Abstract
Photoacoustic agents are widely used in various theranostic applications. By evaluating the biodistribution obtained from photoacoustic images, the effectiveness of theranostic agents in terms of their delivery efficiency and treatment responses can be analyzed. Through this study, we evaluate and summarize the recent advances in photoacoustic-guided phototherapy, particularly in photothermal and photodynamic therapy. This overview can guide the future directions for theranostic development. Because of the recent applications of photoacoustic imaging in clinical trials, theranostic agents with photoacoustic monitoring have the potential to be translated into the clinical world.
1. Introduction
Theragnosis techniques are widely studied as a patient management strategy that combines both biomedical imaging modalities and drug delivery systems [1]. In recent decades, nanoparticles with both diagnostic and therapeutic functions have been demonstrated using various biomedical imaging modalities [2,3,4]. Conventional imaging techniques, such as computed tomography (CT) [5], positron emission tomography (PET) [6], and magnetic resonance imaging (MRI) [7], are generally used to track nanoparticles. Although these imaging modalities have great advantages for the three-dimensional visualization of biological tissues, their high cost and large size may reduce their effectiveness as monitoring tools for theranostic nanoparticles. Particularly, the potential harm in using ionizing radiation or materials creates hurdles for clinical applications in humans.
Recently, optical imaging techniques have been used to monitor optically absorbing theranostic nanoparticles [8,9,10,11]. Compared with other imaging techniques, optical imaging is cost-efficient, easy to implement, capable of real-time imaging, and free from ionizing radiation. Moreover, optical imaging techniques can be used to monitor therapeutic agents for phototherapy, which typically includes photothermal therapy (PTT) [12,13,14] and photodynamic therapy (PDT) [15,16,17]. PTT is based on photothermal energy conversion, which is the energy transfer from absorbed light to heat. The generated heat increases the local temperature and destroys the target cells, typically tumor cells [18]. PDT uses photosensitizing agents to treat diseased cells. Cytotoxic reactive oxygen species (ROS) are generated by activating the photosensitizer using laser illumination [19]. Although optical imaging methods can track the treatment responses of phototherapy, their shallow imaging depth, owing to strong optical scattering in the biological tissues, significantly reduces their potential clinical translation.
Photoacoustic imaging (PAI) is a hybrid imaging technique that visualizes the optical absorption properties of biological tissues with ultrasound (US) resolution [20,21,22]. Compared to pure optical imaging techniques, PAI can significantly increase the imaging depth while maintaining a relatively good spatial resolution [23]. In particular, the resolution and imaging depth of PAI can be tuned between US and fluorescence imaging by adjusting the foci of the ultrasound and light sources (Figure 1) [24,25,26,27]. By inheriting the advantages of optical imaging techniques, PAI can also be used to monitor drug delivery, treatment responses, and agent assessment [28,29,30,31,32]. Moreover, using multispectral photoacoustic (PA) responses, the molecular functional information of biological tissues can be evaluated in vivo [33,34,35,36,37,38]. Therefore, in recent times, various theranostic agents have been developed and evaluated using the PAI technique [39,40,41,42,43,44].
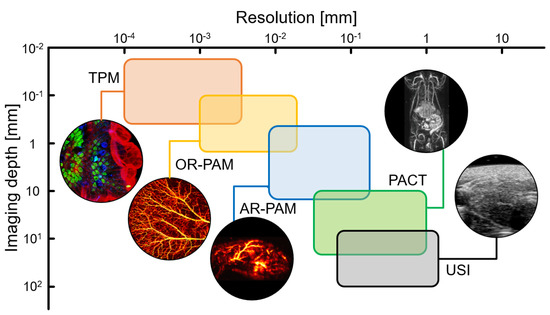
Figure 1.
Resolution and imaging depth of the imaging modalities. TPM, two−photon fluorescence microscopy; OR−PAM, optical−resolution photoacoustic microscopy; AR−PAM, acoustic-resolution photoacoustic microscopy; PACT, photoacoustic computed tomography; USI, ultrasound imaging; The images are reproduced with permission from Refs. [24,25,26,27].
In this paper, we review recent advances in PA agents for theranostic applications, especially in PTT and PDT. First, the principles and feasibility of PAI for monitoring theranostic agents were reviewed. The representative results of PA-guided phototherapy were then evaluated. This overview of PA agents provides a future direction for theranostic applications. By monitoring and evaluating the delivery efficiency and treatment responses, we can assess the potential feasibility of the agents to be translated into the clinical world.
2. Principles of Photoacoustic-Guided Phototherapy
PAI is based on the PA effect, in which light energy is converted into acoustic waves via thermoelastic expansion (Figure 2) [45]. When a short (typically a few nanoseconds) pulsed laser is irradiated, the light energy is absorbed by the target molecule according to its optical absorption characteristics. The electrons of the molecule are excited by the absorbed light energy and release the energy to return to their original ground state. One way to release energy is to use heat, which causes thermoelastic expansion. However, the expanded tissues contract rapidly after pulse duration. This rapid volume change generates vibrations that propagate in the form of acoustic waves, referred to as PA waves. The initial pressure of the PA waves can be described using the following equation:
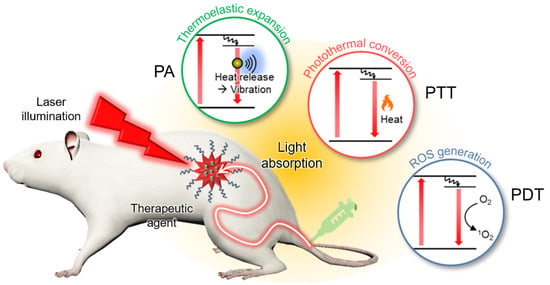
Figure 2.
Schematic illustration of PA-guided phototherapy. PA, photoacoustic; PTT, photothermal therapy; PDT, photodynamic therapy; ROS, reactive oxygen species.
The initial pressure () of the generated PA waves is linearly proportional to four parameters: the Grüneisen parameter ( that depends on the local temperature (); the heat conversion efficiency (), which can be expressed as the residual of the quantum yield; the optical absorption coefficient (), which varies with the wavelength of the excitation laser; and the optical fluence (). Because PA signals are delivered in the form of US waves, conventional US transducers and image reconstruction systems can also be used for PA image generation. However, the information in the resulting PA images is the optical absorption characteristics of the biological tissue, which can be converted into molecular functional information, including hemoglobin oxygen saturation [46,47,48,49], hemodynamic responses [50,51,52], and agent uptake [53,54,55,56,57,58,59,60,61]. Therefore, various PA agents have been demonstrated to possess theranostic applications in vivo. Moreover, recent clinical trials of PAI systems exhibited the feasibility of the technique when translated into human studies [62,63,64,65,66,67,68,69,70,71], especially for cancer diagnosis [72,73,74,75,76].
PTT is a treatment method that causes cell necrosis by rapidly increasing the temperature of the target site. In PTT, near-infrared (NIR) light typically illuminates lesions after delivering light-absorbing agents that have a high photothermal conversion efficiency [77]. When heat is released, normal and diseased cells can be damaged. Therefore, PTT agents must be selectively delivered in lesions. To validate the targeting efficiency of these agents, noninvasive biomedical imaging techniques have been utilized. From this perspective, PAI is a good candidate for visualizing the biodistribution of agents because heat generation from light absorption generates PA waves.
PDT is another type of phototherapy that uses a photochemistry based approach. In PDT, light-activatable chemicals called photosensitizers are used to destroy diseased cells [78]. Photosensitizers are not cytotoxic until they are activated via illumination. After activation using a specific wavelength of light energy, the electrons in the photosensitizers are excited to a very unstable singlet state. Thereafter, excess energy is released in three ways: (1) radiative relaxation, which produces fluorescence; (2) nonradiative decay, which releases heat; and (3) internal conversion, which produces cytotoxic reactive oxygen species (ROS), such as singlet oxygen, superoxide radicals, hydroxyl radicals, and hydrogen peroxide [79]. The photosensitizer concentration at the target site is a key factor for improving the efficacy of PDT [80]. To evaluate the targeting efficiency, PAI has also been used to visualize the biodistribution of photosensitizers.
