Influence of Carbonization Conditions on Structural and Surface Properties of K-Doped Mo2C Catalysts for the Synthesis of Methyl Mercaptan from CO/H2/H2S
Abstract
1. Introduction
2. Experimental Section
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Catalyst Activity Test
2.3.1. Catalytic Performance Tests
2.3.2. Analysis of the Products
3. Results
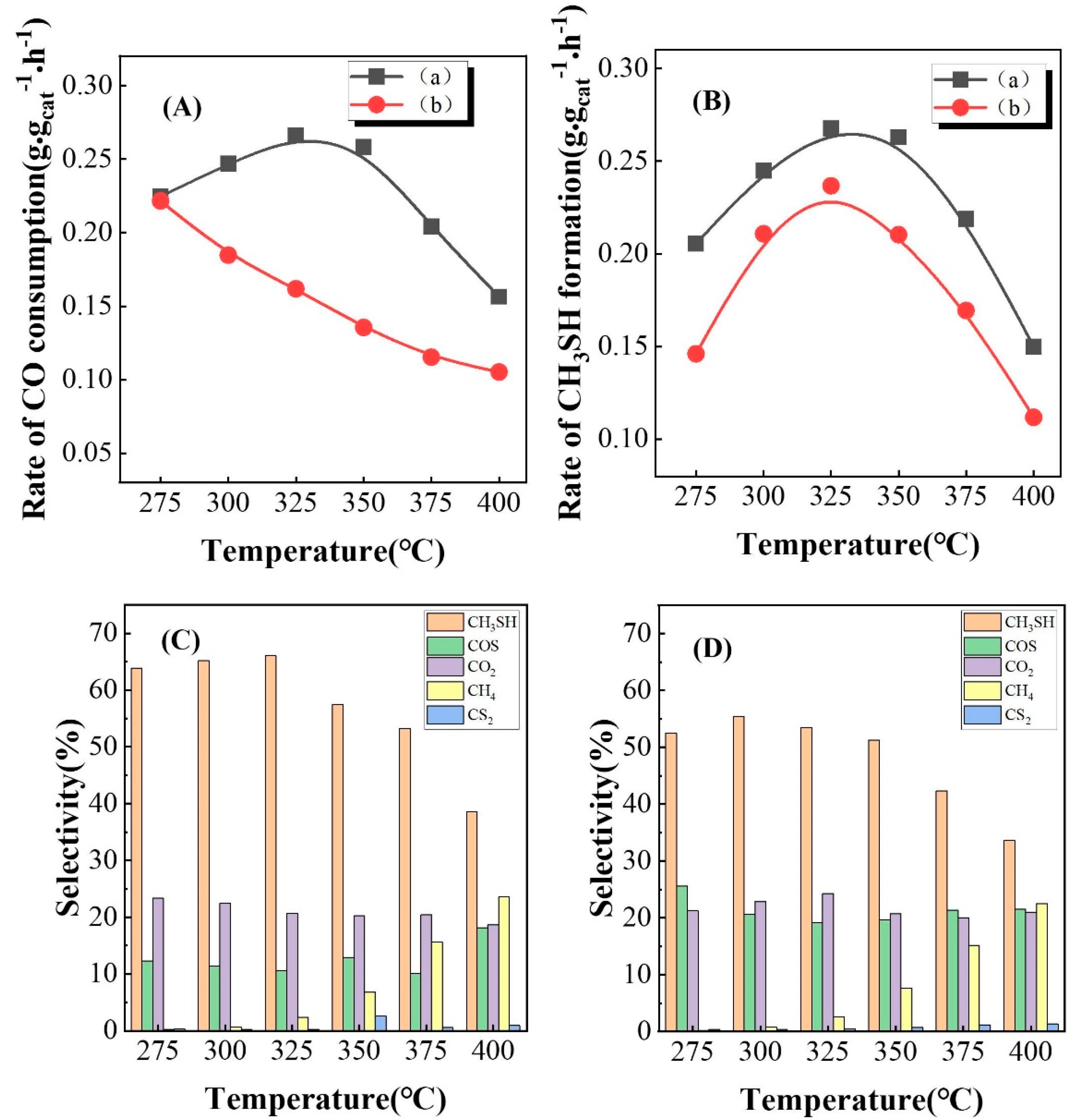
3.1. Performance of Catalysts Prepared at Different Carbonization Temperatures
3.2. The Role of Passivation
3.3. Performance of Catalysts Carbonized under Different Atmospheres
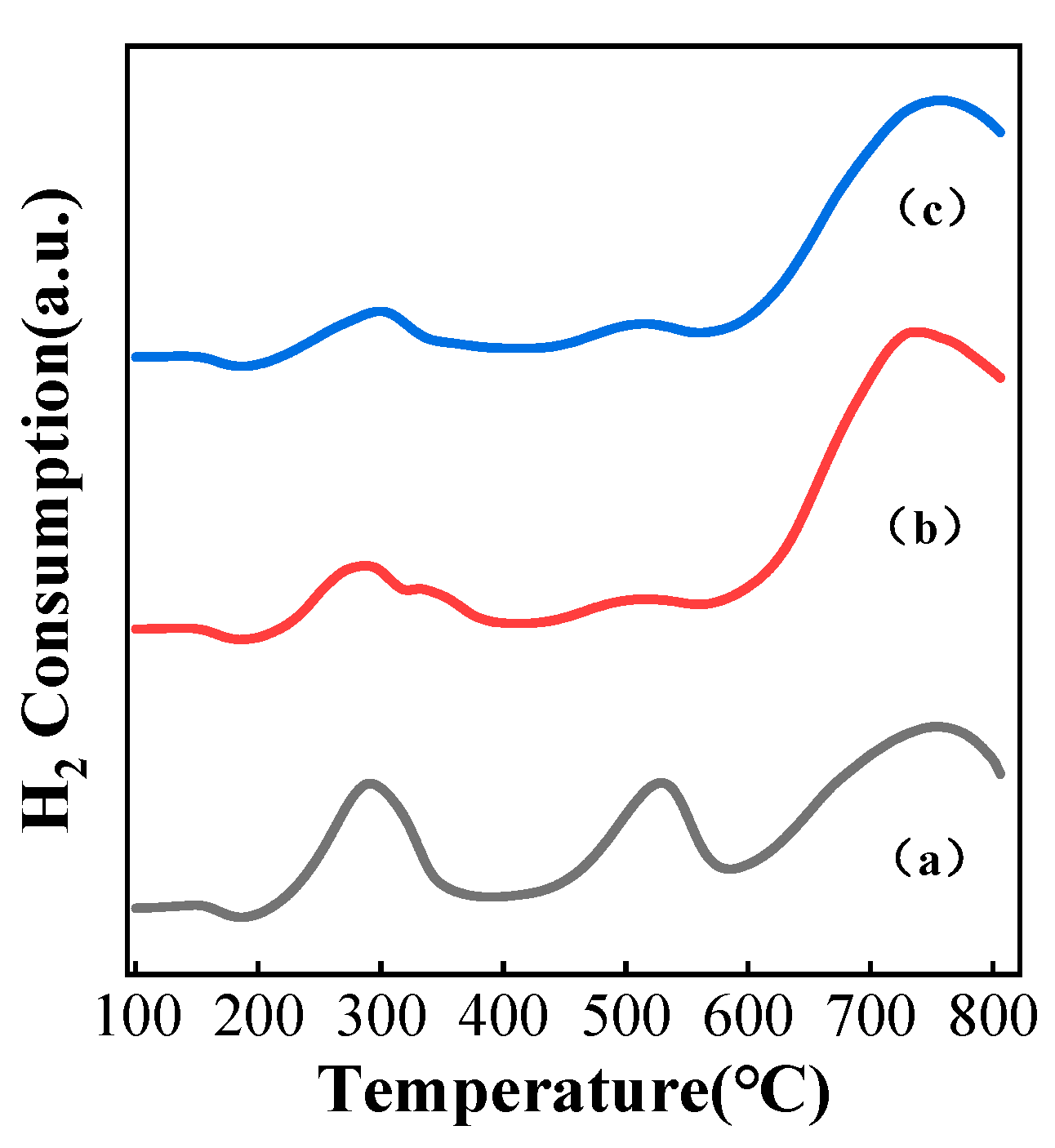
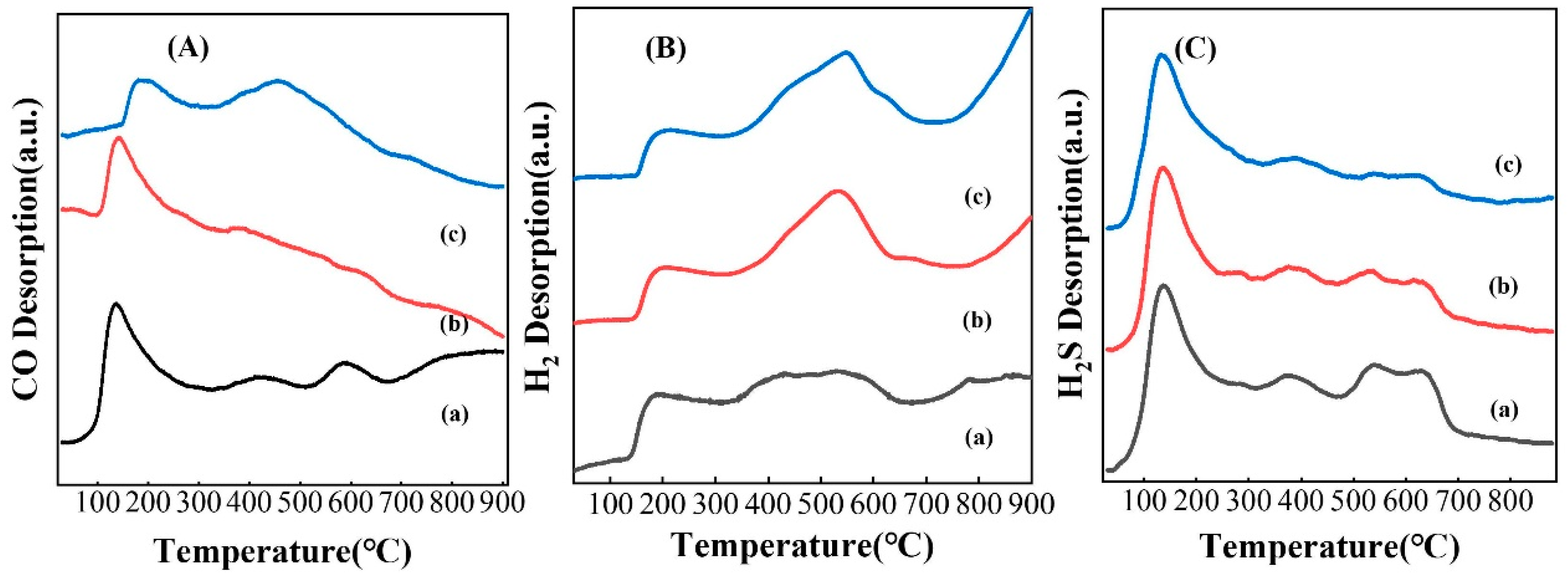
3.4. Characterization of Catalysts Carbonized under Different Atmospheres
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Midilli, A.; Kucuk, H.; Topal, M.E.; Akbulut, U.; Dincer, I. A comprehensive review on hydrogen production from coal gasification: Challenges and Opportunities. Int. J. Hydrogen Energy 2021, 46, 25385–25412. [Google Scholar] [CrossRef]
- Claure, M.T.; Chai, S.-H.; Dai, S.; Unocic, K.A.; Alamgir, F.M.; Agrawal, P.K.; Jones, C.W. Tuning of higher alcohol selectivity and productivity in CO hydrogenation reactions over K/MoS2 domains supported on mesoporous activated carbon and mixed MgAl oxide. J. Catal. 2015, 324, 88–97. [Google Scholar] [CrossRef]
- Wagner, N.J.; Coertzen, M.; Matjie, R.H.; van Dyk, J.C. Chapter 5—Coal Gasification. In Applied Coal Petrology; Suárez-Ruiz, I., Crelling, J.C., Eds.; Elsevier: Burlington, MA, USA, 2008; pp. 119–144. [Google Scholar]
- Renda, S.; Barba, D.; Palma, V. Recent Solutions for Efficient Carbonyl Sulfide Hydrolysis: A Review. Ind. Eng. Chem. Res. 2022, 61, 5685–5697. [Google Scholar] [CrossRef]
- He, B.; Xu, Z.; Cao, X.; Lu, J.; Fang, J.; Li, Y.; Feng, S.; Luo, Y. The influence of lanthanum (La) promoter on Mo/Al2O3 for the catalytic synthesis of methyl mercaptan (CH3SH) from COS/H2/H2S. Fuel Process. Technol. 2022, 238, 107488. [Google Scholar] [CrossRef]
