Synthesis of Nanocrystalline PuO2 by Hydrothermal and Thermal Decomposition of Pu(IV) Oxalate: A Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Physicochemical Characterization
2.2.1. X-ray Diffraction (XRD) Characterization and Evaluation of the Crystallite Size
2.2.2. Microscopic Characterization
2.2.3. Carbon Analysis
3. Results and Discussion
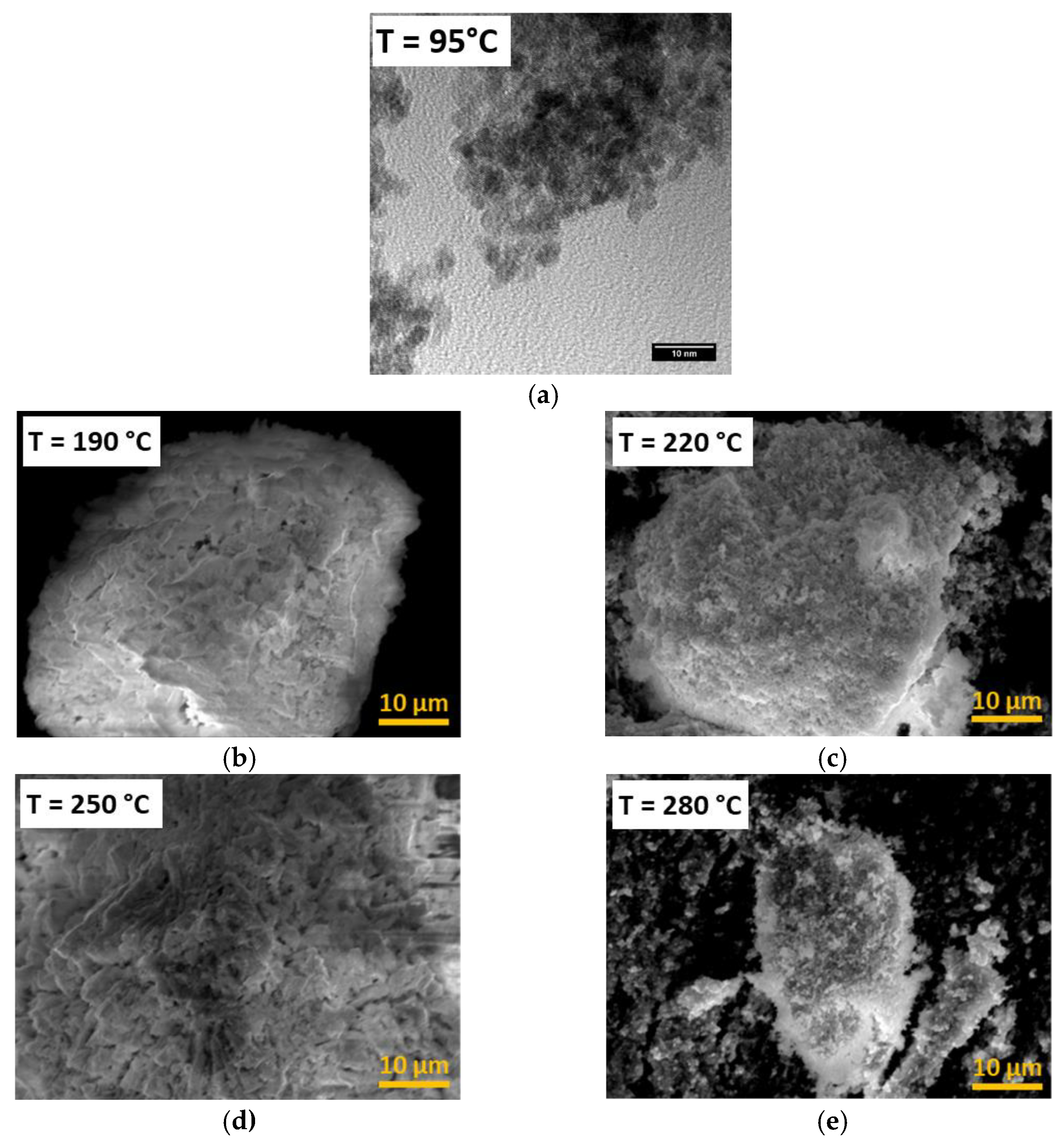
3.1. Effect of Temperature
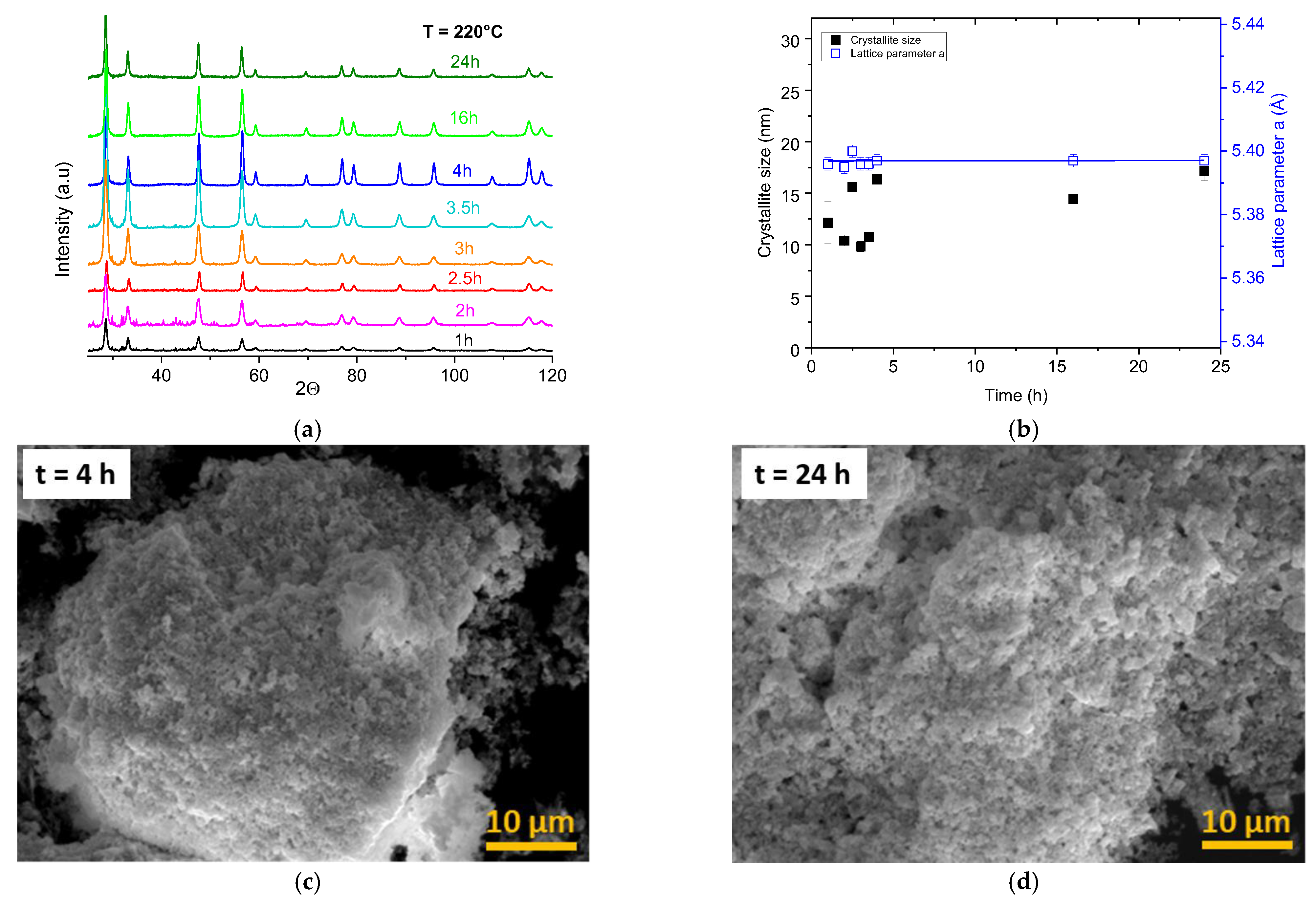
3.2. Effects of Duration
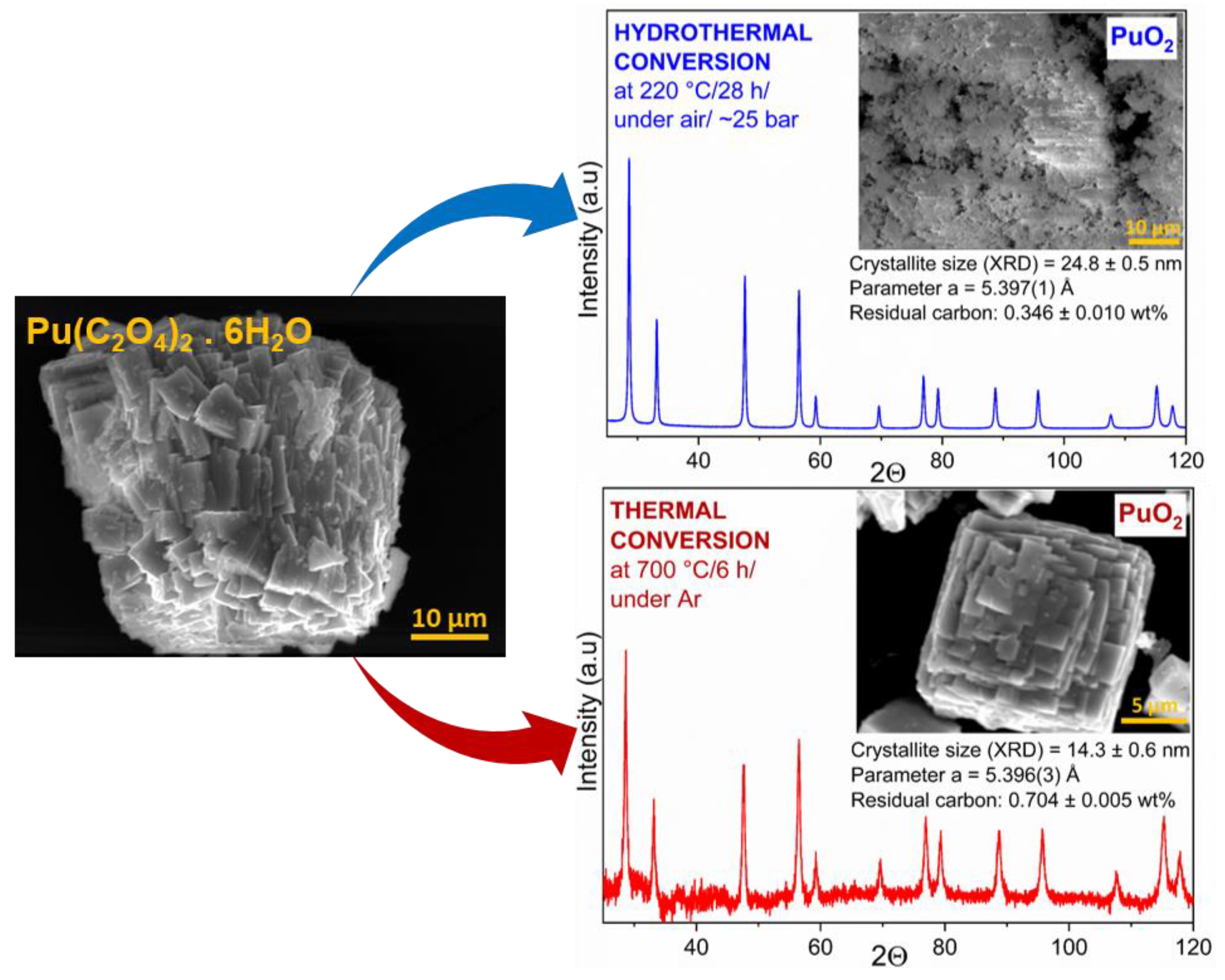
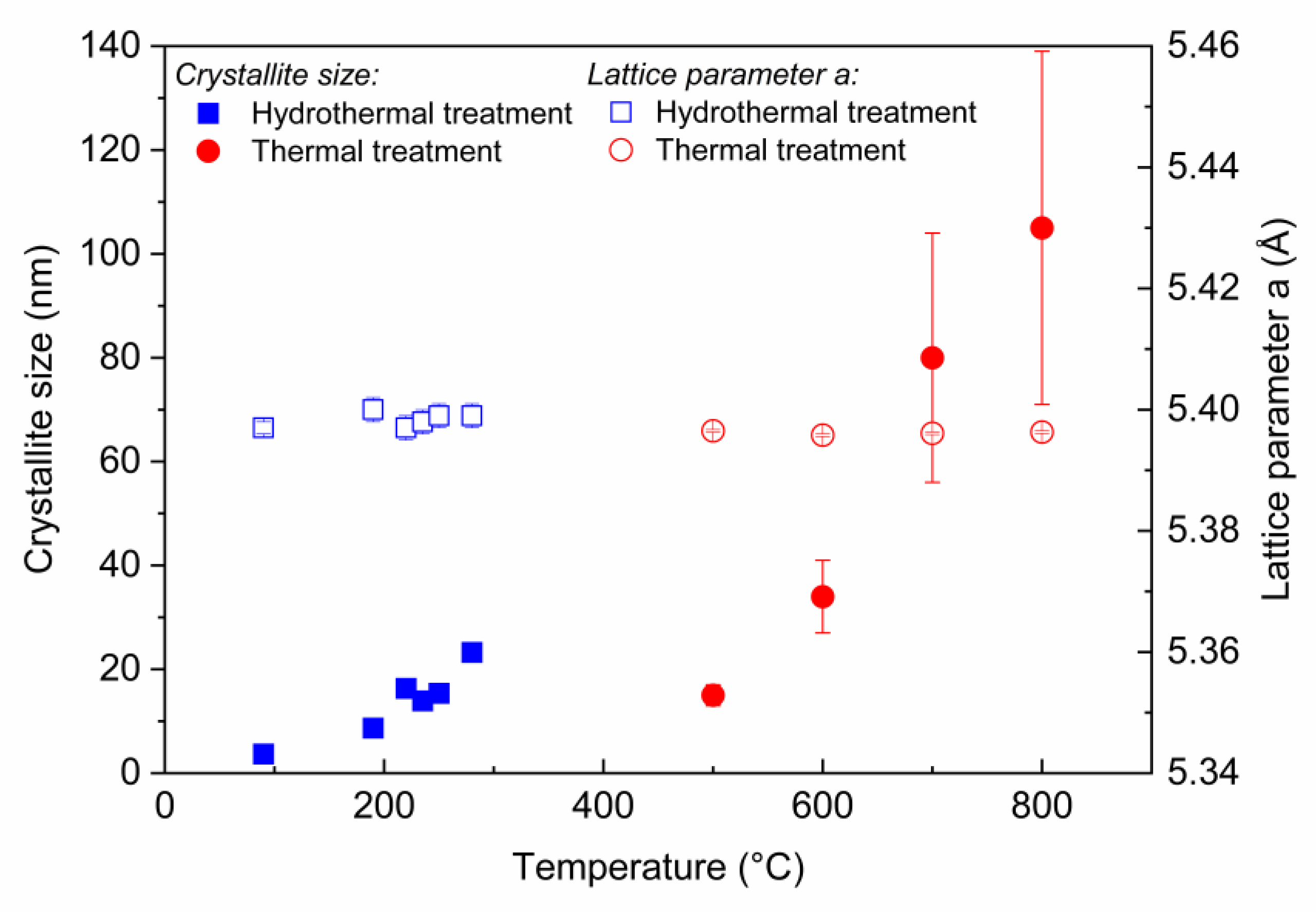
3.3. Hydrothermal vs. Thermal Decomposition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khanal, L.R.; Sundararajan, J.A.; Qiang, Y. Advanced nanomaterials for nuclear energy and nanotechnology. Energy Technol. 2020, 8, 1901070. [Google Scholar] [CrossRef]
- Roy, I.; Krishnan, S.; Kabashin, A.V.; Zavestovskaya, I.N.; Prasad, P.N. Transforming nuclear medicine with nanoradiopharmaceuticals. ACS Nano 2022, 16, 5036–5061. [Google Scholar] [CrossRef] [PubMed]
- Tyrpekl, V.; Vigier, J.-F.; Manara, D.; Wiss, T.; Dieste Blanco, O.; Somers, J. Low-temperature decomposition of U(IV) and Th(IV) oxalates to nanograined oxide powders. J. Nucl. Mater. 2015, 460, 200–208. [Google Scholar] [CrossRef]
- Hudry, D.; Apostolidis, C.; Walter, O.; Gouder, T.; Courtois, E.; Kübel, C.; Meyer, D. Non-aqueous synthesis of isotropic and anisotropic actinide oxide nanocrystals. Chem. Eur. J. 2012, 18, 8283–8287. [Google Scholar] [CrossRef] [PubMed]
