Encapsulation of Olive Leaf Polyphenol-Rich Extract in Polymeric Micelles to Improve Its Intestinal Permeability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Olea europaea L. Extract (OPA40) Preparation
2.3. Chromatography Conditions
2.4. Preparation of Micelles (MM) and Polyphhenol-Extract Loaded Micelles (MM-OPA40)
2.5. Characterization of Micelles
2.5.1. Particle Size, ζ-Potential, and Morphological Characterization
2.5.2. Encapsulation Efficiency
2.5.3. Cloud Point and Critical Micellar Concentration Determination
2.6. Lyophilization
2.7. Storage Stability of MM-OPA40 and Freeze-Dried MM-OPA40
2.8. In Vitro Release Studies
2.9. Parallel Artificial Membrane Permeability Assay (PAMPA)
2.10. Caco-2 Cell Line
2.11. MTT Assay
2.12. Caco-2 Cell Permeability Assay
2.12.1. Formation of the Cell Monolayer and the Evaluation of Membrane Integrity
2.12.2. Transmembrane Transport Study
3. Results and Discussion
3.1. Characterization of the Extract and Preparation of Mixed Micelles
3.2. Determination of the Cloud Point
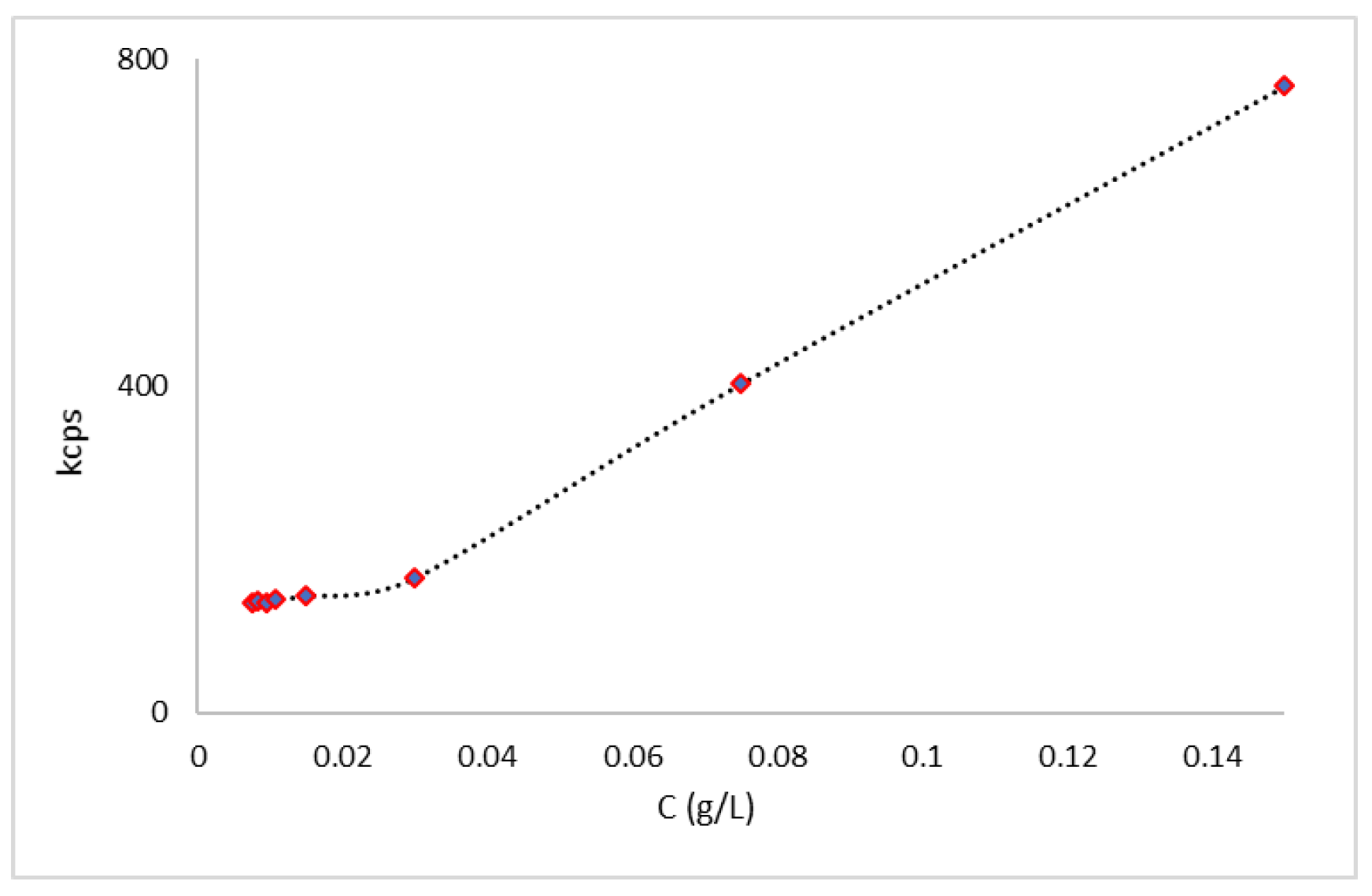
3.3. Determination of Critical Micellar Concentration via Light Scattering Techniques
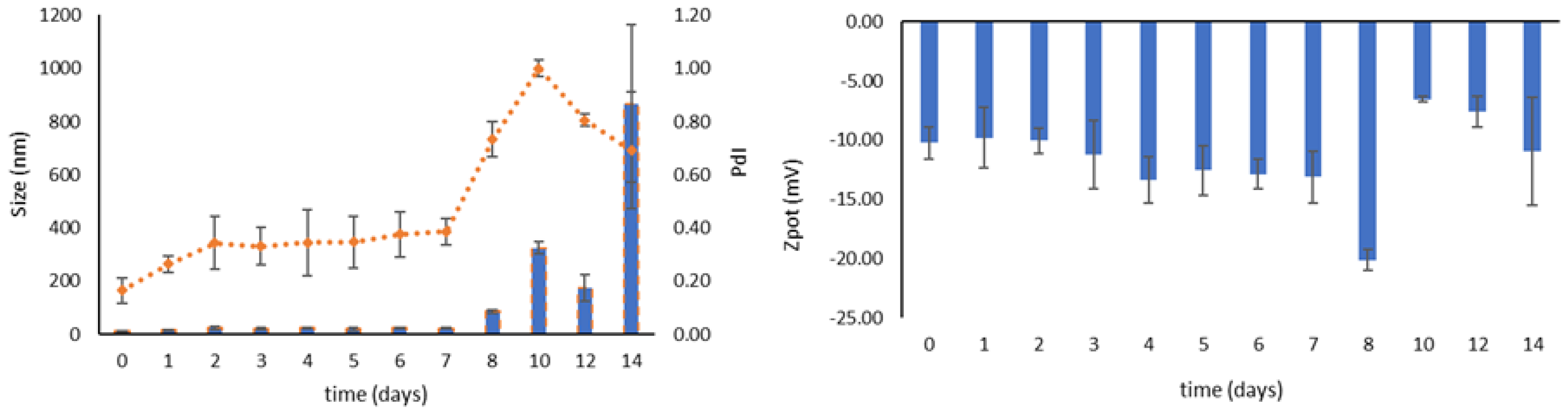
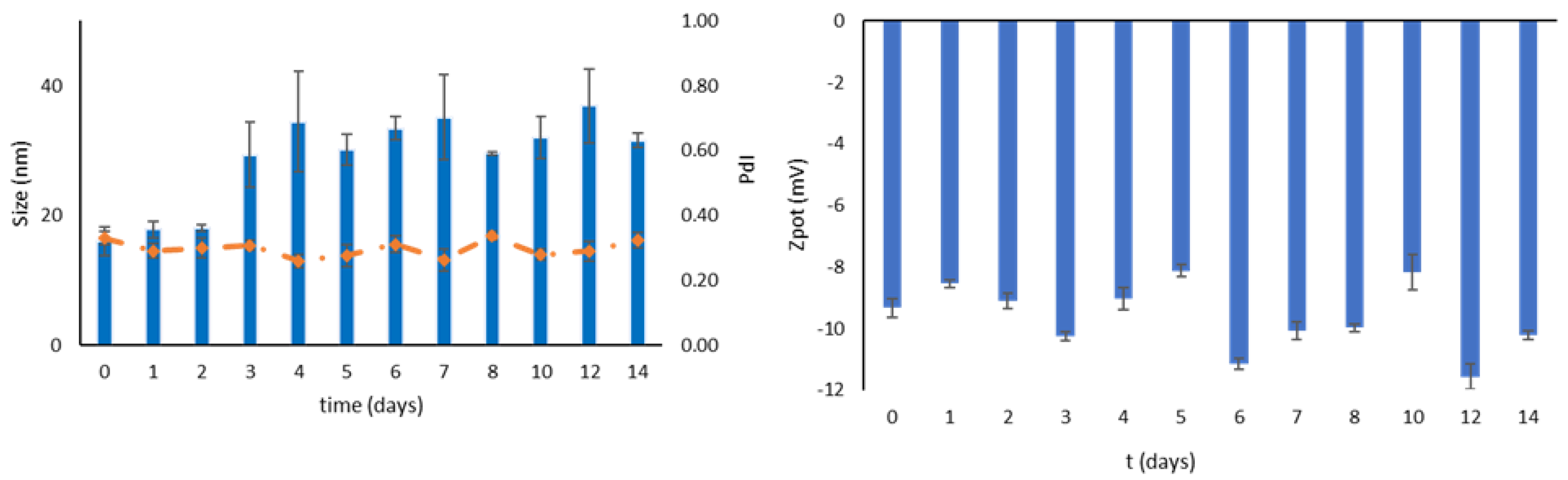
3.4. Stability during Storage
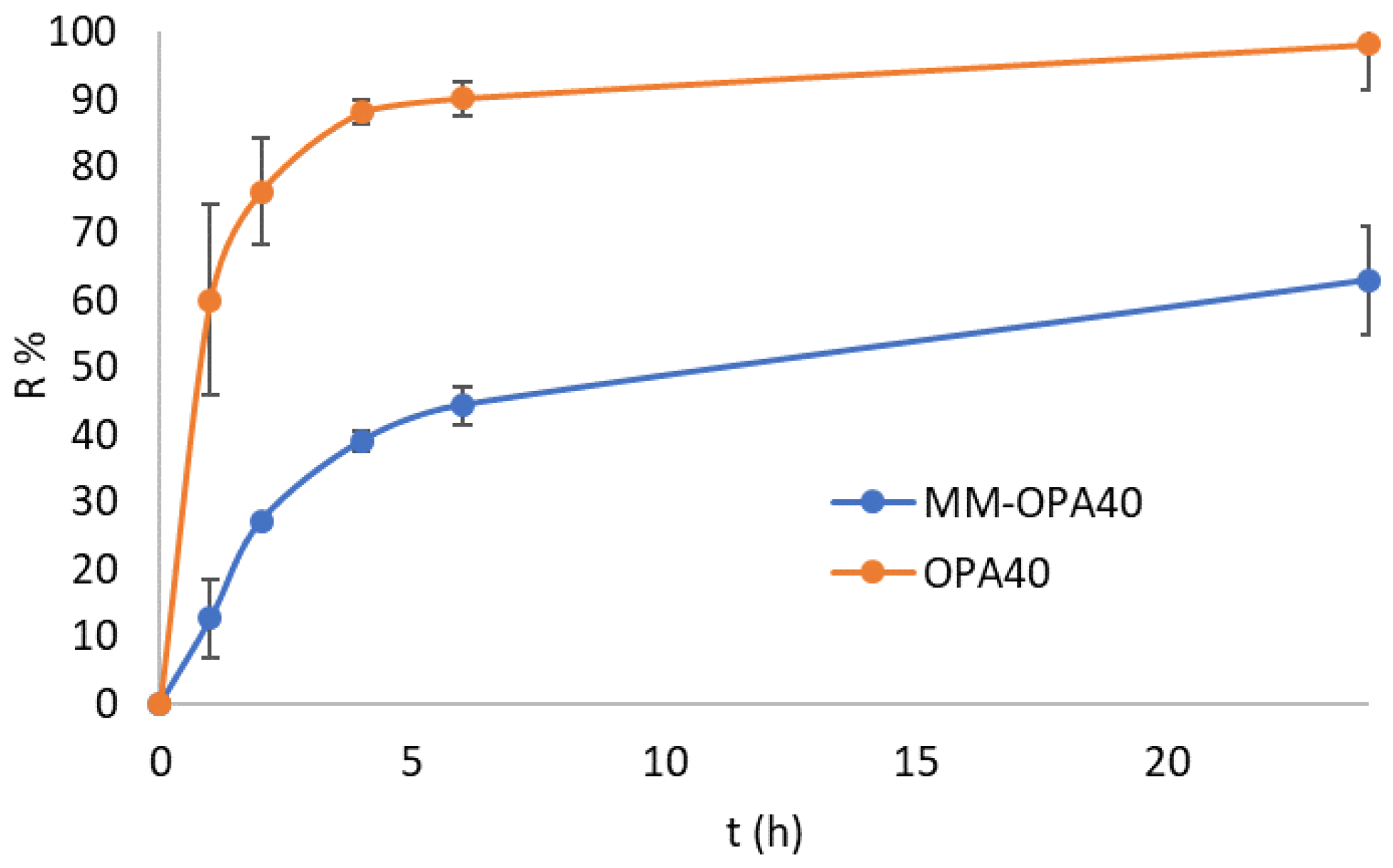
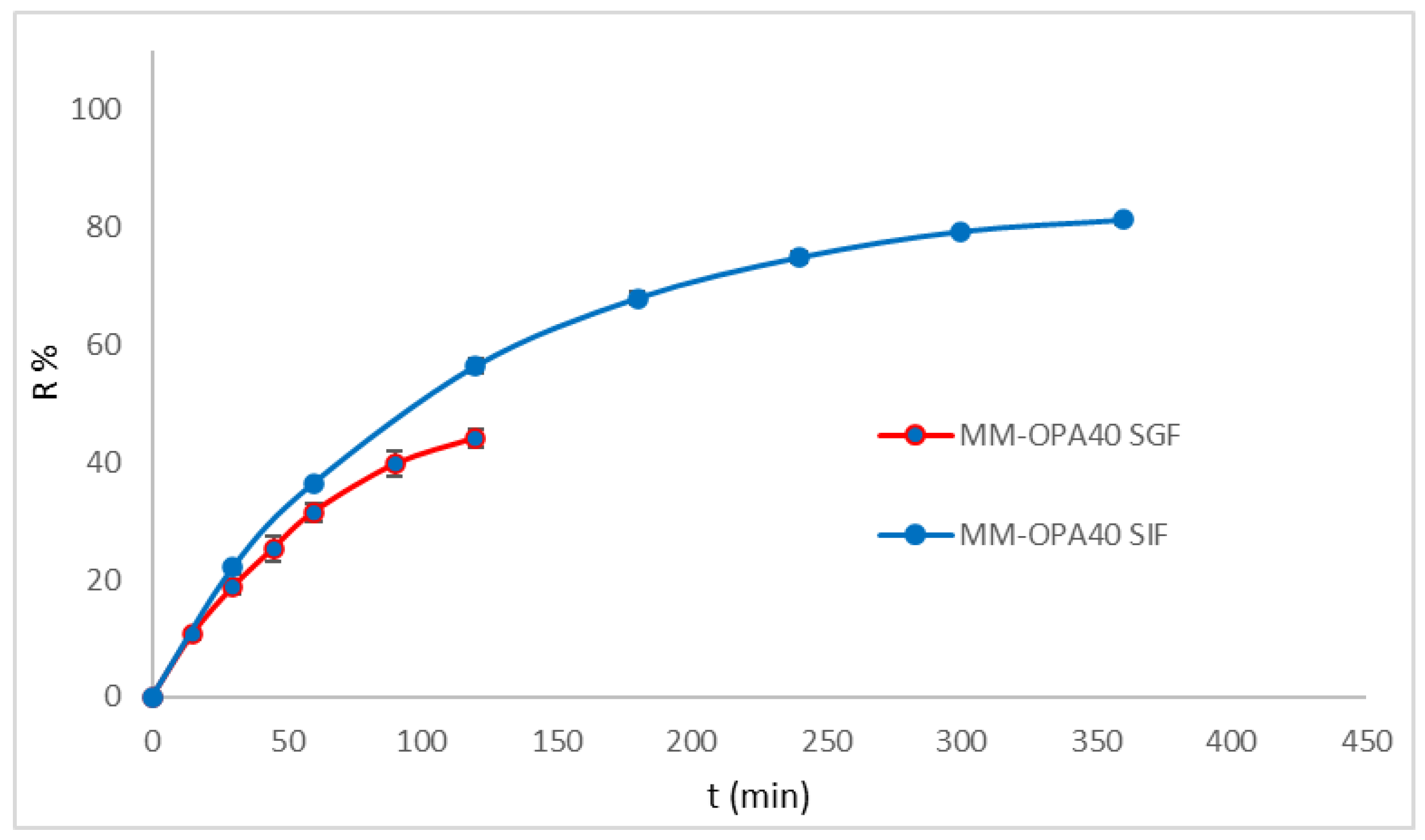
3.5. In Vitro Release Study
3.6. Parallel Artificial Membrane Permeability Assay (PAMPA)
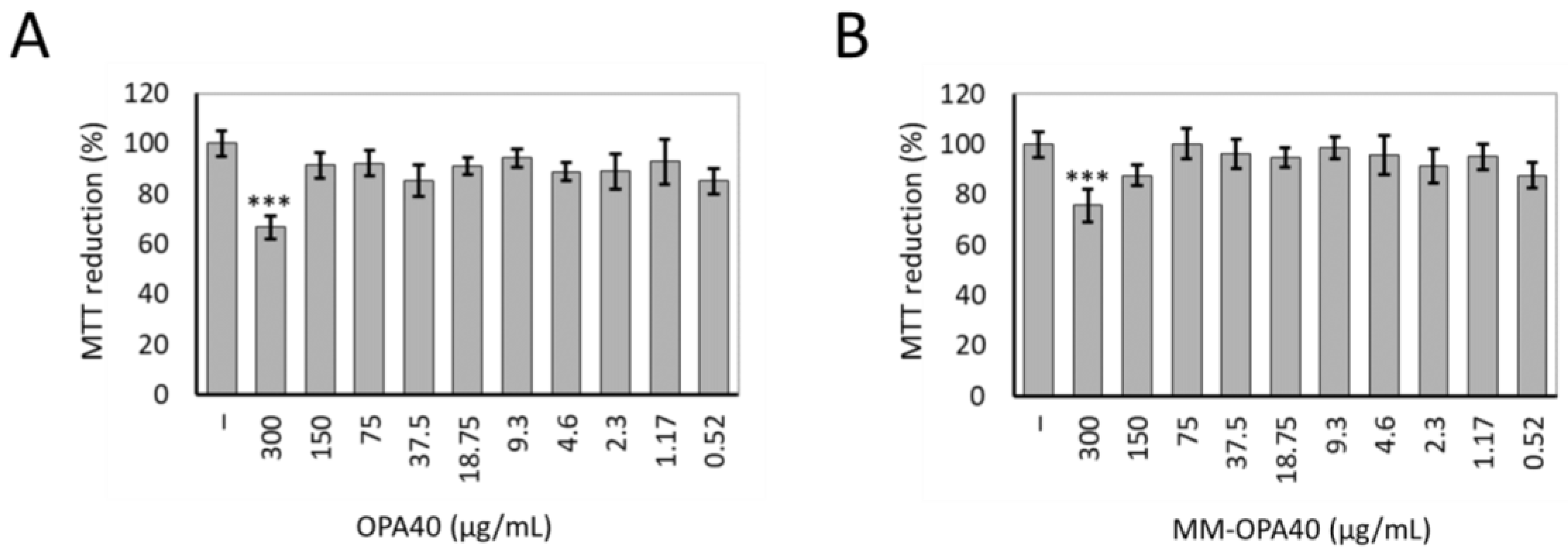
3.7. Effect of OLE40 and MM-OPA40 on Caco-2 Cell Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Z.; Jia, X.; Zheng, Z. Chemical composition and nutritional function of olive (Olea europaea L.): A review. Phytochem. Rev. 2018, 17, 1091–1110. [Google Scholar] [CrossRef]
- Markhali, F.S.; Teixeira, J.A.; Rocha, C.M.R. Olive Tree Leaves—A Source of Valuable Active Compounds. Processes 2020, 8, 1177. [Google Scholar] [CrossRef]
- Barbaro, B.; Toietta, G.; Maggio, R.; Arciello, M.; Tarocchi, M.; Galli, A.; Balsano, C. Effects of the Olive-Derived Polyphenol Oleuropein on Human Health. Int. J. Mol. Sci. 2014, 15, 18508–18524. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.H.; Saari, N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)—A review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef] [PubMed]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological activities of the natural antioxidant oleuropein: Exceeding the expectation—A mini-review. J. Funct. Foods 2015, 18, 926–940. [Google Scholar] [CrossRef]
