Cytotoxicity of PEG-Coated Gold and Gold–Iron Alloy Nanoparticles: ROS or Ferroptosis?
Abstract
:1. Introduction
2. Materials and Methods
2.1. NPs Synthesis
2.2. NPs Characterization
2.3. Toxicity Dose–Response Curve
2.4. Crystal Violet Assay
2.5. ROS Scavengers
2.6. Ferroptosis Assay
2.7. Statistical Analysis
3. Results
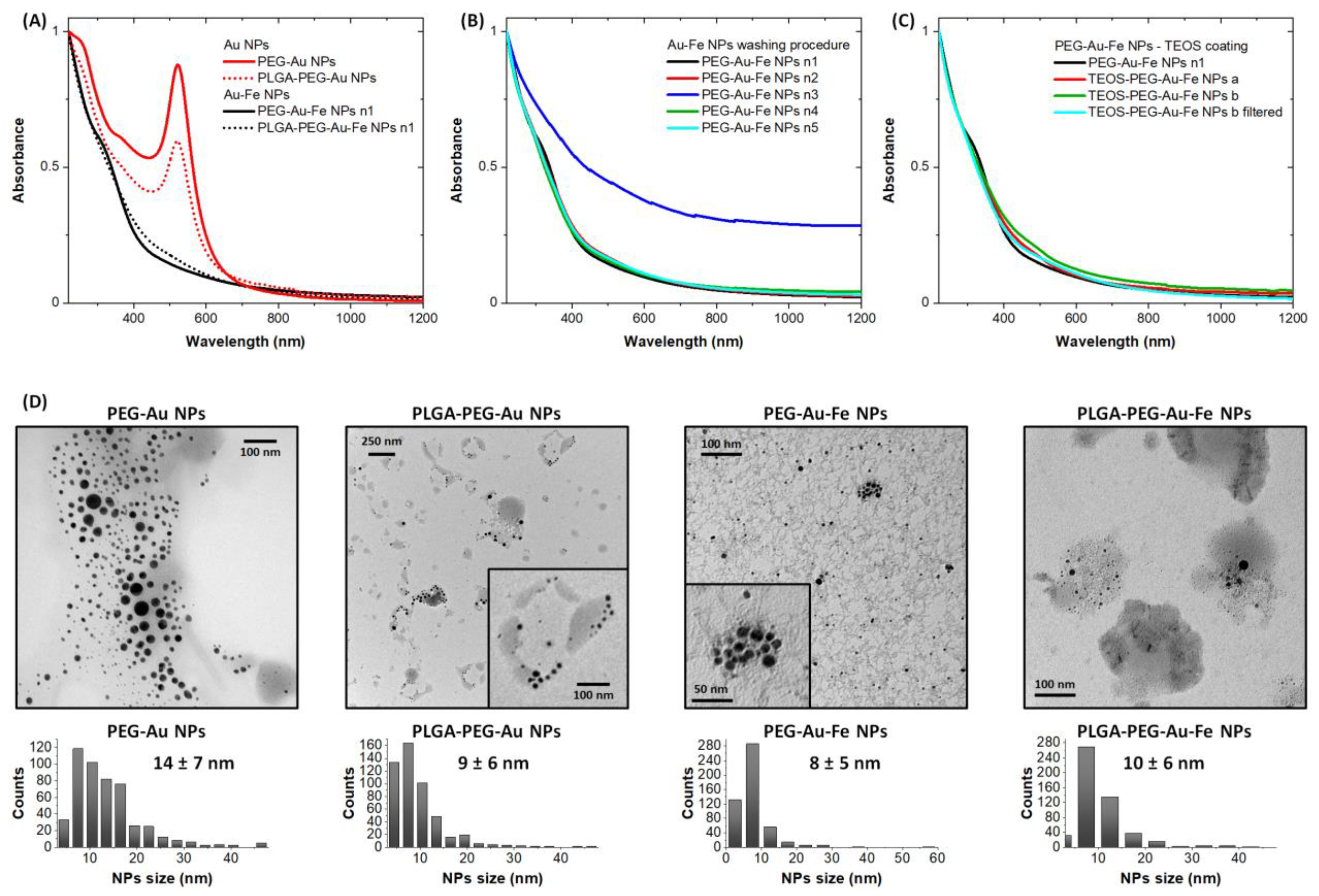
3.1. Characterization of NPs Samples
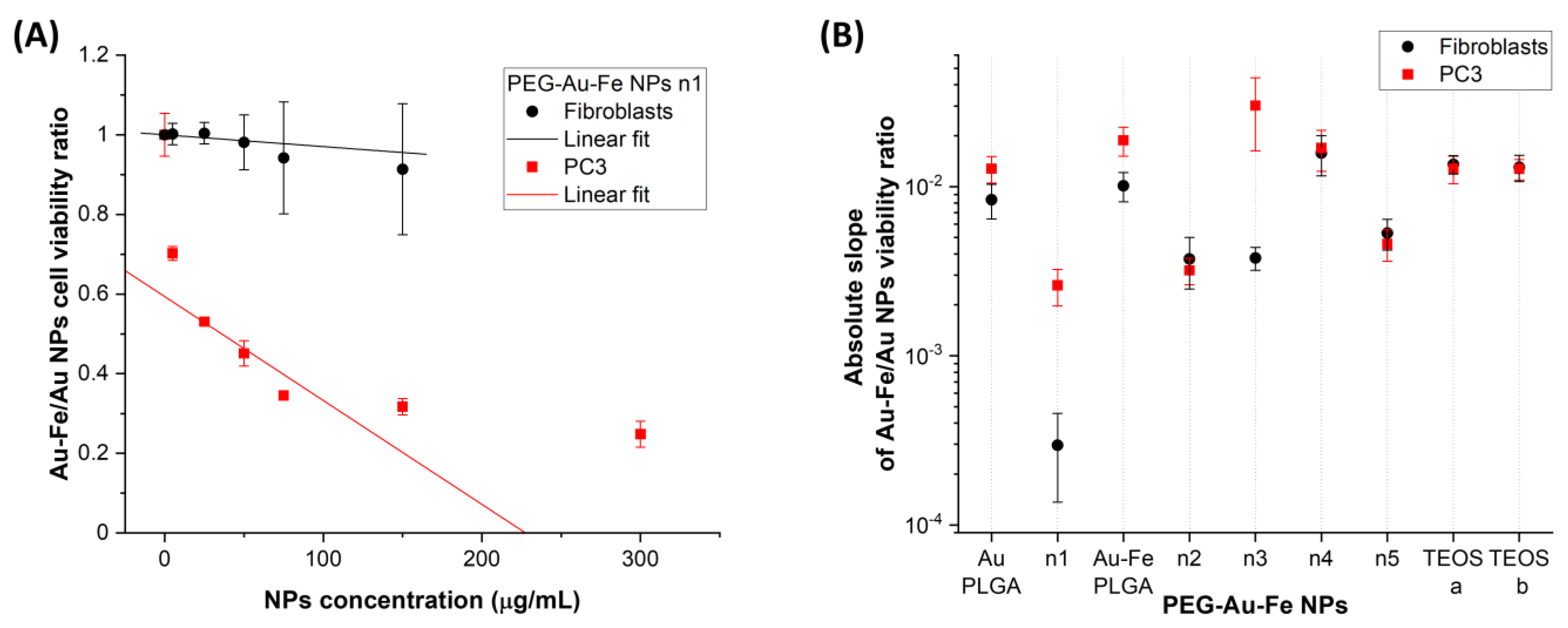
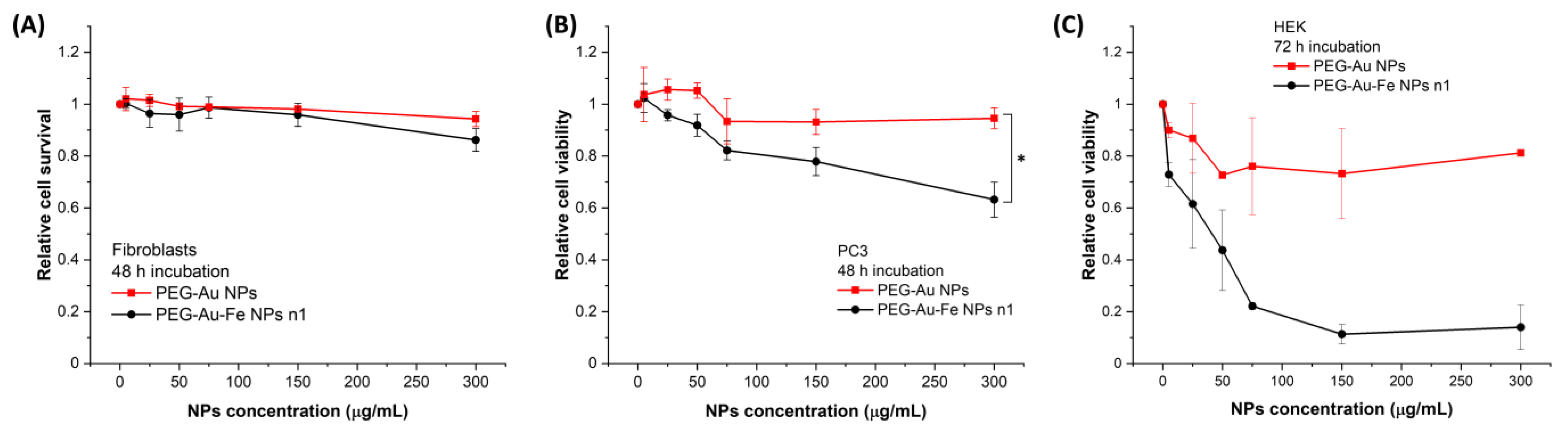
3.2. Assessment of NPs Toxicity
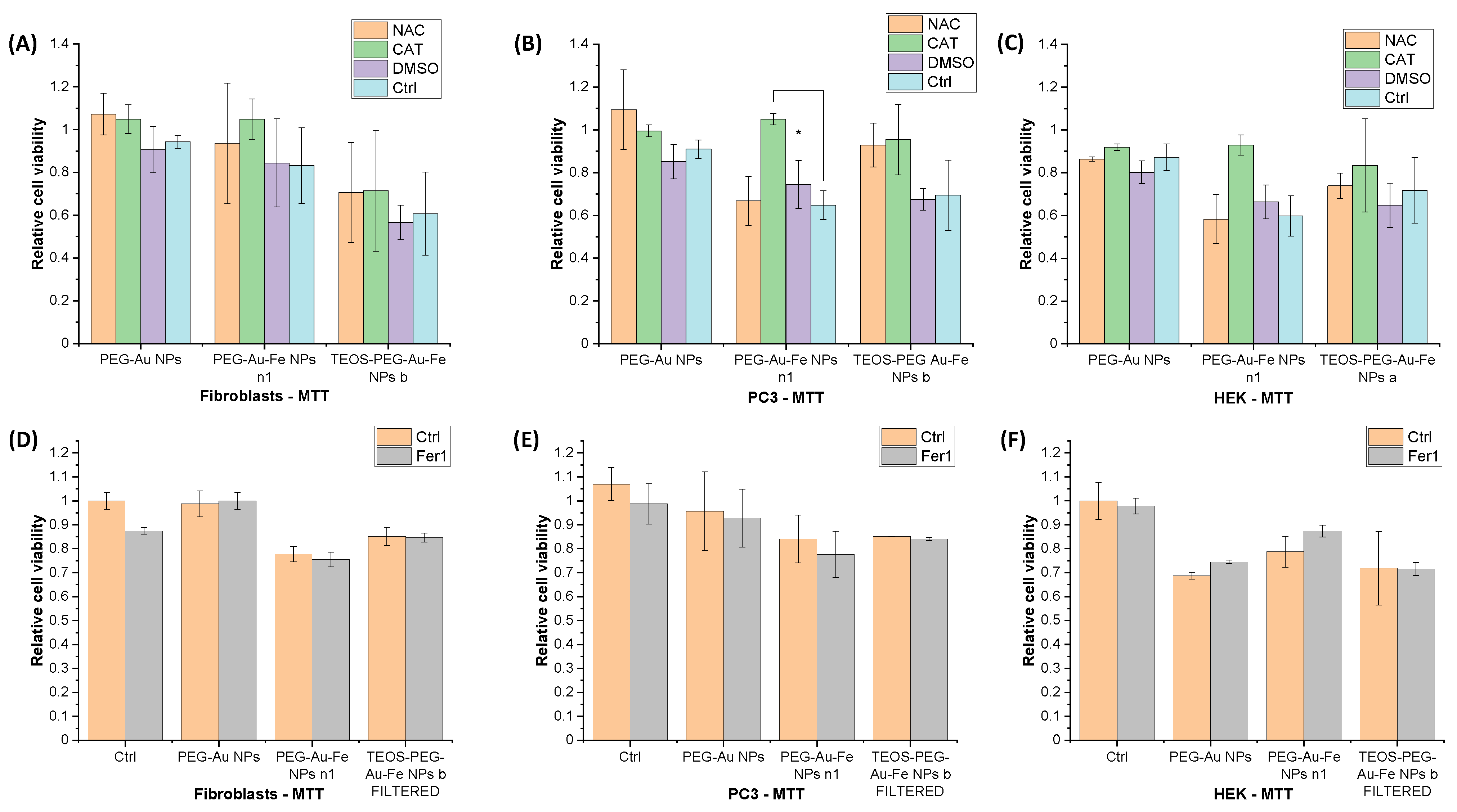
3.3. Origin of NPs Toxicity: ROS or Ferroptosis?
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer Nanomedicine: Progress, Challenges and Opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Yun, W.S.; Kim, J.; Lim, D.K.; Kim, D.H.; Jeon, S.I.; Kim, K. Recent Studies and Progress in the Intratumoral Administration of Nano-Sized Drug Delivery Systems. Nanomaterials 2023, 13, 2225. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yu, L.; Chen, Y. Engineering Self-Assembled Nanomedicines Composed of Clinically Approved Medicines for Enhanced Tumor Nanotherapy. Nanomaterials 2023, 13, 2499. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Singh, A.; Swihart, M.T.; Zavestovskaya, I.N.; Prasad, P.N. Laser-Processed Nanosilicon: A Multifunctional Nanomaterial for Energy and Healthcare. ACS Nano 2019, 13, 9841–9867. [Google Scholar] [CrossRef] [PubMed]
- Hamidu, A.; Pitt, W.G.; Husseini, G.A. Recent Breakthroughs in Using Quantum Dots for Cancer Imaging and Drug Delivery Purposes. Nanomaterials 2023, 13, 2566. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Cámara, J.A.; Boullosa, A.M.; Gustà, M.F.; Mondragón, L.; Schwartz, S.; Casals, E.; Abasolo, I.; Bastús, N.G.; Puntes, V. Nanoceria as Safe Contrast Agents for X-ray CT Imaging. Nanomaterials 2023, 13, 2208. [Google Scholar] [CrossRef]
- Lopes, T.S.; Alves, G.G.; Pereira, M.R.; Granjeiro, J.M.; Leite, P.E.C. Advances and Potential Application of Gold Nanoparticles in Nanomedicine. J. Cell. Biochem. 2019, 120, 16370–16378. [Google Scholar] [CrossRef]
