Abstract
The increasing use of natural gas as an efficient, reliable, affordable, and cleaner energy source, compared with other fossil fuels, has brought the catalytic CH4 complete oxidation reaction into the spotlight as a simple and economic way to control the amount of unconverted methane escaping into the atmosphere. CH4 emissions are a major contributor to the ‘greenhouse effect’, and therefore, they need to be effectively reduced. Catalytic CH4 oxidation is a promising method that can be used for this purpose. Detailed studies of the activity, oxidative thermal aging, and the time-on-stream (TOS) stability of pristine La1−xSrxMnO3 perovskites (LSXM; X = % substitution of La with Sr = 0, 30, 50 and 70%) and iridium-loaded Ir/La1−xSrxMnO3 (Ir/LSXM) perovskite catalysts were conducted in a temperature range of 400–970 °C to achieve complete methane oxidation under excess oxygen (lean) conditions. The effect of X on the properties of the perovskites, and thus, their catalytic performance during heating/cooling cycles, was studied using samples that were subjected to various pretreatment conditions in order to gain an in-depth understanding of the structure–activity/stability correlations. Large (up to ca. 300 °C in terms of T50) inverted volcano-type differences in catalytic activity were found as a function of X, with the most active catalysts being those where X = 0%, and the least active were those where X = 50%. Inverse hysteresis phenomena (steady-state rate multiplicities) were revealed in heating/cooling cycles under reaction conditions, the occurrence of which was found to depend strongly on the employed catalyst pre-treatment (pre-reduction or pre-oxidation), while their shape and the loop amplitude were found to depend on X and the presence of Ir. All findings were consistently interpreted, which involved a two-term mechanistic model that utilized the synergy of Eley–Rideal and Mars–van Krevelen kinetics.
1. Introduction
The replacement of traditional fossil fuels with cleaner and/or sustainable energy sources is currently imperative due to the recent global energy and environmental crisis. Regarding the global transition toward so-called “low carbon footprint energy technologies” and successful sustainability, humanity has exhibited an ever-increasing dependence upon natural gas (NG); gas is widely considered to be the ‘bridge fuel’ during this transition period [1,2,3,4]. NG typically contains ~85–95% methane [4,5,6]. The high efficiency of methane as a fuel, as well as the associated low emissions of NOx, CO, and particulate pollutants when producing thermal energy in urban tissues and industries, or when producing mechanical energy for vehicles, make it a greener alternative compared with other fossil fuels; this is because it produces reliable and affordable energy, and it promotes the development of other “green” energy sources [4]. In addition, its use in added-value chemical production [7,8,9], synthesis gas and/or H2 production (with the simultaneous recycling of CO2 emissions via dry reforming processes [10,11,12,13,14,15]), as well as its direct (internal) electrochemical conversion which produces electricity using solid oxide fuel cells (SOFCs) [16,17,18,19,20], are some additional important applications of CH4 in cleaner energy production technologies. However, as the use of methane in this plethora of applications expands, so do the emissions of unconverted methane, most of which have a low methane content of 0.1–1.0% [21,22,23,24,25,26,27,28]. Such emissions must be urgently and effectively mitigated given that CH4 is one of the main greenhouse gases; it has a greenhouse efficiency that is 25 times greater than CO2, otherwise, the climate benefits of its use could be offset [1,2,3].
As CH4 contains a very strong C–H bond (450 kJ/mol), its thermal combustion with air requires very high temperatures (ca. 1500 °C) compared with other HCs. Moreover, it simultaneously produces higher amounts of NOx as by-products of the oxidation of N2 (found in the air) in the burners operating at such high temperatures [4,5]. Therefore, the catalytic oxidation of methane is considered advantageous for controlling its end-of-pipe emissions due to the relatively low operating temperatures required; this is associated with minimal NOx emissions. However, it is still true that the strong C–H bond in the methane molecule makes its catalytic activation, and thus its oxidation, relatively more difficult compared with other hydrocarbons. In recent years, various families of materials have been proposed as catalysts for the deep (complete) oxidation of methane, emphasizing on the activity and thermal stability of the materials, as well as their dependence on the chemical composition of the materials and the preparation method used [4,5,21,22,23,24,25,26,27,28].
In this regard, catalysts based on noble metal (NM) nanoparticles that are dispersed on oxide or mixed oxide supports are typically more active than other families of materials, but they have the disadvantages of being priced at a high cost, and they tend to gradual deactivate during operation due to the aggregation of the noble metal nanoparticles [22,27,28]. Among NMs, iridium has shown quite an encouraging performance in emission control catalysis, which involves the oxidation of CO and volatile organic compounds (VOCs), de-NOx, and de-N2O reactions [29,30,31,32,33,34], as well as in CH4 reforming reactions [14,15,35,36], but their application in real systems is limited due to the high aggregation propensity of Ir nanoparticles under oxidizing conditions at elevated temperatures [37,38,39]. However, we have recently demonstrated that sensitive to agglomeration metal nanoparticles can be effectively stabilized through metal-support interactions induced by supports possessing a high oxygen storage capacity (OSC) and high oxygen ions mobility (labile lattice oxygen) [39,40,41]. A model that could consistently interpret these findings was developed. It was based on the effect of thermally driven O2− ions moving from the supports onto catalyst NPs in order to create an Oδ− layer on their surfaces. Then, as a protective coat, it resists the main particle agglomeration mechanisms, namely, the Particle Migration and Coalescence (PMC) and Ostwald Ripening (OR) mechanisms [40,41].
On the other hand, mixed metal oxides, especially those of the perovskite class (ABO3), combine high thermal and chemical stabilities with the adequate activity of oxidation reactions, while keeping their cost relatively low [42,43,44,45,46]. Perovskites based on the combination of La and Mn in the A and B sites, respectively, are among the most popular materials in the family. The partial substitution of La3+ by Sr2+ in the perovskite structure (i.e., La1−xSrxMnO3) can enhance the redox properties of the material by increasing the oxygen vacancies and the oxidation state of the B cation (Mn), introducing significant changes to their catalytic performance and their thermal stability [42,43,44,45,46,47,48,49]. Especially after the inspiring discovery of Nishihata et al. [50] and Tanaka et al. [51], who demonstrated the self-regenerative function of noble metal substituted perovskites, a phenomenon that was later called “redox exclusion” [52,53], as well as methods that determine the nanostructure of the perovskite itself, thus improving the surface area and other catalysis-related properties [54,55], new horizons were opened for the use of perovskites in various environmental- [54,56,57,58,59] and energy- [55,60,61,62,63,64] related catalytic processes, which resulted in great success.
However, the classical partial substitution of the A and/or B sites of an ABO3 perovskite with other cations, with the same or different valences, to obtain substituted (as called) A1−yA’yB1−xB’xO3±δ perovskites, remains a popular methodology for controlling the performance of perovskites due to its high flexibility. The bulk, surface, and redox properties of the original ABO3 perovskite can be easily tailored on demand [43,44,45,46,47]. Emphasis is always placed on the oxygen storage capacity (OSC), oxygen ion mobility, and population of the surface oxygen vacancies (O defects) of the substituted perovskites; these properties play a key role in catalysis via oxides [56,57,58,59,60,61,62,63,64,65,66,67], as well as in catalysis via dispersed metal nanoparticles on oxide supports [13,14,15,68,69,70,71,72,73,74,75]. Regarding the latter, these properties are critical for the development of desirable metal–support interactions. It is therefore obvious why perovskites are a class of materials that attract high and ever-increasing interest with regard to heterogeneous catalysis; they are either used as pristine materials, exploiting their own advantageous characteristics for catalysis, or as effective supports for metal nanoparticles, as they endow them with favorable metal-support interactions.
In a previous study, the effect of the degree of the substitution of La by Sr on La1−xSrxMnO3 perovskite and their counterpart, iridium-loaded catalysts (Ir/La1−xSrxMnO3), was studied in CO oxidation with excess O2 reaction [30]. Both catalyst series were found to be effective in the reaction, with the Ir/La1−xSrxMnO3 catalysts significantly outperforming their pristine La1−xSrxMnO3 counterparts. Interesting inverse hysteresis phenomena were observed during heating/cooling cycles, depending on both the degree of substitutional Sr (x), and the pretreatment (pre-oxidation/pre-reduction) of the catalysts. The results were part of a project to decipher the effectiveness of perovskite-based catalysts, in combination with the relatively cheap noble metal, Ir, in reactions related to the control of NG-powered vehicle emissions. Traffic caused by NG-fueled vehicles is increasing rapidly (ca. 23 million worldwide, ranging from heavy-duty to light-duty cars, with an annual increase of 20%) [4]. Catalysis of CH4 combustion is also of particular interest because unburnt CH4 also exists in the exhaust stream of NG-powered engines and processes [21,22,23,24,25,26,27,28,64,65,66,67,68]. Complete methane combustion is also a useful side process in the very demanding application of natural-gas (NG) fueled gas-turbines (GT), as they operate alongside the catalytically stabilized hybrid combustion concept [76].
In the present work, lean CH4 combustion on pristine La1−xSrxMnO3 (LSXM; X = 0, 30, 50 and 70% substitution of La with Sr) perovskites and their 2 wt% iridium loaded counterparts (Ir/LSXM) is comparatively studied in a temperature range of 400–970 °C. The impact of the degree of substitution of the A-site (La) of the perovskite with Sr, on its catalytic behavior is explored. This occurs after an exploration into the catalysts’ various pre-treatment protocols, such as pre-reduction, pre-oxidation, and thermal aging, which were implemented to obtain a complete overview of their CH4 combustion performance and how it correlates with the morphological characteristics and properties of the materials. Complex hysteresis phenomena recorded for the first time using this catalyst/reaction system during heating/cooling cycles were therefore consistently interpreted. Although LSM perovskites are possibly among the most studied materials for their catalytic performance in various reactions, not excluding some studies concerning the oxidation of CH4, the highly systematic activity and stability studies performed herein revealed new phenomena and findings that could be both of specific and general interest for catalysis research.
2. Materials and Methods
2.1. LSxM and Ir/LSxM Catalysts Synthesis
The unloaded and 2 wt% Ir loaded perovskite-type La1−xSrxMnO3 (denoted hereafter as LSxM, where x = 0, 30, 50 and 70 expresses the % replacement of La with Sr in the perovskite formula) catalysts were prepared using the co-precipitation method described by Haron et al. [77]. The nitrate salts, La(NO3)3∙6H2O (VWR Chemicals, 99.9%), Sr(NO3)2 (Sigma Aldrich, St. Louis, MO, USA, 99.0%) and Mn(NO3)2∙6H2O (Panreac, Darmstadt, Germany, 98.0%), were used as metal precursors. In brief, appropriate amounts of the nitrate salts were diluted in distilled water and added to a NaOH (VWR Chemicals, Radnor, PA, USA, 98.9%) precipitating agent solution for co-precipitation. The obtained suspension was filtered, washed, dried, de-agglomerated, and finally calcined in air at 1000 °C for 6 h for the final perovskite structure to be obtained. A series of LS00M, LS30M, LS50M and LS70M perovskites were produced.