- Omar, S.H. Oleuropein in Olive and its Pharmacological Effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Miller, A.; Tardugno, R.; Pergolizzi, S. Chemical analysis, biological and therapeutic activities of Olea europaea L. extracts. Nat. Prod. Res. 2022, 36, 2932–2945. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, A.; Martínez-Ortega, A.J.; Remón-Ruiz, P.J.; Piñar-Gutiérrez, A.; Pereira-Cunill, J.L.; García-Luna, P.P. Therapeutic properties and use of extra virgin olive oil in clinical nutrition: A narrative review and literature update. Nutrients 2022, 14, 1440. [Google Scholar] [CrossRef]
- Parkinson, L.; Cicerale, S. The Health Benefiting Mechanisms of Virgin Olive Oil Phenolic Compounds. Molecules 2016, 21, 1734. [Google Scholar] [CrossRef]
- Micheli, L.; Bertini, L.; Bonato, A.; Villanova, N.; Caruso, C.; Caruso, M.; Bernini, R.; Tirone, F. Role of Hydroxytyrosol and Oleuropein in the Prevention of Aging and Related Disorders: Focus on Neurodegeneration, SkeletalMuscle Dysfunction and Gut Microbiota. Nutrients 2023, 15, 1767. [Google Scholar] [CrossRef]
- Malliou, F.; Andriopoulou, C.E.; Gonzalez, F.J.; Kofinas, A.; Skaltsounis, A.L.; Konstandi, M. Oleuropein-Induced Acceleration of Cytochrome P450-Catalyzed Drug Metabolism: Central Role for Nuclear Receptor Peroxisome Proliferator-Activated Receptor α. Drug Metab. Dispos. 2021, 49, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R.; Vella, N.; Rosignoli, P. Epigenetic Modifications Induced by Olive Oil and Its Phenolic Compounds: A Systematic Review. Molecules 2021, 26, 273. [Google Scholar] [CrossRef] [PubMed]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a Bioactive Compound from Olea europaea L., as a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Markopoulos, C.; Vertzoni, M.; Agalias, A.; Magiatis, P.; Reppas, C. Stability of oleuropein in the human proximal gut. J. Pharm. Pharmacol. 2009, 61, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Navarro, M.E.; Cebrián-Tarancón, C.; Oliva, J.; Salinas, M.R.; Alonso, G.L. Oleuropein Degradation Kinetics in Olive Leaf and Its Aqueous Extracts. Antioxidants 2021, 10, 1963. [Google Scholar] [CrossRef] [PubMed]
