Asymmetric Coordination Environment Engineering of Atomic Catalysts for CO2 Reduction
Abstract
1. Introduction
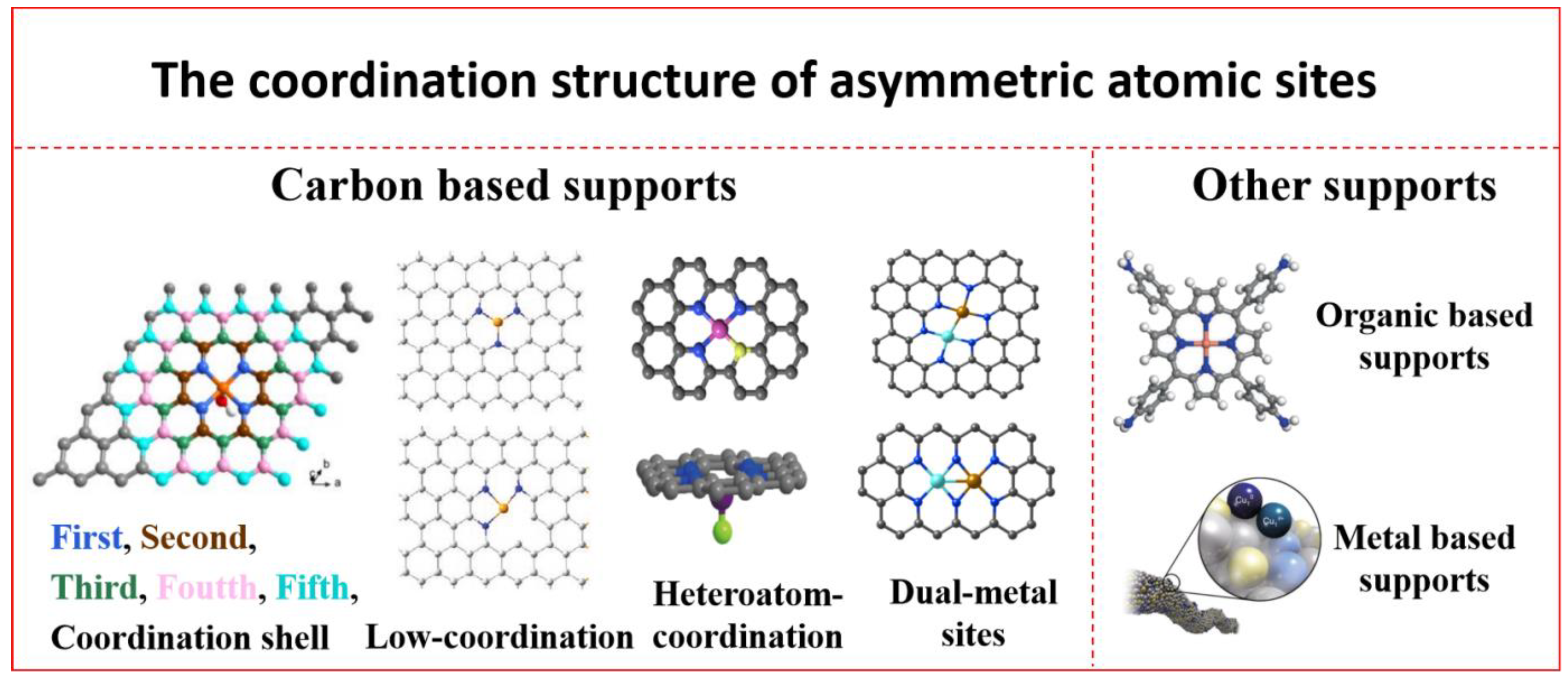
2. Advantages of Asymmetrically Coordinated SACs

3. The Synthesis Strategies for Breaking MN4
4. The Characterization of Asymmetric Atom Sites

5. Asymmetric Atom Sites for CO2RR
5.1. Low-Coordination Structure

5.2. Lateral Heteroatom Coordination Structure

5.3. Axial Heteroatom Coordination Structure
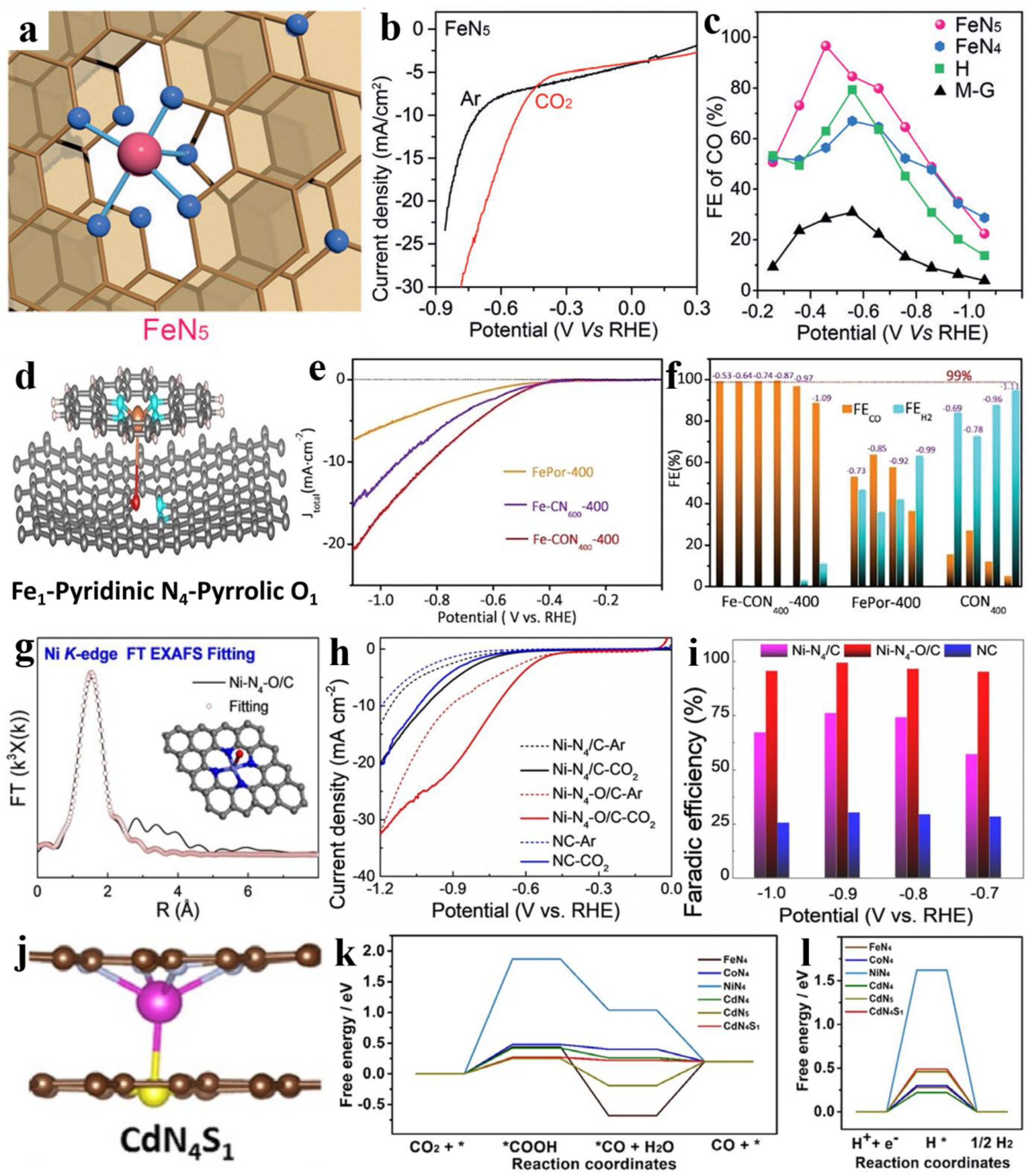
5.4. Dual-Metal Coordination Structure

5.5. Asymmetric Atom Sites of Organic/Metal-Based Supports for CO2RR
6. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.L.; Mu, J.E.; McCarl, B.A.; Yu, J.L. The impact of climate change on global energy use. Mitig. Adapt. Strateg. Glob. Change 2022, 27, 9. [Google Scholar] [CrossRef]
- Wang, L.G.; Duan, X.X.; Liu, X.J.; Gu, J.; Si, R.; Qiu, Y.; Qiu, Y.M.; Shi, D.E.; Chen, F.H.; Sun, X.M.; et al. Atomically Dispersed Mo Supported on Metallic Co9S8 Nanoflakes as an Advanced Noble-Metal-Free Bifunctional Water Splitting Catalyst Working in Universal pH Conditions. Adv. Energy Mater. 2020, 10, 1903137. [Google Scholar] [CrossRef]
- Liang, C.; Shuaibo, Z.; Waqar, A.; Jing, Z.; Yue, Z.; Wensheng, Y.; Yongfang, L. High-performance large-area perovskite photovoltaic modules. Nano Res. Energy 2022, 1, e9120024. [Google Scholar]
- Khan, S.A.R.; Ponce, P.; Yu, Z.; Ponce, K. Investigating economic growth and natural resource dependence: An asymmetric approach in developed and developing economies. Resour. Policy 2022, 77, 102672. [Google Scholar] [CrossRef]
- Sen, W.; Jiaxin, M.; Xiaoyu, S.; Yuanyuan, Z.; Zhong-Shuai, W. Recent status and future perspectives of ultracompact and customizable micro-supercapacitors. Nano Res. Energy 2022, 1, e9120018. [Google Scholar]
- Winter, R.A. Innovation and the dynamics of global warming. J. Environ. Econ. Manag. 2014, 68, 124–140. [Google Scholar] [CrossRef]
- Guojin, L.; Xinliang, L.; Yanbo, W.; Shuo, Y.; Zhaodong, H.; Qi, Y.; Donghong, W.; Binbin, D.; Minshen, Z.; Chunyi, Z.; et al. Building durable aqueous K-ion capacitors based on MXene family. Nano Res. Energy 2022, 1, e9120002. [Google Scholar]
- Kirikkaleli, D.; Sowah, J.K. Time-frequency dependency of temperature and sea level: A global perspective. Environ. Sci. Pollut. Res. 2021, 28, 58787–58798. [Google Scholar] [CrossRef]
- Adebayo, T.S.; Onifade, S.T.; Alola, A.A.; Muoneke, O.B. Does it take international integration of natural resources to ascend the ladder of environmental quality in the newly industrialized countries? Resour. Policy 2022, 76, 102616. [Google Scholar] [CrossRef]
- Jie, Z.; Leyu, B.; Yuanhang, C.; Baomin, X.; Alex, K.Y.J. Self-assembled monolayer enabling improved buried interfaces in blade-coated perovskite solar cells for high efficiency and stability. Nano Energy 2022, 1, e9120004. [Google Scholar]
- Hou, J.; Peng, X.; Sun, J.; Zhang, S.; Liu, Q.; Wang, X.; Luo, J.; Liu, X. Accelerating hydrazine-assisted hydrogen production kinetics with Mn dopant modulated CoS2 nanowire arrays. Inorg. Chem. Front. 2022, 9, 3047–3058. [Google Scholar] [CrossRef]
- Strmcnik, D.; Lopes, P.P.; Genorio, B.; Stamenkovic, V.R.; Markovic, N.M. Design principles for hydrogen evolution reaction catalyst materials. Nano Energy 2016, 29, 29–36. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.; Liu, Q.; Yu, P.; Luo, J.; Hu, G.; Liu, X. CoSe2 nanocrystals embedded into carbon framework as efficient bifunctional catalyst for alkaline seawater splitting. Inorg. Chem. Commun. 2022, 146, 110170. [Google Scholar] [CrossRef]
- Peng, L.S.; Yang, N.; Yang, Y.Q.; Wang, Q.; Xie, X.Y.; Sun-Waterhouse, D.; Shang, L.; Zhang, T.R.; Waterhouse, G.I.N. Atomic Cation-Vacancy Engineering of NiFe-Layered Double Hydroxides for Improved Activity and Stability towards the Oxygen Evolution Reaction. Angew. Chem. 2021, 60, 24612–24619. [Google Scholar] [CrossRef]
- Wang, H.J.; Wang, W.X.; Yu, H.J.; Mao, Q.Q.; Xu, Y.; Li, X.N.; Wang, Z.Q.; Wang, L. Interface engineering of polyaniline-functionalized porous Pd metallene for alkaline oxygen reduction reaction. Appl. Catal. B Environ. 2022, 307, 121172. [Google Scholar] [CrossRef]
- Qi, G.C.; Zhao, Q.R.; Liu, Q.J.; Fang, D.Y.; Liu, X.J. Biomass-derived carbon frameworks for oxygen and carbon dioxide electrochemical reduction. Ionics 2021, 27, 3579–3586. [Google Scholar] [CrossRef]
- Lulu, L.; ul Hasan, I.M.; Farwa; Ruinan, H.; Luwei, P.; Nengneng, X.; Nabeel Khan, N.; Jia-Nan, Z.; Jinli, Q. Copper as a single metal atom based photo-, electro-, and photoelectrochemical catalyst decorated on carbon nitride surface for efficient CO2 reduction: A review. Nano Res. Energy 2022, 1, e9120015. [Google Scholar]
- Gao, S.; Wang, T.; Jin, M.; Zhang, S.; Liu, Q.; Hu, G.; Yang, H.; Luo, J.; Liu, X. Bifunctional Nb-N-C atomic catalyst for aqueous Zn-air battery driving CO2 electrolysis. Sci. China Mater. 2022, 65, 1–11. [Google Scholar] [CrossRef]
- Ge, M.; Mengmeng, J.; Tianran, W.; Qian, L.; Shusheng, Z.; Xianyun, P.; Jun, L.; Xijun, L. MoC nanocrystals confined in N-doped carbon nanosheets toward highly selective electrocatalytic nitric oxide reduction to ammonia. Nano Res. 2022, 15, 8890–8896. [Google Scholar]
- Qian, X.; Ma, C.Q.; Shahid, U.B.; Sun, M.J.; Zhang, X.L.; Tian, J.; Shao, M.H. Synergistic Enhancement of Electrocatalytic Nitrogen Reductionover Few-Layer MoSe2-Decorated Ti3C2Tx MXene. ACS Catal. 2022, 12, 6385–6393. [Google Scholar] [CrossRef]
- Defeng, Q.; Fang, L.; Tianran, W.; Mengmeng, J.; Ge, M.; Shusheng, Z.; Qian, L.; Wenxian, L.; Dui, M.; Mohamed, S.; et al. High-efficiency electrocatalytic NO reduction to NH3 by nanoporous VN. Nano Res. Energy 2022, 1, e9120022. [Google Scholar]
- Geng, J.; Ji, S.; Jin, M.; Zhang, C.; Xu, M.; Wang, G.; Liang, C.; Zhang, H. Ambient Electrosynthesis of Urea with Nitrate and Carbon Dioxide over Iron-Based Dual-Sites. Angew. Chem. 2023, e202210958. [Google Scholar] [CrossRef]
- Jiawei, G.; Yi, P.; Ting, Z.; Jiao, M.; Huan, P.; Yusuke, Y. Porphyrin-based framework materials for energy conversion. Nano Res. Energy 2022, 1, e9120009. [Google Scholar]
- Ding, J.; Yang, H.; Zhang, S.; Liu, Q.; Cao, H.; Luo, J.; Liu, X. Advances in the Electrocatalytic Hydrogen Evolution Reaction by Metal Nanoclusters-based Materials. Small 2022, 18, 2204524. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, D.; Li, Y. Single-atom catalysis for carbon neutrality. Carbon Energy 2022, 4, 1021–1079. [Google Scholar] [CrossRef]
- Yu, X.; Deng, J.; Liu, Y.; Jing, L.; Hou, Z.; Pei, W.; Dai, H. Single-Atom Catalysts: Preparation and Applications in Environmental Catalysis. Catalysts 2022, 12, 1239. [Google Scholar] [CrossRef]
- Guo, X.; Wan, X.; Shui, J. Molybdenum-based materials for electrocatalytic nitrogen reduction reaction. Cell Rep. Phys. Sci. 2021, 2, 100447. [Google Scholar] [CrossRef]