3. Photoacoustic Agents for Theragnosis
3.1. Photoacoustic Agents for Photothermal Therapy
Organic materials are widely used for contrast-enhanced PAI because of their good photostability and nontoxicity. Organic semiconductor polymers have been studied for PA-guided PTT by tuning their optical absorption characteristics. Chen et al. demonstrated NIR-absorbing semiconductor polymer nanoparticles for both contrast-enhanced PAI and PTT [81]. The developed polymers were synthesized with different molecular weights based on diketopyrrolopyrrole–dithiophenes (DPP-DTs), the sizes of which could be tuned by controlling the concentrations of the initial polymers (Figure 3a). Remarkably, the absorption peak of DDP-DT red-shifted with an increase in the particle size (Figure 3b). The results showed the potential of DDP-DTs for multispectral PAI with broadly tunable absorption peaks (630–811 nm) by adjusting the particle size. Moreover, DDP-DTs were PEGylated with carboxyl groups (PS-PEG-COOH) to function as PTT agents (Figure 3c). The encapsulated semiconductor polymer dots (DPP-DT Pdots) were delivered into H22 hepatocellular tumor-bearing mice, and PA images were obtained at an excitation wavelength of 808 nm (Figure 3d). The resulting images showed contrast enhancement (a 2.6-fold increase compared to the control group) in the tumor, verifying the accumulation of DPP-DT Pdots. The PTT efficiency was also evaluated by measuring the temperature increase under laser illumination at the tumor site (Figure 3e). The temperature was increased by 26 °C within 5 min in the DPP-DT-Pdot group, whereas insignificant temperature increases were observed in the control groups. Furthermore, when comparing the tumor volumes, the prognosis in the PTT-treated group with DPP-DT Pdots showed a significant therapeutic ability compared to the other groups (Figure 3f).
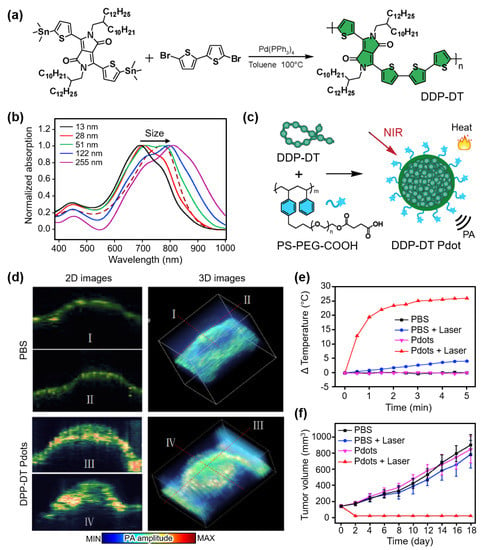
Figure 3.
(a) Synthesis of DPP-DT. (b) Absorption spectra of DPP-DTs with varying particle sizes. (c) Schematic illustration for photothermal and PA wave generation of DPP-DT Pdots. (d) In vivo PA images of tumor-bearing mice after injection of saline and DPP-DT Pdots. (e) Temperature increase at the tumor site under laser illumination. (f) Averaged tumor volume after treatment. DPP-DT, diketopyrrolopyrrole-dithiophenes; PA, photoacoustic; Pdot, semiconductor polymer dot; PBS, phosphate buffered saline. The images are reproduced with permission from Ref. [81].
Phthalocyanine (Pc) is another type of organic material that has been widely used as a functional dye owing to its easily adjustable photochemical properties for strong absorption in the NIR region. While Pc dyes have mainly been studied as photosensitizers for PDT, Li et al. reported a Pc-based theranostic agent that showed a high PA signal and PTT ability [82]. They fabricated a supramolecular assembly using zinc Pc tetrasubstituted with 4-sulfonatophenoxy (PcS4) and 3-(N,N,N-trimethylammonium) phenoxy (PcN4) groups (Figure 4a). The developed agent exhibited strong PA signals at an excitation wavelength of ~700 nm (Figure 4b). To evaluate the in vivo contrast enhancement, PA images were obtained from 4T1 breast tumor xenografted mice after the intraperitoneal injection of the developed PcS4-PcN4 with a 200 μM concentration and 200 μL volume (Figure 4c). From the whole-body PA images, the signal enhancement at the tumor site was observed after the injection of the PcS4-PcN4 supramolecules. In contrast, no remarkable signal enhancement was observed in the mice injected with PcS4 or PcN4. The results showed that supramolecular interactions between PcS4 and PcN4 significantly enhanced the visibility of PA images. Moreover, the photothermal efficiency of PcS4-PcN4 was evaluated by monitoring the temperature under the 660 nm laser illumination (Figure 4d). Compared to the control group, the PcS4-PcN4-delivered site showed a temperature increase of approximately 25 °C. Accordingly, the relative tumor volume after treatment showed the treatment ability of PcS4-PcN4-assisted PTT, with a much slower size increase (Figure 4e).
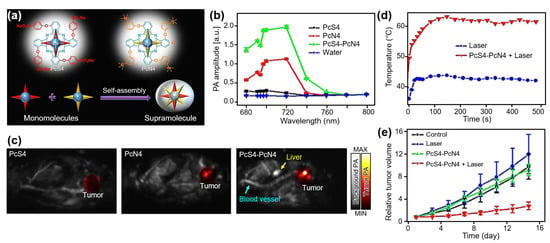
Figure 4.
(a) Schematic illustration of supramolecular assembly. (b) PA amplitudes of 25 uM PcS4, PcN4, PcS4-PcN4 supramolecule, and water. (c) In vivo PA images of tumor-bearing mice at 72 h after injection. (d) Temperature increase at the tumor site under laser irradiation. (e) Relative tumor volume after treatment. PcS4, zinc Pc tetrasubstituted with 4-sulfonatophenoxy groups; PcN4, zinc Pc tetrasubstituted with 3-(N,N,N-trimethylammonium) phenoxy groups; PA, photoacoustic. The images are reproduced with permission from Ref. [82].
Contrast agents that target specific tumors have also been studied. Fan et al. demonstrated PA-guided anticancer therapy using melanin nanoparticles (MNPs) coupled with poly-L-lysine (PLL) to target laryngeal squamous cell carcinoma (LSCC) [83]. They also added microRNAs (miRNAs), which can suppress tumor growth, to the MNP-functionalized PPL. The strategy in this study was as follows: (1) release miRNA to downregulate cancer-associated genes and (2) destroy tumor cells through PTT. To verify the treatment strategy, miRNA-bonded MNP-PLLs were delivered into Hep2 tumor xenografts via intratumoral injection. The PA images of the mice showed the accumulation of nanoparticles in the tumor region. Under 808 nm laser illuminations, the photothermal heat releases miRNAs by breaking molecular interactions between the nanocarriers and miRNAs. The infrared thermal images showed a temperature increase of 22.6 °C in the miRNA-bonded MNP-PLL-delivered mice, whereas a 3.0 °C increase was observed in the phosphate buffer saline (PBS)-delivered mice. Additionally, the released miRNAs suppressed tumor growth. The results showed a significant antitumor effect by combining PTT and gene therapy.
Metallic nanoparticles have received considerable attention because of their relative ease of tuning optical responses. Recently, Wang et al. introduced gold-based nanocomposites, named AuNSPHs, that exhibited high photothermal conversion efficiency [84]. AuNSPHs were based on gold nanostars (AuNSs) coated with polyaniline (PANI) using 1-dodecylmercaptan (DDT) as a linker (Figure 5a). To add targeting ability to tumors, hyaluronic acid coating was applied through electrostatic interactions with poly(diallyldimethylammonium chloride) (PDDA). The resulting AuNSPHs showed up to 78.6% photothermal conversion efficiency and strong PA signal generation at 850 nm (Figure 5b). For in vivo evaluation, PA images of 4T1 tumor-bearing mice were analyzed after the intravenous injection of AuNSPHs (Figure 5c). After 8 h of injection, the PA signals in the tumor region increased 2.3 times, showing an accumulation in AuNSPHs. Under 808 nm laser illumination, the temperature increased by 18.9 °C in the AuNSPH-delivered site, whereas only 3.7 °C was increased in the control group (Figure 5d). The relative tumor volume after PTT also showed the treatment efficacy of AuNSPHs (Figure 5e). Based on their outstanding photothermal conversion efficiency (78.6%), AuNSPHs can be potentially used as theranostic agents.