- Micheli, L.; Di Cesare Mannelli, L.; Mosti, E.; Ghelardini, C.; Bilia, A.R.; Bergonzi, M.C. Antinociceptive Action of Thymoquinone-Loaded Liposomes in an In Vivo Model of Tendinopathy. Pharmaceutics 2023, 15, 1516. [Google Scholar] [CrossRef] [PubMed]
- Vasarri, M.; Degl’Innocenti, D.; Albonetti, L.; Bilia, A.R.; Bergonzi, M.C. Pentacyclic Triterpenes from Olive Leaves Formulated in Microemulsion: Characterization and Role in De Novo Lipogenesis in HepG2 Cells. Int. J. Mol. Sci. 2023, 24, 12113. [Google Scholar] [CrossRef]
- Chavda, V.P.; Patel, A.B.; Mistry, K.J.; Suthar, S.F.; Wu, Z.-X.; Chen, Z.-S.; Hou, K. Nano-Drug Delivery Systems Entrapping Natural Bioactive Compounds for Cancer: Recent Progress and Future Challenges. Front. Oncol. 2022, 12, 867655. [Google Scholar] [CrossRef]
- Suchiwa, P.-O.; Piyameth, D.; Waree, T. Trends in advanced oral drug delivery system for curcumin: A systematic review. J. Control. Release 2022, 348, 335–345. [Google Scholar] [CrossRef]
- Bergonzi, M.C.; Vasarri, M.; Marroncini, G.; Barletta, E.; Degl’Innocenti, D. Thymoquinone-Loaded Soluplus®-Solutol® HS15 Mixed Micelles: Preparation, In Vitro Characterization, and Effect on the SH-SY5Y Cell Migration. Molecules 2020, 25, 4707. [Google Scholar] [CrossRef]
- Piazzini, V.; Landucci, E.; Urru, M.; Chiarugi, A.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Bergonzi, M.C. Enhanced dissolution, permeation and oral bioavailability of aripiprazole mixed micelles: In vitro and in vivo evaluation. Int. J. Pharm. 2020, 583, 119361. [Google Scholar] [CrossRef] [PubMed]
- Cagel, M.; Tesan, F.C.; Bernabeu, E.; Salgueiro, M.J.; Zubillaga, M.B.; Moretton, M.A.; Chiappetta, D.A. Polymeric Mixed Micelles as Nanomedicines: Achievements and Perspectives. Eur. J. Pharm. Biopharm. 2017, 113, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, G.; Satturwar, P.; Jones, M.C.; Furtos, A.; Leroux, J.-C. Polymeric micelles for oral drug delivery. Eur. J. Pharm. Biopharm. 2010, 76, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine Scale-up Technologies: Feasibilities and Challenges. AAPS PharmSciTech 2014, 15, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Edgecombe, S.C.; Stretch, G.L.; Hayball, P.J. Oleuropein, an antioxidant polyphenol from olive oil, is poorly absorbed from isolated perfused rat intestine. J. Nutr. 2000, 130, 2996–3002. [Google Scholar] [CrossRef]