- Lu, J.; Liu, P.; Xu, Z.; He, S.; Luo, Y. Investigation of the reaction pathway for synthesizing methyl mercaptan (CH3SH) from H2S-containing syngas over K–Mo-type materials. RSC Adv. 2018, 8, 21340–21353. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lu, J.; Xu, Z.; Liu, F.; Chen, D.; Yu, J.; Liu, J.; He, S.; Wan, G.; Luo, Y. The effect of alkali metals on the synthesis of methanethiol from CO/H2/H2S mixtures on the SBA-15 supported Mo-based catalysts. Mol. Catal. 2017, 442, 39–48. [Google Scholar] [CrossRef]
- Cordova, A.; Blanchard, P.; Lancelot, C.; Frémy, G.; Lamonier, C. Probing the Nature of the Active Phase of Molybdenum-Supported Catalysts for the Direct Synthesis of Methylmercaptan from Syngas and H2S. ACS Catal. 2015, 5, 2966–2981. [Google Scholar] [CrossRef]
- Gutiérrez, O.Y.; Kaufmann, C.; Hrabar, A.; Zhu, Y.; Lercher, J.A. Synthesis of methyl mercaptan from carbonyl sulfide over sulfide K2MoO4/SiO2. J. Catal. 2011, 280, 264–273. [Google Scholar] [CrossRef]
- Gutierrez, O.Y.; Zhong, L.; Zhu, Y.; Lercher, J.A. Synthesis of Methanethiol from CS2 on Ni-, Co-, and K-Doped MoS2/SiO2 Catalysts. Chemcatchem 2013, 5, 3249–3259. [Google Scholar] [CrossRef]
- Gutiérrez, O.Y.; Kaufmann, C.; Lercher, J.A. Synthesis of Methanethiol from Carbonyl Sulfide and Carbon Disulfide on (Co)K-Promoted Sulfide Mo/SiO2 Catalysts. ACS Catal. 2011, 1, 1595–1603. [Google Scholar] [CrossRef]
- Paskach, T.J.; Schrader, G.L.; McCarley, R.E. Synthesis of Methanethiol from Methanol over Reduced Molybdenum Sulfide Catalysts Based on the Mo6S8 Cluster. J. Catal. 2002, 211, 285–295. [Google Scholar]
- Kramer, R.L.; Reid, E.E. The catalytic preparation of mercaptans.1. J. Am. Chem. Soc. 1921, 43, 880–890. [Google Scholar] [CrossRef]