Half of each perovskite was then impregnated under continuous stirring conditions at 75 °C in an appropriate amount of aqueous solution comprising IrCl3·H2O (Abcr GmbH & Co KG) with 2 mg Ir/mL in order to achieve the corresponding 2 wt% Ir/LSXM catalyst series. After the water evaporated, the obtained suspensions were dried at 110 °C for 12 h; then, they were subjected to reduction at 400 °C for 3 h in a 50 mL/min flow of 25% H2/He to remove residual chlorine and to avoid the formation of large Ir crystallites [32,33]. The as-prepared LSXM and Ir/LSXM catalyst series are listed in Table 1.

Table 1.
The LSΧM (Χ = % substitution of La with Sr) and 2wt% Ir/LSΧM series of catalysts studied. A summary of their chemical formulas, textural, morphological, and oxygen storage capacity characteristics [30].
2.2. Catalyst Characterization Methods
The textural and structural characteristics and redox properties of the LSΧM and Ir/LSΧM catalysts were determined using the N2 physical adsorption BET–BJH method, powder X-ray diffraction (XRD), isothermal pulse H2-chemisorption (H2-Chem.), and Temperature programmed reduction using hydrogen (H2-TPR).
More specifically, the Brunauer–Emmett-Teller (BET) method and Barrett–Joyner–Halenda (BJH) model were used to analyze N2 adsorption–desorption isotherms obtained at −196 °C, and relative pressures in the range of 0.05–0.3, using a Quantachrome Nova 2200 e instrument. Subsequently, 150 mg of the sample placed in the instrument holder was degassed for 12 h using a vacuum at 350 °C, prior to measurements. The total specific surface area (SBET), the pore volume, and the average pore size diameter of the materials were obtained.
The powder X-ray diffraction (PXRD) patterns of pre-oxidized samples (calcinated in the air at 400 °C for 1 h before the XRD measurements) were collected with a BrukerAXS D8 Advance diffractometer operating at 35 kV and 35 mA, using Cu Kα radiation and a LynxEye detector with a Ni-filter, to determine the crystalline structure of the materials. Measurements were carried out in a 2θ angle range of 4–70 degrees, with a scanning speed of 0.5 degrees per min. The identification and quantification of the phases were performed using BrukerAXS Topas software (COD, Crystallography Open Database) and the Rietveld method.
Pulse hydrogen chemisorption (H2-chemisorption) experiments were performed at 25 °C on a Quantachrome/ChemBet Pulsar TPR/TPD instrument equipped with an Omnistar/Pfeiffer Vacuum mass spectrometer to determine iridium dispersion and mean iridium particle size. An amount of 150 mg of the material was loaded on the instrument holder, which was pretreated with a 5% H2/He mixture (15 mL/min) at 550 °C for 1 h. Then, it was flushed with N2 (15 mL/min) at the same temperature for 30 min, and cooled to 25 °C using a N2 flow, before consecutive pulse injections of pure hydrogen (280 µL H2 per pulse) were imposed until saturation was reached. This was carried out in order to measure the total H2-uptake (Vchem). The Vchem values were then used to calculate iridium dispersion, DIr (dimensionless, H/Ir ratio), and mean Ir crystallite size, dIr (in nm) via the following set of Equations (1) and (2) [15,30]:
where, VChem (mL/g) is the H2-uptake in the chemisorption experiment, Fs is the hydrogen to metal correlation factor (=2 assuming the one-to-one correlation of adsorbed H atoms with metal sites (i.e., H–Ir)), AIr is the atomic weight of iridium (192.22 g/mol), Vmol is the molar volume of an ideal gas at room temperature and 1 atm pressure (ca. 24,450 mL/mol), XIr is the iridium content of the catalyst (gIr/gcat), ρIr is the Ir metal density (22.5 g/mL), αIr is the cross-sectioned area of the Ir atom (0.12 nm2/atom), NAV = 6.023 × 1023 molecules/mol is the Avogadro number, and 1020 is a unit conversion factor when the units of the parameters in Equations (1) and (2) are used as indicated above.
Temperature-programmed reduction (H2-TPR) was performed using the same instrumentation for H2-chemisorption experiments in order to obtain the reducibility characteristics and determine the total oxygen storage capacity (t-OSC) of the materials. The samples (150 mg) were oxidized in situ at 750 °C for 30 min (20% O2/He flow), then, they were cooled to 25 °C using the same flow, purged for 10 min with He flow. Next, the TPR experiment was performed with a linear (10 °C/min) increase in temperature, up to 750 °C, using 15 mL/min of 1% H2/He flow. The time integral of the H2-TPR profile determines the total oxygen storage capacity (t-OSC) of the material [13,15,30].
2.3. Catalytic Activity and Stability Evaluation Experiments
The catalytic activity and thermal stability experiments were performed in a continuous flow experimental apparatus (Figure S1) consisting of the following: (i) a feed unit utilizing MKS-247 mass flow meters, (ii) a reactor unit with a tubular fixed-bed type reactor (quartz, ID = 3 mm), and (iii) an analysis unit equipped with online gas chromatography (SHIMADZU GC-14B, a thermal conductivity detector, He carrier gas, Porapak-N, and Molecular Sieve 5A columns connected in parallel). The reactor was loaded with mcat = 50 mg of a catalyst in the form of a powder (particle size 180–250 μm). A K-thermocouple, centered in the catalyst bed, was used to measure the reaction temperature. The volume of the catalytic bed was ca. 0.03 cm3.
Catalysts were comparatively evaluated for the CH4 combustion reaction under conditions of excess O2 (1% CH4 + 5% O2, balance He at 1 bar) at a weight-basis Gas Hourly Space Velocity (WGHSV) equal to 90,000 mL/g∙h (total flowrate FT = 75 mL/min), in the temperature range of 400–970 °C; this corresponds with the operating temperature window of vehicle emission control systems (in particular, of close-coupled catalytic converters). The residence time of the reactants is ca. τ = 0.023 s (i.e., a gas hourly space velocity, GHSV, of ca. 156,500 h−1). The ‘light-off’ behavior of the catalysts (CH4 conversion versus temperature in constant reactor feed conditions) was obtained by increasing the temperature stepwise (~30 °C/step); in other words, they remained at each temperature for ~20 min to ensure a steady-state operation.
Both the pre-reduced and pre-oxidized states of the catalyst were evaluated by respectively applying the following pretreatment conditions, prior to light-off measurements: (i) pre-reduction at 600 °C for 2 h with a 50 mL/min flow of 25% H2/He, and (ii) pre-oxidation at 400 °C for 1 h with a 50 mL/min flow of 20% O2/He. In addition, 12 h of time-on-stream (TOS) stability experiments were performed at a constant temperature, which was different for each catalyst, and equal to that of its corresponding T50 (temperature for 50% CH4 conversion). Finally, resistance against the deactivation of the LSΧM and Ir/LSΧM counterpart catalysts, after the imposition of stressed oxidative thermal aging conditions, was studied by imposing 5 h in situ oxidation at 750 °C with a 20% O2/He flow; then, a re-evaluation of catalyst performances under the same reaction conditions that were previously applied took place. All the above steps, and the order in which they were performed, are shown in Scheme 1.
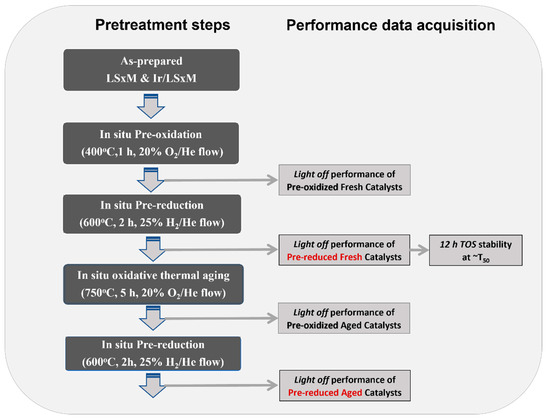
Scheme 1.
Experimental procedure followed for LSXM and Ir/LSXM catalysts efficiency and stability evaluation under the complete oxidation of CH4 reaction. The evaluation was always performed under reaction conditions comprising 1.0% CH4 + 5.0% O2 + 94% He, p = 1 bar, and WGHSV = 90,000 cm3/g∙h (FT = 75 cm3/min, mcat = 50 mg).
The conversion of CH4 (XCH4) is calculated using Equation (3), as follows:
where F is the total gas flow rate (mL/min), [CH4] is the v/v fraction of CH4 in the gas stream, and the subscripts “in” and “out” indicate the reactor inlet and outlet gas streams, respectively.
3. Results and Discussion
3.1. Catalyst Characteristics and Physicochemical Properties
A thorough characterization of the LSXM and Ir/LSXM materials has been previously performed and can be found in [30]. The textural, morphological, and total oxygen storage capacity (t-OSC) characteristics are summarized in Table 1 and described in detail below.
The specific surface area (SBET) values of the materials were between 6.8–12 m2/g, which are quite low, but typical for perovskite-type materials. The substitution of La with Sr, up to when X = 50%, decreased SBET monotonically from 12 to 6.8 m2/g, which then increased again for when X = 70%, as it approached the initial value. Perovskites are considered to be ionic materials consisting of closed-packed arrays of relatively large oxygen ions. Under these conditions the rate controlling step for densification during sintering comprises the diffusion of the oxygen anions; this takes place through oxygen vacancies rather than through interstitial sites. The addition of the substituted Sr, was compensated with Mn4+ cations, the reduction of which, upon heating at 1000 °C, creates oxygen vacancies. These enhance the oxygen anion diffusion rate, and consequently, the densification rate. This explains the gradual SBET reduction, along with the increasing Sr content. Τhe subsequent increase in SBET, at higher Sr contents, indicates that at Sr contents higher than 0.5, the defect chemistry of the system changes and is not dictated by the same rules. In addition, the presence of secondary phases, even at small amounts, is able to significantly influence densification during sintering at 1000 °C.
The addition of Ir via impregnation reduced the value of SBET due to the partial blocking of the LSXM small-sized pores, caused by the Ir nanoparticles. This is evidenced by the slightly higher average pore size values of Ir/LSXM compared with their LSXM counterparts (Table 1).
The average size of Ir nanoparticles was estimated to be in the order of 1.0–1.2 nm (Table 1) using Equations (1) and (2) fitted with the H2-uptake values obtained from the isothermal H2-chemisorption experiments. The corresponding Ir dispersion values (DIr) ranged between 61% and 73%.
The structural characteristics of the materials obtained via the analysis of their XRD patterns confirmed the development of the lanthanum manganate perovskite structure at an angle of 2θ~32.4–33.1° (Figure S2 in the Supplementary Materials file). Other secondary phases of single or mixed oxides, appearing mainly in the material with the higher substitutions of La with Sr (X = 70%), can be seen in Figure S2. The main peak of the perovskite structure, at about 33°, is magnified in Figure S3, wherein a gradual transition from a rhombohedral to a cubic structure (with increasing Sr content) is clearly distinguishable from the splitting of the peak at a diffraction angle 2θ~32–33°. In addition, there is a shift to larger angles as the substitution of La with Sr increases; this indicates that the unit cell is being contracted [47,48]. This cell contraction most likely resulted from the oxidation of Mn3+ to Mn4+ when the Sr content of the material increased, rather than from the creation of oxygen vacancies, which, if they occurred, would lead to unit cell expansion [47,48]. Moreover, the appearance of other crystalline phases of single or mixed oxides, along with the perovskite structure, appeared in materials with higher substitutions of La with Sr (x = 70%). On the other hand, no Ir species were detected in the XRD diffractograms of Ir/LSΧM catalysts, which is most likely due to its small-sized nanoparticles (~1 nm); this indicates successful dispersion.
