Polyindole Embedded Nickel/Zinc Oxide Nanocomposites for High-Performance Energy Storage Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
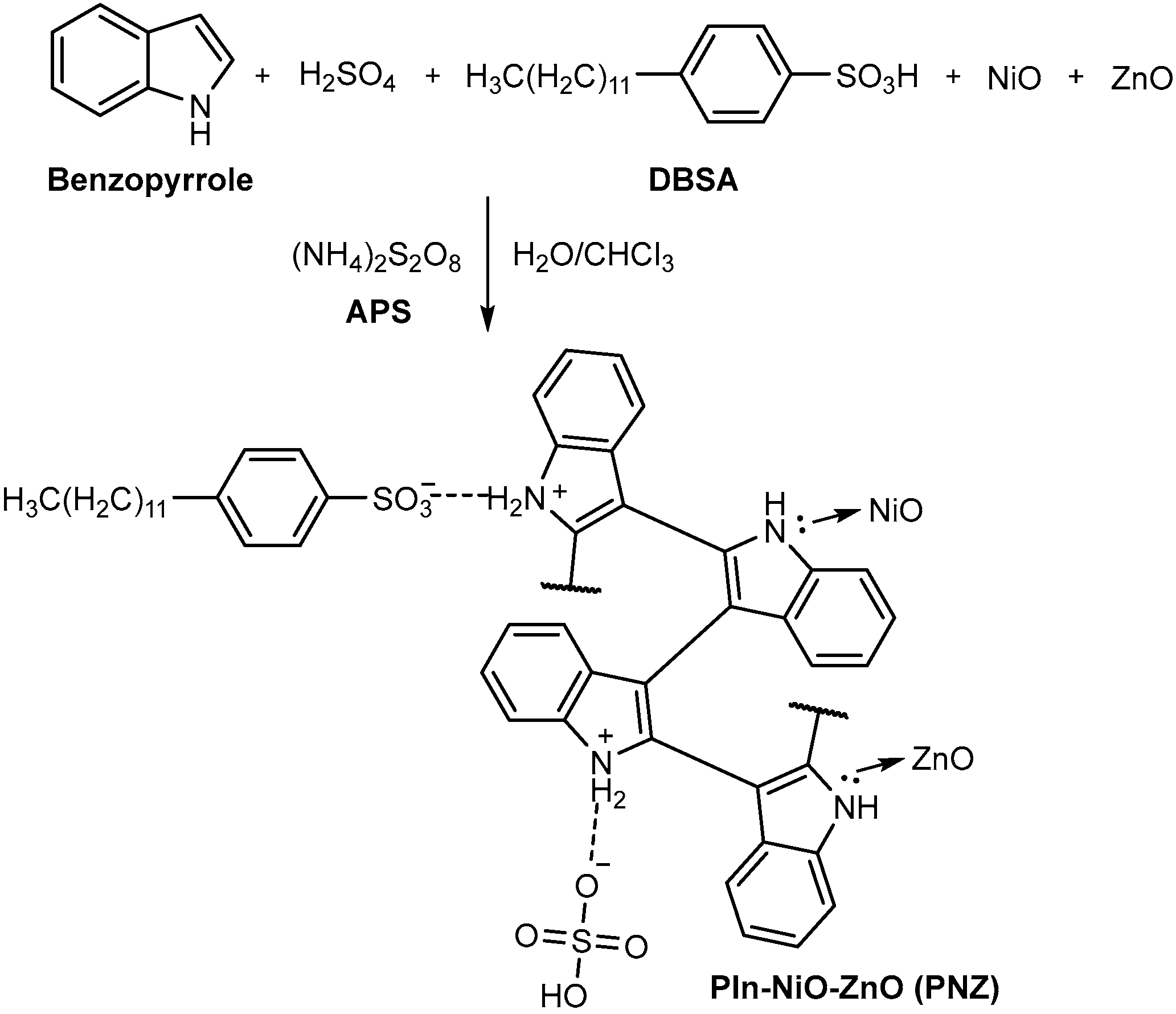
2.2. One-Pot Synthesis of the Polyindole/Metal Oxide Composites
2.3. Material Characterisation
2.4. Electrochemical Characterization
3. Results and Discussion
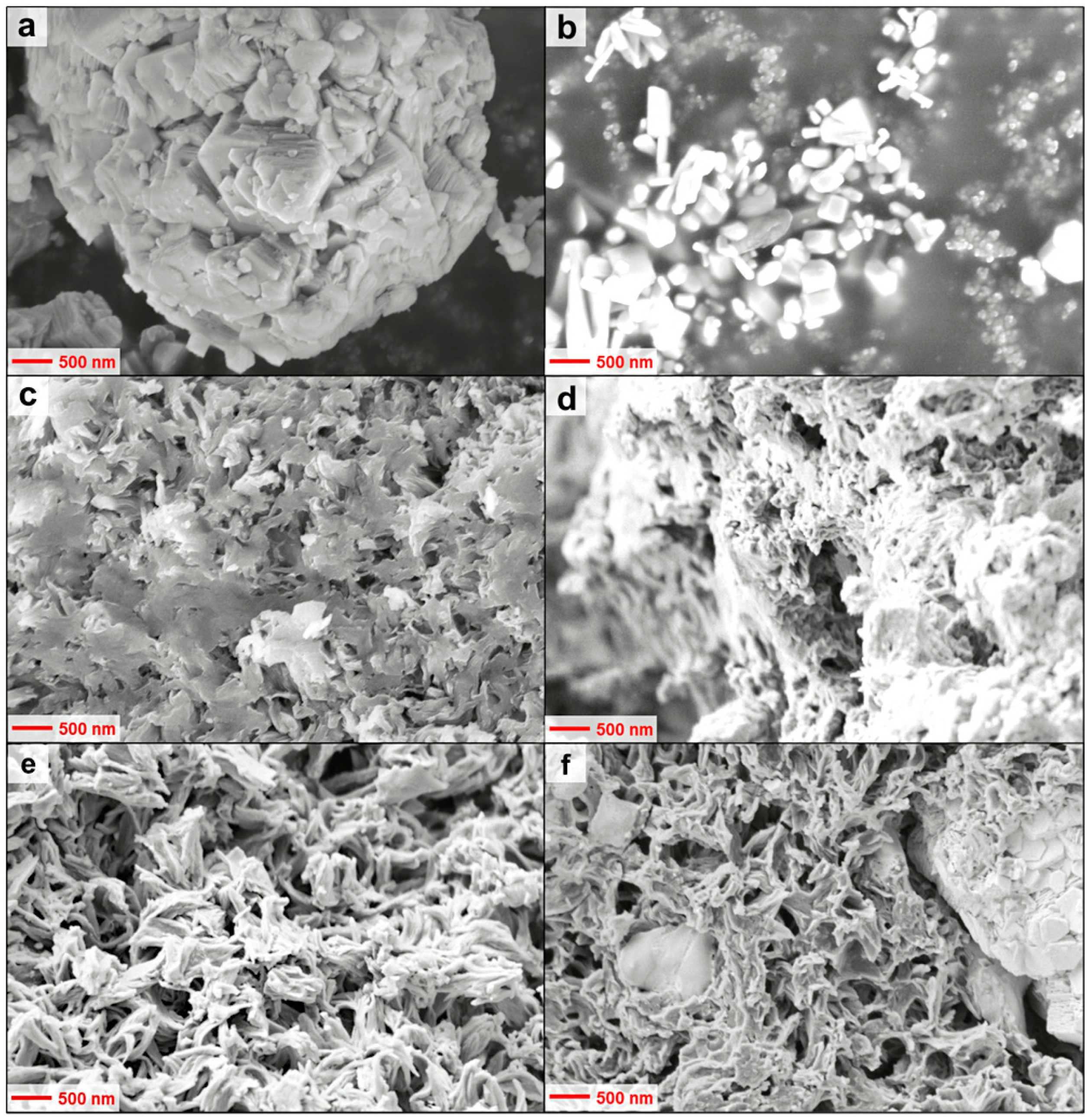
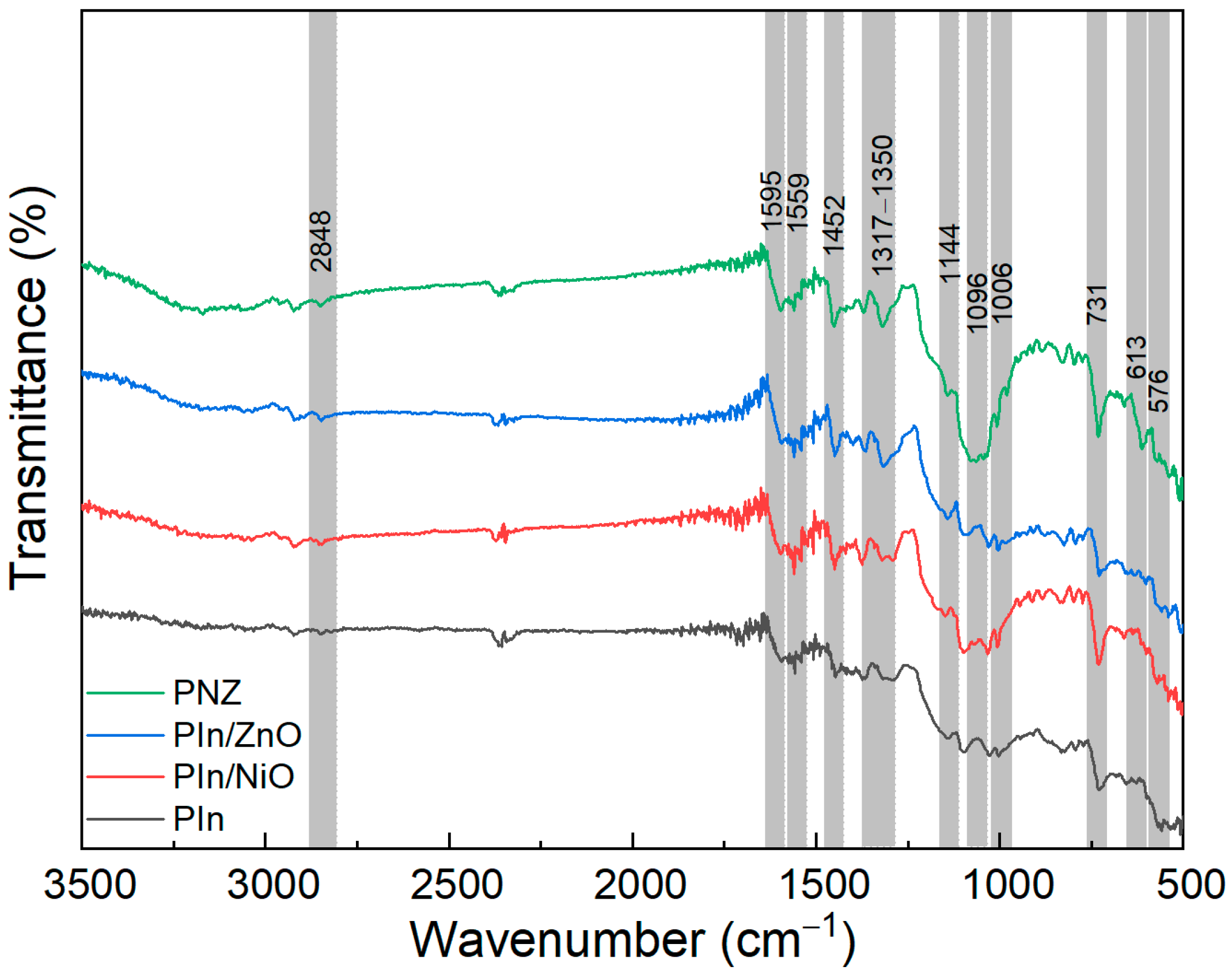
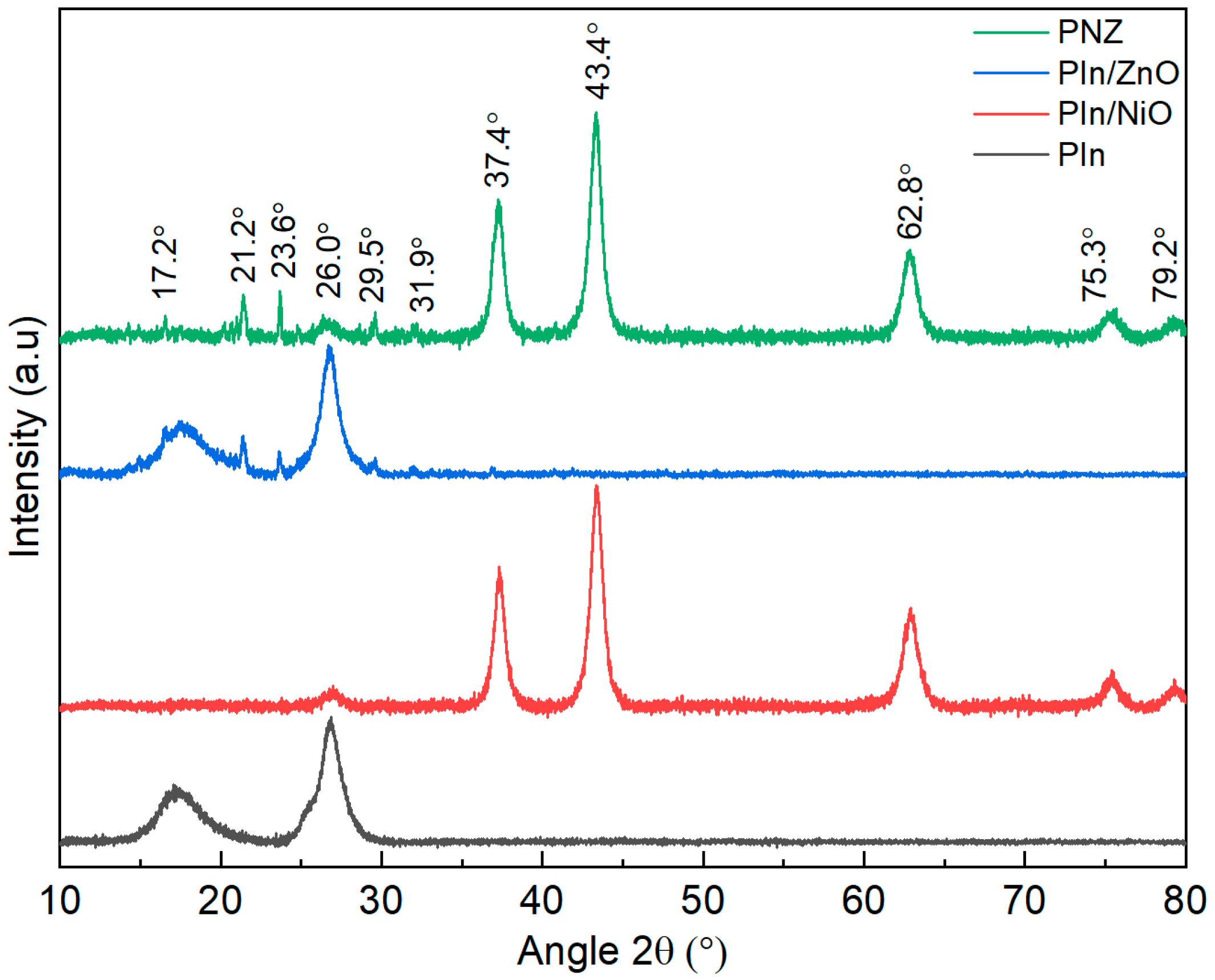
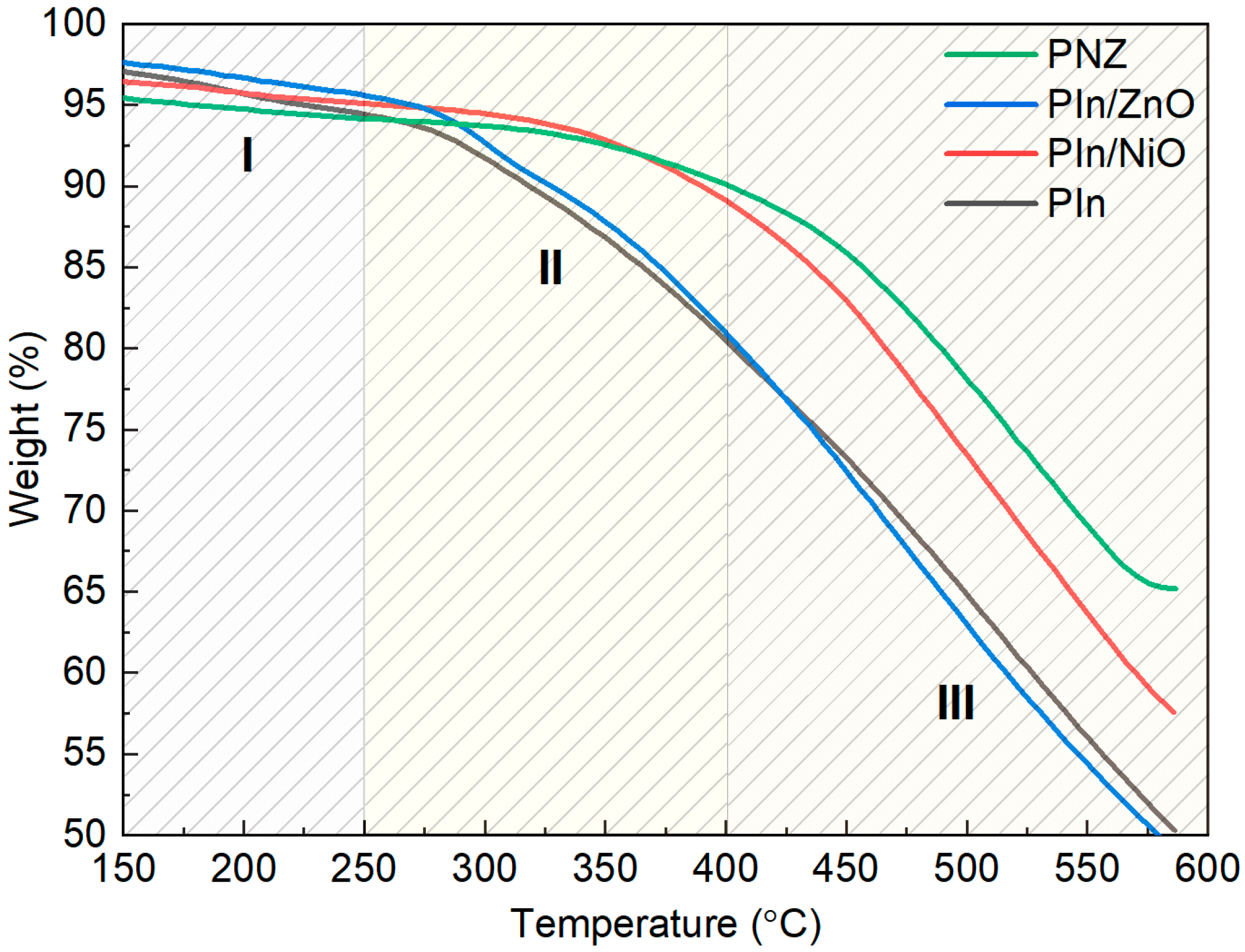
3.1. Physico-Chemical Characterisation of the Polyindole Composites
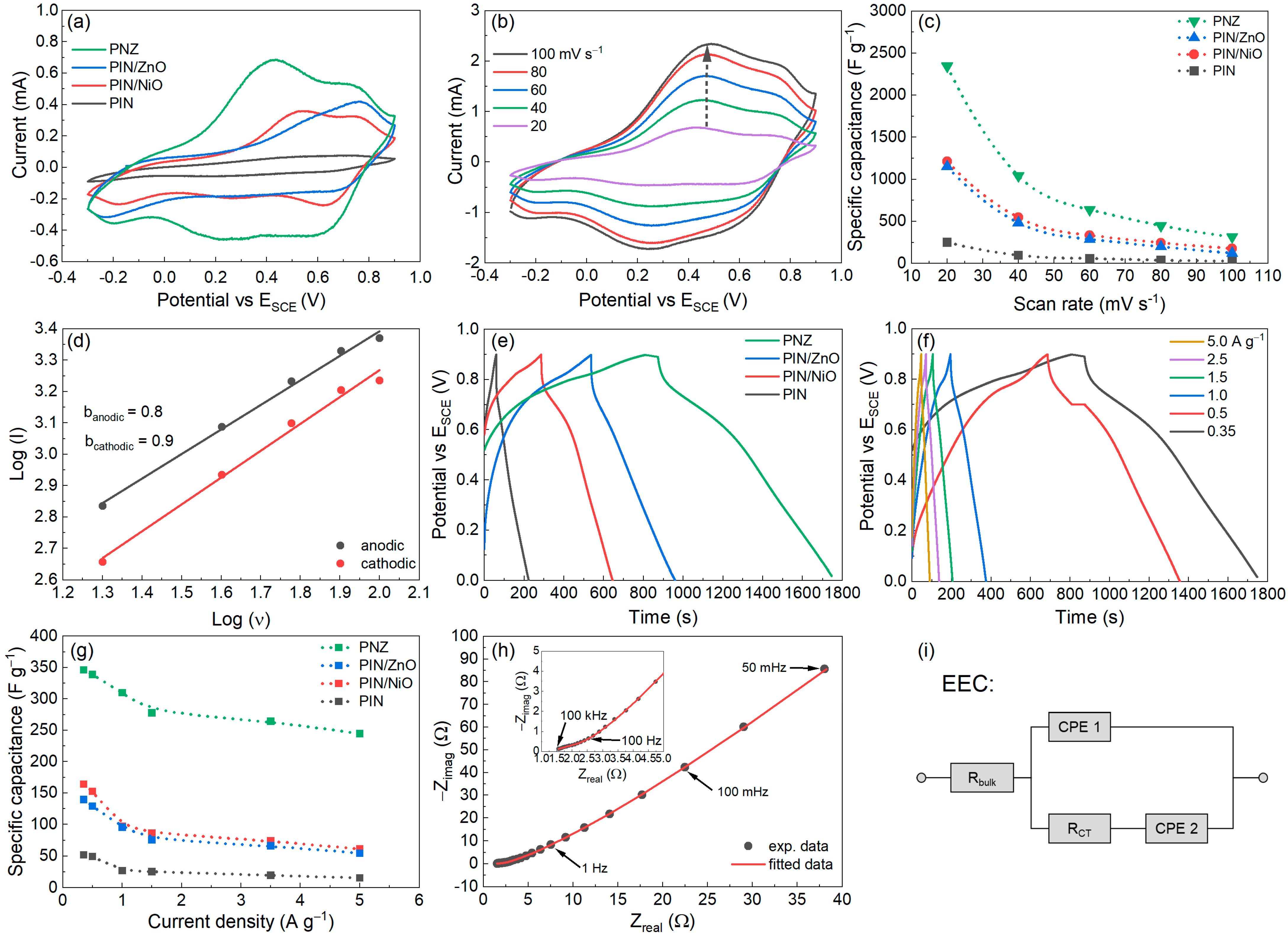
3.2. Electrochemical Characterisation of the Polyindole Composites
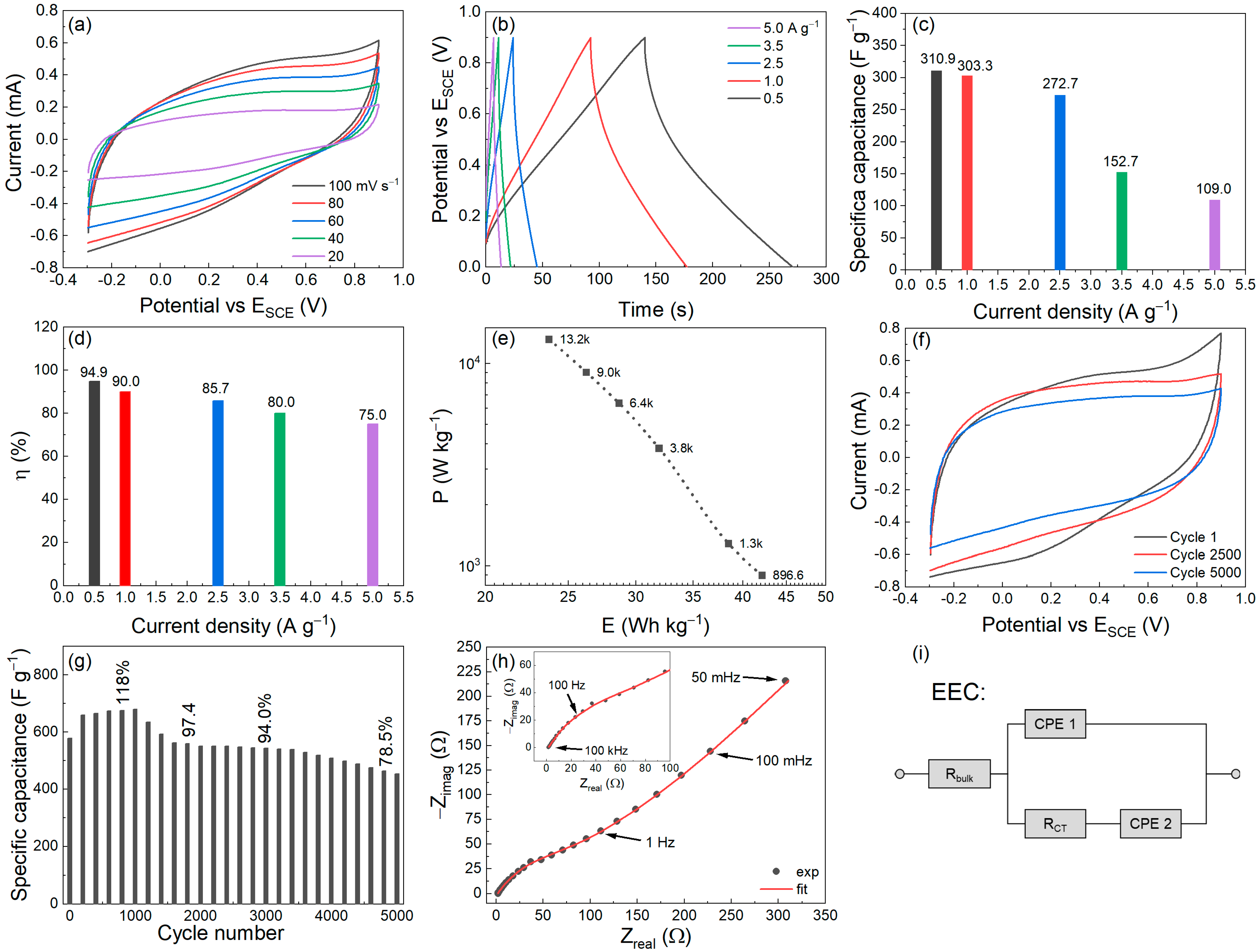
3.3. Analysis of the PNZ Composite as Faradaic Supercapacitor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kubra, K.T.; Javaid, A.; Patil, B.; Sharif, R.; Salman, A.; Shahzadi, S.; Siddique, S.; Ghani, S. Synthesis and characterization of novel Pr6O11/Mn3O4 nanocomposites for electrochemical supercapacitors. Ceram. Int. 2019, 45, 6819–6827. [Google Scholar]
- Zhao, X.; Mao, L.; Cheng, Q.; Li, J.; Liao, F.; Yang, G.; Xie, L.; Zhao, C.; Chen, L. Two-dimensional spinel structured Co-based materials for high performance supercapacitors: A Critical Review. Chem. Eng. J. 2020, 387, 124081. [Google Scholar] [CrossRef]
- Huang, C.; Hao, C.; Zheng, W.; Zhou, S.; Yang, L.; Wang, X.; Zhu, L. Synthesis of polyaniline/nickel ox-ide/sulfonated graphene ternary composite for all-solid-state asymmetric supercapacitor. Appl. Surf. Sci. 2020, 505, 144589. [Google Scholar] [CrossRef]