- Hudry, D.; Apostolidis, C.; Walter, O.; Gouder, T.; Janssen, A.; Courtois, E.; Kübel, C.; Meyer, D. Synthesis of transuranium-based nanocrystals via the thermal decomposition of actinyl nitrates. RSC Adv. 2013, 3, 18271–18274. [Google Scholar] [CrossRef]
- Hudry, D.; Apostolidis, C.; Walter, O.; Gouder, T.; Courtois, E.; Kübel, C.; Meyer, D. Controlled synthesis of thorium and uranium oxide nanocrystals. Chem. Eur. J. 2013, 19, 5297–5305. [Google Scholar] [CrossRef] [PubMed]
- Hudry, D.; Apostolidis, C.; Walter, O.; Janssen, A.; Manara, D.; Griveau, J.-C.; Colineau, E.; Vitova, T.; Prüssmann, T.; Wang, D.; et al. Ultra-small plutonium oxide nanocrystals: An innovative material in plutonium science. Chem. Eur. J. 2014, 20, 10431–10438. [Google Scholar] [CrossRef] [PubMed]
- Hudry, D.; Griveau, J.-C.; Apostolidis, C.; Walter, O.; Colineau, E.; Rasmusen, G.; Wang, D.; Chakravadhaluna, V.S.K.; Courtois, E.; Kübel, C.; et al. Thorium/uranium mixed oxide nanocrystals: Synthesis, structural characterization and magnetic properties. Nano Res. 2014, 7, 119–131. [Google Scholar] [CrossRef]
- Nenoff, T.M.; Jacobs, B.W.; Robinson, D.B.; Provencio, P.P.; Huang, J.; Ferreira, S.; Hanson, D.J. Synthesis and low temperature in situ sintering of uranium oxide nanoparticles. Chem. Mater. 2011, 23, 5185–5190. [Google Scholar] [CrossRef]