The reducibility characteristics of the LSXM perovskites are shown in the H2-TPR profiles of Figure 1. The total oxygen storage capacity (t-OSC) of each perovskite, obtained from the integrated area of their respective TPR profiles in the time interval of the experiment [13,30], are given in Table 1. Values range from 670 to 1350 μmol O2/g, increasing systematically with increasing levels of X.
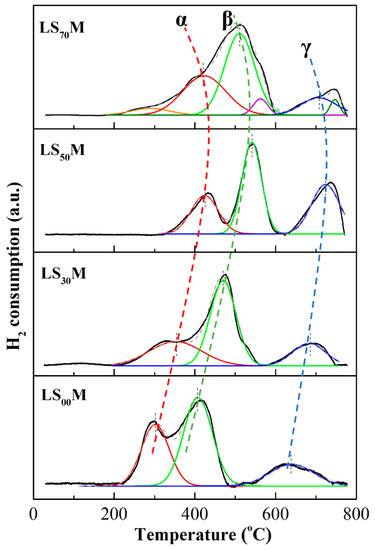
Figure 1.
H2-TPR profiles for the LSXM catalysts and main peak deconvolutions. Peak (α) → Oads; peak (β) → Mn4+ to Mn3+ reduction; peak (γ) → Mn3+ to Mn2+ reduction.
The deconvolution of the broad overlapping peaks in the TPR profiles of LSXM reveals three main peaks (α, β, and γ) for the perovskites with compositions where Χ = 0–50%, whereas additional, lower intensity peaks appear when X = 70%. The three main α, β, and γ kinds of oxygen are placed at temperature regions of ca. 200–450 °C, 350–600 °C, and 600–800 °C, respectively; their reduction regions gradually shift to higher temperatures by increasing X until X = 50%, then, they shift again to lower temperatures when X = 70%, in accordance with Ponce et al. [78].
In accordance with the literature, peak α is attributed to adsorbed surface oxygen species, Oads; peak β is attributed to the labile lattice oxygen, thus reflecting the Mn4+/Mn3+ redox couple (Equation (4)) caused by the reduction of high-valence Mn4+ to Mn3+; and finally, peak γ is attributed to the labile lattice oxygen reflecting the Mn3+/Mn2+ redox couple (Equation (5)) caused by the reduction of Mn3+ to Mn2+ [25,44,78,79,80,81,82,83]. Equations (2) and (3) are written in accordance with the Kröger–Vink notation, as follows [84]:
where the reference valence for manganese is considered to be +3.
As we shall see below, LSXM perovskites are active during lean CH4 combustion in the entire temperature region of ca. 250–800 °C, indicating that depending on the operation temperature, all these oxygen types could potentially contribute to the overall methane consumption rate.
Considering the combined XRD and H2-TPR findings, it may be concluded that the higher the X, the higher the M4+ state content in pre-oxidized LSXM, and consequently, the higher the OSC of the material (Table 1). On the other hand, the tendency to reduce manganese (i.e., the lability of lattice oxygen, peaks β and γ) becomes progressively more difficult with increasing levels of X, up to 50%; then, for X = 70%, this is reversed (Figure 1). This can be understood if one considers that gradually increasing quantities of Mn4+ are needed to compensate for the Sr2+ additions; this finding is in accordance with the results reported in the literature [78], and it is reflected by the fact that the equilibrium constant of the reaction (4) is reported to decrease with increasing levels of X [85].
The addition of Ir to LSXM shifts the reducibility peaks of the obtained Ir/LSXM material to temperatures as low as ca. 150–400 °C, in which narrow temperature range encompassing all peaks (reflecting Ir4+ → Ir0, Mn4+ → Mn3+ and Mn3+ → Mn2+ reductions) they substantially overlap (Figure S4). This noble metal-induced enhancement of the support’s reducibility is due to an increased spillover of hydrogen atoms from the noble metal particles to the reducible support, thus promoting the reducibility of the latter [74].
The additional, low-intensity peaks that appeared at temperatures of ca. 280, 560, and 750 °C on the LS70M H2-TPR spectrum presumably originate from the reduction of the additional oxide phases that appear to be present in the XRD pattern of this perovskite (Figure S2). For example, manganate oxide phases can present with reduction peaks at low and intermediate temperature regions following successive reduction processes (MnO2 → Mn2O3 → Mn3O4 → MnO) [86]. However, the low amount of these phases detected on LS70M only marginally affects the overall qualitative/quantitative reducibility behavior of the material, which is still mainly determined by the α, β, and γ peaks (Figure 1).
3.2. Light-Off/Light-Out Performance of LSXM and Ir/LSXM Catalysts
Following the experimental schedule described in Scheme 1 for catalytic performance data acquisition, the light-off (heating)/light-out (cooling) temperature cycles were conducted after different pre-treatment stages, involving fresh or aged (at 750 °C), pre-oxidized or pre-reduced, LSXM and Ir/LSXM catalysts, respectively. The results of the catalytic behavior, in the order they were obtained (Scheme 1), are discussed below (i.e., first those concerning fresh catalysts are discussed, followed by those concerning thermally aged catalysts, and so on).
3.2.1. Pre-Oxidized Fresh LSXM and Ir/LSXM Catalysts
Figure 2 shows the performance of fresh pre-oxidized LSXM (Figure 2a) and Ir/LSXM (Figure 2b) catalysts during a heating/cooling cycle. The corresponding T50 values (temperature for 50% conversion of CH4) are presented in Figure 2c.
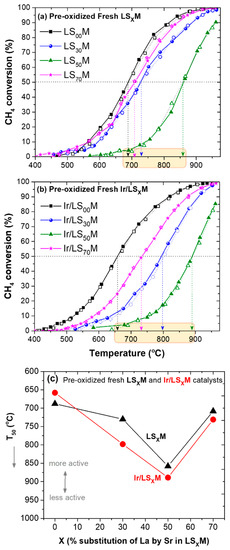
Figure 2.
Light-off/light-out behavior of pre-oxidized fresh LSXM (a) and Ir/LSXM (b) catalysts, and the corresponding T50 values as a function of X (c). Feed conditions: 1.0% CH4 + 5.0% O2, He balance at 1 atm, FT = 75 mL/min, mcat = 50 mg, WGHSV = 90,000 mL/g∙h. Solid symbols and lines in (a,b) show the heating path, and the open symbols and dashed lines show the cooling path of the cycle.
It is apparent that the light-off (heating) and light-out (cooling) performances of the CH4 conversion coincide to a reasonable extent. Therefore, no hysteresis phenomena were observed. However, the activity of the catalysts appears to be strongly dependent on the La substitution with Sr (X) in the perovskite, which can be seen more clearly with the variation in T50 versus Χ (Figure 2c). This dependence, which is qualitatively similar for the two series of materials (LSXM and Ir/LSXM), is not monotonic, showing inverted volcanic behavior (with respect to activity), with the LS50M and Ir/LS50M pair appearing to be the less active. Furthermore, and rather unexpectedly, the iridium-loaded perovskites (Ir/LSXM) appear typically less active than their pristine perovskite counterparts (LSXM).
3.2.2. Pre-Reduced Fresh LSXM and Ir/LSXM Catalysts
The light-off (heating)/light-out (cooling) behaviors for the two sets of fresh catalysts, LSXM and Ir/LSXM, starting from their pre-reduced state, are depicted in Figure 3. After this pretreatment, the behavior of the catalysts appears more complex than their pre-oxidized counterparts, as it involved hysteresis phenomena (steady-state rate multiplicity) [30,87,88,89,90]. Both kinds of hysteresis were obtained for the pre-reduced samples during heating/cooling cycles (i.e., normal (counterclockwise) or inverse (clockwise) hysteresis as commonly described in the literature [87], although the latter is clearer (more intense)). In particular, the pair (LS00M, Ir/LS00M), when X = 0%, follows normal hysteresis (with the activity possessing higher values upon cooling), whereas those with intermediate values, regarding the Sr substitution (X = 30 and 50%), obey inverse hysteresis. The hysteresis behavior of the pair (LS70M, Ir/LS70M), with the maximum (X = 70%) substitution of La with Sr, is complicated; the former catalyst follows normal hysteresis, and the latter follows inverse hysteresis, although, the amplitude of the hysteresis loop in both cases is limited. Interestingly, the hysteresis loops between the counterpart catalysts, LSXM and Ir/LSXM, are mirrored with respect to their qualitative characteristics, suggesting that hysteresis mainly originates from the perovskite compartment of the catalyst, rather than from Ir. The latter mainly affects the hysteresis amplitude.
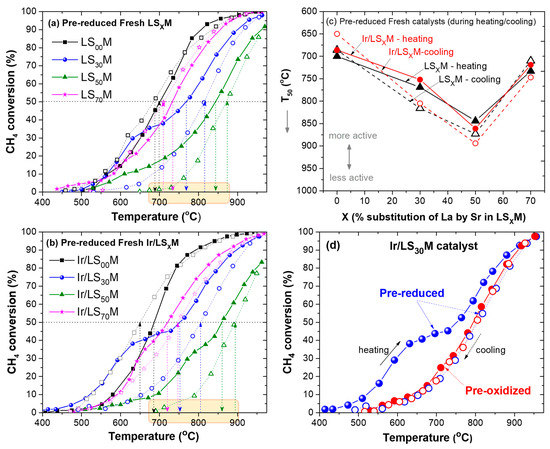
Figure 3.
Light-off/light-out behavior of pre-reduced, fresh, LSXM (a) and Ir/LSXM (b) catalysts and the corresponding T50 values as a function of X (c). A selected comparison of the heating/cooling cycles obtained from the pre-reduced (blue symbols and lines) and pre-oxidized (red symbols and lines) Ir/LS30M catalysts (d). Feed conditions, as shown in Figure 2. Solid symbols and lines show the heating path of the cycles, open symbols and dashed lines show the cooling path of the cycles.
Regarding the activity ranking of the pre-reduced catalysts, this can be followed best using their T50 behavior, as depicted in Figure 3c. Similar to that observed for the pre-oxidized samples (Figure 2c), an inverted volcano-type behavior of the activity with increasing substitutions (X) of La with Sr is once again clear (Figure 3c). Thus, LSXM and Ir/LSXM, when X = 0%, outperformed those when X = 30%, to a greater extent than those when X = 50% (the least active). Then, catalysts with X = 70% appeared more active than those with intermediate X values, approaching the optimal activity of those when X = 0%. Moreover, during the heating part of the light-off/light-out cycle, Ir/LSXM catalysts appeared to exhibit a slightly superior performance compared with their LSXM counterparts, except in the case of the LS50M—Ir/LS50M pair (Figure 3c); these behaviors are almost opposite to those recorded for the pre-oxidized samples (Figure 2c). The same is true for the catalysts where X = 0 and 30% (low Sr content), but not for cases where X = 50% and 70% (high Sr content) during the cooling part of the cycle.