- Daniel, M.C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Hu, Q.; Fang, Z.; Ge, J.; Li, H. Nanotechnology for Cardiovascular Diseases. Innovation 2022, 3, 293–346. [Google Scholar] [CrossRef]
- Sivasubramanian, M.; Chu, C.H.; Hsia, Y.; Chen, N.T.; Cai, M.T.; Tew, L.S.; Chuang, Y.C.; Chen, C.T.; Aydogan, B.; De Liao, L.; et al. Illuminating and Radiosensitizing Tumors with 2DG-Bound Gold-Based Nanomedicine for Targeted CT Imaging and Therapy. Nanomaterials 2023, 13, 1790. [Google Scholar] [CrossRef] [PubMed]
- Alhussan, A.; Jackson, N.; Calisin, R.; Morgan, J.; Beckham, W.; Chithrani, D.B. Utilizing Gold Nanoparticles as Prospective Radiosensitizers in 3D Radioresistant Pancreatic Co-Culture Model. Int. J. Mol. Sci. 2023, 24, 12523. [Google Scholar] [CrossRef] [PubMed]
- Laprise-Pelletier, M.; Simão, T.; Fortin, M.-A. Gold Nanoparticles in Radiotherapy and Recent Progress in Nanobrachytherapy. Adv. Healthc. Mater. 2018, 7, 1701460. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.; Mertz, D. Iron Oxide@Mesoporous Silica Core-Shell Nanoparticles as Multimodal Platforms for Magnetic Resonance Imaging, Magnetic Hyperthermia, Near-Infrared Light Photothermia, and Drug Delivery. Nanomaterials 2023, 13, 1342. [Google Scholar] [CrossRef] [PubMed]
- Arsalani, S.; Arsalani, S.; Isikawa, M.; Guidelli, E.J.; Mazon, E.E.; Ramos, A.P.; Bakuzis, A.; Pavan, T.Z.; Baffa, O.; Carneiro, A.A.O. Hybrid Nanoparticles of Citrate-Coated Manganese Ferrite and Gold Nanorods in Magneto-Optical Imaging and Thermal Therapy. Nanomaterials 2023, 13, 434. [Google Scholar] [CrossRef] [PubMed]
- Farinha, P.; Coelho, J.M.P.; Reis, C.P.; Gaspar, M.M. A Comprehensive Updated Review on Magnetic Nanoparticles in Diagnostics. Nanomaterials 2021, 11, 3432. [Google Scholar] [CrossRef] [PubMed]
- Ezealigo, B.N.; Ezealigo, U.S.; Ighodalo, K.I.; Ezema, F.I. Iron Oxide Nanoparticles: Current and Future Applications in Nanomedicine. Fundam. Ind. Appl. Magn. Nanoparticles 2022, 11, 349–392. [Google Scholar] [CrossRef]
