Abstract
A sulfur-containing metal–organic framework, donated as UiO-66-NSMe, was prepared by the post-synthetic modification (PSM) of UiO-66-NH2 with 2-(Methylthio)benzaldehyde, and the successful synthesis of PSM was confirmed by X-ray photoelectron spectroscopy (XPS), FT-IR and 1H NMR studies. According to the characteristics of mercury thiophilic, UiO-66-NSMe could be used as a luminescent sensor for Hg2+ detection with a high selectivity and sensitivity (Ksv = 2.5 × 104 M−1; LOD = 20 nM), which could be attributed to the coordination between sulfur sites and Hg2+ based on XPS results. In practical applications, UiO-66-NSMe yielded satisfactory recovery rates (ranging from 96.1% to 99.5%) when it was employed for detecting Hg2+ in spiked environmental samples. Furthermore, UiO-66-NSMe was successfully employed to detect mercury (II) residues with the in situ rapid nondestructive imaging in simulated fresh agricultural products. Thus, this PSM strategy could provide good guidance for environmental protection methodologies in the future.
1. Introduction
Rapid industrialization has released significant amounts of pollutants, including heavy metal ions, into the environment. Among the most toxic and hazardous contaminants, mercury is extensively used in batteries, barometers, float valves, thermometers, and other devices [1]. However, when introduced into natural water environments through microbial processes, ionic mercury readily converts into neurotoxic methylmercury. Even trace levels of mercury bioaccumulation can lead to organ and health issues, such as digestive, kidney, and nervous system disorders [2]. Therefore, the detection of mercury ions in aqueous solutions is of paramount importance. Numerous techniques have been employed to detect mercury, including atomic absorption spectrometry (AAS), cold vapor atomic fluorescence spectroscopy (AFS), mass spectrometry (MS), and liquid chromatography (LC) [3,4]. However, these methods encounter challenges related to intricate sample preparation and costly equipment, which restrict their extensive in-field application. Consequently, achieving prompt response and the highly selective and sensitive detection of Hg2+ in water remains a formidable challenge.
Metal–organic frameworks (MOFs) [5,6,7] materials have garnered significant attention in the last two decades due to their high surface area, exceptional stability, adjustable porosity, customizable chemical functionality, and potential applications, particularly in gas storage and separation [8,9], heterogeneous catalysis [10,11], chemical sensing [12,13,14,15], proton conduction [16], and drug delivery [17]. Specifically, as fluorescent sensors, MOFs offer the advantage of precise structural design and control by selecting different metal ions and organic ligands to meet the needs of various analytes. Simultaneously, they can selectively adsorb molecules with specific sizes or properties through the sieving action of their pores, thereby concentrating the analytes within MOFs. This can enhance the sensitivity of detecting target molecules. Some fluorescent sensors based on MOFs for detecting Hg2+ have been documented [18,19,20]. For example, Wen et al. developed two amino-decorated MOFs for the selective and quantitative detection of Hg2+, attributed to the chelating interaction between Hg2+ and the pendant amino motif of the ligand [21]. Our group reported the first example of novel dual-emission MOF-implicated ratiometric sensor for mercury (II) in pure water with an LOD of about 2 nM, attributed to Hg2+-induced structural collapse [22]. More recently, Wang et al. designed and synthesized several FRET-based MOFs with different donor/acceptor ratios. The MOF with an optimized donor/acceptor ratio of 7.0 was employed as an efficient fluorescence turn-on sensor for Hg (II) ions, demonstrating good sensitivity and selectivity [23].
However, certain challenges, such as the compatibility of the functional groups, limited linker solubility, and inadequate chemical, or thermal stability in some cases, can hinder direct synthesis. Moreover, there are several factors that restrict their widespread use, including their inability to function in pure water, susceptibility to interference from other metal ions, and the inability to achieve precise metal ion detection specificity. Cohen and Burrows, among others, have established that the post-synthetic modification (PSM) of MOFs can yield new active sites as an effective and flexible strategy [24,25]. This approach enhances selectivity and augments detection sensitivity, offering a promising solution to the aforementioned problems. As an example, Lang and his colleagues utilized PSM in a two-dimensional metal–organic framework through photodimerization. The resulting PSM product displayed the capability to detect Al3+ ions through a luminescent quenching mechanism, offering significantly enhanced selectivity and sensitivity [26].
In this study, we focus on the exceptional water stability of UiO-series MOFs, attributed to the robust interaction between zirconium (IV) (Zr4+) clusters and carboxylic ligands [27]. To this end, we synthesized a conventional UiO-66-type MOF (UiO-66-NH2) utilizing a microwave-assisted method, which yields primary amine groups suitable for subsequent post-synthetic functionalization. Through the post-synthetic modification of UiO-66-NH2 with 2-(Methylthio)benzaldehyde, a novel sulfur-containing MOF (UiO-66-NSMe) was created. Notably, when tested in the HEPES buffer, UiO-66-NSMe exhibited a significantly improved ability to selectively detect Hg2+ compared to the original compound. Furthermore, we delve into the mechanistic understanding behind this enhanced selectivity. The in situ imaging of UiO-66-NSMe for mercury (II) ion detection has also been developed.
