A Diamond/Graphene/Diamond Electrode for Waste Water Treatment
Abstract
:1. Introduction
2. Experimental Details
2.1. Preparation of the BDD and DGD Electrodes
2.2. Characterization
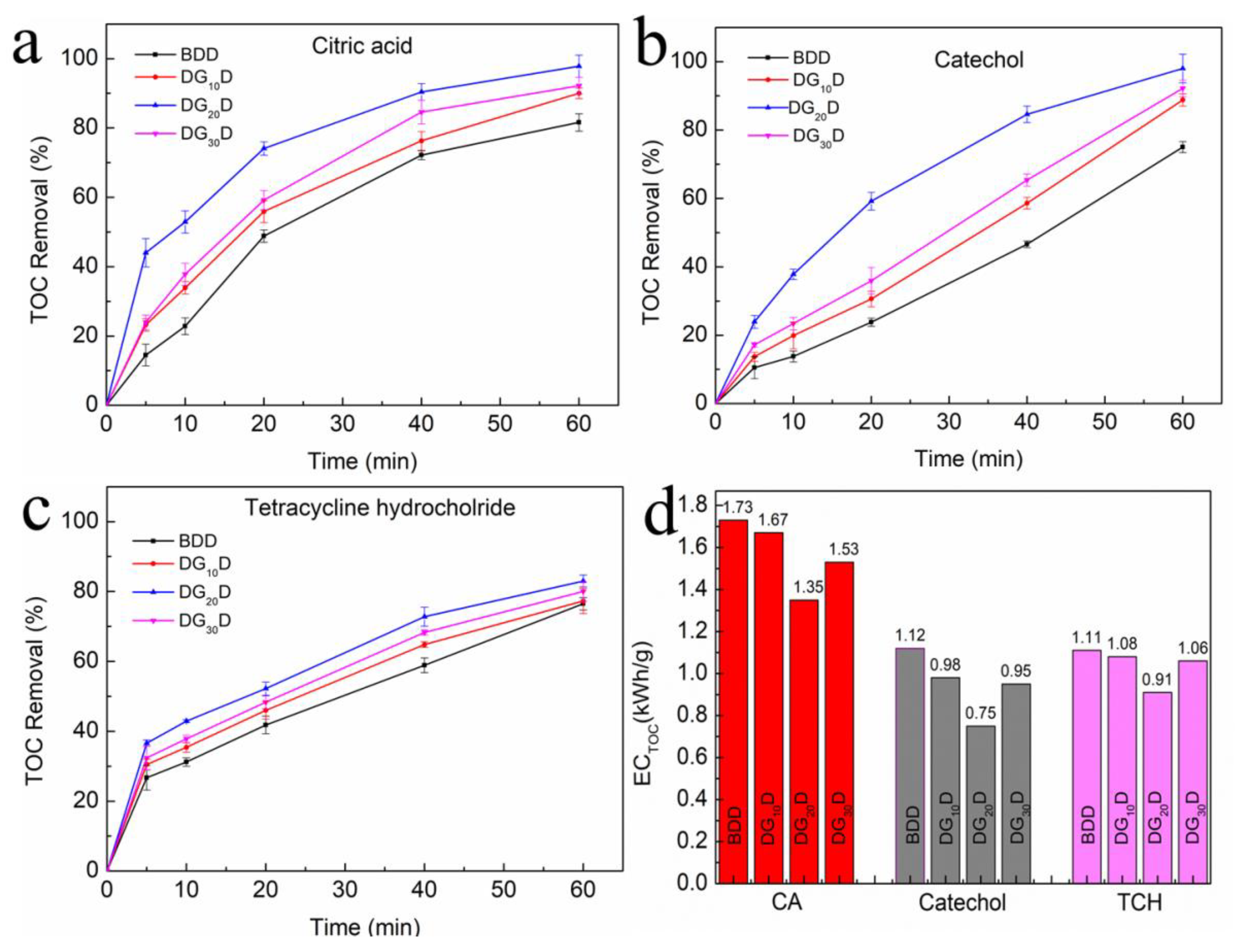
3. Result and Discussion
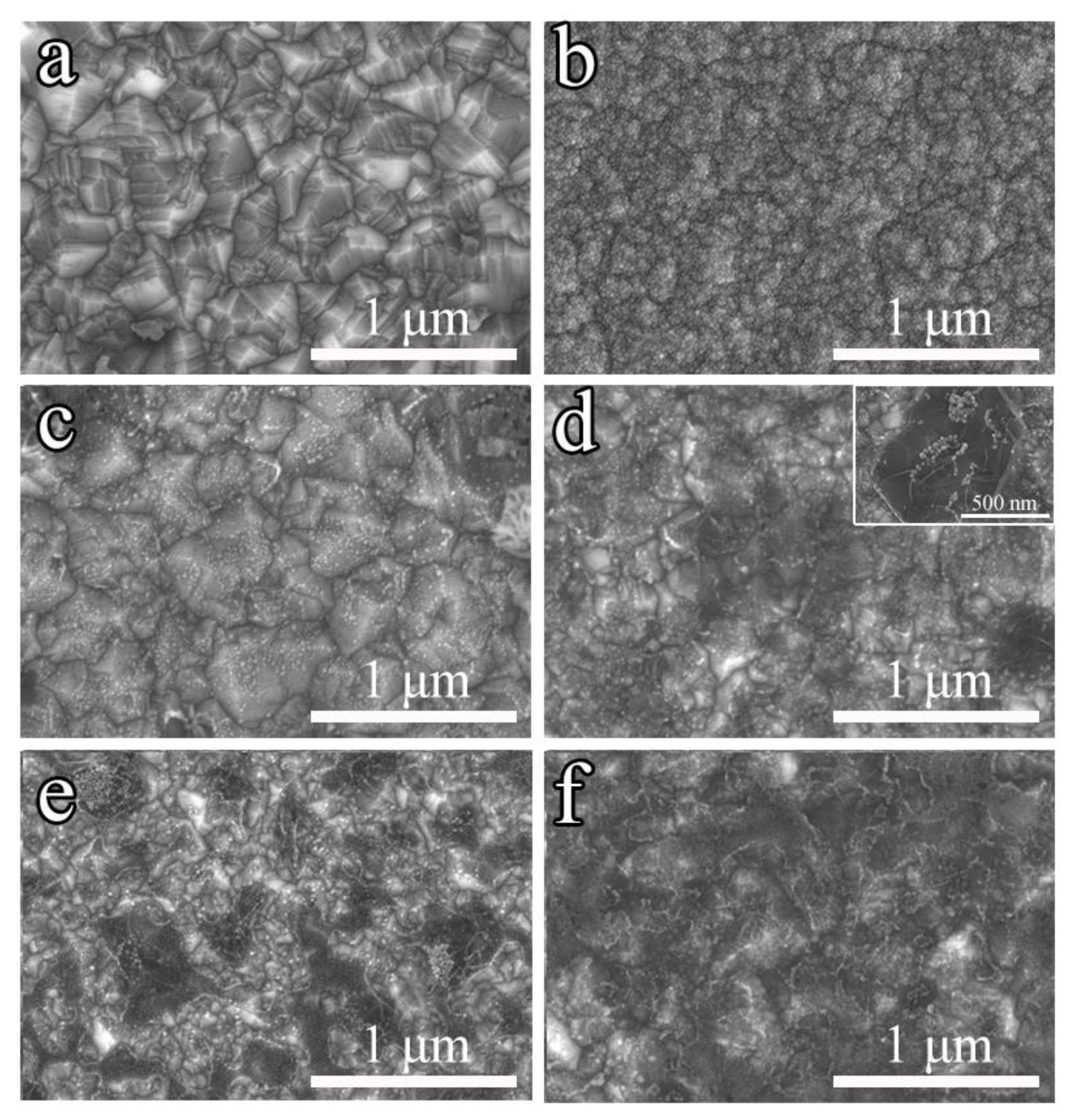
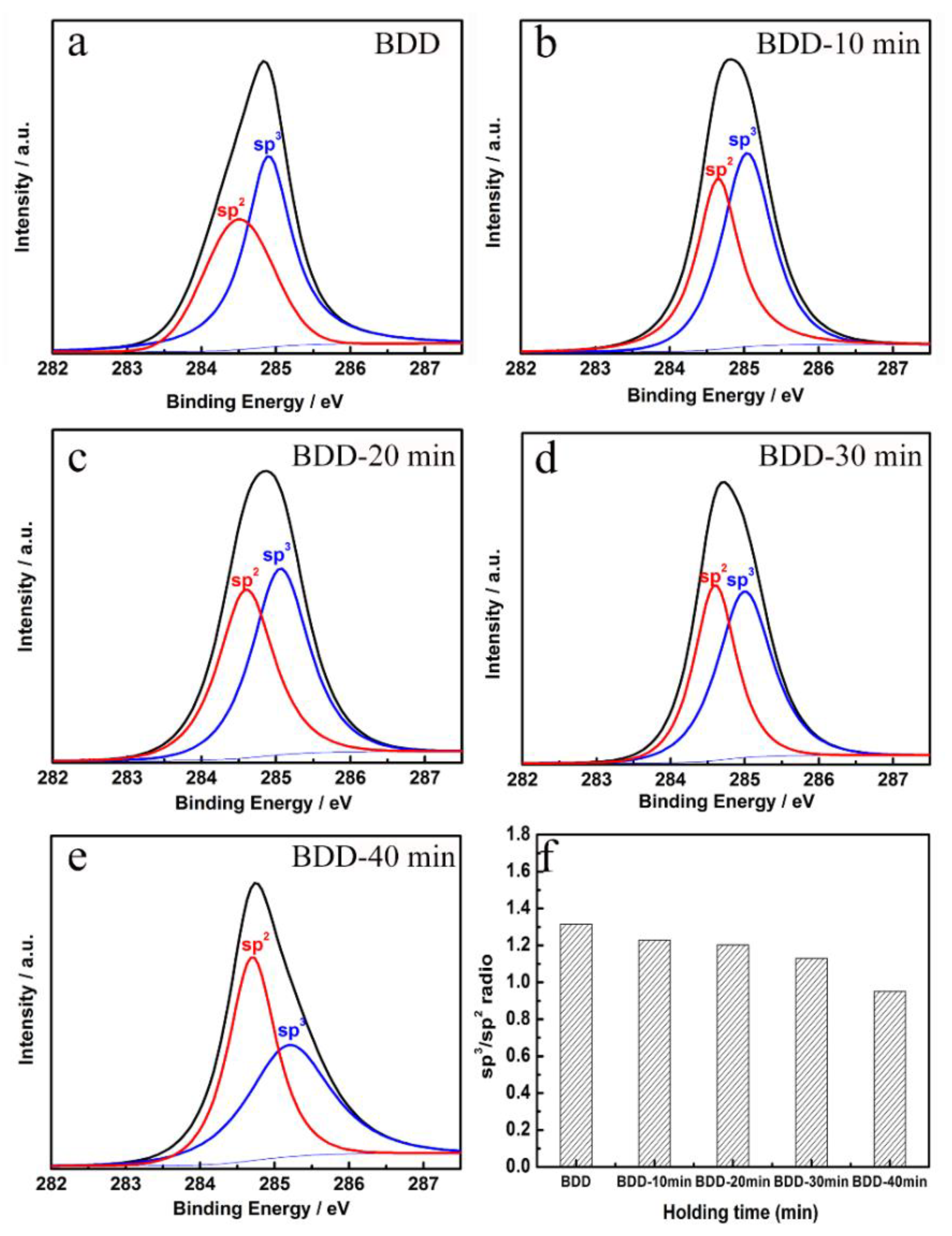
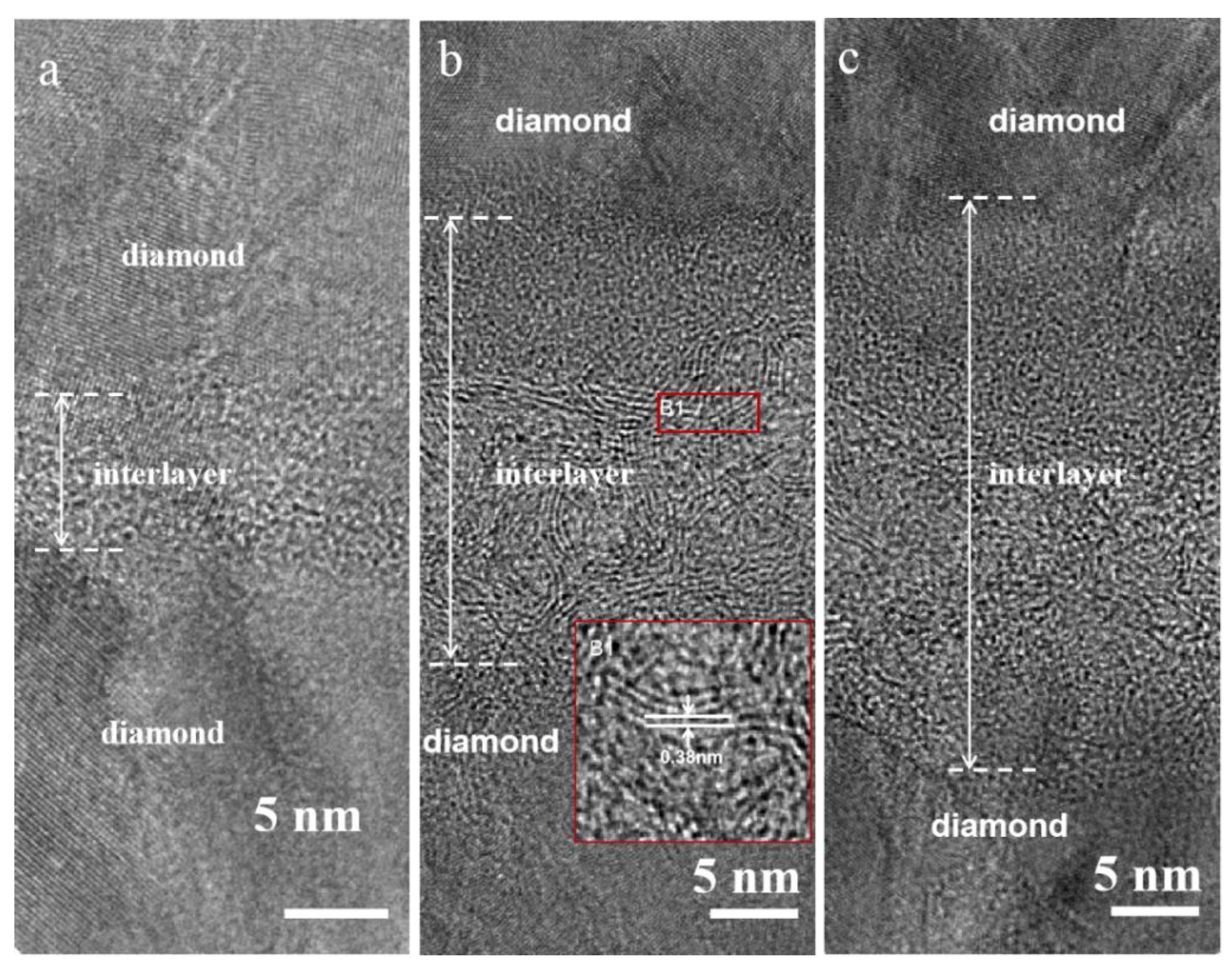
3.1. Material Characterization
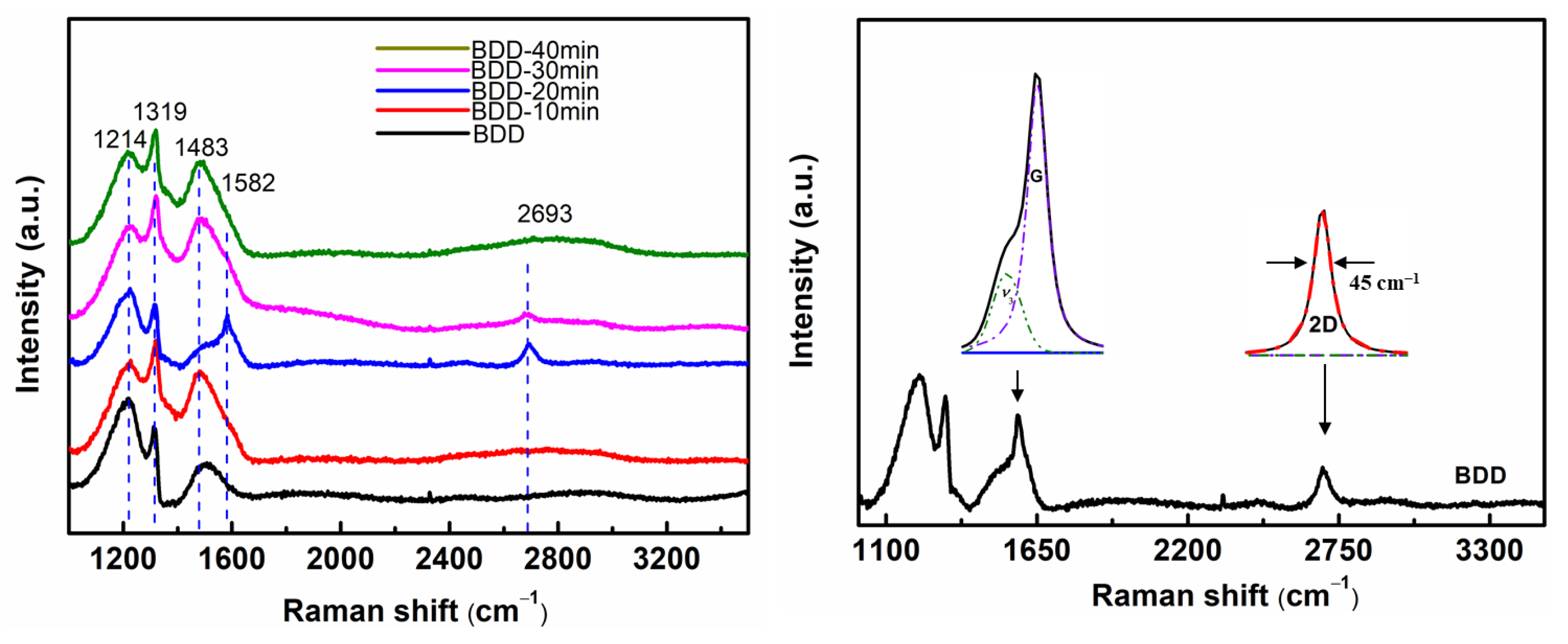
3.2. Raman Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aguiar, T.; Baumann, L.; Albuquerque, A.; Teixeira, L.; de Souza Gil, E.; Scalize, P. Application of Electrocoagulation for the Removal of Transition Metals in Water. Sustainability 2023, 15, 1492. [Google Scholar] [CrossRef]
- Xie, Q.; Ren, B. Pollution and risk assessment of heavy metals in rivers in the antimony capital of Xikuangshan. Sci. Rep. 2022, 12, 14393. [Google Scholar] [CrossRef] [PubMed]
- Puljko, A.; Milakovic, M.; Krizanovic, S.; Kosic-Vuksic, J.; Babic, I.; Petric, I.; Maravic, A.; Jelic, M.; Udikovic-Kolic, N. Prevalence of enteric opportunistic pathogens and extended-spectrum cephalosporin- and carbapenem-resistant coliforms and genes in wastewater from municipal wastewater treatment plants in Croatia. J. Hazard. Mater. 2022, 427, 128155. [Google Scholar] [CrossRef] [PubMed]
