Laser-Induced Synthesis of Electrocatalytically Active Ag, Pt, and AgPt/Polyaniline Nanocomposites for Hydrogen Evolution Reactions
Abstract
1. Introduction
2. Experimental procedures
2.1. Materials and Methods
2.2. PANI Synthesis
2.3. LID
2.4. Sample Characterization
2.5. Electrochemistry
3. Results and Discussion
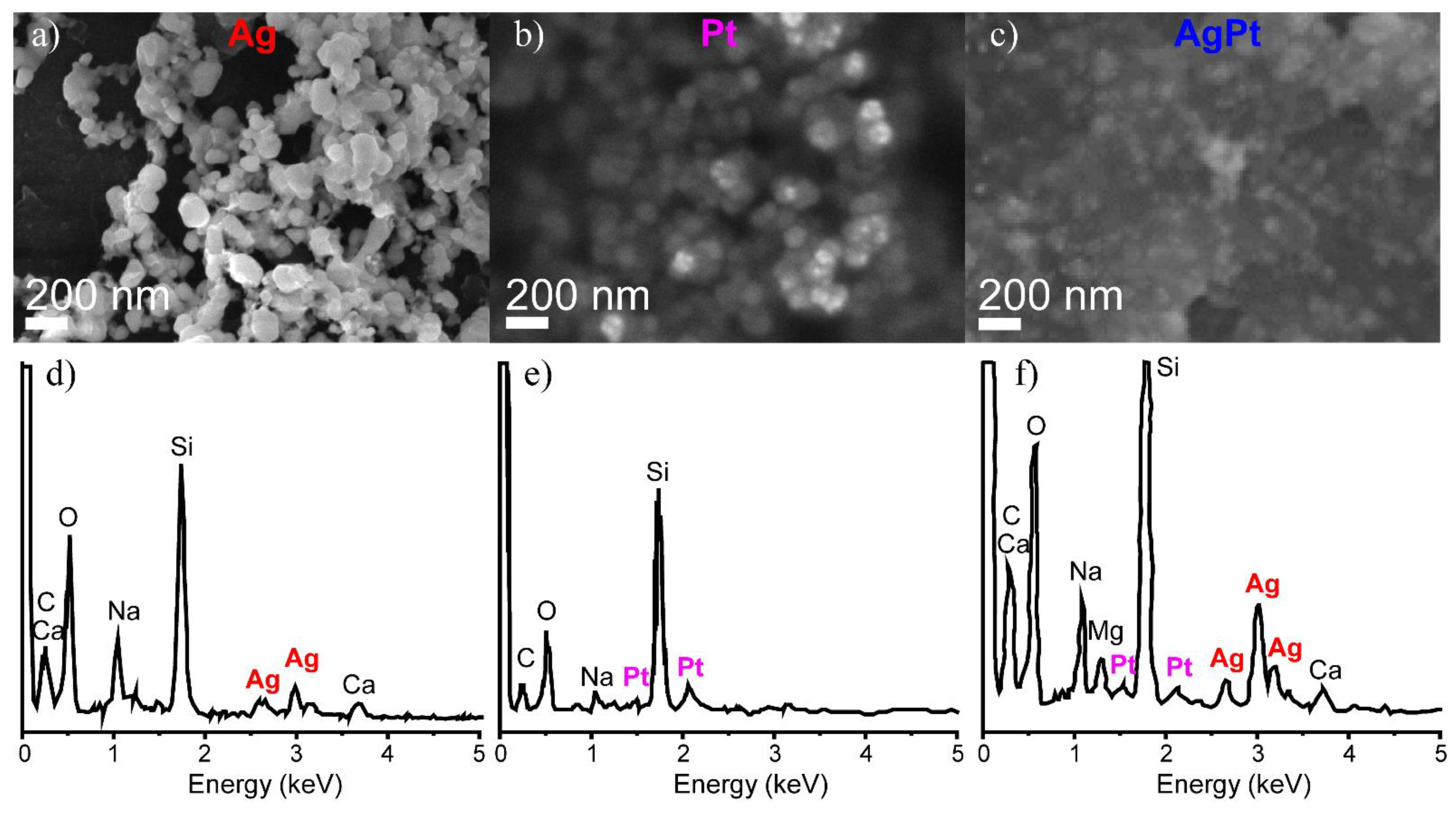
3.1. Characterization of the Samples
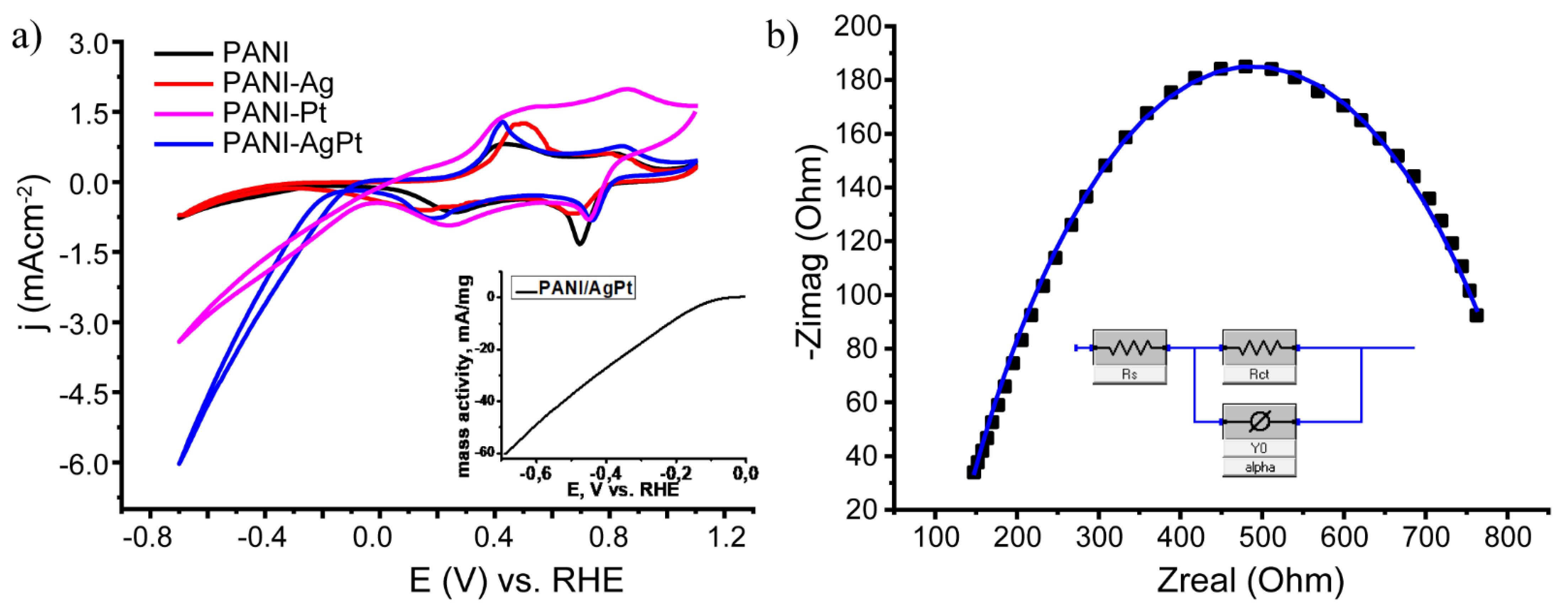
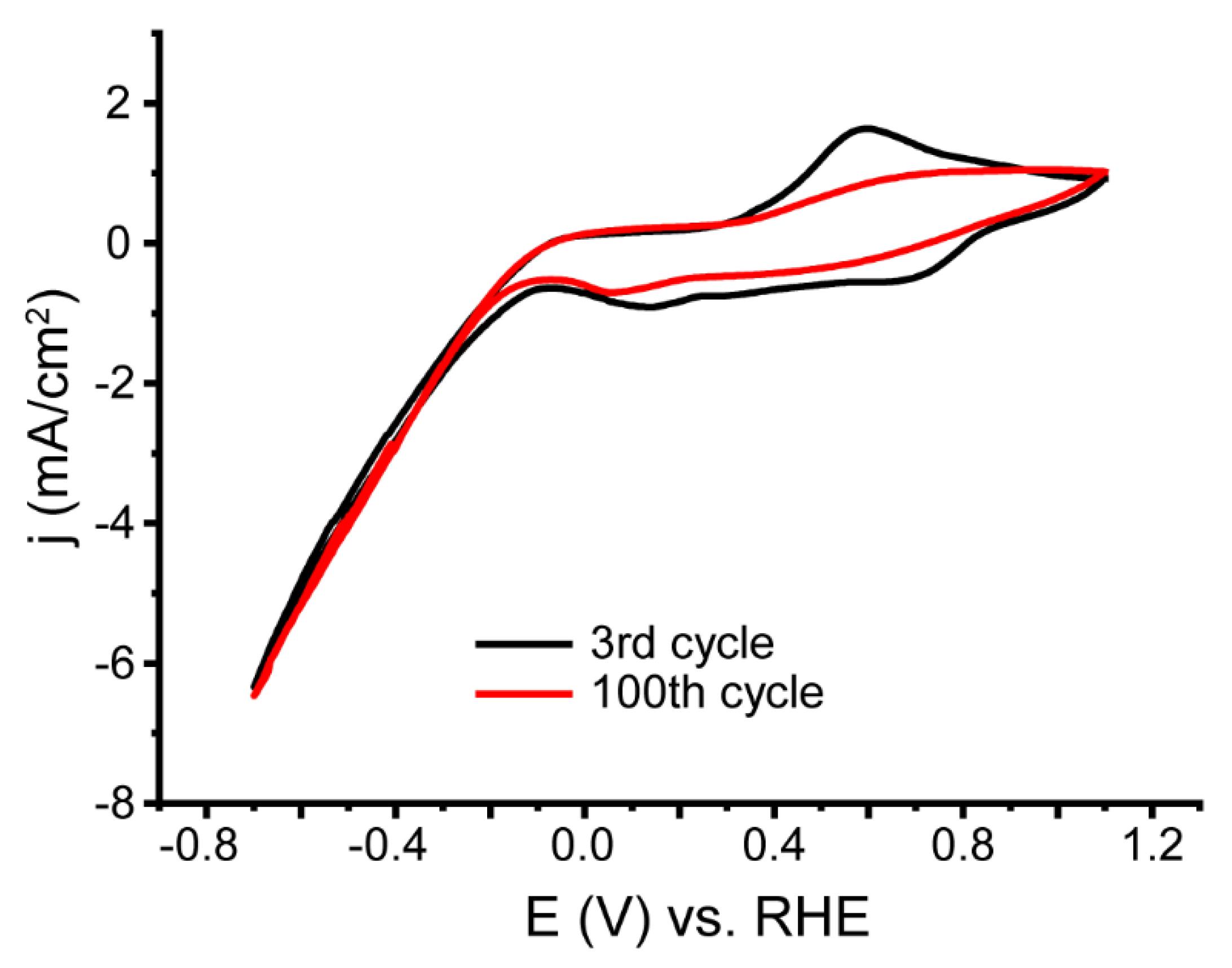
3.2. Electrochemical Studies of the Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, G.; Rui, K.; Dou, S.X.; Sun, W. Heterostructures for Electrochemical Hydrogen Evolution Reaction: A Review. Adv. Funct. Mater. 2018, 28, 1803291. [Google Scholar] [CrossRef]
- Yu, F.; Zhou, H.; Huang, Y.; Sun, J.; Qin, F.; Bao, J.; Goddard, W.A.; Chen, S.; Ren, Z. High-Performance Bifunctional Porous Non-Noble Metal Phosphide Catalyst for Overall Water Splitting. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Banis, M.N.; Ren, Z.; Adair, K.R.; Doyle-Davis, K.; Meira, D.M.; Finfrock, Y.Z.; Zhang, L.; Kong, F.; Sham, T. Unveiling the Nature of Pt Single-Atom Catalyst during Electrocatalytic Hydrogen Evolution and Oxygen Reduction Reactions. Small 2021, 17, 2007245. [Google Scholar] [CrossRef] [PubMed]
- Nazir, R.; Fageria, P.; Basu, M.; Gangopadhyay, S.; Pande, S. Decoration of Pd and Pt Nanoparticles on a Carbon Nitride (C 3 N 4) Surface for Nitro-Compounds Reduction and Hydrogen Evolution Reaction. New J. Chem. 2017, 41, 9658–9667. [Google Scholar] [CrossRef]
- Abbaspour, A.; Mirahmadi, E. Electrocatalytic Hydrogen Evolution Reaction on Carbon Paste Electrode Modified with Ni Ferrite Nanoparticles. Fuel 2013, 104, 575–582. [Google Scholar] [CrossRef]
- Wan, W.; Wei, S.; Li, J.; Triana, C.A.; Zhou, Y.; Patzke, G.R. Transition Metal Electrocatalysts Encapsulated into N-Doped Carbon Nanotubes on Reduced Graphene Oxide Nanosheets: Efficient Water Splitting through Synergistic Effects. J. Mater. Chem. A 2019, 7, 15145–15155. [Google Scholar] [CrossRef]