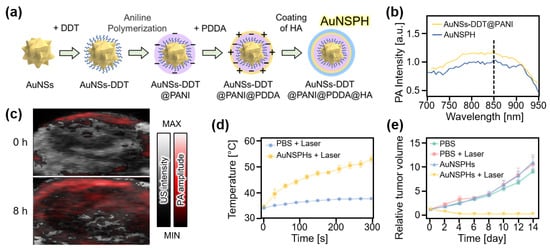
Figure 5.
(a) Schematic illustration of the synthesis of AuNSPHs. (b) PA amplitude of AuNSPHs. (c) In vivo overlaid PA and US images of tumor region in mice at 0 and 8 h after injection of AuNSPHs. (d) Temperature increase at the tumor region under the illumination of the laser. (e) Relative tumor volume after treatment. AuNS, gold nanostar; DDT, 1-dodecylmercaptan; PANI, polyaniline; PDDA, poly(diallyldimethylammonium chloride); HA, hyaluronic acid; AuNSPH, AuNSs-DDT@PANI@PDDA@HA; PA, photoacoustic; US, ultrasound; PBS, phosphate buffered saline. The images are reproduced with permission from Ref. [84].
3.2. Photoacoustic Agents for Photodynamic Therapy
In recent decades, two-dimensional nanomaterials have been used to improve the efficiency of drug delivery systems. Lin et al. synthesized two-dimensional tellurium (Te) nanosheets that could produce ROS under light exposure, making them suitable for PA-guided PDT [85]. The Te nanosheets were initially synthesized using the sonication of raw Te powder and then functionalized using glutathione (GHS) conjugations, which made the resulting nanosheets (TeNS@GHS) stable and biocompatible. The ROS generation of TeNS@GHS was verified by measuring the electron spin resonance signals under 670 nm light illumination. To evaluate the feasibility of the PA-guided therapy, in vivo PA images were obtained after the intratumoral injection of TeNS@GHSs into HepG2 tumor-bearing mice. After injection, the accumulation of the TeNS@GHSs showed a ~21-fold increase in the PA signals at the tumor site. Additionally, the relative tumor volume was monitored after illumination (670 nm, 160 mW/cm2) for 10 min. The results showed significantly suppressed tumor growth in the treated group compared to that in the other groups.
Zhang et al. reported mesoporous platinum (mPt) nanoplatforms that efficiently catalyzed the conversion of hydrogen peroxide to oxygen and generated ROS under laser illumination [86]. They connected the polyethylene glycol-modified photosensitizer chlorin e6 (PEG-Ce6) onto mPt nanoparticles to construct PDT nanoplatforms named Pt@PEG-Ce6. The developed nanoplatforms possessed efficient PDT ability by releasing oxygen in a hypoxic tumor environment. The multispectral in vivo PA images of 4T1 xenografts in mice, acquired after the intravenous injection of Pt@PEG-Ce6s, verified the oxygen release from an increase in the oxygen saturation level. The monitored tumor volume after treatment evaluated the efficacy of PDT, showing suppressed tumor growth in the treated group compared to that in the control group.
Xavierselwan et al. also demonstrated theranostic perfluoropentane (PFP) nanodroplets that can carry three agents, i.e., benzoporphyrin derivative photosensitizer (BPD), indocyanine green (ICG), and oxygen (Figure 6a) [87]. Under laser illumination, ICG absorbs light energy and initiates the vaporization of PFP nanodroplets, which release oxygen. Therefore, the developed droplets can be used as light-triggering drug delivery platforms. To test the ability of PDT, the treatment laser beam (690 nm, 150 mW/cm2) was irradiated on FaDu xenografts in mice after the tail vein injection of the PFP droplets. Multispectral PAI verified the oxygen release at the tumor site by measuring a ~9.1-fold increase in the oxygen saturation level (Figure 6b). After PDT, the tumor growth was successfully suppressed by the nanodroplets (Figure 6c). Tumor prognosis showed that PFP containing BPD and ICG at a ratio of 1:1 efficiently performed PDT.
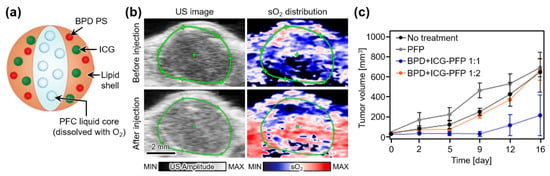
Figure 6.
(a) Schematic illustration of theranostic PFP nanodroplets. (b) In vivo US images and corresponding sO2 distribution map at tumor region in mice before and after injection of PFP nanodroplets. (c) Measured tumor volume after treatment. PFP, perfluoropentane; BPD, benzoporphyrin derivative photosensitizer; ICG, indocyanine green; PFC, perfluorocarbon; US, ultrasound; sO2, oxygen saturation level. The images are reproduced with permission from Ref. [87].
3.3. Photoacoustic Agents for Combined Photothermal and Photodynamic Therapy
Studies have attempted to perform both PTT and PDT using a single agent. Tang et al. developed an organic semiconducting nanodroplet for PAI-guided PTT and PDT (Figure 7a) [88]. The nanodroplet PS-PDI-PAnD was synthesized using encapsulating a ZnF16Pc photosensitizer with perfluorocarbons (PFCs) stabilized via light-absorbing perylene diimides (PDIs). Under laser illumination, PDIs effectively absorb energy and possess a high photothermal conversion efficiency. Moreover, the temperature increase activates the vaporization of PFC molecules, releasing the oxygen that can be consumed by the photosensitizer to produce cytotoxic singlet oxygen for PDT. Furthermore, vaporized PFCs generate bubbles that can be visualized using US imaging (USI). To validate the feasibility of image-guided therapy, these agents were intravenously injected into U87MG tumor-bearing mice. The in vivo tumor images showed contrast enhancement in both PA and US images owing to the accumulation of PS-PDI-PAnDs (Figure 7b). After the ten-minute irradiation laser (671 nm, 0.5 W/cm2), the relative tumor volume was significantly suppressed in the PS-PDI-PAnD-delivered group, whereas other groups failed to suppress the tumor growth (Figure 7c). Nanoparticles (PDI-PAnP), which were not encapsulated in the photosensitizers, also showed therapeutic effects owing to the photothermal conversion of the PDI shells. However, the absence of PDT resulted in a lower therapeutic effect compared to the PS-PDI-PAnD-delivered group.
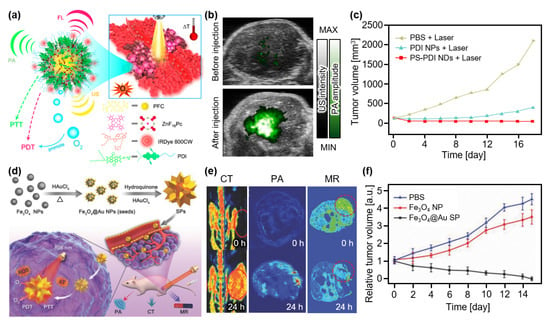
Figure 7.
(a) Schematic illustration of phototherapy with the PS-PDI NDs. (b) Overlaid PA and US images of tumor region in mice before and after injection of the PS-PDI NDs. (c) Measured tumor volume after treatment. (a–d) Schematic illustration of the Fe3O4@Au SPs. (e) In vivo CT, PA, and MR images of tumor-bearing mice at 0 and 24 h after injection of the Fe3O4@Au SPs. (f) Relative tumor volume after treatment. Fe3O4@Au, gold-coated iron oxide; NP, nanoparticle; SP, supraparticle; PA, photoacoustic; CT, X-ray computed tomography; MR, magnetic resonance; US, ultrasound; PTT, photothermal therapy; PDT, photodynamic therapy; PBS, phosphate buffered saline; PS, photosensitizer; PDI, perylene diimides; ND, nanodroplet. The images are reproduced with permission from Refs. [88,89].
