Synthesis of MnSe-Based GO Composites as Effective Photocatalyst for Environmental Remediations
Abstract
1. Introduction
2. Preparation of the Samples
2.1. GO Preparation
2.2. MnSe Preparation
2.3. GO-MnSe Nanocomposites
2.4. Characterization
2.5. Photocatalytic Activity
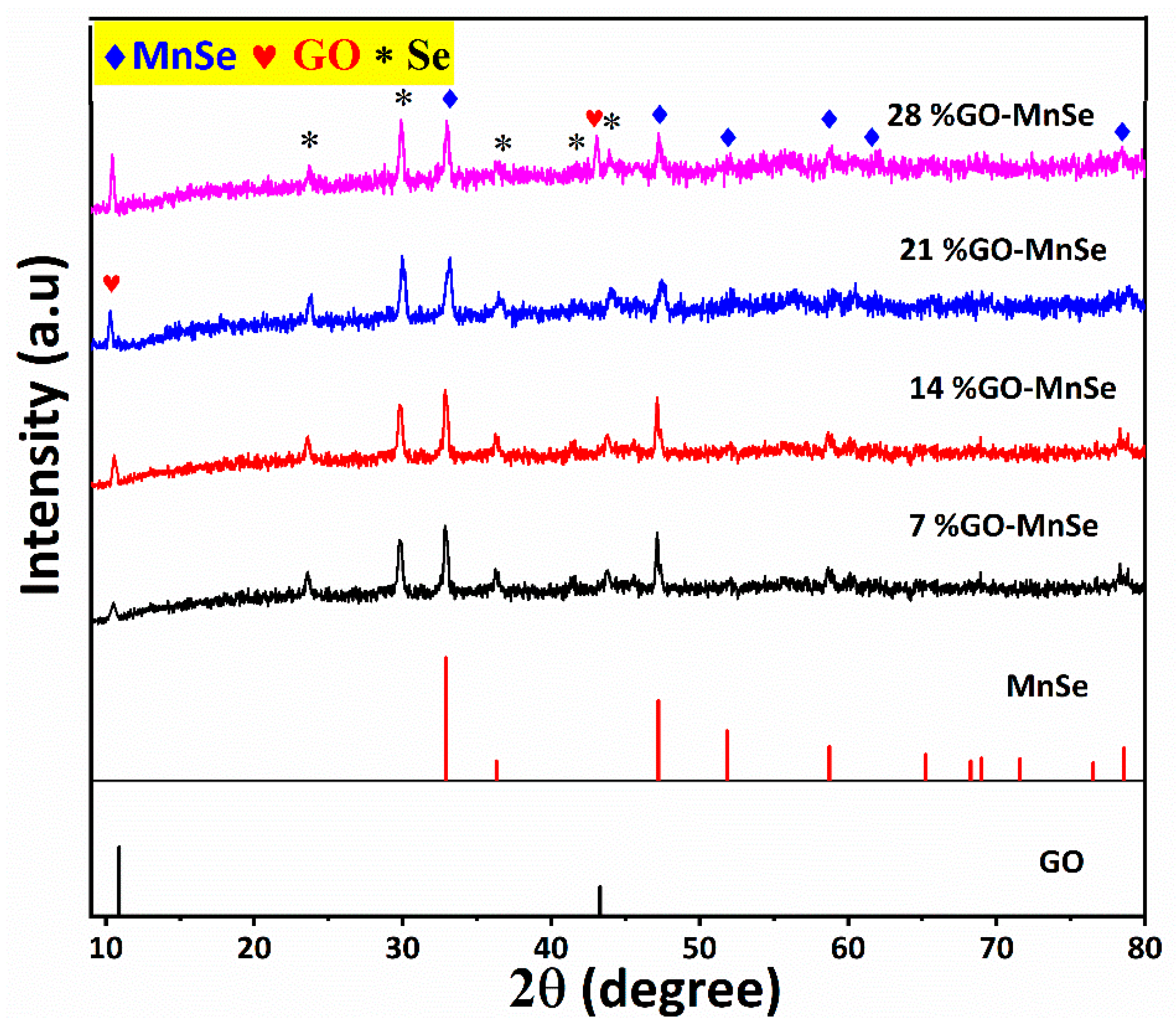
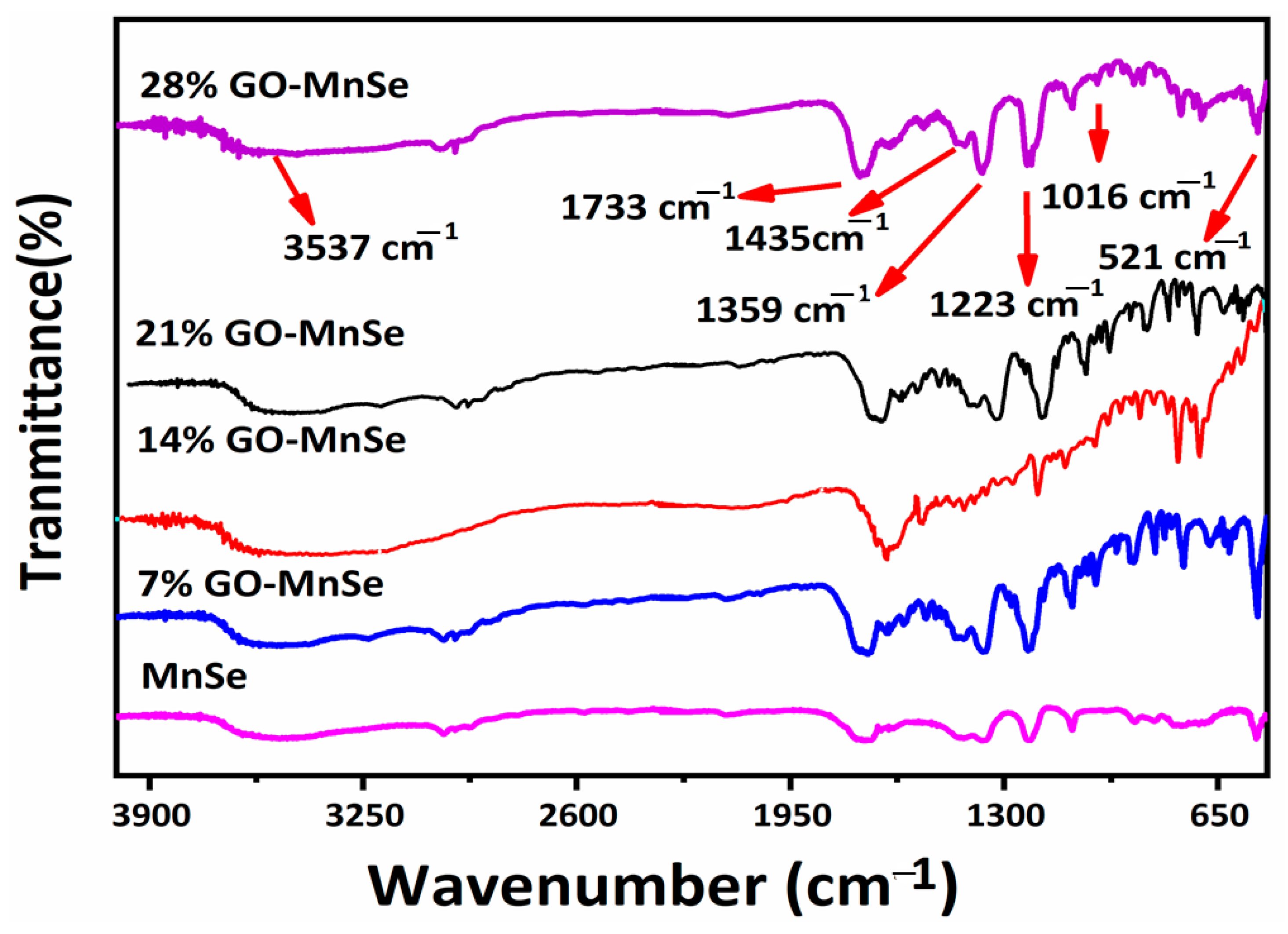
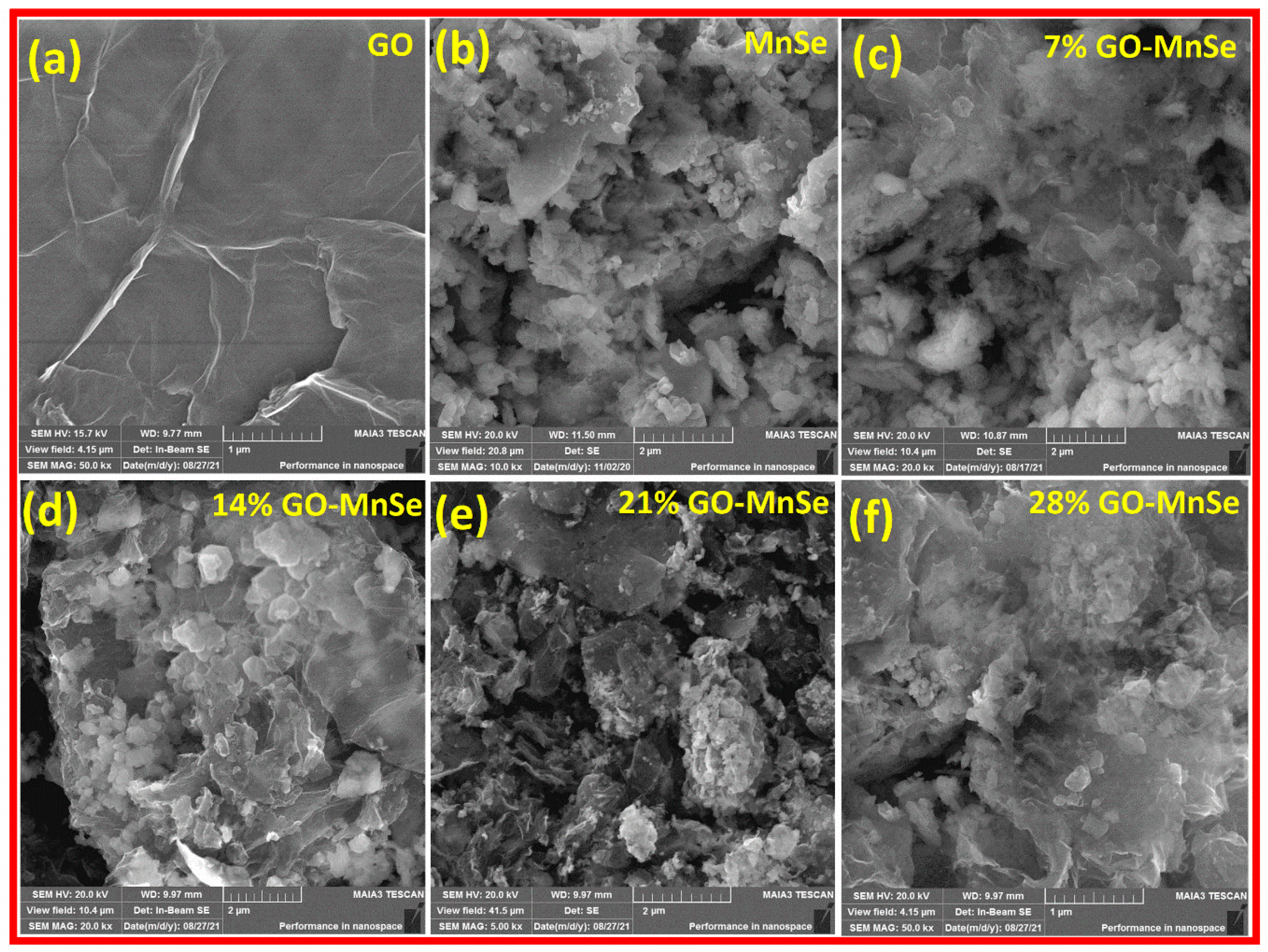
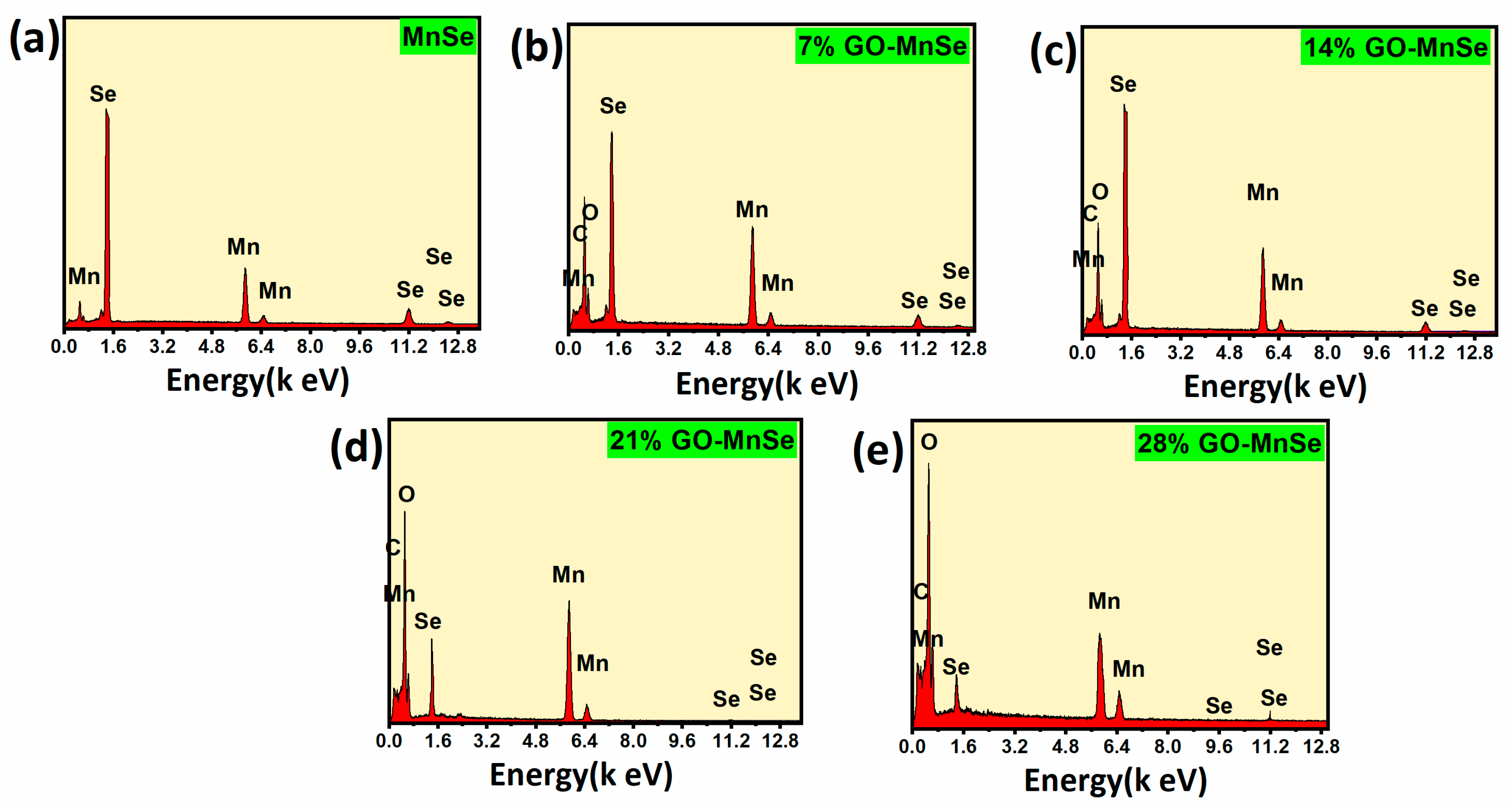
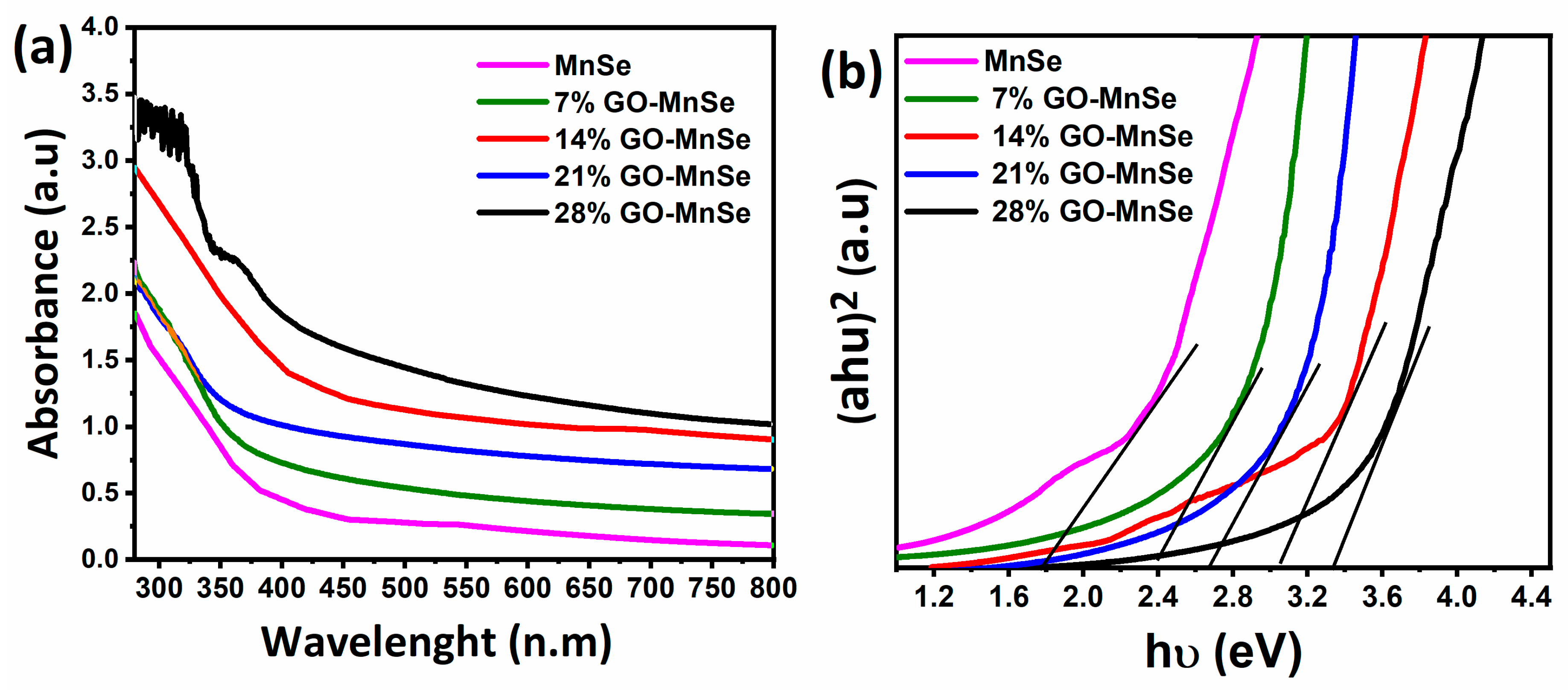
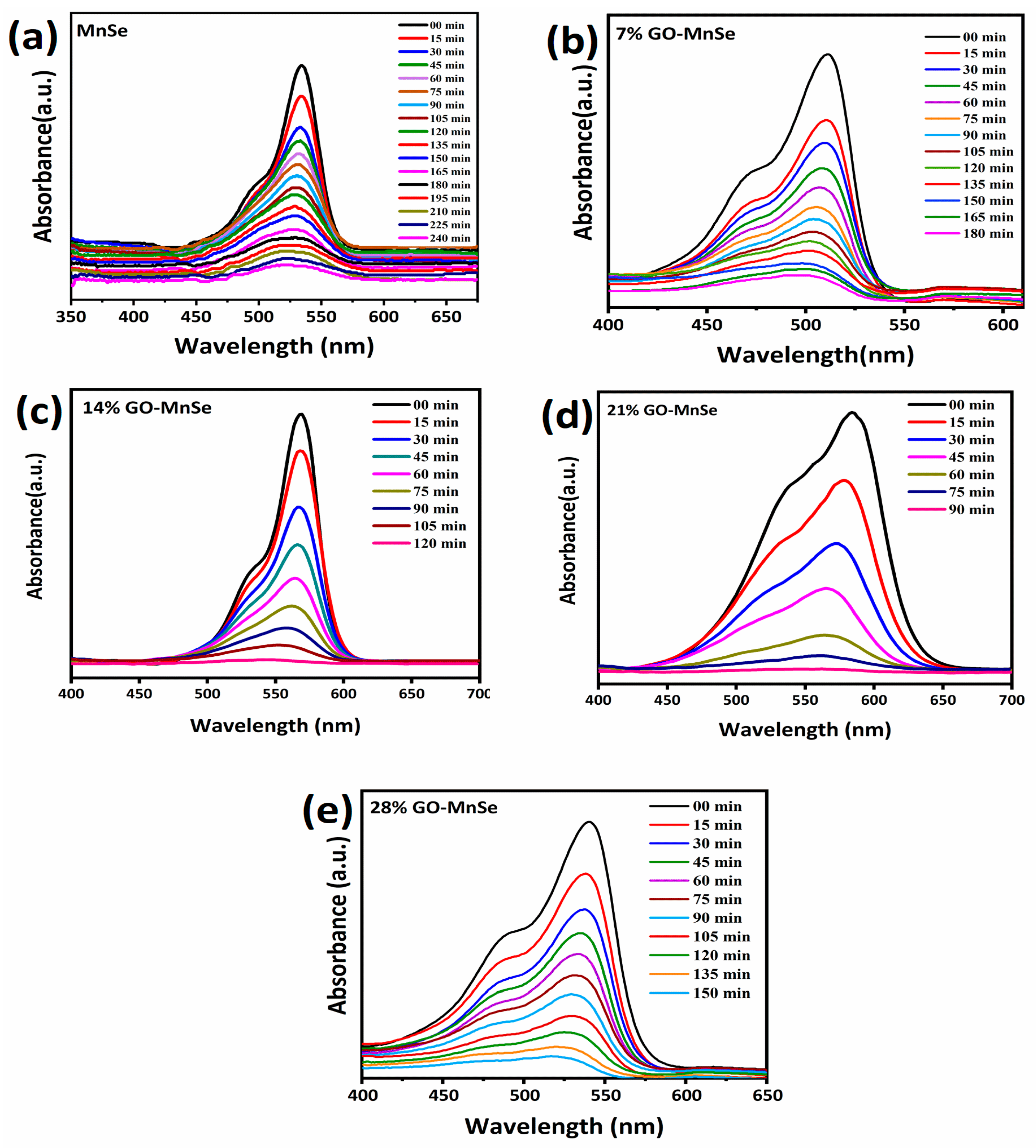
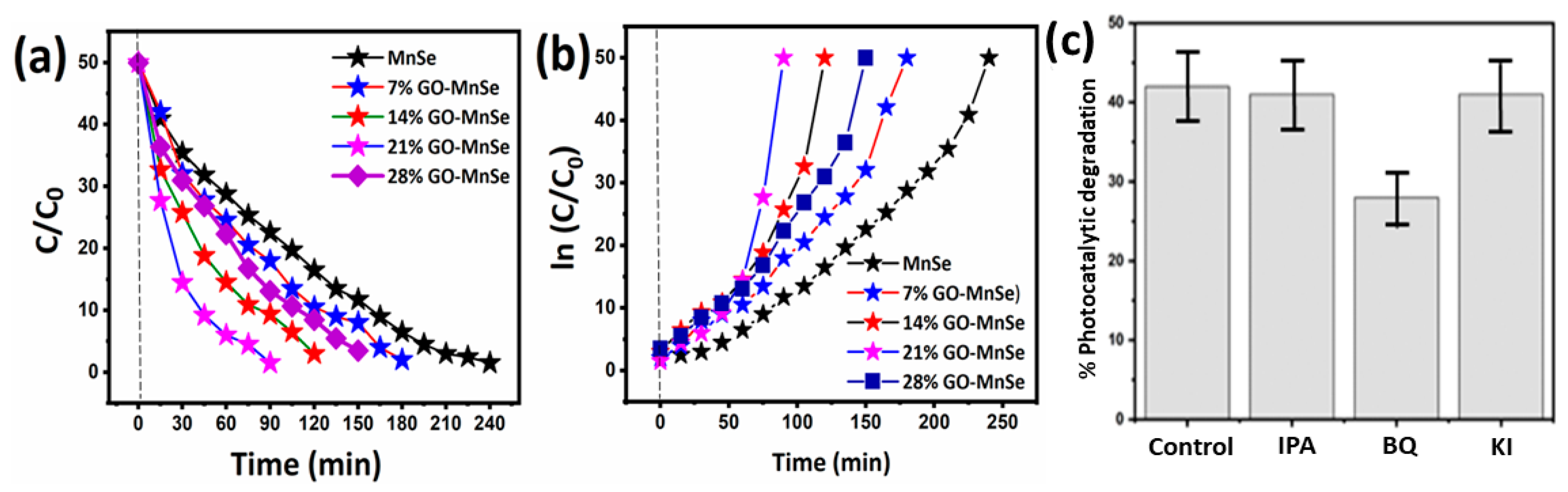
3. Results and Discussion
- The covalent MB molecules might connect to huge aromatic domains on GO layers via p-p stacking, favoring a significant increase in reactivity [60] and efficiently improving light absorption in the visible region.
- Our excellent photocatalytic and repeatable performance might be attributed to the tight coupling between MnSe and GO, which enhances interface charge transfer (in GO as an acceptor) and prevents electron-hole coupling [61].
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shan, C.; Liu, Z.; Hark, S. CdSe nanowires with controllable growth orientations. Appl. Phys. Lett. 2007, 90, 193123. [Google Scholar] [CrossRef]
- Matsune, K.; Oda, H.; Toyama, T.; Okamoto, H.; Kudriavysevand, Y.; Asomoza, R. 15% Efficiency CdS/CdTe thin film solar cells using CdS layers doped with metal organic compounds. Sol. Energy Mater. Sol. Cells 2006, 90, 3108–3114. [Google Scholar] [CrossRef]
- Duan, X.; Huang, Y.; Agarwal, R.; Lieber, C.M. Single-nanowire electrically driven lasers. Nature 2003, 421, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.-F. Effects of quantum coupling on the performance of metal-oxide-semiconductor field transistors. Pramana 2009, 72, 407–414. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Zeng, J.; Shi, Y.; Lin, S.; Huang, G.; Wang, H.; Kong, Z.; Xi, J.; Ji, Z. MnS coupled with ultrathin MoS2 nanolayers as heterojunction photocatalyst for high photocatalytic and photoelectrochemical activities. J. Alloys Compd. 2019, 771, 364–372. [Google Scholar] [CrossRef]