- Gao, P.; Chen, Z.; Gong, Y.; Zhang, R.; Liu, H.; Tang, P.; Chen, X.; Passerini, S.; Liu, J. The role of cation vacancies in electrode materials for enhanced electrochemical energy storage: Synthesis, advanced characterization, and fundamentals. Adv. Energy Mater. 2020, 10, 1903780. [Google Scholar]
- Ramesan, M.T.; Anjitha, T.; Parvathi, K.; Anilkumar, T.; Mathew, G. Nano zinc ferrite filler incorporated polyindole/poly(vinyl alcohol) blend: Preparation, characterization, and investigation of electrical properties. Adv. Polym. Technol. 2018, 37, 3639–3649. [Google Scholar] [CrossRef]
- Yu, M.; Qiu, W.; Wang, F.; Zhai, T.; Fang, P.; Lu, X.; Tong, Y. Three dimensional architectures: Design, assembly and application in electrochemical capacitors. J. Mater. Chem. A 2015, 3, 15792–15823. [Google Scholar]
- Isa, N.M.; Baharin, R.; Majid, R.A.; Rahman, W.A.W.A. Optical properties of conjugated polymer: Review of its change mechanism for ionizing radiation sensor. Polym. Adv. Technol. 2017, 28, 1559–1572. [Google Scholar] [CrossRef]
- Milan, B.P.; Changho, Y.; Han, J.K. Synthesis of conducting bifunctional polyaniline@Mn-TiO2 nanocomposites for supercapacitor electrode and visible light driven photocatalyst. Catalysts 2020, 10, 546. [Google Scholar]
- Yanik, M.O.; Yigit, E.A.; Akansu, Y.E.; Sahmetlioglu, E. Magnetic conductive polymer-graphene nanocomposites based supercapacitors for energy storage. Energy. 2017, 138, 883–889. [Google Scholar]
- Zhou, Q.; Zhu, D.; Ma, X.; Xu, J.; Zhou, W.; Zhao, F. High-performance capacitive behavior of layered reduced graphene oxide and polyindole nanocomposite materials. RSC Adv. 2016, 6, 29840–29847. [Google Scholar] [CrossRef]
