Recent Progress of Photothermal Therapy Based on Conjugated Nanomaterials in Combating Microbial Infections
Abstract
1. Introduction
2. PTT Based on Conjugated Nanomaterials
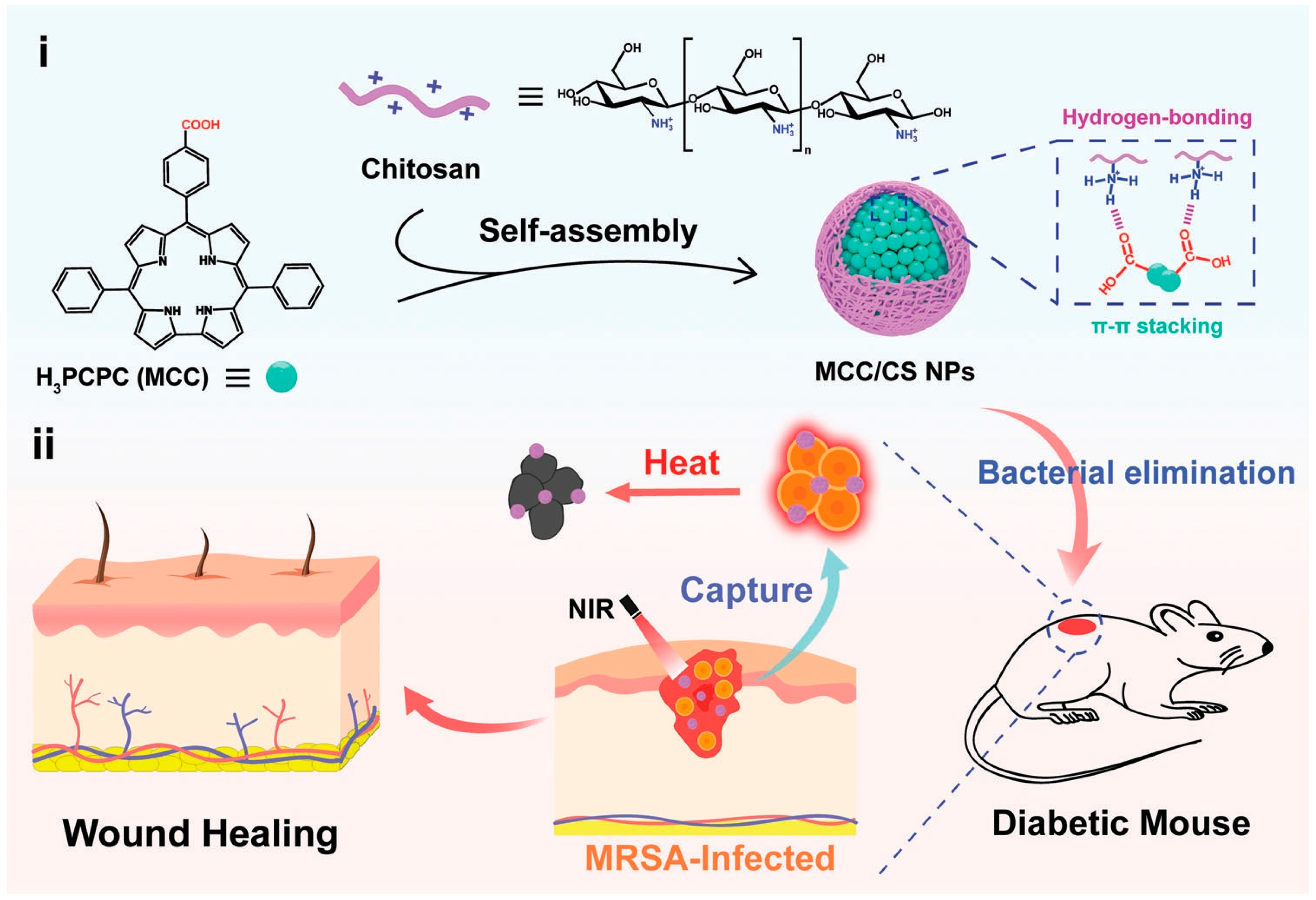
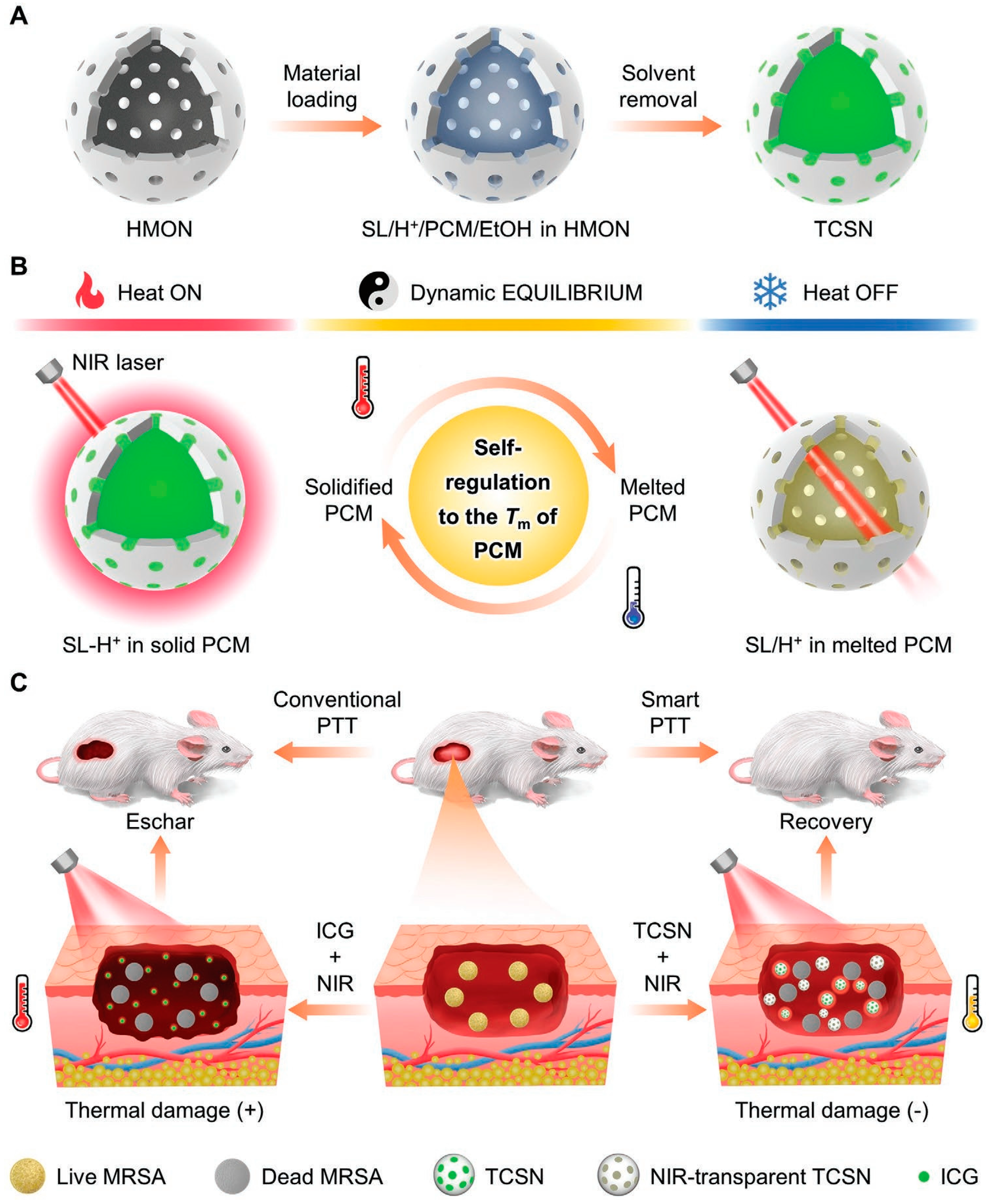
2.1. Conjugated Small Molecules
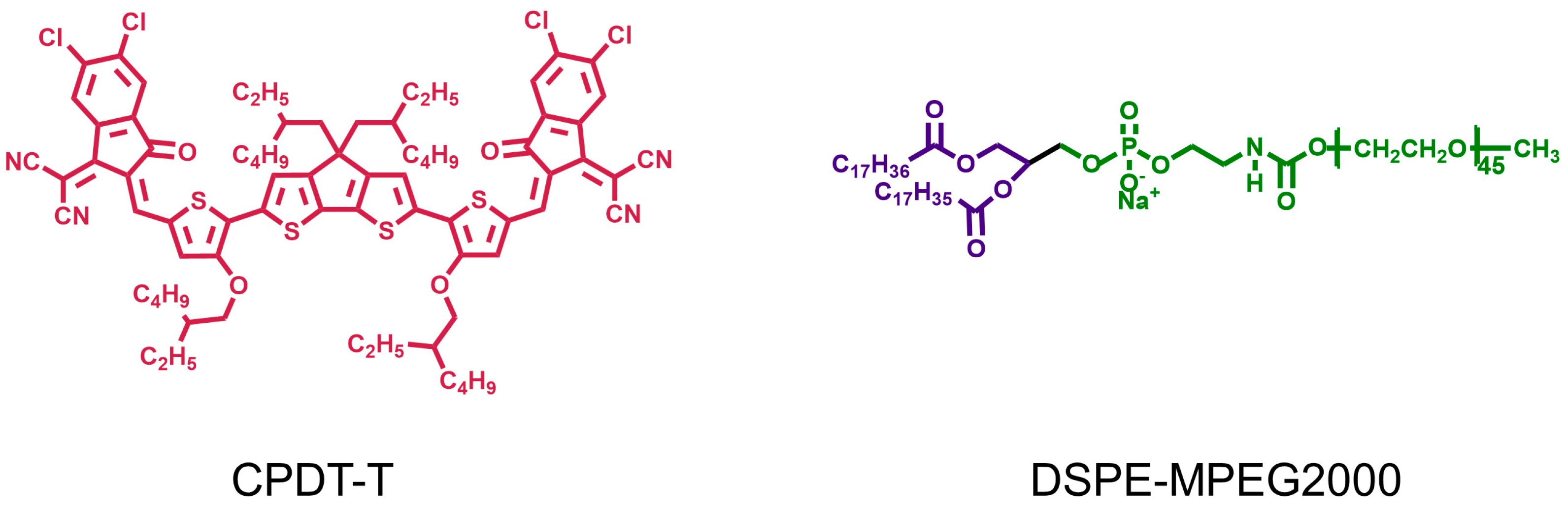
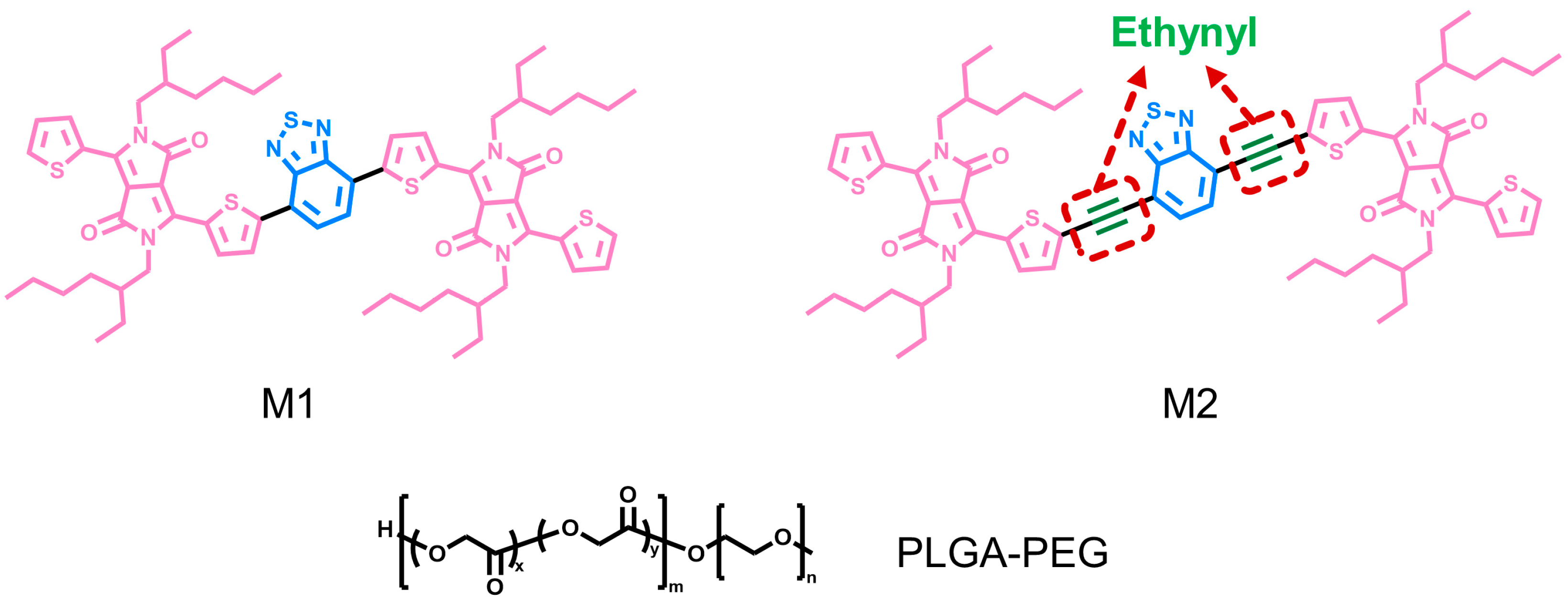
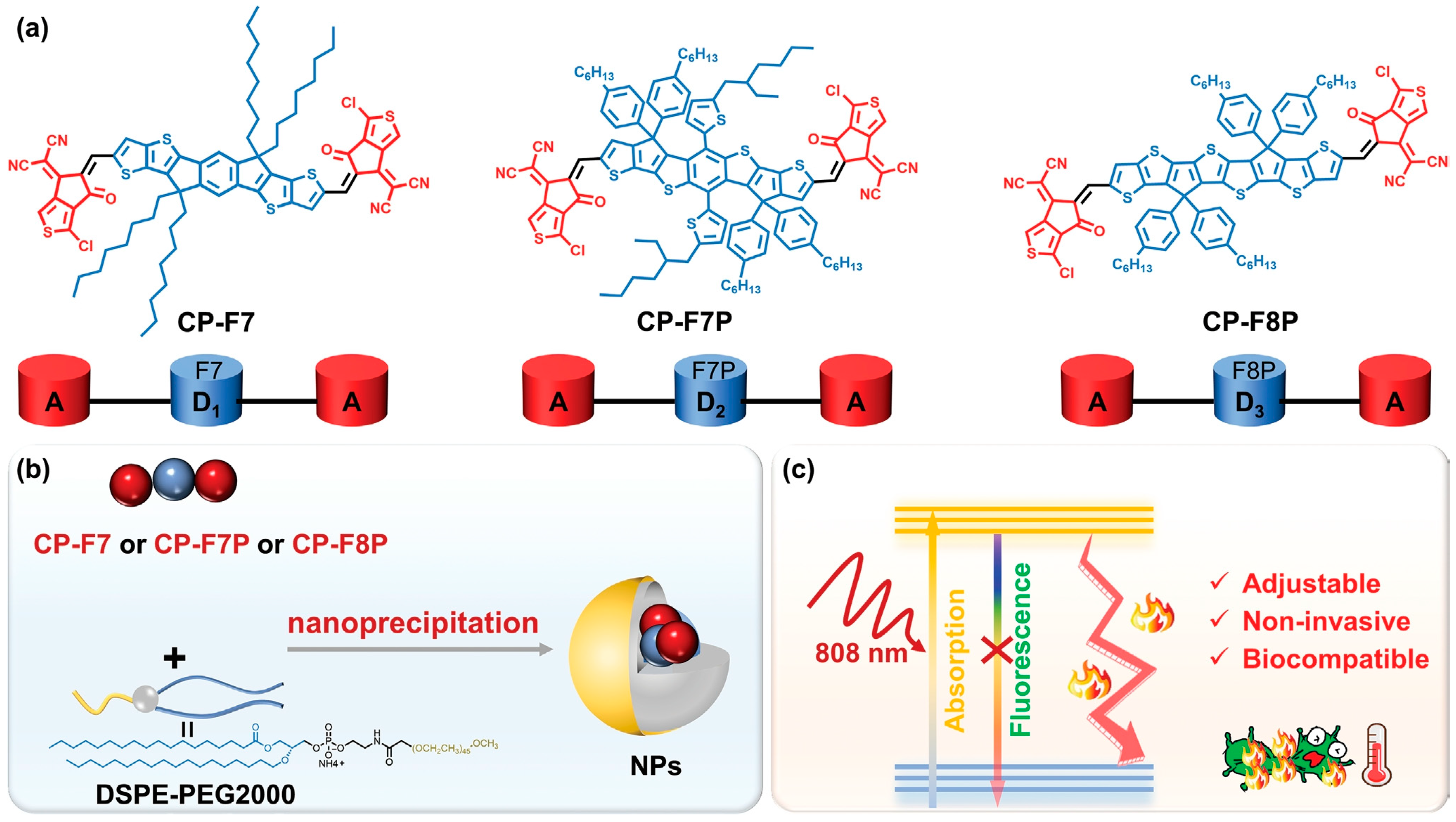
2.2. Conjugated Oligomers
2.3. Conjugated Polymers
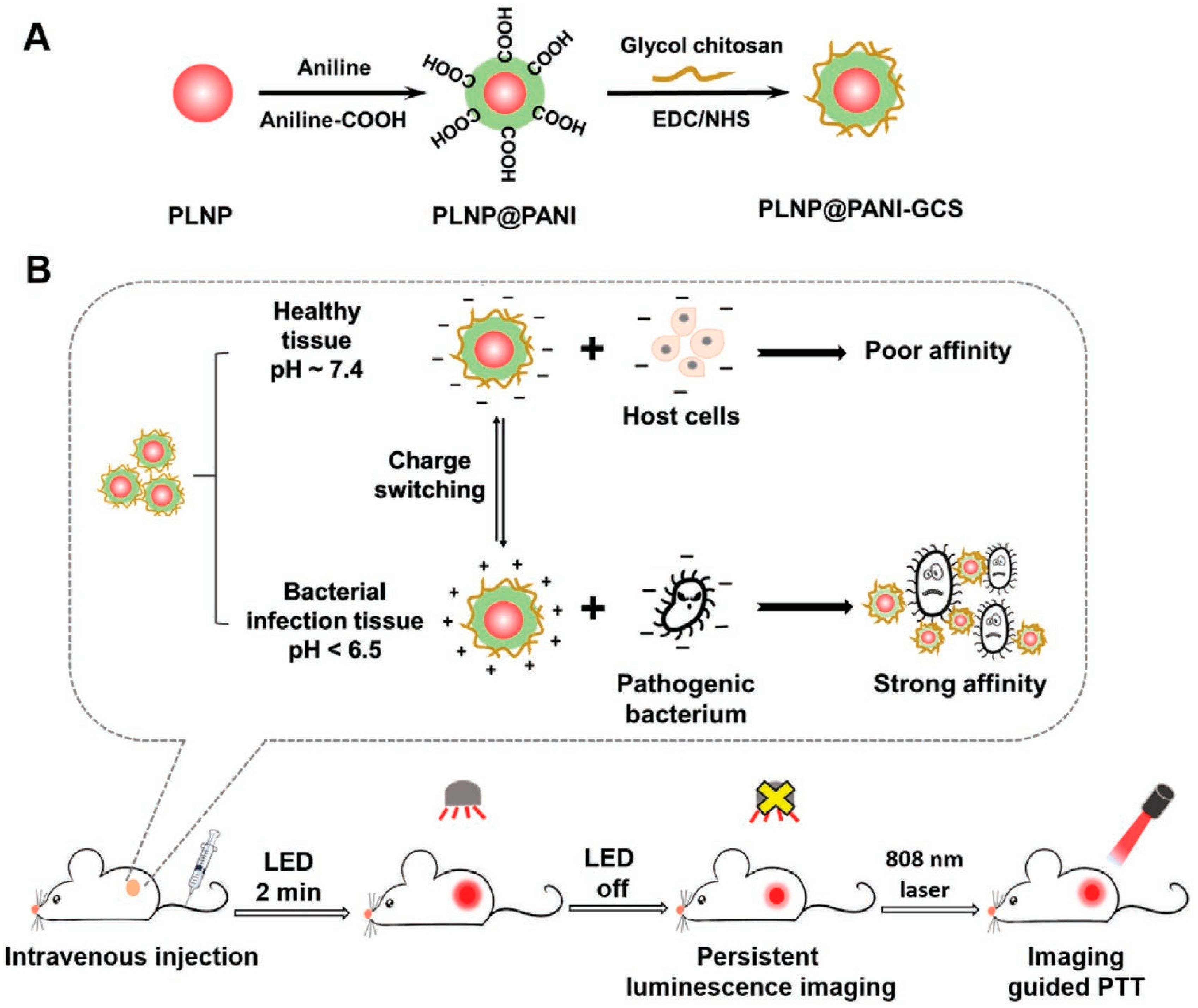
2.3.1. Polyaniline (PANI)
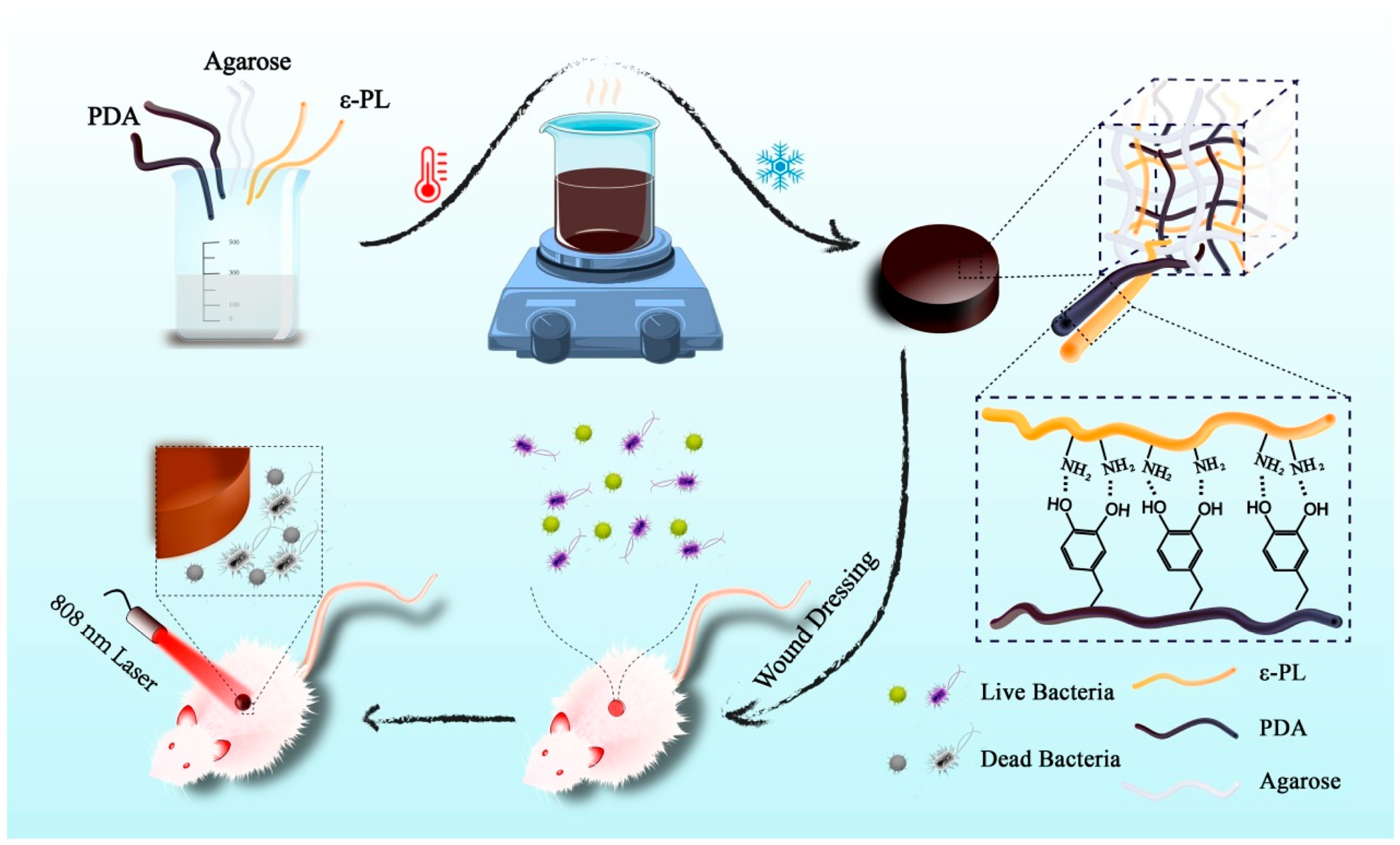
2.3.2. Polydopamine
2.3.3. Polypyrrole
| Conjugated Nanomaterials | Examples | Irradiation Conditions | Temperature of the Wound Reached (°C) | PCE | Antimicrobial Activity | Reference | |
|---|---|---|---|---|---|---|---|
| Conjugated small molecules | MCC/CS NPs | 606 nm; 500 mW·cm−2; 5 min | 51.3 | 66.4% | E. coli, MRSA ≈ 100% | [68] | |
| TCSN (SL, MA, BPA) | 1.5 W·cm−2; 5 min | 50 | - | MRSA = 99.99% | [74] | ||
| Conjugated oligomers | M2-NPs (BTD introduced with ethynyl groups) | 660 nm; 750 mW·cm−2; 8 min | 52 | 47.2% | C. albicans = 99%; S. aureus, E. coli = 97%; | [83] | |
| CP-F8P NPs | 808 nm; 1 W·cm−2; 5 min | 49.5 | 81.6% | S. aureus, C. albicans > 99%; Ampr E. coli = 99.9% | [84] | ||
| Conjugated polymers | PANI | Me-PANI NPs | 808 nm; 1.5 W·cm−2; 10 min | 58 | - | S. aureus, E. coli ≈ 100% | [92] |
| PLNP@PANI-GCS | 808 nm; 1.5 W·cm−2; 5 min | 52.2 | - | MRSA, S. aureus, E. coli > 99% | [24] | ||
| PDA | ADPH (agarose/PDA/ε-PL hydrogel) | 808 nm; 1 W·cm−2; 10 min | 59.4 | 63.9% | S. aureus, E. coli ≈ 100% | [105] | |
| B@MPDA-Mal | 808 nm; 1 W·cm−2; 10 min | 44.3 | - | MRSA = 95% | [107] | ||
| PPy | PVA-PPy NPs | 808 nm; 7 W·cm−2; 4 min | - | - | S. aureus = 98.3%; E. coli = 97.2% | [114] | |
| BP-SA | 808 nm; 10 min | 58.2 | - | S. aureus = 99.3%; E. coli = 99.5% | [119] | ||
| Pseudo-conjugated polymers | pPCP-NPs | 1064 nm; 1 W·cm−2; 10 min | 52.2 | 51.5% | ABS-SA, ABS-EC ≈ 100% | [28] | |
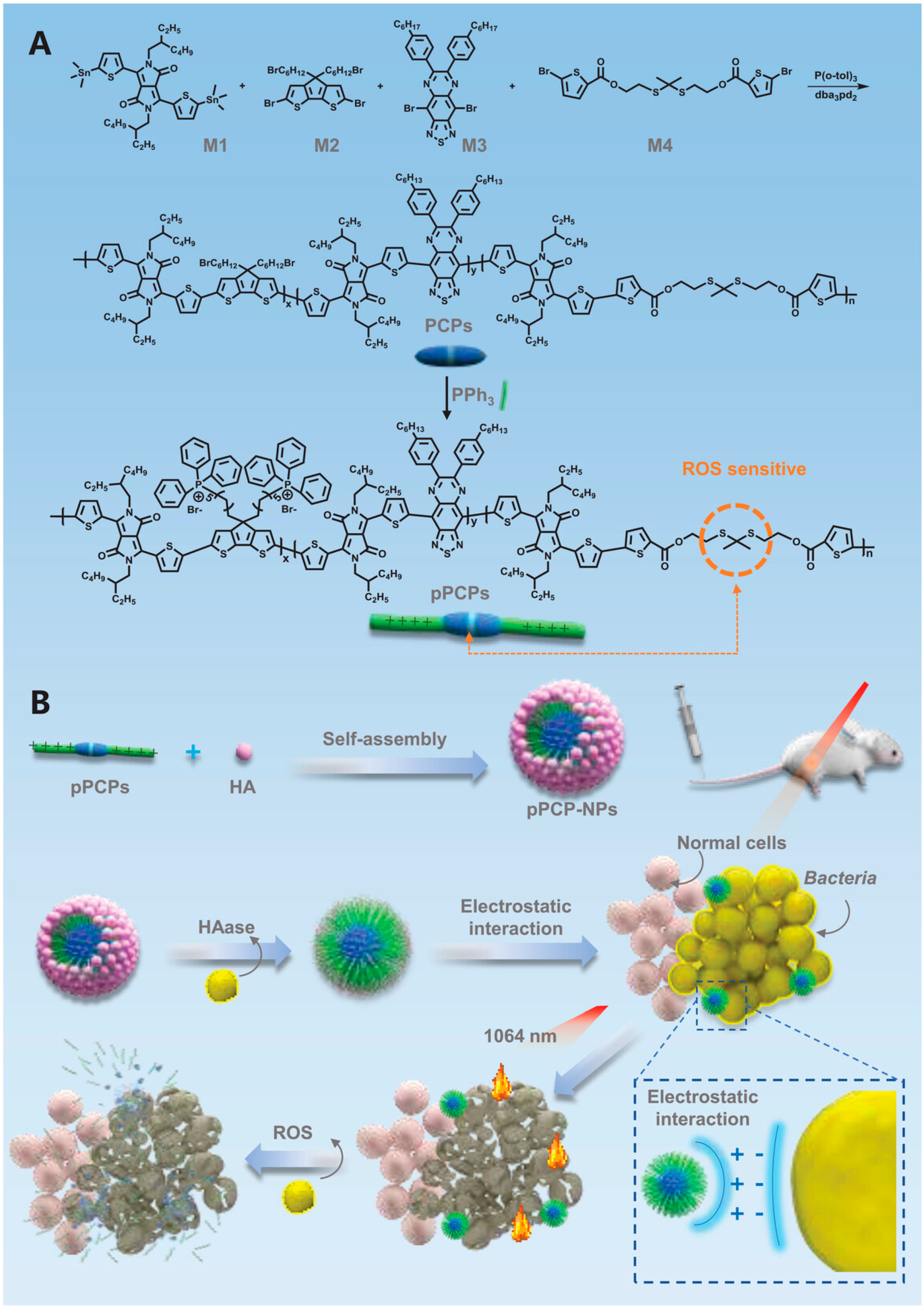
2.4. Pseudo-Conjugated Polymers
3. Combination Therapy of PTT and PDT Based on Conjugated Nanomaterials
4. Summary and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehrabi, M.R.; Soltani, M.; Chiani, M.; Raahemifar, K.; Farhangi, A. Nanomedicine: New Frontiers in Fighting Microbial Infections. Nanomaterials 2023, 13, 483. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, T.R.; Wells, T.J.; Souza-Fonseca-Guimaraes, F. Towards efficient immunotherapy for bacterial infection. Trends Microbiol. 2022, 30, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Baran, A.; Kwiatkowska, A.; Potocki, L. Antibiotics and Bacterial Resistance-A Short Story of an Endless Arms Race. Int. J. Mol. Sci. 2023, 24, 5777. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.U.; Ahmad, M.M. Nanomaterials Aiming to Tackle Antibiotic-Resistant Bacteria. Pharmaceutics 2022, 14, 582. [Google Scholar] [CrossRef] [PubMed]
- Idris, F.N.; Nadzir, M.M. Multi-drug resistant ESKAPE pathogens and the uses of plants as their antimicrobial agents. Arch. Microbiol. 2023, 205, 115. [Google Scholar] [CrossRef] [PubMed]
- Gajdacs, M.; Albericio, F. Antibiotic Resistance: From the Bench to Patients. Antibiotics 2019, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Yang, Y.; Zheng, W.; Huang, Y.; Xu, F.; Bao, Z. Synergistic photothermal antibacterial therapy enabled by multifunctional nanomaterials: Progress and perspectives. Mater. Chem. Front. 2023, 7, 355–380. [Google Scholar] [CrossRef]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef]
- Roemhild, R.; Bollenbach, T.; Andersson, D.I. The physiology and genetics of bacterial responses to antibiotic combinations. Nat. Rev. Microbiol. 2022, 20, 478–490. [Google Scholar] [CrossRef]
- Nichols, R.J.; Sen, S.; Choo, Y.J.; Beltrao, P.; Zietek, M.; Chaba, R.; Lee, S.; Kazmierczak, K.M.; Lee, K.J.; Wong, A.; et al. Phenotypic landscape of a bacterial cell. Cell 2011, 144, 143–156. [Google Scholar] [CrossRef]