- Wang, Z.; Qiao, R.; Tang, N.; Lu, Z.; Wang, H.; Zhang, Z.; Xue, X.; Huang, Z.; Zhang, S.; Zhang, G.; et al. Active Targeting Theranostic Iron Oxide Nanoparticles for MRI and Magnetic Resonance-Guided Focused Ultrasound Ablation of Lung Cancer. Biomaterials 2017, 127, 25–35. [Google Scholar] [CrossRef]
- Torresan, V.; Forrer, D.; Guadagnini, A.; Badocco, D.; Pastore, P.; Casarin, M.; Selloni, A.; Coral, D.; Ceolin, M.; Fernández van Raap, M.B.; et al. 4D Multimodal Nanomedicines Made of Nonequilibrium Au-Fe Alloy Nanoparticles. ACS Nano 2020, 14, 12840–12853. [Google Scholar] [CrossRef]
- Li, B.; Lane, L.A. Probing the Biological Obstacles of Nanomedicine with Gold Nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1542. [Google Scholar] [CrossRef]
- Avramescu, M.L.; Chénier, M.; Beauchemin, S.; Rasmussen, P. Dissolution Behaviour of Metal-Oxide Nanomaterials in Various Biological Media. Nanomaterials 2023, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wang, X.; Zeng, S.; Ramamurthy, G.; Burda, C.; Basilion, J.P. Prostate-Specific Membrane Antigen Targeted Gold Nanoparticles for Prostate Cancer Radiotherapy: Does Size Matter for Targeted Particles? Chem. Sci. 2019, 10, 8119–8128. [Google Scholar] [CrossRef] [PubMed]
- Schuemann, J.; Bagley, A.F.; Berbeco, R.; Bromma, K.; Butterworth, K.T.; Byrne, H.L.; Chithrani, B.D.; Cho, S.H.; Cook, J.R.; Favaudon, V.; et al. Roadmap for Metal Nanoparticles in Radiation Therapy: Current Status, Translational Challenges, and Future Directions. Phys. Med. Biol. 2020, 65, 21RM02. [Google Scholar] [CrossRef] [PubMed]
- Bravin, C.; Amendola, V. Wide Range Detection of C-Reactive Protein with a Homogeneous Immunofluorimetric Assay Based on Cooperative Fluorescence Quenching Assisted by Gold Nanoparticles. Biosens. Bioelectron. 2020, 169, 112591. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Guadagnini, A.; Agnoli, S.; Badocco, D.; Pastore, P.; Fracasso, G.; Gerosa, M.; Vurro, F.; Busato, A.; Marzola, P. Polymer-Coated Silver-Iron Nanoparticles as Efficient and Biodegradable MRI Contrast Agents. J. Colloid Interface Sci. 2021, 596, 332–341. [Google Scholar] [CrossRef]