- Piazzini, V.; Rosseti, C.; Bigagli, E.; Luceri, C.; Bilia, A.R.; Bergonzi, M.C. Prediction of permeation and cellular transport of Silybum marianum extract formulated in a nanoemulsion by using PAMPA and Caco-2 cell models. Planta Med. 2017, 83, 1184–1193. [Google Scholar] [CrossRef]
- Ottaviani, G.; Martel, S.; Carrupt, P.-A. Parallel Artificial Membrane Permeability Assay: A New Membrane for the Fast Prediction of Passive Human Skin Permeability. J. Med. Chem. 2006, 49, 3948–3954. [Google Scholar] [CrossRef]
- Angelis, I.D.; Turco, L. Caco-2 Cells as a Model for Intestinal Absorption. Curr. Prot. Toxicol. 2011, 47, 20–26. [Google Scholar] [CrossRef]
- Ding, X.; Hu, X.; Chen, Y.; Xie, J.; Ying, M.; Wang, Y.; Yu, Q. Differentiated Caco-2 cell models in food-intestine interaction study: Current applications and future trends. Trends Food Sci. Technol. 2021, 107, 455–465. [Google Scholar] [CrossRef]
- García-Estévez, I.; Alcalde-Eon, C.; Escribano-Bailón, M.T. Flavanol Quantification of Grapes via Multiple Reaction Monitoring Mass Spectrometry. Application to Differentiation among Clones of Vitis vinifera L. cv. Rufete Grapes. J. Agric. Food Chem. 2017, 65, 6359–6368. [Google Scholar] [CrossRef]
- Quirantes-Piné, R.; Lozano-Sánchez, J.; Herrero, M.; Ibáñez, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC–ESI–QTOF–MS as a Powerful Analytical Tool for Characterising Phenolic Compounds in Olive-leaf Extracts. Phytochem. Anal. 2013, 24, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Nicolì, F.; Negro, C.; Vergine, M.; Aprile, A.; Nutricati, E.; Sabella, E.; Miceli, A.; Luvisi, A.; De Bellis, L. Evaluation of Phytochemical and Antioxidant Properties of 15 Italian Olea europaea L. Cultivar Leaves. Molecules 2019, 24, 1998. [Google Scholar] [CrossRef] [PubMed]
- Laguerre, M.; Lόpez Giraldo, L.J.; Piombo, G.; Figueroa-Espinoza, M.C.; Pina, M.; Benaissa, M.; Combe, A.; Rossignol Castera, A.; Lecomte, J.; Villeneuve, P. Characterization of Olive-Leaf Phenolics by ESI-MS and Evaluation of their Antioxidant Capacities by the CAT Assay. J. Am. Oil Chem. Soc. 2009, 86, 1215–1225. [Google Scholar] [CrossRef]