- Minato, Y.; Dawadi, S.; Kordus, S.L.; Sivanandam, A.; Aldrich, C.C.; Baughn, A.D. Mutual potentiation drives synergy between trimethoprim and sulfamethoxazole. Nat. Commun. 2018, 9, 1003. [Google Scholar] [CrossRef] [PubMed]
- Songca, S.P.; Adjei, Y. Applications of Antimicrobial Photodynamic Therapy against Bacterial Biofilms. Int. J. Mol. Sci. 2022, 23, 3209. [Google Scholar] [CrossRef] [PubMed]
- Yougbare, S.; Chou, H.L.; Yang, C.H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Kuo, T.R. Facet-dependent gold nanocrystals for effective photothermal killing of bacteria. J. Hazard. Mater. 2021, 407, 124617. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, L.; Gong, F.; Yang, X.; Han, Z.; Wang, Y.; Ge, J.; Gao, X.; Li, Y.; Zhong, X.; et al. PtCu Nanosonosensitizers with Inflammatory Microenvironment Regulation for Enhanced Sonodynamic Bacterial Elimination and Tissue Repair. Adv. Funct. Mater. 2023, 33, 2212489. [Google Scholar] [CrossRef]
- Chen, L.; Xing, S.; Lei, Y.; Chen, Q.; Zou, Z.; Quan, K.; Qing, Z.; Liu, J.; Yang, R. A Glucose-Powered Activatable Nanozyme Breaking pH and H(2) O(2) Limitations for Treating Diabetic Infections. Angew. Chem. Int. Ed. 2021, 60, 23534–23539. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Shi, Q.Q.; Zha, Z.B.; Zhu, D.D.; Zheng, L.R.; Shi, L.X.; Wei, X.W.; Lian, L.; Wu, K.L.; Cheng, L. Copper single-atom catalysts with photothermal performance and enhanced nanozyme activity for bacteria-infected wound therapy. Bioact. Mater. 2021, 6, 4389–4401. [Google Scholar] [CrossRef]
- Yu, C.H.; Sui, S.Y.; Yu, X.T.; Huang, W.L.; Wu, Y.F.; Zeng, X.; Chen, Q.M.; Wang, J.; Peng, Q. Ti3C2Tx MXene loaded with indocyanine green for synergistic photothermal and photodynamic therapy for drug-resistant bacterium. Colloids Surf. B-Biointerfaces 2022, 217, 112663. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.Y.; Ling, M.J.; Sun, R.; Zhu, M.Y.; Chen, J.J.; Yu, M.; Peng, Z.W.; Yu, Z.Q.; Liu, X.Q. Near-Infrared II Light-Triggered Robust Carbon Radical Generation for Combined Photothermal and Thermodynamic Therapy of Hypoxic Tumors. Adv. Funct. Mater. 2021, 31, 2101709. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.P.; Qian, C.G.; Guo, Y.X.; Li, C.Z.; Gao, F.; Yang, Y.; Wang, K.K.; Oupicky, D.; Sun, M.J. Near-infrared light-activated IR780-loaded liposomes for anti-tumor angiogenesis and Photothermal therapy. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2283–2294. [Google Scholar] [CrossRef]
- He, W.; Wang, Z.Y.; Bai, H.T.; Zhao, Z.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Highly efficient photothermal nanoparticles for the rapid eradication of bacterial biofilms. Nanoscale 2021, 13, 13610–13616. [Google Scholar] [CrossRef]
- Shanmugam, V.; Selvakumar, S.; Yeh, C.S. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem. Soc. Rev. 2014, 43, 6254–6287. [Google Scholar] [CrossRef]
- Korupalli, C.; Kalluru, P.; Nuthalapati, K.; Kuthala, N.; Thangudu, S.; Vankayala, R. Recent Advances of Polyaniline-Based Biomaterials for Phototherapeutic Treatments of Tumors and Bacterial Infections. Bioengineering 2020, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Han, X.; Wang, M.; Li, C.; Jia, T.; Zhao, X. Recent advances in the development of near-infrared organic photothermal agents. Chem. Eng. J. 2021, 417, 128844. [Google Scholar] [CrossRef]
- Yan, L.X.; Chen, L.J.; Zhao, X.; Yan, X.P. pH Switchable Nanoplatform for In Vivo Persistent Luminescence Imaging and Precise Photothermal Therapy of Bacterial Infection. Adv. Funct. Mater. 2020, 30, 1909042. [Google Scholar] [CrossRef]
