Abstract
(1) Dental caries, periodontitis, or peri-implantitis are commensal infections related to oral biofilm former bacteria. Likewise, magnesium oxide nanoparticles (MgO-NPs) were studied to introduce them to the antibacterial properties of a few microorganisms. Considering this, the purpose of the present investigation was to determine the antibacterial properties of MgO-NPs on representative oral strains. (2) Methods: MgO-NPs with a cubic crystal structure were obtained by magnesium hydroxide mechanical activation. After synthesis, the MgO-NPs product was annealed at 800 °C (2 h). The MgO-NPs obtained were tested against ten oral ATCC strains at ten serial concentrations (1:1 20.0–0.039 mg/mL per triplicate) using the micro-broth dilution method to determine the minimal inhibitory concentration (MIC) or minimal bactericidal concentration (MIB). Measures of OD595 were compared against each positive control with a Student’s t-test. Viability was corroborated by colony-forming units. (3) Results: The polycrystalline structure had an average size of 21 nm as determined by X-ray diffraction and transmission electron microscopy (high resolution). Antimicrobial sensitivity was observed in Capnocytophaga gingivalis (MIB/MIC 10–5 mg/mL), Eikenella corrodens (MIB 10 mg/mL), and Streptococcus sanguinis (MIB 20 mg/mL) at high concentrations of the MgO-NPs and at lower concentrations of the MgO-NPs in Actinomyces israelii (MIB 0.039 mg/mL), Fusobacterium nucleatum subsp. nucleatum (MIB/MIC 5–2.5 mg/mL), Porphyromonas gingivalis (MIB 20 mg/mL/MIC 2.5 mg/mL), Prevotella intermedia (MIB 0.625 mg/mL), Staphylococcus aureus (MIC 2.5 mg/mL), Streptococcus mutans (MIB 20 mg/mL/MIC 0.321 mg/mL), and Streptococcus sobrinus (MIB/MIC 5–2.5 mg/mL). (4) Conclusions: The MgO-NPs’ reported antibacterial properties in all oral biofilm strains were evaluated for potential use in dental applications.
1. Introduction
The use of magnesium oxide as a nanomaterial (MgO-NPs) has increased, especially in the medical field, such as in diagnostics, detection, and biosensors of molecular behavior [1,2]. The antibacterial effect of nanoparticles based on magnesium oxide has shown effective properties on a wide range of microorganisms, for whom the antibacterial mechanism of action is due to the size [2,3] and dosage dependence [4,5]. There are different approximations to explain the antibacterial mechanism of MgO-NPs. The interaction of nanoparticles with bacteria increases the production of reactive oxygen species inside bacteria cells and Ca2+ ions concentrations. Additionally, the interaction of MgO-NPs with specific molecular sites of bacteria in the planktonic state can trigger membrane disruptions, leading to bacteria death and the cytoplasm bacteria alkalinization of pH from 7 to 10 with high concentrations of Mg2+ ions [5,6]. Other toxicity effects of MgO-NPs indicate the potential number of reactive groups dependent on superficial oxygen and the weak points on the bacterial surfaces [3,5]. Despite these studies, and unlike other metal oxide nanoparticles, there is not much information on MgO nanoparticles in biological and antimicrobial applications [2,7].
Therefore, it is important to deepen the study of MgO-NPs and to look for new forms of synthesis for their possible oral biological applications, given the excellent chemical and biocompatible properties of MgO [7,8,9]. Likewise, dental biofilm is compounded by around 400 species, with representative genera Actinomyces, Capnocytophaga, Eikenella, and Streptococcus [10,11,12]. Some of them are strongly involved in biofilm formation conjoint with dental problems such as caries or periodontitis. For example, Streptococcus mutans is considered an antimicrobial target for caries. Nevertheless, other acidogenic and lactic acid producers are strongly associated with dental demineralization, including Lactobacillus sp., Actinomyces sp., and Veillonella sp., among other cariogenic bacteria [13,14,15]. Furthermore, there is an association between periodontal diseases and a pathogenic biofilm compound made up of a high proportion of certain genera, such as Porphyromonas, Prevotella, and Fusobacterium [16]. On the other hand, oral and body commensals like Staphylococcus aureus are considered some of the most resistant strains and biofilm formers in medical devices. Additionally, the literature demonstrates their role in nosocomial and oral infections such as peri-implantitis [17,18,19].
Applications of nanoparticles in the dental field are possible, and the materials can be used for oral-disease preventive antiseptics and dental biomaterials to improve their properties and the quality of treatments [20,21,22]. Thus, some reports have evaluated the obtention of nano-material available for sale by testing the toxicity effects in humans. [23,24,25]. Therefore, it is necessary to seek no toxic elements that are also biocompatible for clinically safe use. In this work, we present MgO-NPs obtained by the mechanochemical method to evaluate their antibacterial activity against representative bacteria related to biofilm formation and recognized as commensal strains involved in oral infections such as dental caries or periodontal diseases.
2. Materials and Methods
2.1. Nanoparticles Materials
Magnesium hydroxide tetrahydrate, Mg(OH)2·4H2O (98%), and acetone CO(CH3)2 (99.5%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All elements were used without further preparation. Ultrapure water (ddH2O, 18 MΩ/cm) obtained from a Barnstead E-pure deionization system has been used.