- Ram, B.C.; Sarfaraz, A. Mesoporous complexion and multi-channeled charge storage action of PIn-rGO-TiO2 ternary hybrid materials for supercapacitor applications. J. Energy Storage 2022, 46, 103912. [Google Scholar]
- Majumder, M.; Choudhary, R.B.; Koiry, S.P.; Thakur, A.K.; Kumar, U. Gravimetric and volumetric capacitive performance of polyindole/carbon black/MoS2 hybrid electrode material for supercapacitor applications. Electrochim. Acta 2017, 248, 98–111. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Q.; Wang, A.; Xu, J.; Wu, S.; Shen, J. The bamboo-like composites of V2O5/polyindole and activated carbon cloth as electrodes for all-solid-state flexible asymmetric supercapacitors. Appl. Mater. Interfaces 2016, 8, 3776–3783. [Google Scholar] [CrossRef] [PubMed]
- Ramesan, M.T.; Santhi, V. Synthesis, characterization, conductivity and sensor application study of polypyrrole/silver doped nickel oxide nanocomposites. Compos. Interfaces 2018, 25, 725–741. [Google Scholar]
- Xie, Y.; Sha, X. Electrochemical cycling stability of nickel (II) coordinated polyaniline. Synth. Met. 2018, 237, 29–39. [Google Scholar] [CrossRef]
- Fua, L.; Fub, Z. Plectranthus amboinicus leaf extract assisted biosynthesis of ZnO nanoparticles and their photocatalytic activity. Ceram. Int. 2015, 41, 2492–2496. [Google Scholar] [CrossRef]
- Thadathil, A.; Pradeep, H.; Joshy, D.; Ismail, Y.A.; Periyat, P. Polyindole and polypyrrole as a sustainable platform for environmental remediation and sensor applications. Mater. Adv. 2022, 3, 2990–3022. [Google Scholar]