- Lapotko, D.O. Nanophotonics and Theranostics: Will Light Do the Magic? Theranostics 2013, 3, 138–140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qin, Z.J.; Ren, T.B.; Zhou, H.J.; Zhang, X.X.; He, L.; Li, Z.; Zhang, X.B.; Yuan, L. NIRII-HDs: A Versatile Platform for Developing Activatable NIR-II Fluorogenic Probes for Reliable In Vivo Analyte Sensing. Angew. Chem. Int. Ed. 2022, 61, e202201541. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Huang, Y.X.; Huang, X.Y.; Wang, F.; Wei, X.B. Copolymerized carbon nitride nanoparticles for near-infrared II photoacoustic-guided synergistic photothermal/radiotherapy. Front. Chem. 2023, 11, 1124559. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Tang, D.; Kang, X.; Yuan, H.; Yu, Y.; Xiong, X.; Wu, N.; Chen, F.; Wang, X.; Xiao, H.; et al. Degradable Pseudo Conjugated Polymer Nanoparticles with NIR-II Photothermal Effect and Cationic Quaternary Phosphonium Structural Bacteriostasis for Anti-Infection Therapy. Adv. Sci. 2022, 9, e2200732. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Zhen, X.; Xie, C.; Pu, K. Dual-Peak Absorbing Semiconducting Copolymer Nanoparticles for First and Second Near-Infrared Window Photothermal Therapy: A Comparative Study. Adv. Mater. 2018, 30, 1705980. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Li, J.Y.; Mu, F.; Wang, J.; Shen, C.; Wang, H.; Huang, F.; Chen, B.; Luo, Z.M.; et al. DNA-Templated Ag@Pd Nanoclusters for NIR-II Photoacoustic Imaging-Guided Photothermal-Augmented Nanocatalytic Therapy. Adv. Healthc. Mater. 2023, 2300267. [Google Scholar] [CrossRef]
- Shi, W.Q.; Han, Q.R.; Wu, J.J.; Ji, C.Y.; Zhou, Y.Q.; Li, S.H.; Gao, L.P.; Leblanc, R.M.; Peng, Z.L. Synthesis Mechanisms, Structural Models, and Photothermal Therapy Applications of Top-Down Carbon Dots from Carbon Powder, Graphite, Graphene, and Carbon Nanotubes. Int. J. Mol. Sci. 2022, 23, 1456. [Google Scholar] [CrossRef] [PubMed]
- Qie, X.W.; Zan, M.H.; Gui, P.; Chen, H.Y.; Wang, J.K.; Lin, K.C.; Mei, Q.; Ge, M.F.; Zhang, Z.Q.; Tang, Y.G.; et al. Design, Synthesis, and Application of Carbon Dots With Synergistic Antibacterial Activity. Front. Bioeng. Biotechnol. 2022, 10, 894100. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Chu, G.; Jin, J.; Liu, C.; Cheng, L.; Guo, Y.; Deng, Z.; Wang, Y. A Combined Cyanine/Carbomer Gel Enhanced Photodynamic Antimicrobial Activity and Wound Healing. Nanomaterials 2022, 12, 2173. [Google Scholar] [CrossRef]
- Shi, J.P.; Li, J.; Wang, Y.; Cheng, J.J.; Zhang, C.Y. Recent advances in MoS2-based photothermal therapy for cancer and infectious disease treatment. J. Mater. Chem. B 2020, 8, 5793–5807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.W.; Kuang, Z.; Song, P.; Li, W.Z.; Gui, L.; Tang, C.C.; Tao, Y.G.; Ge, F.; Zhu, L.B. Synthesis of a Two-Dimensional Molybdenum Disulfide Nanosheet and Ultrasensitive Trapping of Staphylococcus Aureus for Enhanced Photothermal and Antibacterial Wound-Healing Therapy. Nanomaterials 2022, 12, 1865. [Google Scholar] [CrossRef] [PubMed]
- Li, C.N.; Lin, W.H.; Liu, S.; Sun, T.T.; Xie, Z.G. Structural optimization of organic fluorophores for highly efficient photothermal therapy. Mater. Chem. Front. 2021, 5, 284–292. [Google Scholar] [CrossRef]
- Song, X.J.; Chen, Q.; Liu, Z. Recent advances in the development of organic photothermal nano-agents. Nano Res. 2015, 8, 340–354. [Google Scholar] [CrossRef]
- Liu, L.C.; Wang, W.F.; Hong, W.H.; Jin, Y.Y.; Wang, L.C.; Liu, S.J.; Wang, A.L.; Liu, X.S. Photothermal 2D Nanosheets Combined with Astragaloside IV for Antibacterial Properties and Promoting Angiogenesis to Treat Infected Wounds. Front. Bioeng. Biotechnol. 2022, 9, 826011. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, S.; Li, J.; Wei, J.; Muellen, K.; Yin, M. A Water-Soluble, NIR-Absorbing Quaterrylenediimide Chromophore for Photoacoustic Imaging and Efficient Photothermal Cancer Therapy. Angew. Chem. Int. Ed. 2019, 58, 1638–1642. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, M.; Liu, W.; Zeng, X.; Song, X.; Yang, X.; Zhang, X.; Feng, J. Metal Ion/Tannic Acid Assembly as a Versatile Photothermal Platform in Engineering Multimodal Nanotheranostics for Advanced Applications. ACS Nano 2018, 12, 3917–3927. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Zhou, J.; Chen, X. Photothermal Janus PPy-SiO2@PAN/F-SiO2@PVDF-HFP membrane for high-efficient, low energy and stable desalination through solar membrane distillation. Chem. Eng. J. 2023, 451, 138473. [Google Scholar] [CrossRef]
