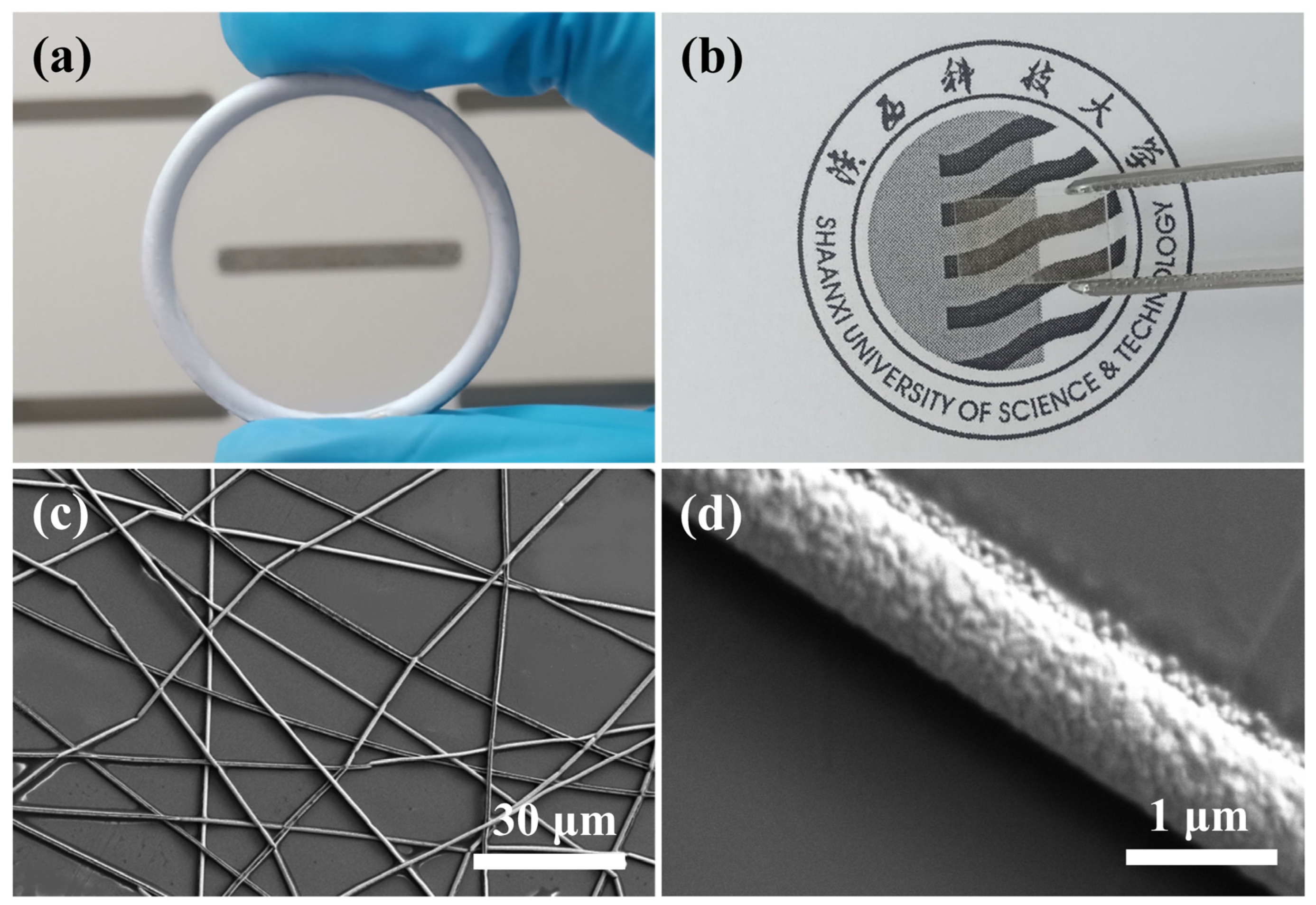
3.1. The Ag Nanonetwork TCF Prepared Using Template Method
Figure 2a shows the obtained network of AgNWs attached to the metal collecting ring. It can be seen that there is a very homogeneous and almost invisible film inside the ring, which can reach a size of more than 8 cm. The metal films attached to the rings can be easily transferred to any substrate, which is the advantage of this particular method.