- Che, H.; Che, G.; Jiang, E.; Liu, C.; Dong, H.; Li, C. A novel Z-Scheme CdS/Bi3O4Cl heterostructure for photocatalytic degradation of antibiotics: Mineralization activity, degradation pathways and mechanism insight. J. Taiwan Inst. Chem. Eng. 2018, 91, 224–234. [Google Scholar] [CrossRef]
- Tariq, F.; Hussain, R.; Noreen, Z.; Javed, A.; Shah, A.; Mahmood, A.; Sajjad, M.; Bokhari, H.; ur Rahman, S. Enhanced antibacterial activity of visible light activated sulfur-doped TiO2 nanoparticles against Vibrio cholerae. Mater. Sci. Semicond. Process. 2022, 147, 106731. [Google Scholar] [CrossRef]
- Xie, Z.; Zhou, Y.; Guan, L.; Muhammad, S.; Jiang, Y.; Zhang, S.; Yu, C.; Jiao, Y.; Zhang, S.; Ren, Y. Effect of impurity in Cu2O nanowires on the degradation of methyl orange. J. Mater. Sci. Mater. Electron. 2020, 31, 3817–3824. [Google Scholar] [CrossRef]
- Lahmar, H.; Benamira, M.; Akika, F.Z.; Trari, M. Reduction of chromium (VI) on the hetero-system CuBi2O4/TiO2 under solar light. J. Phys. Chem. Solids 2017, 110, 254–259. [Google Scholar] [CrossRef]
- Slama, H.B.; Chenari Bouket, A.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Olisah, C.; Adams, J.B.; Rubidge, G. The state of persistent organic pollutants in South African estuaries: A review of environmental exposure and sources. Ecotoxicol. Environ. Saf. 2021, 219, 112316. [Google Scholar] [CrossRef] [PubMed]
- Amran, F.; Zaini, M.A.A. Green Nanocomposite Adsorbents for Dyes Removal. In Biorenewable Nanocomposite Materials, Vol. 2: Desalination and Wastewater Remediation; American Chemical Society: New York, NY, USA, 2022. [Google Scholar]
- American Chemical Society. ACS Symposium Series: Ibnu-Sina Institute for Scientific & Industrial Research, 81310 UTM Johor Bahru, Johor, Malaysia; American Chemical Society: New York, NY, USA, 2022; Volume 1411, pp. 165–188. [Google Scholar]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Birhanli, A.; Ozmen, M. Evaluation of the toxicity and teratogenity of six commercial textile dyes using the frog embryo teratogenesis assay-Xenopus. Drug Chem. Toxicol. 2005, 28, 51–65. [Google Scholar] [CrossRef]