- Guinda, Á.; Castellano, J.M.; Santos-Lozano, J.M.; Delgado-Hervás, T.; Gutiérrez-Adánez, P.; Rada, M. Determination of major bioactive compounds from olive leaf. LWT—Food Sci. Technol. 2015, 64, 431–438. [Google Scholar] [CrossRef]
- Cecchi, L.; Piazzini, V.; D’Ambrosio, M.; Luceri, C.; Rocco, F.; Innocenti, M.; Vanti, G.; Mulinacci, N.; Bergonzi, M.C. Formulation of a Phenol-Rich Extract from Unripe Olives (Olea europaea L.) in Microemulsion to Improve Its Solubility and Intestinal Permeability. Molecules 2020, 25, 3198. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Fang, X.; Zhou, D.; Wang, Y.; Chen, M. TPGS-g-PLGA/Pluronic F68 mixed micelles for tanshinone IIA delivery in cancer therapy. Int. J. Pharm. 2014, 476, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.J.; Li, J.; Nation, R.L.; Boyd, B.J. Drug Release from Nanomedicines: Selection of Appropriate Encapsulation and Release Methodology. Drug Deliv. Transl. Res. 2012, 2, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Cagel, M.; Bernabeu, E.; Gonzalez, L.; Lagomarsino, E.; Zubillaga, M.; Moretton, M.A.; Chiappetta, D.A. Mixed micelles for encapsulation of doxorubicin with enhanced in vitro cytotoxicity on breast and ovarian cancer cell lines versus Doxil®. Biomed. Pharmacother. 2017, 95, 894–903. [Google Scholar] [CrossRef]
- Topel, Ö.; Çakır, B.A.; Budama, L.; Hoda, N. Determination of Critical Micelle Concentration of Polybutadiene-Block-Poly(Ethyleneoxide) Diblock Copolymer by Fluorescence Spectroscopy and Dynamic Light Scattering. J. Mol. Liq. 2013, 177, 40–43. [Google Scholar] [CrossRef]
- Nekkanti, V.; Wang, Z.; Betageri, G.V. Pharmacokinetic Evaluation of Improved Oral Bioavailability of Valsartan: Proliposomes Versus Self-Nanoemulsifying Drug Delivery System. AAPS PharmSciTech. 2015, 17, 851–862. [Google Scholar] [CrossRef]
- Chiappetta, D.; Sosnik, A. Poly (ethylene oxide)-poly (propylene oxide) block copolymer micelles as drug delivery agents: Improved hydrosolubility, stability and bioavailability of drugs. Eur. J. Pharm. Biopharm. 2007, 66, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Tavares Luiz, M.; Delello Di Filippo, L.; Alves, R.C.; Hugo Sousa Araújo, V.; Lobato Duarte, J.; Maldonado Marchetti, J.; Chorilli, M. The use of TPGS in drug delivery systems to overcome biological barriers. Eur. Polym. J. 2021, 142, 110129. [Google Scholar] [CrossRef]