- Olin, J.F.; Bernard, B.; Goshorn, R.H.J.F. Process for Preparation of Methyl mercaptan. US Patent 3070632, 25 December 1962. [Google Scholar]
- Cordova, A.; Blanchard, P.; Salembier, H.; Lancelot, C.; Frémy, G.; Lamonier, C. Direct synthesis of methyl mercaptan from H2/CO/H2S using tungsten based supported catalysts: Investigation of the active phase. Catal. Today 2017, 292, 143–153. [Google Scholar] [CrossRef]
- Zhang, B.; Taylor, S.H.; Hutchings, G.J. Catalytic synthesis of methanethiol from CO/H2/H2S mixtures using α-Al2O3. New J. Chem. 2004, 28, 471–476. [Google Scholar] [CrossRef]
- Zhang, B.; Taylor, S.H.; Hutchings, G.J. Synthesis of Methyl Mercaptan and Thiophene from CO/H2/H2S Using α-Al2O3. Catal. Lett. 2003, 91, 181–183. [Google Scholar] [CrossRef]
- Wang, Q.; Hao, Y.; Chen, A.; Yang, Y. Effect of Thermal Treatment on Structure and Catalytic Performance of K2MoO4-NiO/SiO2 Catalyst for One-Step Synthesis of Methanethiol from High H2S-Containing Syngas. Chin. J. Catal. 2010, 31, 242–247. [Google Scholar]
- Chen, A.; Wang, Q.; Hao, Y.; Fang, W.; Yang, Y. The promoting effect of tellurium on K2MoO4/SiO2 catalyst for methanethiol synthesis from high H2S-Containing syngas. Catal. Lett. 2007, 118, 295–299. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.Q.; Dai, S.J.; Yuan, Y.Z.; Lin, R.C.; Tang, D.L.; Zhang, H.B. The promoting effects of La2O3 and CeO2 on K2MoS4/SiO2 catalyst for methanthiol synthesis from syngas blending with H2S. Appl. Catal. A Gen. 2000, 192, 175–180. [Google Scholar] [CrossRef]
- Dai, S.J.; Yang, Y.Q.; Yuan, Y.Z.; Tang, D.L.; Lin, R.C.; Zhang, H.B. On methanethiol synthesis from H2S-containing syngas over K2MoS4/SiO2 catalysts promoted with transition metal oxides. Catal. Lett. 1999, 61, 157–160. [Google Scholar] [CrossRef]
