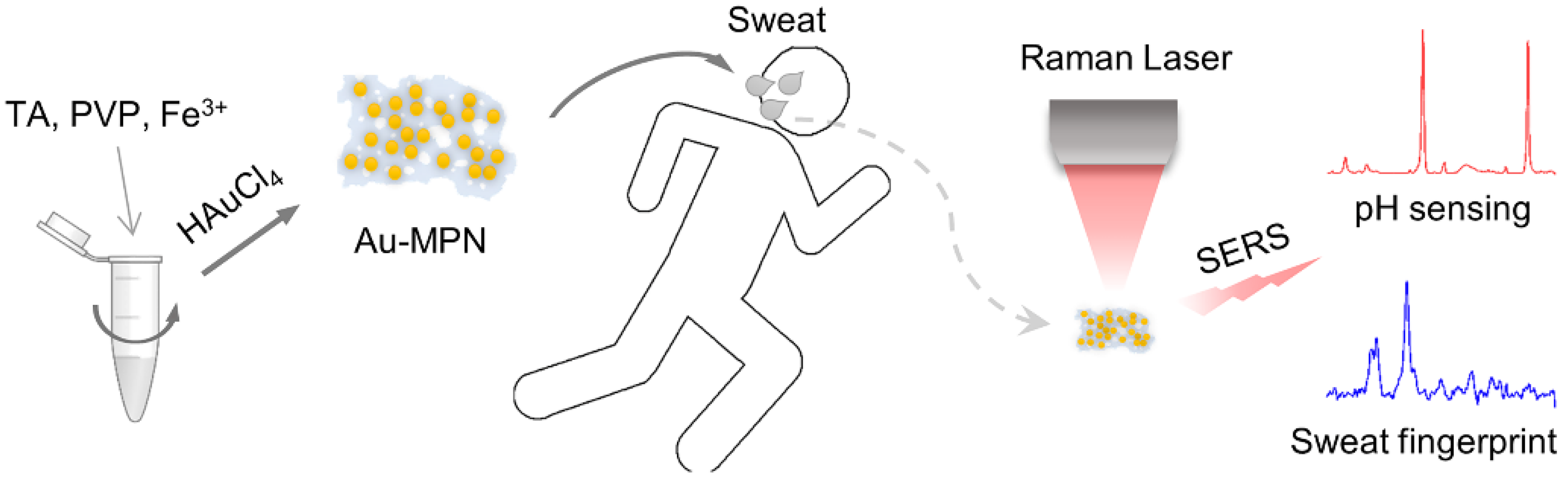
Fast Synthesis of Au Nanoparticles on Metal–Phenolic Network for Sweat SERS Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of MPN
2.3. Preparation of Au-MPN
2.4. Preparation of pH SERS Probe
2.5. Characterization
2.6. SERS Experiments
2.7. SERS Fingerprint Analysis of Sweat
2.8. SERS pH Detection of Sweat
3. Results and Discussion
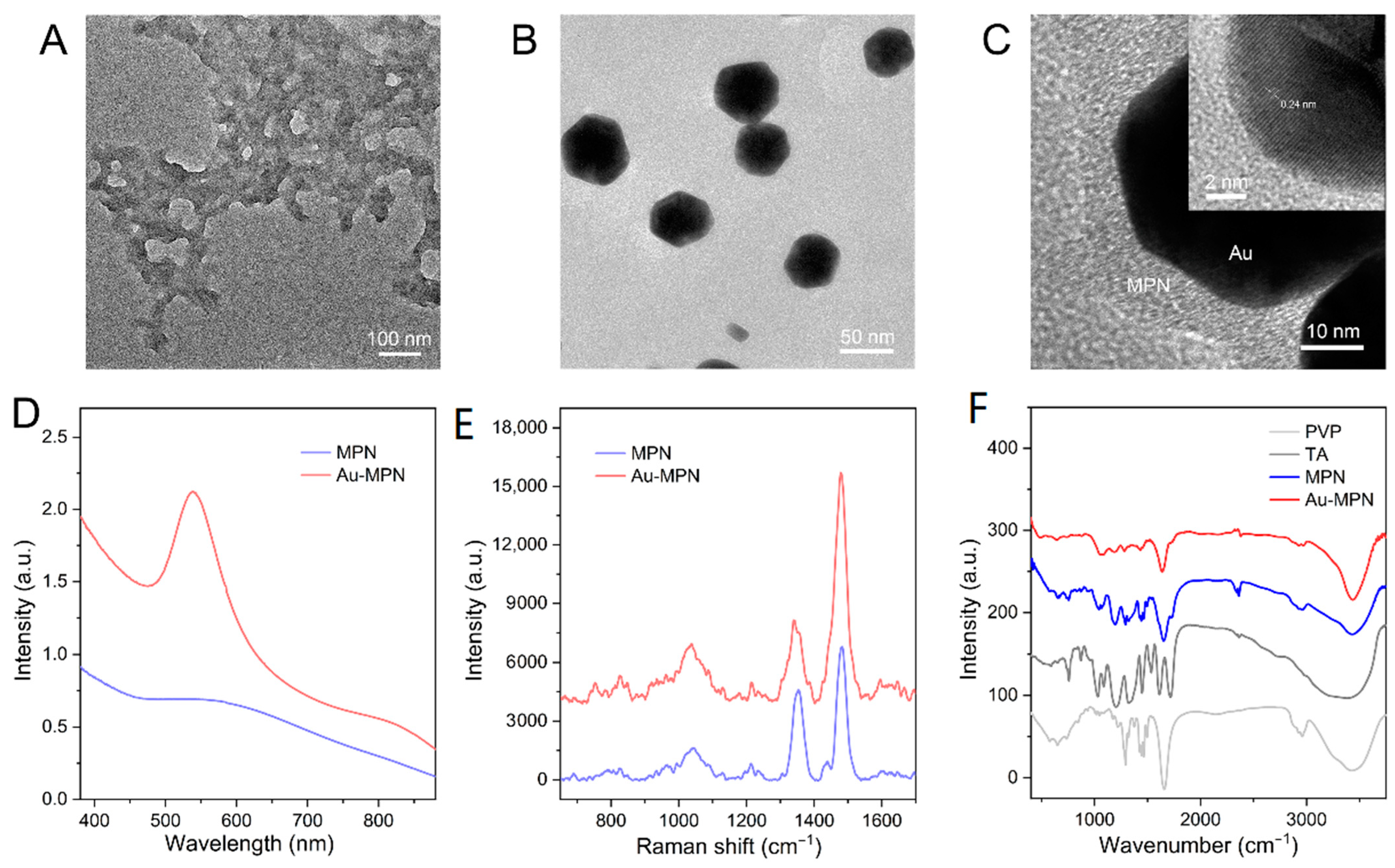
3.1. Characterization of the Nanostructures
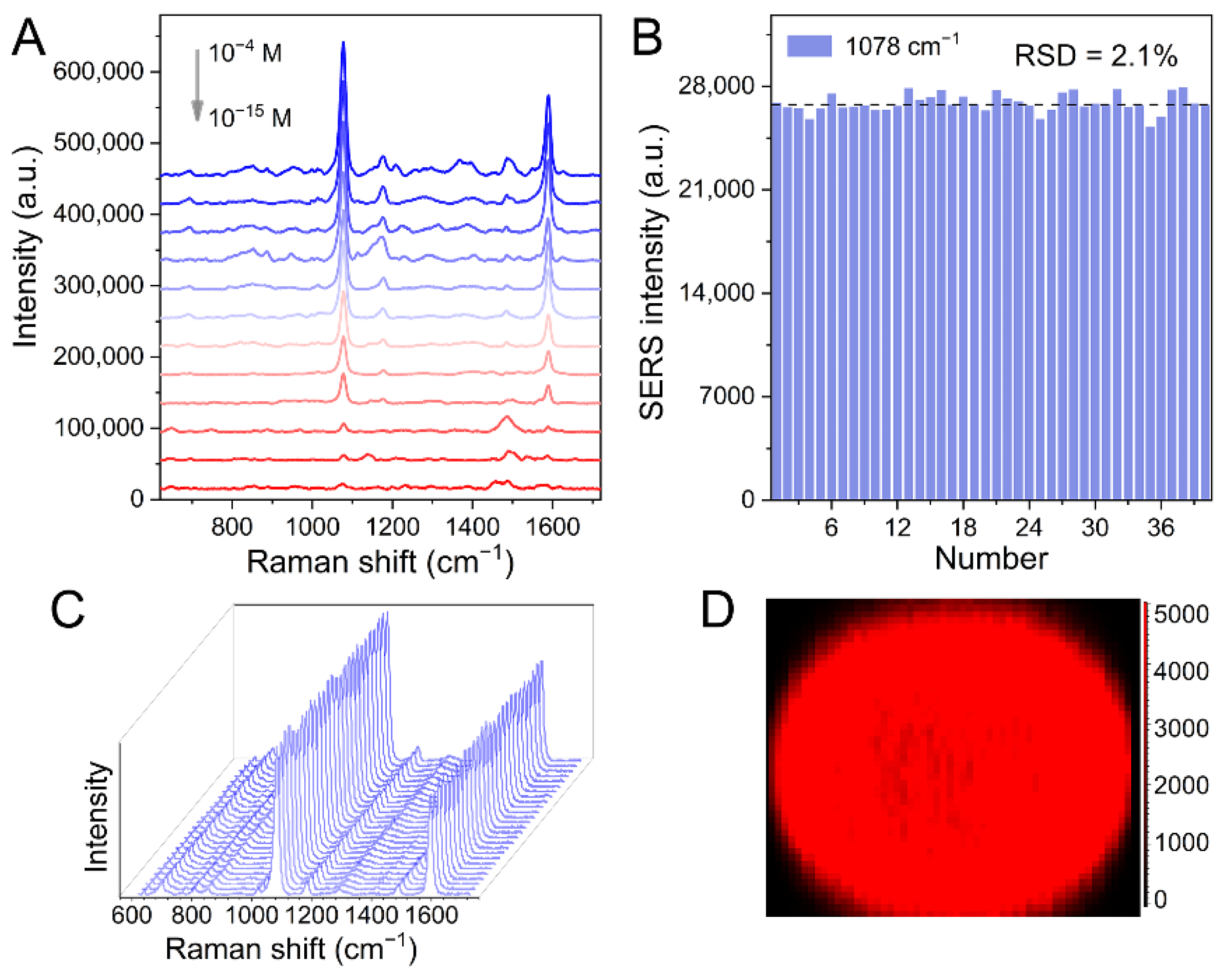
3.2. SERS Activities of Au-MPN
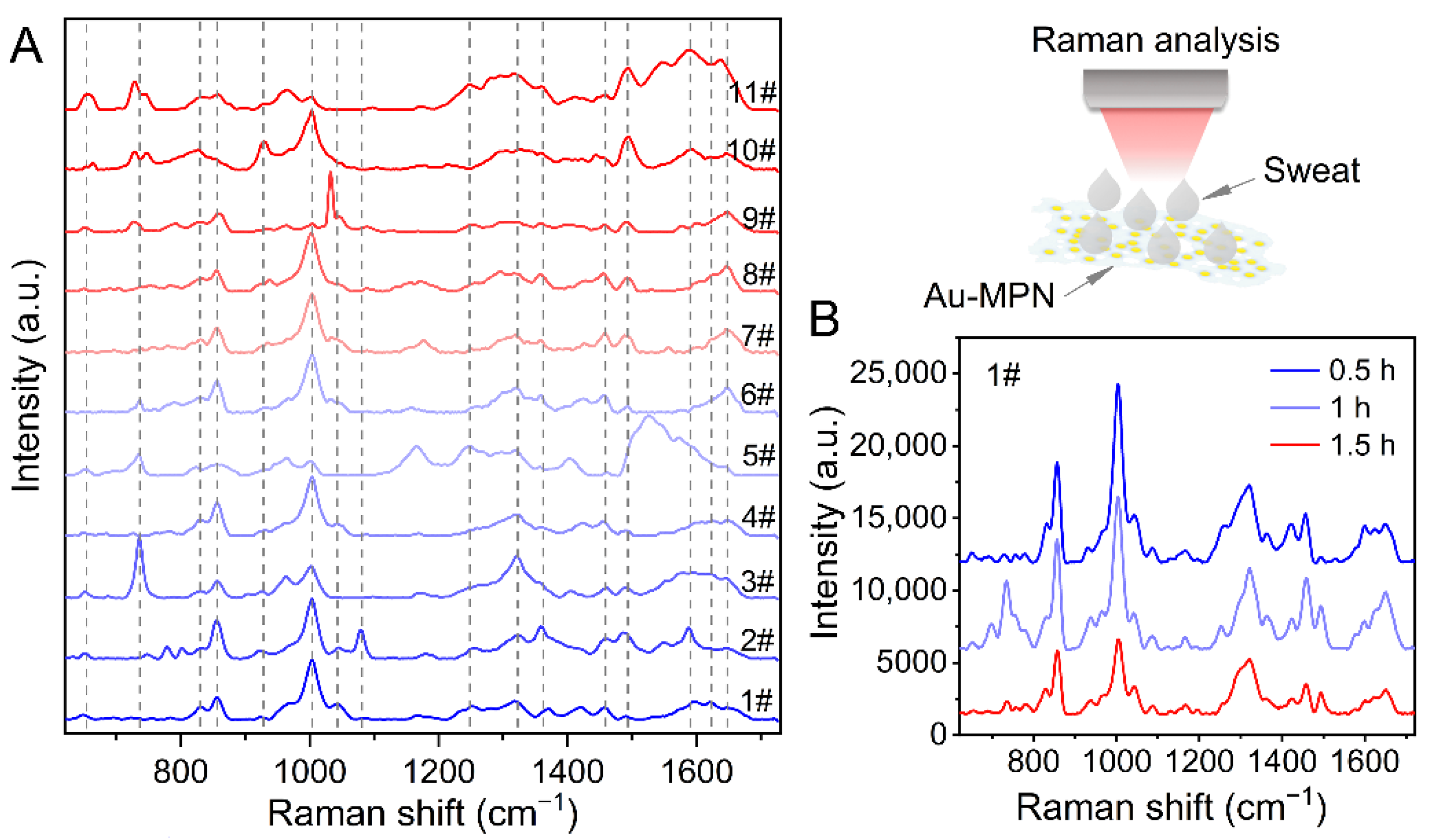
3.3. Molecular Fingerprint of Human Sweat Induced by Au-MPN
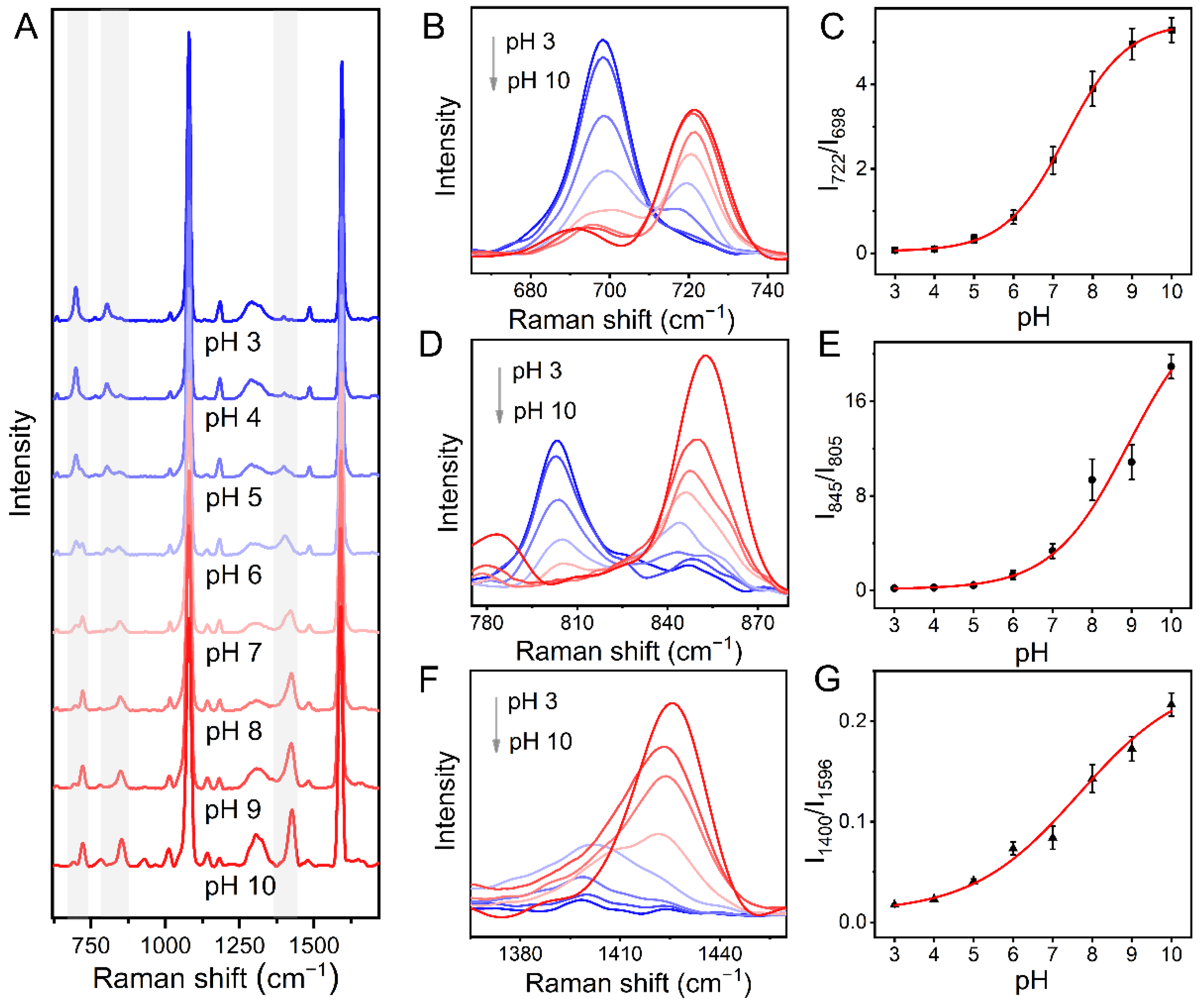
3.4. pH Sensing Based on Au-MPN
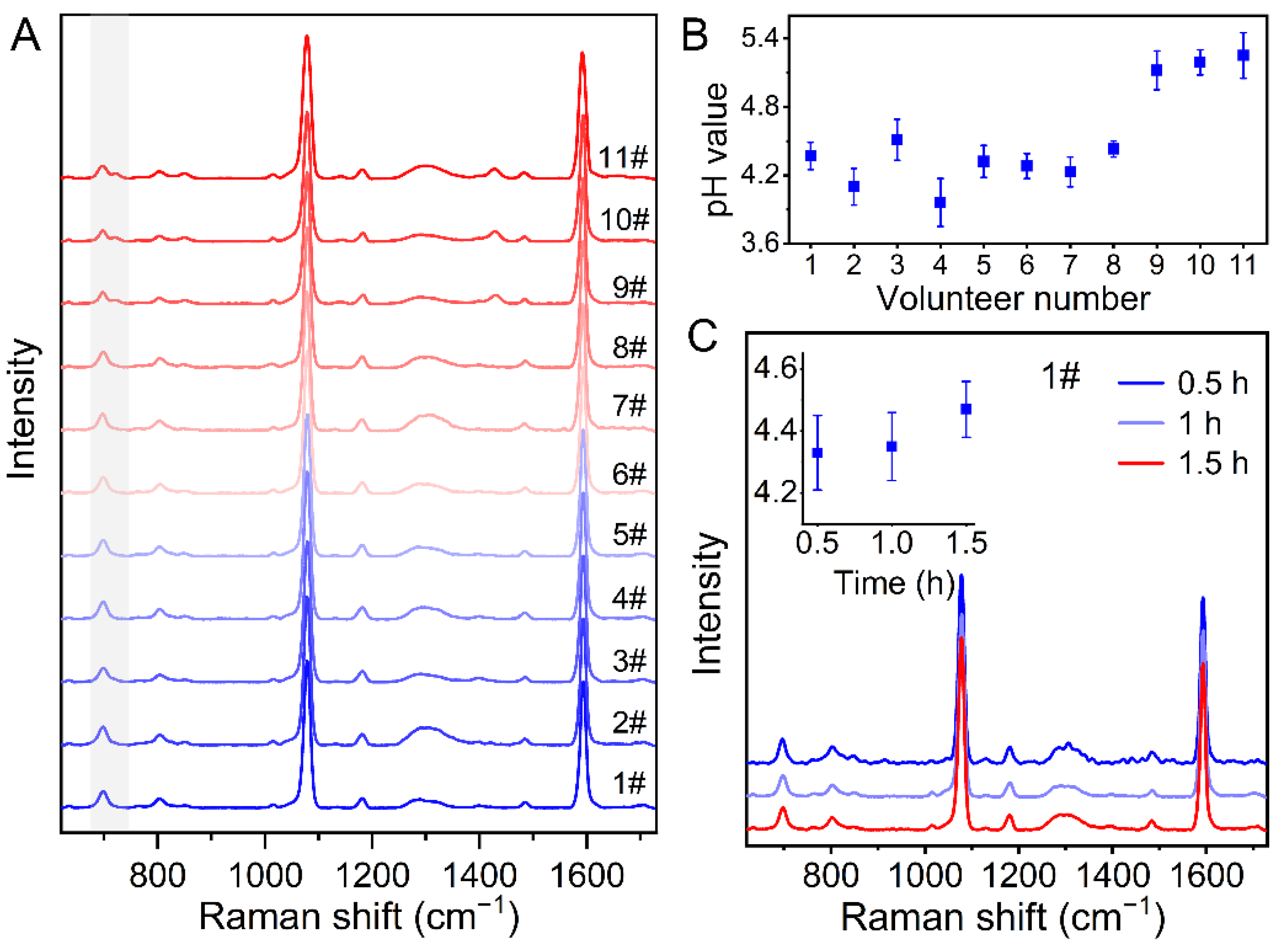
3.5. pH Monitoring of Human Sweat after Exercise
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.-L.; Kuan, W.-H.; Liu, C.-L. Comparative Study of the Composition of Sweat from Eccrine and Apocrine Sweat Glands During Exercise and in Heat. Int. J. Environ. Res. Public Health 2020, 17, 3377. [Google Scholar] [CrossRef] [PubMed]
- Bourque, C.W. Central Mechanisms of Osmosensation and Systemic Osmoregulation. Nat. Rev. Neurosci. 2008, 9, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Saat, M.; Sirisinghe, R.G.; Singh, R.; Tochihara, Y. Effects of Short-Term Exercise in the Heat on Thermoregulation, Blood Parameters, Sweat Secretion and Sweat Composition of Tropic-Dwelling Subjects. J. Physiol. Anthropol. Appl. Hum. Sci. 2005, 24, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.M.; Patterson, M.J.; Nimmo, M.A. Acute Effects of Dehydration on Sweat Composition in Men during Prolonged Exercise in the Heat. Acta Psychiatr. Scand. 2004, 182, 37–43. [Google Scholar] [CrossRef]
