperature. Adv. Funct. Mater. 2018, 28, 1801035. [Google Scholar] [CrossRef]
- Rathi, K.; Kumar, A.N.; Pal, K. Fabrication of flexible La-MoS2 hybrid-heterostructure based sensor for NO2 gas sensing at room temperature. Nanotechnology 2020, 31, 395504. [Google Scholar] [CrossRef]
- Cho, B.; Kim, A.R.; Kim, D.J.; Chung, H.-S.; Choi, S.Y.; Kwon, J.-D.; Park, S.W.; Kim, Y.; Lee, B.H.; Lee, K.H. Two-dimensional atomic-layered alloy junctions for high-performance wearable chemical sensor. ACS Appl. Mater. Interfaces 2016, 8, 19635–19642. [Google Scholar] [CrossRef]
- Cha, J.-H.; Choi, S.-J.; Yu, S.; Kim, I.-D. 2D WS 2-edge functionalized multi-channel carbon nanofibers: Effect of WS 2 edge-abundant structure on room temperature NO2 sensing. J. Mater. Chem. A 2017, 5, 8725–8732. [Google Scholar] [CrossRef]
- Yue, N.; Weicheng, J.; Rongguo, W.; Guomin, D.; Yifan, H. Hybrid nanostructures combining graphene–MoS 2 quantum dots for gas sensing. J. Mater. Chem. A 2016, 4, 8198–8203. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, J.; Li, P.; Cao, Y. Room-temperature SO2 gas-sensing properties based on a metal-doped MoS 2 nanoflower: An experimental and density functional theory investigation. J. Mater. Chem. A 2017, 5, 20666–20677. [Google Scholar] [CrossRef]
- Asres, G.A.; Baldoví, J.J.; Dombovari, A.; Järvinen, T.; Lorite, G.S.; Mohl, M.; Shchukarev, A.; Pérez Paz, A.; Xian, L.; Mikkola, J.-P. Ultrasensitive H2S gas sensors based on p-type WS2 hybrid materials. Nano Res. 2018, 11, 4215–4224. [Google Scholar] [CrossRef]
- Parangusan, H.; Bhadra, J.; Ahmad, Z.; Mallick, S.; Touati, F.; Al-Thani, N. Investigation of the structural, optical and gas sensing properties of PANI coated Cu–ZnS microsphere composite. RSC Adv. 2020, 10, 26604–26612. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tai, H.; Zhang, P.; Ye, Z.; Su, Y.; Jiang, Y. Enhanced ammonia-sensing properties of PANI-TiO2-Au ternary self-assembly nanocomposite thin film at room temperature. Sens. Actuators B Chem. 2017, 246, 85–95. [Google Scholar] [CrossRef]
- Öztas, M.; Bedir, M. Thickness dependence of structural, electrical and optical properties of sprayed ZnO: Cu films. Thin Solid Film. 2008, 516, 1703–1709. [Google Scholar] [CrossRef]
- Khuspe, G.; Bandgar, D.; Sen, S.; Patil, V. Fussy nanofibrous network of polyaniline (PANi) for NH3 detection. Synth. Met. 2012, 162, 1822–1827. [Google Scholar] [CrossRef]