- Arman, N.; Salmiati, S.; Aris, A.; Salim, M.; Nazifa, T.; Muhamad, M.; Marpongahtun, M. A Review on Emerging Pollutants in the Water Environment: Existences, Health Effects and Treatment Processes. Water 2021, 13, 3258. [Google Scholar] [CrossRef]
- Al-Rashdi, B.; Johnson, D.; Hilal, N. Removal of heavy metal ions by nanofiltration. Desalination 2013, 315, 2–17. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Khan, S.U.; Khalid, M.; Hashim, K.; Jamadi, M.H.; Mousazadeh, M.; Basheer, F.; Farooqi, I.H. Efficacy of Electrocoagulation Treatment for the Abatement of Heavy Metals: An Overview of Critical Processing Factors, Kinetic Models and Cost Analysis. Sustainability 2023, 15, 1708. [Google Scholar] [CrossRef]
- Gallo-Cordova, A.; Castro, J.J.; Winkler, E.L.; Lima, E., Jr.; Zysler, R.D.; del Puerto Morales, M.; Ovejero, J.G.; Streitwieser, D.A. Improving degradation of real wastewaters with self-heating magnetic nanocatalysts. J. Clean. Prod. 2021, 308, 127385. [Google Scholar] [CrossRef]
- Iovino, P.; Chianese, S.; Fenti, A.; Blotevogel, J.; Musmarra, D. An innovative approach for atrazine electrochemical oxidation modelling: Process parameter effect, intermediate formation and kinetic constant assessment. Chem. Eng. J. 2023, 474, 146022. [Google Scholar] [CrossRef]
- Liu, Z.; Hosseinzadeh, S.; Wardenier, N.; Verheust, Y.; Chys, M.; Van Hulle, S. Combining ozone with UV and H2O2 for the degradation of micropollutants from different origins: Lab-scale analysis and optimization. Environ. Technol. 2019, 40, 3773–3782. [Google Scholar] [CrossRef]