2. Materials and Methods
2.1. Materials
All chemicals used in this study were of reagent-grade quality and were obtained from commercial sources without the need for additional purification. Specifically, 2-aminoterephthalic acid (99%) and acetic acid (99.5%) were purchased from the Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). N,N-Dimethylformamide (DMF, 99.5%), methanol (99.5%), Zirconium (IV) chloride (ZrCl4, 98%), 2-(Methylthio)benzaldehyde (97%), and HEPES (99%) were purchased from Beijing Innochem Science & Technology Co., Ltd. (Beijing, China). Mercury (II) nitrate hydrate (98%) and the other metal salts were purchased from Shanghai Fourth Chemical Reagent Company (Shanghai, China). Stock solution (2 × 10−2 M) of the aqueous nitrate salts of K+, Na+, Li+, Cd2+, Zn2+, Mn2+, Fe2+, Cu2+, Ba2+, Sr2+, Ca2+, Mg2+, Ni2+, Co2+, Pb2+, Ag+, Fe3+, Cr3+, Al3+, and Hg2+ were prepared for further experiments. Deionized water generated from the purification chain was used for all experiments. Lettuce was purchased at the Zhaishan Farmers’ Market (No. 8 Chengnan Avenue, Quanshan District, Xuzhou, China).
2.2. Characterization Methods
The X-ray powder diffraction (XRD) patterns of UiO-66-NH2, UiO-66-NSMe, and UiO-66-NSMe after Hg2+ detection were recorded on a Bruker D8 Advance X-ray powder diffractometer using Cu–Kα (λ = 1.5405 Ǻ) radiation. FT-IR spectra were recorded as KBr pellets on Bruker Optics TENSOR 27 FT-IR spectrophotometer (Bruker, Billerica, MA, USA). X-ray photoelectron spectroscopy (XPS) experiments were performed using a PHI QUANTUM2000 surface analysis instrument. The morphologies of the prepared samples were recorded by a Field Emission Scanning Electron Microscopy (SEM) by Hitachi (SU8010) (Tokyo, Japan). Samples were treated via Pt sputtering for 90 s before observation. Nitrogen sorption isotherms were recorded on an Autosorb-IQ2 instrument at 77 K. 1H NMR spectra were recorded using Me4Si as an internal standard on a Bruker-400 spectrometer. The Zr4+ contents before and after Hg2+ detection were measured by Inductively Coupled Plasma Spectrometer (Perkin Elmer, Waltham, MA, USA). The solution fluorescence spectra were measured on JASCO FP6500. Both excitation and emission slit widths were 5 nm, and fluorescence measurements were carried out in a 1 cm quartz cuvette while stirring the suspension of UiO-66-NH2 or UiO-66-NSMe.
2.3. Synthesis of UiO-66-NH2
Following the methodology outlined in the references [28], the synthesis of UiO-66-NH2 was achieved through a microwave-assisted procedure. Initially, a combination of 2-aminoterephthalic acid (0.75 mmol, 0.135 g) and ZrCl4 (0.75 mmol, 0.175 g) was introduced into a solution composed of DMF (20 mL) and acetic acid (2.4 mL). This mixture was then subjected to microwave reactor and heating at 120 °C for a duration of 1 h. After cooling to room temperature, the solution underwent centrifugation, resulting in a precipitate that underwent a thorough washing process using DMF and MeOH, which was repeated three times, to effectively remove any unreacted ligand/metal. The resulting gray-colored powder was collected and subsequently air-dried.
2.4. Synthesis of UiO-66-NSMe
A quantity of UiO-66-NH2 weighing 100 mg was dispersed within a solution of 2-(Methylthio)benzaldehyde (1.2 mmol, 0.178 g) in MeOH (50 mL), along with two drops of acetic acid. The resulting mixture was subjected to stirring at 75 °C for a duration of 24 h. Following the completion of the reaction, the resultant product, UiO-66-NSMe, was obtained in a colorless form. The obtained products were collected through a filtration process, subsequently washed with MeOH, and finally air-dried.
2.5. Digestion and 1H NMR on UiO-66-NSMe
Approximately 10 mg of UiO-66-NSMe materials was desiccated under vacuum at 100 °C for 8 h. Subsequently, it were subjected to digestion by d6-DMSO (500 μL) and 48% HF (30 μL) via sonication for 1 h until a clear solution was achieved. The resulting solution was then subjected to 1H NMR analysis.
2.6. Detection of Hg2+
In the typical procedure, 1 mg of finely ground UiO-66-NSMe sample was carefully dispersed in 3 mL of HEPES buffer with a pH of 7.4. Following a 5 min sonication period to ensure thorough mixing, the initial fluorescence spectra of UiO-66-NSMe were recorded. Subsequently, the fluorescence spectra of UiO-66-NSMe were measured upon the addition of Hg2+ ions, using an excitation wavelength of 360 nm. To assess the selectivity of UiO-66-NSMe for Hg2+, similar experiments were conducted with various other metal ions, each undergoing a comparable sensing procedure in the solution.
In the analysis of Hg2+ in spiked samples, we employed lake water, tap water, and rainwater devoid of Hg2+ as representative sample matrices. Spiked lake water, tap water, and rainwater were subjected to direct detection immediately following spiking. To simulate the direct detection of Hg2+ residue on the surfaces of garden produce, we sprayed lettuce samples with solutions containing varying concentrations of Hg2+. After a 2 min interval, a UiO-66-NSMe water suspension was applied to the smeared surfaces, followed by another 2 min wait period. Subsequently, we observed the samples under UV light. To prepare the fluorescent test paper, a standard light-yellow wood-colored filter paper was cut into a square measuring 2 cm in both length and width. Subsequently, the filter paper was immersed in a UiO-66-NSMe solution and allowed to air-dry naturally.