- Lang, L.; Shi, Y.; Wang, J.; Wang, F.-B.; Xia, X.-H. Hollow Core–shell Structured Ni–Sn@ C Nanoparticles: A Novel Electrocatalyst for the Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2015, 7, 9098–9102. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, H.; Chen, J.; Zhang, B.; Zhang, M.; Wang, L.; Du, M. Small and Well-Dispersed Cu Nanoparticles on Carbon Nanofibers: Self-Supported Electrode Materials for Efficient Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2016, 41, 18044–18049. [Google Scholar] [CrossRef]
- Cao, S.; Jiang, J.; Zhu, B.; Yu, J. Shape-Dependent Photocatalytic Hydrogen Evolution Activity over a Pt Nanoparticle Coupled gC 3 N 4 Photocatalyst. Phys. Chem. Chem. Phys. 2016, 18, 19457–19463. [Google Scholar] [CrossRef]
- Li, Z.; Qi, Z.; Wang, S.; Ma, T.; Zhou, L.; Wu, Z.; Luan, X.; Lin, F.-Y.; Chen, M.; Miller, J.T. In Situ Formed Pt3Ti Nanoparticles on a Two-Dimensional Transition Metal Carbide (MXene) Used as Efficient Catalysts for Hydrogen Evolution Reactions. Nano Lett. 2019, 19, 5102–5108. [Google Scholar] [CrossRef]
- Jiang, L.-Y.; Huang, X.-Y.; Wang, A.-J.; Li, X.-S.; Yuan, J.; Feng, J.-J. Facile Solvothermal Synthesis of Pt 76 Co 24 Nanomyriapods for Efficient Electrocatalysis. J. Mater. Chem. A 2017, 5, 10554–10560. [Google Scholar] [CrossRef]
- Li, J.; Sun, S. Intermetallic Nanoparticles: Synthetic Control and Their Enhanced Electrocatalysis. Acc. Chem. Res. 2019, 52, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Liang, X.; Li, W.; Yang, Y.; Miao, Y. Synthesis of Ultrafine Pt/Pd Bimetallic Nanoparticles and Their Decoration on MWCNTs for Hydrogen Evolution. J. Electrochem. Soc. 2015, 162, H415. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Regner, J.; Gusmão, R.; Sofer, Z. Bismuth-Noble Metal Alloy Nanostructures Prepared by Colloidal Chemical Routes for Use in Hydrogen Evolution and Oxygen Reduction Electrocatalysis: Bi-Pt Versus Bi-Pd. Adv. Sustain. Syst. 2022, 6, 2200163. [Google Scholar] [CrossRef]
- Huang, H.; Fu, L.; Kong, W.; Ma, H.; Zhang, X.; Cai, J.; Wang, S.; Xie, Z.; Xie, S. Equilibrated PtIr/IrOx Atomic Heterojunctions on Ultrafine 1D Nanowires Enable Superior Dual-Electrocatalysis for Overall Water Splitting. Small 2022, 18, 2201333. [Google Scholar] [CrossRef] [PubMed]
- Pelicano, C.M.; Saruyama, M.; Takahata, R.; Sato, R.; Kitahama, Y.; Matsuzaki, H.; Yamada, T.; Hisatomi, T.; Domen, K.; Teranishi, T. Bimetallic Synergy in Ultrafine Cocatalyst Alloy Nanoparticles for Efficient Photocatalytic Water Splitting. Adv. Funct. Mater. 2022, 32, 2202987. [Google Scholar] [CrossRef]