- Lu, J.; Fang, J.; Xu, Z.; He, D.; Feng, S.; Li, Y.; Wan, G.; He, S.; Wu, H.; Luo, Y. Facile synthesis of few-layer and ordered K-promoted MoS2 nanosheets supported on SBA-15 and their potential application for heterogeneous catalysis. J. Catal. 2020, 385, 107–119. [Google Scholar] [CrossRef]
- Yu, M.; Chang, M.-W.; Kosinov, N.; Hensen, E.J.M. Alkali catalyzes methanethiol synthesis from CO and H2S. J. Catal. 2022, 405, 116–128. [Google Scholar] [CrossRef]
- Hua, W.; Sun, H.-H.; Xu, F.; Wang, J.-G. A review and perspective on molybdenum-based electrocatalysts for hydrogen evolution reaction. Rare Met. 2020, 39, 335–351. [Google Scholar] [CrossRef]
- Alexander, A.-M.; Hargreaves, J.S.J. Alternative catalytic materials: Carbides, nitrides, phosphides and amorphous boron alloys. Chem. Soc. Rev. 2010, 39, 4388–4401. [Google Scholar] [CrossRef]
- Hwu, H.H.; Chen, J.G.G. Surface chemistry of transition metal carbides. Chem. Rev. 2005, 105, 185–212. [Google Scholar] [CrossRef]
- Xiao, T.-C.; York, A.P.E.; Al-Megren, H.; Williams, C.V.; Wang, H.-T.; Green, M.L.H. Preparation and Characterisation of Bimetallic Cobalt and Molybdenum Carbides. J. Catal. 2001, 202, 100–109. [Google Scholar] [CrossRef]
- Schaidle, J.A.; Lausche, A.C.; Thompson, L.T. Effects of sulfur on Mo2C and Pt/Mo2C catalysts: Water gas shift reaction. J. Catal. 2010, 272, 235–245. [Google Scholar] [CrossRef]
- Lewandowski, M.; Szymańska-Kolasa, A.; Da Costa, P.; Sayag, C. Catalytic performances of platinum doped molybdenum carbide for simultaneous hydrodenitrogenation and hydrodesulfurization. Catal. Today 2007, 119, 31–34. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, C.; Chen, B.; Zhang, Y.; Zhu, Y.; Qiu, J.; Au, C. Catalytic role of β-Mo2C in DRM catalysts that contain Ni and Mo. Catal. Today 2015, 258, 676–683. [Google Scholar] [CrossRef]
- Zhang, A.; Zhu, A.; Chen, B.; Zhang, S.; Au, C.; Shi, C. In-situ synthesis of nickel modified molybdenum carbide catalyst for dry reforming of methane. Catal. Commun. 2011, 12, 803–807. [Google Scholar] [CrossRef]