2.2. Synthesis and Characterization of MgO-NPs
The synthesis was performed and described in a previous study [26], using a chemical reaction to obtain MgO-NPs expressed like this:
The X-ray diffraction patterns had been carried with radiation (CuKαλ = 1.5406 Å) in a diffractometer D5000 Siemens; the intensity of diffraction was analyzed as being between 2.5° and 70°, with 2θ steps of 0.02°, for 0.8 s each point. MgO-NPs’ average size (D) was calculated using their diffractograms by the Debye–Scherer equation, D = κλ/βcosθ, where κ the shape factor is equal to 0.9, CuKα radiation is λ, β is the full width at half the maximum intensity of the selected peaks (FWHM), and the Bragg angle is θ. FT-IR scanning was performed on the molecular materials in the form of KBr pellets and on films deposited on silicon wafers using a Nicolet spectrometer. Transmission electron micrographs were performed by transmission electron microscopy (high resolution), obtained through a JEOL 2010 FasTEM analysis microscope (Tokyo, Japan) at 200 kV by depositing a drop of the powdered MgO-NPs dispersed in ethanol on 300 mesh Cu grids coated in a carbon layer.
2.3. Antibacterial Susceptibility Testing
The bacterial species applied were acquired from the American Type Culture Collection (Rockville, MD, USA) from lyophilized stocks. The antibacterial susceptibility test was performed in a certified quality management system ISO: 9001:2015 from the Molecular Genetics Laboratory, at the dentistry school at UNAM.
The selection of oral commensals was based on resistance biofilm strains, and early and later biofilm colonizers, described in Table 1, all represent dental biofilm bacteria. All of the strains were cultured in an anaerobic chamber with an 80% N2, 10% CO2, and 10% H2 anaerobic environment, rehydrated in Mycoplasma broth base, and cultured in trypticase soy agar (TSA) enriched with 5 mL hemin, 0.05%, 500 mL distilled water UV/UF, 5 mL of menadione 0.005%, and 25 mL of defibrinated ram blood at 35 °C for 3–7 days. Each strain was harvested from TSA in pure cultures and resuspended in 1.8 µL microcentrifuge tubes with trypticase soy broth (TSB) enriched as well as TSA, and the optical density (OD) of each strain was read with the Eppendorf® Uvette® in a UV–visible spectrophotometer (Eppendorf) with each reader at an optical density λ = 600 nm (OD600). Every strain was adjusted to one to obtain 109 cells/mL. Then decimal 1:1 dissolution was performed to obtain a concentration with 106 cells/mL, and the solution was then transferred to 96-well clear polystyrene microplates (Corning®, Glendale, AZ, USA) for microbial susceptibility testing.

Table 1.
Oral species evaluated.
The bacterial susceptibility testing of oral strains was evaluated by the micro broth dilution test. The ten serial dissolutions (1:1) from 20 to 0.039 mg/mL of the MgO-NPs were tested against each evaluated bacteria (100 µL at 106 cells). Then 100 µL of each dispersion was added per well for a final volume of 200 µL. For the negative control of the inhibition it was used the TSB culture media with each strain, and then amoxicillin was added (1 mg/mL). The microplates of each bacteria strain were exposed to the MgO-NPs incubated in an anaerobic environment as previously described at 35 °C in an orbital that was shaken at 160 rpm for 72 h, except for P. gingivalis and P. intermedia with 120 h culture and additionally enrichment media with hemin, 0.05%. After incubation, each microplate was analyzed for absorbance reading. The absorption was measured in a spectrophotometer ultraviolet–visible (UV–Vis) Filter Max F5 multi-mode microplate reader; a wavelength of 595 nm was taken as the standard value for bacteria. An extra plate was done with all the serial dispersions for the MgO-NPs (in the absence of bacteria) with the purpose of subtracting the absorbance’s nanoparticles. For the determination of growth inhibition, to confirm cell viability after the susceptibility test, we recovered an aliquot per dispersion evaluated and performed an anaerobic re-culture from 5–7 days in a TSA-enriched solution to determine the resistance or susceptibility of each tested bacteria by the counting of colony-forming units (CFUs).
2.4. Data Analysis
Each triplicated assay was averaged, and data results were compared for each positive bacteria control with the Student’s t-test (IC at 95%) in SPSS software. For strict statistics for the results, the analysis was adjusted with multiple comparisons as follows, described in the formula p of 0.05 = 1 – (1 − k)3 [27], k = using the individual p-value of 0.0168.
A colony counter and a stereomicroscope were used for each re-culture sample in TSA enriched for the viability test by CFUs (Fisherbrand™). The interpretation of bacteriostatic sensitivity by minimum inhibitory concentration (MIC) was determined by the counting of visible colonies; the minimum bactericidal concentration (MBC) was reported when there was no growth, with CFUs = 0 and the resistance evaluations reported as CFUs = + (positive and uncountable growing) or as non-visible inhibition as previously reported [28].