- Chong, M.N.; Cho, Y.J.; Poh, P.E.; Jin, B. Evaluation of Titanium dioxide photocatalytic technology for the treatment of reactive Black 5 dye in synthetic and real greywater effluents. J. Clean. Prod. 2015, 89, 196–202. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, P.; Qian, J.; Ao, Y.; Wang, C.; Hou, J. Phosphate group grafted twinned BiPO4 with significantly enhanced photocatalytic activity: Synergistic effect of improved charge separation efficiency and redox ability. Appl. Catal. B Environ. 2018, 234, 90–99. [Google Scholar] [CrossRef]
- Kabra, K.; Chaudhary, R.; Sawhney, R.L. Treatment of Hazardous Organic and Inorganic Compounds through Aqueous-Phase Photocatalysis: A Review. Ind. Eng. Chem. Res. 2004, 43, 7683–7696. [Google Scholar] [CrossRef]
- Shao, B.; Liu, X.; Liu, Z.; Zeng, G.; Zhang, W.; Liang, Q.; Liu, Y.; He, Q.; Yuan, X.; Wang, D. Synthesis and characterization of 2D/0D g-C3N4/CdS-nitrogen doped hollow carbon spheres (NHCs) composites with enhanced visible light photodegradation activity for antibiotic. Chem. Eng. J. 2019, 374, 479–493. [Google Scholar] [CrossRef]
- Klimov, V.I. Detailed-balance power conversion limits of nanocrystal-quantum-dot solar cells in the presence of carrier multiplication. Appl. Phys. Lett. 2006, 89, 123118. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, Q.; Uchaker, E.; Gao, R.; Qu, X.; Zhang, S.; Cao, G. Architectured ZnO photoelectrode for high efficiency quantum dot sensitized solar cells. Energy Environ. Sci. 2013, 6, 3542–3547. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Sridevi, D.; Vadivel, R.; Perumal, S.; Sundaravadivel, E.; Sivaramakrishnan, V. A facile synthesis of Mn-doped ZnSe nanoparticles for an enhanced photocatalytic activity and biological applications. Ceram. Int. 2022, 48, 29394–29402. [Google Scholar]
- Cun, W.; Jincai, Z.; Xinming, W.; Bixian, M.; Guoying, S.; Ping’an, P.; Jiamo, F. Preparation, characterization and photocatalytic activity of nano-sized ZnO/SnO2 coupled photocatalysts. Appl. Catal. B Environ. 2002, 39, 269–279. [Google Scholar] [CrossRef]
