Wearable and Washable MnO2−Zn Battery Packaged by Vacuum Sealing
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of MnO2 Yarn Cathode, Zn Yarn Anode, Gel Electrolyte, and Textile Battery
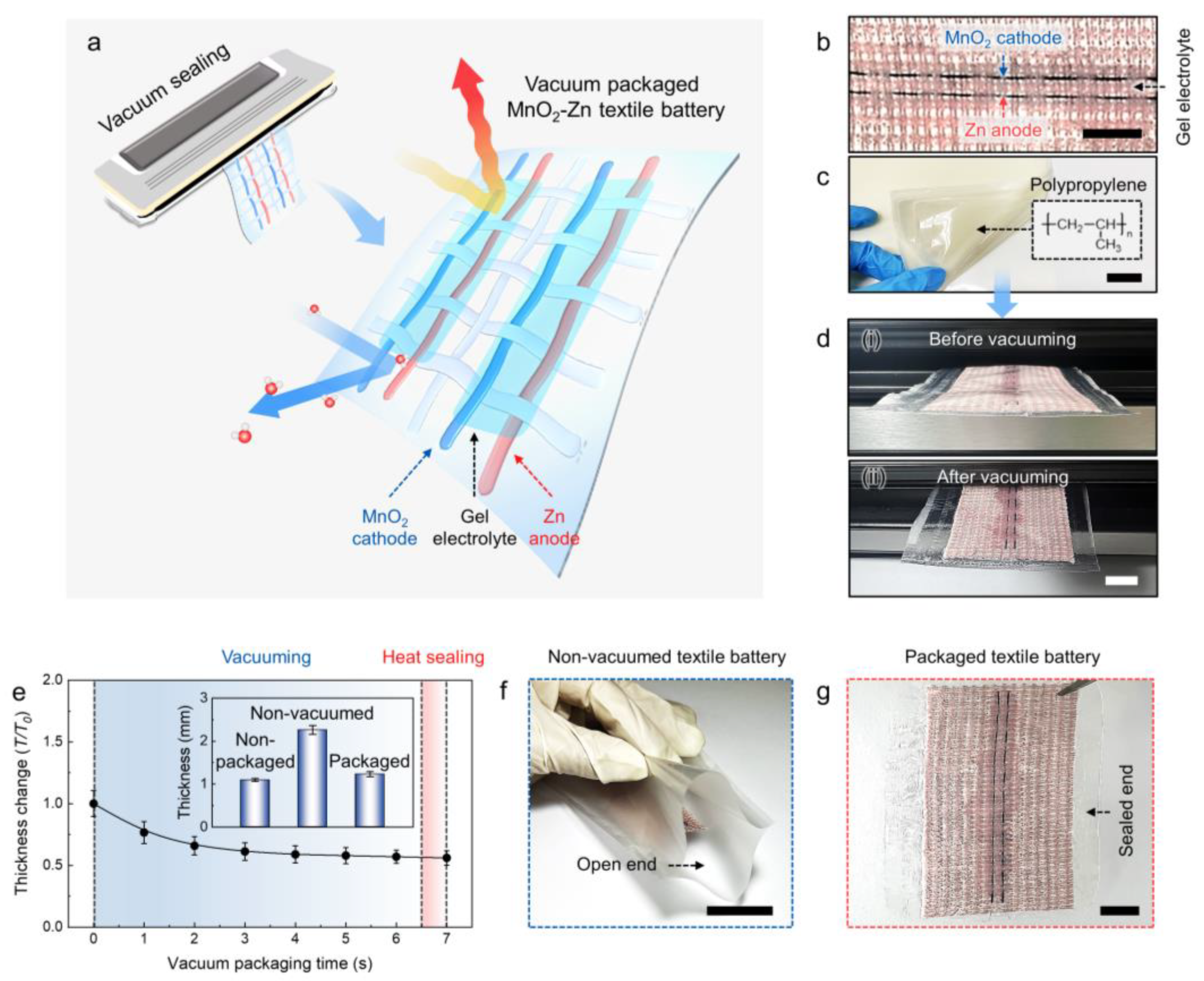
2.3. Textile Battery Packaging by Vacuum Sealing
2.4. Characterization
2.5. Calculation
3. Results
3.1. MnO2−Zn Textile Battery Packaged by Vacuum Sealing
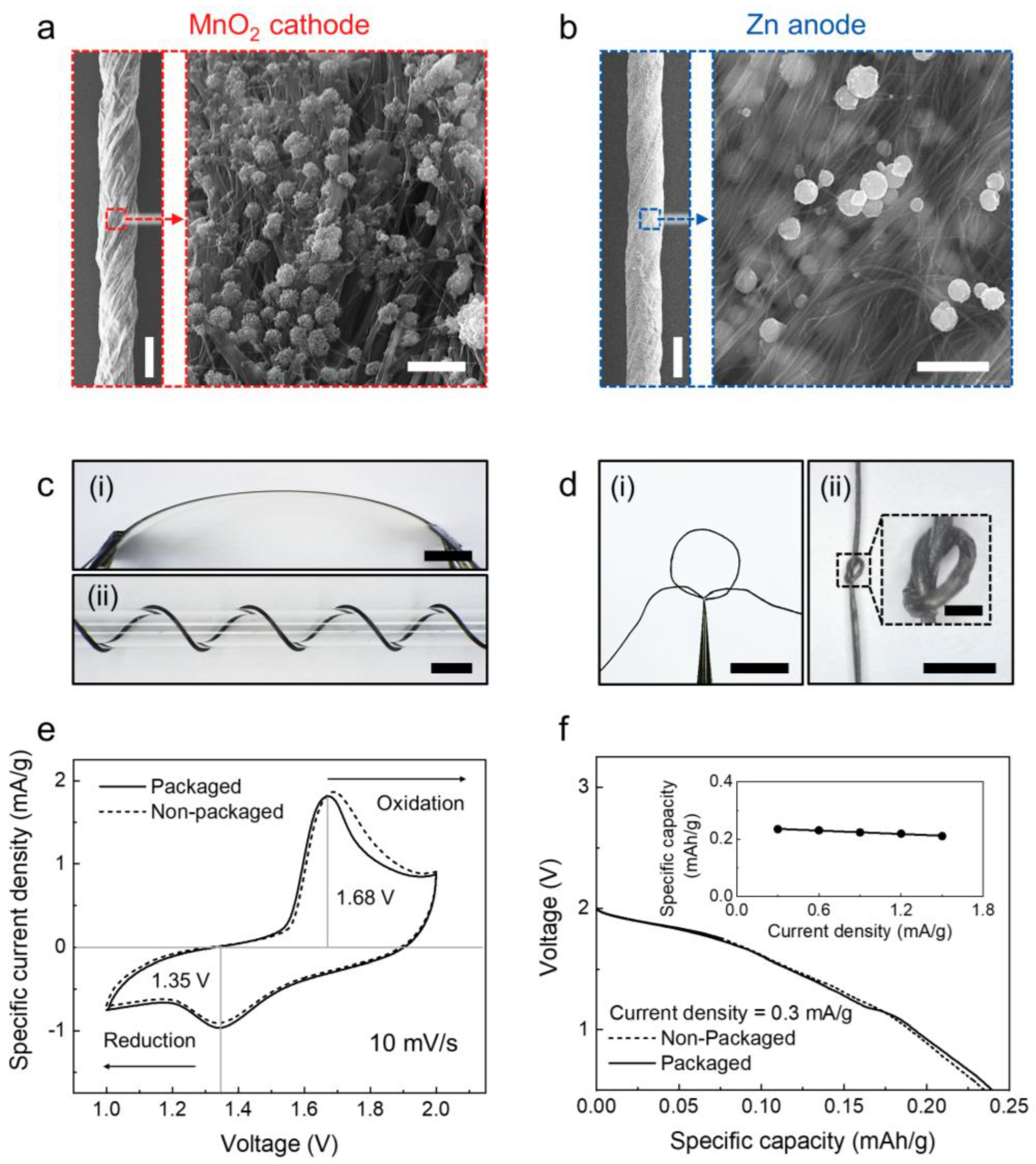
3.2. Electrochemical Performance of the Yarn and Textile MnO2−Zn Battery
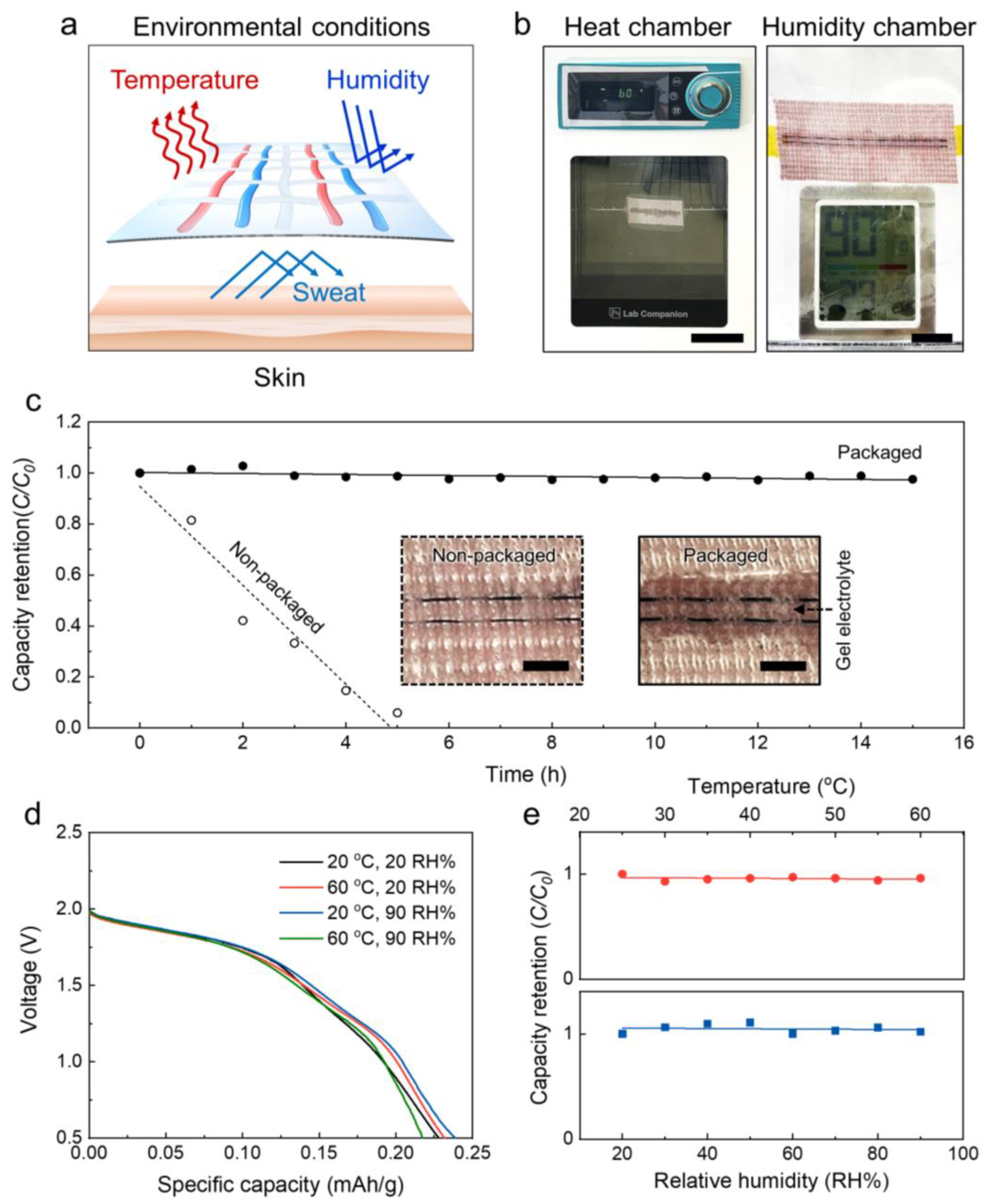
3.3. Electrochemical Performances under Various Environmental Conditions
3.4. Electrochemical Performances under Various Mechanical Deformations
3.5. Electrochemical Performances during Washing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, J.; Emadi, A. A new battery ultracapacitor hybrid energy storage system for electrichybid and plug-in hybrid electric vehicles. IEEE Trans. Power Electron. 2012, 27, 122–132. [Google Scholar]
- Erickson, E.M.; Ghanty, C.; Aurbach, D. New horizons for Conventional lituium ion battery technology. J. Phys. Chem. Lett. 2014, 5, 3313. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, H.; Zhu, M.; Huang, Y.; Tang, Z.; Pei, Z.; Wang, Z.; Shi, Z.; Liu, J.; Huang, Y.; et al. Towards wearable electronic devices: Aquasi-solid-state aqueous lithium-ion battery with outstanding stability, flexibility, safety and breathability. Nano Energy 2018, 44, 164–173. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, C.Z.; Yuan, H.; Chen, Y.; Zhang, W.; Huang, J.Q.; Yu, D.; Liu, Y.; Titirci, M.M.; Chueh, Y.L.; et al. A review of rechargeable batteries for portable electronic devices. InfoMat 2019, 1, 6–32. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, X.; Qin, R.; Liu, X.; Fang, P.; Zheng, D.; Tong, Y.; Lu, X. Dendrite-free zinc deposition induced by multifunctional CNT frameworks for stable flexible Zn-ion batteries. Adv. Mater. 2019, 31, 1903675. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kim, J.S.; Noh, J.; Lee, I.; Kim, H.J.; Choi, S.; Seo, J.; Jeon, S.; Kim, T.S.; Lee, J.Y.; et al. Wearable textile battery rechargeable by solar energy. Nano Lett. 2013, 13, 5753–5761. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, D.; Zheng, Z. Textile-Based electrochemical energy storage devices. Adv. Energy Mater. 2016, 6, 160783. [Google Scholar] [CrossRef]
- Tao, X.; Koncar, V.; Huang, T.H.; Shen, C.L.; Ko, Y.C.; Jou, G.T. How to make reliable, washable, and wearable textronic devices. Sensors 2017, 17, 673. [Google Scholar] [CrossRef]
- Wang, D.; Chang, J.; Huang, Q.; Chen, D.; Li, P.; Yu, Y.W.D.; Zheng, Z. Crumpled, high power, and safe wearable lithium-ion battery enabled by nanostructured metallic textiles. Fundam. Res. 2021, 1, 399–407. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Y.; Liu, X.; Lv, C.; Li, Y.; Wei, D.; Liu, Z. Carbon-nanomaterial-based flexible batteries for wearable electronics. Adv. Mater. 2019, 31, 1800716. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ip, W.S.; Lau, Y.Y.; Sun, J.; Zeng, J.; Yeung, N.S.S.; Ng, W.S.; Li, H.; Pei, Z.; Xue, Q.; et al. Weavable, conductive yarn-based NiCo//Zn textile battery with high energy density and rate capability. ACS Nano 2017, 11, 8953–8961. [Google Scholar] [CrossRef]
- Fan, X.; Liu, B.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Flexible and wearable power sources for next-generation wearble electronics. Batter. Supercaps 2020, 3, 1262–1274. [Google Scholar] [CrossRef]