3. Results
3.1. Characterization of MgO-NPs
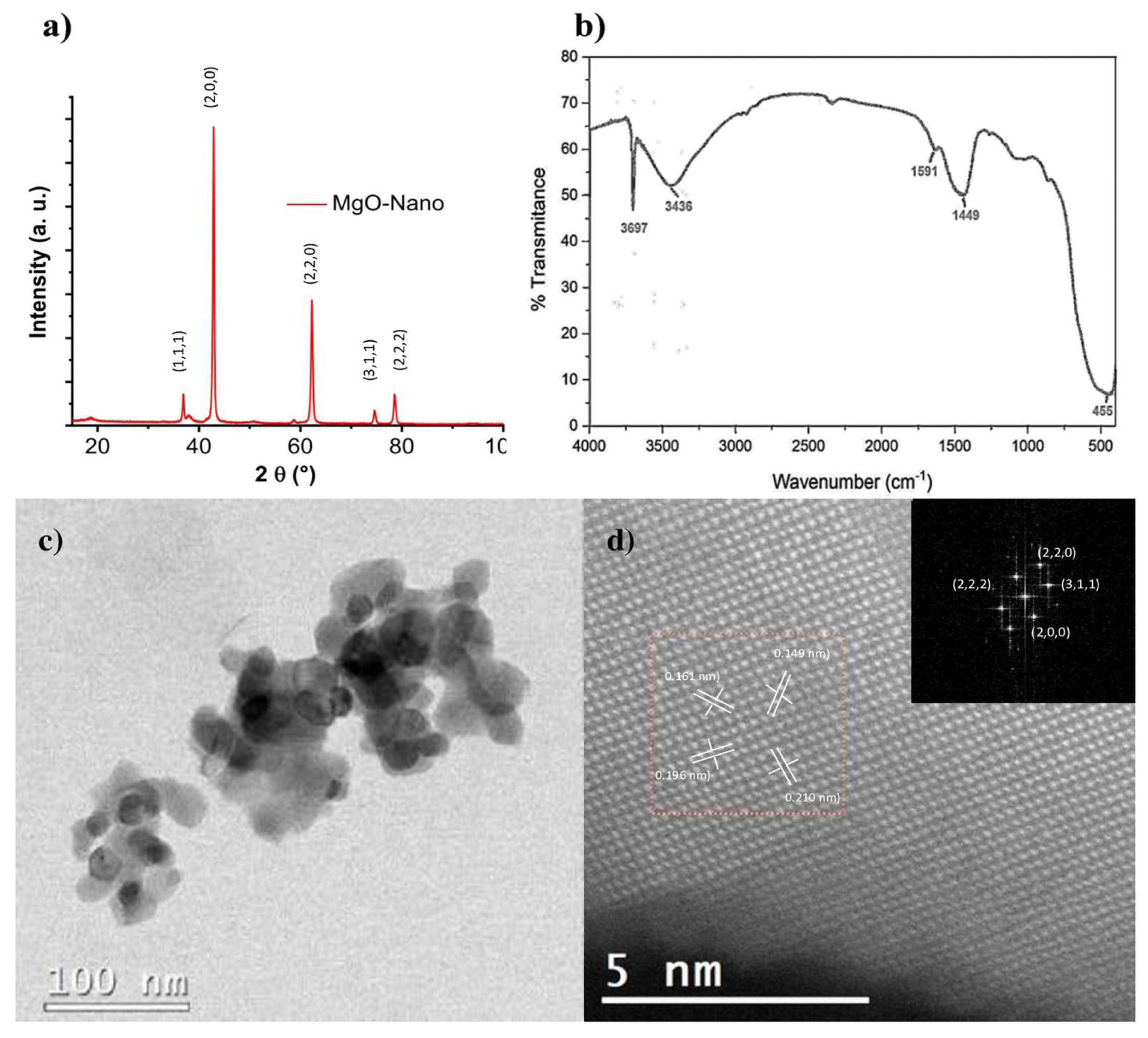
The X-ray diffraction patterns obtained from the MgO-NPs show a face-centered cubic (fcc) lattice with the space group Fm3m (Figure 1a). The peaks observed were indexed according to the Joint Committee on Powder Diffraction Standards (JCPDS) with the card number: 89-7746, and the corresponding crystal planes of MgO were indexed as (111), (200), (220), (311), and (222). The peaks in the XRD patterns corresponding to the single crystalline phase and the average crystallite sizes were calculated by the Scherrer equation, with the crystal plane at 200; the estimated size was 21 nm. Respecting the FT-IR spectrum (Figure 1b), the signals at 3697 cm−1 and 3436 cm−1 are characteristic of the O–H stretching band. The peaks at 1449–1591 cm−1 correspond to the bending vibration of the O–H; these strong bands appear due to the hygroscopic nature of MgO, whereas the strong peak around 455 cm−1 is due to the stretching vibrations of the bond between Mg and O in MgO-NPs. HRTEM micrographs (Figure 1c,d) indicate the shapes to be polyhedric, with sizes smaller than or close to 100 nm. Likewise, the analysis results in high resolution showed that the interplanar distances coincide with the reflections of the MgO crystal structure, as observed in the XRD results (Figure 1a).
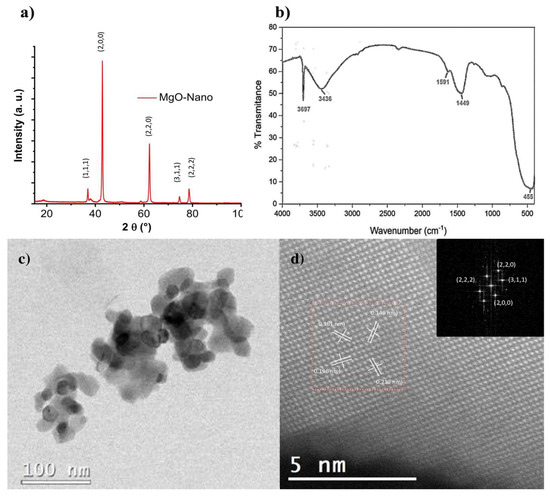
Figure 1.
Characterization of MgO-NPs. (a) In X-ray diffraction, the peaks observed were indexed according to the JCPDS with the card number: 89-7746, and the corresponding crystal planes of MgO were indexed to (111), (200), (220), (311) and (222). (b) In FT-IR spectra, the signals at 3697 cm−1 and 3436 cm−1 are characteristic of the O–H stretching band. The peaks at 1449–1591 cm−1 correspond to the bending vibration of the O–H; these strong bands appear due to the hygroscopic nature of MgO, whereas the strong peak around 455 cm−1 is due to the stretching vibrations of the bond between Mg and O. (c) Transmission electron microscopy (high resolution) of MgO-NPs indicate the shapes to be polyhedric, with sizes smaller than or close to 100 nm. Likewise, the results of the analysis conducted in high resolution (d), showed that the interplanar distances coincide with the reflections of the crystal structure of the MgO.