- Sarkar, S.; Levi-Polyachenko, N. Conjugated polymer nano-systems for hyperthermia, imaging and drug delivery. Adv. Drug Deliv. Rev. 2020, 163, 40–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Zhang, S.Y.; Zhang, Z.H.; Ji, L.L.; Zhang, J.M.; Wang, Q.H.; Guo, T.; Ni, S.M.; Cai, R.; Mu, X.Y.; et al. Recent Progress on NIR-II Photothermal Therapy. Front. Chem. 2021, 9, 728066. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, F.; Guo, Z.; Xiao, Y.; Zhang, Y.; Sun, X.; Zhe, T.; Cao, Y.; Wang, L.; Lu, Q.; et al. Silver nanoparticle-embedded hydrogel as a photothermal platform for combating bacterial infections. Chem. Eng. J. 2020, 382, 122990. [Google Scholar] [CrossRef]
- Jung, H.S.; Verwilst, P.; Sharma, A.; Shin, J.; Sessler, J.L.; Kim, J.S. Organic molecule-based photothermal agents: An expanding photothermal therapy universe. Chem. Soc. Rev. 2018, 47, 2280–2297. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Yu, Y.; Huang, Y.; Jiang, Y.; Zhao, Y.; Nie, Z.; Wang, F.; Ma, W.; Yu, Z.; Huang, Y.; et al. A Near-Infrared-II Polymer with Tandem Fluorophores Demonstrates Superior Biodegradability for Simultaneous Drug Tracking and Treatment Efficacy Feedback. ACS Nano 2021, 15, 5428–5438. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Wang, Z.; Feng, L. Photothermal Conjugated Polymers and Their Biological Applications in Imaging and Therapy. ACS Appl. Polym. Mater. 2020, 2, 4222–4240. [Google Scholar] [CrossRef]
- Qi, J.; Ou, H.; Liu, Q.; Ding, D. Gathering brings strength: How organic aggregates boost disease phototheranostics. Aggregate 2021, 2, 95–113. [Google Scholar] [CrossRef]
- Lin, F.; Duan, Q.-Y.; Wu, F.-G. Conjugated Polymer-Based Photothermal Therapy for Killing Microorganisms. ACS Appl. Polym. Mater. 2020, 2, 4331–4344. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, L.; Wang, C.; Peng, R.; Liu, Z. Conjugated polymers for photothermal therapy of cancer. Polym. Chem. 2014, 5, 1573–1580. [Google Scholar] [CrossRef]
- Ponzio, R.A.; Ibarra, L.E.; Achilli, E.E.; Odella, E.; Chesta, C.A.; Martinez, S.R.; Palacios, R.E. Sweet light o? mine: Photothermal and photodynamic inactivation of tenacious pathogens using conjugated polymers. J. Photochem. Photobiol. B-Biol. 2022, 234, 112510. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Huang, Y.Y.; Zhang, Z.C.; Quan, H.; Yao, Y. Organic conjugated small molecules with donor-acceptor structures: Design and application in the phototherapy of tumors. Mater. Chem. Front. 2022, 6, 2968–2993. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, H.-M.; Song, G.; Li, Z.; Zhang, X.-B. Conjugated-Polymer-Based Nanomaterials for Photothermal Therapy. ACS Appl. Polym. Mater. 2020, 2, 4258–4272. [Google Scholar] [CrossRef]
- Geng, J.; Sun, C.; Liu, J.; Liao, L.-D.; Yuan, Y.; Thakor, N.; Wang, J.; Liu, B. Biocompatible Conjugated Polymer Nanoparticles for Efficient Photothermal Tumor Therapy. Small 2015, 11, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Shirakawa, H.; Ikeda, S. Simultaneous polymerization and formation of polyacetylene film on the surface of concentrated soluble Ziegler-type catalyst solution. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 2533–2542. [Google Scholar] [CrossRef]
- Jia, W.; Huang, F.; Zhang, Q.; Zhao, L.; Li, C.; Lu, Y. Novel conjugated small molecule-based nanoparticles for NIR-II photothermal antibacterial therapy. Chem. Commun. 2022, 58, 6340–6343. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wen, K.; Chen, H.; Jiang, S.; Wu, X.; Lv, L.; Peng, A.; Zhang, S.; Huang, H. Achieving High-Performance Photothermal and Photodynamic Effects upon Combining D-A Structure and Nonplanar Conformation. Small 2020, 16, 2000909. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Fu, J.; Zhou, Y.; Shi, Y.; Wang, J.; Feng, X.; Zhao, Y.; Zhou, G.; Lu, C.; Quan, G.; et al. Metal-Organic Framework-Based Chemo-Photothermal Combinational System for Precise, Rapid, and Efficient Antibacterial Therapeutics. Pharmaceutics 2019, 11, 463. [Google Scholar] [CrossRef]
- Ting, C.-W.; Chou, Y.-H.; Huang, S.-Y.; Chiang, W.-H. Indocyanine green-carrying polymeric nanoparticles with acid-triggered detachable PEG coating and drug release for boosting cancer photothermal therapy. Colloids Surf. B-Biointerfaces 2021, 208, 112048. [Google Scholar] [CrossRef]