- Lu, Y.; Park, K. Polymeric Micelles and Alternative Nanonized Delivery Vehicles for Poorly Soluble Drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Tsui, H.W.; Wang, J.H.; Hsu, Y.H.; Chen, L.-J. Study of heat of micellization and phase separation for Pluronic aqueous solutions by using a high sensitivity differential scanning calorimetry. Colloid Polym. Sci. 2010, 288, 1687–1696. [Google Scholar] [CrossRef]
- Bernabeu, E.; Gonzalez, L.; Cagel, M.; Gergic, E.P.; Moretton, M.A.; Chiappetta, D.A. Novel Soluplus®—TPGS mixed micelles for encapsulation of paclitaxel with enhanced in vitro cytotoxicity on breast and ovarian cancer cell lines. Colloids Surf. B 2016, 140, 403–411. [Google Scholar] [CrossRef]
- Tarhanlı, I.; Senses, E. Cellulose nanocrystal and Pluronic L121-based thermo-responsive composite hydrogels. Carbohydr. Polym. 2023, 321, 121281. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.C.; Chan, D.P.; Shoichet, M.S. Polymeric micelle stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Rel. 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Yang, T.-F.; Chen, C.-N.; Chen, M.-C.; Lai, C.-H.; Liang, H.-F.; Sung, H.-W. Shell-crosslinked Pluronic L121 micelles as a drug delivery vehicle. Biomaterials 2007, 28, 725–734. [Google Scholar] [CrossRef]
- Gyulai, G.; Magyar, A.; Rohonczy, J.; Orosz, J.; Yamasaki, M.; Bősze, S.; Kiss, E. Preparation and characterization of cationic Pluronic for surface modification and functionalization of polymeric drug delivery nanoparticles. Express Polym. Lett. 2016, 10, 216. [Google Scholar] [CrossRef]
- Parmar, A.; Chavda, S.; Bahadur, P. Pluronic–cationic surfactant mixed micelles: Solubilization and release of the drug hydrochlorothiazide. Colloids Surf. A 2014, 441, 389–397. [Google Scholar] [CrossRef]
- Pankaj, S.; Shruti, C.; Rakesh, K.M. A systematic physicochemical investigation on solubilization and in vitro release of poorly water-soluble oxcarbazepine drug in pluronic micelles. Colloids Surf. A 2016, 504, 479–488. [Google Scholar] [CrossRef]
- Kazemi, M.; Varshosaz, J.; Tabbakhian, M. Preparation and evaluation of lipid-based liquid crystalline formulation of fenofibrate. Adv. Biomed. Res. 2018, 7, 126. [Google Scholar]