- Jiang, D.; Xu, C.; Zhang, Q.; Ye, Y.; Cai, Y.; Li, K.; Li, Y.; Huang, X.; Wang, Y. In-Situ Preparation of Lactate-Sensing Membrane for the Noninvasive and Wearable Analysis of Sweat. Biosens. Bioelectron. 2022, 210, 114303. [Google Scholar] [CrossRef]
- Steijlen, A.S.M.; Jansen, K.M.B.; Bastemeijer, J.; French, P.J.; Bossche, A. Low-Cost Wearable Fluidic Sweat Collection Patch for Continuous Analyte Monitoring and Offline Analysis. Anal. Chem. 2022, 94, 6893–6901. [Google Scholar] [CrossRef]
- Liao, J.; Zhang, X.; Sun, Z.; Chen, H.; Fu, J.; Si, H.; Ge, C.; Lin, S. Laser-Induced Graphene-Based Wearable Epidermal Ion-Selective Sensors for Noninvasive Multiplexed Sweat Analysis. Biosensors 2022, 12, 397. [Google Scholar] [CrossRef]
- Nunes, M.J.; Cordas, C.M.; Moura, J.J.G.; Noronha, J.P.; Branco, L.C. Screening of Potential Stress Biomarkers in Sweat Associated with Sports Training. Sports Med. Open 2021, 7, 8. [Google Scholar] [CrossRef]
- Harshman, S.W.; Browder, A.B.; Davidson, C.N.; Pitsch, R.L.; Strayer, K.E.; Schaeublin, N.M.; Phelps, M.S.; O’Connor, M.L.; Mackowski, N.S.; Barrett, K.N.; et al. The Impact of Nutritional Supplementation on Sweat Metabolomic Content: A Proof-of-Concept Study. Front. Chem. 2021, 9, 659583. [Google Scholar] [CrossRef]
- Patterson, M.J.; Galloway, S.D.R.; Nimmo, M.A. Effect of Induced Metabolic Alkalosis on Sweat Composition in Men. Acta Psychiatr. Scand. 2002, 174, 41–46. [Google Scholar] [CrossRef]
- Patterson, M.J.; Galloway, S.D.R.; Nimmo, M.A. Variations in Regional Sweat Composition in Normal Human Males. Exp.Physiol. 2000, 85, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Bennet, D.; Khorsandian, Y.; Pelusi, J.; Mirabella, A.; Pirrotte, P.; Zenhausern, F. Molecular and Physical Technologies for Monitoring Fluid and Electrolyte Imbalance: A Focus on Cancer Population. Clin. Trans. Med. 2021, 11, e461. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Li, J.; Xu, H.; Xu, Z.; Meng, J.; Chen, X.; Li, F. Scientific Athletics Training: Flexible Sensors and Wearable Devices for Kineses Monitoring Applications. Sci. Sin. Inform. 2022, 52, 54–74. [Google Scholar] [CrossRef]