Wang et al. developed spiky supraparticles (SPs) that showed good therapeutic effects with multimodal imaging capabilities [89]. They synthesized SPs via a simple seed-mediated growth method, using gold-coated iron oxide (Fe3O4@Au) as the seed (Figure 7d). Fe3O4@Au SP demonstrated great feasibility as a multimodal imaging agent for CT, MRI, and PAI (Figure 7e). Under the laser illumination (808 nm, 0.5 W/cm2), photothermal conversion was successfully monitored with a conversion efficiency of 31% and a temperature increase of 65.7 °C. Moreover, ROS generation was verified by measuring fluorescence signals, which is evidence of the generation of singlet oxygen, after laser illumination with the same wavelength (808 nm). The therapeutic ability of Fe3O4@Au SPs was evaluated in HeLa cancer-xenografted mice. After the intravenous injection of the agents, the treatment laser (808 nm, 0.5 W/cm2) was irradiated for 5 min. The resulting relative tumor volume showed that the Fe3O4@Au SPs succeeded in curing the tumor, whereas other controls failed to suppress tumor growth (Figure 7f).
4. Discussion
Recent studies on PAI-guided phototherapy are summarized in Table 1. Because both PAI and phototherapy can be derived from the optical absorption of nanomaterials, PAI can be used as a monitoring tool with minimal modification of the therapeutic agents. By monitoring a series of PA images, we can monitor the delivery of agents before treatment, which can assess their targeting ability for efficient treatment. In addition to the contrast agents discussed in this review, various NIR dyes can be utilized for synthesizing therapeutic agents to monitor the targeting efficiency. Particularly, fluorescent dyes [90,91,92] with an adequate quantum yield can expand their application area to PAI [23]. This overview of therapeutic agents can guide future directions for the development of image-guided therapy. Based on recent clinical trials of PAI, theranostic agents with PA monitoring would have great potential for translation into the clinical world.

Table 1.
Summary of PAI-guided phototherapy. , wavelength for PAI; , wavelength for treatment; PTT, photothermal therapy; PDT, photodynamic therapy; SPN, semiconductor polymer nanoparticle; NP, nanoparticle; DDP-DT, diketopyrrolopyrrole-dithiophene; Pc, phthalocyanine; SP, supramolecule; Au, gold; PLL, poly-L-lysine; MNP, melanin nanoparticle; Te, tellurium; mPt, mesoporous platinum; PFP, perfluoropentane; Fe3O4, iron oxide; PAI, photoacoustic imaging; USI, ultrasound imaging; FLI, fluorescence imaging; MRI, magnetic resonance imaging; CT, X-ray computed tomography.
To improve the treatment efficiency, the optimal synthesis of both PTT and PDT agents is required. In PTT, increasing the photothermal conversion efficiency is key for efficient treatment. There have been studies conducted to determine the optimal size of nanomaterials and apply various shapes of nanomaterials, including nanostars and spiky supraparticles. In PDT, overcoming the hypoxic conditions in the tumor region can improve the treatment efficiency because the generation of ROS typically consumes oxygen. Nanodroplets that can deliver oxygen in addition to photosensitizers are a good option to overcome the hypoxic condition of the tumor site. Under laser irradiation, oxygen is released, and ROS generation is activated. Further, computational studies for evaluating state dynamics can be applied to design efficient therapeutic agents [93].
Recently, agents that absorb lasers in the second near-infrared (NIR-II) region (1000–1700 nm) have been widely studied for PA contrast. In this region, the photon scattering in biological tissues is significantly less than that in the first near-infrared (NIR-I) region (650–1000 nm), which is mainly presented in this paper. Although PDT may be limited due to the lower efficiency of energy transfer to oxygen in NIR-II compared to the visible and NIR-I ranges, the less scattering of light is highly beneficial in biomedical studies as it enhances the penetration depth [94], allowing higher laser energy delivery to the skin [95] and stronger PA signal generation. Therefore, therapeutic agents that absorb in the NIR-II region can be used for drug delivery to deep tissues [96,97,98].
Advances in PAI systems may also be beneficial for biomedical applications of therapeutic agents. To visualize the biodistribution of agents, whole-body PAI systems equipped with multiple or tunable laser wavelengths have been widely developed [22]. Recently, three-dimensional PA computed tomography (PACT) systems have shown great potential for monitoring the biodistribution of small animals in vivo [26]. In addition, advanced deep-learning algorithms have been reported to achieve better image quality [99,100,101,102,103,104]. The continuous improvement of the PAI system can potentially translate the PAI-guided phototherapy technique to clinical applications.
In addition to the monitoring of the delivery, techniques to control the release of drugs have also been studied [105,106,107]. The key to a controlled release is the triggering mechanism caused by external stimuli or environmental change. The representative external stimuli related to PAI are light [108], temperature [109], and US waves [110]. Monitoring those drugs using PAI can improve the efficiency of the release by determining the optimal time for triggering. In recent years, the controlled release of hydrogen (H2) gases has been studied [111,112,113], showing synergetic treatment with phototherapies. The difference in the biological environments such as pH level [114], enzymatic reactions [115], and redox reactions [116] in the diseased region also can be used to trigger drug release. When the agents meet the environmental change, the shape of the agents is transformed to release the drugs, causing shifted optical absorption characteristics. In this perspective, PAI can noninvasively verify the drug release in the target tissues in vivo [117,118,119].
Author Contributions
Conceptualization, C.K. and J.K.; investigation, S.H. and T.N.; resources, S.H. and T.N.; writing—original draft preparation, S.H., T.N. and M.K.; writing—review and editing, M.K., C.K. and J.K.; visualization, S.H. and T.N.; supervision, J.K.; project administration, C.K. and J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation (NRF) grants (2021R1A5A1032937, 2021M3C1C3097624, 2020R1A6A1A03047902), the Korea Medical Device Development Fund grant (1711137875, RS-2020-KD000008), and BK21 FOUR projects (Pusan National University; Pohang University of Science and Technology) funded by the Korean government (the Ministry of Science and ICT; the Ministry of Education; the Ministry of Trade, Industry and Energy).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
C.K. has financial interests in OPTICHO, which did not support this work.