- Wang, C.; He, T.; Cheng, J.; Guan, Q.; Wang, B. Bioinspired interface design of sewable, weavable, and washable fiber zinc batteries for wearable power textiles. Adv. Funct. Mater. 2020, 30, 2004430. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, C.; Li, Q.; Pan, Z.; Sun, J.; Zhou, Z.; He, B.; Man, P.; Xie, L.; Kang, L.; et al. Flexible and high-voltage coaxial-fiber aqueous rechargeable zinc-ion battery. Nano Lett. 2019, 19, 4035–4042. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Ju, J.; Lu, H.; Shi, X.; Wang, X.; Wang, W.; Xia, Q.; Zhou, G.; Suun, W.; Li, M.C.; et al. A weavable and scalable cotton-yarn based battery activated by battery activated by human sweat for textile electronics. Adv. Sci. 2022, 9, 2103822. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, K.; Shen, G. Flexible fiber energy storage and integrated devices: Recent progress and perspectives. Mater. Today Commun. 2015, 5, 18. [Google Scholar] [CrossRef]
- Zeng, Y.; Meng, Y.; Lai, Z.; Zhang, X.; Yu, M.; Fang, P.; Wu, M.; Tong, Y.; Lu, X. An ultrastable and high-performance flexible fiber-shaped Ni-Zn battery based on a Ni-NiO heterostructured nanosheet cathode. Adv. Mater. 2017, 29, 1702698. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Yang, K.; Fan, X.; Tong, Y.; Zhang, Z.; Lu, X.; Mai, K.; Ni, Q.; Zhang, M.; et al. Ultrahigh energy fiber-shaped supercapacitors based on porous hollow conductive polymer composite fiber electrodes. J. Mater. Chem. A 2018, 6, 12250. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, C.; Kim, J.H.; Andrade, M.J.; Baughman, R.H.; Kim, S.J. Biscrolled carbon nanotube yarn structured silver-zinc battery. Sci. Rep. 2018, 8, 11150. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Z.; Liang, G.; Huang, Y.; Zhu, M.; Pei, Z.; Xue, Q.; Tang, Z.; Wang, Y.; Li, B.; et al. Waterproof and tailorable elastic rechargeable yarn zinc ion batteries by a cross-linked polyacrylamide elctrolyte. ACS Nano. 2018, 12, 3140–3148. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, A.M.; Zamarayeva, A.M.; Rousseau, J.; Chu, H.; Derin, I.; Steingar, D.A. Highly stretchable alkaline batteries based on an embedded conductive fabric. Adv. Mater. 2012, 24, 5071–5076. [Google Scholar] [CrossRef]
- Tan, P.; Chen, B.; Xu, H.; Cai, W.; He, W.; Liu, M.; Sha, Z.; Ni, M. Co3O4 Nanosheets as active material for hybrid Zn batteries. Small 2018, 4, 1800225. [Google Scholar] [CrossRef]
- Pullanchiyodan, A.; Manjakkal, L.; Dervin, S.; Shakthivel, D.; Dahiya, R. Metal coated conductive fabrics with graphite electrodes and biocompatiable gel electrolyte for wearable supercapacitors. Adv. Mater. Technol. 2020, 5, 1901107. [Google Scholar] [CrossRef]
- Wang, L.; Pan, Y.; He, D.; Qian, L.; Cao, X.; He, B.; Li, J. Conductive polyester fabrics with high washability as electrocardiogram textile electrodes. ACS Appl. Polym. Mater. 2022, 4, 1440–1447. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, H.; Liu, H.; Nie, Z.; Xu, F.; Zhao, Y.; Zhu, J.; Huang, W. Electrolyte dynamics engineering for flexbile fiber-shaped aqueous zinc-ion battery with ultralong stability. Nano Lett. 2021, 21, 9651–9660. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.D.; Fang, S.; Lepró, X.; Lewis, C.; Ovalle-Robbes, R.; Carretero-González, J.; Castillo-Martí nez, E.; Kozlov, M.E.; Oh, J.; Rawat, N.; et al. Biscrolling nanotube sheets and functional guests into yarns. Science 2011, 331, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, S.; Deng, S.; Wu, W.; Zeng, Y.; Xia, X.; Pan, G.; Tong, Y.; Lu, X. 3D CNTs networks enable MnO2 cathodes with high capacity and superior rate capability for flexible rechargeable Zn-MnO2 batteries. Small Methods 2019, 3, 1900525. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, X.; Meng, Y.; Yu, M.; Yi, J.; Wu, Y.; Lu, X.; Tong, Y. Achieving ultrahigh energy density and long durability in a flexible rechargeable quasi-solid-state Zn-MnO2 battery. Adv. Mater. 2017, 29, 1700274. [Google Scholar] [CrossRef]
- Stoševski, I.; Bonakdarpour, A.; Cuadra., F.; Wilkinson, D.P. Highly crystalline ramsdellite as a cathode material for near-neutral aqueous MnO2/Zn batteries. Chem. Commun. 2019, 55, 2082. [Google Scholar] [CrossRef]
- Wang, X.; Wang, F.; Wang, L.; Li, M.; Wang, Y.; Chen, B.; Zhu, Y.; Fu, L.; Zha, L.; Zhang, L.; et al. An aqueous rechargeable Zn//Co3O4 battery with high energy density and good cycling behavior. Adv. Mater. 2016, 28, 4904–4911. [Google Scholar] [CrossRef]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reverisible aqueous zinc/manganese oxide energy storage from converision reaction. Nat. Energy 2016, 1, 16039–16045. [Google Scholar] [CrossRef]
- Su, S.; Yu, Y.; Wang, Y.; Wang, X.; Shi, L.; Wu, D.; Zou, P.; Nairan, A.; Lin, Z.; Kang, F.; et al. Holey nickel nanotube reticular network scaffold for high-performance flexible rechargeable Zn/MnO2 batteries. Chem. Eng. J. 2019, 370, 330–336. [Google Scholar] [CrossRef]