Figure 2b shows a photo of the AgNW network after it has been attached to a quartz substrate. In addition, this strategy is applicable to the preparation of flexible TCF, which only requires the selection of a flexible substrate. A small amount of alcohol dripped on the substrate during the transfer process can effectively increase the adhesion between the film and the substrate. The PVP template does not significantly affect the light transmission and electrical conductivity of the film and can be removed on a case-by-case basis. As shown in
Figure 2c,d, we have characterized the microscopic morphology of AgNWs using SEM. The AgNWs tightly attached to the substrate surface have three significant advantages: (1) the length of AgNWs can reach the centimeter scale (due to experimental constraints, we can only measure the approximate scale), which is much larger than the samples prepared using chemical or conventional methods; (2) AgNWs have a high purity. This physical template method does not introduce other substances, including metal ions, organic substances, and oxides, except for impurities in the target material itself; (3) It can be seen from some of the intersections in the image that different NWs can be perfectly cross-linked at the intersection. All the above three points have favorable effects on the electrical conductivity of the nanonetworks. The diameter of the AgNWs obtained under the given experimental conditions is 946 nm (as shown in
Figure S1a in Supplementary Materials). The sputtering time for this sample was 3 min.
The light transmission and electrical conductivity of the prepared AgTCF are affected by the diameter and density of AgNWs. We obtained a series of AgTCFs with different properties by varying the electrospinning time. As shown in
Figure 3a, the sheet resistance of AgTCFs increased continuously from 10
−1 Ω/sq to 10
1 Ω/sq as the NW density decreased, and the light transmittance of the film rapidly saturated to about 80%. When applied to DSSCs and photoelectrochemical UV detectors, we can choose the data at the inflection point of the curve as the optimal electrode conditions.
Figure 3b shows the transmittance curves of various TCFs in the UV–visible region. As is well-known, conductive oxide based TCFs such as FTO and ITO have strong absorption for UV light below 400 nm and cannot transmit light below 300 nm, which limits their application in UV devices. The transmittance in the visible region of FTO/ITO has weak fluctuations. The bare Ag nanonetworks we prepared have excellent transmission throughout 200–900 nm with a transmittance of about 94%. After transfer to the quartz substrate, the transmittance exhibits a slight decrease to about 80%. Compared with FTO/ITO, AgTCF has a more stable transmission across the entire wavelength band. It is important to note that the above data are just some of the typical results we obtained. The properties of AgTCF obtained using this method are influenced by the diameter, density, and length of the NWs, and the films can be optimized by changing the spinning substance, spinning time, spinning temperature, and sputtering parameters. In view of the advantages of AgNWs in three aspects, length, purity and connection method, we believe that the AgTCF obtained using this strategy can achieve the perfect transparent conductive properties.
Heat-resistance temperature is another key indicator of TCFs. The TCFs based on metal NWs have been widely studied; however, to the best of our knowledge, few of them have been successfully applied to photoelectrochemical devices. In addition to chemical instability and carrier recombination, the inability to withstand high temperatures is an important reason. Therefore, we investigated the heat resistance of AgTCFs by observing the changes in sheet resistance and microscopic morphology at different temperatures.
Due to the changes in surface energy, defect and face-to-volume ratio caused by the reduction in size, the heat resistance of AgNW will decrease with the decrease in diameter [
14]. At the same time, the metal itself has impurities, twin boundaries, dislocation and other defects [
15]; therefore, in general, the thermal behavior of AgNWs is not completely identical. The binding energy of the atoms on the surface of the NWs is smaller than that of the atoms on the interior, with fewer nearest neighbor atoms and weaker bonds; therefore, the melting of AgNWs starts from the surface [
16]. When the temperature rises to a certain value, the NWs gradually melt and break. Eventually, to ensure the lowest surface energy, it starts to turn into spherical clusters.