- Hairong, X.; Hao, G.; Yusuke, Y.; Takayoshi, S.; Renzhi, M. Photo-enhanced rechargeable high-energy-density metal batteries for solar energy conversion and storage. Nano Res. Energy 2022, 1, e9120007. [Google Scholar]
- Yang, M.S.; Liu, S.; Sun, J.Q.; Jin, M.M.; Fu, R.; Zhang, S.S.; Li, H.Y.; Sun, Z.Y.; Luo, J.; Liu, X.J.; et al. Highly dispersed Bi clusters for efficient rechargeable Zn-CO2 batteries. Appl. Catal. B Environ. 2022, 307, 121145. [Google Scholar] [CrossRef]
- Han, L.L.; Song, S.J.; Liu, M.J.; Yao, S.Y.; Liang, Z.X.; Cheng, H.; Ren, Z.H.; Liu, W.; Lin, R.Q.; Qi, G.C.; et al. Stable and Efficient Single-Atom Zn Catalyst for CO2 Reduction to CH4. J. Am. Chem. Soc. 2020, 142, 12563–12567. [Google Scholar] [CrossRef]
- Sun, T.; Mitchell, S.; Li, J.; Lyu, P.; Wu, X.; Perez-Ramirez, J.; Lu, J. Design of Local Atomic Environments in Single-Atom Electrocatalysts for Renewable Energy Conversions. Adv. Mater. 2021, 33, 2003075. [Google Scholar] [CrossRef]
- Shen, T.; Wang, S.; Zhao, T.; Hu, Y.; Wang, D. Recent Advances of Single-Atom-Alloy for Energy Electrocatalysis. Adv. Energy Mater. 2022, 12, 2201823. [Google Scholar] [CrossRef]
- Qiao, B.T.; Wang, A.Q.; Yang, X.F.; Allard, L.F.; Jiang, Z.; Cui, Y.T.; Liu, J.Y.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef]
- Liu, H.; Peng, X.; Liu, X. Single-Atom Catalysts for the Hydrogen Evolution Reaction. ChemElectroChem 2018, 5, 2963–2974. [Google Scholar] [CrossRef]
- Li, R.Z.; Wang, D.S. Understanding the structure-performance relationship of active sites at atomic scale. Nano Res. 2022, 15, 6888–6923. [Google Scholar] [CrossRef]
- Liang, Z.W.; Shen, J.D.; Xu, X.J.; Li, F.K.; Liu, J.; Yuan, B.; Yu, Y.; Zhu, M. Advances in the Development of Single-Atom Catalysts for High-Energy-Density Lithium-Sulfur Batteries. Adv. Mater. 2022, 34, 2200102. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S.; Luo, Y.; Liu, Q.; Luo, J.; Chu, P.; Liu, X. Preparation of high entropy alloys and application to catalytical water electrolysis. APL Mater. 2022, 10, 070701. [Google Scholar] [CrossRef]
- Zheng, X.B.; Li, B.B.; Wang, Q.S.; Wang, D.S.; Li, Y.D. Emerging low-nuclearity supported metal catalysts with atomic level precision for efficient heterogeneous catalysis. Nano Res. 2022, 15, 7806–7839. [Google Scholar] [CrossRef]
- Wu, D.H.; He, B.L.; Wang, Y.Y.; Lv, P.; Ma, D.W.; Jia, Y. Double-atom catalysts for energy-related electrocatalysis applications: A theoretical perspective. J. Phys. D Appl. Phys. 2022, 55, 203001. [Google Scholar] [CrossRef]
- Mi, Y.Y.; Qiu, Y.; Liu, Y.F.; Peng, X.N.; Hu, M.; Zhao, S.Z.; Cao, H.Q.; Zhuo, L.C.; Li, H.Y.; Ren, J.Q.; et al. Cobalt-Iron Oxide Nanosheets for High-Efficiency Solar-Driven CO2-H2O Coupling Electrocatalytic Reactions. Adv. Funct. Mater. 2020, 30, 2003438. [Google Scholar] [CrossRef]
- Liu, H.; Fu, J.; Li, H.; Sun, J.; Liu, X.; Qiu, Y.; Peng, X.; Liu, Y.; Bao, H.; Zhuo, L.; et al. Single palladium site in ordered porous heteroatom-doped carbon for high-performance alkaline hydrogen oxidation. Appl. Catal. B Environ. 2022, 306, 121029. [Google Scholar] [CrossRef]
- Zhu, P.; Xiong, X.; Wang, D.S. Regulations of active moiety in single atom catalysts for electrochemical hydrogen evolution reaction. Nano Res. 2022, 15, 5792–5815. [Google Scholar] [CrossRef]
- Li, J.H.; Li, Q.L.; Zhao, L.; Zhang, J.H.; Tang, X.; Gu, L.X.; Guo, Q.; Ma, H.X.; Zhou, Q.; Liu, Y.; et al. Rapid screening of Zr-containing particles from Chang’e-5 lunar soil samples for isotope geochronology: Technical roadmap for future study. Geosci. Front. 2022, 13, 101367. [Google Scholar] [CrossRef]
- Meng, G.; Cao, H.; Wei, T.; Liu, Q.; Fu, J.; Zhang, S.; Luo, J.; Liu, X. Highly dispersed Ru clusters toward an efficient and durable hydrogen oxidation reaction. Chem. Commun. 2022, 58, 11839–11842. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.W.; Dai, C.; Wang, X.; Hu, J.Y.; Zhang, J.Y.; Zheng, L.X.; Mao, L.; Zheng, H.J.; Zhu, M.S. Protruding Pt single-sites on hexagonal ZnIn2S4 to accelerate photocatalytic hydrogen evolution. Nat. Commun. 2022, 13, 1287. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.S.; Liu, Y.F.; Sun, J.Q.; Zhang, S.S.; Liu, X.J.; Luo, J. Integration of partially phosphatized bimetal centers into trifunctional catalyst for high-performance hydrogen production and flexible Zn-air battery. Sci. China Mater. 2022, 65, 1176–1186. [Google Scholar] [CrossRef]
- Peng, X.Y.; Zhao, S.Z.; Mi, Y.Y.; Han, L.L.; Liu, X.J.; Qi, D.F.; Sun, J.Q.; Liu, Y.F.; Bao, H.H.; Zhuo, L.C.; et al. Trifunctional Single-Atomic Ru Sites Enable Efficient Overall Water Splitting and Oxygen Reduction in Acidic Media. Small 2020, 16, 2002888. [Google Scholar] [CrossRef]
- Yan, H.; Zhao, M.Y.; Feng, X.; Zhao, S.M.; Zhou, X.; Li, S.F.; Zha, M.H.; Meng, F.Y.; Chen, X.B.; Liu, Y.B.; et al. PO43− Coordinated Robust Single-Atom Platinum Catalyst for Selective Polyol Oxidation. Angew. Chem. 2022, 134, e202116059. [Google Scholar] [CrossRef]
- Xia, T.; Wan, J.W.; Yu, R.B. Progress of the Structure-property Correlation of Heteroatomic Coordination Structured Carbon-based Single-atom Electrocatalysts. Chem. J. Chin. Univ. 2022, 43, 20220162. [Google Scholar]
- Gu, H.; Wu, J.; Zhang, L. Recent advances in the rational design of single-atom catalysts for electrochemical CO2 reduction. Nano Res. 2022, 15, 9747–9763. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, X.; Lee, J.M.; Wang, X. Tailoring of Active Sites from Single to Dual Atom Sites for Highly Efficient Electrocatalysis. ACS Nano 2022, 16, 17572. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Jia, B.; Wang, X.; Jiang, S.; Ma, T. Isolating Single and Few Atoms for Enhanced Catalysis. Adv. Mater. 2022, 34, e2201796. [Google Scholar] [CrossRef]
- Peng, L.; Shang, L.; Zhang, T.; Waterhouse, G.I.N. Recent Advances in the Development of Single-Atom Catalysts for Oxygen Electrocatalysis and Zinc–Air Batteries. Adv. Energy Mater. 2020, 10, 2003018. [Google Scholar] [CrossRef]
- Li, R.; Wang, D. Superiority of Dual-Atom Catalysts in Electrocatalysis: One Step Further Than Single-Atom Catalysts. Adv. Energy Mater. 2022, 12, 2103564. [Google Scholar] [CrossRef]
- Tang, T.; Wang, Z.; Guan, J. Optimizing the Electrocatalytic Selectivity of Carbon Dioxide Reduction Reaction by Regulating the Electronic Structure of Single-Atom M-N-C Materials. Adv. Funct. Mater. 2022, 32, 2111504. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Shi, J.; Tan, D.; Liu, L.; Zhang, F.; Lu, C.; Su, Z.; Tan, X.; Cheng, X.; et al. Manganese acting as a high-performance heterogeneous electrocatalyst in carbon dioxide reduction. Nat. Commun. 2019, 10, 2980. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, L.Z.; Li, S.; Huang, X.; Chang, J.N.; Wang, J.H.; Zhou, J.; Li, S.L.; Lan, Y.Q. Coordination environment dependent selectivity of single-site-Cu enriched crystalline porous catalysts in CO2 reduction to CH4. Nat. Commun. 2021, 12, 6390. [Google Scholar] [CrossRef]