When comparing the behaviors of pre-reduced and pre-oxidized LSxM (Figure S5a) and Ir/LSXM (Figure S5b) catalysts, a clear trend is revealed; in the vast majority of cases, the cooling part of the pre-reduced catalysts better approximates, or even coincides with, the behavior of their pre-oxidized counterparts. A typical example (for the Ir/LS30M catalyst) is shown in Figure 3d.
3.3. Light-Off/Light-Out Performance of LSXM and Ir/LSXM Catalysts Aged at 750 °C
Herein, the behavior of CH4 conversion as a function of temperature during heating/cooling cycles between 400 and 900 °C is presented for LSXM and Ir/LSXM catalysts that were previously subjected to oxidative thermal aging for 5 h, as described in Scheme 1. Both the pre-oxidized and pre-reduced states of the samples at the beginning of the cycle were investigated.
3.3.1. Pre-Oxidized Catalysts Aged at 750 °C
Figure 4 presents the CH4 conversion results obtained during the heating/cooling cycle for the pre-oxidized LSXM (Figure 4a) and the Ir/LSXM (Figure 4b) catalysts that were aged at 750°C, whereas Figure 4c depicts the corresponding T50 behavior of the catalysts as a function of X. As for fresh catalysts (Figure 2), no hysteresis phenomena were observed during the cycles over the pre-oxidized, aged at 750°C samples. It was also observed that pure perovskites (LSXM) slightly outperformed their Ir-doped counterparts (Ir/LSXM), except for LS00M (Figure 4c).
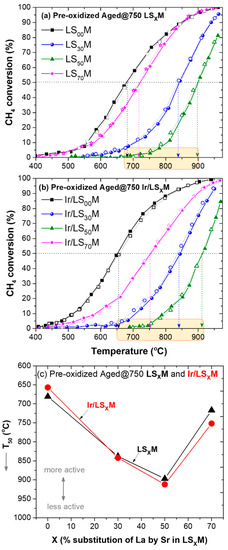
Figure 4.
Light-off/light-out behavior of pre-oxidized, aged at 750 °C, LSXM (a) and Ir/LSXM (b) catalysts, and their corresponding T50 values as a function of X (c). Feed conditions are shown, as in Figure 2. Solid symbols and lines in (a,b) show the heating path and open symbols, and the dashed lines show the cooling path of the cycle.
Regarding the dependence of the activity on the Sr content of LSXM, the aged catalysts maintained the same behavior for their fresh counterparts (Figure 2). It is once again an inverted volcanic-type dependent activity competing against X, with LS50M and Ir/LS50M catalysts exhibiting inferior performances, with T50 values approximately 250 °C higher than those for LS00M and Ir/LS00M (Figure 4c).
3.3.2. Pre-Reduced Catalysts Aged at 750 °C
The performance of the pre-reduced LSXM and Ir/LSXM catalysts that were aged at 750 °C, and their impact on the lean CH4 oxidation reaction during heating/cooling cycles, are depicted in Figure 5a,b, respectively. There are no significant differences compared with what was already observed in the behavior of their fresh counterparts. Again, hysteresis phenomena, which are more pronounced for the intermediate values of the percentages where La was substituted for Sr (X = 30 and 50%), were observed.
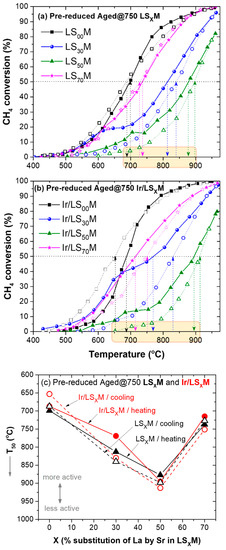
Figure 5.
Light-off/light-out behavior of pre-reduced, aged at 750 °C, LSXM (a) and Ir/LSXM (b) catalysts, and their corresponding T50 values as a function of X (c). Feed conditions are shown, as in Figure 2, Figure 3 and Figure 4. Solid symbols and lines show the heating path, and open symbols and dashed lines show the cooling path of the cycle.
The CH4 conversion efficiency performance versus X of the pre-reduced catalysts aged at 750 °C, in terms of T50, is shown in Figure 5c. There are some variations in T50 values between the heating and cooling parts of the cycle due to the hysteresis, which generally indicates more active catalysts in the heating part of the loop. Since the thermal cycle starts with pre-reduced catalysts, it is possible that in this part of the cycle, the pre-imposed metallic Ir0 is partially oxidized by the oxidizing environment of the reactants (lean conditions), creating an IrO2/Ir0 complex state. As demonstrated by Rui and co-workers [91], this offers favorable sites for the overall methane oxidation reaction, compared with metallic Ir0 or stoichiometric IrO2. In the same vein as the results of Schick et al. [31], regarding the total oxidation of VOCs over Ir/SiO2 catalysts, which showed that surface Ir3+ species are associated with improved catalyst performance as well as size–activity relationship; regarding the latter, this is because as the size of the Ir nanoparticles decreases, the defective Ir3+ active sites increase.
However, when the system achieves 100% CH4 conversion at a high temperature, i.e., it operates in net oxidizing conditions, (due to the remained unconsumed O2, the Ir nanoparticles are fully oxidized into stoichiometric IrO2, therefore, they become less active in the reaction, and during its return to low temperatures (cooling) the system exhibits lower activity.
Nevertheless, the effect of substituting La with Sr (X) remains the main cause of strong changes in terms of the efficiency of the catalysts. Changes in the order of ~60 °C max. were obtained between the heating and cooling sections of the hysteresis loop; these are insignificant compared with changes of 250 °C, caused by the 50% replacement of La with Sr.
3.4. Thermal Aging and Time-on-Stream Stability of Catalysts
In order to evaluate the thermal stability of the materials after prolonged exposure to thermal aging at high temperatures under oxidizing conditions (5 h at 750 °C in a 20% O2/He flow), Figure 6 was constructed, which presents the efficiency (in terms of T50) of fresh and aged (at 750 °C) LSXM (Figure 6a–c) and Ir/LSXM (Figure 6d–f) catalyst series.
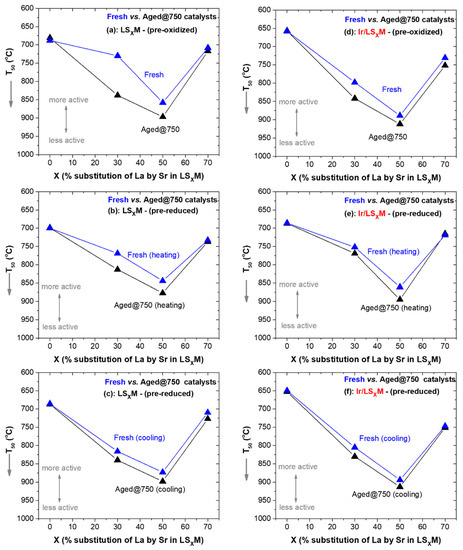
Figure 6.
Comparison of fresh and aged at 750 °C LSXM and Ir/LSXM catalysts in T50 vs. X diagrams. (a–c) correspond with LSXM; (d–f) correspond with Ir/LSXM. (a,d) compare fresh vs. aged (at 750 °C) pre-oxidized LSXM and Ir/LSXM catalysts, respectively; (b,c) compare fresh vs. aged (at 750 °C) pre-reduced LSXM and Ir/LSXM catalysts during heating, respectively; (c,f) compare fresh vs. aged (at 750 °C) pre-reduced LSXM and Ir/LSXM catalysts during cooling, respectively. Feed conditions are shown, as in Figure 2, Figure 3, Figure 4 and Figure 5.
For the pre-oxidized catalysts of each series, LSXM and Ir/LSXM, which do not show hysteresis after such a pretreatment, one diagram is sufficient for their comparison (Figure 6a for LSXM and Figure 6d for Ir/LSXM). However, for pre-reduced catalysts exhibiting hysteresis, a comparison is made with respect to both the heating and cooling parts of the hysteresis loops (Figure 6b,c for LSXM and Figure 6e,f for Ir/LSXM).
It is evident from Figure 6 that aging causes the degradation of the catalytic efficiency of the studied materials, but this is rather insignificant, as in most cases, this results in only slight increases in T50 (ca. +20 °C). Furthermore, in the case of catalysts with X = 0 and 70%, the activity of the catalysts, either of unloaded or Ir-loaded perovskites, remains unaffected after aging. The thermal aging stability of perovskites under oxidizing conditions is well established, and its confirmation it was not surprising to obtain this result for the pristine LSxM (Figure 6a–c). Moreover, the strong susceptibility and tendency to aggregate, regarding the supported iridium nanoparticles under oxidative thermal aging conditions, is also known [37,38], and thus, it was the focus of the stability experiments. However, the results of Figure 6d–f are similar to those of the pristine perovskites; the Ir/LSXM catalysts exhibited good stability, and an explanation for this is given below.
Regarding the time-on-stream stability (TOS) of our catalysts, experiments were performed for a period of 12 h (Figure 7) under constant temperatures that corresponded with the T50 exhibited by each of them. The less active catalysts, LS50M and Ir/LS50M, with high T50 values (~900 °C), were operated at temperatures of 850 °C; at such temperatures, they have an initial CH4 conversion rate of about 20–25% (Figure 7). All catalysts showed very good stability, since only a slight degradation, in the order of 5%, was recorded after 12 h of operation. It is worth noting that the catalyst pair, LS00M and Ir/LS00M, was the most active compared with all others investigated, and it also offered the best TOS stability, with zero degradation in terms of their efficiency, over the 12 h period.
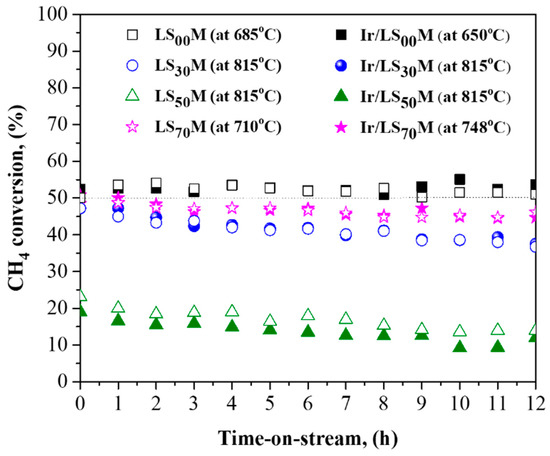
Figure 7.
A 12 h time-on-stream stability test for the LSXM (open symbols) and Ir/LSXM (closed symbols) catalysts. Conditions: 1.0% CH4 + 5.0% O2, He balance at 1 atm, FT = 75 mL/min, mcat = 50 mg, WGHSV = 90,000 mL/g∙h. Regarding the temperature (indicated in figure inset), it was close to T50 for each catalyst, except for the less active pair, LS50M and Ir/LS50M.