- Chung, M.; Skinner, W.H.; Robert, C.; Campbell, C.J.; Rossi, R.M.; Koutsos, V.; Radacsi, N. Fabrication of a Wearable Flexible Sweat pH Sensor Based on SERS-Active Au/TPU Electrospun Nanofibers. ACS Appl. Mater. Interfaces 2021, 13, 51504–51518. [Google Scholar] [CrossRef] [PubMed]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; van Koeverden, M.P.; Such, G.K.; Cui, J.; Caruso, F. One-Step Assembly of Coordination Complexes for Versatile Film and Particle Engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef]
- Chen, J.; Pan, S.; Zhou, J.; Lin, Z.; Qu, Y.; Glab, A.; Han, Y.; Richardson, J.J.; Caruso, F. Assembly of Bioactive Nanoparticles via Metal–Phenolic Complexation. Adv. Mater. 2022, 34, 2108624. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, L.; Ju, Y.; Dai, Y. Recent Advances in Metal–Phenolic Networks for Cancer Theranostics. Small 2021, 17, 2100314. [Google Scholar] [CrossRef]
- Xie, L.; Li, J.; Wang, G.; Sang, W.; Xu, M.; Li, W.; Yan, J.; Li, B.; Zhang, Z.; Zhao, Q.; et al. Phototheranostic Metal–Phenolic Networks with Antiexosomal PD-L1 Enhanced Ferroptosis for Synergistic Immunotherapy. J. Am. Chem. Soc. 2022, 144, 787–797. [Google Scholar] [CrossRef]
- Xu, C.; Peng, C.; Yang, X.; Zhang, R.; Zhao, Z.; Yan, B.; Zhang, J.; Gong, J.; He, X.; Kwok, R.T.K.; et al. One-Pot Synthesis of Customized Metal–Phenolic-Network-Coated Aie Dots for in Vivo Bioimaging. Adv. Sci. 2022, 9, 2104997. [Google Scholar] [CrossRef]
- Zhou, M.; Zhao, C.; Li, Y.; Guo, Y.; Liu, H.; Zhang, Y.; Liu, Z. Facile Synthesis of Metal-Phenolic-Coated Gold Nanocuboids for Surface-Enhanced Raman Scattering. Appl. Opt. 2020, 59, 6124–6130. [Google Scholar] [CrossRef]