3.2. Antibacterial Susceptibility Testing
The microbial susceptibility of biofilm species is described in Table 2, with the OD595 values, the Student’s t-test significance after the micro broth dilution assays under ten MgO-NPs dissolutions (20–0.039 mg/mL), and the CFUs’ reported recovery of viability. The MgO-NPs exhibited bactericidal (MIB) and/or bacteriostatic (MIC) effects on all biofilms’ former strains.

Table 2.
Microbial susceptibility of biofilm former species under MgO-NPs.
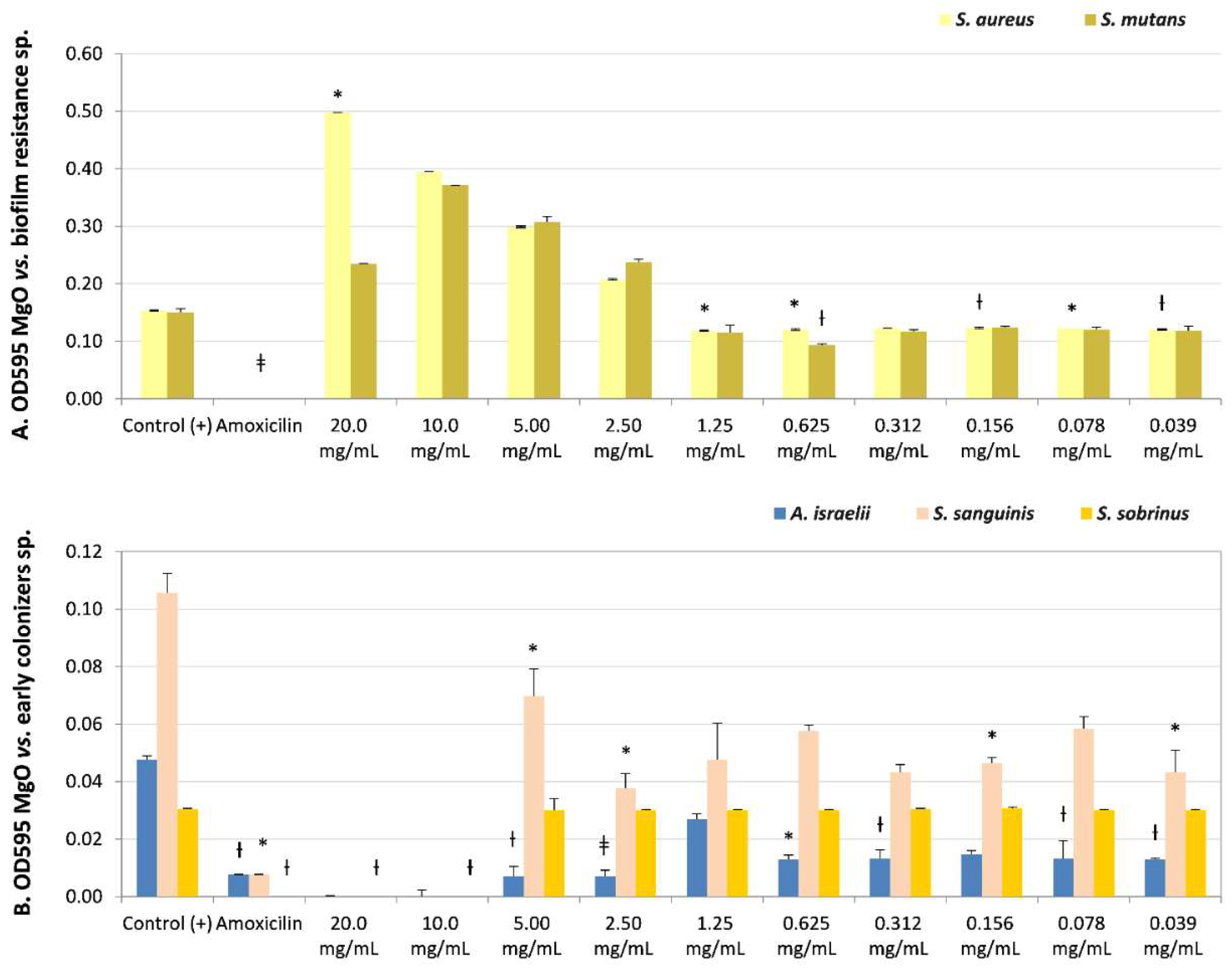
The lower sensitivity to the MgO-NPs at the maximum concentration evaluated (20 mg/mL) was in the biofilm-resistance strains S. aureus and S. mutans. However, both presented bactericidal (CFUs = 0) or bacteriostatic effects (CFUs = 15), respectively. S. mutans presented bacteriostatic sensibility (MIC) at lower concentrations (0.312 mg/mL, non-significant, NS, and CFUs = 0). Additionally, S. aureus presented an MIC of 2.5 mg/mL (NS, recovery of viability CFUs = 0) (Table 2 and Figure 2A) even though it had not presented a MIB at any dissolution.
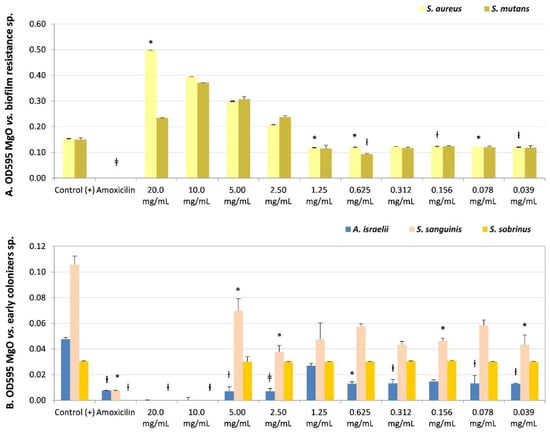
Figure 2.