- Sambaza, S.; Maity, A.; Pillay, K. Visible light degradation of ibuprofen using PANI coated WO3@TiO2 photocatalyst. J. Saudi Chem. Soc. 2022, 26, 101563. [Google Scholar] [CrossRef]
- Han, D.; Chu, Y.; Yang, L.; Liu, Y.; Lv, Z. Reversed micelle polymerization: A new route for the synthesis of DBSA–polyaniline nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2005, 259, 179–187. [Google Scholar] [CrossRef]
- Liu, I.-T.; Hon, M.-H.; Teoh, L.G. The preparation, characterization and photocatalytic activity of radical-shaped CeO2/ZnO microstructures. Ceram. Int. 2014, 40, 4019–4024. [Google Scholar] [CrossRef]
- Sutar, D.; Padma, N.; Aswal, D.; Deshpande, S.; Gupta, S.; Yakhmi, J. Preparation of nanofibrous polyaniline films and their application as ammonia gas sensor. Sens. Actuators B Chem. 2007, 128, 286–292. [Google Scholar] [CrossRef]
- Sarf, F. Metal Oxide Gas Sensors by Nanostructures. In Gas Sensors; IntechOpen: London, UK, 2020; Volume 1. [Google Scholar]
- Fan, C.; Sun, F.; Wang, X.; Huang, Z.; Keshvardoostchokami, M.; Kumar, P.; Liu, B. Synthesis of ZnO hierarchical structures and their gas sensing properties. Nanomaterials 2019, 9, 1277. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-F.; Blum, F.D.; Bertino, M.F.; Kim, C.-S.; Pillalamarri, S.K. One-step fabrication of a polyaniline nanofiber vapor sensor. Sens. Actuators B Chem. 2008, 134, 31–35. [Google Scholar] [CrossRef]
- Burman, D.; Ghosh, R.; Santra, S.; Ray, S.K.; Guha, P.K. Role of vacancy sites and UV-ozone treatment on few layered MoS2 nanoflakes for toxic gas detection. Nanotechnology 2017, 28, 435502. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, B.; Wu, X.; Gu, D.; Li, X. Enhanced ammonia sensing properties of rGO/WS2 heterojunction based chemiresistive sensor by marginal sulfonate decoration. Sens. Actuators B Chem. 2021, 337, 129776. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, A.; Kaur, D. Room temperature ammonia gas sensing properties of MoS2 nanostructured thin film. AIP Conf. Proc. 2018, 1953, 030261. [Google Scholar]
- Järvinen, T.; Lorite, G.S.; Peräntie, J.; Toth, G.; Saarakkala, S.; Virtanen, V.K.; Kordas, K. WS2 and MoS2 thin film gas sensors with high response to NH3 in air at low temperature. Nanotechnology 2019, 30, 405501. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Li, Z.; Wang, J.; Zhang, J. WS2 nanoflakes based selective ammonia sensors at room temperature. Sens. Actuators B Chem. 2017, 240, 273–277. [Google Scholar] [CrossRef]