2.7. Typical Procedure for Cyanosilylation of Aldehydes
Into a 3 mL CH2Cl2 mixture containing 1.2 mmol of Trimethylsilyl cyanide ((CH3)3SiCN, TMSCN) and 0.5 mmol of aromatic aldehyde, UiO-66-NSMe or UiO-66-NSMe⊃Hg2+ (5 mg) was introduced. The resulting mixture was stirred at room temperature for a duration of 3 h. The progression of the reaction was monitored via thin-layer chromatography (TLC), and conversions were quantified through 1H-NMR analysis.
3. Results
3.1. Characterization of PSM Product UiO-66-NSMe
A reaction involving ZrCl4 and 2-aminoterephthalic acid in DMF utilizing microwave synthesis yielded UiO-66-NH2. The resulting material exhibited powder X-ray diffraction (PXRD) patterns that were notably congruent with those previously reported for the UiO-66 topology (Figure 1a). Additionally, scanning electron microscopy (SEM) observations displayed a consistently uniform octahedral morphology (Figure 1b), aligning with the findings in the existing literature [29,30], all confirming the successful fabrication of UiO-66-NH2. Subsequently, sulfur-containing functional groups were covalently incorporated into the framework by exploiting Schiff base reactions between amino groups and aldehyde groups within the framework structure.
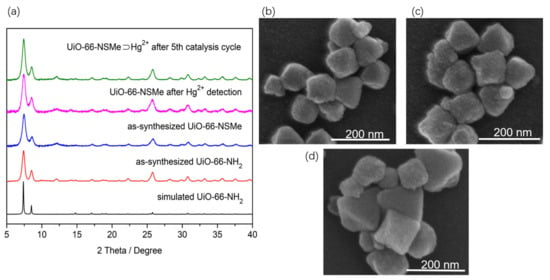
Figure 1.
(a) Powder X-ray diffraction (XRD) profiles for the simulated (black) and as-synthesized UiO-66-NH2 (red), as-synthesized UiO-66-NSMe (blue), UiO-66-NSMe after Hg2+ detection (magenta), and UiO-66-NSMe⊃Hg2+ after the fifth catalysis cycle (olive). (b) SEM imaging of UiO-66-NH2. (c) SEM imaging of UiO-66-NSMe. (d) SEM imaging of UiO-66-NSMe after Hg2+ detection.
By conducting a reflux reaction involving UiO-66-NH2 and 2-(Methylthio)benzaldehyde with glacial acetic acid as the catalyst, UiO-66-NSMe was obtained. The synthesized material underwent filtration, washing, and drying steps. The PXRD patterns demonstrated that the structural integrity of UiO-66-NMSe remained unchanged during the process of post-synthetic modification (Figure 1a). Simultaneously, the morphology of the samples was examined using SEM. As depicted in Figure 1c, the images of UiO-66-NSMe exhibited a uniformly dispersed octahedral structure. These observations are in line with the PXRD analysis, indicating that the PSM process applied to UiO-66-NH2 did not exert a discernible influence on its microstructure.
The successful synthesis of UiO-66-NSMe was subjected to a comprehensive analysis. A comparison of the FT-IR spectra of UiO-66-NSMe with UiO-66-NH2, depicted in Figure 2a, revealed new peaks at approximately 2972–2924 cm−1. These peaks correspond to the characteristic C-H stretching vibrations of the methyl group in UiO-66-NSMe. Importantly, a new peak appeared at 1618 cm−1, which is indicative of the characteristic C=N stretching vibrations specific to UiO-66-NSMe. Simultaneously, the peak at 1334 cm−1, which was attributed to the C-N stretching vibration of UiO-66-NH2, nearly disappeared. The alterations in the FT-IR spectra collectively demonstrated that the structure of UiO-66-NSMe is characterized by the absence of free amino groups, replaced by the creation of new C=N groups via the Schiff base reaction. In addition, methyl groups associated with -SMe are unambiguously identified within the structure of UiO-66-NSMe.
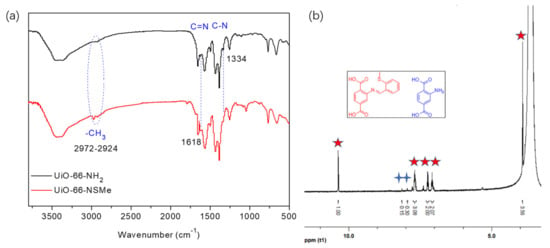
Figure 2.
(a) FT-IR spectra of UiO-66-NH2 and UiO-66-NSMe. (b) 1H NMR spectra of UiO-66-NSMe digested in DMSO-d6/HF.