- Giordano, C.; Antonietti, M. Synthesis of crystalline metal nitride and metal carbide nanostructures by sol–gel chemistry. Nano Today 2011, 6, 366–380. [Google Scholar] [CrossRef]
- Giordano, C.; Erpen, C.; Yao, W.; Antonietti, M. Synthesis of Mo and W Carbide and Nitride Nanoparticles via a Simple “Urea Glass” Route. Nano Lett. 2008, 8, 4659–4663. [Google Scholar] [CrossRef]
- Gu, Y.; Li, Z.; Chen, L.; Ying, Y.; Qian, Y. Synthesis of nanocrystalline Mo2C via sodium co-reduction of MoCl5 and CBr4 in benzene. Mater. Res. Bull. 2003, 38, 1119–1122. [Google Scholar] [CrossRef]
- Liao, L.; Wang, S.; Xiao, J.; Bian, X.; Zhang, Y.; Scanlon, M.D.; Hu, X.; Tang, Y.; Liu, B.; Girault, H.H. A nanoporous molybdenum carbide nanowire as an electrocatalyst for hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 387–392. [Google Scholar] [CrossRef]
- Morishita, T.; Soneda, Y.; Hatori, H.; Inagaki, M. Carbon-coated tungsten and molybdenum carbides for electrode of electrochemical capacitor. Electrochim. Acta 2007, 52, 2478–2484. [Google Scholar] [CrossRef]
- Nartowski, A.M.; Parkin, I.P.; Mackenzie, M.; Craven, A.J. Solid state metathesis: Synthesis of metal carbides from metal oxides. J. Mater. Chem. 2001, 11, 3116–3119. [Google Scholar] [CrossRef]
- Lin, Z.; Wan, W.; Yao, S.; Chen, J.G. Cobalt-modified molybdenum carbide as a selective catalyst for hydrodeoxygenation of furfural. Appl. Catal. B Environ. 2018, 233, 160–166. [Google Scholar] [CrossRef]
- Liu, X.; Kunkel, C.; Ramírez de la Piscina, P.; Homs, N.; Viñes, F.; Illas, F. Effective and Highly Selective CO Generation from CO2 Using a Polycrystalline α-Mo2C Catalyst. ACS Catal. 2017, 7, 4323–4335. [Google Scholar] [CrossRef]