- Neodo, S.; Nie, M.; Wharton, J.A.; Stokes, K.R. Nickel-ion detection on a boron-doped diamond electrode in acidic media. Electrochim. Acta 2013, 88, 718–724. [Google Scholar] [CrossRef]
- Fierro, S.; Watanabe, T.; Akai, K.; Einaga, Y. Highly sensitive detection of Cr6+ on boron doped diamond electrodes. Electrochim. Acta 2012, 82, 9–11. [Google Scholar] [CrossRef]
- Arantes, T.M.; Sardinha, A.; Baldan, M.R.; Cristovan, F.H.; Ferreira, N.G. Lead detection using micro/nanocrystalline boron-doped diamond by square-wave anodic stripping voltammetry. Talanta 2014, 128, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Leng, B.; Yang, N.; Yang, B.; Liu, L.; Huang, N.; Jiang, X. Rational Construction of 3D-Networked Carbon Nanowalls/Diamond Supporting CuO Architecture for High-Performance Electrochemical Biosensors. Small 2019, 15, 1901527. [Google Scholar] [CrossRef]
- Mei, X.; Wei, Q.; Long, H.; Yu, Z.; Deng, Z.; Meng, L.; Wang, J.; Luo, J.; Lin, C.-T.; Ma, L.; et al. Long-term stability of Au nanoparticle-anchored porous boron-doped diamond hybrid electrode for enhanced dopamine detection. Electrochim. Acta 2018, 271, 84–91. [Google Scholar] [CrossRef]
- Moura de Salles Pupo, M.; Albahaca Oliva, J.M.; Barrios Eguiluz, K.I.; Salazar-Banda, G.R.; Radjenovic, J. Characterization and comparison of Ti/TiO2-NT/SnO2–SbBi, Ti/SnO2–SbBi and BDD anode for the removal of persistent iodinated contrast media (ICM). Chemosphere 2020, 253, 126701. [Google Scholar] [CrossRef] [PubMed]