- Zou, Y.; Goei, R.; Ong, S.A.; Ong, A.J.; Huang, J.; Tok, A.I.Y. Development of Core-Shell Rh@ Pt and Rh@ Ir Nanoparticle Thin Film Using Atomic Layer Deposition for HER Electrocatalysis Applications. Processes 2022, 10, 1008. [Google Scholar] [CrossRef]
- Zhang, E.; Ma, F.; Liu, J.; Sun, J.; Chen, W.; Rong, H.; Zhu, X.; Liu, J.; Xu, M.; Zhuang, Z. Porous Platinum–silver Bimetallic Alloys: Surface Composition and Strain Tunability toward Enhanced Electrocatalysis. Nanoscale 2018, 10, 21703–21711. [Google Scholar] [CrossRef]
- Belgherbi, O.; Chouder, D.; Lakhdari, D.; Dehchar, C.; Laidoudi, S.; Lamiri, L.; Hamam, A.; Seid, L. Enzyme-Free Glucose Sensor Based on Star-Like Copper Particles-Polyaniline Composite Film. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2499–2508. [Google Scholar] [CrossRef]
- Salahandish, R.; Ghaffarinejad, A.; Naghib, S.M.; Niyazi, A.; Majidzadeh-A, K.; Janmaleki, M.; Sanati-Nezhad, A. Sandwich-Structured Nanoparticles-Grafted Functionalized Graphene Based 3D Nanocomposites for High-Performance Biosensors to Detect Ascorbic Acid Biomolecule. Sci. Rep. 2019, 9, 1226. [Google Scholar] [CrossRef]
- Chu, T.-X.; Vu, V.-P.; Tran, H.-T.; Tran, T.-L.; Tran, Q.-T.; Manh, T. Le Molecularly Imprinted Polyaniline Nanowire-Based Electrochemical Biosensor for Chloramphenicol Detection: A Kinetic Study of Aniline Electropolymerization. J. Electrochem. Soc. 2020, 167, 27527. [Google Scholar] [CrossRef]
- Oueiny, C.; Berlioz, S.; Perrin, F. Carbon Nanotube–polyaniline Composites Review Article. Prog. Polym. Sci. 2013, 39, 707–748. [Google Scholar] [CrossRef]
- Tabrizi, A.G.; Arsalani, N.; Mohammadi, A.; Ghadimi, L.S.; Ahadzadeh, I.; Namazi, H. A New Route for the Synthesis of Polyaniline Nanoarrays on Graphene Oxide for High-Performance Supercapacitors. Electrochim. Acta 2018, 265, 379–390. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.; Yang, T.; Zhang, P.; Wei, X.; Zhang, L.; Li, H. Polyaniline/graphene Hybrid Fibers as Electrodes for Flexible Supercapacitors. Synth. Met. 2020, 268, 116484. [Google Scholar] [CrossRef]