- Mehsenpour, M.; Naderi, M.; Arash, G.; Aghabararpour, M.; Haghshenas, D.F. Fabrication of paper-based carbon/graphene/ZnO aerogel composite decorated by polyaniline nanostructure: Investigation of electrochemical properties. Ceram. Int. 2021, 47, 29908–29918. [Google Scholar]
- Rashti, A.; Wang, B.; Hassani, E.; Feyzbar-Khalkhali-Nejad, F.; Zhang, X.; Oh, T.-S. Electrophoretic deposition of nickel cobaltite/polyaniline/rGO composite electrode for high performance all-solid-state asymmetric supercapacitors. Energy Fuels 2020, 5, 6448–6461. [Google Scholar]
- Rajesh, M.; Manikandan, R.; Kim, B.C.; Becuwe, M.; Yu, K.H.; Raj, C.J. Electrochemical polymerization of chloride doped PEDOT hierarchical porous nanostructure on graphite as A potential electrode for high performance supercapacitor. Electrochim. Acta 2020, 354, 136669. [Google Scholar]
- Waikar, M.R.; Rasal, A.S.; Shinde, N.S.; Dhas, S.D.; Moholkar, A.V.; Shirsat, M.D.; Chakarvarti, S.K.; Sonkawade, R.G. Electro-chemical performance of Polyaniline based symmetrical energy storage device. Mater. Sci. Semicond. Proc. 2020, 120, 105291. [Google Scholar]
- Begum, B.; Bilal, S.; Shah, A.H.A.; Röse, P. Synthesis, characterization and electrochemical performance of a redox-responsive polybenzopyrrole @ nickel oxide nanocomposite for robust and efficient faradaic. Nanomaterials 2022, 12, 513. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Abdallah, Y.; Ali, M.A.; Masum, M.M.I.; Li, B.; Sun, G.; Meng, Y.; Wang, Y.; An, Q. Lemon-fruit-based green synthesis of zinc oxide nanoparticles and titanium dioxide nanoparticles against soft rot bacterial pathogen dickey dadantii. Biomolecules 2019, 9, 863. [Google Scholar]