- Flores, N.; Cabot, P.L.; Centellas, F.; Garrido, J.A.; Rodríguez, R.M.; Brillas, E.; Sirés, I. 4-Hydroxyphenylacetic acid oxidation in sulfate and real olive oil mill wastewater by electrochemical advanced processes with a boron-doped diamond anode. J. Hazard. Mater. 2017, 321, 566–575. [Google Scholar] [CrossRef]
- Iniesta, J.; Michaud, P.A.; Panizza, M.; Cerisola, G.; Aldaz, A.; Comninellis, C. Electrochemical oxidation of phenol at boron-doped diamond electrode. Electrochim. Acta 2001, 46, 3573–3578. [Google Scholar] [CrossRef]
- Miao, D.; Li, Z.; Chen, Y.; Liu, G.; Deng, Z.; Yu, Y.; Li, S.; Zhou, K.; Ma, L.; Wei, Q. Preparation of macro-porous 3D boron-doped diamond electrode with surface micro structure regulation to enhance electrochemical degradation performance. Chem. Eng. J. 2022, 429, 132366. [Google Scholar] [CrossRef]
- Yang, W.; Deng, Z.; Wang, Y.; Ma, L.; Zhou, K.; Liu, L.; Wei, Q. Porous boron-doped diamond for efficient electrocatalytic elimination of azo dye Orange G. Sep. Purif. Technol. 2022, 293, 121100. [Google Scholar] [CrossRef]
- Lan, G.; Qiu, Y.; Fan, J.; Wang, X.; Tang, H.; Han, W.; Liu, H.; Liu, H.; Song, S.; Li, Y. Defective graphene@diamond hybrid nanocarbon material as an effective and stable metal-free catalyst for acetylene hydrochlorination. Chem. Commun. 2019, 55, 1430–1433. [Google Scholar] [CrossRef] [PubMed]