- Ganesan, R.; Gedanken, A. Organic–inorganic Hybrid Materials Based on polyaniline/TiO2 Nanocomposites for Ascorbic Acid Fuel Cell Systems. Nanotechnology 2008, 19, 435709. [Google Scholar] [CrossRef]
- Mondal, S.K.; Raman, R.K.; Shukla, A.K.; Munichandraiah, N. Electrooxidation of Ascorbic Acid on Polyaniline and Its Implications to Fuel Cells. J. Power Sources 2005, 145, 16–20. [Google Scholar] [CrossRef][Green Version]
- Ding, L.; Su, B. A Non-Enzymatic Hydrogen Peroxide Sensor Based on Platinum Nanoparticle–polyaniline Nanocomposites Hosted in Mesoporous Silica Film. J. Electroanal. Chem. 2015, 736, 83–87. [Google Scholar] [CrossRef]
- Lakhdari, D.; Guittoum, A.; Benbrahim, N.; Belgherbi, O.; Berkani, M.; Vasseghian, Y.; Lakhdari, N. A Novel Non-Enzymatic Glucose Sensor Based on NiFe (NPs)–polyaniline Hybrid Materials. Food Chem. Toxicol. 2021, 151, 112099. [Google Scholar] [CrossRef]
- Chiu, W.-T.; Chang, T.-F.M.; Sone, M.; Tixier-Mita, A.; Toshiyoshi, H. Roles of TiO2 in the Highly Robust Au Nanoparticles-TiO2 Modified Polyaniline Electrode towards Non-Enzymatic Sensing of Glucose. Talanta 2020, 212, 120780. [Google Scholar] [CrossRef]
- Essousi, H.; Barhoumi, H.; Bibani, M.; Ktari, N.; Wendler, F.; Al-Hamry, A.; Kanoun, O. Ion-Imprinted Electrochemical Sensor Based on Copper Nanoparticles-Polyaniline Matrix for Nitrate Detection. J. Sensors 2019, 2019, 4257125. [Google Scholar] [CrossRef]
- Manshina, A.A.; Grachova, E.V.; Povolotskiy, A.V.; Povolotckaia, A.V.; Petrov, Y.V.; Koshevoy, I.O.; Makarova, A.A.; Vyalikh, D.V.; Tunik, S.P. Laser-Induced Transformation of Supramolecular Complexes: Approach to Controlled Formation of Hybrid Multi-Yolk-Shell Au-Ag@a-C:H Nanostructures. Sci. Rep. 2015, 5, 12027. [Google Scholar] [CrossRef] [PubMed]
- Avilova, E.A.; Khairullina, E.M.; Shishov, A.Y.; Eltysheva, E.A.; Mikhailovskii, V.; Sinev, D.A.; Tumkin, I.I. Direct Laser Writing of Copper Micropatterns from Deep Eutectic Solvents Using Pulsed near-IR Radiation. Nanomaterials 2022, 12, 1127. [Google Scholar] [CrossRef] [PubMed]
- Khairullina, E.; Ratautas, K.; Panov, M.; Andriianov, V.; Mickus, S.; Manshina, A.; Račiukaitis, G.; Tumkin, I. Laser-Assisted Surface Activation for the Fabrication of Flexible Non-Enzymatic Cu-Based Sensors. Mikrochim Acta 2022, 189, 259. [Google Scholar] [CrossRef] [PubMed]