- Scaramuzza, S.; de Faria, C.M.G.; Coviello, V.; Forrer, D.; Artiglia, L.; Badocco, D.; Pastore, P.; Ghigna, P.; Postuma, I.; Cansolino, L.; et al. A Laser Synthesis Route to Boron-Doped Gold Nanoparticles Designed for X-Ray Radiotherapy and Boron Neutron Capture Therapy Assisted by CT Imaging. Adv. Funct. Mater. 2023, 33, 2303366. [Google Scholar] [CrossRef]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 356901. [Google Scholar] [CrossRef]
- Reda, M.; Bagley, A.F.; Zaidan, H.Y.; Yantasee, W. Augmenting the Therapeutic Window of Radiotherapy: A Perspective on Molecularly Targeted Therapies and Nanomaterials. Radiother. Oncol. 2020, 150, 225–235. [Google Scholar] [CrossRef]
- Laux, P.; Riebeling, C.; Booth, A.M.; Brain, J.D.; Brunner, J.; Cerrillo, C.; Creutzenberg, O.; Estrela-Lopis, I.; Gebel, T.; Johanson, G.; et al. Biokinetics of Nanomaterials: The Role of Biopersistence. NanoImpact 2017, 6, 69–80. [Google Scholar] [CrossRef]
- Sharma, S.; Parmar, A.; Kori, S.; Sandhir, R. PLGA-Based Nanoparticles: A New Paradigm in Biomedical Applications. TrAC Trends Anal. Chem. 2016, 80, 30–40. [Google Scholar] [CrossRef]
- Amendola, V.; Scaramuzza, S.; Litti, L.; Meneghetti, M.; Zuccolotto, G.; Rosato, A.; Nicolato, E.; Marzola, P.; Fracasso, G.; Anselmi, C.; et al. Magneto-Plasmonic Au-Fe Alloy Nanoparticles Designed for Multimodal SERS-MRI-CT Imaging. Small 2014, 10, 2476–2486. [Google Scholar] [CrossRef] [PubMed]
- AAT Bioquest Inc. Quest GraphTM IC50 Calculator. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 22 November 2023).
- Popov, A.A.; Swiatkowska-Warkocka, Z.; Marszalek, M.; Tselikov, G.; Zelepukin, I.V.; Al-Kattan, A.; Deyev, S.M.; Klimentov, S.M.; Itina, T.E.; Kabashin, A.V. Laser-Ablative Synthesis of Ultrapure Magneto-Plasmonic Core-Satellite Nanocomposites for Biomedical Applications. Nanomaterials 2022, 12, 649. [Google Scholar] [CrossRef]