Microbial susceptibility of MgO-NPs against (A) resistant biofilm strains Staphylococcus aureus and Streptococcus mutans. (B) Early colonizers were Actinomyces israelii, Streptococcus sanguinis, and Streptococcus sobrinus. OD595: optical density λ at 595 nm averaged triplicated values; Control (+): bacteria positive control, Amoxicillin: inhibition control, and MgO dissolution used (20 mg/mL to 0.039 mg/mL). Paired differences were determined by Student’s t-test after adjusting per triplicate results as previously described by [27]. The difference between each strain Control + versus each plate dilution is shown as * p < 0.05; ƚ p < 0.01; ǂ p < 0.001.
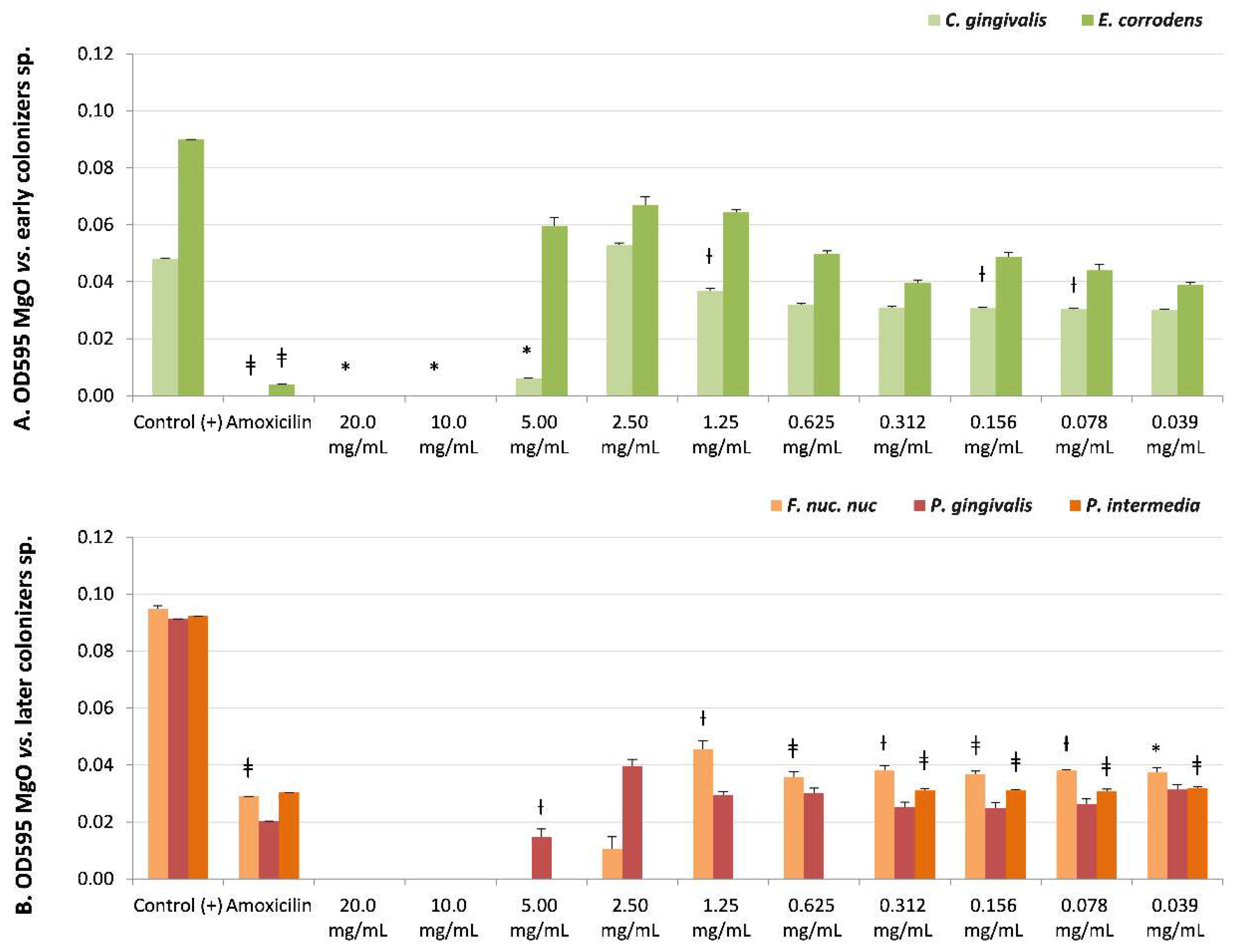
S. sanguinis (Table 2 and Figure 2B) (early colonizer), with one of the later colonizer biofilm strains P. gingivalis or a periodontopathogen strain (Table 2 and Figure 3B), reported no recovery of viability with CFUs = 0 until the maximum concentration evaluated (20 mg/mL). However, P. gingivalis presented bacteriostatic sensibility at a lower concentration of 2.50 mg/mL (NS, CFUs = 300).
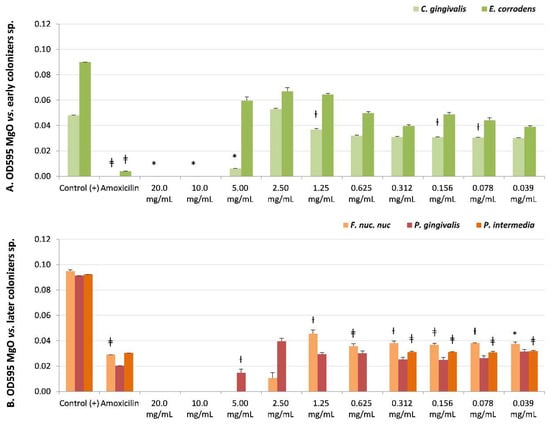
Figure 3.