- Varela, A.S.; Ranjbar Sahraie, N.; Steinberg, J.; Ju, W.; Oh, H.S.; Strasser, P. Metal-Doped Nitrogenated Carbon as an Efficient Catalyst for Direct CO2 Electroreduction to CO and Hydrocarbons. Angew. Chem. 2015, 54, 10758–10762. [Google Scholar] [CrossRef]
- Shen, J.; Kortlever, R.; Kas, R.; Birdja, Y.Y.; Diaz-Morales, O.; Kwon, Y.; Ledezma-Yanez, I.; Schouten, K.J.P.; Mul, G.; Koper, M.T.M.; et al. Electrocatalytic reduction of carbon dioxide to carbon monoxide and methane at an immobilized cobalt protoporphyrin. Nat. Commun. 2015, 6, 8177. [Google Scholar] [CrossRef]
- Bi, W.; Li, X.; You, R.; Chen, M.; Yuan, R.; Huang, W.; Wu, X.; Chu, W.; Wu, C.; Xie, Y. Surface Immobilization of Transition Metal Ions on Nitrogen-Doped Graphene Realizing High-Efficient and Selective CO2 Reduction. Adv. Mater. 2018, 30, 1706617. [Google Scholar] [CrossRef]
- Tripkovic, V.; Vanin, M.; Karamad, M.; Björketun, M.E.; Jacobsen, K.W.; Thygesen, K.S.; Rossmeisl, J. Electrochemical CO2 and CO Reduction on Metal-Functionalized Porphyrin-like Graphene. J. Phys. Chem. C 2013, 117, 9187–9195. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, S.; Wu, J.; Liu, M.; Yazdi, S.; Ren, M.; Sha, J.; Zhong, J.; Nie, K.; Jalilov, A.S.; et al. Electrochemical CO2 Reduction with Atomic Iron-Dispersed on Nitrogen-Doped Graphene. Adv. Energy Mater. 2018, 8, 1703487. [Google Scholar] [CrossRef]
- Huan, T.N.; Ranjbar, N.; Rousse, G.; Sougrati, M.; Zitolo, A.; Mougel, V.; Jaouen, F.; Fontecave, M. Electrochemical Reduction of CO2 Catalyzed by Fe-N-C Materials: A Structure–Selectivity Study. ACS Catal. 2017, 7, 1520–1525. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, W.; Zhang, L.H.; Chen, D.; Zhan, J.; Wang, X.; Shiju, N.R.; Yu, F. Control over Electrochemical CO2 Reduction Selectivity by Coordination Engineering of Tin Single-Atom Catalysts. Adv. Sci. 2021, 8, 2102884. [Google Scholar] [CrossRef] [PubMed]
- Yun, R.; Zhan, F.; Wang, X.; Zhang, B.; Sheng, T.; Xin, Z.; Mao, J.; Liu, S.; Zheng, B. Design of Binary Cu–Fe Sites Coordinated with Nitrogen Dispersed in the Porous Carbon for Synergistic CO2 Electroreduction. Small 2021, 17, 2006951. [Google Scholar] [CrossRef]
- Wang, T.; Sang, X.; Zheng, W.; Yang, B.; Yao, S.; Lei, C.; Li, Z.; He, Q.; Lu, J.; Lei, L.; et al. Gas Diffusion Strategy for Inserting Atomic Iron Sites into Graphitized Carbon Supports for Unusually High-Efficient CO2 Electroreduction and High-Performance Zn–CO2 Batteries. Adv. Mater. 2020, 32, 2002430. [Google Scholar] [CrossRef]
- Feng, M.; Wu, X.; Cheng, H.; Fan, Z.; Li, X.; Cui, F.; Fan, S.; Dai, Y.; Lei, G.; He, G. Well-defined Fe–Cu diatomic sites for efficient catalysis of CO2 electroreduction. J. Mater. Chem. A 2021, 9, 23817–23827. [Google Scholar] [CrossRef]
- Li, S.; Zhao, S.; Lu, X.; Ceccato, M.; Hu, X.M.; Roldan, A.; Catalano, J.; Liu, M.; Skrydstrup, T.; Daasbjerg, K.; et al. Low-Valence Znδ+ (0<δ<2) Single-Atom Material as Highly Efficient Electrocatalyst for CO2 Reduction. Angew. Chem. 2021, 60, 22826–22832. [Google Scholar]
- Liu, X.; Zheng, L.; Han, C.; Zong, H.; Yang, G.; Lin, S.; Kumar, A.; Jadhav, A.R.; Tran, N.Q.; Hwang, Y.; et al. Identifying the Activity Origin of a Cobalt Single-Atom Catalyst for Hydrogen Evolution Using Supervised Learning. Adv. Funct. Mater. 2021, 31, 2100547. [Google Scholar] [CrossRef]
- Qiu, H.J.; Ito, Y.; Cong, W.; Tan, Y.; Liu, P.; Hirata, A.; Fujita, T.; Tang, Z.; Chen, M. Nanoporous Graphene with Single-Atom Nickel Dopants: An Efficient and Stable Catalyst for Electrochemical Hydrogen Production. Angew. Chem. 2015, 54, 14031–14035. [Google Scholar] [CrossRef]
- Rong, X.; Wang, H.J.; Lu, X.L.; Si, R.; Lu, T.B. Controlled Synthesis of a Vacancy-Defect Single-Atom Catalyst for Boosting CO2 Electroreduction. Angew. Chem. 2020, 59, 1961–1965. [Google Scholar] [CrossRef]
- Jia, C.; Li, S.; Zhao, Y.; Hocking, R.K.; Ren, W.; Chen, X.; Su, Z.; Yang, W.; Wang, Y.; Zheng, S.; et al. Nitrogen Vacancy Induced Coordinative Reconstruction of Single-Atom Ni Catalyst for Efficient Electrochemical CO2 Reduction. Adv. Funct. Mater. 2021, 31, 2107072. [Google Scholar] [CrossRef]
- He, G.; Yan, M.; Gong, H.; Fei, H.; Wang, S. Ultrafast synthetic strategies under extreme heating conditions toward single-atom catalysts. Int. J. Extrem. Manuf. 2022, 4, 032003. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Zeng, Y.; Chen, J.; Qiu, L.; Zhou, H.; Sun, C.; Yu, Y.; Zhu, C.; Zhu, Z.; et al. Single Fe Atom on Hierarchically Porous S, N-Codoped Nanocarbon Derived from Porphyra Enable Boosted Oxygen Catalysis for Rechargeable Zn-Air Batteries. Small 2019, 15, 1900307. [Google Scholar] [CrossRef]
- Jing, H.; Zhao, Z.; Zhang, C.; Liu, W.; Wu, D.; Zhu, C.; Hao, C.; Zhang, J.; Shi, Y. Tuned single atom coordination structures mediated by polarization force and sulfur anions for photovoltaics. Nano Res. 2021, 14, 4025–4032. [Google Scholar] [CrossRef]
- Hu, B.; Huang, A.; Zhang, X.; Chen, Z.; Tu, R.; Zhu, W.; Zhuang, Z.; Chen, C.; Peng, Q.; Li, Y.; et al. Atomic Co/Ni dual sites with N/P-coordination as bifunctional oxygen electrocatalyst for rechargeable zinc-air batteries. Nano Res. 2021, 14, 3482–3488. [Google Scholar] [CrossRef]
- Yuan, K.; Lützenkirchen-Hecht, D.; Li, L.; Shuai, L.; Li, Y.; Cao, R.; Qiu, M.; Zhuang, X.; Leung, M.K.H.; Chen, Y.; et al. Boosting Oxygen Reduction of Single Iron Active Sites via Geometric and Electronic Engineering: Nitrogen and Phosphorus Dual Coordination. J. Am. Chem. Soc. 2020, 142, 2404–2412. [Google Scholar] [CrossRef]
- Li, Z.; Wu, R.; Xiao, S.; Yang, Y.; Lai, L.; Chen, J.S.; Chen, Y. Axial chlorine coordinated iron-nitrogen-carbon single-atom catalysts for efficient electrochemical CO2 reduction. Chem. Eng. J. 2022, 430, 132882. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y.; Xu, R.; Chen, W.; Zheng, L.; Han, A.; Zhu, Y.; Zhang, J.; Zhang, H.; Luo, J.; et al. Electronic structure engineering to boost oxygen reduction activity by controlling the coordination of the central metal. Energy Environ. Sci. 2018, 11, 2348–2352. [Google Scholar] [CrossRef]
- Singh, K.P.; Song, M.Y.; Yu, J.S. Iodine-treated heteroatom-doped carbon: Conductivity driven electrocatalytic activity. J. Mater. Chem. A 2014, 2, 18115–18124. [Google Scholar] [CrossRef]
- Wang, N.; Li, X.; Tu, Z.; Zhao, F.; He, J.; Guan, Z.; Huang, C.; Yi, Y.; Li, Y. Synthesis and Electronic Structure of Boron-Graphdiyne with an sp-Hybridized Carbon Skeleton and Its Application in Sodium Storage. Angew. Chem. Int. Ed. 2018, 57, 3968–3973. [Google Scholar] [CrossRef]
- Liu, S.; Jin, M.; Sun, J.; Qin, Y.; Gao, S.; Chen, Y.; Zhang, S.; Luo, J.; Liu, X. Coordination environment engineering to boost electrocatalytic CO2 reduction performance by introducing boron into single-Fe-atomic catalyst. Chem. Eng. J. 2022, 437, 135294. [Google Scholar] [CrossRef]
- Ni, W.; Gao, Y.; Lin, Y.; Ma, C.; Guo, X.; Wang, S.; Zhang, S. Nonnitrogen Coordination Environment Steering Electrochemical CO2-to-CO Conversion over Single-Atom Tin Catalysts in a Wide Potential Window. ACS Catal. 2021, 11, 5212–5221. [Google Scholar] [CrossRef]