- Torresan, V.; Guadagnini, A.; Badocco, D.; Pastore, P.; Muñoz Medina, G.A.; Fernàndez van Raap, M.B.; Postuma, I.; Bortolussi, S.; Bekić, M.; Čolić, M.; et al. Biocompatible Iron–Boron Nanoparticles Designed for Neutron Capture Therapy Guided by Magnetic Resonance Imaging. Adv. Healthc. Mater. 2020, 10, e2001632. [Google Scholar] [CrossRef] [PubMed]
- Gurbatov, S.; Puzikov, V.; Modin, E.; Shevlyagin, A.; Gerasimenko, A.; Mitsai, E.; Kulinich, S.A.; Kuchmizhak, A. Ag-Decorated Si Microspheres Produced by Laser Ablation in Liquid: All-in-One Temperature-Feedback SERS-Based Platform for Nanosensing. Materials 2022, 15, 8091. [Google Scholar] [CrossRef] [PubMed]
- Fromme, T.; Tintrop, L.K.; Reichenberger, S.; Schmidt, T.C.; Barcikowski, S. Impact of Chemical and Physical Properties of Organic Solvents on the Gas and Hydrogen Formation during Laser Synthesis of Gold Nanoparticles. ChemPhysChem 2023, 24, e202300089. [Google Scholar] [CrossRef] [PubMed]
- Hesabizadeh, T.; Sung, K.; Park, M.; Foley, S.; Paredes, A.; Blissett, S.; Guisbiers, G. Synthesis of Antibacterial Copper Oxide Nanoparticles by Pulsed Laser Ablation in Liquids: Potential Application against Foodborne Pathogens. Nanomaterials 2023, 13, 2206. [Google Scholar] [CrossRef]
- Crivellaro, S.; Guadagnini, A.; Arboleda, D.M.D.M.; Schinca, D.; Amendola, V. A System for the Synthesis of Nanoparticles by Laser Ablation in Liquid That Is Remotely Controlled with PC or Smartphone. Rev. Sci. Instrum. 2019, 90, 033902. [Google Scholar] [CrossRef] [PubMed]
- Khairani, I.Y.; Mínguez-Vega, G.; Doñate-Buendía, C.; Gökce, B. Green Nanoparticle Synthesis at Scale: A Perspective on Overcoming the Limits of Pulsed Laser Ablation in Liquids for High-Throughput Production. Phys. Chem. Chem. Phys. 2023, 25, 19380–19408. [Google Scholar] [CrossRef]