References
- Lee, D.-E.; Koo, H.; Sun, I.-C.; Ryu, J.H.; Kim, K.; Kwon, I.C. Multifunctional Nanoparticles for Multimodal Imaging and Theragnosis. Chem. Soc. Rev. 2012, 41, 2656–2672. [Google Scholar] [CrossRef]
- Ryu, J.H.; Koo, H.; Sun, I.-C.; Yuk, S.H.; Choi, K.; Kim, K.; Kwon, I.C. Tumor-Targeting Multi-Functional Nanoparticles for Theragnosis: New Paradigm for Cancer Therapy. Adv. Drug Deliv. Rev. 2012, 64, 1447–1458. [Google Scholar] [CrossRef]
- Kang, M.S.; Lee, S.Y.; Kim, K.S.; Han, D.-W. State of the Art Biocompatible Gold Nanoparticles for Cancer Theragnosis. Pharmaceutics 2020, 12, 701. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, J.H.; Park, H.; Kim, Y.-S.; Park, K.; Nam, H.; Lee, S.; Park, J.H.; Park, R.-W.; Kim, I.-S. Tumor-Homing Multifunctional Nanoparticles for Cancer Theragnosis: Simultaneous Diagnosis, Drug Delivery, and Therapeutic Monitoring. J. Control. Release 2010, 146, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, C. CuGeO3 Nanoparticles: An Efficient Photothermal Theragnosis Agent for CT Imaging-Guided Photothermal Therapy of Cancers. Front. Bioeng. Biotechnol. 2020, 8, 590518. [Google Scholar] [CrossRef] [PubMed]
- Eo, J.S.; Paeng, J.C.; Lee, D.S. Nuclear Imaging for Functional Evaluation and Theragnosis in Liver Malignancy and Transplantation. World J. Gastroenterol. 2014, 20, 5375. [Google Scholar] [CrossRef]
- Caspani, S.; Magalhães, R.; Araújo, J.P.; Sousa, C.T. Magnetic Nanomaterials as Contrast Agents for MRI. Materials 2020, 13, 2586. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, T.S.; Song, I.H.; Cho, Y.L.; Chae, J.R.; Yun, M.; Kang, H.; Lee, J.H.; Lim, J.H.; Cho, W.G. Hybridization-based Aptamer Labeling using Complementary Oligonucleotide Platform for PET and Optical Imaging. Biomaterials 2016, 100, 143–151. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef]
- Guo, Q.; Kuang, L.; Cao, H.; Li, W.; Wei, J. Self-Assembled mPEG-PCL-g-PEI Micelles for Multifunctional Nanoprobes of Doxorubicin Delivery and Magnetic Resonance Imaging and Optical Imaging. Colloids Surf. B Biointerfaces 2015, 136, 687–693. [Google Scholar] [CrossRef]
- Sun, I.C.; Eun, D.K.; Koo, H.; Ko, C.Y.; Kim, H.S.; Yi, D.K.; Choi, K.; Kwon, I.C.; Kim, K.; Ahn, C.H. Tumor-Targeting Gold Particles for Dual Computed Tomography/Optical Cancer Imaging. Angew. Chem. 2011, 50, 9348–9351. [Google Scholar] [CrossRef]
- Liu, S.; Pan, X.; Liu, H. Two-Dimensional Nanomaterials for Photothermal Therapy. Angew. Chem. 2020, 132, 5943–5953. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, H.-M.; Song, G.; Li, Z.; Zhang, X.-B. Conjugated-Polymer-based Nanomaterials for Photothermal Therapy. ACS Appl. Polym. Mater. 2020, 2, 4258–4272. [Google Scholar] [CrossRef]
- Zhi, D.; Yang, T.; O’hagan, J.; Zhang, S.; Donnelly, R.F. Photothermal Therapy. J. Control. Release 2020, 325, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Gazzi, A.; Fusco, L.; Khan, A.; Bedognetti, D.; Zavan, B.; Vitale, F.; Yilmazer, A.; Delogu, L.G. Photodynamic Therapy based on Graphene and MXene in Cancer Theranostics. Front. Bioeng. Biotechnol. 2019, 7, 295. [Google Scholar] [CrossRef]
- Radhi, H.; Farag, A.; Al-Allaq, T.; Virdee, P.; Almudamgha, R.; Al-Hadad, M.; Fashid, A.; Alani, A.F.; Awda, M.; Alnajar, Z. Photodynamic Therapy as a Treatment Option for Oral Cancer and Dysplasia. Ann. Med. Health Sci. Res. 2018, 8, 59–64. [Google Scholar]
- dos Santos, A.l.F.; De Almeida, D.R.Q.; Terra, L.F.; Baptista, M.c.S.; Labriola, L. Photodynamic Therapy in Cancer Treatment—An Update Review. J. Cancer Metastasis Treat. 2019, 5, 25. [Google Scholar] [CrossRef]
- Zou, L.; Wang, H.; He, B.; Zeng, L.; Tan, T.; Cao, H.; He, X.; Zhang, Z.; Guo, S.; Li, Y. Current Approaches of Photothermal Therapy in Treating Cancer Metastasis with Nanotherapeutics. Theranostics 2016, 6, 762. [Google Scholar] [CrossRef] [PubMed]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Xu, M.; Wang, L.V. Photoacoustic Imaging in Biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef]
- Kim, C.; Favazza, C.; Wang, L.V. In Vivo Photoacoustic Tomography of Chemicals: High-Resolution Functional and Molecular Optical Imaging at New Depths. Chem. Rev. 2010, 110, 2756–2782. [Google Scholar] [CrossRef]
- Kye, H.; Song, Y.; Ninjbadgar, T.; Kim, C.; Kim, J. Whole-Body Photoacoustic Imaging Techniques for Preclinical Small Animal Studies. Sensors 2022, 22, 5130. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.; Kim, H.-H.; Kim, C.-S.; Kim, J. Review on Optical Imaging Techniques for Multispectral Analysis of Nanomaterials. Nanotheranostics 2022, 6, 50. [Google Scholar] [CrossRef]
- Razansky, D.; Klohs, J.; Ni, R. Multi-Scale Optoacoustic Molecular Imaging of Brain Diseases. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4152–4170. [Google Scholar] [CrossRef]
- Lee, H.; Han, S.; Park, S.; Cho, S.; Yoo, J.; Kim, C.; Kim, J. Ultrasound-Guided Breath-Compensation in Single-Element Photoacoustic Imaging for Three-Dimensional Whole-Body Images of Mice. Front. Phys. 2022, 10, 457. [Google Scholar] [CrossRef]
- Choi, S.; Yang, J.; Lee, S.Y.; Kim, J.; Lee, J.; Kim, W.J.; Lee, S.; Kim, C. Deep Learning Enhances Multiparametric Dynamic Volumetric Photoacoustic Computed Tomography In Vivo (DL-PACT). Adv. Sci. 2023, 10, 2202089. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, B.; Ha, J.; Steinberg, I.; Hooper, S.M.; Jeong, C.; Park, E.-Y.; Choi, W.; Liang, T.; Bae, J.-S.; et al. Multiparametric Photoacoustic Analysis of Human Thyroid Cancers In Vivo. Cancer Res. 2021, 81, 4849–4860. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Park, S.; Kim, J.; Kim, C. Listening to Drug Delivery and Responses via Photoacoustic Imaging. Adv. Drug Deliv. Rev. 2022, 184, 114235. [Google Scholar] [CrossRef]
- Kim, C.; Razansky, D. Listening to Light and Seeing Through: Biomedical Photoacoustic Imaging. IEEE Pulse 2015, 6, 3–4. [Google Scholar] [CrossRef]
- Fu, Q.; Zhu, R.; Song, J.; Yang, H.; Chen, X. Photoacoustic Imaging: Contrast Agents and Their Biomedical Applications. Adv. Mater. 2019, 31, 1805875. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.Y.; Kang, M.S.; Lee, H.; Lee, J.H.; Kim, J.; Han, D.-W.; Kim, K.S. Recent Trends in Photoacoustic Imaging Techniques for 2D Nanomaterial-Based Phototherapy. Biomedicines 2021, 9, 80. [Google Scholar] [CrossRef]
- Kang, M.S.; Lee, H.; Jeong, S.J.; Eom, T.J.; Kim, J.; Han, D.-W. State of the Art in Carbon Nanomaterials for Photoacoustic Imaging. Biomedicines 2022, 10, 1374. [Google Scholar] [CrossRef]
- Razansky, D. Multispectral Optoacoustic Tomography—Volumetric Color Hearing in Real Time. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1234–1243. [Google Scholar] [CrossRef]
- Ma, R.; Taruttis, A.; Ntziachristos, V.; Razansky, D. Multispectral Optoacoustic Tomography (MSOT) Scanner for Whole-Body Small Animal Imaging. Opt. Express 2009, 17, 21414–21426. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.; Kim, J.; Kim, C. Multiplane Spectroscopic Whole-Body Photoacoustic Imaging of Small Animals In Vivo. Med. Biol. Eng. Comput. 2016, 54, 283–294. [Google Scholar] [CrossRef]