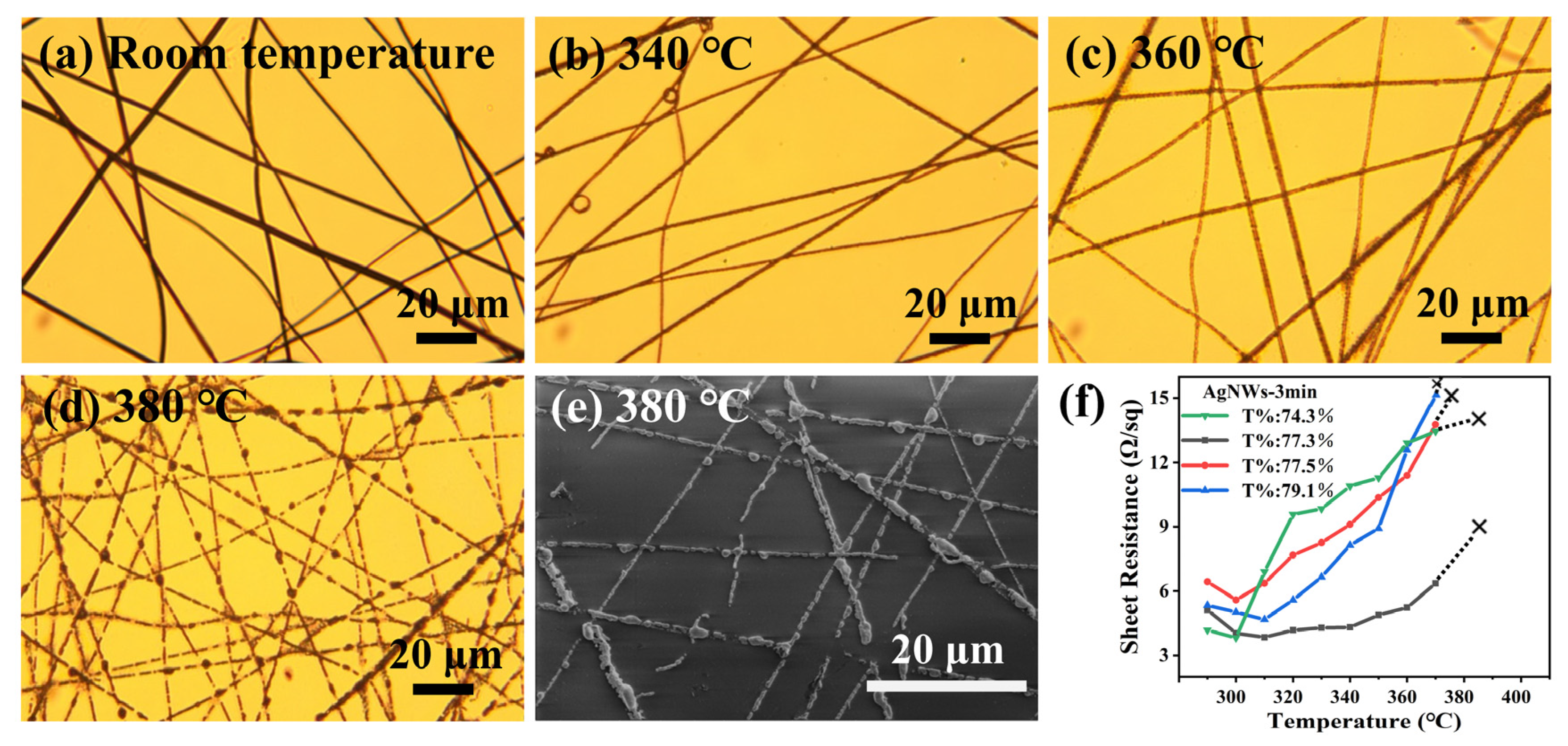
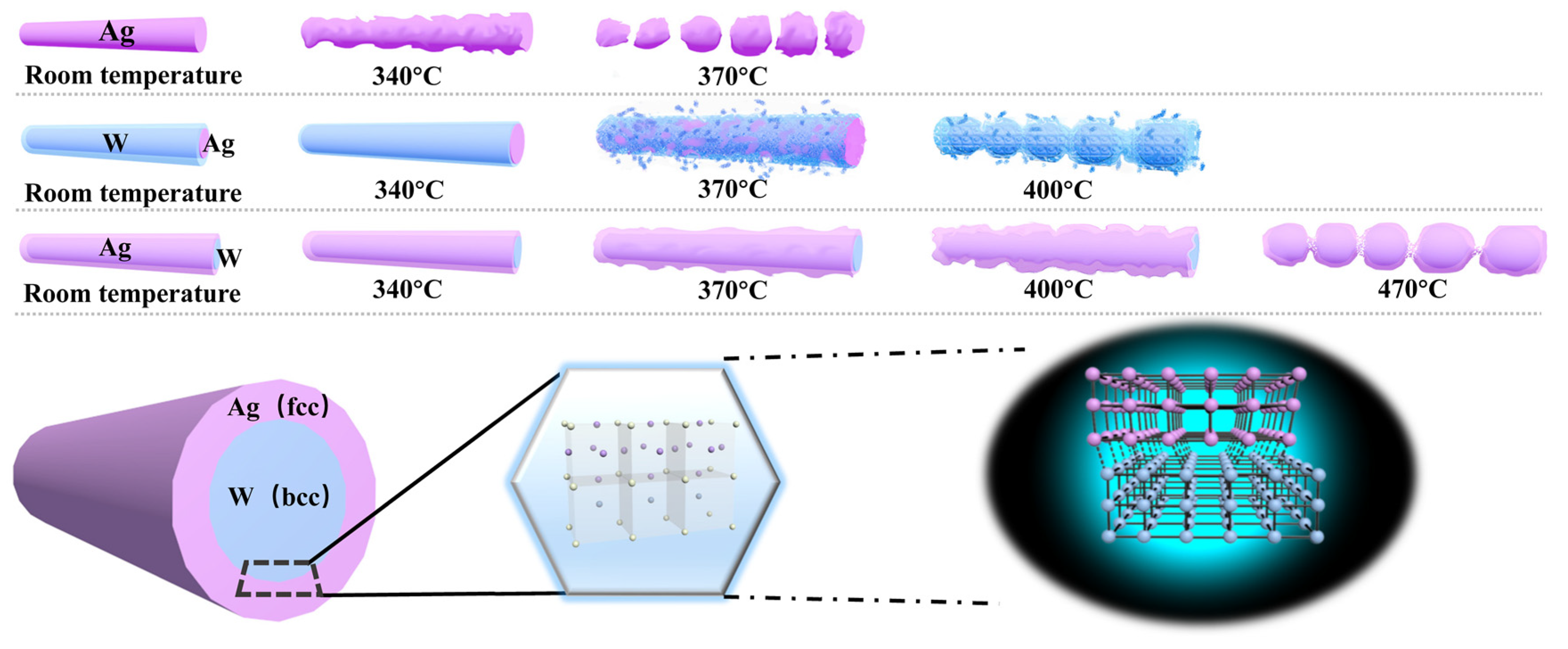
It can be seen from
Figure 4a–d that the morphology of AgNWs starts to show slight changes when the heat treatment temperature reaches 360 °C. When the heat treatment temperature reached 380 °C, the AgNWs had completely fractured and agglomeration appeared. The SEM image shown in
Figure 4e gives a clearer demonstration. The sheet resistance of AgNWs was measured with four probes. As shown in
Figure 4f, the sheet resistance of the sample changes continuously with the increase in temperature and cannot be measured when the temperature exceeds 370 °C. The results showed that the heat-resistance temperature of AgNWs is about 370 °C. In order to obtain more accurate results, four samples with different transmittance were selected and measured, and a uniform conclusion, which is the same as above, was obtained.
3.2. The Ag/W and W/Ag Composite Nanonetwork TCFs
The heat-resistance temperature of the prepared AgTCF (370 °C) does not reach the phase transition temperature of common semiconductor materials used in photoelectrochemical devices; therefore, it needs to be further improved. Compositing with high melting point metal materials is an effective strategy.
