Advantages of Ta-Doped Sb3Te1 Materials for Phase Change Memory Applications
Abstract
:1. Introduction
2. Experiment
3. Results and discussion
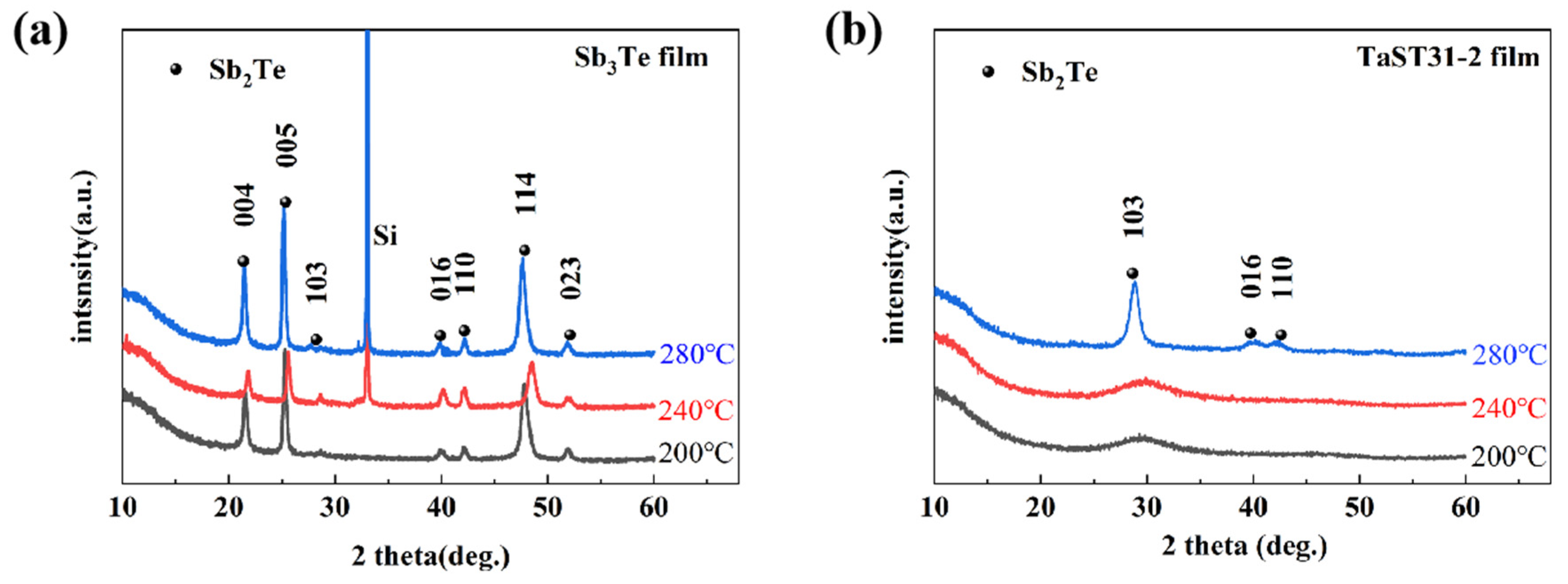
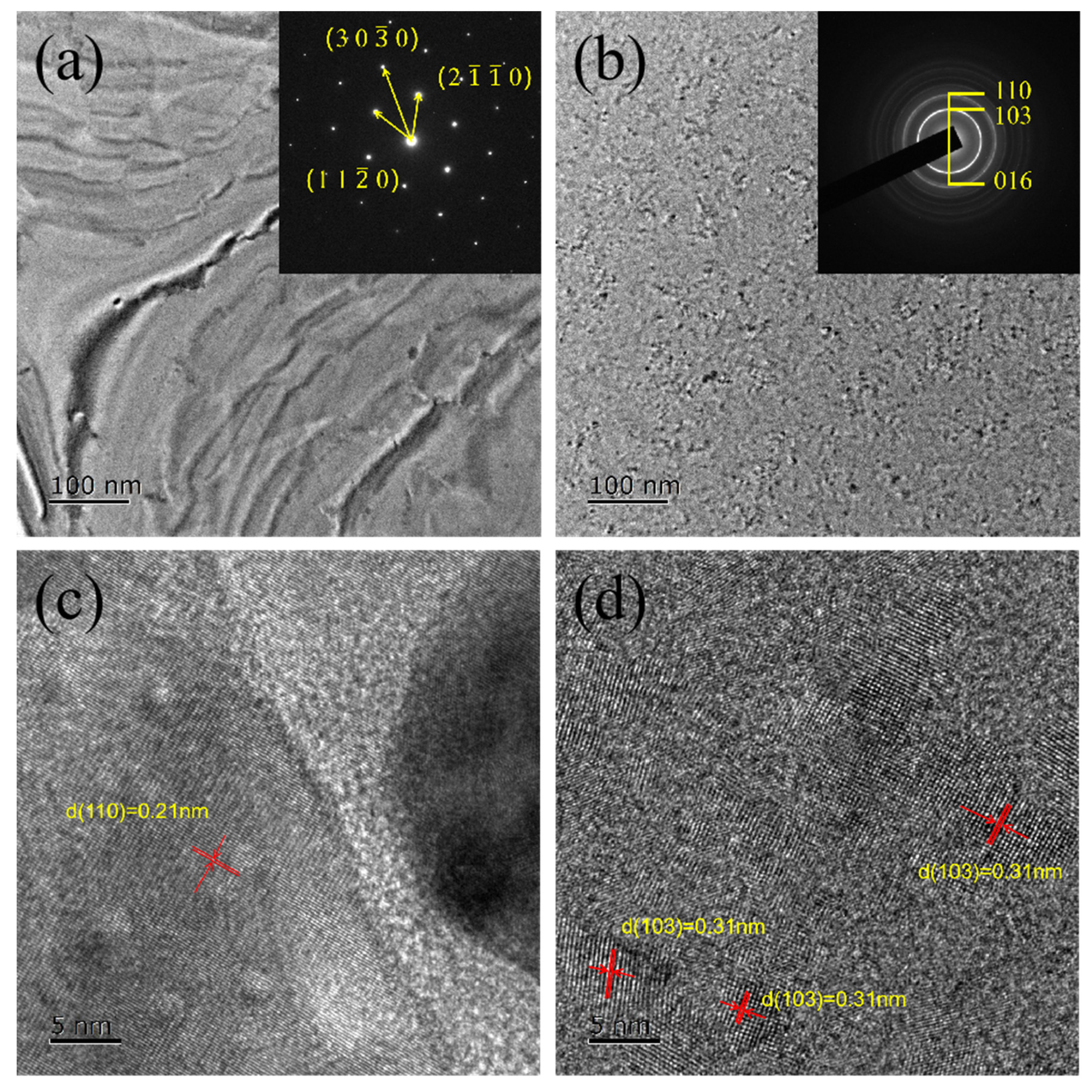
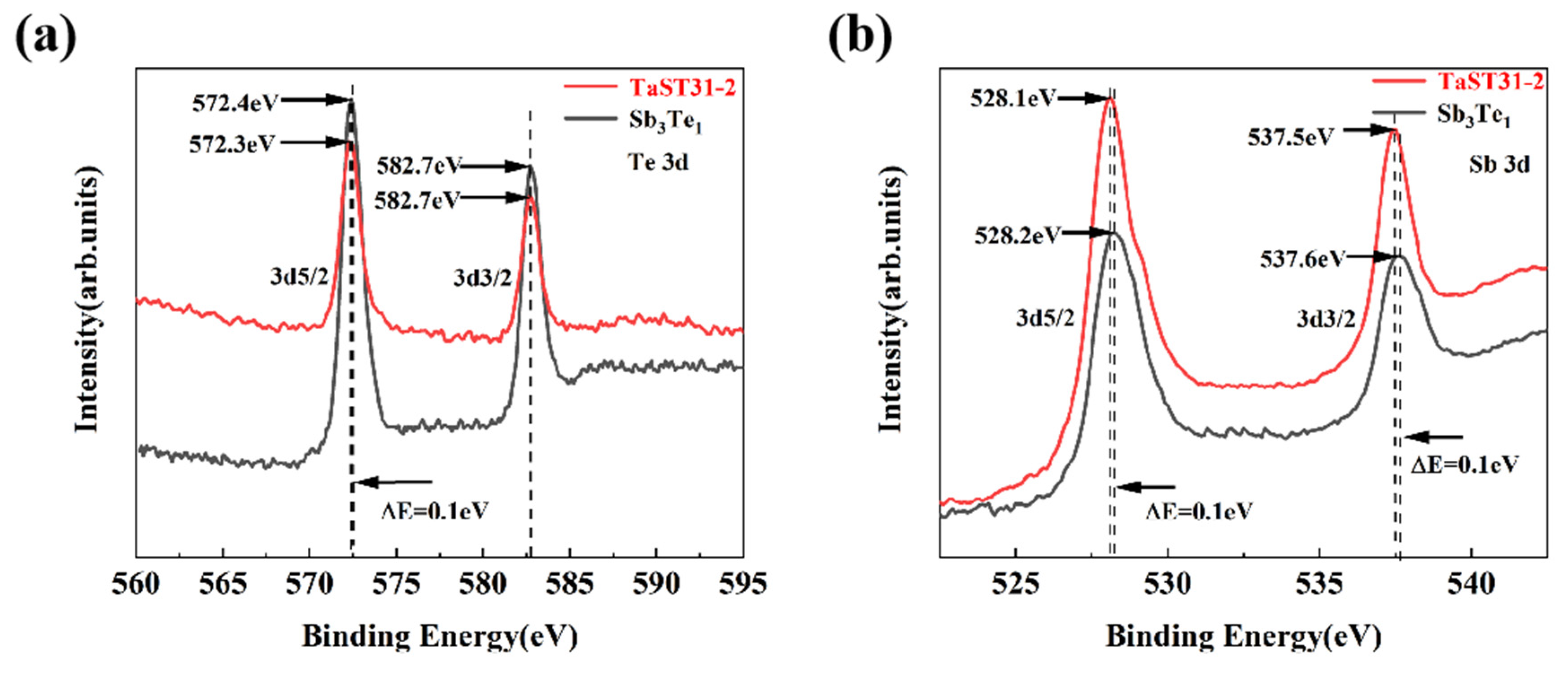
3.1. Effect of Ta Doping on Crystalline Properties and Microstructure
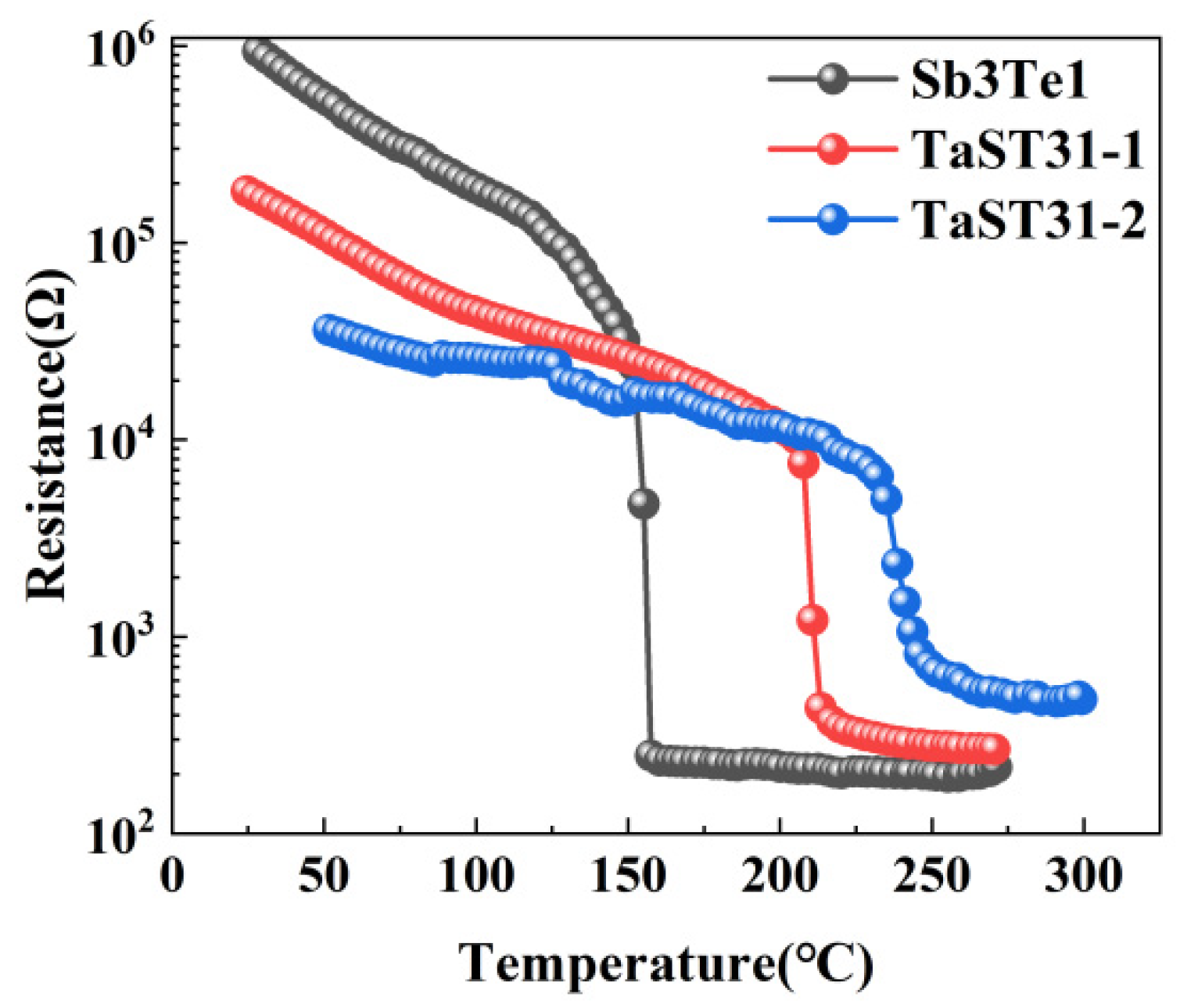
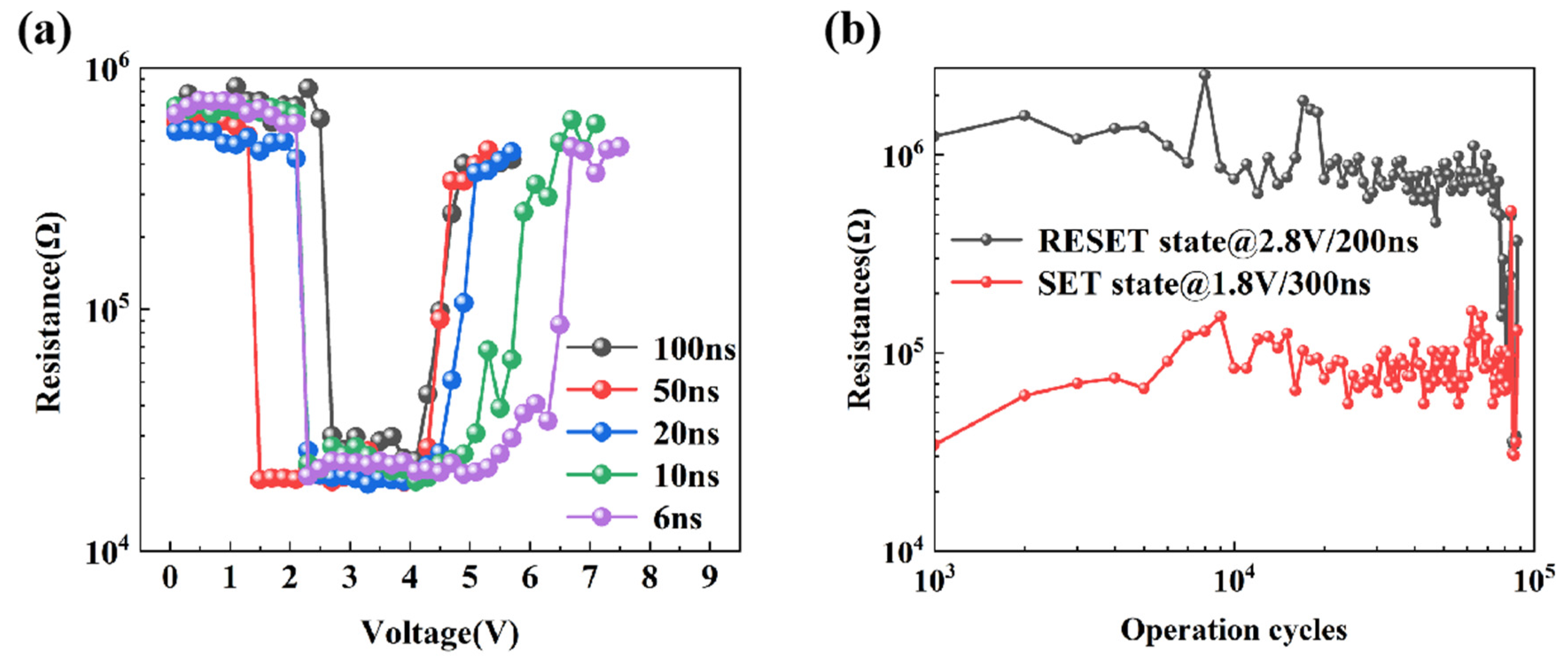
3.2. Device Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Burr, G.W.; Breitwisch, M.J.; Franceschini, M.; Garetto, D.; Gopalakrishnan, K.; Jackson, B.; Kurdi, B.; Lam, C.; Lastras, L.A.; Padilla, A.; et al. Phase change memory technology. J. Vac. Sci. Technol. B 2010, 28, 223. [Google Scholar] [CrossRef]
- Raoux, S.; Wełnic, W.; Ielmini, D. Phase change materials and their application to nonvolatile memories. Chem. Rev. 2010, 110, 240–267. [Google Scholar] [CrossRef]
- Liu, B.; Song, Z.; Feng, S.; Chen, B. Chen, Characteristics of chalcogenide nonvolatile memory nano-cell-element based on Sb2Te3 material. Microelectron. Eng. 2005, 82, 168–174. [Google Scholar] [CrossRef]