- Guo, J.; Ping, Y.; Ejima, H.; Alt, K.; Meissner, M.; Richardson, J.J.; Yan, Y.; Peter, K.; von Elverfeldt, D.; Hagemeyer, C.E.; et al. Engineering Multifunctional Capsules through the Assembly of Metal–Phenolic Networks. Angew. Chem. Int. Ed. 2014, 53, 5546–5551. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhou, J.; Qu, Y.; Pan, S.; Han, Y.; Lafleur, R.P.M.; Chen, J.; Cortez-Jugo, C.; Richardson, J.J.; Caruso, F. Luminescent Metal-Phenolic Networks for Multicolor Particle Labeling. Angew. Chem. Int. Ed. 2021, 60, 24968–24975. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Meng, J.; Chen, S.; Zhang, S.; Liu, T.; Li, C.; Wang, F. A Soft Metal-Polyphenol Capsule-Based Ultrasensitive Immunoassay for Electrochemical Detection of Epstein-Barr (EB) Virus Infection. Biosens. Bioelectron. 2020, 164, 112310. [Google Scholar] [CrossRef] [PubMed]
- Yun, G.; Besford, Q.A.; Johnston, S.T.; Richardson, J.J.; Pan, S.; Biviano, M.; Caruso, F. Self-Assembly of Nano- to Macroscopic Metal–Phenolic Materials. Chem. Mater. 2018, 30, 5750–5758. [Google Scholar] [CrossRef]
- Yun, G.; Pan, S.; Wang, T.-Y.; Guo, J.; Richardson, J.J.; Caruso, F. Synthesis of Metal Nanoparticles in Metal-Phenolic Networks: Catalytic and Antimicrobial Applications of Coated Textiles. Adv. Healthc. Mater. 2018, 7, 1700934. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Lu, J.; Xie, W.; Li, X.; Wu, H.; Zhao, L. Facile Preparation of Recyclable Fe@Metal Phenolic Networks-Au System for Catalytic Reduction of 4-Nitrophenol. Mater. Chem. Phys. 2022, 281, 125907. [Google Scholar] [CrossRef]