- Sahu, A.; Choi, W.I.; Lee, J.H.; Tae, G. Graphene oxide mediated delivery of methylene blue for combined photodynamic and photothermal therapy. Biomaterials 2013, 34, 6239–6248. [Google Scholar] [CrossRef]
- Lv, Y.; Li, P.; Su, R.; Cai, J.; Zhong, H.; Wen, F.; Su, W. Methylene Blue/Carbon Dots Composite with Photothermal and Photodynamic Properties: Synthesis, Characterization, and Antibacterial Application. Photochem. Photobiol. 2023, 99, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, C.; Lu, B.; Yao, Y. Enhancing the Stability and Photothermal Conversion Efficiency of ICG by Pillar 5 arene-Based Host-Guest Interaction. Front. Chem. 2021, 9, 775436. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zhang, Z.; Jin, D.; Yuan, X.; Wang, J.; Ding, Y.; Wang, Y.; Yao, Y. A-DA′D-A fused-ring small molecule-based nanoparticles for combined photothermal and photodynamic therapy of cancer. Chem. Commun. 2021, 57, 12020–12023. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dai, W.; Peng, Y.; Niu, Z.; Sun, Q.; Shan, C.; Yang, H.; Verma, G.; Wojtas, L.; Yuan, D.; et al. A Corrole-Based Covalent Organic Framework Featuring Desymmetrized Topology. Angew. Chem. Int. Ed. 2020, 59, 4354–4359. [Google Scholar] [CrossRef]
- You, L.; Shen, H.; Shi, L.; Zhang, G.; Liu, H.; Wang, H.; Ji, L. Photophysical properties of the Corrole photosensitizers. Sci. China-Phys. Mech. Astron. 2010, 53, 1491–1496. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, R.-X.; Liu, H.-Y.; Chang, C.K. Corrole-based photodynamic antitumor therapy. J. Chin. Chem. Soc. 2019, 66, 1090–1099. [Google Scholar] [CrossRef]
- Shao, W.; Wang, H.; He, S.; Shi, L.; Peng, K.; Lin, Y.; Zhang, L.; Ji, L.; Liu, H. Photophysical Properties and Singlet Oxygen Generation of Three Sets of Halogenated Corroles. J. Phys. Chem. B 2012, 116, 14228–14234. [Google Scholar] [CrossRef]
- Yu, Y.; Tian, R.; Zhao, Y.; Qin, X.; Hu, L.; Zou, J.-J.; Yang, Y.-W.; Tian, J. Self-Assembled Corrole/Chitosan Photothermal Nanoparticles for Accelerating Infected Diabetic Wound Healing. Adv. Healthc. Mater. 2022, 12, 2201651. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, Z.; Sun, W.; Lin, X.; Wang, R.; Li, C.; Zong, L.; Fu, Z.; Liu, H.; Xu, S. Robust emission in near-infrared II of lanthanide nanoprobes conjugated with Au (LNPs-Au) for temperature sensing and controlled photothermal therapy. Chem. Eng. J. 2023, 452, 139504. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, G.; Ruan, Y.; Feng, K.; Gao, M.; Liu, Y.; Sun, X. Temperature-Responsive Nanoassemblies for Self-Regulated Photothermal Therapy and Controlled Copper Release to Accelerate Chronic Wound Healing. ACS Appl. Bio Mater. 2023, 6, 2003–2013. [Google Scholar] [CrossRef]
- Xia, Y.; Li, C.; Cao, J.; Chen, Z.; Wang, J.; Wu, Y.; Zhang, X. Liposome-templated gold nanoparticles for precisely temperature-controlled photothermal therapy based on heat shock protein expression. Colloids Surf. B-Biointerfaces 2022, 217, 112686. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Feng, L.; Qi, S.; Cao, J.; Ge, Y.; Wu, L.; Wang, S. Reversible Thermochromic Nanoparticles Composed of a Eutectic Mixture for Temperature-Controlled Photothermal Therapy. Nano Lett. 2020, 20, 2137–2143. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; He, X.; Obeng, E.; Ye, Z.; Shen, J.; Ding, X. Two-dimensional NbS2 nanosheets with hyperthermia for killing bacteria to promote infected wound healing. Mater. Des. 2022, 223, 111124. [Google Scholar] [CrossRef]
- Wang, J.X.; Hao, B.Y.; Xue, K.; Fu, H.; Xiao, M.H.; Zhang, Y.X.; Shi, L.Q.; Zhu, C.L. A Smart Photothermal Nanosystem with an Intrinsic Temperature-Control Mechanism for Thermostatic Treatment of Bacterial Infections. Adv. Mater. 2022, 34, 2205653. [Google Scholar] [CrossRef]
- Shao, W.; Wei, Q.; Wang, S.; Li, F.; Wu, J.; Ren, J.; Cao, F.; Liao, H.; Gao, J.-Q.; Zhou, M.; et al. Molecular engineering of D-A-D conjugated small molecule nanoparticles for high performance NIR-II photothermal therapy. Mater. Horiz. 2020, 7, 1379–1386. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, X.; Zhang, H.; Ou, H.; Lam, J.W.Y.; Liu, Y.; Shi, L.; Ding, D.; Tang, B.Z. Molecular Motion in Aggregates: Manipulating TICT for Boosting Photothermal Theranostics. J. Am. Chem. Soc. 2019, 141, 5359–5368. [Google Scholar] [CrossRef]