- Sun, Z.; Zhou, J.; Pan, Y.; Song, Z.; Mao, H.K.; Ahuja, R. Pressure-induced reversible amorphization and an amorphous-amorphous transition in Ge2Sb2Te5 phase change memory material. Proc. Natl. Acad. Sci. USA 2011, 108, 10410–10414. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, S.; Yagi, S.; Yamazaki, H.; Funakoshi, N. Crystallization process of Sb-Te alloy films for optical storage. J. Appl. Phys. 1988, 64, 1000–1004. [Google Scholar] [CrossRef]
- Meinders, E.R.; Lankhorst, M.H.R. Determination of the crystallisation kinetics of fast-growth phase-change materials for mark-formation prediction. Jpn. J. Appl. Phys. 2003, 42, 809–812. [Google Scholar] [CrossRef]
- Lankhorst, M.H.R.; Van Pieterson, L.; Van Schijndel, M.; Jacobs, B.A.J.; Rijpers, J.C.N. Prospects of doped Sb–Te phase-change materials for high-speed recording. Jpn. J. Appl. Phys. 2003, 42, 863–868. [Google Scholar] [CrossRef]
- Van Pieterson, L.; Lankhorst, M.H.R.; van Schijndel, M.; Kuiper, A.E.T.; Roosen, J.H.J. Phase-change recording materials with a growth-dominated crystallization mechanism: A materials overview. J. Appl. Phys. 2005, 97, 083520. [Google Scholar] [CrossRef]