- Hao, Q.; Zhong, H.X.; Wang, J.Z.; Liu, K.H.; Yan, J.M.; Ren, Z.H.; Zhou, N.; Zhao, X.; Zhang, H.; Liu, D.X.; et al. Nickel dual-atom sites for electrochemical carbon dioxide reduction. Nat. Synth. 2022, 1, 719–728. [Google Scholar] [CrossRef]
- Ding, T.; Liu, X.; Tao, Z.; Liu, T.; Chen, T.; Zhang, W.; Shen, X.; Liu, D.; Wang, S.; Pang, B.; et al. Atomically Precise Dinuclear Site Active toward Electrocatalytic CO2 Reduction. J. Am. Chem. Soc. 2021, 143, 11317–11324. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, X.; Kang, Y.; Ye, C.; Jin, R.; Yan, H.; Lin, R.; Yang, J.; Xu, Q.; Wang, Y.; et al. A Supported Pd2 Dual-Atom Site Catalyst for Efficient Electrochemical CO2 Reduction. Angew. Chem. Int. Ed. 2021, 60, 13388–13393. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, W.; Yuan, P.; Yang, G.; Mu, S.; Liang, J.; Xia, H.; Guo, K.; Liu, M.; Zhao, S.; et al. Boosting Nitrogen Reduction to Ammonia on FeN4 Sites by Atomic Spin Regulation. Adv. Sci. 2021, 8, 2102915. [Google Scholar] [CrossRef]
- Yang, G.G.; Zhu, J.W.; Yuan, P.F.; Hu, Y.F.; Qu, G.; Lu, B.A.; Xue, X.Y.; Yin, H.B.; Cheng, W.Z.; Cheng, J.Q.; et al. Regulating Fe-spin state by atomically dispersed Mn-N in Fe-N-C catalysts with high oxygen reduction activity. Nat. Commun. 2021, 12, 1734. [Google Scholar] [CrossRef]
- Zhang, L.; Si, R.; Liu, H.; Chen, N.; Wang, Q.; Adair, K.; Wang, Z.; Chen, J.; Song, Z.; Li, J.; et al. Atomic layer deposited Pt-Ru dual-metal dimers and identifying their active sites for hydrogen evolution reaction. Nat. Commun. 2019, 10, 4936. [Google Scholar] [CrossRef]
- Xu, W.; Tang, H.; Gu, H.; Xi, H.; Wu, P.; Liang, B.; Liu, Q.; Chen, W. Research progress of asymmetrically coordinated single-atom catalysts for electrocatalytic reactions. J. Mater. Chem. A 2022, 10, 14732–14746. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, Y.; Gao, G.; Yan, X.; Chen, N.; Chen, J.; Soo, M.T.; Wood, B.; Yang, D.; Du, A.; et al. Graphene Defects Trap Atomic Ni Species for Hydrogen and Oxygen Evolution Reactions. Chem 2018, 4, 285–297. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D.; Huang, K.; Dong, J.; Liao, J.; Dai, S.; Tang, X.; Yan, M.; Gong, H.; Liu, J.; et al. Iodine-Doping-Induced Electronic Structure Tuning of Atomic Cobalt for Enhanced Hydrogen Evolution Electrocatalysis. ACS Nano 2021, 15, 18125–18134. [Google Scholar] [CrossRef]
- Zhou, Y.; Song, E.; Chen, W.; Segre, C.U.; Zhou, J.; Lin, Y.-C.; Zhu, C.; Ma, R.; Liu, P.; Chu, S.; et al. Dual-Metal Interbonding as the Chemical Facilitator for Single-Atom Dispersions. Adv. Mater. 2020, 32, 2003484. [Google Scholar] [CrossRef]
- Cui, X.; Gao, L.; Lu, C.H.; Ma, R.; Yang, Y.; Lin, Z. Rational coordination regulation in carbon-based single-metal-atom catalysts for electrocatalytic oxygen reduction reaction. Nano Converg. 2022, 9, 34. [Google Scholar] [CrossRef]
- Sui, R.; Pei, J.; Fang, J.; Zhang, X.; Zhang, Y.; Wei, F.; Chen, W.; Hu, Z.; Hu, S.; Zhu, W.; et al. Engineering Ag–Nx Single-Atom Sites on Porous Concave N-Doped Carbon for Boosting CO2 Electroreduction. ACS Appl. Mater. Interfaces 2021, 13, 17736–17744. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Zhao, X.; Yao, T.; Chen, W.; You, R.; Zhao, C.; Wu, G.; Wang, J.; Huang, W.; et al. Regulation of Coordination Number over Single Co Sites: Triggering the Efficient Electroreduction of CO2. Angew. Chem. 2018, 57, 1944–1948. [Google Scholar] [CrossRef]
- Guo, Y.; Yao, S.; Xue, Y.; Hu, X.; Cui, H.; Zhou, Z. Nickel single-atom catalysts intrinsically promoted by fast pyrolysis for selective electroreduction of CO2 into CO. Appl. Catal. B Environ. 2022, 304, 120997. [Google Scholar] [CrossRef]
- Lin, L.; Li, H.; Wang, Y.; Li, H.; Wei, P.; Nan, B.; Si, R.; Wang, G.; Bao, X. Temperature-Dependent CO2 Electroreduction over Fe-N-C and Ni-N-C Single-Atom Catalysts. Angew. Chem. 2021, 60, 26582–26586. [Google Scholar] [CrossRef]
- Ma, W.C.; He, X.Y.; Wang, W.; Xie, S.J.; Zhang, Q.H.; Wang, Y. Electrocatalytic reduction of CO2 and CO to multi-carbon compounds over Cu-based catalysts. Chem. Soc. Rev. 2021, 50, 12897–12914. [Google Scholar] [CrossRef]
- Guan, A.; Chen, Z.; Quan, Y.; Peng, C.; Wang, Z.; Sham, T.-K.; Yang, C.; Ji, Y.; Qian, L.; Xu, X.; et al. Boosting CO2 Electroreduction to CH4 via Tuning Neighboring Single-Copper Sites. ACS Energy Lett. 2020, 5, 1044–1053. [Google Scholar] [CrossRef]
- Feng, J.; Gao, H.; Zheng, L.; Chen, Z.; Zeng, S.; Jiang, C.; Dong, H.; Liu, L.; Zhang, S.; Zhang, X.; et al. A Mn-N3 single-atom catalyst embedded in graphitic carbon nitride for efficient CO2 electroreduction. Nat. Commun. 2020, 11, 4341. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Dai, X.; Yao, T.; Chen, W.; Wang, X.; Wang, J.; Yang, J.; Wei, S.; Wu, Y.; Li, Y.; et al. Ionic Exchange of Metal–Organic Frameworks to Access Single Nickel Sites for Efficient Electroreduction of CO2. J. Am. Chem. Soc. 2017, 139, 8078–8081. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.N.; Jiao, L.; Qian, Y.; Pan, C.Y.; Zheng, L.; Cai, X.; Liu, B.; Yu, S.H.; Jiang, H.L. Regulating the Coordination Environment of MOF-Templated Single-Atom Nickel Electrocatalysts for Boosting CO2 Reduction. Angew. Chem. 2020, 59, 2705–2709. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cheng, J.; Yang, X.; Xu, Y.; Sun, W.; Liu, N.; Liu, J. Boosting Electrochemical CO2 Reduction by Controlling Coordination Environment in Atomically Dispersed Ni@NxCy Catalysts. ACS Sustain. Chem. Eng. 2021, 9, 6438–6445. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y. Unveiling the Active Structure of Single Nickel Atom Catalysis: Critical Roles of Charge Capacity and Hydrogen Bonding. J. Am. Chem. Soc. 2020, 142, 5773–5777. [Google Scholar] [CrossRef]
- Zu, X.; Li, X.; Liu, W.; Sun, Y.; Xu, J.; Yao, T.; Yan, W.; Gao, S.; Wang, C.; Wei, S.; et al. Efficient and Robust Carbon Dioxide Electroreduction Enabled by Atomically Dispersed Snδ+ Sites. Adv. Mater. 2019, 31, 1808135. [Google Scholar] [CrossRef]
- Hou, Y.; Qiu, M.; Kim, M.G.; Liu, P.; Nam, G.; Zhang, T.; Zhuang, X.; Yang, B.; Cho, J.; Chen, M.; et al. Atomically dispersed nickel–nitrogen–sulfur species anchored on porous carbon nanosheets for efficient water oxidation. Nat. Commun. 2019, 10, 1392. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Hu, Y.; Yang, S.; Yang, J.; Chen, W.; Zhou, H.; Zhou, F.; Wang, L.; Du, J.; et al. Simultaneous diffusion of cation and anion to access N, S co-coordinated Bi-sites for enhanced CO2 electroreduction. Nano Res. 2021, 14, 2790–2796. [Google Scholar] [CrossRef]