- Begum, B.; Bilal, S.; Shah, A.U.H.A.; Röse, P. Physical, chemical, and electrochemical properties of redox-responsive polybenzopyrrole as electrode material for faradaic energy storage. Polymers 2021, 13, 2883. [Google Scholar] [PubMed]
- Hajera, G.; Anwar-ul-Haq, S.; Ulrike, K.; Salma, B. Study on direct synthesis of energy efficient multifunctional polyanilinegraphene oxide nanocomposite and its application in aqueous symmetric supercapacitor devices. Nanomaterials 2020, 10, 118. [Google Scholar]
- Yang, H.; Xu, H.; Li, M.; Zhang, L.; Huang, Y.; Hu, X. Assembly of NiO/Ni(OH)2/PEDOT nanocomposites on contra wires for fiber-shaped flexible asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 1774–1779. [Google Scholar]
- Batool, A.; Kanwal, F.; Imran, M.; Jamil, T.; Siddiqi, S.A. Synthesis of polypyrrole/zinc oxide composites and study of their structural, thermal and electrical properties. Synth. Met. 2012, 161, 2753–2758. [Google Scholar] [CrossRef]
- Inamuddin; Shakeel, N.; Ahamed, M.I.; Kanchi, S.; Kashmery, H.A. Green synthesis of ZnO nanoparticles decorated on polyindole functionalized-MCNTs and used as anode material for enzymatic biofuel cell applications. Sci. Rep. 2020, 10, 5052. [Google Scholar]
- Verma, C.J.; Keshari, A.S.; Dubey, P.; Prakash, R. Polyindole modifed g-C3N4 nanohybrids via in-situ chemical polymerization for its improved electrochemical performance. Vacuum 2020, 177, 109363. [Google Scholar]