- Kabir, H.; Zhu, H.; May, J.; Hamal, K.; Kan, Y.; Williams, T.; Echeverria, E.; McIlroy, D.N.; Estrada, D.; Davis, P.H.; et al. The sp2-sp3 carbon hybridization content of nanocrystalline graphite from pyrolyzed vegetable oil, comparison of electrochemistry and physical properties with other carbon forms and allotropes. Carbon 2019, 144, 831–840. [Google Scholar] [CrossRef]
- Yuan, Q.; Liu, Y.; Ye, C.; Sun, H.; Dai, D.; Wei, Q.; Lai, G.; Wu, T.; Yu, A.; Fu, L.; et al. Highly stable and regenerative graphene–diamond hybrid electrochemical biosensor for fouling target dopamine detection. Biosens. Bioelectron. 2018, 111, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qin, J.; Li, M.; Li, C.; Xu, S.; Qian, L.; Yang, B. Gold-nanoparticle-decorated boron-doped graphene/BDD electrode for tumor marker sensor. Sens. Actuators B Chem. 2020, 302, 127209. [Google Scholar] [CrossRef]
- Sen, D.; Das, B.K.; Saha, S.; Roy, R.; Mitra, A.; Chattopadhyay, K.K. sp3 bonded 2-dimensional allotrope of carbon: A first-principles prediction. Carbon 2019, 146, 430–437. [Google Scholar] [CrossRef]
- Pei, J.; Yu, X.; Zhang, Z.; Zhang, J.; Wei, S.; Boukherroub, R. In-situ graphene modified self-supported boron-doped diamond electrode for Pb(II) electrochemical detection in seawater. Appl. Surf. Sci. 2020, 527, 146761. [Google Scholar] [CrossRef]