- Yun, G.; Richardson, J.J.; Capelli, M.; Hu, Y.; Besford, Q.A.; Weiss, A.C.G.; Lee, H.; Choi, I.S.; Gibson, B.C.; Reineck, P.; et al. The Biomolecular Corona in 2D and Reverse: Patterning Metal–Phenolic Networks on Proteins, Lipids, Nucleic Acids, Polysaccharides, and Fingerprints. Adv. Funct. Mater. 2020, 30, 1905805. [Google Scholar] [CrossRef]
- Sun, J.; Xue, D.; Shan, W.; Liu, R.; Liu, R.; Zhao, H.; Li, T.; Wang, Z.; Zhang, J.; Shao, B. In Situ Growth Large Area Silver Nanostructure on Metal Phenolic Network Coated Nano Film and Its SERS Sensing Application for Monofluoroacetic Acid. ACS Sens. 2021, 6, 2129–2135. [Google Scholar] [CrossRef]
- Wang, X.; Huang, S.-C.; Hu, S.; Yan, S.; Ren, B. Fundamental Understanding and Applications of Plasmon-Enhanced Raman Spectroscopy. Nat. Rev. Phys. 2020, 2, 253–271. [Google Scholar] [CrossRef]
- Choi, H.K.; Lee, K.S.; Shin, H.H.; Koo, J.J.; Yeon, G.J.; Kim, Z.H. Single-Molecule Surface-Enhanced Raman Scattering as a Probe of Single-Molecule Surface Reactions: Promises and Current Challenges. Acc. Chem. Res. 2019, 52, 3008–3017. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguie, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Yin, L.; Huang, Q.; You, R.; Feng, S.; Lu, Y. An Endoscope-Like SERS Probe Based on the Focusing Effect of Silica Nanospheres for Tyrosine and Urea Detection in Sweat. Nanomaterials 2022, 12, 421. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, H.J.; Lee, J.; Lee, T.; Yun, J.; Lee, G.; Hong, Y. Hand-Held Raman Spectrometer-Based Dual Detection of Creatinine and Cortisol in Human Sweat Using Silver Nanoflakes. Anal. Chem. 2021, 93, 14996–15004. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Cui, Q.; Xu, L.; Tian, Y.; Jiao, A.; Wang, C.; Zhang, M.; Li, S.; Chen, M. Silk Fibroin Fibers Decorated with Urchin-Like Au/Ag Nanoalloys: A Flexible Hygroscopic SERS Sensor for Monitoring of Folic Acid in Human Sweat. Opt. Express 2021, 29, 30892–30904. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, C.; Wang, J.; Luo, X.; Xie, L.; Zhan, S.; Kim, J.; Wang, X.; Liu, X.; Ying, Y. Wearable Plasmonic-Metasurface Sensor for Noninvasive and Universal Molecular Fingerprint Detection on Biointerfaces. Sci. Adv. 2021, 7, eabe4553. [Google Scholar] [CrossRef]
- Mogera, U.; Guo, H.; Namkoong, M.; Rahman, M.S.; Nguyen, T.; Tian, L. Wearable Plasmonic Paper-Based Microfluidics for Continuous Sweat Analysis. Sci. Adv. 2022, 8, eabn1736. [Google Scholar] [CrossRef]
- Koh, E.H.; Lee, W.-C.; Choi, Y.-J.; Moon, J.-I.; Jang, J.; Park, S.-G.; Choo, J.; Kim, D.-H.; Jung, H.S. A Wearable Surface-Enhanced Raman Scattering Sensor for Label-Free Molecular Detection. ACS Appl. Mater. Interfaces 2021, 13, 3024–3032. [Google Scholar] [CrossRef]
- Saroj, A.L.; Singh, R.K.; Chandra, S. Studies on Polymer Electrolyte Poly(Vinyl) Pyrrolidone (PVP) Complexed with Ionic Liquid: Effect of Complexation on Thermal Stability, Conductivity and Relaxation Behaviour. Mater. Sci. Eng. B 2013, 178, 231–238. [Google Scholar] [CrossRef]
- Xu, W.; Pan, S.; Noble, B.B.; Chen, J.; Lin, Z.; Han, Y.; Zhou, J.; Richardson, J.J.; Yarovsky, I.; Caruso, F. Site-Selective Coordination Assembly of Dynamic Metal-Phenolic Networks. Angew. Chem. Int. Ed. 2022, 61, e202208037. [Google Scholar] [CrossRef]
- Sikirzhytski, V.; Sikirzhytskaya, A.; Lednev, I.K. Multidimensional Raman Spectroscopic Signature of Sweat and Its Potential Application to Forensic Body Fluid Identification. Anal. Chim. Acta 2012, 718, 78–83. [Google Scholar] [CrossRef]
- Westley, C.; Xu, Y.; Thilaganathan, B.; Carnell, A.J.; Turner, N.J.; Goodacre, R. Absolute Quantification of Uric Acid in Human Urine Using Surface Enhanced Raman Scattering with the Standard Addition Method. Anal. Chem. 2017, 89, 2472–2477. [Google Scholar] [CrossRef] [PubMed]