- Shyamala, S.; Muthuchudarkodi, R.R. Polyindole based zinc oxide nanocomposite-synthesis and characterization. Syst. Evol. Microbiol. 2018, 4, 588–595. [Google Scholar]
- Shah, A.H.A.; Yasmeen, N.; Rahman, G.; Bilal, S. High electrocatalytic behavior of Ni impregnated conducting polymer coated platinum and graphite electrodes for electrooxidation of methanol. Electrochim. Acta 2017, 224, 468–474. [Google Scholar]
- Cai, X.; Cui, X.; Zu, L.; Zhang, Y.; Gao, X.; Lian, H.; Liu, Y.; Wang, X. Ultra-high electrical performance of nano nickel oxide and polyaniline composite materials. Polymers 2017, 9, 288. [Google Scholar] [CrossRef]
- Mohsen, R.J.; Morsi, S.M.M.; Selim, M.M. Electrical, thermal, morphological, and antibacterial studies of synthesized polyaniline/zinc oxide nanocomposites. Polym. Bull. 2019, 76, 1–21. [Google Scholar]
- Li, X.; Zhang, C.; Xin, S.; Yang, Z.; Li, Y.; Zhang, D.; Yao, P. Facile synthesis of MoS2/reduced graphene oxide@ polyaniline for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 21373–21380. [Google Scholar] [PubMed]
- Bilal, S.; Begum, B.; Gul, S.; Shah, A.H.A. PANI/DBSA/H2SO4; A promising and highly efficient electrode material for aqueous supercapacitors. Synth. Met. 2018, 235, 1–15. [Google Scholar]
- Syduly, B.S.; Palaniappan, S.; Sirnivas, P. Nano fibre polyaniline containing long chain and small molecule dopants and carbon composites for supercapacitors. Electrochim. Acta 2013, 95, 251–259. [Google Scholar]