- Schlicht, S.; Kireev, A.; Vasileva, A.; Grachova, E.V.; Tunik, S.P.; Manshina, A.A.; Bachmann, J. A Model Electrode of Well-Defined Geometry Prepared by Direct Laser-Induced Decoration of Nanoporous Templates with Au-Ag@C Nanoparticles. Nanotechnology 2017, 28, 065405. [Google Scholar] [CrossRef]
- Vasileva, A.; Haschke, S.; Mikhailovskii, V.; Gitlina, A.; Bachmann, J.; Manshina, A. Direct Laser-Induced Deposition of AgPt@ C Nanoparticles on 2D and 3D Substrates for Electrocatalytic Glucose Oxidation. Nano-Struct. Nano-Objects 2020, 24, 100547. [Google Scholar] [CrossRef]
- Manshina, A.; Ivanova, T.; Povolotskiy, A. Laser-Induced Deposition of Hetero-Metallic Structures from Liquid Phase. Laser Phys. 2010, 20, 1532–1536. [Google Scholar] [CrossRef]
- Manshina, A.a.; Povolotskiy, A.V.; Povolotskaya, A.V.; Ivanova, T.Y.; Koshevoy, I.O.; Tunik, S.P.; Suvanto, M.; Pakkanen, T.a. Laser-Induced Heterometallic Phase Deposition from Solutions of Supramolecular Complexes. Surf. Coatings Technol. 2012, 206, 3454–3458. [Google Scholar] [CrossRef]
- Manshina, A.; Povolotskiy, A.; Ivanova, T.; Kurochkin, A.Y.; Tver’yanovich, Y.; Kim, D.; Kim, M.; Kwon, S. Laser-assisted metal deposition from CuSO4-based electrolyte solution. Laser Phys. Lett 2007, 4, 163–167. [Google Scholar] [CrossRef]
- Man’shina, A.A.; Povolotskiy, A.V.; Ivanova, T.Y.; Kurochkin, A.V.; Tver’yanovich, Y.S.; Kim, D.; Kim, M.; Kwon, S. Laser-Induced Copper Deposition on the Surface of an Oxide Glass from an Electrolyte Solution. Glas. Phys. Chem. 2007, 33, 209–213. [Google Scholar] [CrossRef]
- Bashouti, M.Y.; Povolotckaia, A.V.; Povolotskiy, A.V.; Tunik, S.P.; Christiansen, S.H.; Leuchs, G.; Manshina, A.A. Spatially-Controlled Laser-Induced Decoration of 2D and 3D Substrates with Plasmonic Nanoparticles. RSC Adv. 2016, 6, 75681–75685. [Google Scholar] [CrossRef]