- Wu, Q.; Zhu, Y.; Fang, X.; Hao, X.; Jiao, L.; Hao, E.; Zhang, W. Conjugated BODIPY Oligomers with Controllable Near-Infrared Absorptions as Promising Phototheranostic Agents through Excited-State Intramolecular Rotations. ACS Appl. Mater. Interfaces 2020, 12, 47208–47219. [Google Scholar] [CrossRef]
- Jia, S.; Li, Z.; Shao, J.; Yuan, H.; Li, L.; Xu, L. Acceptor Regulation of Acceptor-Donor-Acceptor Type Conjugated Oligomer for Photothermal Combating of Resistant Bacteria. ACS Appl. Polym. Mater. 2022, 4, 5275–5280. [Google Scholar] [CrossRef]
- Zhu, C.L.; Yang, Q.O.; Liu, L.B.; Lv, F.T.; Li, S.Y.; Yang, G.Q.; Wang, S. Multifunctional Cationic Poly(p-phenylene vinylene) Polyelectrolytes for Selective Recognition, Imaging, and Killing of Bacteria Over Mammalian Cells. Adv. Mater. 2011, 23, 4805–4810. [Google Scholar] [CrossRef]
- Zhou, L.; Lv, F.; Liu, L.; Wang, S. Water-Soluble Conjugated Organic Molecules as Optical and Electrochemical Materials for Interdisciplinary Biological Applications. Acc. Chem. Res. 2019, 52, 3211–3222. [Google Scholar] [CrossRef]
- He, Y.; Li, N.; Yang, S.; Tan, X.; Tang, L.; Yang, Q. Near-Infrared Molecular Photosensitizer Decorated with Quaternary Ammonium for High-Efficiency Photothermal Treatment of Bacterial Infections. Chemosensors 2023, 11, 164. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Li, S.; Wan, Y.; Chen, J.-X.; Tian, S.; Huang, Z.; Xiao, Y.-F.; Cui, X.; Xiang, C.; et al. Biodegradable pi-Conjugated Oligomer Nanoparticles with High Photothermal Conversion Efficiency for Cancer Theranostics. ACS Nano 2019, 13, 12901–12911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, L.; Wang, Y.; Feng, L. Molecular engineering to boost the photothermal effect of conjugated oligomer nanoparticles. Biomater. Sci. 2021, 9, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, Z.; Zhao, Q.; Jia, S.; Wang, T.; Xu, L.; Yuan, H.; Li, S. Molecular Evolution of Acceptor-Donor-Acceptor-type Conjugated Oligomer Nanoparticles for Efficient Photothermal Antimicrobial Therapy. Adv. Funct. Mater. 2023, 33, 2213209. [Google Scholar] [CrossRef]
- Della Pina, C.; Falletta, E. Advances in Polyaniline for Biomedical Applications. Curr. Med. Chem. 2022, 29, 329–357. [Google Scholar] [CrossRef]
- Hong, Y.; Cho, W.; Kim, J.; Hwng, S.; Lee, E.; Heo, D.; Ku, M.; Suh, J.S.; Yang, J.; Kim, J.H. Photothermal ablation of cancer cells using self-doped polyaniline nanoparticles. Nanotechnology 2016, 27, 185104. [Google Scholar] [CrossRef]
- Wang, J.P.; Yan, R.; Guo, F.; Yu, M.; Tan, F.P.; Li, N. Targeted lipid-polyaniline hybrid nanoparticles for photoacoustic imaging guided photothermal therapy of cancer. Nanotechnology 2016, 27, 285102. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, Z.G.; Zhu, X.J.; Wang, X.J.; Liao, Y.; Ma, Z.F.; Li, F.Y. NIR photothermal therapy using polyaniline nanoparticles. Biomaterials 2013, 34, 9584–9592. [Google Scholar] [CrossRef]
- Bidez, P.R.; Li, S.X.; MacDiarmid, A.G.; Venancio, E.C.; Wei, Y.; Lelkes, P.I. Polyaniline, an electroactive polymer, supports adhesion and proliferation of cardiac myoblasts. J. Biomater. Sci. Polym. Ed. 2006, 17, 199–212. [Google Scholar] [CrossRef]
- Ghahremanloo, A.; Zare, E.N.; Salimi, F.; Makvandi, P. Electroconductive and photoactive poly(phenylenediamine)s with antioxidant and antimicrobial activities for potential photothermal therapy. New J. Chem. 2022, 46, 6255–6266. [Google Scholar] [CrossRef]
- Yang, J.; Choi, J.; Bang, D.; Kim, E.; Lim, E.K.; Park, H.; Suh, J.S.; Lee, K.; Yoo, K.H.; Kim, E.K.; et al. Convertible Organic Nanoparticles for Near-Infrared Photothermal Ablation of Cancer Cells. Angew. Chem. Int. Ed. 2011, 50, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Wu, K.H.; Jiang, Z.L.; Shi, Z.W.; Si, Z.Z.; Wang, Q.; Cao, Y.H.; Hou, R.X.; Zhu, Y.B. A Polyaniline Nanoparticles Crosslinked Hydrogel with Excellent Photothermal Antibacterial and Mechanical Properties for Wound Dressing. Macromol. Biosci. 2022, 22, 2100386. [Google Scholar] [CrossRef] [PubMed]