- Park, E.-Y.; Park, S.; Lee, H.; Kang, M.; Kim, C.; Kim, J. Simultaneous Dual-Modal Multispectral Photoacoustic and Ultrasound Macroscopy for Three-Dimensional Whole-Body Imaging of Small Animals. Photonics 2021, 8, 13. [Google Scholar] [CrossRef]
- Srivatsan, A.; Jenkins, S.V.; Jeon, M.; Wu, Z.; Kim, C.; Chen, J.; Pandey, R.K. Gold nanocage-photosensitizer conjugates for dual-modal image-guided enhanced photodynamic therapy. Theranostics 2014, 4, 163. [Google Scholar] [CrossRef]
- Park, B.; Lee, K.M.; Park, S.; Yun, M.; Choi, H.-J.; Kim, J.; Lee, C.; Kim, H.; Kim, C. Deep Tissue Photoacoustic Imaging of Nickel (II) Dithiolene-Containing Polymeric Nanoparticles in the Second Near-Infrared Window. Theranostics 2020, 10, 2509–2521. [Google Scholar] [CrossRef]
- Guo, T.; Tang, Q.; Guo, Y.; Qiu, H.; Dai, J.; Xing, C.; Zhuang, S.; Huang, G. Boron Quantum Dots for Photoacoustic Imaging-Guided Photothermal Therapy. ACS Appl. Mater. Interfaces 2020, 13, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.-H.; Wang, H.; Yang, H.; Li, Z.; Zhen, L.; Xu, C.-Y. Glucose-Derived Carbonaceous Nanospheres for Photoacoustic Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2016, 8, 15904–15910. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Dou, D.; Yang, L. Porphyrin Photosensitizers in Photodynamic Therapy and Its Applications. Oncotarget 2017, 8, 81591. [Google Scholar] [CrossRef] [PubMed]
- Kwon, N.; Kim, K.H.; Park, S.; Cho, Y.; Park, E.-Y.; Lim, J.; Çetindere, S.; Tümay, S.O.; Kim, W.J.; Li, X. Hexa-BODIPY-Cyclotriphosphazene based Nanoparticle for NIR Fluorescence/Photoacoustic Dual-Modal Imaging and Photothermal Cancer Therapy. Biosens. Bioelectron. 2022, 216, 114612. [Google Scholar] [CrossRef]
- Cao, T.G.N.; Kang, J.H.; Kim, W.; Lim, J.; Kang, S.J.; You, J.Y.; Hoang, Q.T.; Kim, W.J.; Rhee, W.J.; Kim, C. Engineered Extracellular Vesicle-based Sonotheranostics for Dual Stimuli-Sensitive Drug Release and Photoacoustic Imaging-Guided Chemo-Sonodynamic Cancer Therapy. Theranostics 2022, 12, 1247. [Google Scholar]
- Chen, Y.; Xu, C.; Cheng, Y.; Cheng, Q. Photostability Enhancement of Silica-Coated Gold Nanostars for Photoacoustic Imaging Guided Photothermal Therapy. Photoacoustics 2021, 23, 100284. [Google Scholar] [CrossRef]
- Bell, A.G. The Photophone. Science 1880, 1, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Nasiriavanaki, M.; Xia, J.; Wan, H.; Bauer, A.Q.; Culver, J.P.; Wang, L.V. High-Resolution Photoacoustic Tomography of Resting-State Functional Connectivity in the Mouse Brain. Proc. Natl. Acad. Sci. USA 2014, 111, 21–26. [Google Scholar] [CrossRef]
- Lee, C.; Jeon, M.; Jeon, M.Y.; Kim, J.; Kim, C. In Vitro Photoacoustic Measurement of Hemoglobin Oxygen Saturation using a Single Pulsed Broadband Supercontinuum Laser Source. Appl. Opt. 2014, 53, 3884–3889. [Google Scholar] [CrossRef]
- Zhang, H.F.; Maslov, K.; Sivaramakrishnan, M.; Stoica, G.; Wang, L.V. Imaging of Hemoglobin Oxygen Saturation Variations in Single Vessels In Vivo using Photoacoustic Microscopy. Appl. Phys. Lett. 2007, 90, 053901. [Google Scholar] [CrossRef]
- Wang, Y.; Jhang, D.-F.; Tsai, C.-H.; Chiang, N.-J.; Tsao, C.-H.; Chuang, C.-C.; Chen, L.-T.; Chang, W.-S.W.; Liao, L.-D. In Vivo Assessment of Hypoxia Levels in Pancreatic Tumors using a Dual-Modality Ultrasound/Photoacoustic Imaging System. Micromachines 2021, 12, 668. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, L.; Yang, J.-M.; Maslov, K.I.; Wong, T.T.; Li, L.; Huang, C.-H.; Zou, J.; Wang, L.V. High-Speed Label-Free Functional Photoacoustic Microscopy of Mouse Brain in Action. Nat. Methods 2015, 12, 407–410. [Google Scholar] [CrossRef]
- Lin, L.; Xia, J.; Wong, T.T.; Li, L.; Wang, L.V. In Vivo Deep Brain Imaging of Rats using Oral-Cavity Illuminated Photoacoustic Computed Tomography. J. Biomed. Opt. 2015, 20, 016019. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, L.V. Photoacoustic Brain Imaging: From Microscopic to Macroscopic Scales. Neurophotonics 2014, 1, 011003. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Lee, C.; Kim, J.Y.; Kim, C. Organic Nanostructures for Photoacoustic Imaging. ChemNanoMat 2015, 2, 156–166. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.; Zhang, Y.; Jeon, M.; Liu, C.; Song, L.; Lovell, J.F.; Kim, C. Dual-Color Photoacoustic Lymph Node Imaging using Nanoformulated Naphthalocyanines. Biomaterials 2015, 73, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lee, D.; Kim, S.; Kim, H.-H.; Jeong, S.; Kim, J. Contrast Agents for Photoacoustic Imaging: A Review Focusing on the Wavelength Range. Biosensors 2022, 12, 594. [Google Scholar] [CrossRef]
- Lee, D.; Beack, S.; Yoo, J.; Kim, S.K.; Lee, C.; Kwon, W.; Hahn, S.K.; Kim, C. In Vivo Photoacoustic Imaging of Livers Using Biodegradable Hyaluronic Acid-Conjugated Silica Nanoparticles. Adv. Funct. Mater. 2018, 28, 1800941. [Google Scholar] [CrossRef]
- Singh, S.; Giammanco, G.; Hu, C.-H.; Bush, J.; Cordova, L.S.; Lawrence, D.J.; Moran, J.L.; Chitnis, P.V.; Veneziano, R. Size-Tunable ICG-based Contrast Agent Platform for Targeted Near-Infrared Photoacoustic Imaging. Photoacoustics 2022, 29, 100437. [Google Scholar] [CrossRef] [PubMed]
- Kilian, H.I.; Ma, C.; Zhang, H.; Chen, M.; Nilam, A.; Quinn, B.; Tang, Y.; Xia, J.; Yao, J.; Lovell, J.F. Intraperitoneal Administration for Sustained Photoacoustic Contrast Agent Imaging. Photoacoustics 2022, 28, 100406. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Ding, Y.; Lovell, J.F.; Zhang, Y. Design and Application of Organic Contrast Agents for Molecular Imaging in the Second Near Infrared (NIR-II) Window. Photoacoustics 2022, 28, 100426. [Google Scholar] [CrossRef]
- Vonk, J.; Kukačka, J.; Steinkamp, P.; de Wit, J.; Voskuil, F.; Hooghiemstra, W.; Bader, M.; Jüstel, D.; Ntziachristos, V.; van Dam, G. Multispectral Optoacoustic Tomography for In Vivo Detection of Lymph Node Metastases in Oral Cancer Patients using an EGFR-Targeted Contrast Agent and Intrinsic Tissue Contrast: A Proof-of-Concept Study. Photoacoustics 2022, 26, 100362. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Park, B.; Choi, S.; Oh, D.; Kim, J.; Kim, C. Recent Advances in Contrast-enhanced Photoacoustic Imaging: Overcoming the Physical and Practical Challenges. Chem. Rev. 2023. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-Y.; Lee, H.; Han, S.; Kim, C.; Kim, J. Photoacoustic Imaging Systems Based on Clinical Ultrasound Platform. Exp. Biol. Med. 2022, 247, 551–560. [Google Scholar] [CrossRef]
- Kim, J.; Park, E.-Y.; Park, B.; Choi, W.; Lee, K.J.; Kim, C. Towards Clinical Photoacoustic and Ultrasound Imaging: Probe Improvement and Real-Time Graphical User Interface. Exp. Biol. Med. 2020, 245, 321–329. [Google Scholar] [CrossRef]
- Steinberg, I.; Huland, D.M.; Vermesh, O.; Frostig, H.E.; Tummers, W.S.; Gambhir, S.S. Photoacoustic Clinical Imaging. Photoacoustics 2019, 14, 77–98. [Google Scholar] [CrossRef]
- Choi, W.; Park, E.-Y.; Jeon, S.; Kim, C. Clinical Photoacoustic Imaging Platforms. Biomed. Eng. Lett. 2018, 8, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.; Jung, Y.; Chang, S.; Park, J.; Zhang, Y.; Lovell, J.F.; Kim, C. Programmable Real-time Clinical Photoacoustic and Ultrasound Imaging System. Sci. Rep. 2016, 6, 35137. [Google Scholar] [CrossRef]