- Wang, C.; Xu, B.-Q.; Wang, X.; Zhao, J. Preparation and photocatalytic activity of ZnO/TiO2/SnO2 mixture. J. Solid State Chem. 2005, 178, 3500–3506. [Google Scholar] [CrossRef]
- Shah, M.Z.U.; Sajjad, M.; Hou, H.; ur Rahman, S.; Mahmood, A.; Aziz, U.; Shah, A. A new CuO/TiO2 nanocomposite: An emerging and high energy efficient electrode material for aqueous asymmetric supercapacitors. J. Energy Storage 2022, 55, 105492. [Google Scholar] [CrossRef]
- Shah, M.Z.U.; Sajjad, M.; Hou, H.; ur Rahman, S.; Shah, A. Copper sulfide nanoparticles on titanium dioxide (TiO2) nanoflakes: A new hybrid asymmetrical Faradaic supercapacitors with high energy density and superior lifespan. J. Energy Storage 2022, 55, 105651. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y. Facile synthesis and photocatalytic activity of ZnO–CuO nanocomposite. Superlattices Microstruct. 2010, 47, 615–623. [Google Scholar] [CrossRef]
- Zhu, L.; Li, H.; Liu, Z.; Xia, P.; Xie, Y.; Xiong, D. Synthesis of the 0D/3D CuO/ZnO heterojunction with enhanced photocatalytic activity. J. Phys. Chem. C 2018, 122, 9531–9539. [Google Scholar] [CrossRef]
- BiBi, S.; Shah, M.Z.U.; Sajjad, M.; Shafi, H.Z.; Amin, B.; Bajaber, M.A.; Shah, A. A new ZnO-ZnS-CdS heterostructure on Ni substrate: A binder-free electrode for advanced asymmetric supercapacitors with improved performance. Electrochim. Acta 2022, 430, 141031. [Google Scholar] [CrossRef]
- Qiao, F.; Qi, Q.; Wang, Z.; Xu, K.; Ai, S. MnSe-loaded g-C3N4 nanocomposite with synergistic peroxidase-like catalysis: Synthesis and application toward colorimetric biosensing of H2O2 and glucose. Sens. Actuators B Chem. 2016, 229, 379–386. [Google Scholar] [CrossRef]
- Sobhani, A.; Salavati-Niasari, M. Transition metal selenides and diselenides: Hydrothermal fabrication, investigation of morphology, particle size and and their applications in photocatalyst. Adv. Colloid Interface Sci. 2021, 287, 102321. [Google Scholar] [CrossRef]
- Chatterjee, M.J.; Ahamed, S.T.; Mitra, M.; Kulsi, C.; Mondal, A.; Banerjee, D. Visible-light influenced photocatalytic activity of polyaniline-bismuth selenide composites for the degradation of methyl orange, rhodamine B and malachite green dyes. Appl. Surf. Sci. 2019, 470, 472–483. [Google Scholar] [CrossRef]