- Jia, C.; Tan, X.; Zhao, Y.; Ren, W.; Li, Y.; Su, Z.; Smith, S.C.; Zhao, C. Sulfur-Dopant-Promoted Electroreduction of CO2 over Coordinatively Unsaturated Ni-N2 Moieties. Angew. Chem. Int. Ed. 2021, 60, 23342–23348. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, L.H.; Du, J.; Wang, H.; Guo, J.; Zhan, J.; Li, F.; Yu, F. A Tandem Strategy for Enhancing Electrochemical CO2 Reduction Activity of Single-Atom Cu-S1N3 Catalysts via Integration with Cu Nanoclusters. Angew. Chem. 2021, 60, 24022–24027. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Kao, C.-W.; Luo, T.; Chen, K.; Fu, J.; Ma, C.; Li, H.; Li, M.; Chan, T.S.; et al. Unveiling the Proton-Feeding Effect in Sulfur-Doped Fe-N-C Single-Atom Catalyst for Enhanced CO2 Electroreduction. Angew. Chem. 2022, 61, e202206233. [Google Scholar]
- Gu, X.; Jiao, Y.; Wei, B.; Xu, T.; Zhai, P.; Wei, Y.; Zuo, J.; Liu, W.; Chen, Q.; Yang, Z.; et al. Boron bridged NiN4B2Cx single-atom catalyst for superior electrochemical CO2 reduction. Mater. Today 2022, 54, 63–71. [Google Scholar] [CrossRef]
- Sun, X.; Tuo, Y.; Ye, C.; Chen, C.; Lu, Q.; Li, G.; Jiang, P.; Chen, S.; Zhu, P.; Ma, M.; et al. Phosphorus Induced Electron Localization of Single Iron Sites for Boosted CO2 Electroreduction Reaction. Angew. Chem. 2021, 60, 23614–23618. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Xi, S.; Du, Y.; Hai, X.; Wang, J.; Xu, H.; Wu, G.; Zhang, J.; Lu, J.; et al. A Graphene-Supported Single-Atom FeN5 Catalytic Site for Efficient Electrochemical CO2 Reduction. Angew. Chem. Int. Ed. 2019, 58, 14871–14876. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, A.; Yu, K.; Cui, T.; Zhuang, Z.; Liu, S.; Li, J.; Tu, R.; Sun, K.; Tan, X.; et al. Fe1N4–O1 site with axial Fe–O coordination for highly selective CO2 reduction over a wide potential range. Energy Environ. Sci. 2021, 14, 3430–3437. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Sang, X.; Zheng, W.; Zhang, S.; Shuai, L.; Yang, B.; Li, Z.; Chen, J.; Lei, L.; et al. Dynamic Activation of Adsorbed Intermediates via Axial Traction for the Promoted Electrochemical CO2 Reduction. Angew. Chem. 2021, 60, 4192–4198. [Google Scholar] [CrossRef]
- Huang, M.; Deng, B.; Zhao, X.; Zhang, Z.; Li, F.; Li, K.; Cui, Z.; Kong, L.; Lu, J.; Dong, F.; et al. Template-Sacrificing Synthesis of Well-Defined Asymmetrically Coordinated Single-Atom Catalysts for Highly Efficient CO2 Electrocatalytic Reduction. ACS Nano 2022, 16, 2110–2119. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, C.; Yan, X.; Sun, X.; Zhu, Q.; Li, P.; Li, Y.; Liu, S.; Ma, J.; Huang, Y.; et al. Boosting CO2 Electroreduction over a Cadmium Single-Atom Catalyst by Tuning of the Axial Coordination Structure. Angew. Chem. 2021, 133, 20971–20978. [Google Scholar] [CrossRef]
- Huang, J.R.; Qiu, X.F.; Zhao, Z.H.; Zhu, H.L.; Liu, Y.C.; Shi, W.; Liao, P.Q.; Chen, X.M. Single-Product Faradaic Efficiency for Electrocatalytic of CO2 to CO at Current Density Larger than 1.2 A cm−2 in Neutral Aqueous Solution by a Single-Atom Nanozyme. Angew. Chem. 2022, 61, e202210985. [Google Scholar]
- Xie, W.; Li, H.; Cui, G.; Li, J.; Song, Y.; Li, S.; Zhang, X.; Lee, J.Y.; Shao, M.; Wei, M. NiSn Atomic Pair on an Integrated Electrode for Synergistic Electrocatalytic CO2 Reduction. Angew. Chem. 2021, 133, 7458–7464. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, L.; Wulan, B.; Tan, D.; Chen, Q.; Ma, J.; Zhang, J. Atomic Bridging Structure of Nickel—Nitrogen—Carbon for Highly Efficient Electrocatalytic Reduction of CO2. Angew. Chem. 2022, 61, e202113918. [Google Scholar]
- Li, Y.; Shan, W.; Zachman, M.J.; Wang, M.; Hwang, S.; Tabassum, H.; Yang, J.; Yang, X.; Karakalos, S.; Feng, Z.; et al. Atomically Dispersed Dual-Metal Site Catalysts for Enhanced CO2 Reduction: Mechanistic Insight into Active Site Structures. Angew. Chem. Int. Ed. 2022, 61, e202205632. [Google Scholar]
- Zhu, J.; Xiao, M.; Ren, D.; Gao, R.; Liu, X.; Zhang, Z.; Luo, D.; Xing, W.; Su, D.; Yu, A.; et al. Quasi-Covalently Coupled Ni–Cu Atomic Pair for Synergistic Electroreduction of CO2. J. Am. Chem. Soc. 2022, 144, 9661–9671. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Zhuang, Z.; Hao, J.; Wang, C.; Lu, S.; Duan, F.; Xu, F.; Du, M.; Zhu, H. Interatomic Electronegativity Offset Dictates Selectivity When Catalyzing the CO2 Reduction Reaction. Adv. Energy Mater. 2022, 12, 2200579. [Google Scholar] [CrossRef]
- Zeng, Z.; Gan, L.Y.; Bin Yang, H.; Su, X.; Gao, J.; Liu, W.; Matsumoto, H.; Gong, J.; Zhang, J.; Cai, W.; et al. Orbital coupling of hetero-diatomic nickel-iron site for bifunctional electrocatalysis of CO2 reduction and oxygen evolution. Nat. Commun. 2021, 12, 4088. [Google Scholar] [CrossRef]
- Jiao, L.; Zhu, J.; Zhang, Y.; Yang, W.; Zhou, S.; Li, A.; Xie, C.; Zheng, X.; Zhou, W.; Yu, S.-H.; et al. Non-Bonding Interaction of Neighboring Fe and Ni Single-Atom Pairs on MOF-Derived N-Doped Carbon for Enhanced CO2 Electroreduction. J. Am. Chem. Soc. 2021, 143, 19417–19424. [Google Scholar] [CrossRef]
- Ren, W.; Tan, X.; Yang, W.; Jia, C.; Xu, S.; Wang, K.; Smith, S.C.; Zhao, C. Isolated Diatomic Ni-Fe Metal–Nitrogen Sites for Synergistic Electroreduction of CO2. Angew. Chem. 2019, 58, 6972–6976. [Google Scholar] [CrossRef]
- Cheng, H.; Wu, X.; Feng, M.; Li, X.; Lei, G.; Fan, Z.; Pan, D.; Cui, F.; He, G. Atomically Dispersed Ni/Cu Dual Sites for Boosting the CO2 Reduction Reaction. ACS Catal. 2021, 11, 12673–12681. [Google Scholar] [CrossRef]
- Yi, J.D.; Gao, X.; Zhou, H.; Chen, W.; Wu, Y. Design of Co-Cu Diatomic Site Catalysts for High-efficiency Synergistic CO2 Electroreduction at Industrial-level Current Density. Angew. Chem. 2022, 61, e202212329. [Google Scholar] [CrossRef]
- Li, Y.; Wei, B.; Zhu, M.; Chen, J.; Jiang, Q.; Yang, B.; Hou, Y.; Lei, L.; Li, Z.; Zhang, R.; et al. Synergistic Effect of Atomically Dispersed Ni–Zn Pair Sites for Enhanced CO2 Electroreduction. Adv. Mater. 2021, 33, 2102212. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, L.; Liu, S.; Li, A.; Yuan, X.; Hu, C.; Zhang, G.; Deng, W.; Zang, K.; Luo, J.; et al. Enhanced CO2 Electroreduction on Neighboring Zn/Co Monomers by Electronic Effect. Angew. Chem. 2020, 59, 12664–12668. [Google Scholar] [CrossRef]
- Qiu, X.F.; Huang, J.R.; Yu, C.; Zhao, Z.H.; Zhu, H.L.; Ke, Z.; Liao, P.Q.; Chen, X.M. A Stable and Conductive Covalent Organic Framework with Isolated Active Sites for Highly Selective Electroreduction of Carbon Dioxide to Acetate. Angew. Chem. 2022, 61, e202206470. [Google Scholar]
- Shao, P.; Zhou, W.; Hong, Q.L.; Yi, L.; Zheng, L.; Wang, W.; Zhang, H.X.; Zhang, H.; Zhang, J. Synthesis of a Boron–Imidazolate Framework Nanosheet with Dimer Copper Units for CO2 Electroreduction to Ethylene. Angew. Chem. 2021, 60, 16687–16692. [Google Scholar] [CrossRef]
- Zhu, H.L.; Chen, H.Y.; Han, Y.X.; Zhao, Z.H.; Liao, P.Q.; Chen, X.M. A Porous π–π Stacking Framework with Dicopper (I) Sites and Adjacent Proton Relays for Electroreduction of CO2 to C2+ Products. J. Am. Chem. Soc. 2022, 144, 13319–13326. [Google Scholar] [CrossRef]
- Lin, L.; Li, H.; Yan, C.; Li, H.; Si, R.; Li, M.; Xiao, J.; Wang, G.; Bao, X. Synergistic Catalysis over Iron-Nitrogen Sites Anchored with Cobalt Phthalocyanine for Efficient CO2 Electroreduction. Adv. Mater. 2019, 31, 1903470. [Google Scholar] [CrossRef]