- Kralj, S.; Makovec, D.; Čampelj, S.; Drofenik, M. Producing Ultra-Thin Silica Coatings on Iron-Oxide Nanoparticles to Improve Their Surface Reactivity. J. Magn. Magn. Mater. 2010, 322, 1847–1853. [Google Scholar] [CrossRef]
- Čampelj, S.; Makovec, D.; Drofenik, M. Functionalization of Magnetic Nanoparticles with 3-Aminopropyl Silane. J. Magn. Magn. Mater. 2009, 321, 1346–1350. [Google Scholar] [CrossRef]
- Ali, Z.; Andreassen, J.-P.; Bandyopadhyay, S. Fine-Tuning of Particle Size and Morphology of Silica Coated Iron Oxide Nanoparticles. Ind. Eng. Chem. Res. 2023, 62, 4831–4839. [Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 2016, pdb.prot087379. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Chen, H.; Dong, Y.; Luo, X.; Yu, H.; Moore, Z.; Bey, E.A.; Boothman, D.A.; Gao, J. Superparamagnetic Iron Oxide Nanoparticles: Amplifying ROS Stress to Improve Anticancer Drug Efficacy. Theranostics 2013, 3, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.L.; Liu, H.X. The Double-Edged Roles of ROS in Cancer Prevention and Therapy. Theranostics 2021, 11, 4839–4857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yin, M.; Huang, L.; Wang, J.; Gong, L.; Liu, J.; Sun, B. Evaluation of the Cellular and Animal Models for the Study of Antioxidant Activity: A Review. J. Food Sci. 2017, 82, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Simunkova, M.; Barbierikova, Z.; Jomova, K.; Hudecova, L.; Lauro, P.; Alwasel, S.H.; Alhazza, I.; Rhodes, C.J.; Valko, M. Antioxidant vs. Prooxidant Properties of the Flavonoid, Kaempferol, in the Presence of Cu(II) Ions: A ROS-Scavenging Activity, Fenton Reaction and DNA Damage Study. Int. J. Mol. Sci. 2021, 22, 1619. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Z.; Li, X.; Zhang, Y.; Yin, M.; Li, J.; Song, H.; Shi, J.; Ling, D.; Wang, L.; et al. Deciphering Active Biocompatibility of Iron Oxide Nanoparticles from Their Intrinsic Antagonism. Nano Res. 2018, 11, 2746–2755. [Google Scholar] [CrossRef]