- Ruppel, S.S.; Liang, J. Tunable Properties of Polydopamine Nanoparticles and Coated Surfaces. Langmuir 2022, 38, 5020–5029. [Google Scholar] [CrossRef]
- Bi, Z.; Teng, H.; Li, Q.; Zhang, S. Enhanced Carboxymethylcellulose Sponge for Hemostasis and Wound Repair. Front. Mater. 2022, 9, 944274. [Google Scholar] [CrossRef]
- Barclay, T.G.; Hegab, H.M.; Clarke, S.R.; Ginic-Markovic, M. Versatile Surface Modification Using Polydopamine and Related Polycatecholamines: Chemistry, Structure, and Applications. Adv. Mater. Interfaces 2017, 4, 1601192. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Chinchulkar, S.A.; Patra, P.; Dehariya, D.; Yu, A.; Rengan, A.K. Polydopamine nanocomposites and their biomedical applications: A review. Polym. Adv. Technol. 2022, 33, 3935–3956. [Google Scholar] [CrossRef]
- Alkan-Tas, B.; Berksun, E.; Tas, C.E.; Unal, S.; Unal, H. NIR-responsive waterborne polyurethane-polydopamine coatings for light-driven disinfection of surfaces. Prog. Org. Coat. 2022, 164, 106669. [Google Scholar] [CrossRef]
- Wang, C.; Bai, J.; Liu, Y.; Jia, X.; Jiang, X. Polydopamine Coated Selenide Molybdenum: A New Photothermal Nanocarrier for Highly Effective Chemo-Photothermal Synergistic Therapy. ACS Biomater. Sci. Eng. 2016, 2, 2011–2017. [Google Scholar] [CrossRef]
- Cao, H.; Jiang, B.; Yang, Y.; Zhao, M.; Sun, N.; Xia, J.; Gao, X.; Li, J. Cell membrane covered polydopamine nanoparticles with two-photon absorption for precise photothermal therapy of cancer. J. Colloid Interface Sci. 2021, 604, 596–603. [Google Scholar] [CrossRef]
- Fan, S.; Lin, W.; Huang, Y.; Xia, J.; Xu, J.-F.; Zhang, J.; Pi, J. Advances and Potentials of Polydopamine Nanosystem in Photothermal-Based Antibacterial Infection Therapies. Front. Pharmacol. 2022, 13, 829712. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Chen, X.F.; Yang, P.; Liang, G.J.; Yang, Y.; Gu, Z.P.; Li, Y.W. Regulating the absorption spectrum of polydopamine. Sci. Adv. 2020, 6, eabb4696. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Huang, Y.; You, S.; Xiang, Y.; Cai, E.; Mao, R.; Pan, W.; Tong, X.; Dong, W.; Ye, F.; et al. Engineering Robust Ag-Decorated Polydopamine Nano-Photothermal Platforms to Combat Bacterial Infection and Prompt Wound Healing. Adv. Sci. 2022, 9, 2106015. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; You, S.; Mao, R.; Xiang, Y.; Cai, E.; Deng, H.; Shen, J.; Qi, X. Architecting polyelectrolyte hydrogels with Cu-assisted polydopamine nanoparticles for photothermal antibacterial therapy. Mater. Today Bio 2022, 15, 100264. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Pan, W.; Tong, X.; Gao, T.; Xiang, Y.; You, S.; Mao, R.; Chi, J.; Hu, R.; Zhang, W.; et al. ε-Polylysine-stabilized agarose/polydopamine hydrogel dressings with robust photothermal property for wound healing. Carbohydr. Polym. 2021, 264, 118046. [Google Scholar] [CrossRef]
- Mohamed Salleh, N.A.b.; Tanaka, Y.; Sutarlie, L.; Su, X. Detecting bacterial infections in wounds: A review of biosensors and wearable sensors in comparison with conventional laboratory methods. Analyst 2022, 147, 1756–1776. [Google Scholar] [CrossRef]
- Lv, K.; Li, G.; Pan, X.; Liu, L.; Chen, Z.; Zhang, Y.; Xu, H.; Ma, D. Bacteria-Targeted Combined with Photothermal/NO Nanoparticles for the Treatment and Diagnosis of MRSA Infection In Vivo. Adv. Healthc. Mater. 2023, 2300247. [Google Scholar] [CrossRef]
- Tan, Y.; Ghandi, K. Kinetics and mechanism of pyrrole chemical polymerization. Synth. Met. 2013, 175, 183–191. [Google Scholar] [CrossRef]
- Kang, H.; Lee, W.; Oh, J.; Kim, T.; Lee, C.; Kim, B.J. From Fullerene-Polymer to All-Polymer Solar Cells: The Importance of Molecular Packing, Orientation, and Morphology Control. Acc. Chem. Res. 2016, 49, 2424–2434. [Google Scholar] [CrossRef]