Microbial susceptibility of MgO-NPs against (A) early colonizer strains Capnocytophaga gingivalis and Eikenella corrodens and (B) later colonizers F. nuc. nuc: Fusobacterium nucleatum subsp. nucleatum, Porphyromonas gingivalis, and Prevotella intermedia. OD595: optical density λ at 595 nm averaged triplicated values; Control (+): bacteria positive control; Amoxicillin: inhibition control, and MgO dissolution used (20 mg/mL to 0.039 mg/mL). Paired differences were determined by Student’s t-test after adjusting per triplicate results as previously described by [27]. The difference between each strain Control + versus each plate dilution is shown as * p < 0.05; ƚ p < 0.01; ǂ p < 0.001.
The strain with the opposite result, the most sensitivity to MgO-NPs, was the early colonizer Actinomyces israelii, with an MIB at 0.039 mg/mL (p < 0.01, CFUs = 0) (Table 2 and Figure 2B).
There was sensitivity to MgO-NPs at intermediate concentrations against early colonizers, such as Capnocytophaga gingivalis, with MIB at 10 mg/mL (p < 0.05, CFUs = 0) and MIC at 5 mg/mL (p < 0.05, CFUs = 6); Eikenella corrodens, with MIB at 10 mg/mL (NS, CFUs = 0) (Table 2 and Figure 3A); and Streptococcus sobrinus, with MIB at 5 mg/mL (NS, CFUs = 0) and MIC at 2.5 mg/mL (NS, CFUs = 3) (Table 2, and Figure 2B).
The highest susceptibility to the MgO-NPs was reported against the later colonizers or putative pathogenic strains, such as Fusobacterium nucleatum subsp. nucleatum, with MIB at 5 mg/mL (NS, CFUs = 0) and MIC at 2.5 mg/mL (NS, CFUs = 10), and Prevotella intermedia with MIB at 0.625 mg/mL (NS, CFUs = 0) (Table 2, and Figure 3B).
4. Discussion
The characteristics of MgO-NPs by mechanosynthesis are consistent with other studies that include the results of FT-IR [29,30,31] and HRTEM [32]. These characteristics contribute to the antibacterial properties of nanoparticles based on MgO affecting bacterial viability, especially due to nanoparticle size and concentration [2,3,33].
Concerning oral strains playing the role of biofilm formers as cariogenic or periodontal pathogens [11,16], the antimicrobial effect of MgO-NPs has been previously reported [2,3,7], as has as its cell biocompatibility at low concentrations [34]. However, the antibacterial properties of nanostructures based on MgO itself against subgingival strains have been poorly evaluated, finding little or no information [35,36,37].
Our study analyzed the effect of MgO-NPs without any other component, showing good antibacterial properties against cariogenic bacteria, such as S. mutans, against periodontopathogenic bacteria or later colonizers, and against S. aureus, one of the resistant biofilm strains of medical devices and commensal infections in peri-implantitis [17,18,19]. On the other hand, MgO NPs presented less sensitivity to early colonizers of subgingival biofilms, such as C. gingivalis, E. corrodens, and S. sobrinus, suggesting a selective antibacterial effect against pathogenic strains. The only exception was the A. israelii strain with the highest sensitivity to the MgO-NPs. Despite the benefits of A. israelii being an early colonizer of the dental biofilm, it has been a recognized commensal pathogen related to dental caries [13,15] and actinomycosis with odontogenic-originating infections [38]; hence, their higher sensitivity to MgO-NPs could be a positive feature to introduce in dental materials.
Another selective antibacterial effect was on the cariogenic strain S. mutans over the less cariogenic bacteria S. sanguinis. Competition between acidogenic and lactic acid bacteria continuously coexists in the dental caries process [12]. In this case, if the MgO-NPs had presented more sensitivity for S. mutans than S. salivarius, it could promote a less cariogenic microbiota in infants. Herein is the suggestion to apply MgO-NPs to dental materials before S. mutans infectivity until children are three years old or before the window of infectivity by the cariogenic strain is closed [39].
However, only one study reported a significant antibacterial effect on a recent cement, a glass ionomer material based on MgO-NPs, in the biofilm activity of cariogenic bacteria [36]; in this case, the glass ionomer cement has an antibacterial effect itself, and the nanoparticle size is similar to our study. Other dental material compounds with MgO-NPs promote MgO nanocellulose membranes introduced for periodontal tissue regeneration [40] with antibacterial properties against strains such as E. coli and A. actinomycetemcomitans. Some other dental types of cement modified with zein-MgO nanoparticles showed significant antimicrobial properties against the fungal species C. albicans and the bacteria strain S. aureus [35]. In the present study, the best antibacterial susceptibility was observed in MgO-NPs themselves, without combination with another material or substance, on periodontal or putative pathogens or later colonizers of the subgingival biofilm P. gingivalis, P. intermedia, and F. nucleatum subsp. nucleatum.
The application of nano-composed dental materials may be a solution to disturb biofilm formation [41] in commensal infections, such as caries, periodontitis, or peri-implantitis, and also as a therapeutic tool to combat microbial resistance, for example, periodontal treatments coadjutant as antiseptics, since the resistance of used antiseptics or antibiotics has no selectivity to periodontal pathogen strains [42]. The present research results proposed the possibility of introducing these MgO-NPs to antiseptics or dental materials due to the antibacterial sensitivity to cariogenic strains such as S. mutans, to periodontal pathogens as later colonizers of dental biofilm, and to biofilm-resistant strains such as S. aureus.
5. Conclusions
The MgO-NPs had reported antibacterial properties in all oral biofilm strains evaluated for potential use in dental applications due to the antibacterial sensitivity at low concentrations for the cariogenic strain S. mutans over S. sanguinis, for the periodontal and putative pathogens over early biofilm colonizers, and for S. aureus, at the evaluated concentrations.