3.5. Main Observations and Material Properties—Catalytic Efficiency Correlations
The main observations of the above comparative studies of catalytic behavior (efficiency and stability) of the two series of LSXM and Ir/LSXM catalysts for the complete CH4 oxidation reaction under lean conditions, can be summarized as follows:
- (i)
- The most important determining factor of the materials, for all cases (unloaded or Ir-loaded LSXM, pre-oxidized or pre-reduced, and fresh or aged materials), in terms of efficiency, was the composition of the LSXM perovskite, specifically, the amount of La that was substituted with Sr (X = 0, 30, 50, and 70%). This key factor typically causes shifts in T50 of up to ca. 300 °C, whereas other parameters appeared capable of shifts in T50 that were one order of magnitude lower (ca. 30 °C) (Figure 2, Figure 3, Figure 4 and Figure 5).
- (ii)
- X variation produced an inverted volcanic-type effect in terms of catalytic efficiency, with the most active in all cases the catalysts that did not contain Sr, i.e., X = 0% (LS00M = LaMnO3 and Ir/LS00M = Ir/LaMnO3), and less active the catalysts with X = 50% (i.e., LS50M and Ir/LS50M). Catalysts with larger X value (X = 70%; La0.3Sr0.7MnO3 and Ir/La0.3Sr0.7MnO3), tended to be similar to the behavior of the optimal catalysts (LaMnO3 and Ir/LaMnO3) (Figure 2c, Figure 3c, Figure 4c, Figure 5c and Figure 6).
- (iii)
- The addition of Ir nanoparticles onto the LSXM surface did not perform as expected. It had a negative effect on the efficiency of pre-oxidized catalysts (Ir/LSXM show T50 values that were ~30°C higher than those of their non-loaded LSXM counterparts) (Figure 2c and Figure 4c), whereas the pre-reduced catalysts exhibited a small positive effect at high temperatures, but it was more noticeable for lower temperatures (Figure 3c and Figure 5c).
- (iv)
- (v)
- (vi)
- The oxidative thermal aging of the materials caused marginal decreases in their efficiency (i.e., less than ~40 °C shifts of T50 towards higher temperatures). However, for the most active catalysts (with X = 0 and 70%), no activity deterioration was recorded (Figure 6).
- (vii)
- The time-on-stream stability of the materials was generally good, as the efficiency (methane conversion) only declined by 5–10%; this was observed after 12 h of operation. Notably, no degradation in the catalytic efficiency of the optimal LS00M (LaMnO3) and Ir/LS00M (Ir/LaMnO3) catalysts was recorded (Figure 7).
After a close comparison of the LSXM characteristics (and how these change in accordance with the extent (X) to which La was substituted with Sr) with the catalytic results, we can readily conclude that the main factors affecting the activity of the perovskites during the lean CH4 combustion are the total surface area (SBET) and the reducibility of the perovskite. Indeed, as shown in Figure 8a and b, respectively, the efficiency of the LSXM (in terms of T50) faithfully mimics the changes in both the total surface area (SBET) and reducibility (expressed in terms of the peak temperature of Mn4+ → Mn3+ reduction in the TPR profile of Figure 1) when the level of substituted Sr (X) in the perovskite increases.
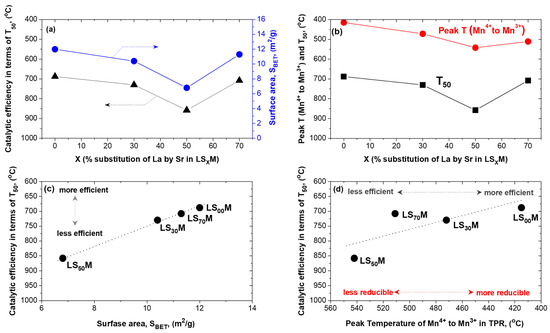
Figure 8.
Correlations between the LSXM lean CH4 combustion efficiency and its textural characteristics and properties, which were mainly changed as a result of the degree of Sr substitution (X). (a) Variation in LSXM surface area and efficiency (T50) as a function of X; (b) variation of LSXM reducibility (in terms of peak temperature of the Mn4+ → Mn3+ reduction in the TPR profile) and efficiency (T50) as a function of X; (c) direct correlation of LSXM catalytic efficiency with its surface area; and (d) direct correlation of LSXM catalytic efficiency with its reducibility.
Studies of the literature, regarding lean CH4 combustion over perovskite-type materials, typically describe the reaction mechanism via the Eley–Rideal (ER), Mars–van Krevelen (MVK), and/or both (two-term) kinetic models [63,67,79]. The ER model involves the direct reaction of gas phase methane with adsorbed oxygen species (Oads) on the perovskite surface. The MVK model implicates the reaction of gas phase CH4 with surface lattice oxygen (Equation (6)), the latter being continuously replenished by the dissociative chemisorption of O2 on surface oxygen vacancies (Equation (7)), as follows:
Performing Density Functional Theory (DFT) calculations for CH4 combustion on LSM perovskites, Wang et al. [92] also indicated that CH4 adsorption and activation (CH4* → CH3* + H*) occurs on Mn sites. They argued that this activation, revealed to be more facile on the (001) rather than (110) crystallographic plane, can cause further enhancements to catalytic methane oxidation after a reaction concerning such methane-derived species with neighbor lattice oxygen atoms takes place.
It is therefore obvious from the above considerations that perovskite activity, regarding the reaction under consideration, will be directly proportional to the population of labile oxygen species on the perovskite surface, and thus, to the perovskite surface area itself. At the same time, the degree of lability of these lattice oxygen species would act synergistically upon the enhancement of the activity.
Observations (i) and (ii) that are related to the entire catalytic efficiency data, either obtained on fresh or aged, or on pre-oxidized or pre-reduced, catalysts, they exhibit an activity order (LS00M ~ LS70M > LS30M > LS50M) that is fully consistent with the above. The higher the perovskite surface area and lattice oxygen lability, the higher its efficiency in terms of lean CH4 combustion (Figure 8c,d). As we have seen here, increasing the amount of substituted Sr in LSXM, up to X = 50%, gradually degrades both of these activity-determining parameters; for X = 70%, however, the formation of other simple oxide phases (e.g., Mn2O3, see XRD results in Figure S2) may be the cause of the recovery of high values in said properties, and therefore, the return of efficiency to high values.
The unexpected inhibitory effect of adding Ir to the surface of LSXM perovskites, regarding their catalytic efficiency (observation iii), is still uncertain. As shown in Table 1, the Ir/LSXM catalysts typically have a lower surface area compared with their LSXM counterparts due to their partially blocked pores, as explained in Section 3.1. Therefore, part of the surface of the perovskite support, which is intrinsically active in the reaction, is rendered inaccessible to the reactants mixture. The catalyst thus loses active centers, which, in the case of pre-oxidized samples, are not replenished by the presence of, relatively inactive to the reaction, IrO2 phase. In the case of the pre-reduced Ir/LSXM catalysts, this undesirable effect of the iridium species is partially compensated with the creation of an active for the reaction IrO2/Ir0 phase (partially oxidized iridium) [31,91]. This occurs after the exposure of the Ir0 species of the pre-reduced catalysts to the excess O2 conditions. However, as our results show, this favorable partially oxidized surface of the iridium particles (IrO2/Ir0) created on the pre-reduced catalysts is not expected to remain in high temperatures and under excess O2 reaction conditions. It transforms to the less active IrO2 which moderates positive effects (Figure 3 and Figure 5).
The consistent interpretations of observations (iv) and (v), which are related to inverse hysteresis phenomena (Figure 3 and Figure 5), and are recorded in this study, are as follows:
Why is hysteresis observed only on the pre-reduced, and not the pre-oxidized, catalysts, and why is it of the inverse type (i.e., clockwise, the heating part outperforms its cooling part)? The light-off (heating part) behavior of the pre-reduced catalysts (Figure 3 and Figure 5) is very similar to the behavior first observed by Arai et al. [67], who studied CH4 combustion on La0.8Sr0.2MnO3 and La0.6Sr0.4MnO3 perovskites using a reaction mixture of 2% v/v CH4 in air. It exhibits a characteristic “hump” in the low-temperature region, then, it changed curvature at higher temperatures, as also found herein (Figure 3 and Figure 5). We agree with the explanation given by the authors, as follows. The initial high rate of CH4 consumption is the result of the contribution to the overall rate of adsorbed oxygen species (Oads) on the perovskite surface which is then limited by increasing temperatures (at high temperatures the Oads desorb rapidly); however, at high temperatures the participation of lattice oxygen () in methane oxidation becomes dominant [67].
Regarding the behavior of the cooling (light-out) part of the hysteresis loops (Figure 3 and Figure 5), we can give the following rational explanation. When approaching ~100% methane conversion at the upper-temperature limit, the catalyst experiences pure oxidative conditions (~100% CH4 conversion, excess O2). It is therefore oxidized under such conditions, and by lowering the temperature, it follows a path of lower activity, similar to the results obtained in pre-oxidized samples (Figure 2 and Figure 4). This explains why the cooling part of the hysteresis loops was always similar to the light-off curves of the pre-oxidized catalysts, in addition to the “inverse” character of the hysteresis.
In accordance with the above, hysteresis mainly occurs due to the favorable contribution of Oads species to the total methane consumption rate. However, the reason why this was found to only work for pre-reduced samples, but not pre-oxidized samples, can be interpreted as follows. The pre-reduction process of LSxM materials is expected to enhance the population of oxygen vacancies on their surface due to a partial Mn4+ → Mn3+ reduction. When such a pre-treated LSXM perovskite experiences in methane oxidation environments, the gas phase dioxygen interacts with oxygen vacancies, providing two atomic oxygens, one of which is bound by the vacancy that becomes the lattice oxygen, and the other can diffuse to the perovskite surface in the form of adsorbed Oads. Thus, a mechanism of continuous replacement concerning both lattice and adsorbed oxygen species is at work during the reaction on pre-reduced materials. Conversely, the pre-oxidized perovskites, due to the lack of oxygen vacancies on their surface to activate the dissociative chemisorption of gaseous dioxygen in this temperature range, have a limited amount of Oads that impose low reaction rates. Thus, for per-oxidized samples, both the heating and cooling parts of the light-off experiments coincide since the samples work for both paths in their oxidized state.
The presence of Ir in the catalyst formulations (Ir/LSXM) appears to have some noticeable effect on the hysteresis phenomena, especially on loop amplitude, regarding CH4 conversion. It appears strongly enlarged (Figure 5b) compared with that recorded in the corresponding LSXM catalysts (Figure 5a). A reasonable explanation is that the partially oxidized IrO2/Ir0 sites (their creation has been described above), which are favorable for the dissociative adsorption of gaseous dioxygen, further facilitate this necessary mechanistic step of the reaction. The as-derived oxygen atoms can then directly react with the methane, and/or spillover to the perovskite surface, thus replenishing the lattice oxygen consumed by the reaction, enhancing methane combustion.