- Shah, M.Z.U.; Sajjad, M.; Hou, H.; BiBi, S.; Shah, A. Hydrothermal synthesis of ZnO@ ZnS heterostructure on Ni foam: A binder free electrode for high power and stable hybrid supercapacitors. Mater. Lett. 2022, 326, 132910. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Zhao, X.; Wang, X.; Yin, L.; Liang, C.; Wang, M.; Li, Y.; Liu, J.; Wu, Q. Uniform wurtzite MnSe nanocrystals with surface-dependent magnetic behavior. Nano Res. 2013, 6, 275–285. [Google Scholar] [CrossRef]
- Huo, P.; Zhou, M.; Tang, Y.; Liu, X.; Ma, C.; Yu, L.; Yan, Y. Incorporation of N–ZnO/CdS/Graphene oxide composite photocatalyst for enhanced photocatalytic activity under visible light. J. Alloys Compd. 2016, 670, 198–209. [Google Scholar] [CrossRef]
- Ran, R.; Meng, X.; Zhang, Z. Facile preparation of novel graphene oxide-modified Ag2O/Ag3VO4/AgVO3 composites with high photocatalytic activities under visible light irradiation. Appl. Catal. B Environ. 2016, 196, 1–15. [Google Scholar] [CrossRef]
- Ji, Z.; Shen, X.; Yang, J.; Xu, Y.; Zhu, G.; Chen, K. Graphene Oxide Modified Ag2O Nanocomposites with Enhanced Photocatalytic Activity under Visible-Light Irradiation. Eur. J. Inorg. Chem. 2013, 2013, 6119–6125. [Google Scholar] [CrossRef]
- Qi, H.-P.; Wang, H.-L.; Zhao, D.-Y.; Jiang, W.-F. Preparation and photocatalytic activity of Ag-modified GO-TiO2 mesocrystals under visible light irradiation. Appl. Surf. Sci. 2019, 480, 105–114. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, H.; Wang, Y.; Wu, K.; Huang, H.; Tang, G.; Yang, J. Synthesis and characterization of graphene oxide modified AgBr nanocomposites with enhanced photocatalytic activity and stability under visible light. Appl. Surf. Sci. 2014, 319, 306–311. [Google Scholar] [CrossRef]