Metal W is a typical high heat resistance metal; its melting point among all pure metals is extremely high, up to 3410 °C, but the electrical conductivity of WNWs is not outstanding. In this work, we successively sputtered Ag and W materials on PVP templates to form composite nanonetworks to optimize the heat resistance of the films. The samples obtained by sputtering Ag first and then W are referred to as Ag/WNWs (and accordingly, Ag/WTCFs). The samples obtained by sputtering W first and then Ag are referred to as W/AgNWs (and accordingly, W/AgTCFs). The light transmission, electrical conductivity and heat resistance of the composite TCFs were investigated (as shown in
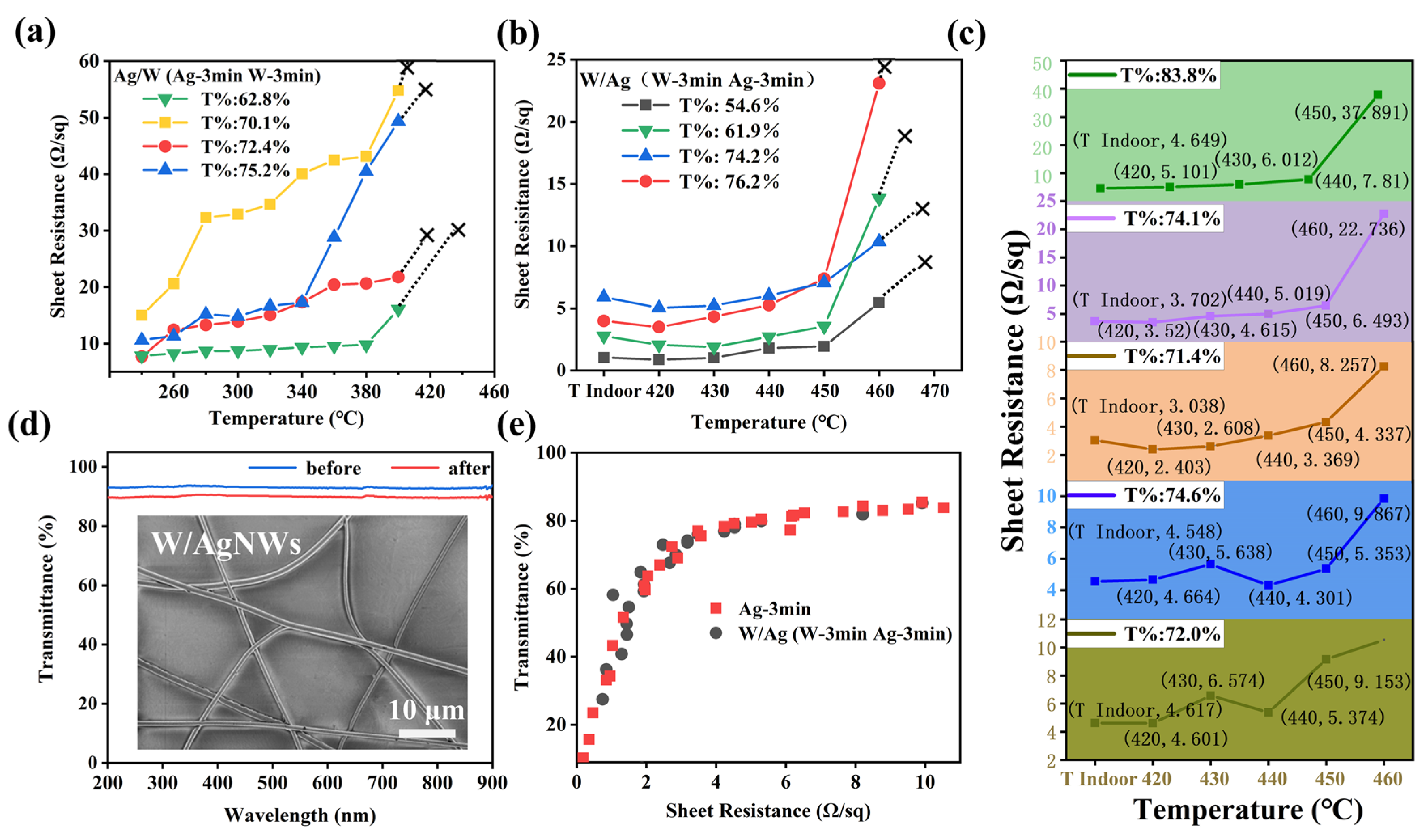
Figure 5). When the heat treatment temperature reaches a certain value, the melting state of metal layer changes, and the sheet resistance increases gradually with the increase in temperature until it cannot be measured with four probes.