Another solid evidence supporting the formation of Schiff base reaction products is derived from 1H NMR studies. In DMSO-d6/HF, UiO-66-NSMe displayed a resonance signal at 10.37 ppm, corresponding to the –CH=N- proton, together with a methyl proton signal at 3.92 ppm. The integration area between these signals is consistent with the anticipated product, affirming the success of the PSM process (Figure 2b). A modification rate of up to 87% was determined by calculating the ratio of aromatic region at PSM products (Figure 2b, red star) to the proton signal of 2-aminoterephthalic acid (Figure 2b, blue cross) in 1H NMR after the digestion of UiO-66-NSMe. Furthermore, the XPS full spectra of UiO-66-NSMe (Figure 4) unveiled a new peak at 163.07 eV in the S 2p region, providing further confirmation of successful PSM and the resultant formation of UiO-66-NSMe. At the same time, the elemental composition obtained by XPS for UiO-66-NSMe indicated that PSM extent is calculated as 84%, which is quite close to the 1H NMR result (Table S1).
N2 sorption isotherms were subsequently measured at 77 K on the as-synthesized UiO-66-NH2 and UiO-66-NSMe samples. It was observed that the surface area had decreased from 963 m2 g−1 to 425 m2 g−1, further confirming the successful formation of PSM products (Figure 3). Nonetheless, UiO-66-NSMe still maintains its pores, ensuring the ingress and egress of guest molecules or ions. All these data collectively highlight the successful covalent post-synthetic modification of UiO-66-NH2, in which a Schiff base reaction was effectively established between the amino group within the MOF and the aldehyde group, all while maintaining the structural integrity.
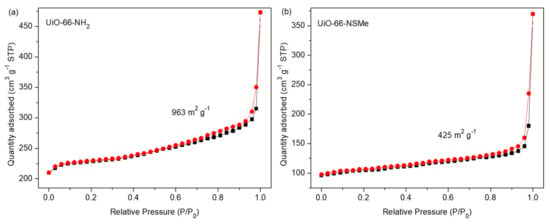
Figure 3.
N2 sorption isotherms for compounds UiO-66-NH2 (a) and UiO-66-NSMe (b) at 77 K. The adsorption and desorption branches are shown with black square and red circle, respectively.
3.2. Luminescent Detection of Metal Ions in HEPES Buffer Based on UiO-66-NSMe
Considering the presence of sulfur atoms within the UiO-66-NSMe framework, we have delved into its metal-ion-sensing capabilities, particularly its exploitation of the thiophilic nature of mercury ions [31,32,33]. Under excitation at 360 nm, the UiO-66-NSMe suspension in HEPES buffer exhibited a pronounced fluorescence emission peak at approximately 450 nm (Figure 4a). Luminescence titrations involving Hg2+ were then conducted with the UiO-66-NSMe suspension to assess its capacity for detecting mercury (II) ions. Notably, rapid and substantial fluorescence quenching was observed upon the addition of Hg2+ solution to the UiO-66-NSMe suspension, reaching concentrations up to 0.56 mM (Figure 4a). This quenching effect can be quantified using the Stern–Volmer equation, I0/I = 1 + Ksv[M], where I denotes the luminescence intensity after Hg2+ addition, I0 is the initial luminescence intensity of UiO-66-NSMe, [M] represents the molar concentration of the introduced Hg2+, and Ksv signifies the Stern–Volmer constant. Through calculation based on the experimental data in Figure 3a, a Ksv value of 2.5 × 104 M−1 was determined (Figure 4b). Following the 3δ IUPAC criteria, the limit of detection for Hg2+ is approximately 20 nM. Therefore, UiO-66-NSMe proves to be a proficient material for detecting mercury (II) ions, with its heightened sensitivity for Hg2+ attributed to the affinity-driven interaction between Hg2+ and the sulfur atoms present.
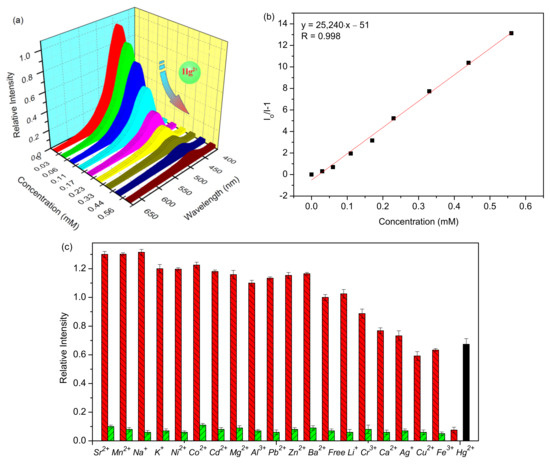
Figure 4.
(a) Luminescence spectra of UiO-66-NSMe upon incremental addition of Hg2+ up to 0.56 mM in HEPES buffer, with excitation at 360 nm. (b) Stern–Volmer plot of UiO-66-NSMe quenched by Hg2+. (c) The red bars stand for the luminescence intensities of UiO-66-NSMe when the selected metal ions (1.12 mM) are present. The green ones stand for the change of the emission when continuing to add 0.56 mM Hg2+ (The black bar represents the fluorescence intensity of UiO-66-NH2 upon adding 0.56 mM of Hg2+).