- Pan, Y.; Lin, R.; Chen, Y.; Liu, S.; Zhu, W.; Cao, X.; Chen, W.; Wu, K.; Cheong, W.-C.; Wang, Y.; et al. Design of Single-Atom Co–N5 Catalytic Site: A Robust Electrocatalyst for CO2 Reduction with Nearly 100% CO Selectivity and Remarkable Stability. J. Am. Chem. Soc. 2018, 140, 4218–4221. [Google Scholar] [CrossRef]
- Wang, J.; Huang, X.; Xi, S.; Lee, J.M.; Wang, C.; Du, Y.; Wang, X. Linkage Effect in the Heterogenization of Cobalt Complexes by Doped Graphene for Electrocatalytic CO2 Reduction. Angew. Chem. 2019, 58, 13532–13539. [Google Scholar] [CrossRef]
- Jiao, J.; Lin, R.; Liu, S.; Cheong, W.-C.; Zhang, C.; Chen, Z.; Pan, Y.; Tang, J.; Wu, K.; Hung, S.-F.; et al. Copper atom-pair catalyst anchored on alloy nanowires for selective and efficient electrochemical reduction of CO2. Nat. Chem. 2019, 11, 222–228. [Google Scholar] [CrossRef]
- Han, L.L.; Liu, X.J.; He, J.; Liang, Z.X.; Wang, H.T.; Bak, S.M.; Zhang, J.M.; Hunt, A.; Waluyo, I.; Pong, W.F.; et al. Modification of the Coordination Environment of Active Sites on MoC for High-Efficiency CH4 Production. Adv. Energy Mater. 2021, 11, 2100044. [Google Scholar] [CrossRef]
- Chen, Y.; Qiao, S.; Tang, Y.; Du, Y.; Zhang, D.; Wang, W.; Xie, H.; Liu, C. Precise and scalable fabrication of metal pair-site catalysts enabled by intramolecular integrated donor atoms. J. Mater. Chem. A 2022, 10, 25307–25318. [Google Scholar] [CrossRef]
- Wei, T.; Bao, H.; Wang, X.; Zhang, S.; Liu, Q.; Luo, J.; Liu, X. Ionic Liquid-assisted electrocatalytic NO reduction to NH3 by P-doped MoS2. ChemCatChem 2023, e202201411. [Google Scholar] [CrossRef]
- Zhang, L.H.; Han, L.L.; Liu, H.X.; Liu, X.J.; Luo, J. Potential-Cycling Synthesis of Single Platinum Atoms for Efficient Hydrogen Evolution in Neutral Media. Angew. Chem. 2017, 56, 13694–13698. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, H.; Zhang, X.; Zhang, Y.; He, Q.; Chen, H.; Cheng, Y.; Peng, M.; Qin, X.; Ji, H.; et al. Building up libraries and production line for single atom catalysts with precursor-atomization strategy. Nat. Commun. 2022, 13, 5721. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhang, S.; Liu, Q.; Qiu, Y.; Luo, J.; Liu, X. Oxygen Vacancy-Rich Amorphous Copper Oxide Enables Highly Selective Electroreduction of Carbon Dioxide to Ethylene. Acta Phys. Chim. Sin. 2023, 39, 202207026. [Google Scholar] [CrossRef]
- Bo, L.; Zhen, L.; Xin, W.; Zonglong, Z. Interface functionalization in inverted perovskite solar cells: From material perspective. Nano Res. Energy 2022, 1, e9120011. [Google Scholar]
- Ahui, H.; Xin, W.; Xiaofang, L.; Ronghai, Y.; Jianglan, S. Inorganic microporous membranes for hydrogen separation: Challenges and solutions. Nano Res. Energy 2022, 1, e9120013. [Google Scholar]
- Li, J.; Chen, G.; Zhu, Y.; Liang, Z.; Pei, A.; Wu, C.-L.; Wang, H.; Lee, H.R.; Liu, K.; Chu, S.; et al. Efficient electrocatalytic CO2 reduction on a three-phase interface. Nat. Catal. 2018, 1, 592–600. [Google Scholar] [CrossRef]
- Gao, S.S.; Liu, Y.F.; Xie, Z.Y.; Qiu, Y.; Zhuo, L.C.; Qin, Y.J.; Ren, J.Q.; Zhang, S.S.; Hu, G.Z.; Luo, J.; et al. Metal-Free Bifunctional Ordered Mesoporous Carbon for Reversible Zn-CO2 Batteries. Small Methods 2021, 5, 2001039. [Google Scholar] [CrossRef]
- Wei, T.; Liu, W.; Zhang, S.; Liu, Q.; Luo, J.; Liu, X. A dual-functional Bi-doped Co3O4 nanosheet array towards high efficiency 5-hydroxymethylfurfural oxidation and hydrogen production. Chem. Commun. 2023, 59, 442–445. [Google Scholar] [CrossRef]
- Lv, F.; Han, N.; Qiu, Y.; Liu, X.J.; Luo, J.; Li, Y.G. Transition metal macrocycles for heterogeneous electrochemical CO2 reduction. Coord. Chem. Rev. 2020, 422, 213435. [Google Scholar] [CrossRef]
- Wang, K.; Wu, Y.; Cao, X.; Gu, L.; Hu, J. A Zn–CO2 Flow Battery Generating Electricity and Methane. Adv. Funct. Mater. 2020, 30, 1908965. [Google Scholar] [CrossRef]
- Wang, X.Z.; Liu, S.; Zhang, H.; Zhang, S.S.; Meng, G.; Liu, Q.; Sun, Z.Y.; Luo, J.; Liu, X.J. Polycrystalline SnSx nanofilm enables CO2 electroreduction to formate with high current density. Chem. Commun. 2022, 58, 7654–7657. [Google Scholar] [CrossRef]
- Leverett, J.; Daiyan, R.; Gong, L.L.; Iputera, K.; Tong, Z.Z.; Qu, J.T.; Ma, Z.P.; Zhang, Q.R.; Cheong, S.S.; Cairney, J.; et al. Designing Undercoordinated Ni-N-x and Fe-N-x on Holey Graphene for Electrochemical CO2 Conversion to Syngas. ACS Nano 2021, 15, 12006–12018. [Google Scholar] [CrossRef]
- Zou, X.Y.; Ma, C.; Li, A.; Gao, Z.; Shadike, Z.; Jiang, K.; Zhang, J.L.; Huang, Z.; Zhu, L. Nanoparticle-Assisted Ni-Co Binary Single-Atom Catalysts Supported on Carbon Nanotubes for Efficient Electroreduction of CO2 to Syngas with Controllable CO/H2 Ratios. ACS Appl. Energy Mater. 2021, 4, 9572–9581. [Google Scholar] [CrossRef]
- Cheng, M.J.; Clark, E.; Pham, H.; Bell, A.; Head-Gordon, M. Quantum mechanical screening of single-atom bimetallic alloys for the selective reduction of CO2 to C1 hydrocarbons. ACS Catal. 2016, 6, 7769–7777. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, J.X.; Li, F.Y.; Chen, Z.F. Copper Dimer Supported on a C2N Layer as an Efficient Electrocatalyst for CO2 Reduction Reaction: A Computational Study. J. Phys. Chem. C 2018, 122, 19712–19721. [Google Scholar] [CrossRef]
- Danye, L.; Qing, Z.; Chaoquan, H.; Dong, C.; Hui, L.; Yongsheng, H.; Lin, X.; Qingbo, Z.; Jun, Y. Light doping of tungsten into copper-platinum nanoalloys for boosting their electrocatalytic performance in methanol oxidation. Nano Res. Energy 2022, 1, e9120017. [Google Scholar]
- Lin, L.; Liu, T.; Xiao, J.; Li, H.; Wei, P.; Gao, D.; Nan, B.; Si, R.; Wang, G.; Bao, X.; et al. Enhancing CO2 Electroreduction to Methane with a Cobalt Phthalocyanine and Zinc–Nitrogen–Carbon Tandem Catalyst. Angew. Chem. 2020, 59, 22408–22413. [Google Scholar] [CrossRef]
- Zengxia, P. Symmetric is nonidentical: Operation history matters for Zn metal anode. Nano Res. Energy 2022, 1, e9120023. [Google Scholar]
- Wang, X.; Huang, G.; Pan, Z.; Kang, S.; Ma, S.; Shen, P.K.; Zhu, J. One-pot synthesis of Mn2P-Mn2O3 heterogeneous nanoparticles in a P, N -doped three-dimensional porous carbon framework as a highly efficient bifunctional electrocatalyst for overall water splitting. Chem. Eng. J. 2022, 428, 131190. [Google Scholar] [CrossRef]
- Ju, W.; Bagger, A.; Hao, G.P.; Varela, A.S.; Sinev, I.; Bon, V.; Roldan Cuenya, B.; Kaskel, S.; Rossmeisl, J.; Strasser, P.; et al. Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO2. Nat. Commun. 2017, 8, 944. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Jiang, E.; Wang, X.; Pan, Z.; Xu, X.; Ma, S.; Kang Shen, P.; Pan, L.; Eguchi, M.; Nanjundan, A.K.; et al. Gram-Scale production of Cu3P-Cu2O Janus nanoparticles into nitrogen and phosphorous doped porous carbon framework as bifunctional electrocatalysts for overall water splitting. Chem. Eng. J. 2022, 427, 130946. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Hou, M.; Wang, W.; Hu, S.; Cen, W.; Cao, X.; Qiao, S.; Han, B.H. Pristine, metal ion and metal cluster modified conjugated triazine frameworks as electrocatalysts for hydrogen evolution reaction. J. Mater. Chem. A 2021, 9, 10146–10159. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Z.; Li, S.; Yang, M.; Ma, R.; Wang, J. One stone two birds: Vanadium doping as dual roles in self-reduced Pt clusters and accelerated water splitting. J. Energy Chem. 2022, 66, 493–501. [Google Scholar] [CrossRef]