- Liu, A.; Lv, S.; Jiang, L.; Liu, F.; Zhao, L.; Wang, J.; Hu, X.; Yang, Z.; He, J.; Wang, C. The gas sensor utilizing polyaniline/MoS2 nanosheets/SnO2 nanotubes for the room temperature detection of ammonia. Sens. Actuators B Chem. 2021, 332, 129444. [Google Scholar] [CrossRef]
- Jha, R.K.; Wan, M.; Jacob, C.; Guha, P.K. Ammonia vapour sensing properties of in situ polymerized conducting PANI-nanofiber/WS 2 nanosheet composites. New J. Chem. 2018, 42, 735–745. [Google Scholar] [CrossRef]


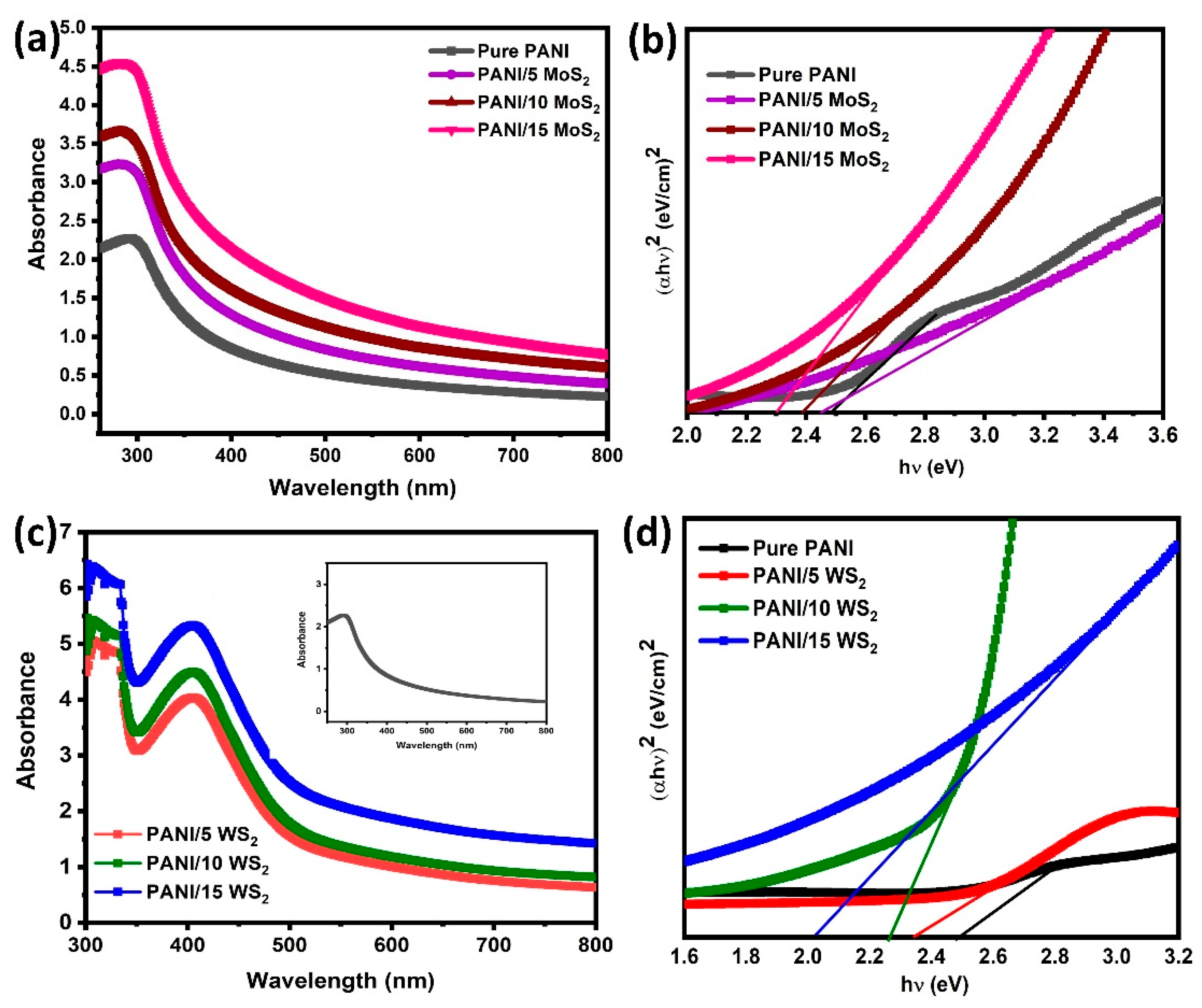
| Samples | Microstrain × 10−3 | Dislocation Density × 1014 Lines/m2 | Crystallite Size (nm) | Band Gap Eg (eV) |
|---|---|---|---|---|
| Pure PANI | 0.8286 | 5.7145 | 41.83 | 2.50 |
| PANI/5 MoS2 | 1.0777 | 10.6666 | 22.86 | 2.45 |
| PANI/10 MoS2 | 1.6613 | 22.9719 | 20.16 | 2.38 |
| PANI/15MoS2 | 2.6730 | 59.4644 | 18.96 | 2.29 |
| PANI/5WS2 | 1.6098 | 21.1864 | 21.18 | 2.35 |
| PANI/10WS2 | 2.0169 | 33.5701 | 17.53 | 2.26 |
| PANI/15WS2 | 2.1420 | 38.8556 | 16.18 | 2.02 |
| SI. No | Material | Gas | Response Time | Recovery Time | Ref |
|---|---|---|---|---|---|
| 1 | rGO-WS2 | NH3 | 60 s | 300 min | [37] |
| 2 | MoS2 thin film | NH3 | 22 s | 30 s | [38] |
| 3 | MoS2, WS2 | NH3 | 5 min | 30 min | [39] |
| 4 | WS2 nanoflakes | NH3 | 120 s | 150 s | [40] |
| 5 | PANI/MoS2/SnO2 | NH3 | 21 s | 130 s | [41] |
| 6 | PANI/WS2 | NH3 | 260 s | 790 s | [42] |
| 7 | PANI/TMDs | NH3 | 10 s | 16 s | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parangusan, H.; Bhadra, J.; Al-Qudah, R.A.; Elhadrami, E.C.; Al-Thani, N.J. Comparative Study on Gas-Sensing Properties of 2D (MoS2, WS2)/PANI Nanocomposites-Based Sensor. Nanomaterials 2022, 12, 4423. https://doi.org/10.3390/nano12244423
Parangusan H, Bhadra J, Al-Qudah RA, Elhadrami EC, Al-Thani NJ. Comparative Study on Gas-Sensing Properties of 2D (MoS2, WS2)/PANI Nanocomposites-Based Sensor. Nanomaterials. 2022; 12(24):4423. https://doi.org/10.3390/nano12244423
Chicago/Turabian StyleParangusan, Hemalatha, Jolly Bhadra, Razen Amer Al-Qudah, Elhassen Cheikh Elhadrami, and Noora Jabor Al-Thani. 2022. "Comparative Study on Gas-Sensing Properties of 2D (MoS2, WS2)/PANI Nanocomposites-Based Sensor" Nanomaterials 12, no. 24: 4423. https://doi.org/10.3390/nano12244423
APA StyleParangusan, H., Bhadra, J., Al-Qudah, R. A., Elhadrami, E. C., & Al-Thani, N. J. (2022). Comparative Study on Gas-Sensing Properties of 2D (MoS2, WS2)/PANI Nanocomposites-Based Sensor. Nanomaterials, 12(24), 4423. https://doi.org/10.3390/nano12244423