High melting point metal coating low melting point metal, that is, W coating Ag (Ag/W) is the first composite structure we thought of. As shown in
Figure 5a, Ag/WTCFs have the highest tolerance temperature of 400 °C. The heat-resistance temperature of the Ag/WTCFs obtained is 30 °C higher than that of the single-phase AgNW, indicating that the addition of metal W can improve the heat resistance. To our surprise, the W/AgTCFs exhibited better heat resistance, with an increase of 100 °C to 460 °C. It shows that the internal W plays an important role in improving the film. We therefore proceeded to vary the sputtering time of W (2, 3, 4, 5 and 6 min, respectively). The results are shown in
Figure 5c. The change of W layer thickness did not significantly affect the heat resistance of the whole film, and only a slight decrease of 10 °C in the heat-resistance temperature was observed when the sputtering time of W was 2 min.
Figure 5d shows that the transmittance of W/AgNWs is slightly lower than that of AgNWs due to the slight increase in diameter blocking more light (as shown in
Figure S1b). The inset of
Figure 5d shows that the morphology of W/AgNWs did not change significantly. We further tested the synergistic relationship between light transmission and electrical conductivity of W/AgTCFs (
Figure 5e), and the results showed that it was not significantly different from the bare AgTCF; we believe that the above synergistic relationship depends mainly on the density of the electrospun template and the thickness of the Ag layer.
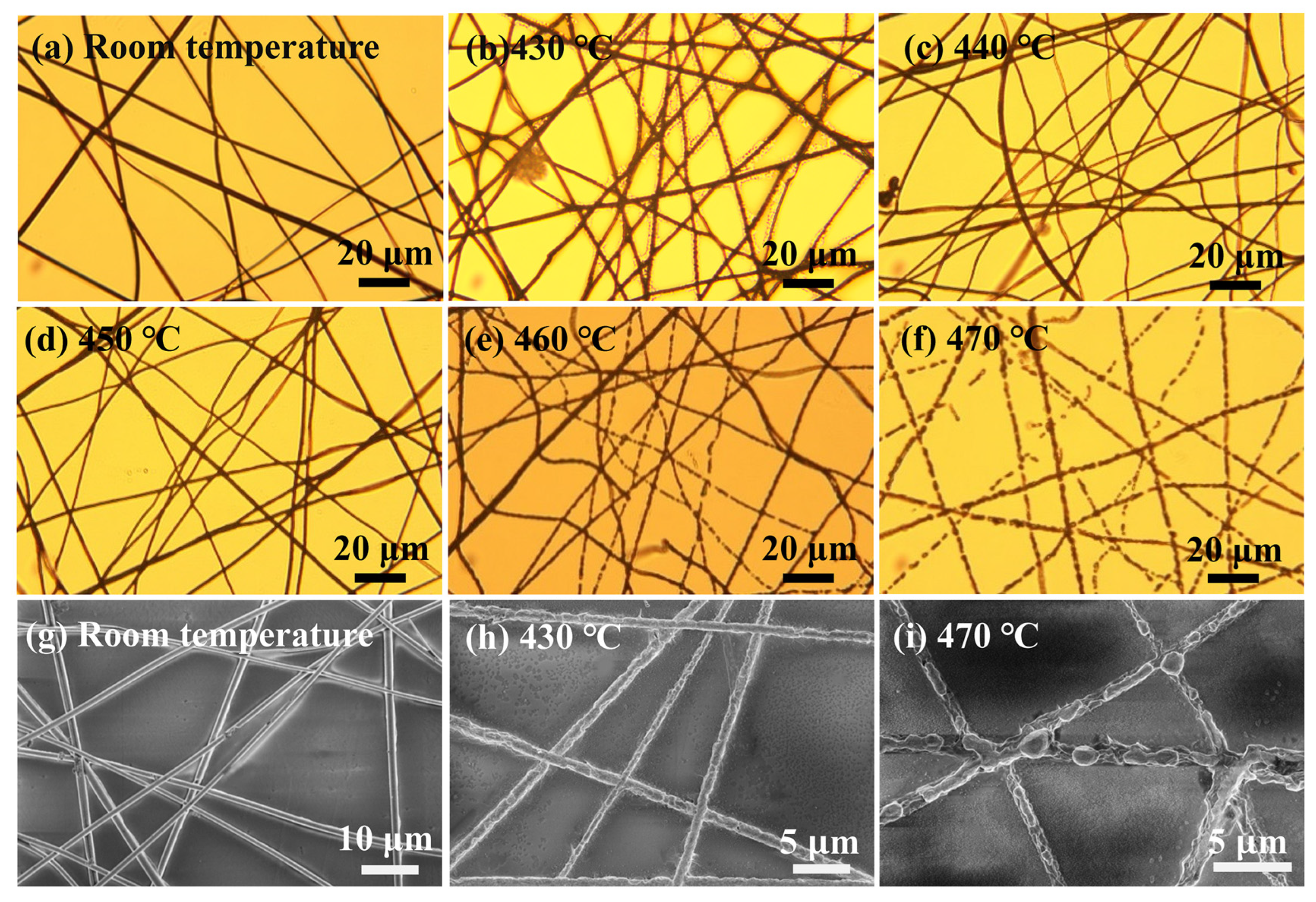
As shown in
Figure 6, we observed the morphological changes of W/AgNWs at different heat treatment temperatures using optical microscopy and SEM, and confirmed that the heat-resistance temperature of W/AgNWs is about 460 °C. Below 450 °C (including 450 °C), the NW still remains linear and continuous. When the temperature reached 460 °C, it was obvious that the morphology of some NWs began to melt and even appeared to break. When the heat treatment temperature reaches 470 °C, all of the NWs are fused.
3.3. Analysis of Thermal Stability
Bare AgNWs, Ag/WNWs and W/AgNWs exhibit different thermal stability and melting processes. As shown in
Figure 7, we have drawn a schematic diagram of the melting processes and structures of different films. The results obtained in the above thermal stability study are analyzed theoretically.
As shown in