- Zhang, H.; Qiu, Y.; Zhang, S.S.; Liu, Q.; Luo, J.; Liu, X.J. Nitrogen-incorporated iron phosphosulfide nanosheets as efficient bifunctional electrocatalysts for energy-saving hydrogen evolution. Ionics 2022, 28, 3927–3934. [Google Scholar] [CrossRef]
- Chen, W.; Li, X.; Wei, X.; Liao, G.; Wang, J.; Li, L. Activation of peroxymonosulfate for degrading ibuprofen via single atom Cu anchored by carbon skeleton and chlorine atom: The radical and non-radical pathways. Sci. Total Environ. 2023, 858, 160097. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, Y.; Chen, Y.; Luan, D.; Gao, S.; Lou, X.W. Mesoporous N-rich Carbon with Single-Ni Atoms as a Multifunctional Sulfur Host for Li-S Batteries. Angew. Chem. 2022, 61, e202212680. [Google Scholar]
- Liu, W.; Feng, J.; Wei, T.; Liu, Q.; Zhang, S.; Luo, Y.; Luo, J.; Liu, X. Active-site and interface engineering of cathode materials for aqueous Zn-Gas batteries. Nano Res. 2022, 1–22. [Google Scholar] [CrossRef]
- Arandia, A.; Yim, J.; Warraich, H.; Leppakangas, E.; Bes, R.; Lempelto, A.; Gell, L.; Jiang, H.; Meinander, K.; Viinikainen, T.; et al. Effect of atomic layer deposited zinc promoter on the activity of copper-on-zirconia catalysts in the hydrogenation of carbon dioxide to methanol. Appl. Catal. B Environ. 2023, 321, 122046. [Google Scholar] [CrossRef]
- Schmelz, D.; Gerold, K.; Kasebier, T.; Sergeev, N.; Szeghalmi, A.; Zeitner, U.D. Optical properties of black silicon structures ALD-coated with Al2O3. Nanotechnology 2023, 34, 015704. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, Y.; Chu, P.; Liu, Q.; Liu, X.; Zhang, S.; Luo, J.; Wang, X.; Hu, G. Recent advances in non-noble metal-based bifunctional electrocatalysts for overall seawater splitting. J. Alloys Compd. 2022, 922, 166113. [Google Scholar] [CrossRef]
- Zhang, C.X.; Liu, H.X.; Liu, Y.F.; Liu, X.J.; Mi, Y.Y.; Guo, R.J.; Sun, J.Q.; Bao, H.H.; He, J.; Qiu, Y.; et al. Rh2S3/N-Doped Carbon Hybrids as pH-Universal Bifunctional Electrocatalysts for Energy-Saving Hydrogen Evolution. Small Methods 2020, 4, 2000208. [Google Scholar] [CrossRef]
- Eid, K.; Sliem, M.H.; Al-Ejji, M.; Abdullah, A.M.; Harfouche, M.; Varma, R.S. Hierarchical Porous Carbon Nitride-Crumpled Nanosheet-Embedded Copper Single Atoms: An Efficient Catalyst for Carbon Monoxide Oxidation. ACS Appl. Mater. Interfaces 2022, 14, 40749–40760. [Google Scholar] [CrossRef]
- Cheng, Y.; Song, H.; Yu, J.; Chang, J.; Waterhouse, G.I.N.; Tang, Z.; Yang, B.; Lu, S. Carbon dots-derived carbon nanoflowers decorated with cobalt single atoms and nanoparticles as efficient electrocatalysts for oxygen reduction. Chin. J. Catal. 2022, 43, 2443–2452. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, C.X.; Liu, H.X.; Sun, J.Q.; Xie, R.C.; Qiu, Y.; Lu, F.; Liu, Y.F.; Zhuo, L.C.; Liu, X.J.; et al. Amorphous MoOX-Stabilized single platinum atoms with ultrahigh mass activity for acidic hydrogen evolution. Nano Energy 2020, 70, 104529. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, X.; Bian, W.; Yang, Y.; Huang, P.; Hofer, W.A.; Huang, H.; Lin, H.; Li, Y.; Lee, S.T.; et al. High-loading Fe1 sites on vanadium disulfides: A scalable and non-defect-stabilized single atom catalyst for electrochemical nitrogen reduction. J. Mater. Chem. A 2022, 10, 21142–21148. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, L.; Dai, J.; Wang, J.; Fang, C.; Zhan, G.; Zheng, Q.; Hou, W.; Zhang, L. Single Atom Ru Monolithic Electrode for Efficient Chlorine Evolution and Nitrate Reduction. Angew. Chem. 2022, 61, e202208215. [Google Scholar] [CrossRef]
- Peng, X.Y.; Mi, Y.Y.; Liu, X.J.; Sun, J.Q.; Qiu, Y.; Zhang, S.S.; Ke, X.X.; Wang, X.Z.; Luo, J. Self-driven dual hydrogen production system based on a bifunctional single-atomic Rh catalyst. J. Mater. Chem. A 2022, 10, 6134–6145. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Z.; Liu, Q.; Sun, P.; Wang, Y.; Chou, S.; Hu, Z.; Zhang, Z. Single-atom Ru anchored in nitrogen-doped MXene (Ti3C2Tx) as an efficient catalyst for the hydrogen evolution reaction at all pH values. J. Mater. Chem. A 2020, 8, 24710–24717. [Google Scholar] [CrossRef]
- Xue, Y.; Huang, B.; Yi, Y.; Guo, Y.; Zuo, Z.; Li, Y.; Jia, Z.; Liu, H.; Li, Y. Anchoring zero valence single atoms of nickel and iron on graphdiyne for hydrogen evolution. Nat. Commun. 2018, 9, 1460. [Google Scholar] [CrossRef]
- Peng, X.Y.; Hou, J.R.; Mi, Y.Y.; Sun, J.Q.; Qi, G.C.; Qin, Y.J.; Zhang, S.S.; Qiu, Y.; Luo, J.; Liu, X.J.; et al. Bifunctional single-atomic Mn sites for energy-efficient hydrogen production. Nanoscale 2021, 13, 4767–4773. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.F.; Liu, S.; Chen, H.H.; Lai, S.H.; Qin, Y.J.; Qiu, Y.; Dai, S.; Zhang, S.S.; Luo, J.; Liu, X.J. Rh nanoparticle functionalized heteroatom-doped hollow carbon spheres for efficient electrocatalytic hydrogen evolution. Mater. Chem. Front. 2021, 5, 3125–3131. [Google Scholar] [CrossRef]
- Zou, L.; Wei, Y.S.; Hou, C.C.; Li, C.; Xu, Q. Single-Atom Catalysts Derived from Metal-Organic Frameworks for Electrochemical Applications. Small 2021, 17, e2004809. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Rebollar, D.; He, H.; Chong, L.; Liu, Y.; Liu, C.; Sun, C.-J.; Li, T.; Muntean, J.V.; Winans, R.E.; et al. Highly selective electrocatalytic CO2 reduction to ethanol by metallic clusters dynamically formed from atomically dispersed copper. Nat. Energy 2020, 5, 623–632. [Google Scholar] [CrossRef]
- Peng, X.N.; Bao, H.H.; Sun, J.Q.; Mao, Z.Y.; Qiu, Y.; Mo, Z.J.; Zhuo, L.C.; Zhang, S.S.; Luo, J.; Liu, X.J.; et al. Heteroatom coordination induces electric field polarization of single Pt sites to promote hydrogen evolution activity. Nanoscale 2021, 13, 7134–7139. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, S.Q.; Liang, S.; An, Q.; Fu, J.; Hu, J.S. Coordination anchoring synthesis of high-density single-metal-atom sites for electrocatalysis. Coord. Chem. Rev. 2022, 466, 214603. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, W.; Cao, D.; Cheng, D. Carbon-based material-supported single-atom catalysts for energy conversion. iScience 2022, 25, 104367. [Google Scholar] [CrossRef]
- Liu, X.J.; Xi, W.; Li, C.; Li, X.B.; Shi, J.; Shen, Y.L.; He, J.; Zhang, L.H.; Xie, L.; Sun, X.M.; et al. Nanoporous Zn-doped Co3O4 sheets with single-unit-cell-wide lateral surfaces for efficient oxygen evolution and water splitting. Nano Energy 2018, 44, 371–377. [Google Scholar] [CrossRef]