- Choi, W.; Park, E.-Y.; Jeon, S.; Yang, Y.; Park, B.; Ahn, J.; Cho, S.; Lee, C.; Seo, D.-K.; Cho, J.-H.; et al. Three-Dimensional Multistructural Quantitative Photoacoustic and US Imaging of Human Feet In Vivo. Radiology 2022, 303, 467–473. [Google Scholar] [CrossRef]
- Ahn, J.; Baik, J.W.; Kim, Y.; Choi, K.; Park, J.; Kim, H.; Kim, J.Y.; Kim, H.H.; Nam, S.H.; Kim, C. Fully Integrated Photoacoustic Microscopy and Photoplethysmography of Human In Vivo. Photoacoustics 2022, 27, 100374. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, J.Y.; Choi, W.; Kim, C. High-Resolution Functional Photoacoustic Monitoring of Vascular Dynamics in Human Fingers. Photoacoustics 2021, 23, 100282. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Choi, W.; Kim, J.; Kim, C. Three-Dimensional Clinical Handheld Photoacoustic/Ultrasound Scanner. Photoacoustics 2020, 18, 100173. [Google Scholar] [CrossRef]
- Baik, J.W.; Kim, H.; Son, M.; Choi, J.; Kim, K.G.; Baek, J.H.; Park, Y.H.; An, J.; Choi, H.Y.; Ryu, S.Y.; et al. Intraoperative Label-Free Photoacoustic Histopathology of Clinical Specimens. Laser Photonics Rev. 2021, 15, 2100124. [Google Scholar] [CrossRef]
- Kothapalli, S.-R.; Sonn, G.A.; Choe, J.W.; Nikoozadeh, A.; Bhuyan, A.; Park, K.K.; Cristman, P.; Fan, R.; Moini, A.; Lee, B.C.; et al. Simultaneous Transrectal Ultrasound and Photoacoustic Human Prostate Imaging. Sci. Transl. Med. 2019, 11, eaav2169. [Google Scholar] [CrossRef]
- Park, B.; Bang, C.H.; Lee, C.; Han, J.H.; Choi, W.; Kim, J.; Park, G.S.; Rhie, J.W.; Lee, J.H.; Kim, C. 3D Wide-Field Multispectral Photoacoustic Imaging of Human Melanomas In Vivo: A Pilot Study. J. Eur. Acad. Dermatol. Venereol. 2020, 35, 669–676. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.H.; Park, B.; Seo, H.M.; Bang, C.H.; Park, G.S.; Park, Y.M.; Rhie, J.W.; Lee, J.H.; Kim, C. Multispectral Ex Vivo Photoacoustic Imaging of Cutaneous Melanoma for Better Selection of the Excision Margin. Br. J. Dermatol. 2018, 179, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lee, H.; Kim, C.; Kim, J. Review on Multispectral Photoacoustic Analysis of Cancer: Thyroid and Breast. Metabolites 2022, 12, 382. [Google Scholar] [CrossRef] [PubMed]
- Neuschler, E.I.; Butler, R.; Young, C.A.; Barke, L.D.; Bertrand, M.L.; Böhm-Vélez, M.; Destounis, S.; Donlan, P.; Grobmyer, S.R.; Katzen, J. A Pivotal Study of Optoacoustic Imaging to Diagnose Benign and Malignant Breast Masses: A New Evaluation Tool for Radiologists. Radiology 2017, 287, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, Y.; Chang, R.; Xing, R.; Yan, X. Supramolecular Photothermal Nanomaterials as an Emerging Paradigm Toward Precision Cancer Therapy. Adv. Funct. Mater. 2019, 29, 1806877. [Google Scholar] [CrossRef]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and Photodynamic Therapy: Mechanisms, Monitoring, and Optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef]
- Donnelly, R.F.; McCarron, P.A.; Tunney, M.M. Antifungal Photodynamic Therapy. Microbiol. Res. 2008, 163, 1–12. [Google Scholar] [CrossRef]
- Jerjes, W.; Upile, T.; Hamdoon, Z.; Alexander Mosse, C.; Morcos, M.; Hopper, C. Photodynamic Therapy Outcome for T1/T2 N0 Oral Squamous Cell Carcinoma. Lasers Surg. Med. 2011, 43, 463–469. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; Chang, K.; Men, X.; Fang, X.; Zhou, L.; Li, D.; Gao, D.; Yin, S.; Zhang, X. Highly Absorbing Multispectral Near-Infrared Polymer Nanoparticles from One Conjugated Backbone for Photoacoustic Imaging and Photothermal Therapy. Biomaterials 2017, 144, 42–52. [Google Scholar] [CrossRef]
- Li, X.; Park, E.Y.; Kang, Y.; Kwon, N.; Yang, M.; Lee, S.; Kim, W.J.; Kim, C.; Yoon, J. Supramolecular Phthalocyanine Assemblies for Improved Photoacoustic Imaging and Photothermal Therapy. Angew. Chem. 2020, 132, 8708–8712. [Google Scholar] [CrossRef]
- Fan, B.; Yang, X.; Li, X.; Lv, S.; Zhang, H.; Sun, J.; Li, L.; Wang, L.; Qu, B.; Peng, X. Photoacoustic-Imaging-Guided Therapy of Functionalized Melanin Nanoparticles: Combination of Photothermal Ablation and Gene Therapy Against Laryngeal Squamous Cell Carcinoma. Nanoscale 2019, 11, 6285–6296. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Yang, L.; Lin, Y.; Tian, Y.; Ni, Q.; Wang, S.; Ju, H.; Guo, J.; Lu, G. Gold Nanostar@ Polyaniline Theranostic Agent with High Photothermal Conversion Efficiency for Photoacoustic Imaging-Guided Anticancer Phototherapy at a Low Dosage. ACS Appl. Mater. Interfaces 2022, 14, 28570–28580. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, Y.; Wang, R.; Tao, G.; Luo, P.-F.; Lin, X.; Huang, G.; Li, J.; Yang, H.-H. Two-Dimensional Tellurium Nanosheets for Photoacoustic Imaging-Guided Photodynamic Therapy. Chem. Commun. 2018, 54, 8579–8582. [Google Scholar] [CrossRef]
- Zhang, L.; Li, M.; Zhou, Q.; Dang, M.; Tang, Y.; Wang, S.; Fu, J.; Teng, Z.; Lu, G. Computed Tomography and Photoacoustic Imaging Guided Photodynamic Therapy Against Breast Cancer based on Mesoporous Platinum with insitu Oxygen Generation Ability. Acta Pharm. Sin. B 2020, 10, 1719–1729. [Google Scholar] [CrossRef]
- Xavierselvan, M.; Cook, J.; Duong, J.; Diaz, N.; Homan, K.; Mallidi, S. Photoacoustic Nanodroplets for Oxygen Enhanced Photodynamic Therapy of Cancer. Photoacoustics 2022, 25, 100306. [Google Scholar] [CrossRef]
- Tang, W.; Yang, Z.; Wang, S.; Wang, Z.; Song, J.; Yu, G.; Fan, W.; Dai, Y.; Wang, J.; Shan, L. Organic Semiconducting Photoacoustic Nanodroplets for Laser-Activatable Ultrasound Imaging and Combinational Cancer Therapy. ACS Nano 2018, 12, 2610–2622. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hao, C.; Sun, M.; Xu, L.; Xu, C.; Kuang, H. Spiky Fe3O4@ Au Supraparticles for Multimodal in vivo Imaging. Adv. Funct. Mater. 2018, 28, 1800310. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Yuan, Z.; Zhao, G. AIE-Based Fluorescence Probes with Different Torsional Configurations for Monitoring Human Serum Albumin. Sens. Actuators B Chem. 2023, 380, 133320. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Wang, Y.; Wang, C.; Guo, Y.; Yuan, Z.; Jia, Y.; Li, P.; Sun, S.; Zhao, G. A Highly Selective Red-Emitting Fluorescent Probe and Its Micro-Nano-Assembly for Imaging Endogenous Peroxynitrite (ONOO−) in Living Cells. Anal. Chim. Acta 2023, 1241, 340778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jia, Y.; Xing, X.; Qin, M.; Wu, Z.; Zhong, Y.; Liu, L.; Sun, S.; Li, P.; Wang, H. A Novel Lysosome-Localized Fluorescent Probe with Aggregation-Induced Emission without Alkalinizing Effect. SmartMat 2021, 2, 554–566. [Google Scholar] [CrossRef]
- Zhao, G.-J.; Han, K.-L. Hydrogen Bonding in the Electronic Excited State. Acc. Chem. Res. 2012, 45, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Antaris, A.L.; Dai, H. Near-Infrared Fluorophores for Biomedical Imaging. Nat. Biomed. Eng. 2017, 1, 0010. [Google Scholar] [CrossRef]
- ANSI Z136.1; American National Standard for the Safe Use of Lasers. American National Standards Institute: New York, NY, USA, 2014.