- Riudavets, J.; Salas, I.; Pons, M.J. Damage characteristics produced by insect pests in packaging film. J. Stored Prod. Res. 2007, 43, 564–570. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Zhang, M.; Fang, S.; Zakhidov, A.A.; Lee, S.B.; Aliev, A.E.; Williams, C.D.; Atkinson, K.R.; Baughman, R.H. Strong, tranparent, multifunctional MWNT sheets. Science 2005, 309, 1215. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, X.; Sun, X.; Li, C.; Liu, W.; Li, Q.; Ma, Y. High-performance cable-type flexible rechargeable Zn battery based on MnO2@CNT fiber microelectrode. ACS Appl. Mater. Interfaces 2018, 10, 24573–24582. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Chun, S.; Son, W.; Suh, D.; Kim, S.H.; Kim, H.; Lee, D.; Kim, Y.; Kim, Y.K.; Lim, S.K.; et al. DNA-inspired, highly packed supercoil battery for ultra-high stretchability and capacity. Nano Energy 2021, 85, 106034. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, J.H.; Oh, M.; Kang, S.; Lee, H.S.; Hong, Y.J.; Park, C.; Lee, R.; Choi, C. Wearable and Washable MnO2−Zn Battery Packaged by Vacuum Sealing. Nanomaterials 2023, 13, 265. https://doi.org/10.3390/nano13020265
Noh JH, Oh M, Kang S, Lee HS, Hong YJ, Park C, Lee R, Choi C. Wearable and Washable MnO2−Zn Battery Packaged by Vacuum Sealing. Nanomaterials. 2023; 13(2):265. https://doi.org/10.3390/nano13020265
Chicago/Turabian StyleNoh, Jun Ho, Myoungeun Oh, Sunjin Kang, Hyeong Seok Lee, Yeong Jun Hong, Chaeyeon Park, Raeyun Lee, and Changsoon Choi. 2023. "Wearable and Washable MnO2−Zn Battery Packaged by Vacuum Sealing" Nanomaterials 13, no. 2: 265. https://doi.org/10.3390/nano13020265
APA StyleNoh, J. H., Oh, M., Kang, S., Lee, H. S., Hong, Y. J., Park, C., Lee, R., & Choi, C. (2023). Wearable and Washable MnO2−Zn Battery Packaged by Vacuum Sealing. Nanomaterials, 13(2), 265. https://doi.org/10.3390/nano13020265