- Liu, X.J.; He, J.; Zhao, S.Z.; Liu, Y.P.; Zhao, Z.; Luo, J.; Hu, G.Z.; Sun, X.M.; Ding, Y. Self-powered H2 production with bifunctional hydrazine as sole consumable. Nat. Commun. 2018, 9, 4365. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, Y.; Lin, Q.; Zhang, L.; Zhang, X.; Wang, H. Noble-metal single-atoms in thermocatalysis, electrocatalysis, and photocatalysis. Energy Environ. Sci. 2021, 14, 2954–3009. [Google Scholar] [CrossRef]
- Han, L.; Ren, Z.; Ou, P.; Cheng, H.; Rui, N.; Lin, L.; Liu, X.; Zhuo, L.; Song, J.; Sun, J.; et al. Modulating Single-Atom Palladium Sites with Copper for Enhanced Ambient Ammonia Electrosynthesis. Angew. Chem. 2021, 60, 345–350. [Google Scholar] [CrossRef]
- Zeng, X.; Tu, Z.; Yuan, Y.; Liao, L.; Xiao, C.; Wen, Y.; Xiong, K. Two-Dimensional Transition Metal-Hexaaminobenzene Monolayer Single-Atom Catalyst for Electrocatalytic Carbon Dioxide Reduction. Nanomaterials 2022, 12, 4005. [Google Scholar] [CrossRef]
- Meng, G.; Wei, T.; Liu, W.; Li, W.; Zhang, S.; Liu, W.; Liu, Q.; Bao, H.; Luo, J.; Liu, X.; et al. NiFe layered double hydroxide nanosheet array for high-efficiency electrocatalytic reduction of nitric oxide to ammonia. Chem. Commun. 2022, 58, 8097–8100. [Google Scholar] [CrossRef]
- Gao, S.; Wei, T.; Sun, J.; Liu, Q.; Ma, D.; Liu, W.; Zhang, S.; Luo, J.; Liu, X. Atomically Dispersed Metal-Based Catalysts for Zn–CO2 Batteries. Small Struct. 2022, 3, 2200086. [Google Scholar] [CrossRef]
- Zhong, M.; Tran, K.; Min, Y.; Wang, C.; Wang, Z.; Dinh, C.-T.; De Luna, P.; Yu, Z.; Rasouli, A.S.; Brodersen, P.; et al. Accelerated discovery of CO2 electrocatalysts using active machine learning. Nature 2020, 581, 178–183. [Google Scholar] [CrossRef]
- Liu, H.X.; Liu, X.J.; Mao, Z.Y.; Zhao, Z.; Peng, X.Y.; Luo, J.; Sun, X.M. Plasma-activated Co3(PO4)2 nanosheet arrays with Co3+-Rich surfaces for overall water water splitting. J. Power Sources 2018, 400, 190–197. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.H.; Liu, R.R.; Cao, Z.; Sun, X.M.; Liu, X.J.; Luo, J. WO3@alpha-Fe2O3 Heterojunction Arrays with Improved Photoelectrochemical Behavior for Neutral pH Water Splitting. ChemCatChem 2016, 8, 2765–2770. [Google Scholar] [CrossRef]
- Li, P.S.; Wang, S.Y.; Samo, I.A.; Zhang, X.H.; Wang, Z.L.; Wang, C.; Li, Y.; Du, Y.Y.; Zhong, Y.; Cheng, C.T.; et al. Common-Ion Effect Triggered Highly Sustained Seawater Electrolysis with Additional NaCl Production. Research 2020, 2020, 2872141. [Google Scholar] [CrossRef]
- Jiang, E.; Li, J.; Li, X.; Ali, A.; Wang, G.; Ma, S.; Kang Shen, P.; Zhu, J. MoP-Mo2C quantum dot heterostructures uniformly hosted on a heteroatom-doped 3D porous carbon sheet network as an efficient bifunctional electrocatalyst for overall water splitting. Chem. Eng. J. 2022, 431, 133719. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, D.J.; Qi, G.C.; Liu, X.J. Fe-doped MoS2 nanosheets array for high-current-density seawater electrolysis. Nanotechnology 2021, 32, 415403. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Li, M.; Liu, X.; Luo, J. Transition-metal-based electrocatalysts for hydrazine-assisted hydrogen production. Mater. Today Adv. 2020, 7, 100083. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, H.; Liu, Y.; Pan, Y. Research progress of precise structural regulation of single atom catalyst for accelerating electrocatalytic oxygen reduction reaction. J. Energy Chem. 2022, 72, 56–72. [Google Scholar] [CrossRef]
- Liu, H.X.; Peng, X.Y.; Liu, X.J.; Qi, G.C.; Luo, J. Porous Mn-Doped FeP/Co3(PO4)2 Nanosheets as Efficient Electrocatalysts for Overall Water Splitting in a Wide pH Range. ChemSusChem 2019, 12, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, F.; Jin, M.; Zhao, D.; Fan, X.; Li, Z.; Luo, Y.; Zheng, D.; Li, T.; Wang, Y.; et al. V-doped TiO2 nanobelt array for high-efficiency electrocatalytic nitrite reduction to ammonia. Mater. Today Phys. 2023, 30, 100944. [Google Scholar] [CrossRef]
- Wang, J.; Xu, R.; Li, Y.; Li, Y.; Yang, M.; Yang, G.; Zhao, Y.; Gao, F. Recent progress, developing strategies, theoretical insights, and perspectives towards high-performance copper single atom electrocatalysts. Mater. Today Energy 2021, 21, 100761. [Google Scholar] [CrossRef]
- Yang, M.; Sun, J.; Qin, Y.; Yang, H.; Zhang, S.; Liu, X.; Luo, J. Hollow CoFe-layered double hydroxide polyhedrons for highly efficient CO2 electrolysis. Sci. China Mater. 2022, 65, 536–542. [Google Scholar] [CrossRef]
- Anuratha, K.S.; Rinawati, M.; Wu, T.H.; Yeh, M.H.; Lin, J.Y. Recent Development of Nickel-Based Electrocatalysts for Urea Electrolysis in Alkaline Solution. Nanomaterials 2022, 12, 2970. [Google Scholar] [CrossRef]

| Catalyst | Active Site | Electrolyte | Product, FE (%) | Current Density (mA cm−2) (E vs. RHE) | Ref. |
|---|---|---|---|---|---|
| Fe1NC/S1–800 | FeN4 | 0.5 M KHCO3 | CO, 82 | ~2.9 (−0.5 V) | [66] |
| NiN4 | NiN4 | 0.5 M KHCO3 | CO, ~80 | ~15 (−0.9 V) | [71] |
| Fe1NC/S1–1000 | FeN3 | 0.5 M KHCO3 | CO, 96 | 6.4 (−0.5 V) | [66] |
| NiN3V | NiN3 | 0.5 M KHCO3 | CO, >90 | ~60 (−0.9 V) | [71] |
| Mn–C3N4/CNT | MnN3 | 0.5 M KHCO3 | CO, 98.8 | 14 (−0.55 V) | [101] |
| Ni SAs/N-C | NiN3C1 | 0.5 M KHCO3 | CO, 71.9 | 10.48 (−1.0 V) | [102] |
| NiSA-N2-C | NiN2C2 | 0.5 M KHCO3 | CO, ~100 | ~12 (−0.8 V) | [103] |
| Single-atom Snδ+ on N-doped graphene | SnN2C2 | 0.25 M KHCO3 | Formate, 74.3 | 11.7 (−1.6 VSCE) | [106] |
| Sn-NOC | SnN3O1 | 0.1 M KHCO3 | CO, 94 | 13.9 (−0.7 V) | [64] |
| Bi-SAs-NS/C | BiN3S1 | 0.5 M KHCO3 | CO, 98.3 | ~10 (−0.8 V) | [108] |
| FeN5 | FeN4N1 | 0.1 M KHCO3 | CO, 97 | ~5 (−0.46 V) | [114] |
| Fe-CON400–400 | FeN4O1 | 0.1 M KHCO3 | CO, ~100 | ~15 (−0.56~−0.87 V) | [115] |
| Ni-N4-O/C | NiN4O1 | 0.5 M KHCO3 | CO, >90 | ~30 (−0.5~−0.1.1 V) | [116] |
| NiSn-APC | NiN4-SnN4 | 0.5 M KHCO3 | Formate, 86.1 | ~22 (−0.82 V) | [120] |
| ZIF-NC-Ni-Fe | 2N-bridged FeNiN6 | 0.1 M KHCO3 | CO, >93 | ~22 (−0.3~−1.0) | [122] |
| Ni/Cu-N-C | Non-bridged NiCuN6 | 0.5 M KHCO3 | CO, 97.7 | ~13.7 (−0.6 V) | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, X.; Ding, J.; Liu, W.; Zhang, S.; Luo, J.; Liu, X. Asymmetric Coordination Environment Engineering of Atomic Catalysts for CO2 Reduction. Nanomaterials 2023, 13, 309. https://doi.org/10.3390/nano13020309
Hou X, Ding J, Liu W, Zhang S, Luo J, Liu X. Asymmetric Coordination Environment Engineering of Atomic Catalysts for CO2 Reduction. Nanomaterials. 2023; 13(2):309. https://doi.org/10.3390/nano13020309
Chicago/Turabian StyleHou, Xianghua, Junyang Ding, Wenxian Liu, Shusheng Zhang, Jun Luo, and Xijun Liu. 2023. "Asymmetric Coordination Environment Engineering of Atomic Catalysts for CO2 Reduction" Nanomaterials 13, no. 2: 309. https://doi.org/10.3390/nano13020309
APA StyleHou, X., Ding, J., Liu, W., Zhang, S., Luo, J., & Liu, X. (2023). Asymmetric Coordination Environment Engineering of Atomic Catalysts for CO2 Reduction. Nanomaterials, 13(2), 309. https://doi.org/10.3390/nano13020309