- Shen, B.; Ji, Z.; Lin, Q.; Gong, P.; Xuan, N.; Chen, S.; Liu, H.; Huang, Z.; Xiao, T.; Sun, Z. Graphenization of Diamond. Chem. Mater. 2022, 34, 3941–3947. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Gai, Z.; Zhang, M.; Liu, S.; Guo, F.; Yang, N.; Jiang, X. Synthesis of graphene interlayer diamond films for enhanced electrochemical performance. Carbon 2022, 196, 602–611. [Google Scholar] [CrossRef]
- Watanabe, T.; Yoshioka, S.; Yamamoto, T.; Sepehri-Amin, H.; Ohkubo, T.; Matsumura, S.; Einaga, Y. The local structure in heavily boron-doped diamond and the effect this has on its electrochemical properties. Carbon 2018, 137, 333–342. [Google Scholar] [CrossRef]
- Xu, J.; Yokota, Y.; Wong, R.A.; Kim, Y.; Einaga, Y. Unusual Electrochemical Properties of Low-Doped Boron-Doped Diamond Electrodes Containing sp2 Carbon. J. Am. Chem. Soc. 2020, 142, 2310–2316. [Google Scholar] [CrossRef]
- Bogdanowicz, R.; Ficek, M.; Malinowska, N.; Gupta, S.; Meek, R.; Niedziałkowski, P.; Rycewicz, M.; Sawczak, M.; Ryl, J.; Ossowski, T. Electrochemical performance of thin free-standing boron-doped diamond nanosheet electrodes. J. Electroanal. Chem. 2020, 862, 114016. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, Z.; Liu, F.; Pei, J.-X.; Tian, X.-Y. Effect of interfacial layer on graphene structure in-situ grown on cemented carbide. J. Alloys Compd. 2019, 806, 1309–1314. [Google Scholar] [CrossRef]