To assess the selectivity of UiO-66-NSMe towards Hg2+, its luminescence responses were examined upon the introduction of various other metal ions, including Cr3+, Al3+, K+, Co2+, Sr2+, Cd2+, Na+, Mg2+, Ba2+, Pb2+, Ni2+, Cu2+, Zn2+, Ag+, Ca2+, Fe3+, Li+, and Mn2+ (Figure 4c and Figure S1). The fluorescence spectra of UiO-66-NSMe exhibited minimal changes in the presence of K+, Co2+, Mg2+, Ba2+, Pb2+, Ni2+, Al3+, Li+, Cd2+, and Zn2+. Slight fluorescence enhancement was observed with Sr2+, Mn2+, and Na+. In UiO-66-NSMe, the fluorescence quenching induced by Cr3+, Ca2+, Ag2+, Cu2+, and Fe3+ was only 40% of their highest quenching efficiency. In sharp contrast, the addition of Hg2+ ions (0.56 mM) led to a fluorescence quenching exceeding 93%. Comparatively, the introduction of Hg2+ ions into the original UiO-66-NH2 suspension resulted in only a 33% fluorescence quench. Meanwhile, the anti-interference capability of UiO-66-NSMe for Hg2+ detection was further explored by competing experiments (Figure 4c, green bars). The experimental result reveals that the coexistence of interferers had no obvious interference on the Hg2+ detection. This distinction highlights the remarkable selectivity of UiO-66-NSMe for Hg2+. The framework modification through PSM, leading to the introduction of sulfur (S) moieties, emerges as an effective approach to enhance the detection of mercury (II) ions.
To further validate the detection mechanism of Hg2+ on UiO-66-NSMe, the PXRD patterns of the UiO-66-NSMe samples following Hg2+ detection matched well with the original pattern (Figure 1a). This alignment demonstrates that the primary framework structure remained unchanged. Moreover, inductively coupled plasma (ICP) measurements revealed minimal Zr4+ content present in the filtrate (Table S2), effectively excluding the possibilities of compound dissolution and the exchange of Hg2+ and Zr4+ within the lattice, which could lead to the drastic luminescence quenching observed. These findings are additionally supported by the sustained SEM morphology even after detecting Hg2+ ions (Figure 1d). Collectively, these results reinforce the notion that the significant luminescence quenching in response to Hg2+ is not attributed to structural changes or dissolution but rather is due to specific interaction between Hg2+ and UiO-66-NSMe.
Furthermore, the X-ray photoelectron spectroscopy (XPS) spectra of UiO-66-NSMe were measured both before and after Hg2+ detection. After Hg2+ detection, a new peak corresponding to Hg 4f at a binding energy of approximately 100.91 eV emerged in the full spectrum of UiO-66-NSMe. This unequivocally confirms the adsorption of Hg2+ within the channels of UiO-66-NSMe (Figure 5). Intriguingly, a shift from 163.07 eV to 164.04 eV in the binding energy of the S 2p peak was observed before and after Hg2+ detection. Such a shift implies an augmented electron density around the sulfur (S) atoms, likely stemming from the coordination interaction between Hg2+ and S atoms within UiO-66-NSMe. Thus, combined with the structure of UiO-66-NSMe, it is possible that Hg2+ ions are chelating coordinated by the S and N sites in UiO-66-NSMe.
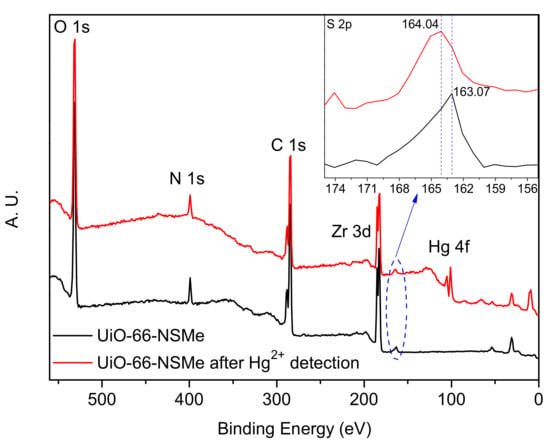
Figure 5.
XPS spectra of UiO-66-NSMe before and after Hg2+ detection.
As the UiO-66-NSMe detection of Hg2+ relies on the coordination between mercury and sulfur sites, the material can be recycled after detecting Hg2+. This newly coordinated Hg2+ material, donated as UiO-66-NSMe⊃Hg2+, possesses additional metal centers compared to the original material, thereby enhancing its Lewis acidic properties. In its role as a catalyst, UiO-66-NSMe⊃Hg2+ demonstrates improved catalytic activity for Lewis acid-catalyzed cyanosilane reactions. To illustrate this enhancement, we conducted a model reaction using benzaldehyde and (CH3)3SiCN at room temperature in CH2Cl2 for 3 h. With UiO-66-NSMe⊃Hg2+ (5 mg) as catalysts, the expected product, 2-phenyl-2-((trimethylsilyl)oxy)acetonitrile, was obtained in >99% yield. In contrast, when UiO-66-NSMe was used as the catalyst, it yielded a 58% 2-phenyl-2-((trimethylsilyl)oxy)acetonitrile with no observed byproducts. This substantial difference can be attributed to the enhanced Lewis acid properties conferred by UiO-66-NSMe⊃Hg2+.