- Sullivan, M.M.; Held, J.T.; Bhan, A. Structure and site evolution of molybdenum carbide catalysts upon exposure to oxygen. J. Catal. 2015, 326, 82–91. [Google Scholar] [CrossRef]
- Vitale, G.; Frauwallner, M.L.; Hernandez, E.; Scott, C.E.; Pereira-Almao, P. Low temperature synthesis of cubic molybdenum carbide catalysts via pressure induced crystallographic orientation of MoO3 precursor. Appl. Catal. A Gen. 2011, 400, 221–229. [Google Scholar] [CrossRef]
- Xiao, T.-C.; York, A.P.E.; Williams, V.C.; Al-Megren, H.; Hanif, A.; Zhou, X.-Y.; Green, M.L.H. Preparation of Molybdenum Carbides Using Butane and Their Catalytic Performance. Chem. Mater. 2000, 12, 3896–3905. [Google Scholar] [CrossRef]
- Pérez-Martínez, D.J.; Eloy, P.; Gaigneaux, E.M.; Giraldo, S.A.; Centeno, A. Study of the selectivity in FCC naphtha hydrotreating by modifying the acid–base balance of CoMo/γ-Al2O3 catalysts. Appl. Catal. A Gen. 2010, 390, 59–70. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, Y.; Chen, A.; Fang, W.; Yang, Y. Study on Methanethiol Synthesis from H2S-Rich Syngas Over K2MoO4 Catalyst Supported on Electrolessly Ni-Plated SiO2. Catal. Lett. 2009, 129, 486–492. [Google Scholar] [CrossRef]
- Blanchard, P.; Lamonier, C.; Griboval, A.; Payen, E. New insight in the preparation of alumina supported hydrotreatment oxidic precursors: A molecular approach. Appl. Catal. A Gen. 2007, 322, 33–45. [Google Scholar] [CrossRef]
- Le Bihan, L.; Blanchard, P.; Fournier, M.; Grimblot, J.; Payen, E. Raman spectroscopic evidence for the existence of 6-molybdoaluminate entities on an Mo/Al2O3 oxidic precursor. J. Chem. Soc. Faraday Trans. 1998, 94, 937–940. [Google Scholar] [CrossRef]
- Carrier, X.; Lambert, J.F.; Che, M. Ligand-Promoted Alumina Dissolution in the Preparation of MoOX/γ-Al2O3 Catalysts: Evidence for the Formation and Deposition of an Anderson-type Alumino Heteropolymolybdate. J. Am. Chem. Soc. 1997, 119, 10137–10146. [Google Scholar] [CrossRef]