Author Contributions
Conceptualization and methodology, A.-P.R.-H., A.L.V.-J. and L.-A.X.-F.; validation and formal analysis A.-P.R.-H. and A.L.V.-J., investigation and resources, A.-P.R.-H., A.L.V.-J. and A.R.V.-O.; writing—original draft preparation and writing—review and editing, A.-P.R.-H., A.L.V.-J., A.R.V.-O., M.O.-M. and L.-A.X.-F.; funding acquisition, A.-P.R.-H., A.L.V.-J., A.R.V.-O., M.O.-M. and L.-A.X.-F. All authors have read and agreed to the published version of the manuscript.
Funding
UNAM-PAPIIT grant projects IA104823 and IA203622.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors declare that they have followed the protocols of the School of Dentistry of the National Autonomous University of Mexico (UNAM). The authors are the owners of this document, as well as the data results when requested
Acknowledgments
All authors gratefully acknowledge the support for this research from the Dentistry Postgraduate School by the UNAM-PAPIIT grant projects (IA104823 and IA203622) and by the Molecular Genetics Laboratory with Certificate Management System ISO:9001:2015.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhattarai, P.; Hameed, S. Basics of biosensors and nanobiosensors. In Nanobiosensors: From Design to Applications; Wiley: Hoboken, NJ, USA, 2020; pp. 1–22. [Google Scholar]
- Raghunath, A.; Perumal, E. Metal oxide nanoparticles as antimicrobial agents: A promise for the future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef]
- Tang, Z.-X.; Lv, B.-F. MgO nanoparticles as antibacterial agent: Preparation and activity. Braz. J. Chem. Eng. 2014, 31, 591–601. [Google Scholar] [CrossRef]
- An, Y.; Zhang, K.; Wang, F.; Lin, L.; Guo, H. Removal of organic matters and bacteria by nano-MgO/GAC system. Desalination 2011, 281, 30–34. [Google Scholar] [CrossRef]
- Nguyen, N.-Y.; Grelling, N.; Wetteland, C.L.; Rosario, R.; Liu, H. Antimicrobial activities and mechanisms of magnesium oxide nanoparticles (nMgO) against pathogenic bacteria, yeasts, and biofilms. Sci. Rep. 2018, 8, 1–23. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227. [Google Scholar] [CrossRef]
- Muñiz-Diaz, R.; Cardoso-Avila, P.E.; Pérez Tavares, J.A.; Patakfalvi, R.; Villa-Cruz, V.; Pérez-Ladrón-de-Guevara, H.; Gutiérrez-Coronado, O.; Arteaga-Garibay, R.I.; Saavedra-Arroyo, Q.E.; Marañón-Ruiz, V.F. Two-step triethylamine-based synthesis of MgO nanoparticles and their antibacterial effect against pathogenic bacteria. Nanomaterials 2021, 11, 410. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Manivannan, G.; Kim, S.J.; Jeyasubramanian, K.; Premanathan, M. Antibacterial activity of MgO nanoparticles based on lipid peroxidation by oxygen vacancy. J. Nanoparticle Res. 2012, 14, 1–10. [Google Scholar] [CrossRef]
- Jain, N.; Marwaha, N.; Verma, R.; Gupta, B.K.; Srivastava, A.K. Facile synthesis of defect-induced highly-luminescent pristine MgO nanostructures for promising solid-state lighting applications. RSC Adv. 2016, 6, 4960–4968. [Google Scholar] [CrossRef]
- Xiao, C.; Ran, S.; Huang, Z.; Liang, J. Bacterial diversity and community structure of supragingival plaques in adults with dental health or caries revealed by 16S pyrosequencing. Front. Microbiol. 2016, 7, 1145. [Google Scholar] [CrossRef]
- Becker, M.R.; Paster, B.J.; Leys, E.J.; Moeschberger, M.L.; Kenyon, S.G.; Galvin, J.L.; Boches, S.K.; Dewhirst, F.E.; Griffen, A.L. Molecular analysis of bacterial species associated with childhood caries. J. Clin. Microbiol. 2002, 40, 1001–1009. [Google Scholar] [CrossRef]
- Kreth, J.; Merritt, J.; Shi, W.; Qi, F. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J. Bacteriol. 2005, 187, 7193–7203. [Google Scholar] [CrossRef]
- Beighton, D. Can the ecology of the dental biofilm be beneficially altered? Adv. Dent. Res. 2009, 21, 69–73. [Google Scholar] [CrossRef]
- Kara, D.; Luppens, S.B.; Ten Cate, J.M. Differences between single-and dual-species biofilms of Streptococcus mutans and Veillonella parvula in growth, acidogenicity and susceptibility to chlorhexidine. Eur. J. Oral Sci. 2006, 114, 58–63. [Google Scholar] [CrossRef]
- Takahashi, N.; Nyvad, B. The role of bacteria in the caries process: Ecological perspectives. J. Dent. Res. 2011, 90, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D. Periodontal microbial ecology. Periodontol 2000 2005, 38, 135–187. [Google Scholar] [CrossRef] [PubMed]
- Archer, N.; Mazaitis, M.; Costerton, J.; Leid, J.; Powers, M.; Shirtliff, M. Staphylococcus aureus biofilms: Properties, regulation, and roles in human disease. Virulence 2011, 2, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Persson, G.R.; Renvert, S. Cluster of bacteria associated with peri-implantitis. Clin. Implant. Dent. Relat. Res. 2014, 16, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Albertini, M.; López-Cerero, L.; O’Sullivan, M.G.; Chereguini, C.F.; Ballesta, S.; Ríos, V.; Herrero-Climent, M.; Bullón, P. Assessment of periodontal and opportunistic flora in patients with peri-implantitis. Clin. Oral Implant. Res. 2015, 26, 937–941. [Google Scholar] [CrossRef]