- Chou, S.W.; Liu, C.L.; Liu, T.M.; Shen, Y.F.; Kuo, L.C.; Wu, C.H.; Hsieh, T.Y.; Wu, P.C.; Tsai, M.R.; Yang, C.C.; et al. Infrared-Active Quadruple Contrast FePt Nanoparticles for Multiple Scale Molecular Imaging. Biomaterials 2016, 85, 54–64. [Google Scholar] [CrossRef]
- Yang, H.; Li, X.; Zhou, H.; Zhuang, Y.; Hu, H.; Wu, H.; Yang, S. Monodisperse Water-Soluble Fe–Ni Nanoparticles for Magnetic Resonance Imaging. J. Alloys Compd. 2011, 509, 1217–1221. [Google Scholar] [CrossRef]
- Sousa, F.; Sanavio, B.; Saccani, A.; Tang, Y.; Zucca, I.; Carney, T.M.; Mastropietro, A.; Jacob Silva, P.H.; Carney, R.P.; Schenk, K.; et al. Superparamagnetic Nanoparticles as High Efficiency Magnetic Resonance Imaging T2 Contrast Agent. Bioconjugate Chem. 2017, 28, 161–170. [Google Scholar] [CrossRef]
- Vega-Avila, E.; Pugsley, M.K. An Overview of Colorimetric Assay Methods Used to Assess Survival or Proliferation of Mammalian Cells. Proc. West. Pharmacol. Soc. 2011, 54, 10–14. [Google Scholar] [PubMed]
- Zheng, H.; Jiang, J.; Xu, S.; Liu, W.; Xie, Q.; Cai, X.; Zhang, J.; Liu, S.; Li, R. Nanoparticle-Induced Ferroptosis: Detection Methods, Mechanisms and Applications. Nanoscale 2021, 13, 2266–2285. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B. NAC, NAC, Knockin’ on Heaven’s Door: Interpreting the Mechanism of Action of N-Acetylcysteine in Tumor and Immune Cells. Redox Biol. 2022, 57, 102497. [Google Scholar] [CrossRef] [PubMed]
- Pedre, B.; Barayeu, U.; Ezeriņa, D.; Dick, T.P. The Mechanism of Action of N-Acetylcysteine (NAC): The Emerging Role of H2S and Sulfane Sulfur Species. Pharmacol. Ther. 2021, 228, 107916. [Google Scholar] [CrossRef] [PubMed]
- Mlejnek, P. Direct Interaction between N-Acetylcysteine and Cytotoxic Electrophile—An Overlooked In Vitro Mechanism of Protection. Antioxidants 2022, 11, 1485. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Chen, T.; Furst, A.; Chien, P.K. The Effects of Cadmium and Iron on Catalase Activities in Tubifex. Int. J. Toxicol. 1994, 13, 112–120. [Google Scholar] [CrossRef]