- Peng, Z.; Yan, Z.; Sun, Z.; Tour, J.M. Direct growth of bilayer graphene on SiO2 substrates by carbon diffusion through nickel. ACS Nano 2011, 5, 8241–8247. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Lin, T.; Bi, H.; Huang, F.; Xie, X.; Chen, I.-W.; Jiang, M. Autonomously Controlled Homogenous Growth of Wafer-Sized High-Quality Graphene via a Smart Janus Substrate. Adv. Funct. Mater. 2012, 22, 1033–1039. [Google Scholar] [CrossRef]
- Zhai, Z.; Huang, N.; Yang, B.-P.; Wang, C.; Liu, L.; Qiu, J.; Shi, D.; Yuan, Z.; Lu, Z.-G.; Song, H.; et al. Insight into the Effect of the Core–Shell Microstructure on the Electrochemical Properties of Undoped 3D-Networked Conductive Diamond/Graphite. J. Phys. Chem. C 2019, 123, 6018–6029. [Google Scholar] [CrossRef]
- Hu, X.; Chen, C.; Lu, S. High mobility n-type conductive ultrananocrystalline diamond and graphene nanoribbon hybridized carbon films. Carbon 2016, 98, 671–680. [Google Scholar] [CrossRef]
- Kamata, T.; Kato, D.; Ida, H.; Niwa, O. Structure and electrochemical characterization of carbon films formed by unbalanced magnetron (UBM) sputtering method. Diam. Relat. Mater. 2014, 49, 25–32. [Google Scholar] [CrossRef]
- Li, X.; Fan, S.; Jin, C.; Gao, M.; Zhao, Y.; Guo, L.; Ji, J.; She, Z. Electrochemical degradation of tetracycline hydrochloride in sulfate solutions on boron-doped diamond electrode: The accumulation and transformation of persulfate. Chemosphere 2022, 305, 135448. [Google Scholar] [CrossRef]