- Emami, J.; Mohiti, H.; Hamishehkar, H.; Varshosaz, J. Formulation and optimization of solid lipid nanoparticle formulation for pulmonary delivery of budesonide using Taguchi and Box-Behnken design. Res. Pharm. Sci. 2015, 10, 17–33. [Google Scholar]
- Salimi, A.; Sharif Makhmal Zadeh, B.; Kazemi, M. Preparation and optimization of polymeric micelles as an oral drug delivery system for deferoxamine mesylate: In vitro and ex vivo studies. Res. Pharm. Sci. 2019, 14, 293–307. [Google Scholar] [CrossRef]
- Dahmani, F.Z.; Yang, H.; Zhou, J.; Yao, J.; Zhang, T.; Zhang, Q. Enhanced oral bioavailability of paclitaxel in pluronic/LHR mixed polymeric micelles: Preparation, in vitro and in vivo evaluation. Eur. J. Pharm. Sci. 2012, 47, 179–189. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, J.; Tan, S.; Otieno, B.O.; Zhang, Z. The applications of Vitamin E TPGS in drug delivery. Eur. J. Pharm. Sci. 2013, 49, 175–186. [Google Scholar] [CrossRef]
- Collnot, E.-M.; Baldes, C.; Schaefer, U.F.; Edgar, K.J.; Wempe, M.F.; Lehr, C.-M. Vitamin E TPGS P-Glycoprotein Inhibition Mechanism: Influence on Conformational Flexibility, Intracellular ATP Levels, and Role of Time and Site of Access. Mol. Pharm. 2010, 7, 642–651. [Google Scholar] [CrossRef]
- Zhang, X.N.; Xu, J.; Tang, L.H.; Gong, J.; Yan, X.Y.; Zhang, Q. Influence on intestinal mucous permeation of paclitaxel of absorption enhancers and dosage forms based on electron spin resonance spectroscopy. Pharmazie 2007, 62, 368–371. [Google Scholar] [CrossRef]

| Sample | Size (nm) ± SD | PdI ± SD |
|---|---|---|
| L121 | 23.30 ± 1.02 | 0.25 ± 0.02 |
| freeze-dried product | 44.93 ± 1.88 | 0.58 ± 0.01 |
| L121 + TPGS 4:1 | 16.09 ± 0.19 | 0.12 ± 0.05 |
| freeze-dried product | 22.60 ± 3.19 | 0.23 ± 0.09 |
| L121 + TPGS 3:2 | 14.78 ± 0.27 | 0.15 ± 0.07 |
| freeze-dried product | 60.36 ± 1.55 | 0.39 ± 0.14 |
| L121 + TPGS 2:3 | 15.08 ± 0.46 | 0.29 ± 0.00 |
| freeze-dried product | 76.98 ± 1.51 | 0.32 ± 0.04 |
| L121 + TPGS 1:1 | 940.5 ± 561.0 | 0.72 ± 0.25 |
| OPA40 (mg/mL) | Size (nm) ± SD | PdI ± SD | EE% ± SD | |
|---|---|---|---|---|
| 5 | before freeze-drying | 15.61 ± 0.05 | 0.04 ± 0.02 | 58.81 ± 4.56 |
| after freeze drying | 15.53 ± 0.13 | 0.06 ± 0.02 | 61.86 ± 3.28 | |
| 15 | before freeze-drying | 15.55 ± 0.11 | 0.12 ± 0.07 | 64.91 ± 7.79 |
| after freeze drying | 2166 ± 244 | 1.00 ± 0.56 | 32.98 ± 2.61 | |