- Kokilavani, S.; Syed, A.; Rajeshwari, M.R.; Subhiksha, V.; Elgorban, A.M.; Bahkali, A.H.; Zaghloul, N.S.; Das, A.; Khan, S.S. Decoration of Ag2WO4 on plate-like MnS for mitigating the charge recombination and tuned bandgap for enhanced white light photocatalysis and antibacterial applications. J. Alloys Compd. 2021, 889, 161662. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, Y.; Nie, W.; Chen, P. MoS2–GO nanocomposites synthesized via a hydrothermal hydrogel method for solar light photocatalytic degradation of methylene blue. Appl. Surf. Sci. 2015, 357, 1606–1612. [Google Scholar] [CrossRef]
- Zaaba, N.; Foo, K.; Hashim, U.; Tan, S.; Liu, W.-W.; Voon, C. Synthesis of graphene oxide using modified hummers method: Solvent influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Ismail, J.; Sajjad, M.; Shah, M.Z.U.; Hussain, R.; Khan, B.A.; Maryam, R.; Shah, A.; Mahmood, A.; ur Rahman, S. Comparative capacitive performance of MnSe encapsulated GO based nanocomposites for advanced electrochemical capacitor with rapid charge transport channels. Mater. Chem. Phys. 2022, 284, 126059. [Google Scholar] [CrossRef]
- Sajjad, M.; Tao, R.; Qiu, L. Phosphine based covalent organic framework as an advanced electrode material for electrochemical energy storage. J. Mater. Sci. Mater. Electron. 2021, 32, 1602–1615. [Google Scholar] [CrossRef]
- Onari, S.; Arai, T. Infrared lattice vibrations and dielectric dispersion in antiferromagnetic semiconductor MnSe2. J. Phys. Soc. Jpn. 1979, 46, 184–188. [Google Scholar] [CrossRef]
- Rose, A.; Raghavan, N.; Thangavel, S.; Maheswari, B.U.; Nair, D.P.; Venugopal, G. Investigation of cyclic voltammetry of graphene oxide/polyaniline/polyvinylidene fluoride nanofibers prepared via electrospinning. Mater. Sci. Semicond. Process. 2015, 31, 281–286. [Google Scholar] [CrossRef]
- Vadivel, S.; Kamalakannan, V.; Balasubramanian, N. d-Pencillamine assisted microwave synthesis of Bi2S3 microflowers/RGO composites for photocatalytic degradation—A facile green approach. Ceram. Int. 2014, 40, 14051–14060. [Google Scholar] [CrossRef]
- Dappe, Y.; Basanta, M.A.; Flores, F.; Ortega, J. Weak chemical interaction and van der Waals forces between graphene layers: A combined density functional and intermolecular perturbation theory approach. Phys. Rev. B 2006, 74, 205434. [Google Scholar] [CrossRef]