- Olaetxea, I.; Valero, A.; Lopez, E.; Lafuente, H.; Izeta, A.; Jaunarena, I.; Seifert, A. Machine Learning-Assisted Raman Spectroscopy for pH and Lactate Sensing in Body Fluids. Anal. Chem. 2020, 92, 13888–13895. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Naqvi, T.K.; Tripathi, S.K.; Kulkarni, M.M.; Prasad, N.E.; Dwivedi, P.K. Plasmonic Paper-Based Flexible Sers Biosensor for Highly Sensitive Detection of Lactic and Uric Acid. IEEE Trans. Nanobiosci. 2022, 21, 294–300. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Guo, Y.; Wang, K.; Zhang, Y.; Lan, P.; Guo, J.; Zhang, W.; Zhong, H.; Guo, Z.; et al. Lamellar Hafnium Ditelluride as an Ultrasensitive Surface-Enhanced Raman Scattering Platform for Label-Free Detection of Uric Acid. Photon. Res. 2021, 9, 1039–1047. [Google Scholar] [CrossRef]
- Pallaoro, A.; Braun, G.B.; Reich, N.O.; Moskovits, M. Mapping Local pH in Live Cells Using Encapsulated Fluorescent SERS Nanotags. Small 2010, 6, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Talley, C.E.; Jusinski, L.; Hollars, C.W.; Lane, S.M.; Huser, T. Intracellular pH Sensors Based on Surface-Enhanced Raman Scattering. Anal. Chem. 2004, 76, 7064–7068. [Google Scholar] [CrossRef] [PubMed]
- Aina, V.; Marchis, T.; Laurenti, E.; Diana, E.; Lusvardi, G.; Malavasi, G.; Menabue, L.; Cerrato, G.; Morterra, C. Functionalization of Sol Gel Bioactive Glasses Carrying Au Nanoparticles: Selective Au Affinity for Amino and Thiol Ligand Groups. Langmuir 2010, 26, 18600–18605. [Google Scholar] [CrossRef]
- Schwartz-Duval, A.S.; Konopka, C.J.; Moitra, P.; Daza, E.A.; Srivastava, I.; Johnson, E.V.; Kampert, T.L.; Fayn, S.; Haran, A.; Dobrucki, L.W.; et al. Intratumoral Generation of Photothermal Gold Nanoparticles through a Vectorized Biomineralization of Ionic Gold. Nat. Commun. 2020, 11, 4530. [Google Scholar] [CrossRef]