Figure 5a,b, the heat resistance of the composite TCFs was enhanced regardless of whether the added W metal was the outer or inner layer, which is called overheating phenomenon. In other words, the melt is restrained by some action during the non-uniform nucleation process. This is due to the three following reasons:
(1) A semi-coherent lattice relationship is formed at the interface of W and Ag, which changes the thermal vibration of atoms and inhibits the nucleation [
17,
18] and the growth of melted NWs. According to the mismatch degree of interface atoms, phase interfaces are divided into coherent, semi-coherent and non-coherent interfaces, which is defined as:
where
δ is the mismatch (constant),
aα >
aβ, respectively, are the atomic spacing of crystals parallel to the interface on both sides. When
δ < 0.05, the phase interface is coherent; when 0.05 <
δ < 0.25, the phase interface is a semi-coherent interface; when
δ > 0.25, the phase interface is an incoherent interface. The larger
δ is, the greater the interfacial energy is. The crystal structure of Ag is face-centered cubic (fcc), and the crystal structure of metal W is body-centered cubic (bcc); then, heterogeneous interfaces can be formed. W and Ag are insoluble and cannot form compounds; therefore, sharp interfaces can be formed at atomic scale. According to the formula, the interface atomic mismatch of W and Ag is 0.23; therefore, the interface of the two phases belongs to the semi-coherent interface.
Solid phase transition is achieved through nucleation and growth. The difference of free energy between the old and new phases drives the formation of solid phase transition, while the interface energy and strain energy hinder the formation of solid phase transition [
19,
20]. According to the Lindemann criterion [
21], melting is the vibrational lattice instability released when the root mean sheet displacement (rmsd) of atoms reaches a critical fraction of atomic spacing. When the crystal is in the thermal environment above the melting point, the melting occurs only when the amplitude of the thermal vibration reaches a critical fracture between atoms. The thermal vibration of the atoms on the semi-coherent interface is suppressed by the larger interfacial energy.
(2) When the liquid core is formed in the crystal, the change of the volume will cause the strain in the crystal, and the strain energy will increase, which hinders the occurrence of the solid phase transition [
16,
22,
23,
24]. This occurs mainly in Ag/WNWs.