- Haschke, S.; Pankin, D.; Mikhailovskii, V.; Barr, M.K.S.; Both-Engel, A.; Manshina, A.; Bachmann, J. Nanoporous Water Oxidation Electrodes with a Low Loading of Laser-Deposited Ru/C Exhibit Enhanced Corrosion Stability. Beilstein J. Nanotechnol. 2019, 10, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Mamonova, D.V.; Vasileva, A.A.; Petrov, Y.V.; Koroleva, A.V.; Danilov, D.V.; Kolesnikov, I.E.; Bikbaeva, G.I.; Bachmann, J.; Manshina, A.A. Single Step Laser-Induced Deposition of Plasmonic Au, Ag, Pt Mono-, Bi-and Tri-Metallic Nanoparticles. Nanomaterials 2021, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Armarego, W.L.F. Purification of Laboratory Chemicals. Butterworth-Heinemann: Oxford, UK, 2017; ISBN 0128054565. [Google Scholar]
- Mamonova, D.V.; Vasileva, A.A.; Petrov, Y.V.; Danilov, D.V.; Kolesnikov, I.E.; Kalinichev, A.A.; Bachmann, J.; Manshina, A.A. Laser-Induced Deposition of Plasmonic Ag and Pt Nanoparticles, and Periodic Arrays. Materials. 2020, 14, 10. [Google Scholar] [CrossRef]
- Vasileva, A.; Pankin, D.; Mikhailovskii, V.; Kolesnikov, I.; Mínguez-Bacho, I.; Bachmann, J.; Manshina, A. In Situ Microsynthesis of Polyaniline: Synthesis–structure–conductivity Correlation. New J. Chem. 2021, 45, 15968–15976. [Google Scholar] [CrossRef]
- Cochet, M.; Louarn, G.; Quillard, S.; Buisson, J.P.; Lefrant, S. Theoretical and Experimental Vibrational Study of Emeraldine in Salt Form. Part II. J. Raman Spectrosc. 2000, 31, 1041–1049. [Google Scholar] [CrossRef]
- Lindfors, T.; Ivaska, A. Raman Based pH Measurements with Polyaniline. J. Electroanal. Chem. 2005, 580, 320–329. [Google Scholar] [CrossRef]
- Ciric-Marjanovic, G.; Dragicevic, L.; Milojevic, M.; Mojovic, M.; Mentus, S.; Dojcinovic, B.; Marjanovic, B.; Stejskal, J. Synthesis and Characterization of Self-Assembled Polyaniline Nanotubes/silica Nanocomposites. J. Phys. Chem. B 2009, 113, 7116–7127. [Google Scholar] [CrossRef]
- Trchová, M.; Morávková, Z.; Bláha, M.; Stejskal, J. Raman Spectroscopy of Polyaniline and Oligoaniline Thin Films. Electrochim. Acta 2014, 122, 28–38. [Google Scholar] [CrossRef]
- 50. Rohom, A.B.; Londhe, P.U.; Mahapatra, S.K.; Kulkarni, S.K.; Chaure, N.B. Electropolymerization of polyaniline thin films. High Perform. Polym. 2014, 26, 641–646. [Google Scholar] [CrossRef]
- Nobrega, M.M.; Martins, V.L.; Torresi, R.M.; Temperini, M.L.A. One-Step Synthesis, Characterization, and Properties of Emeraldine Salt Nanofibers Containing Gold Nanoparticles. J. Phys. Chem. C 2014, 118, 4267–4274. [Google Scholar] [CrossRef]