- Bouala, G.I.N.; Clavier, N.; Podor, R.; Cambedouzou, J.; Mesbah, A.; Brau, H.P.; Léchelle, J.; Dacheux, N. Preparation and characterisation of uranium oxides with spherical shapes and hierarchical structures. CrystEngComm 2014, 16, 6944–6954. [Google Scholar] [CrossRef]
- Trillaud, V.; Maynadié, J.; Manaud, J.; Dacheux, N.; Clavier, N. Synthesis of size-controlled UO2 microspheres from the hydrothermal conversion of U(IV) aspartate. CrystEngComm 2018, 20, 7749–7760. [Google Scholar] [CrossRef]
- Tabata, C.; Shirasaki, K.; Sunaga, A.; Sakai, H.; Li, D.; Konaka, M.; Yamamura, T. Supercritical hydrothermal synthesis of UO2+x: Stoichiometry, crystal shape and size, and homogeneity observed using 23Na-NMR spectroscopy of (U,Na)O2+x. CrystEngComm 2021, 23, 8660–8672. [Google Scholar] [CrossRef]
- Tabata, C.; Shirasaki, K.; Sakai, H.; Sunaga, A.; Li, D.; Konaka, M.; Yamamura, T. Influence of additives on low-temperature hydrothermal synthesis of UO2+x and ThO2. CrystEngComm 2022, 24, 3637–3648. [Google Scholar] [CrossRef]
- Shirasaki, K.; Tabata, C.; Sunaga, A.; Sakai, H.; Li, D.; Konaka, M.; Yamamura, T. Homogeneity of (U,M)O2 (M = Th, Np) prepared by supercritical hydrothermal synthesis. J. Nucl. Mater. 2022, 563, 153608. [Google Scholar] [CrossRef]