- Fathi, M.; Saghafi, M.; Mahboubi, F.; Mohajerzadeh, S. Synthesis and electrochemical investigation of polyaniline/unzipped carbon nanotube composites as electrode material in supercapacitors. Synth. Met. 2014, 198, 345–356. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Guan, H.; Zhao, Y.; Zhang, B. Decoration of nickel hydroxide nanoparticles onto polypyrrole nanotubes with enhanced electrochemical performance for supercapacitors. J. Alloys Compd. 2017, 721, 731–740. [Google Scholar]
- Zhou, X.; Wang, A.; Pan, Y.; Yu, C.; Zou, Y.; Zhou, Y.; Chen, Q.; Wu, S. Facile synthesis of a Co3O4@carbon nanotubes/polyindole composite and its application in all-solid-state flexible supercapacitors. J. Mater. Chem. A 2015, 3, 13011–13015. [Google Scholar]
- Shah, A.-u.-H.A.; Khan, M.O.; Bilal, S.; Rahman, G.; Hung, H.V. Electrochemical co-deposition and characterization of polyaniline and manganese oxide nanofibrous composites for energy storage properties. Adv. Polym. Technol 2018, 37, 2230–2237. [Google Scholar]
- Shah, S.S.; Das, H.T.; Barai, H.R.; Aziz, M. Boosting the electrochemical performance of polyaniline by one-step electrochemical deposition on nickel foam for high-performance asymmetric supercapacitor. Polymers 2022, 14, 270. [Google Scholar]
- Majumder, M.; Choudhary, R.B.; Thakur, A.K.; Rout, C.S.; Gupta, G. Rare earth metal oxide (RE2O3; RE = Nd, Gd, and Yb) incorporated polyindole composites: Gravimetric and volumetric capacitive performance for supercapacitor applications. New J. Chem. 2018, 42, 5295–5308. [Google Scholar] [CrossRef]
- Singu, B.S.; Palaniappan, S.; Yoon, K.R. Polyaniline-nickel oxide nanocomposite for supercapacitor. J. Appl. Electrochem. 2016, 46, 1039–1047. [Google Scholar] [CrossRef]