- Rozlivkova, Z.; Trchova, M.; Exnerova, M.; Stejskal, J. The Carbonization of Granular Polyaniline to Produce Nitrogen-Containing Carbon. Synth. Met. 2011, 161, 1122–1129. [Google Scholar] [CrossRef]
- Morávková, Z.; Bober, P. Writing in a Polyaniline Film with Laser Beam and Stability of the Record: A Raman Spectroscopy Study. Int. J. Polym. Sci. 2018, 2018, 1797216. [Google Scholar] [CrossRef]
- Chakraborty, P.; Kothari, A.; Nagarajan, R. Highly Ordered Polyaniline as an Efficient Dye Remover. Adsorpt. Sci. Technol. 2018, 36, 429–440. [Google Scholar] [CrossRef]
- Song, E.; Choi, J.-W. Conducting Polyaniline Nanowire and Its Applications in Chemiresistive Sensing. Nanomaterials 2013, 3, 498–523. [Google Scholar] [CrossRef] [PubMed]
- López, I.A.; Ceballos, M.; Hernández, G.; Acosta LGómez, I. Silver Nanoprisms and Nanospheres for Prosthetic Biomaterials. Revista Mexicana de Física 2015, 61, 77–82. [Google Scholar]
- Nazir, R.; Fageria, P.; Basu, M.; Pande, S. Decoration of Carbon Nitride Surface with Bimetallic Nanoparticles (Ag/Pt, Ag/Pd, and Ag/Au) via Galvanic Exchange for Hydrogen Evolution Reaction. J. Phys. Chem. C 2017, 121, 19548–19558. [Google Scholar] [CrossRef]
- Liu, Q.; He, Y.M.; Weng, X.; Wang, A.J.; Yuan, P.X.; Fang, K.M.; Feng, J.J. One-pot aqueous fabrication of reduced graphene oxide supported porous PtAg alloy nanoflowers to greatly boost catalytic performances for oxygen reduction and hydrogen evolution. J. Colloid Interface Sci. 2018, 513, 455–463. [Google Scholar] [CrossRef]
- Lv, J.J.; Feng, J.X.; Li, S.S.; Wang, Y.Y.; Wang, A.J.; Zhang, Q.L.; Feng, J.J. Ionic liquid crystal-assisted synthesis of PtAg nanoflowers on reduced graphene oxide and their enhanced electrocatalytic activity toward oxygen reduction reaction. Electrochim. Acta 2014, 133, 407–413. [Google Scholar] [CrossRef]
- Weber, I.; Solla-Gullon, J.; Brimaud, S.; Feliu, J.M.; Behm, R.J. Structure, surface chemistry and electrochemical de-alloying of bimetallic PtxAg100-x nanoparticles: Quantifying the changes in the surface properties for adsorption and electrocatalytic transformation upon selective Ag removal. J. Electroanal. Chem. 2017, 793, 164–173. [Google Scholar] [CrossRef]
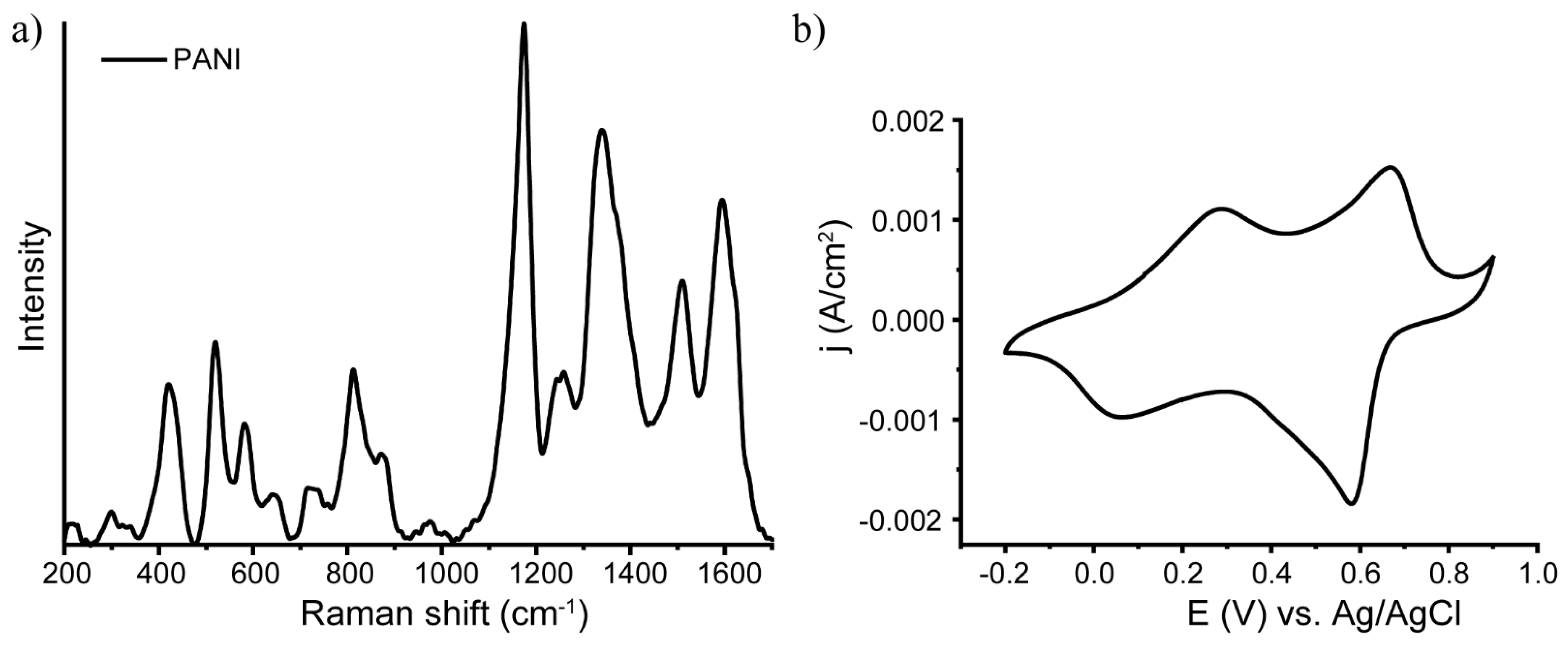
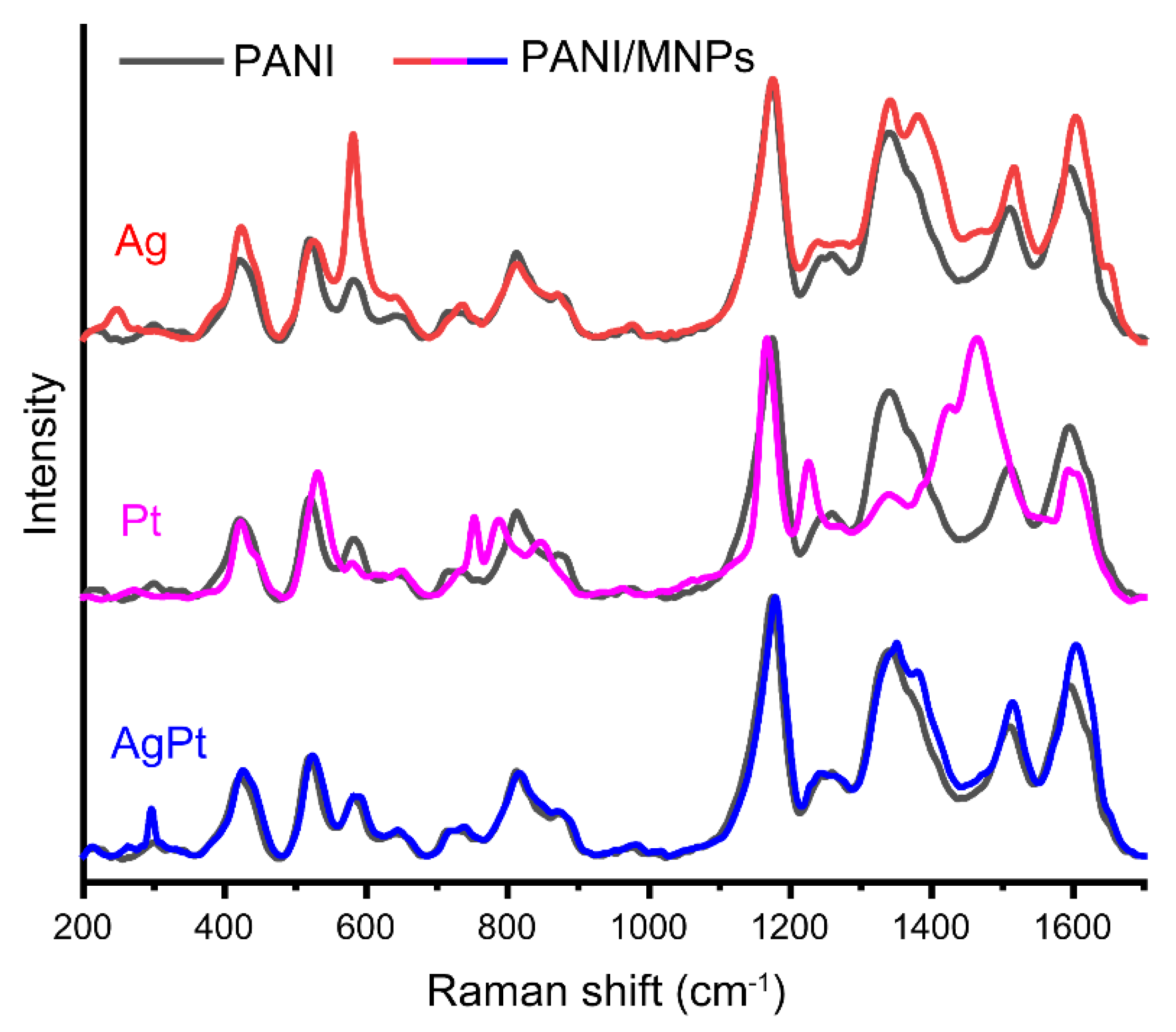