- Ghosh, G. The Sb-Te (antimony-tellurium) system. J. Phase Equilibria 1994, 15, 349–360. [Google Scholar] [CrossRef]
- Solé, S.; Schmetterer, C.; Richter, K.W. A Revision of the Sb-Te Binary Phase Diagram and Crystal Structure of the Modulated γ-Phase Field. J. Phase Equilibria Diffus. 2022, 43, 648–659. [Google Scholar] [CrossRef]
- Boniardi, M.; Redaelli, A.; Cupeta, C.; Pellizzer, F.; Crespi, L.; D’Arrigo, G.; Lacaita, A.L.; Servalli, G. Optimization metrics for phase change memory (PCM) cell architectures. In Proceedings of the 2014 IEEE International Electron Devices Meeting, San Francisco, CA, USA, 15–17 December 2014. [Google Scholar]
- Rao, F.; Ding, K.Y.; Zhou, Y.X.; Zheng, Y.H.; Xia, M.J.; Lv, S.L.; Song, Z.T.; Feng, S.L.; Ronneberger, I.; Mazzarello, R.; et al. Reducing the stochasticity of crystal nucleation to enable subnanosecond memory writing. Science 2017, 358, 1423–1426. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-Y.; Kim, D.; Park, Y.-J.; Yang, T.-Y.; Lee, Y.-Y.; Joo, Y.-C. The phase-change kinetics of amorphous Ge2Sb2Te5 and device characteristics investigated by thin-film mechanics. Acta Mater. 2015, 94, 143–151. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, L.; Rao, F.; Song, Z.; Li, X.; Peng, C.; Zhou, X.; Ren, K.; Yao, D.; Feng, S. N-doped Sb2Te1 phase change materials for higher data retention. J. Alloys Compd. 2011, 509, 10105–10109. [Google Scholar] [CrossRef]
- Wang, W.; Loke, D.; Shi, L.; Zhao, R.; Yang, H.; Law, L.-T.; Ng, L.-T.; Lim, K.-G.; Yeo, Y.-C.; Chong, T.-C.; et al. Enabling Universal Memory by overcoming the contradictory speed and stability nature of phase-change materials. Sci. Rep. 2010, 2, 360. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Cai, D.; Xue, Y.; Guo, T.; Song, S.; Song, Z. Sb-rich CuSbTe material: A candidate for high-speed and high-density phase change memory application. Mater. Sci. Semicond. Process. 2019, 103, 104625. [Google Scholar] [CrossRef]