- Pleskov, Y.V.; Krotova, M.D.; Elkin, V.V.; Ekimov, E.A. Electrochemical Behaviour of Boron-doped Diamond Compacts—A New Electrode Material. Electrochim. Acta 2016, 201, 268–273. [Google Scholar] [CrossRef]


| Film Type | E1 (w) | E2 (w) | E3 (w) | Q |
|---|---|---|---|---|
| BDD | 4.62 | 6.07 | 1.44 | 23.72% |
| DG10D | 2.84 | 3.89 | 1.04 | 26.73% |
| DG20D | 1.83 | 4.29 | 2.46 | 57.34% |
| DG30D | 1.85 | 3.51 | 1.65 | 47.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Gai, Z.; Guo, F.; Zhang, M.; Zhang, L.; Xia, G.; Chai, X.; Ren, Y.; Zhang, X.; Jiang, X. A Diamond/Graphene/Diamond Electrode for Waste Water Treatment. Nanomaterials 2023, 13, 3043. https://doi.org/10.3390/nano13233043
Wang Y, Gai Z, Guo F, Zhang M, Zhang L, Xia G, Chai X, Ren Y, Zhang X, Jiang X. A Diamond/Graphene/Diamond Electrode for Waste Water Treatment. Nanomaterials. 2023; 13(23):3043. https://doi.org/10.3390/nano13233043
Chicago/Turabian StyleWang, Yibao, Zhigang Gai, Fengxiang Guo, Mei Zhang, Lili Zhang, Guangsen Xia, Xu Chai, Ying Ren, Xueyu Zhang, and Xin Jiang. 2023. "A Diamond/Graphene/Diamond Electrode for Waste Water Treatment" Nanomaterials 13, no. 23: 3043. https://doi.org/10.3390/nano13233043
APA StyleWang, Y., Gai, Z., Guo, F., Zhang, M., Zhang, L., Xia, G., Chai, X., Ren, Y., Zhang, X., & Jiang, X. (2023). A Diamond/Graphene/Diamond Electrode for Waste Water Treatment. Nanomaterials, 13(23), 3043. https://doi.org/10.3390/nano13233043