(3) When melting occurs, the dynamic process at the interface is mainly atomic bond breaking [
16]. For the composite nanonetworks, there are strong covalent bonds between the W atoms, which are the matrix. For coherent or semi-coherent interfaces, the lattice mismatch at the interface is small, and the chemical bonds between the atoms at the interface and the matrix have more or less ionic properties, which will lead to the fact that the thermal vibration at the interface is stronger than the inner W, and weaker than that of the outer Ag.
According to the above analysis, the heat-resistance temperature of Ag and W composite NWs should be between bare AgNW and WNW due to the effect of multiple interactions at the interface. The heat-resistance temperature of WNWs is 670 °C (
Figure S2), so 400 °C and 460 °C are reasonable values.
When the magnetron sputtering conditions are the same (namely 150 W, 0.5 Pa, 3 min), the heat-resistance temperature of W/AgNWs (460 °C) is higher than that of Ag/WNWs (400 °C) for the following reasons.
The linear expansion coefficients of Ag and W are 19.2 × 10
−6/K and 4.5 × 10
−6/K, respectively. When the heat treatment is carried out from room temperature to high temperature, the expansion degree of the two phases is different, and the contraction degree is also very different after the temperature drops from high temperature. Compared with W phase, Ag has a larger shrinkage degree and a faster shrinkage speed. Therefore, when the temperature drops, great internal stress is generated at the interface. When the temperature cooling speed is too fast, the internal stress generated cannot be released [
25]. At this time, Ag phase produces residual tensile stress and W phase produces residual compressive stress [
26]. In the case of Ag/WNWs, the inner Ag layer gradually melts and expands under the action of high temperature, then breaks and finally changes into chain ball shape. In this process, the Ag layer expands when heated, and the expansion state is much higher than that of the W layer. This process leads to the premature destruction of the external W layer. In the case of W/AgNWs, when the temperature rises to a certain temperature, AgNWs splits into chain ball ahead of WNWs. The inside WNWs are gradually “pulled” broken by the surface tension of the molten Ag layer. Compared with Ag/WNWs, the overall heat resistance of W/AgNWs shows certain advantages without the dual effects of Ag thermal expansion and surface tension.
In addition, some interesting results should be noticed. (1) As shown in
Figure 4f and
Figure 5b, the sheet resistance of the films showed a certain degree of reduction at the beginning of the heat treatment, both for AgNWs and W/AgNWs. This is mainly because, firstly, the heat treatment improves the crystallinity of the AgNWs; secondly, the heat treatment makes the PVP melt and volatilize, and the AgNWs fuse at the crossover point. However, we did not observe a similar phenomenon in the Ag/WNWs shown in
Figure 5a, which is due to the higher melting temperature of the outer W layer, which remains solid at the beginning of the heat treatment, separating the different AgNWs. This further demonstrates the advantage of W/AgNWs. (2) For Ag/WNWs, the sheet resistance of the film gradually increases when the heat treatment temperature reaches 260 °C, which is not found in AgNWs and W/AgNWs. As analyzed earlier, this is due to the fact that the coefficient of thermal expansion of Ag is much larger than that of W, and the stress generated in the inner layer after the temperature increase destroys the W layer prematurely. (3) In each set of heat treatment experiments, several samples with different characteristics were selected for testing, but the sample heat-resistance temperature did not change significantly, which further indicates that the interface is the most critical factor affecting the heat resistance of the composite system. (4) For W/AgNWs, increasing the thickness of the W layer can slowly increase the heat-resistance temperature of the whole film, but this is not possible in the experiment. The load-bearing capacity of the PVP NW network template is limited, and an excessively thick W layer will destroy the self-support of the template. (5) For all samples, increasing the thickness of Ag can effectively improve the electrical conductivity of the film and obtain a lower sheet resistance without bringing a significant impact on the light transmission. However, this would increase the roughness of the film and bring about an increase in cost. Therefore, we did not control this parameter in our experiments and uniformly selected a sputtering time of 3 min. However, we believe that there is still much room for improving the conductivity of AgNWs-based TCFs.