- Njoroge, W.K.; Wöltgens, H.-W.; Wuttig, M. Density changes upon crystallization of Ge2Sb2.04Te4.74 films. J. Vac. Sci. Technol. B 2002, 20, 230–233. [Google Scholar] [CrossRef]
- Cheng, Y.; Song, Z.; Gu, Y.; Song, S.; Rao, F.; Wu, L.; Liu, B.; Feng, S. Influence of silicon on the thermally-induced crystallization process of Si-Sb4Te phase change materials. Appl. Phys. Lett. 2011, 99, 261914. [Google Scholar] [CrossRef]
- Gordy, W.; Thomas, W.J.O. Electronegativities of the elements. J. Chem. Phys. 1956, 24, 439–444. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, B.; Wang, Q.; Zhang, Z.; Song, S.; Song, Z.; Yao, D.; Xi, W.; Guo, X.; Feng, S. Study on the phase change material Cr-doped Sb 3 Te 1 for application in phase change memory. J. Non-Cryst. Solids 2015, 422, 46–50. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, L.C.; Song, Z.T.; Rao, F.; Cai, D.L.; Peng, C.; Zhou, X.L.; Ren, K.; Song, S.N.; Liu, B.; et al. Ti10Sb60Te30 for phase change memory with high-temperature data retention and rapid crystallization speed. Appl. Phys. Lett. 2012, 100, 122101. [Google Scholar] [CrossRef]