- Hussain, S.; Zaidi, S.A.; Vikraman, D.; Kim, H.-S.; Jung, J. Facile preparation of molybdenum carbide (Mo2C) nanoparticles and its effective utilization in electrochemical sensing of folic acid via imprinting. Biosens. Bioelectron. 2019, 140, 111330. [Google Scholar] [CrossRef]
- Huang, Y.; Gong, Q.; Song, X.; Feng, K.; Nie, K.; Zhao, F.; Wang, Y.; Zeng, M.; Zhong, J.; Li, Y. Mo2C Nanoparticles Dispersed on Hierarchical Carbon Microflowers for Efficient Electrocatalytic Hydrogen Evolution. ACS Nano 2016, 10, 11337–11343. [Google Scholar] [CrossRef]
- Pang, M.; Wang, X.; Xia, W.; Muhler, M.; Liang, C. Mo(VI)–Melamine Hybrid As Single-Source Precursor to Pure-Phase β-Mo2C for the Selective Hydrogenation of Naphthalene to Tetralin. Ind. Eng. Chem. Res. 2013, 52, 4564–4571. [Google Scholar] [CrossRef]
- Malaibari, Z.O.; Croiset, E.; Amin, A.; Epling, W. Effect of interactions between Ni and Mo on catalytic properties of a bimetallic Ni-Mo/Al2O3 propane reforming catalyst. Appl. Catal. A Gen. 2015, 490, 80–92. [Google Scholar] [CrossRef]
- Wang, G.; Schaidle, J.A.; Katz, M.B.; Li, Y.; Pan, X.; Thompson, L.T. Alumina supported Pt–Mo2C catalysts for the water–gas shift reaction. J. Catal. 2013, 304, 92–99. [Google Scholar] [CrossRef]
- Tian, R.; Lu, J.; Xu, Z.; Zhang, W.; Liu, J.; Wang, L.; Xie, Y.; Zhao, Y.; Cao, X.; Luo, Y. Unraveling the Synergistic Reaction and the Deactivation Mechanism for the Catalytic Degradation of Double Components of Sulfur-Containing VOCs over ZSM-5-Based Materials. Environ. Sci. Technol. 2023, 57, 1443–1455. [Google Scholar] [CrossRef]
- Lu, J.; Tian, R.; Zhang, W.; Zhang, Y.; Yang, Y.; Xu, Z.; He, D.; Ai, T.; Luo, Y. An ultra-long stability of lanthanum (La) modified molecular sieve for catalytic degradation of typical sulfur-containing VOCs in a near-real environment. Appl. Catal. B Environ. 2023, 339, 123114. [Google Scholar] [CrossRef]