- Ullah, R.; Dutta, J. Photocatalytic degradation of organic dyes with manganese-doped ZnO nanoparticles. J. Hazard. Mater. 2008, 156, 194–200. [Google Scholar] [CrossRef]
- Shah, M.Z.U.; Javed, M.S.; Sajjad, M.; Shah, A.; Shah, M.S.; ur Rahman, S.; Mahmood, A.; Ahmad, M.; Assiri, M.A.; Hou, H. A novel TiO2/CuSe based nanocomposite for high-voltage asymmetric supercapacitors. J. Sci. Adv. Mater. Devices 2022, 7, 100418. [Google Scholar] [CrossRef]
- Hou, C.; Hu, B.; Zhu, J. Photocatalytic Degradation of Methylene Blue over TiO2 Pretreated with Varying Concentrations of NaOH. Catalysts 2018, 8, 575. [Google Scholar] [CrossRef]
- Chuaicham, C.; Pawar, R.; Sasaki, K. Dye-sensitized Photocatalyst of Sepiolite for Organic Dye Degradation. Catalysts 2019, 9, 235. [Google Scholar] [CrossRef]
- Rout, D.R.; Jena, H.M. Enhanced Cr (VI) adsorption using ZnO decorated graphene composite: Batch and continuous studies. J. Taiwan Inst. Chem. Eng. 2022, 140, 104534. [Google Scholar] [CrossRef]
- Rahmat, S.; Tan, W.; Kawamura, G.; Matsuda, A.; Lockman, Z. Facile fabrication of rGO/Rutile TiO2 nanowires as photocatalyst for Cr (VI) Reduction. Mater. Today Proc. 2019, 17, 1143–1151. [Google Scholar] [CrossRef]
- Xu, L.; Yang, L.; Bai, X.; Du, X.; Wang, Y.; Jin, P. Persulfate activation towards organic decomposition and Cr (VI) reduction achieved by a novel CQDs-TiO2−x/rGO nanocomposite. Chem. Eng. J. 2019, 373, 238–250. [Google Scholar] [CrossRef]
- Doluel, E.C.; Kartal, U.; Dikici, T.; Yurddaskal, M. Effect of Ag content on photocatalytic activity of Ag@ TiO2/rGO hybrid photocatalysts. J. Electron. Mater. 2020, 49, 3849–3859. [Google Scholar] [CrossRef]
- Sonia, S.; Kumar, P.S.; Mangalaraj, D.; Ponpandian, N.; Viswanathan, C. Influence of growth and photocatalytic properties of copper selenide (CuSe) nanoparticles using reflux condensation method. Appl. Surf. Sci. 2013, 283, 802–807. [Google Scholar] [CrossRef]
- Pawar, M.; Topcu Sendoğdular, S.; Gouma, P. A Brief Overview of TiO2 Photocatalyst for Organic Dye Remediation: Case Study of Reaction Mechanisms Involved in Ce-TiO2 Photocatalysts System. J. Nanomater. 2018, 2018, 5953609. [Google Scholar] [CrossRef]