- Walter, O.; Popa, K.; Dieste Blanco, O. Hydrothermal decomposition of actinide(IV) oxalates: A new aqueous route towards reactive actinide oxide nanocrystals. Open Chem. 2016, 14, 170–174. [Google Scholar] [CrossRef]
- Popa, K.; Walter, O.; Dieste Blanco, O.; Guiot, A.; Bouëxière, D.; Colle, J.-Y.; Martel, L.; Naji, M.; Manara, D. A low-temperature synthesis method for AnO2 nanocrystals (An = Th, U, Np, and Pu) and associate solid solutions. CrystEngComm 2018, 20, 4614–4622. [Google Scholar] [CrossRef]
- Vigier, J.-F.; Freis, D.; Walter, O.; Dieste Blanco, O.; Bouëxière, D.; Zuleger, E.; Palina, N.; Vitova, T.; Konings, R.J.M.; Popa, K. Synthesis and characterization of homogeneous (U,Am)O2 and (U,Pu,Am)O2 nanopowders. CrystEngComm 2022, 24, 6338–6348. [Google Scholar] [CrossRef]
- Balice, L.; Bouëxière, D.; Cologna, M.; Cambriani, A.; Vigier, J.-F.; De Bona, E.; Sorarù, G.D.; Kübel, C.; Walter, O.; Popa, K. Nano and micro U1-xThxO2 solid solutions: From powders to pellets. J. Nucl. Mater. 2018, 498, 307–313. [Google Scholar] [CrossRef]
- De Bona, E.; Balice, L.; Cognini, L.; Holzhäuser, M.; Popa, K.; Walter, O.; Cologna, M.; Prieur, D.; Wiss, T.; Baldinozzi, G. Single-step, high pressure, and two-step spark plasma sintering of UO2 nanopowders. J. Eur. Ceram. Soc. 2021, 41, 3655–3663. [Google Scholar] [CrossRef]
- Manaud, J.; Maynadie, J.; Mesbah, A.; Hunault, M.O.J.Y.; Martin, P.M.; Zunino, M.; Dacheux, N.; Clavier, N. Hydrothermal conversion of uranium(IV) oxalate into oxides: A comprehensive study. Inorg. Chem. 2020, 59, 3260–3273. [Google Scholar] [CrossRef]