- Fang, J.; Lu, J.; Xu, Z.; Feng, S.; Li, Y.; He, B.; Luo, M.; Wang, H.; Luo, Y. Modulation the metal-support interactions of potassium molybdenum-based catalysts for tuned catalytic performance of synthesizing CH3SH. Sep. Purif. Technol. 2023, 316, 123815. [Google Scholar] [CrossRef]


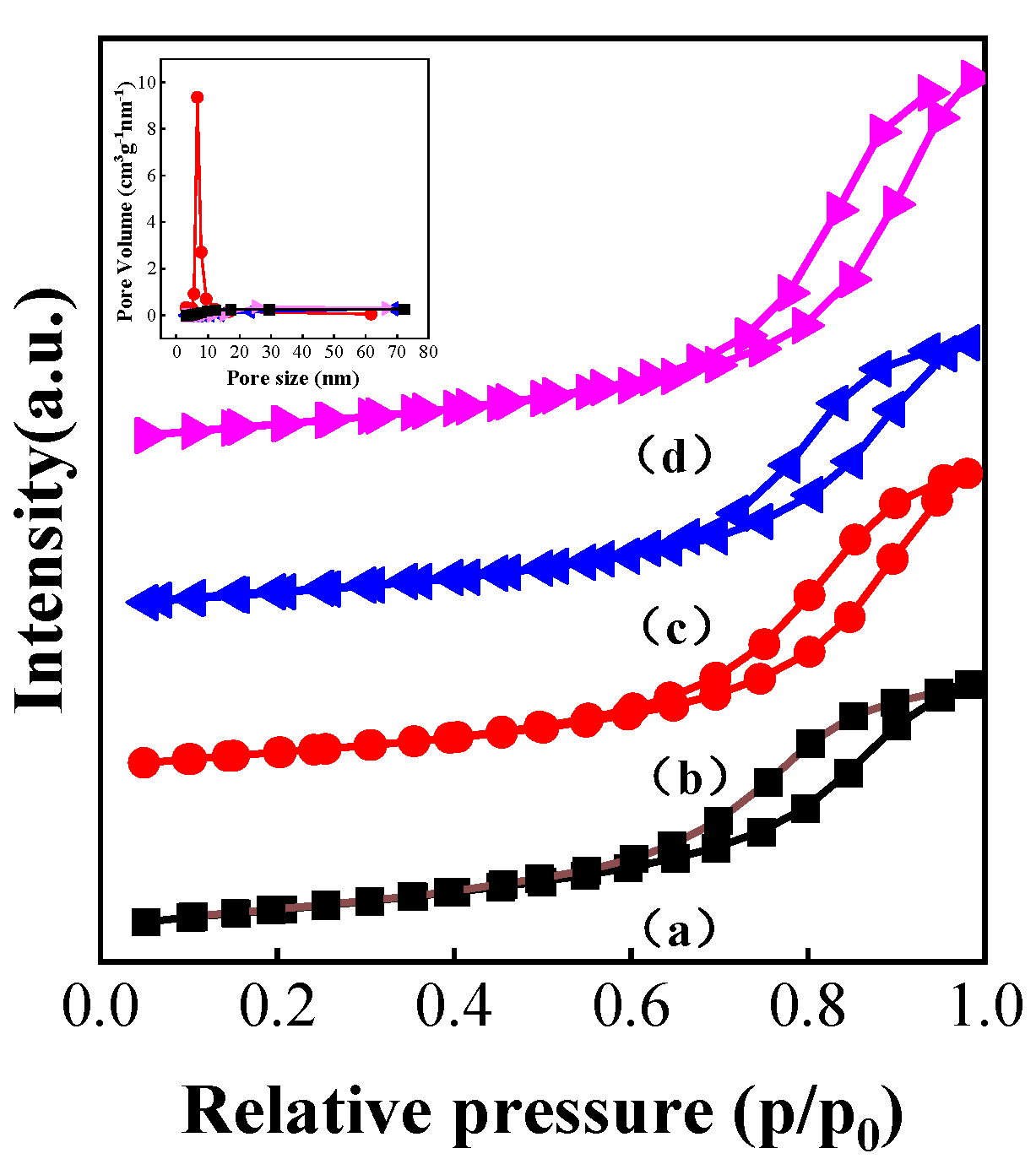

| Sample | Surface Area (m2/g) | Pore Volume (cc/g) | Pore Diameter Dv (d) (nm) |
|---|---|---|---|
| K-Mo/Al2O3 | 116.1 | 0.269 | 7.853 |
| CH4-K-Mo2C/Al2O3 | 103.9 | 0.321 | 9.618 |
| C2H6-K-Mo2C/Al2O3 | 101.3 | 0.290 | 8.598 |
| C3H8-K-Mo2C/Al2O3 | 113.4 | 0.390 | 11.159 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Ai, T.; Hu, Y.; Xu, Z.; Li, Y.; Jiang, H.; Luo, Y. Influence of Carbonization Conditions on Structural and Surface Properties of K-Doped Mo2C Catalysts for the Synthesis of Methyl Mercaptan from CO/H2/H2S. Nanomaterials 2023, 13, 2602. https://doi.org/10.3390/nano13182602
Zheng X, Ai T, Hu Y, Xu Z, Li Y, Jiang H, Luo Y. Influence of Carbonization Conditions on Structural and Surface Properties of K-Doped Mo2C Catalysts for the Synthesis of Methyl Mercaptan from CO/H2/H2S. Nanomaterials. 2023; 13(18):2602. https://doi.org/10.3390/nano13182602
Chicago/Turabian StyleZheng, Xiangqian, Tianhao Ai, Yuhong Hu, Zhizhi Xu, Yubei Li, Huan Jiang, and Yongming Luo. 2023. "Influence of Carbonization Conditions on Structural and Surface Properties of K-Doped Mo2C Catalysts for the Synthesis of Methyl Mercaptan from CO/H2/H2S" Nanomaterials 13, no. 18: 2602. https://doi.org/10.3390/nano13182602
APA StyleZheng, X., Ai, T., Hu, Y., Xu, Z., Li, Y., Jiang, H., & Luo, Y. (2023). Influence of Carbonization Conditions on Structural and Surface Properties of K-Doped Mo2C Catalysts for the Synthesis of Methyl Mercaptan from CO/H2/H2S. Nanomaterials, 13(18), 2602. https://doi.org/10.3390/nano13182602