Importantly, UiO-66-NSMe⊃Hg2+ could be readily recovered from the catalytic system through centrifugation and subsequently reused in fresh conditions. Remarkably, this catalyst exhibited no significant decrease in efficiency; the yield for 2-phenyl-2-((trimethylsilyl)oxy)acetonitrile was 97% for the second run, 98% for the third run, 96% for the fourth run, and 97% for the fifth run. The stability of UiO-66-NSMe⊃Hg2+ was confirmed by XRD after the fifth catalysis cycle (Figure 1a). This exceptional catalytic cycling performance further underscores the robust interaction between UiO-66-NSMe and mercury (II) ions, reinforcing the previous findings of coordination between mercury (II) ions and sulfur sites.
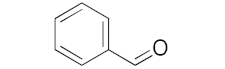
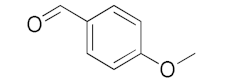
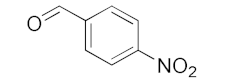
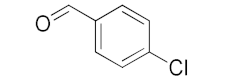
With these impressive catalytic results in hand, we extended our investigation to explore the generality and scope of the current protocol across a variety of aromatic aldehydes in conjunction with (CH3)3SiCN. As presented in Table 1 (Figures S2–S7), when utilizing UiO-66-NSMe⊃Hg2+ as the catalyst, the yields for 4-methoxybenzaldehyde, 4-nitrobenzaldehyde, and 4-chlorobenzaldehyde reached 96%, 95%, and 93%, respectively. These yields were almost twice as high as when UiO-66-NSMe was used as the catalyst. However, as the size of the substrates increased, catalytic efficiency exhibited a substantial decrease. For instance, 1-naphthaldehyde yielded only 51%, while the larger 3,5-di-tert-butylbenzaldehyde resulted in a mere 15% yield when UiO-66-NSMe⊃Hg2+ served as the catalyst. These outcomes underscore that Hg2+-modified UiO-66-NSMe performs well not only in the cyanosilylation of aromatic aldehydes but also displays promising size-selective behavior. This presents a bright prospect for the design of more active catalyst materials and offers an effective means for the reutilization of test materials.

Table 1.
Results of the cyanosilylation reaction catalyzed by UiO-66-NSMe and UiO-66-NSMe⊃Hg2+.
3.3. In situ Imaging Detection of Hg2+ in Real Water, Vegetables, and Test Paper
To evaluate the reliability for practical Hg2+ detection by using the prepared material in real water samples, we employed a “turn-off” fluorescent sensor based on UiO-66-NSMe. Given that Hg2+ ions were not naturally present in rainwater, lake water (Yunlong Lake, Yunlong district, Xuzhou, China), or tap water (No. 1 Beijing South Road, Tongshan district, Xuzhou, China), we conducted recovery experiments using spiked samples. The added amounts of Hg2+ were set at 10 μM, 20 μM, and 30 μM, respectively. The fluorescence-based method for detecting Hg2+, relying on UiO-66-NSMe in real water samples, as indicated in Table 2 (Figures S8 and S9), yielded recovery rates ranging from 96.1% to 99.5%. These outstanding detection results demonstrate that our designed UiO-66-NSMe possesses the capability of reliable detection for Hg2+ in real-world samples.

Table 2.
Spike and recovery test of Hg2+ by using UiO-66-NSMe in real water samples.
Given UiO-66-NSMe’s remarkable ability to rapidly and efficiently detect Hg2+, we conducted further investigations to assess its potential for on-site detection. UiO-66-NSMe was employed to simulate the detection of mercury (II) residue on the surface of garden produce through direct imaging technology. Because vegetables are sensitive agricultural products that require a lot of water to irrigate, exogenous mercury (II) ions can enter them. Since Hg2+ ions were not naturally present on the lettuce we obtained, we simulated mercury (II) contamination by applying varying concentrations of Hg2+ solution to their surfaces. Subsequently, luminescence was observed under UV light at 365 nm following the addition of the UiO-66-NSMe suspension. As illustrated in Figure 5, a significant reduction in UiO-66-NSMe luminescence on the surface of lettuce was observed, progressively diminishing until complete quenching as the Hg2+ concentration increased from 0.04 mM to 0.56 mM (Figure 6). Collectively, these results strongly indicate that UiO-66-NSMe holds great promise for the rapid and on-site detection of mercury (II) residues in real samples through direct imaging techniques.
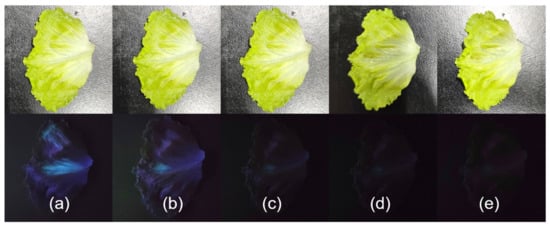
Figure 6.
Noticeable differences in fluorescence became evident when UiO-66-NSMe suspension (0.33 g/L) was introduced to the surface of the lettuce under ultraviolet light, and the change in fluorescence was observed within 30 s. Lettuce was subjected to spraying with solutions of varying concentrations: 0 (a), 0.04 (b), 0.18 (c), 0.35 (d), and 0.56 (e) mM Hg2+, simulating mercury (II) residue.