- Hamouda, I.M. Current perspectives of nanoparticles in medical and dental biomaterials. J. Biomed. Res. 2012, 26, 143–151. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mukherjee, S.; Mishra, M. Nanoparticles used in dentistry: A review. J. Oral Biol. Craniofacial Res. 2018, 8, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Vasiliu, S.; Racovita, S.; Gugoasa, I.A.; Lungan, M.A.; Popa, M.; Desbrieres, J. The benefits of smart nanoparticles in dental applications. Int. J. Mol. Sci. 2021, 22, 2585. [Google Scholar] [CrossRef]
- Vega-Jiménez, A.; Almaguer-Flores, A.; Flores-Castañeda, M.; Camps, E.; Uribe-Ramírez, M.; Aztatzi-Aguilar, O.; De Vizcaya-Ruiz, A. Bismuth subsalicylate nanoparticles with anaerobic antibacterial activity for dental applications. Nanotechnology 2017, 28, 435101. [Google Scholar] [CrossRef]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of nanoparticles and an overview of current experimental models. Iran. Biomed. J. 2016, 20, 1. [Google Scholar]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe nanoparticles: Are we there yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef]
- Vázquez-Olmos, A.R.; Vega-Jiménez, A.L.; Paz-Díaz, B. Mechanosynthesis and antimicrobial effect of nanostructured metal oxides. Mundo Nano Rev. Interdiscip. En Nanociencias Y Nanotecnología 2018, 11, 29–44. [Google Scholar]
- Socransky, S.S.; Haffajee, A.D.; Smith, C.; Dibart, S. Relation of counts of microbial species to clinical status at the sampled site. J. Clin. Periodontol. 1991, 18, 766–775. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), 5–16. [Google Scholar] [CrossRef]
- Modwi, A.; Khezami, L.; Taha, K.K.; Idriss, H. Flower buds like MgO nanoparticles: From characterisation to indigo carmine elimination. Z. Für Nat. A 2018, 73, 975–983. [Google Scholar] [CrossRef]
- Manikandan, S.; Rajan, K.S. Rapid synthesis of MgO nanoparticles & their utilization for formulation of a propylene glycol based nanofluid with superior transport properties. RSC Adv. 2014, 4, 51830–51837. [Google Scholar]
- Choudhury, B.; Choudhury, A. Microstructural, optical and magnetic properties study of nanocrystalline MgO. Mater. Res. Express 2014, 1, 025026. [Google Scholar] [CrossRef]
- Patil, A.B.; Bhanage, B.M. Novel and green approach for the nanocrystalline magnesium oxide synthesis and its catalytic performance in Claisen–Schmidt condensation. Catal. Commun. 2013, 36, 79–83. [Google Scholar] [CrossRef]
- Huang, L.; Li, D.; Lin, Y.; Evans, D.G.; Duan, X. Influence of nano-MgO particle size on bactericidal action against Bacillus subtilis var. niger. Chin. Sci. Bull. 2005, 50, 514–519. [Google Scholar] [CrossRef]
- Kumaran, R.S.; Choi, Y.K.; Singh, V.; Song, H.J.; Song, K.G.; Kim, K.J.; Kim, H.J. In vitro cytotoxic evaluation of MgO nanoparticles and their effect on the expression of ROS genes. Int. J. Mol. Sci. 2015, 16, 7551–7564. [Google Scholar] [CrossRef]
- Naguib, G.H.; Nassar, H.M.; Hamed, M.T. Antimicrobial properties of dental cements modified with zein-coated magnesium oxide nanoparticles. Bioact. Mater. 2022, 8, 49–56. [Google Scholar] [CrossRef]
- Noori, A.J.; Kareem, F.A. The effect of magnesium oxide nanoparticles on the antibacterial and antibiofilm properties of glass-ionomer cement. Heliyon 2019, 5, e02568. [Google Scholar] [CrossRef]
- Naguib, G.H.; Hosny, K.M.; Hassan, A.H.; Al Hazmi, F.; Al Dharrab, A.; Alkhalidi, H.M.; Hamed, M.T.; Alnowaiser, A.M.; Pashley, D.H. Zein based magnesium oxide nanoparticles: Assessment of antimicrobial activity for dental implications. Pak. J. Pharm. Sci. 2018, 31, 245–250. [Google Scholar]
- Dastgir, R.; Sohrabi, M. Periapical Actinomycosis: A Rare Subdivision of Cervicofacial Actinomycosis, Review of the Literature, and a Case Report. Case Rep. Dent. 2022, 2022, 7323268. [Google Scholar] [CrossRef]
- Mattos-Graner, R.O.; Klein, M.I.; Smith, D.J. Lessons learned from clinical studies: Roles of mutans streptococci in the pathogenesis of dental caries. Curr. Oral Health Rep. 2014, 1, 70–78. [Google Scholar] [CrossRef]
- Peng, W.; Ren, S.; Zhang, Y.; Fan, R.; Zhou, Y.; Li, L.; Xu, X.; Xu, Y. MgO Nanoparticles-incorporated PCL/gelatin-derived coaxial electrospinning nanocellulose membranes for periodontal tissue regeneration. Front. Bioeng. Biotechnol. 2021, 9, 668428. [Google Scholar] [CrossRef]
- Shkodenko, L.; Kassirov, I.; Koshel, E. Metal oxide nanoparticles against bacterial biofilms: Perspectives and limitations. Microorganisms 2020, 8, 1545. [Google Scholar] [CrossRef]
- Sreenivasan, P.; Gaffar, A. Antiplaque biocides and bacterial resistance: A review. J. Clin. Periodontol. 2002, 29, 965–974. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).