- Manaud, J.; Maynadie, J.; Mesbah, A.; Hunault, M.O.J.Y.; Martin, P.M.; Zunino, M.; Dacheux, N.; Clavier, N. Hydrothermal conversion of thorium oxalate into ThO2·nH2O oxide. Inorg. Chem. 2020, 59, 14954–14966. [Google Scholar] [CrossRef]
- Ramaniah, M.V. Analytical chemistry of fast reactor fuels—A review. Pure Appl. Chem. 1982, 54, 889–908. [Google Scholar] [CrossRef]
- Orr, R.M.; Sims, H.E.; Taylor, R.J. A review of plutonium oxalate decomposition reactions and effects of decomposition temperature on the surface area of the plutonium dioxide product. J. Nucl. Mater. 2015, 465, 756–773. [Google Scholar] [CrossRef]
- Corbey, J.F.; Sweet, L.E.; Sinkov, S.I.; Reilly, D.D.; Parker, C.M.; Lonergan, J.M.; Johnson, T.J. Quantitative microstructural characterization of plutonium oxalate auto-degradation and evidence for PuO2 nanocrystal formation. Eur. J. Inorg. Chem. 2021, 32, 3277–3291. [Google Scholar] [CrossRef]
- Vigier, N.; Grandjean, S.; Arab-Chapelet, B.; Abraham, F. Reaction mechanisms of the thermal conversion of Pu(IV) oxalate into plutonium oxide. J. Alloys Compd. 2007, 444–445, 594–597. [Google Scholar] [CrossRef]
- De Almeida, L.; Grandjean, S.; Vigier, N.; Patisson, F. Insights into the thermal decomposition of lanthanide(III) and actinide(III) oxalates—From neodymium and cerium to plutonium. Eur. J. Inorg. Chem. 2012, 4986–4999. [Google Scholar] [CrossRef]