| SERS Band/cm−1 | Assignment (Vibrational Mode) | Origin |
|---|---|---|
| 651 | O=C–N– bending | amino acid |
| 736 | N–H bending | uric acid |
| 830 | ρ(CH3) | amino acid, lactic acid |
| 856 | ρ(CH3) | amino acid, lactic acid |
| 927 | ν(C–CO2−), ν(C–CH3), δ(CO2−), ρ(CH3) | amino acid, lactic acid |
| 1003 | ν(C–N), aromatic ring vibration | urea, amino acid |
| 1045 | ν(C–C) | protein |
| 1080 | ν(C–O), δ(C–COH) | lactic acid |
| 1249 | δ(CH), amide III | protein |
| 1322 | δ(CH), amide III | protein |
| 1358 | ν(C–O) | uric acid |
| 1457 | δ(CH3) | lactic acid |
| 1493 | ν(C–N), ν(C–C) | uric acid |
| 1589 | ν(C–N), ν(CO2−) | uric acid, lactic acid |
| 1621 | ν4(NH2) | urea |
| 1648 | ν2(NH2) | urea |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, X.; Ning, M.; Wang, P.; Wang, W.; Zhang, X.; Liu, Z.; Zhang, Y.; Li, S. Fast Synthesis of Au Nanoparticles on Metal–Phenolic Network for Sweat SERS Analysis. Nanomaterials 2022, 12, 2977. https://doi.org/10.3390/nano12172977
Zhang X, Wang X, Ning M, Wang P, Wang W, Zhang X, Liu Z, Zhang Y, Li S. Fast Synthesis of Au Nanoparticles on Metal–Phenolic Network for Sweat SERS Analysis. Nanomaterials. 2022; 12(17):2977. https://doi.org/10.3390/nano12172977
Chicago/Turabian StyleZhang, Xiaoying, Xin Wang, Mengling Ning, Peng Wang, Wen Wang, Xiaozhou Zhang, Zhiming Liu, Yanjiao Zhang, and Shaoxin Li. 2022. "Fast Synthesis of Au Nanoparticles on Metal–Phenolic Network for Sweat SERS Analysis" Nanomaterials 12, no. 17: 2977. https://doi.org/10.3390/nano12172977
APA StyleZhang, X., Wang, X., Ning, M., Wang, P., Wang, W., Zhang, X., Liu, Z., Zhang, Y., & Li, S. (2022). Fast Synthesis of Au Nanoparticles on Metal–Phenolic Network for Sweat SERS Analysis. Nanomaterials, 12(17), 2977. https://doi.org/10.3390/nano12172977