- Ravelli, D.; Dondi, D.; Fagnoni, M.; Albini, A. Photocatalysis. A multi-faceted concept for green chemistry. Chem. Soc. Rev. 2009, 38, 1999–2011. [Google Scholar] [CrossRef]

| Material | Light Source | Irradiation Time (min) | MB Reduction (%) | Ref. |
|---|---|---|---|---|
| ZnO/rGO | Visible light | 120 | 85 | [54] |
| TiO2/GO | UV light | 150 | 88 | [55] |
| CQDs-TiO2-x-rGO | Visible light | 120 | 91 | [56] |
| Ag-TiO2/rGO | Visible light | 100 | 93 | [57] |
| CuSe | Visible light | 90 | 76 | [58] |
| MnSe | Visible light | 90 | -- | This work |
| 7 %MnSe/GO | Visible light | 90 | -- | This work |
| 14 %MnSe/GO | Visible light | 90 | -- | This work |
| 21 %MnSe/GO | Visible light | 90 | -- | This work |
| 28 %MnSe/GO | Visible light | 90 | -- | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jevtovic, V.; Khan, A.U.; Almarhoon, Z.M.; Tahir, K.; Latif, S.; Abdulaziz, F.; Albalawi, K.; Zaki, M.E.A.; Rakic, V. Synthesis of MnSe-Based GO Composites as Effective Photocatalyst for Environmental Remediations. Nanomaterials 2023, 13, 667. https://doi.org/10.3390/nano13040667
Jevtovic V, Khan AU, Almarhoon ZM, Tahir K, Latif S, Abdulaziz F, Albalawi K, Zaki MEA, Rakic V. Synthesis of MnSe-Based GO Composites as Effective Photocatalyst for Environmental Remediations. Nanomaterials. 2023; 13(4):667. https://doi.org/10.3390/nano13040667
Chicago/Turabian StyleJevtovic, Violeta, Afaq Ullah Khan, Zainab M. Almarhoon, Kamran Tahir, Salman Latif, Fahad Abdulaziz, Karma Albalawi, Magdi E. A. Zaki, and Violeta Rakic. 2023. "Synthesis of MnSe-Based GO Composites as Effective Photocatalyst for Environmental Remediations" Nanomaterials 13, no. 4: 667. https://doi.org/10.3390/nano13040667
APA StyleJevtovic, V., Khan, A. U., Almarhoon, Z. M., Tahir, K., Latif, S., Abdulaziz, F., Albalawi, K., Zaki, M. E. A., & Rakic, V. (2023). Synthesis of MnSe-Based GO Composites as Effective Photocatalyst for Environmental Remediations. Nanomaterials, 13(4), 667. https://doi.org/10.3390/nano13040667