- Zhang, L.; Oudeng, G.; Wen, F.; Liao, G. Recent Advances in Near-Infrared-II Hollow Nanoplatforms for Photothermal-Based Cancer Treatment. Biomater. Res. 2022, 26, 61. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Tan, H.; Peng, Q.; Chen, H.; Ma, H.; Wang, L.; Peng, A.; Shi, Q.; Cai, X.; Huang, H. Achieving Efficient NIR-II Type-I Photosensitizers for Photodynamic/Photothermal Therapy upon Regulating Chalcogen Elements. Adv. Mater. 2022, 34, 2108146. [Google Scholar] [CrossRef]
- Yin, S.; Song, J.; Liu, D.; Wang, K.; Qi, J. NIR-II AIEgens with Photodynamic Effect for Advanced Theranostics. Molecules 2022, 27, 6649. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, G.; Li, L.; Zhang, P.; Kim, J.Y.; Kim, Y.; Kim, H.H.; Wang, L.V.; Lee, S.; Kim, C. Deep Learning Acceleration of Multiscale Superresolution Localization Photoacoustic Imaging. Light Sci. Appl. 2022, 11, 131. [Google Scholar] [CrossRef]
- Kim, J.; Lee, D.; Lim, H.; Yang, H.; Kim, J.; Kim, J.; Kim, Y.; Kim, H.H.; Kim, C. Deep Learning Alignment of Bidirectional Raster Scanning in High Speed Photoacoustic Microscopy. Sci. Rep. 2022, 12, 16238. [Google Scholar] [CrossRef]
- Jeon, S.; Choi, W.; Park, B.; Kim, C. A Deep Learning-Based Model that Reduces Speed of Sound Aberrations for Improved In Vivo Photoacoustic Imaging. IEEE Trans. Image Process. 2021, 30, 8773–8784. [Google Scholar] [CrossRef]
- Yang, C.; Lan, H.; Gao, F.; Gao, F. Review of Deep Learning for Photoacoustic Imaging. Photoacoustics 2021, 21, 100215. [Google Scholar] [CrossRef]
- Cho, S.; Baik, J.; Managuli, R.; Kim, C. 3D PHOVIS: 3D Photoacoustic Visualization Studio. Photoacoustics 2020, 18, 100168. [Google Scholar] [CrossRef]
- Gröhl, J.; Schellenberg, M.; Dreher, K.; Maier-Hein, L. Deep Learning for Biomedical Photoacoustic Imaging: A Review. Photoacoustics 2021, 22, 100241. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.A.; Bagheri, R.; Mosafer, J.; Baradaran, B.; Hashemzaei, M.; Baghbanzadeh, A.; de la Guardia, M.; Mokhtarzadeh, A. Recent Advances on Thermosensitive and pH-Sensitive Liposomes Employed in Controlled Release. J. Control. Release 2019, 315, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Yang, X.; Xia, Y. Putting Gold Nanocages to Work for Optical Imaging, Controlled Release and Cancer Theranostics. Nanomedicine 2016, 11, 1715–1728. [Google Scholar] [CrossRef] [PubMed]
- Mathiyazhakan, M.; Upputuri, P.K.; Sivasubramanian, K.; Dhayani, A.; Vemula, P.K.; Zou, P.; Pu, K.; Yang, C.; Pramanik, M.; Xu, C. In Situ Synthesis of Gold Nanostars within Liposomes for Controlled Drug Release and Photoacoustic Imaging. Sci. China Mater. 2016, 59, 892–900. [Google Scholar] [CrossRef]
- Fomina, N.; Sankaranarayanan, J.; Almutairi, A. Photochemical Mechanisms of Light-Triggered Release from Nanocarriers. Adv. Drug Deliv. Rev. 2012, 64, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Ta, T.; Porter, T.M. Thermosensitive Liposomes for Localized Delivery and Triggered Release of Chemotherapy. J. Control. Release 2013, 169, 112–125. [Google Scholar] [CrossRef]
- Klibanov, A.L.; Shevchenko, T.I.; Raju, B.I.; Seip, R.; Chin, C.T. Ultrasound-Triggered Release of Materials Entrapped in Microbubble–Liposome Constructs: A Tool for Targeted Drug Delivery. J. Control. Release 2010, 148, 13–17. [Google Scholar] [CrossRef]
- Yu, S.; Li, G.; Zhao, P.; Cheng, Q.; He, Q.; Ma, D.; Xue, W. NIR-Laser-Controlled Hydrogen-Releasing PdH Nanohydride for Synergistic Hydrogen-Photothermal Antibacterial and Wound-Healing Therapies. Adv. Funct. Mater. 2019, 29, 1905697. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H.; Tu, Q.; He, Q.; Huang, N. New Approaches for Hydrogen Therapy of Various Diseases. Curr. Pharm. Des. 2021, 27, 636–649. [Google Scholar] [CrossRef]
- Zhao, P.; Jin, Z.; Chen, Q.; Yang, T.; Chen, D.; Meng, J.; Lu, X.; Gu, Z.; He, Q. Local Generation of Hydrogen for Enhanced Photothermal Therapy. Nat. Commun. 2018, 9, 4241. [Google Scholar] [CrossRef] [PubMed]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Layered Composite Based on Halloysite and Natural Polymers: A Carrier for the pH Controlled Release of Drugs. New J. Chem. 2019, 43, 10887–10893. [Google Scholar] [CrossRef]
- Thornton, P.D.; Mart, R.J.; Ulijn, R.V. Enzyme-Responsive Polymer Hydrogel Particles for Controlled Release. Adv. Mater. 2007, 19, 1252–1256. [Google Scholar] [CrossRef]
- Hsu, P.-H.; Almutairi, A. Recent Progress of Redox-Responsive Polymeric Nanomaterials for Controlled Release. J. Mater. Chem. B 2021, 9, 2179–2188. [Google Scholar] [CrossRef]
- Yang, Z.; Song, J.; Tang, W.; Fan, W.; Dai, Y.; Shen, Z.; Lin, L.; Cheng, S.; Liu, Y.; Niu, G. Stimuli-Responsive Nanotheranostics for Real-Time Monitoring Drug Release by Photoacoustic Imaging. Theranostics 2019, 9, 526. [Google Scholar] [CrossRef]
- Li, X.; Bottini, M.; Zhang, L.; Zhang, S.; Chen, J.; Zhang, T.; Liu, L.; Rosato, N.; Ma, X.; Shi, X. Core–Satellite Nanomedicines for In Vivo Real-Time Monitoring of Enzyme-Activatable Drug Release by Fluorescence and Photoacoustic Dual-Modal Imaging. ACS Nano 2018, 13, 176–186. [Google Scholar] [CrossRef]
- Duan, Z.; Gao, Y.-J.; Qiao, Z.-Y.; Fan, G.; Liu, Y.; Zhang, D.; Wang, H. A Photoacoustic Approach for Monitoring the Drug Eelease of pH-Sensitive Poly (β-amino ester) s. J. Mater. Chem. B 2014, 2, 6271–6282. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).