- South, C.J.; Roy, L.E. Insights into the thermal decomposition of plutonium(IV) oxalate—DFT study of the intermediate structures. J. Nucl. Mater. 2021, 549, 152864. [Google Scholar] [CrossRef]
- Abraham, F.; Arab-Chapelet, B.; Rivenet, M.; Tamain, C.; Grandjean, S. Actinide oxalates, solid state structures and applications. Coord. Chem. Rev. 2014, 266–267, 28–68. [Google Scholar] [CrossRef]
- Nissen, D.A. The thermal decomposition of plutonium (IV) oxalate hexahydrate. J. Thermal. Anal. 1980, 18, 99–109. [Google Scholar] [CrossRef]
- Bouëxière, D.; Popa, K.; Walter, O.; Cologna, M. Kinetic study on the grain growth of PuO2 nanocrystals. RSC Adv. 2019, 9, 6542–6547. [Google Scholar] [CrossRef]
- Belin, R.C.; Valenza, P.J.; Reynaud, M.A.; Raison, P.E. New hermetic sample holder for radioactive materials fitting to Siemens D5000 and Bruker D8 X-ray diffractometers: Application to the Rietveld analysis of plutonium dioxide. J. App. Cryst. 2004, 37, 1034–1037. [Google Scholar] [CrossRef]
- Kvashnina, K.O.; Romanchuk, A.Y.; Pidchenko, I.; Amidani, L.; Gerber, E.; Trigub, A.; Rossberg, A.; Weiss, S.; Popa, K.; Walter, O.; et al. Novel metastable pentavalent plutonium solid phase on the pathway from aqueous plutonium(VI) to PuO2 nanoparticles. Angew. Chem. Int. Ed. 2019, 58, 17558–17562. [Google Scholar] [CrossRef] [PubMed]