Regarding observation (vi), which concerns the good stability of our materials in spite of the fact that they were subjected to arduous conditions, even those containing Ir (Ir/LSXM), is an impressive finding considering that Ir is a very sensitive catalyst with regard to thermal aging; it has a high aggregation tendency, even at temperatures as low as 450°C [13,14,37,38,39,40,41]. The present results once again confirm our recent experimental findings, theory, and developed model, demonstrating that supports possess a sufficient population of bulk and surface O-defects (oxygen vacancies), and a high mobility of lattice O2− ions effectively act against the thermal sintering of catalyst nanoparticles dispersed on their surface [40,41]. The perovskites used herein as supports for Ir nanoparticles meet these requirements.
Observation (vii), on the other hand, concerning the good time-on-stream stability of our materials, most likely reflects the absence of the accumulative carbon deposition phenomena originating from the methane cracking reaction, the occurrence of which is not thermodynamically excluded in the temperature range used for the reaction in this study.
An additional aspect worth noting in the present findings concerns the fact that, in the literature, it is known that at high temperatures (ca. > 850 °C, reached in the present study) the contribution of the gas phase to the total methane oxidation can be appreciable under certain conditions and should not be ignored. Matzaras and co-workers, in a Cartesian plot of SV (surface-to-volume ratio) versus residence time, delineated the regimes concerning the significant involvement of gas-phase chemistry for given pressures and temperatures [93]. Considering the conditions in which the present experiments were performed (SV > 200,000 and residence time ~0.017 s at a pressure of 1 bar), it seems that the catalytic system operates under conditions that starkly differ with those wherein the contribution of the gas-phase reaction could be noticeable. This view is further strengthened by two additional facts, as follows: first, no slope change tendency of the light-off curves at high temperatures is observed in Figure 2, Figure 3, Figure 4 and Figure 5; second, nor was CO detected in the reaction products (the contribution of the gas phase goes through the reaction sequence: CH4 → CO gas-phase route followed by the catalytic oxidation of the produced gaseous CO to CO2).
Finally, taking into account the small particle size of the catalytic powdered samples (180–250 μm), and the high GHSV used (ca. 156,000 h−1), mass and heat transfer limitations, if any, should be negligible.
4. Conclusions
The complete oxidation of the methane reaction was investigated in detail on a series of LSXM (X = % substitution of La with Sr = 0, 30, 50, and 70%) perovskites, and on their 2 wt% Ir-loaded counterpart catalysts.
The key parameter affecting efficiency for both series concerned the degree (X) of substitutional Sr, rather than the addition of Ir; the latter caused a slight inhibition of activity, rather than promotion, when in its oxidation state (IrO2), and it notably enhanced activity when in a partially oxidized state (IrO2/Ir0).
The main factors through which parameter Χ affects the activity were the changes it induced in the materials’ total surface area and on their reducibility that act synergistically.
The materials’ efficiency followed an inverted volcanic behavior pattern, as a function of X, with the most efficient catalysts being the LS00M—Ir/LS00M pair, and the least efficient being the LS50M—Ir/LS50M pair; this strikingly reflects the effect of X on the total surface area and the reducibility of the materials. Large differences in T50 (ca. 300 °C) were recorded between the most and least active catalysts.
The inhibitory effect on efficiency, caused by the addition of Ir to the perovskite, was attributed to partial pore blocking, and consequently, to a reduction in the number of active sites, in combination (for pre-oxidized catalysts) with the low activity of IrO2 particles. For pre-reduced catalysts, the creation under reaction conditions of a reactive partially oxidized IrO2/Ir0 phase enhanced activity, especially at low temperatures.
Inverse hysteresis phenomena recorded on pre-reduced, but not pre-oxidized, LSxM and Ir/LSXM catalysts during light-off (heating)/light-out (cooling) temperature cycles and overall reaction kinetics were consistently interpreted by assuming that CH4 oxidation was performed in parallel with Oads species (Eley–Rideal model) and lattice oxygens (Mars–van Krevelen model) (i.e., a two-term kinetic model).
Both the LSXM and Ir/LSXM series of materials were found to be very stable in both oxidative thermal aging tests at 750 °C for 5 h, and the 12 h time-on-stream stability tests under the reaction’s conditions.
The great stability of perovskites at high temperatures for the total oxidation of CH4, the convenience they provide for effectively tailoring their performance via the partial substitution of the A or B sites with other cations, and the efficient CH4 conversion they achieve, even without noble metal loading, make them promising materials that are worthy for future studies concerning demanding applications such as exhaust gas treatment in NG-driven vehicles or gas turbines (GTs).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano13152271/s1: Figure S1. Schematic configuration of the continuous flow experimental apparatus equipped with on-line Gas Chromatography; Figure S2. XRD patterns of LSXM perovskites (La1−xSrxMnO3; X = 0, 30, 50 and 70% substitution of La by Sr) and the corresponding 2 wt% Ir/LSXM catalysts; Figure S3. Magnification of the XRD patterns of LSXM perovskites at the region 2θ = 32–34°, where the main peak of the perovskite phase appears; Figure S4. H2 consumption versus temperature (H2-TPR profiles) of Ir/LSXM catalysts. Figure S5. Dependence of T50 on X (% substitution of La by Sr in the perovskite composition) for pre-reduced and pre-oxidized LSXM catalysts (a), and pre-reduced and pre-oxidized Ir/LSXM catalysts (b); Table S1. Temperature for 50% CH4 conversion (T50) of pre-oxidized and pre-reduced, fresh LSXM and Ir/LSXM catalysts; Table S2. Temperature for 50% CH4 conversion (T50) of pre-reduced and pre-oxidized LSxM and Ir/LSxM catalysts that were aged at 750 °C.
Author Contributions
Conceptualization, C.D. and I.V.Y.; methodology, C.D. and I.V.Y.; validation, C.D., I.V.Y. and V.T.Z.; formal analysis, C.D., I.V.Y. and E.N.; investigation, C.D., E.N., T.G. and S.F.; resources, I.V.Y.; data curation, C.D., E.N. and S.F.; writing—original draft preparation, I.V.Y.; writing—review and editing, I.V.Y., C.D., E.N. and V.T.Z.; visualization, C.D. and I.V.Y.; supervision, I.V.Y.; project administration, I.V.Y.; funding acquisition, I.V.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been co-financed by the European Union and Greek national funds under the call “Greece–China Call for Proposals for Joint RT&D Projects” (Project code: T7DKI-00356).
Data Availability Statement
The data are available from the laboratory of origin upon request www.pccplab.tuc.gr (accessed on 1 March 2023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, X.; Myhrvold, N.P.; Hausfather, Z.; Caldeira, K. Climate benefits of natural gas as a bridge fuel and potential delay of near-zero energy systems. Appl. Energy 2016, 167, 317–322. [Google Scholar] [CrossRef]
- Brauers, H. Natural gas as a barrier to sustainability transitions? A systematic mapping of the risks and challenges. Energy Res. Soc. Sci. 2022, 89, 102538. [Google Scholar] [CrossRef]
- Weissman, S. Natural Gas as a Bridge Fuel. Measuring the Bridge. Center for Sustainable Energy. Available online: https://energycenter.org/sites/default/files/docs/nav/policy/research-and-reports/Natural_Gas_Bridge_Fuel.pdf (accessed on 9 February 2023).
- Jiang, D.; Khivantsev, K.; Wang, Y. Low-Temperature Methane Oxidation for Efficient Emission Control in Natural Gas Vehicles: Pd and Beyond. ACS Catal. 2020, 10, 14304–14314. [Google Scholar] [CrossRef]
- Stoian, M.; Roge, V.; Lazar, L.; Maurer, T.; Vedrine, J.C.; Marcu, I.-C.; Fechete, I. Total Oxidation of Methane on Oxide and Mixed Oxide Ceria-Containing Catalysts. Catalysts 2021, 11, 427. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G. Biogas management: Advanced utilization for production of renewable energy and added-value chemicals. Front. Environ. Sci. 2017, 5, 7. [Google Scholar] [CrossRef]
- Jiang, Y.; Yentekakis, I.V.; Vayenas, C.G. Methane to ethylene with 85 percent yield in a gas recycle electrocatalytic reactor-separator. Science 1994, 264, 1563–1566. [Google Scholar] [CrossRef]
- Siakavelas, G.I.; Charisiou, N.D.; AlKhoori, A.; Sebestian, V.; Hinder, S.J.; Baker, M.A.; Yentekakis, I.V.; Polychronopoulou, K.; Goula, M.A. Cerium oxide catalysts for oxidative coupling of methane reaction: Effect of lithium, samarium and lanthanum dopants. J. Environ. Chem. Eng. 2022, 10, 107259. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Dong, F. Grand Challenges for Catalytic Remediation in Environmental and Energy Applications Toward a Cleaner and Sustainable Future. Front. Environ. Chem. 2020, 1, 5. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Panagiotopoulou, P.; Artemakis, G. A review of recent efforts to promote dry reforming of methane (DRM) to syngas production via bimetallic catalyst formulations. Appl. Catal. B Environ. 2021, 296, 120210. [Google Scholar] [CrossRef]
- Osazuwa, O.U.; Abidin, S.Z.; Fan, X.; Amenaghawon, A.N.; Azizan, M.T. An insight into the effects of synthesis methods on catalysts properties for methane reforming. J. Environ. Chem. Eng. 2021, 9, 105052. [Google Scholar] [CrossRef]
- Karam, L.; El Hassan, N. Advantages of mesoporous silica based catalysts in methane reforming by CO2 from kinetic perspective. J. Environ. Chem. Eng. 2018, 6, 4289–4297. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Hatzisymeon, M.; Betsi-Argyropoulou, I.; Botzolaki, G.; Kousi, K.; Kondarides, D.I.; Taylor, M.J.; Parlett, C.M.A.; Osatiashtiani, A.; et al. Effect of support oxygen storage capacity on the catalytic performance of Rh nanoparticles for CO2 reforming of methane. Appl. Catal. B Environ. 2019, 24, 490–501. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Panagiotopoulou, P.; Katsoni, A.; Diamadopoulos, E.; Mantzavinos, D.; Delimitis, A. Dry reforming of methane: Catalytic performance and stability of Ir catalysts supported on γ-Al2O3, Zr0.92Y0.08O2−δ (YSZ) or Ce0.9Gd0.1O2−δ (GDC) supports. Top. Catal. 2015, 58, 1228–1241. [Google Scholar] [CrossRef]
- Nikolaraki, E.; Goula, G.; Panagiotopoulou, P.; Taylor, M.J.; Kousi, K.; Kyriakou, G.; Kondarides, D.I.; Lambert, R.M.; Yentekakis, I.V. Support Induced Effects on the Ir Nanoparticles Activity, Selectivity and Stability Performance under CO2 Reforming of Methane. Nanomaterials 2021, 11, 2880. [Google Scholar] [CrossRef]
- Barelli, L.; Ottaviano, A. Solid oxide fuel cell technology coupled with methane dry reforming: A viable option for high efficiency plant with reduced CO2 emissions. Energy 2014, 71, 118–129. [Google Scholar] [CrossRef]
- Gur, T.M. Comprehensive review of methane conversion in solid oxide fuel cells: Prospects for efficient electricity generation from natural gas. Progr. Energy Combust. Sci. 2016, 54, 1–64. [Google Scholar] [CrossRef]
- Sengodan, S.; Lan, R.; Humphreys, J.; Du, D.; Xu, W.; Wang, H.; Tao, S. Advances in reforming and partial oxidation of hydrocarbons for hydrogen production and fuel cell applications. Renew. Sustain. Energy Rev. 2018, 82, 761–780. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Papadam, T.; Goula, G. Electricity production from wastewater treatment via a novel biogas-SOFC aided process. Solid State Ion. 2008, 179, 15211525. [Google Scholar] [CrossRef]
- Yentekakis, I.V. Open- and closed-circuit study of an intermediate temperature SOFC directly fueled with simulated biogas mixtures. J. Power Sources 2006, 160, 422–425. [Google Scholar] [CrossRef]
- Miniajluk, N.; Trawczynski, J.; Zawadzki, M.; Tylus, W. LaMnO3 (La0.8Sr0.2MnO3) Perovskites for Lean Methane Combustion: Effect of Synthesis Method. Adv. Mater. Phys. Chem. 2018, 8, 193–215. Available online: http://creativecommons.org/licenses/by/4.0/ (accessed on 1 May 2023).