To facilitate simple and portable detection, we developed a fluorescence test paper for the rapid assessment of Hg2+ in aqueous solutions. This test paper was created by immersing a light-yellow wood-colored Whatman filter paper measuring 2.0 × 2.0 cm2 into a dispersion of ground UiO-66-NSMe in water and subsequently allowing it to dry at room temperature. For the detection of minuscule quantities of Hg2+ ions, 1 μL of aqueous solutions containing varying concentrations of Hg2+ ions was placed onto the UiO-66-NSMe test papers. As depicted in Figure 7, when exposed to UV light at 365 nm, the test papers exhibited varying colors of different intensities that were discernible to the naked eye, corresponding to the concentration levels of Hg2+ ions. The minimum detectable amount of Hg2+ ions was calculated to be at the remarkably low level of 13.7 ng. All these practice experimental findings underscore the advantages of UiO-66-NSMe as an efficient, sensitive, and selective sensor for Hg2+ ions.
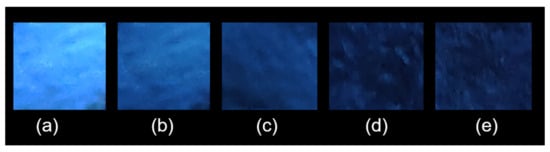
Figure 7.
Photographs depicting the fluorescence quenching of UiO-66-NSMe on test strips for the visual detection of small amounts of Hg2+ under 365 nm UV light: (a) test strip; Hg2+ of different concentrations (b) 0.04 mM, (c) 0.18 mM, (d) 0.35 mM, (e) 0.56 mM.
4. Conclusions
In conclusion, we have successfully synthesized a novel framework with the -SCH3 functional group, designated as UiO-66-NSMe, through post-synthetic modification (PSM). The effective conversion was verified by FT-IR, XPS, and 1H NMR analyses, yielding a PSM rate of up to 87%. Luminescence-based studies of metal ion detection demonstrated that UiO-66-NSMe displays exceptional selectivity and sensitivity towards Hg2+ in HEPES buffer. Additionally, the comprehensive detection mechanism was attributed to the presence of a coordination interaction between Hg2+ and S atoms within UiO-66-NSMe. The MOF sensor was also utilized for the detection of Hg2+ in tap water, rainwater, and river water spiked with the contaminant. Additionally, it was employed to detect mercury (II) residues with in situ rapid nondestructive imaging in simulated fresh agricultural products. Moreover, a straightforward and portable test paper enabled the visual detection of Hg2+ ions at the nanogram level by the naked eye. We believe that this study holds promise for advancing environmental detection methodology in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano13202784/s1, Table S1: XPS elemental compositions (atomic %); Figure S1: The fluorescence spectra of UiO-66-NSMe in HEPES buffer upon the addition of 0.56 mM of various metal ions; Figures S2 to S7: 1H NMR characterization of catalytic products by as-synthesized UiO-66-NSMe⊃Hg2+; Table S2: The ICP result on the filtrate of UiO-66-NSMe after Hg2+ detection; Figure S8: The relative fluorescence intensity of UiO-66-NSMe with varying Hg2+ concentrations; Figure S9: Spike test of Hg2+ by using UiO-66-NSMe in real water samples.
Author Contributions
Conceptualization, P.W. and J.W.; methodology, C.J. and L.P.; software, J.Q. and Y.Z.; validation, C.J., L.P., N.S. and T.Z.; formal analysis, C.J. and L.P.; investigation, J.Q.; data curation, C.J. and L.P.; writing—original draft preparation, J.W.; writing—review and editing, P.W. and J.W.; visualization, N.S.; supervision, P.W. and J.W.; project administration, J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Major Basic Research Project of Natural Science Foundation of the Jiangsu Higher Education Institutions (21KJA150001), the Natural Science Foundation of Jiangsu Province for Outstanding Youth (No. BK20180105), the Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (TAPP), and the Jiangsu Innovation and Entrepreneurship Training Program for College Students (No. 202210320142Y).
Data Availability Statement
All data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a Global Pollutant: Sources, Pathways, and Effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef] [PubMed]
- Nolan, E.M.; Lippard, S.J. Tools and Tactics for the Optical Detection of Mercuric Ion. Chem. Rev. 2008, 108, 3443–3480. [Google Scholar] [CrossRef]
- Liu, J.L.; Han, Q.; Hu, E.Y.; Yang, C.; Yin, M.M. Determination of Trace Mercury in Water Samples by Cloud Point Extraction Coupled with Atomic Fluorescence Spectrometry. J. Anal. Chem. 2023, 78, 303–309. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, G.; Li, H. Selective binding and removal of guests in a microporous metal–organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef]
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.M.; Zhou, W.; Chen, B. Porous Metal–Organic Frameworks for Gas Storage and Separation: What, How, and Why? J. Phys. Chem. Lett. 2014, 5, 3468–3479. [Google Scholar] [CrossRef]