| Electrode Material | E (Wh kg−1) | P (kW kg−1) | Stability/Cycles a | Ref. |
|---|---|---|---|---|
| CNTs/PIn/Co3O4 | 42.9 | 0.4 | 90.2%/5000 (1 A g−1) | [10] |
| PIn/CB/MoS2 | 8.7 | 2.03 | 92.3%/5000 (1 A g−1) | [12] |
| V2O5/PIn@AC | 38.7 | 0.9 | 91.1%/5000 (1 A g−1) | [13] |
| PIn/NiO | 17.5 | 19.3 | 114%/10,000 (1 A g−1) | [22] |
| PIn/Nd2O3 | 8.9 | 1.0 | 97.0%/5000 (3 A g−1) | [42] |
| PIn/Gd2O3 | <8.9 | 1.0 | 85.0%/5000 (3 A g−1) | [43] |
| PNZ | 42.1 | 13.7 | 78.5%/5000 (100 mV s−1) | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Humayun, H.; Begum, B.; Bilal, S.; Shah, A.u.H.A.; Röse, P. Polyindole Embedded Nickel/Zinc Oxide Nanocomposites for High-Performance Energy Storage Applications. Nanomaterials 2023, 13, 618. https://doi.org/10.3390/nano13030618
Humayun H, Begum B, Bilal S, Shah AuHA, Röse P. Polyindole Embedded Nickel/Zinc Oxide Nanocomposites for High-Performance Energy Storage Applications. Nanomaterials. 2023; 13(3):618. https://doi.org/10.3390/nano13030618
Chicago/Turabian StyleHumayun, Huriya, Bushra Begum, Salma Bilal, Anwar ul Haq Ali Shah, and Philipp Röse. 2023. "Polyindole Embedded Nickel/Zinc Oxide Nanocomposites for High-Performance Energy Storage Applications" Nanomaterials 13, no. 3: 618. https://doi.org/10.3390/nano13030618
APA StyleHumayun, H., Begum, B., Bilal, S., Shah, A. u. H. A., & Röse, P. (2023). Polyindole Embedded Nickel/Zinc Oxide Nanocomposites for High-Performance Energy Storage Applications. Nanomaterials, 13(3), 618. https://doi.org/10.3390/nano13030618