- Gelin, P.; Primet, M. Complete oxidation of methane at low temperature over noble metal based catalysts: A review. Appl. Catal. B Environ. 2002, 39, 1–37. [Google Scholar] [CrossRef]
- Li, H.; Fu, R.; Duan, W.; Jiang, Z. The preparation effect on activity and thermal stability of La0.8Ca0.2FeO3 perovskite honeycombs dispersed by MgAl2O4 spinel washcoat for catalytic combustion of dilute methane. J. Environ. Chem. Eng. 2006, 4, 2187–2195. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, J.; Wu, Y.; Hu, W.; Qu, P.; Xiao, X.; Zhang, G.; Liu, X.; Jiao, Y.; Zhong, L.; et al. Particle Size Effects in Stoichiometric Methane Combustion: Structure–Activity Relationship of Pd Catalyst Supported on Gamma-Alumina. ACS Catal. 2020, 10, 10339–10349. [Google Scholar] [CrossRef]
- Cimino, S.; Lisi, L.; Pirone, R.; Russo, G.; Turco, M. Methane combustion on perovskites-based structured catalysts. Catal. Today 2000, 59, 19–31. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, M.; Yang, L.; Li, Z.; Tian, F.-X.; He, Y. Recent progress of catalytic methane combustion over transition metal oxide catalysts. Front. Chem. 2022, 10, 959442. [Google Scholar] [CrossRef]
- Zhang, X.; Long, E.; Li, Y.; Guo, J.; Zhang, L.; Gong, M.; Wang, M.; Chen, Y. CeO2-ZrO2-La2O3-Al2O3 composite oxide and its supported palladium catalyst for the treatment of exhaust of natural gas engined vehicles. J. Nat. Gas Chem. 2009, 18, 139–144. [Google Scholar] [CrossRef]
- Mortensen, R.L.; Noack, H.-D.; Pedersen, K.; Mossin, S.; Mielby, J. Recent Advances in Complete Methane Oxidation using Zeolite-Supported Metal Nanoparticle Catalysts. ChemCatChem 2022, 14, e202101924. [Google Scholar] [CrossRef]
- Santos, V.P.; Carabineiro, S.A.C.; Tavares, P.B.; Pereira, M.F.R.; Orfao, J.J.M.; Figueirdo, J.L. Oxidation of CO, ethanol and toluene over TiO2 supported noble metal catalysts. Appl. Catal. B Environ. 2010, 99, 198–205. [Google Scholar] [CrossRef]
- Drosou, C.; Nikolaraki, E.; Nikolaou, V.; Koilia, E.; Artemakis, G.; Stratakis, A.; Evdou, A.; Charisiou, N.D.; Goula, M.A.; Zaspalis, V.; et al. Activity and Thermal Aging Stability of La1−xSrxMnO3 (x = 0.0, 0.3, 0.5, 0.7) and Ir/La1−xSrxMnO3 Catalysts for CO Oxidation with Excess O2. Nanomaterials 2023, 13, 663. [Google Scholar] [CrossRef]
- Schick, L.; Sanchis, R.; Gonzalez-Alfaro, V.; Agouram, S.; Lopez, J.M.; Torrente-Murciano, L.; Garcia, T.; Solsona, B. Size-activity relationship of iridium particles supported on silica for the total oxidation of volatile organic compounds (VOCs). Chem. Eng. J. 2019, 366, 100–111. [Google Scholar] [CrossRef]
- Pachatouridou, E.; Papista, E.; Iliopoulou, E.F.; Delimitis, A.; Goula, G.; Yentekakis, I.V.; Marnellos, G.E.; Konsolakis, M. Nitrous oxide decomposition over Al2O3 supported noble metals (Pt, Pd, Ir): Effect of metal loading and feed composition. J. Environ. Chem. Eng. 2015, 3, 815–821. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Kampouri, S.; Betsi-Argyropoulou, I.; Panagiotopoulou, P.; Taylor, M.J.; Kyriakou, G.; Lambert, R.M. Ir-catalyzed nitrous oxide (N2O) decomposition: Effect of the Ir particle size and metal-support interactions. Catal. Lett. 2018, 148, 341–347. [Google Scholar] [CrossRef]
- Goula, M.A.; Charisiou, N.D.; Papageridis, K.N.; Delimitis, A.; Papista, E.; Pachatouridou, E.; Iliopoulou, E.F.; Marnellos, G.; Konsolakis, M.; Yentekakis, I.V. A comparative study of the H2-assisted selective catalytic reduction of nitric oxide by propene over noble metal (Pt, Pd, Ir)/γ-Al2O3 catalysts. J. Environ. Chem. Eng. 2016, 4, 1629–1641. [Google Scholar] [CrossRef]
- Li, H.; Hao, C.; Tian, J.; Wang, S.; Zhao, C. Ultra-durable Ni-Ir/MgAl2O4 catalysts for dry reforming of methane enabled by dynamic balance between carbon deposition and elimination. Chem. Catal. 2022, 2, 1748–1763. [Google Scholar] [CrossRef]
- Nakagawa, K.; Ikenaga, N.; Suzuki, T.; Kobayashi, T.; Haruta, M. Partial oxidation of methane to synthesis gas over supported iridium catalysts. Appl. Catal. A 1998, 169, 281–290. [Google Scholar] [CrossRef]
- Fiedorow, R.M.J.; Chahar, B.S.; Wanke, S.E. The sintering of supported metal catalysts. II. Comparison of sintering rates of supported Pt, Ir, and Rh Catalysts in hydrogen and oxygen. J. Catal. 1978, 51, 193–202. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Panagiotopoulou, P.; Kampouri, S.; Taylor, M.J.; Kyriakou, G.; Lambert, R.M. Stabilization of catalyst particles against sintering on oxide supports with high oxygen ion lability exemplified by Ir-catalysed decomposition of N2O. Appl. Catal. B Environ. 2016, 192, 357–364. [Google Scholar] [CrossRef]
- Goula, G.; Botzolaki, G.; Osatiashtiani, A.; Parlett, C.M.A.; Kyriakou, G.; Lambert, R.M.; Yentekakis, I.V. Oxidative Thermal Sintering and Redispersion of Rh Nanoparticles on Supports with High Oxygen Ion Lability. Catalysts 2019, 9, 541. [Google Scholar] [CrossRef]
- Yentekakis, I.V. The effective-double-layer as an efficient tool for the design of sinter-resistant catalysts. In Recent Advances in Electrochemical Promotion of Catalysis; Vayenas, C.G., Vernoux, P., Eds.; Springer-Nature: Berlin/Heidelberg, Germany, 2023; pp. 117–149. Available online: https://link.springer.com/chapter/10.1007/978-3-031-13893-5_4 (accessed on 1 May 2023).