| Band, cm−1 | Vibration | Reference |
|---|---|---|
| 420 | Out-of-plane ring deformation | [49,50] |
| 520 | Out-of-plane ring deformation | [49] |
| 572 | Vibration of phenoxazine-type units | [49,51,52] |
| 720 | Amine deformations reported for the bipolaronic form of emeraldine salt | [49] |
| 780 shoulder | Ring deformation in the emeraldine base | [53] |
| 808 | Substituted benzene ring deformations;out-of-plane C-H deformation of thequinonoid ring; ring deformation of the SQ ring | [48,49] |
| 865 shoulder | Substituted benzene ring deformations | [49] |
| 1170 | Bending vibrations involving C-H bonds in semiquinonoid rings | [48,49,50] |
| 1254 | C–N stretching in quinonoid structures | [47,53] |
| 1335 | Polaron-associated vibrational mode C-N+. | [50,53,54] |
| 1373 | Bipolaron | [53,54] |
| 1506 | N-H deformation in the semiquinonoid structures | [50,53] |
| 1595 | C–C stretching vibration in the semiquinonoid structures | [48,50,53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasileva, A.A.; Mamonova, D.V.; Petrov, Y.V.; Kolesnikov, I.E.; Leuchs, G.; Manshina, A.A. Laser-Induced Synthesis of Electrocatalytically Active Ag, Pt, and AgPt/Polyaniline Nanocomposites for Hydrogen Evolution Reactions. Nanomaterials 2023, 13, 88. https://doi.org/10.3390/nano13010088
Vasileva AA, Mamonova DV, Petrov YV, Kolesnikov IE, Leuchs G, Manshina AA. Laser-Induced Synthesis of Electrocatalytically Active Ag, Pt, and AgPt/Polyaniline Nanocomposites for Hydrogen Evolution Reactions. Nanomaterials. 2023; 13(1):88. https://doi.org/10.3390/nano13010088
Chicago/Turabian StyleVasileva, Anna A., Daria V. Mamonova, Yuri V. Petrov, Ilya E. Kolesnikov, Gerd Leuchs, and Alina A. Manshina. 2023. "Laser-Induced Synthesis of Electrocatalytically Active Ag, Pt, and AgPt/Polyaniline Nanocomposites for Hydrogen Evolution Reactions" Nanomaterials 13, no. 1: 88. https://doi.org/10.3390/nano13010088
APA StyleVasileva, A. A., Mamonova, D. V., Petrov, Y. V., Kolesnikov, I. E., Leuchs, G., & Manshina, A. A. (2023). Laser-Induced Synthesis of Electrocatalytically Active Ag, Pt, and AgPt/Polyaniline Nanocomposites for Hydrogen Evolution Reactions. Nanomaterials, 13(1), 88. https://doi.org/10.3390/nano13010088