- Huang, Y.B.; Liang, J.; Wang, X.S.; Cao, R. Multifunctional metal–organic framework catalysts: Synergistic catalysis and tandem reactions. Chem. Soc. Rev. 2017, 46, 126–157. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.Q.; Jiang, H.L.; Sun, L.B. Metal–organic frameworks for heterogeneous basic catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal–organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal–organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef] [PubMed]
- Yan, B. Luminescence response mode and chemical sensing mechanism for lanthanide-functionalized metal–organic framework hybrids. Inorg. Chem. Front. 2021, 8, 201–233. [Google Scholar] [CrossRef]
- Huangfu, M.; Wang, M.; Lin, C.; Wang, J.; Wu, P. Luminescent metal–organic frameworks as chemical sensors based on “mechanism–response”: A review. Dalton Trans. 2021, 50, 3429–3449. [Google Scholar] [CrossRef]
- Wong, N.E.; Ramaswamy, P.; Lee, A.S.; Gelfand, B.S.; Bladek, K.J.; Taylor, J.M.; Spasyuk, D.M.; Shimizu, G.K.H. Tuning Intrinsic and Extrinsic Proton Conduction in Metal–Organic Frameworks by the Lanthanide Contraction. J. Am. Chem. Soc. 2017, 139, 14676–14683. [Google Scholar] [CrossRef]
- Rocca, J.D.; Liu, D.; Lin, W. Nanoscale Metal–Organic Frameworks for Biomedical Imaging and Drug Delivery. Acc. Chem. Res. 2011, 44, 957–968. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Qin, C.; Shao, K.Z.; Su, Z.M. A fluorescent sensor for highly selective sensing of nitro explosives and Hg(ii) ions based on a 3D porous layer metal–organic framework. CrystEngComm 2016, 18, 4765–4771. [Google Scholar] [CrossRef]
- Wang, M.; Guan, J.; Liu, S.; Chen, K.; Gao, Z.; Liu, Q.; Chen, X. Dual-ligand lanthanide metal–organic framework probe for ratiometric fluorescence detection of mercury ions in wastewater. Microchim. Acta 2023, 190, 359. [Google Scholar] [CrossRef]
- El Taher, B.J.; Sabouni, R.; Ghommem, M. Luminescent metal organic framework for selective detection of mercury in aqueous media: Microwave-based synthesis and evaluation. Colloid. Surface. A 2020, 607, 125477. [Google Scholar] [CrossRef]
- Wen, L.; Zheng, X.; Lv, K.; Wang, C.; Xu, X. Two Amino-Decorated Metal–Organic Frameworks for Highly Selective and Quantitatively Sensing of HgII and CrVI in Aqueous Solution. Inorg. Chem. 2015, 54, 7133–7135. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Liu, Y.; Liu, Y.; Wang, J.; Li, Y.; Liu, W.; Wang, J. Cadmium-Based Metal–Organic Framework as a Highly Selective and Sensitive Ratiometric Luminescent Sensor for Mercury(II). Inorg. Chem. 2015, 54, 11046–11048. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mal, A.; Gui, B.; Wang, C. Tuning Energy Transfer in Metal-Organic Frameworks for Fluorescence Turn-on Sensing of Hg(II) Ions. Chin. J. Chem. 2023, 41, 1051–1056. [Google Scholar] [CrossRef]
- Cohen, S.M. Postsynthetic Methods for the Functionalization of Metal–Organic Frameworks. Chem. Rev. 2012, 112, 970–1000. [Google Scholar] [CrossRef] [PubMed]
- Burrows, A.D.; Frost, C.G.; Mahon, M.F.; Richardson, C. Post-Synthetic Modification of Tagged Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2008, 47, 8482–8486. [Google Scholar] [CrossRef]
- Li, W.X.; Gu, J.H.; Li, H.X.; Dai, M.; Young, D.J.; Li, H.Y.; Lang, J.P. Post-synthetic Modification of a Two-Dimensional Metal–Organic Framework via Photodimerization Enables Highly Selective Luminescent Sensing of Aluminum(III). Inorg. Chem. 2018, 57, 13453–13460. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Ge, J.; Liu, L.; Shen, Y. Facile synthesis of amine-functionalized UiO-66 by microwave method and application for methylene blue adsorption. J. Porous Mater. 2017, 24, 647–655. [Google Scholar] [CrossRef]
- Huang, A.; Wan, L.; Caro, J. Microwave-assisted synthesis of well-shaped UiO-66-NH2 with high CO2 adsorption capacity. Mater. Res. Bull. 2018, 98, 308–313. [Google Scholar] [CrossRef]
- Taddei, M.; Dau, P.V.; Cohen, S.M.; Ranocchiari, M.; van Bokhoven, J.A.; Costantino, F.; Sabatini, S.; Vivani, R. Efficient microwave assisted synthesis of metal–organic framework UiO-66: Optimization and scale up. Dalton Trans. 2015, 44, 14019–14026. [Google Scholar] [CrossRef]
- Sapianik, A.A.; Barsukova, M.O.; Kovalenko, K.A.; Samsonenko, D.G.; Fedin, V.P. Heterometallic MOFs constructed from thiophene and furandicarboxylate ligands for heavy metal luminescence sensing. Dalton Trans. 2021, 50, 2807–2814. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xie, K.; Wang, A.; Lyu, F.; Ge, J.; Zhang, L.; Zhang, H.; Su, W.; Hou, Y.L.; Zhou, C.; et al. High selective detection of mercury (II) ions by thioether side groups on metal-organic frameworks. Anal. Chim. Acta 2019, 1081, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Volynkin, S.S.; Demakov, P.A.; Shuvaeva, O.V.; Kovalenko, K.A. Metal-organic framework application for mercury speciation using solid phase extraction followed by direct thermal release–electrothermal atomization atomic absorption spectrophotometric detection (ETA AAS). Anal. Chim. Acta 2021, 1177, 338795. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).