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskites as substitutes of noble metals for heterogeneous catalysis: Dream or reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar] [CrossRef]
- Zhu, J.; Li, H.; Zhong, L.; Xiao, P.; Xu, X.; Yang, X.; Zhao, Z.; Li, J. Perovskite oxides: Preparation, characterizations, and applications in heterogeneous catalysis. ACS Catal. 2014, 4, 2917–2940. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, P.; Dai, S. Recent Advances of Lanthanum-Based Perovskite Oxides for Catalysis. ACS Catal. 2015, 5, 6370–6385. [Google Scholar] [CrossRef]
- Peña, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Georgiadis, A.G.; Drosou, C.; Charisiou, N.D.; Goula, M.A. Selective Catalytic Reduction of NOx over Perovskite-Based Catalysts Using CxHy(Oz), H2 and CO as Reducing Agents—A Review of the Latest Developments. Nanomaterials 2022, 12, 1042. [Google Scholar] [CrossRef] [PubMed]
- Evdou, A.; Georgitsis, T.; Matsouka, C.; Pachatouridou, E.; Iliopoulou, E.; Zaspalis, V. Defect Chemistry and Chemical Looping Performance of La1−xMxMnO3 (M=Sr, Ca, (x = 0–0.5)) Perovskites. Nanomaterials 2022, 12, 3461. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, M.E.; Jacot, R.; Scheffe, J.; Cooper, T.; Patzke, G.; Steinfeld, A. Physico-chemical changes in Ca, Sr and Al-doped La–Mn–O perovskites upon thermochemical splitting of CO2 via redox cycling. Phys. Chem. Chem. Phys. 2015, 17, 6629–6634. [Google Scholar] [CrossRef] [PubMed]
- Matsouka, C.; Zaspalis, V.; Nalbandian, L. Perovskites as oxygen carriers in chemical looping reforming process—Preparation of dense perovskite membranes and ionic conductivity measurement. Mater. Today Proc. 2018, 5, 27543–27552. [Google Scholar] [CrossRef]
- Nishihata, Y.; Mizuki, J.; Akao, T.; Tanaka, H.; Uenishi, M.; Kimura, M.; Okamoto, T.; Hamada, N. Self-regeneration of a Pd-perovskite catalyst for automotive emissions control. Nature 2002, 418, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Uenishi, M.; Taniguchi, M.; Tan, I.; Narita, K.; Kimura, M.; Kaneko, K.; Nishihata, Y.; Mizuki, J. The intelligent catalyst having the self-regenerative function of Pd, Rh and Pt for automotive emissions control. Catal. Today 2006, 117, 321–328. [Google Scholar] [CrossRef]
- Kousi, K.; Tang, C.; Metcalfe, I.S.; Neagu, D. Emergence and Future of Exsolved Materials. Small 2021, 17, 2006479. [Google Scholar] [CrossRef]
- Cali, E.; Kerherve, G.; Naufal, F.; Kousi, K.; Neagu, D.; Papaioannou, E.I.; Thomas, M.P.; Guiton, B.S.; Metcalfe, I.S.; Irvine, J.T.; et al. Exsolution of Catalytically Active Iridium Nanoparticles from Strontium Titanate. ACS Appl. Mater. Interfaces 2020, 12, 37444–37453. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent Advances in Novel Nanostructuring Methods of Perovskite Electrocatalysts for Energy-Related Applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Konsolakis, M. Three-way Catalysis. In Perovskites and Related Mixed Oxides; Wiley-VCH, Vergal GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 559–586. [Google Scholar] [CrossRef]
- Shen, M.; Zhao, Z.; Chen, J.; Su, Y.; Wang, J.; Wang, X. Effects of calcium substitute in LaMnO3 perovskites for NO catalytic oxidation. J. Rare Earths 2013, 31, 119–123. [Google Scholar] [CrossRef]
- Tarjomannejad, A.; Niaei, A.; Gómez, M.J.I.; Farzi, A.; Salari, D.; Albaladejo-Fuentes, V. NO+CO reaction over LaCu0.7B0.3O3 (B = Mn, Fe, Co) and La0.8A0.2Cu0.7Mn0.3O3 (A = Rb, Sr, Cs, Ba) perovskite-type catalysts. J. Therm. Anal. Calorim. 2017, 129, 671–680. [Google Scholar] [CrossRef]
- Hwang, J.; Rao, R.R.; Giordano, L.; Katayama, Y.; Yu, Y.; Shao-Horn, Y. Perovskites in catalysis and electrocatalysis. Science 2017, 358, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pan, Y.; Ge, L.; Chen, Y.; Mao, X.; Guan, D.; Li, M.; Zhong, Y.; Hu, Z.; Peterson, V.K.; et al. High-Performance Perovskite Composite Electrocatalysts Enabled by Controllable Interface Engineering. Small 2021, 17, 2101573. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, T.; Dong, X.; Li, M.; Wang, H. Effects of Ce substitution at the A-site of LaNi0.5Fe0.5O3 perovskite on the enhanced catalytic activity for dry reforming of methane. Appl. Catal. B Environ. 2018, 224, 214–221. [Google Scholar] [CrossRef]
- Sim, Y.; Kwon, D.; An, S.; Ha, J.M.; Oh, T.S.; Jung, J.C. Catalytic behavior of ABO3 perovskites in the oxidative coupling of methane. Mol. Catal. 2020, 489, 110925. [Google Scholar] [CrossRef]
- Bashan, V.; Ust, Y. Perovskite catalysts for methane combustion: Applications, design, effects for reactivity and partial oxidation. Int. J. Energy Res. 2019, 43, 7755–7789. [Google Scholar] [CrossRef]
- Bhattar, S.; Abedin, M.A.; Kanitkar, S.; Spivey, J.J. A review on dry reforming of methane over perovskite derived catalysts. Catal. Today 2021, 365, 2–23. [Google Scholar] [CrossRef]
- Zou, G.; Wang, Z.; Sun, M.; Luo, X.; Wang, X. A novel solid-gas process to synthesize LaMnO3 perovskite with high surface area and excellent activity for methane combustion. J. Nat. Gas Chem. 2021, 20, 294–298. [Google Scholar] [CrossRef]
- He, L.; Fan, Y.; Bellettre, J.; Yue, J.; Luo, L. A review on catalytic methane combustion at low temperatures: Catalysts, mechanisms, reaction conditions and reactor designs. Renew. Sustain. Energy Rev. 2020, 119, 109589. [Google Scholar] [CrossRef]
- Arai, H.; Yamada, T.; Eguchi, K.; Seiyama, T. Catalytic combustion of methane over various perovskite-type oxides. Appl. Catal. 1986, 26, 265–276. [Google Scholar] [CrossRef]
- Luo, L.; Wang, S.; Wu, Z.; Qin, Z.; Zhu, H.; Fan, W.; Wang, J. Structure and performance of supported iridium catalyst for the lean methane oxidation at low temperature. Appl. Catal. A 2022, 641, 118699. [Google Scholar] [CrossRef]
- Pliangos, A.; Yentekakis, I.V.; Papadakis, V.G.; Vayenas, C.G.; Verykios, X.E. Support-induced promotional effects on the activity of automotive exhaust catalysts: 1. The case of oxidation of light hydrocarbons (C2H4). Appl. Catal. B 1997, 14, 161–173. [Google Scholar] [CrossRef]
- Vayenas, C.G.; Brosda, S.; Pliangos, C. The double-layer approach to promotion, electrocatalysis, electrochemical promotion, and metal–support interactions. J. Catal. 2003, 216, 487–504. [Google Scholar] [CrossRef]
- Vernoux, P.; Lizarraga, L.; Tsampas, M.N.; Sapountzi, F.M.; DeLucas-Consuegra, A.; Valverde, J.L.; Souentie, S.; Vayenas, C.G.; Tsiplakides, D.; Balomenou, S.; et al. Ionically conducting ceramics as active catalyst supports. Chem. Rev. 2013, 113, 8192–8260. [Google Scholar] [CrossRef]
- Papadakis, V.G.; Pliangos, C.A.; Yentekakis, I.V.; Verykios, X.E.; Vayenas, C.G. Development of high performance, Pd-based, three way catalysts. Catal. Today 1996, 29, 71–75. [Google Scholar] [CrossRef]
- Konsolakis, M.; Drosou, C.; Yentekakis, I.V. Support mediated promotional effects of rare earth oxides (CeO2 and La2O3) on N2O decomposition and N2O reduction by CO or C3H6 over Pt/Al2O3 structured catalysts. Appl. Catal. B Environ. 2012, 123–124, 405–413. [Google Scholar] [CrossRef]
- Fornasiero, P.; Di Monte, R.; Ranga Rao, G.; Kaspar, J.; Meriani, S.; Trovarelli, A.; Graziani, M. Rh-loaded CeO2-ZrO2 solid-solutions as highly efficient oxygen exchangers: Dependence of the reduction behavior and the oxygen storage capacity on the structural properties. J. Catal. 1995, 151, 168–177. [Google Scholar] [CrossRef]
- Botzolaki, G.; Goula, G.; Rontogianni, A.; Nikolaraki, E.; Chalmpes, N.; Zygouri, P.; Karakassides, M.; Gournis, D.; Charisiou, N.D.; Goula, M.A.; et al. CO2 Methanation on Supported Rh Nanoparticles: The combined Effect of Support Oxygen Storage Capacity and Rh Particle Size. Catalysts 2020, 10, 944. [Google Scholar] [CrossRef]
- Dalla Betta, R.A.; Rostrup-Nielsen, T. Application of catalytic combustion to a 1.5 MW industrial gas turbine. Catal. Today 1999, 47, 369–375. [Google Scholar] [CrossRef]
- Haron, W.; Wisitsoraat, A.; Wongnawa, S. Comparison of monocrystalline LaMO3 (M = Co, Al) Perovskite Oxide Prepared by Co-Precipitation Method. Int. J. Chem. Eng. Appl. 2014, 5, 123–126. [Google Scholar] [CrossRef]
- Ponce, S.; Peña, M.A.; Fierro, J.L.G. Surface properties and catalytic performance in methane combustion of Sr-substituted lanthanum manganites. Appl. Catal. B Environ. 2000, 24, 193–205. [Google Scholar] [CrossRef]
- Saracco, G.; Geobaldo, F.; Baldi, G. Methane combustion on Mg-doped LaMnO3 perovskite catalysts. Appl. Catal. B Environ. 1999, 20, 277–288. [Google Scholar] [CrossRef]
- Kaliaquine, S.; Van Neste, A.; Szabo, V.; Gallot, J.E.; Bassir, M.; Muzychuk, R. Perovskite-type oxides synthesized by reactive grinding: Part I. Preparation and characterization. Appl. Catal. A 2001, 209, 345–358. [Google Scholar] [CrossRef]
- Patcas, F.; Buciuman, F.C.; Zsako, J. Oxygen non-stoichiometry and reducibility of B-site substituted lanthanum manganites. Thermoch. Acta 2000, 360, 71–76. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Chu, P.; Liu, J.; Wang, M.; Zhang, P.; Duan, E. Different active sites of LaCoO3 and LaMnO3 for CH4 oxidation by regulation of precursor’s ion concentration. Glob. Environ. Eng. 2020, 7, 28–39. [Google Scholar] [CrossRef]
- Nitadori, T.; Kurihara, S.; Misono, M. Catalytic properties of La1−xA′xMnO3 (A′ = Sr, Ce, Hf). J. Catal. 1986, 98, 221–228. [Google Scholar] [CrossRef]
- Kröger, F.A.; Vink, H.J.; Seitz, F.; Turnbull, D. (Eds.) Relations between the concentrations of imperfections in crystalline solids. In Solid State Physics; Academic Press: Cambridge, MA, USA, 1956; Volume 3, pp. 307–435. ISBN 9780126077032. [Google Scholar] [CrossRef]
- Nowotny, j.; Rekas, M. Defect chemistry of (La,Sr)MnO3. J. Am. Ceram. Soc. 1998, 81, 67–80. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Li, D.; Wang, Z.; Liu, G.; Liu, H.; Chen, Y. Oxalate route for promoting activity of manganese oxide catalysts in total VOCs’ oxidation: Effect of calcination temperature and preparation method. J. Mater. Chem. A 2014, 2, 2544–2554. [Google Scholar] [CrossRef]
- Hauptmann, W.; Votsmeier, M.; Gieshoff, J.; Drochner, A.; Vogel, H. Inverse hysteresis during the no oxidation on Pt under lean conditions. Appl. Catal. B Environ. 2009, 93, 22–29. [Google Scholar] [CrossRef]
- Grunwaldt, J.D.; van Vegten, N.; Baiker, A. Insight into the structure of supported palladium catalysts during the total oxidation of methane. Chem. Commun. 2007, 44, 4635–4637. [Google Scholar] [CrossRef] [PubMed]
- Al Soubaihi, R.M.; Saoud, K.M.; Dutta, J. Critical review of low-temperature CO oxidation and hysteresis phenomenon on heterogeneous catalysts. Catalysts 2018, 8, 660. [Google Scholar] [CrossRef]
- Lashina, E.A.; Slavinskaya, E.M.; Chumakova, N.A.; Stadnichenko, A.I.; Salanov, A.N.; Chumakov, G.A.; Boronin, A.I. Inverse temperature hysteresis and self-sustained oscillations in CO oxidation over Pd at elevated pressures of reaction mixture: Experiment and mathematical modeling. Chem. Eng. Sci. 2020, 212, 115312. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Zhang, L.; Rui, Z. Strong metal-support interaction assisted redispersion strategy for obtaining ultrafine and stable IrO2/Ir active sites with exceptional methane oxidation activity. Appl. Catal. B 2021, 297, 120410. [Google Scholar] [CrossRef]
- Wang, Y.; Arandiyan, H.; Tahini, H.A.; Scott, J.; Tan, X.; Dai, H.; Gale, J.D.; Rohl, A.L.; Smith, S.C.; Smith, R. The controlled disassembly of mesostructured perovskites as an avenue to fabricating high performance nanohybrid catalysts. Nat. Commun. 2017, 8, 15553. [Google Scholar] [CrossRef]
- Reinke, M.; Mantzaras, J.; Schaeren, R.; Bombach, R.; Inauen, A.; Schenker, S. High-pressure catalytic combustion of methane over platinum: In situ experiments and detailed numerical predictions. Combust. Flame 2004, 136, 217–240. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).