| 20 | before freeze-drying | 15.93 ± 0.03 | 0.14 ± 0.04 | 71.18 ± 2.34 |
| after freeze drying | 2356 ± 646.80 | 1.00 ± 0.79 | 26.78 ± 3.22 |
| OPA40 (mg/mL) | Size (nm) ± SD | PdI ± SD | EE% ± SD | |
|---|---|---|---|---|
| 0 | before freeze-drying | 15.13 ± 0.11 | 0.10 ± 0.01 | - |
| after freeze drying | 16.01 ± 0.18 | 0.17 ± 0.02 | - | |
| 10 | before freeze-drying | 16.36 ± 0.56 | 0.23 ± 0.03 | 62.51 ± 1.19 |
| after freeze drying | 14.48 ± 0.12 | 0.20 ± 0.03 | 59.89 ± 3.28 | |
| 15 | before freeze-drying | 14.21 ± 0.14 | 0.19 ± 0.05 | 66.21 ± 1.11 |
| after freeze drying | 15.97 ± 1.13 | 0.24 ± 0.04 | 64.71 ± 2.82 | |
| 20 | before freeze-drying | 18.22 ± 1.80 | 0.26 ± 0.03 | 63.91 ± 6.81 |
| after freeze drying | 2345 ± 222.45 | 1.07 ± 0.46 | 39.03 ± 2.52 |
| Release Kinetics | MM-OPA40 |
|---|---|
| Zero order | 0.6958 |
| First order | 0.8270 |
| Korsmeyer–Peppas | 0.6812 |
| Hixson | 0.7852 |
| Higuchi | 0.9144 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergonzi, M.C.; De Stefani, C.; Vasarri, M.; Ivanova Stojcheva, E.; Ramos-Pineda, A.M.; Baldi, F.; Bilia, A.R.; Degl’Innocenti, D. Encapsulation of Olive Leaf Polyphenol-Rich Extract in Polymeric Micelles to Improve Its Intestinal Permeability. Nanomaterials 2023, 13, 3147. https://doi.org/10.3390/nano13243147
Bergonzi MC, De Stefani C, Vasarri M, Ivanova Stojcheva E, Ramos-Pineda AM, Baldi F, Bilia AR, Degl’Innocenti D. Encapsulation of Olive Leaf Polyphenol-Rich Extract in Polymeric Micelles to Improve Its Intestinal Permeability. Nanomaterials. 2023; 13(24):3147. https://doi.org/10.3390/nano13243147
Chicago/Turabian StyleBergonzi, Maria Camilla, Chiara De Stefani, Marzia Vasarri, Emilija Ivanova Stojcheva, Alba María Ramos-Pineda, Francesco Baldi, Anna Rita Bilia, and Donatella Degl’Innocenti. 2023. "Encapsulation of Olive Leaf Polyphenol-Rich Extract in Polymeric Micelles to Improve Its Intestinal Permeability" Nanomaterials 13, no. 24: 3147. https://doi.org/10.3390/nano13243147
APA StyleBergonzi, M. C., De Stefani, C., Vasarri, M., Ivanova Stojcheva, E., Ramos-Pineda, A. M., Baldi, F., Bilia, A. R., & Degl’Innocenti, D. (2023). Encapsulation of Olive Leaf Polyphenol-Rich Extract in Polymeric Micelles to Improve Its Intestinal Permeability. Nanomaterials, 13(24), 3147. https://doi.org/10.3390/nano13243147