- Virot, M.; Dumas, T.; Cot-Auriol, M.; Moisy, P.; Nikitenko, S.I. Synthesis and multi-scale properties of PuO2 nanoparticles: Recent advances and open questions. Nanoscale Adv. 2022, 4, 4938–4971. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumann, V.; Popa, K.; Walter, O.; Rivenet, M.; Senentz, G.; Morel, B.; Konings, R.J.M. Synthesis of Nanocrystalline PuO2 by Hydrothermal and Thermal Decomposition of Pu(IV) Oxalate: A Comparative Study. Nanomaterials 2023, 13, 340. https://doi.org/10.3390/nano13020340
Baumann V, Popa K, Walter O, Rivenet M, Senentz G, Morel B, Konings RJM. Synthesis of Nanocrystalline PuO2 by Hydrothermal and Thermal Decomposition of Pu(IV) Oxalate: A Comparative Study. Nanomaterials. 2023; 13(2):340. https://doi.org/10.3390/nano13020340
Chicago/Turabian StyleBaumann, Viktoria, Karin Popa, Olaf Walter, Murielle Rivenet, Gérald Senentz, Bertrand Morel, and Rudy J.M. Konings. 2023. "Synthesis of Nanocrystalline PuO2 by Hydrothermal and Thermal Decomposition of Pu(IV) Oxalate: A Comparative Study" Nanomaterials 13, no. 2: 340. https://doi.org/10.3390/nano13020340
APA StyleBaumann, V., Popa, K., Walter, O., Rivenet, M., Senentz, G., Morel, B., & Konings, R. J. M. (2023). Synthesis of Nanocrystalline PuO2 by Hydrothermal and Thermal Decomposition of Pu(IV) Oxalate: A Comparative Study. Nanomaterials, 13(2), 340. https://doi.org/10.3390/nano13020340