- Lee, Y.H.; Layman, D.K.; Bell, R.R.; Norton, H.W. Response of Glutathione Peroxidase and Catalase to Excess Dietary Iron in Rats. J. Nutr. 1981, 111, 2195–2202. [Google Scholar] [CrossRef]
- Boateng, F.; Ngwa, W. Delivery of Nanoparticle-Based Radiosensitizers for Radiotherapy Applications. Int. J. Mol. Sci. 2019, 21, 273. [Google Scholar] [CrossRef]
- Hu, J.; Dong, Y.; Ding, L.; Dong, Y.; Wu, Z.; Wang, W.; Shen, M.; Duan, Y. Local Delivery of Arsenic Trioxide Nanoparticles for Hepatocellular Carcinoma Treatment. Signal Transduct. Target. Ther. 2019, 4, 28. [Google Scholar] [CrossRef]
- Caddy, G.; Stebbing, J.; Wakefield, G.; Xu, X.Y. Modelling of Nanoparticle Distribution in a Spherical Tumour during and Following Local Injection. Pharmaceutics 2022, 14, 1615. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Chen, G.; Liang, C.; Xu, J.; Dong, Z.; Han, X.; Wang, C.; Liu, Z. Iron Nanoparticles for Low-Power Local Magnetic Hyperthermia in Combination with Immune Checkpoint Blockade for Systemic Antitumor Therapy. Nano Lett. 2019, 19, 4287–4296. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Faria, C.M.G.; Bissoli, M.; Vago, R.; Spinelli, A.E.; Amendola, V. Cytotoxicity of PEG-Coated Gold and Gold–Iron Alloy Nanoparticles: ROS or Ferroptosis? Nanomaterials 2023, 13, 3044. https://doi.org/10.3390/nano13233044
de Faria CMG, Bissoli M, Vago R, Spinelli AE, Amendola V. Cytotoxicity of PEG-Coated Gold and Gold–Iron Alloy Nanoparticles: ROS or Ferroptosis? Nanomaterials. 2023; 13(23):3044. https://doi.org/10.3390/nano13233044
Chicago/Turabian Stylede Faria, Clara M. G., Michael Bissoli, Riccardo Vago, Antonello E. Spinelli, and Vincenzo Amendola. 2023. "Cytotoxicity of PEG-Coated Gold and Gold–Iron Alloy Nanoparticles: ROS or Ferroptosis?" Nanomaterials 13, no. 23: 3044. https://doi.org/10.3390/nano13233044
APA Stylede Faria, C. M. G., Bissoli, M., Vago, R., Spinelli, A. E., & Amendola, V. (2023). Cytotoxicity of PEG-Coated Gold and Gold–Iron Alloy Nanoparticles: ROS or Ferroptosis? Nanomaterials, 13(23), 3044. https://doi.org/10.3390/nano13233044