- Privitera, S.; Pimini, E.; Zonca, R. Amorphous-to-crystal transition of nitrogenand oxygen-doped Ge2Sb2Te5 films studied by in situ resistance measurements. Appl. Phys. Lett. 2004, 85, 3044–3046. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, M.; Qiao, Y.; Xue, Y.; Song, S.; Song, Z.; Li, X. Advantages of Ta-Doped Sb3Te1 Materials for Phase Change Memory Applications. Nanomaterials 2023, 13, 633. https://doi.org/10.3390/nano13040633
Shao M, Qiao Y, Xue Y, Song S, Song Z, Li X. Advantages of Ta-Doped Sb3Te1 Materials for Phase Change Memory Applications. Nanomaterials. 2023; 13(4):633. https://doi.org/10.3390/nano13040633
Chicago/Turabian StyleShao, Mingyue, Yang Qiao, Yuan Xue, Sannian Song, Zhitang Song, and Xiaodan Li. 2023. "Advantages of Ta-Doped Sb3Te1 Materials for Phase Change Memory Applications" Nanomaterials 13, no. 4: 633. https://doi.org/10.3390/nano13040633
APA StyleShao, M., Qiao, Y., Xue, Y., Song, S., Song, Z., & Li, X. (2023). Advantages of Ta-Doped Sb3Te1 Materials for Phase Change Memory Applications. Nanomaterials, 13(4), 633. https://doi.org/10.3390/nano13040633