- Fan, Y.Z.; Wang, Z.A.; Ren, W.Z.; Liu, G.Y.; Xing, J.; Xiao, T.Z.; Li, W.; Li, Y.J.; Yu, P.; Ning, C.Y.; et al. Space-Confined Synthesis of Thin Polypyrrole Nanosheets in Layered Bismuth Oxychloride for a Photoresponse Antibacterial within the Near-Infrared Window and Accelerated Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 36966–36979. [Google Scholar] [CrossRef]
- Wang, M.Z. Emerging Multifunctional NIR Photothermal Therapy Systems Based on Polypyrrole Nanoparticles. Polymers 2016, 8, 373. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, N.; Akbarinejad, A.; Hosseinkhani, S.; Rabbani, F. Synthesis of highly fluorescent water-soluble polypyrrole for cell imaging and iodide ion sensing. Anal. Chim. Acta 2019, 1084, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Antony, M.J.; Jayakannan, M. Amphiphilic azobenzenesulfonic acid anionic surfactant for water-soluble, ordered, and luminescent polypyrrole nanospheres. J. Phys. Chem. B 2007, 111, 12772–12780. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, X.D.; Cheng, Y.F.; Li, L.; Zhang, K.; Kang, E.T.; Xu, L.Q. Surface co-deposition of polypyrrole nanoparticles and tannic acid for photothermal bacterial eradication. Colloids Surf. B-Biointerfaces 2022, 212, 112381. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.W.; Ke, Q.; Chen, Q.; Zhang, X.Y.; Su, J.Y.; Ning, C.Y.; Fang, L.M. Flexible, Breathable, and Self-Powered Patch Assembled of Electrospun Polymer Triboelectric Layers and Polypyrrole-Coated Electrode for Infected Chronic Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 17641–17652. [Google Scholar] [CrossRef]
- Luo, R.Z.; Dai, J.Y.; Zhang, J.P.; Li, Z. Accelerated Skin Wound Healing by Electrical Stimulation. Adv. Healthc. Mater. 2021, 10, 2100557. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.Z.; Yu, F.; Leng, Q.H.; Zhao, S.Y.; Xu, Y.Y.; Zhang, W.; Zhu, Z.L.; Ye, J.; Wei, Q.; Wang, X.L. Electrical and visible light dual-responsive ZnO nanocomposite with multiple wound healing capability. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 124, 112066. [Google Scholar] [CrossRef]
- Qiao, Z.; Ding, J.; Wu, C.H.; Zhou, T.; Wu, K.; Zhang, Y.S.; Xiao, Z.W.; Wei, D.; Sun, J.; Fan, H.S. One-Pot Synthesis of Bi2S3/TiO2/rGO Heterostructure with Red Light-Driven Photovoltaic Effect for Remote Electrotherapy-Assisted Wound Repair. Small 2023, 19, 2206231. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Xie, J.N.; Xing, J.; Li, C.H.; Wong, T.M.; Yu, H.; Li, Y.X.; Yang, F.B.; Tian, Y.; Zhang, H.; et al. Light-Programmable Nanocomposite Hydrogel for State-Switchable Wound Healing Promotion and Bacterial Infection Elimination. Adv. Healthc. Mater. 2023, 12, 2201565. [Google Scholar] [CrossRef]
- He, Y.L.; Cao, Y.Y.; Wang, Y.P. Progress on Photothermal Conversion in the Second NIR Window Based on Conjugated Polymers. Asian J. Org. Chem. 2018, 7, 2201–2212. [Google Scholar] [CrossRef]
- Yu, Y.; Tang, D.; Liu, C.; Zhang, Q.; Tang, L.; Lu, Y.; Xiao, H. Biodegradable Polymer with Effective Near-Infrared-II Absorption as a Photothermal Agent for Deep Tumor Therapy. Adv. Mater. 2022, 34, 2105976. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Zhou, H.; Cui, M.; Liang, G.; Zhang, H.; Xiao, H. NIR-II Light Accelerated Prodrug Reduction of Pt(IV)-Incorporating Pseudo Semiconducting Polymers for Robust Degradation and Maximized Photothermal/Chemo-Immunotherapy. Adv. Mater. 2023, 35, 2300048. [Google Scholar] [CrossRef] [PubMed]
- Naskar, A.; Kim, K.S. Friends against the Foe: Synergistic Photothermal and Photodynamic Therapy against Bacterial Infections. Pharmaceutics 2023, 15, 1116. [Google Scholar] [CrossRef]
- Hu, X.J.; Zhang, H.; Wang, Y.T.; Shiu, B.C.; Lin, J.H.; Zhang, S.J.; Lou, C.W.; Li, T.T. Synergistic antibacterial strategy based on photodynamic therapy: Progress and perspectives. Chem. Eng. J. 2022, 450, 138129. [Google Scholar] [CrossRef]
- Yin, C.; Wang, Z.; Dai, C.; Yang, B.; Wang, W.; Yang, E.; Guo, F.; Fan, C.; Zhang, P.; Sun, J.; et al. Light-triggered photosynthetic engineered bacteria for enhanced-photodynamic therapy by relieving tumor hypoxic microenvironment. Theranostics 2023, 13, 1632–1648. [Google Scholar] [CrossRef]
- Wang, D.; Li, H.J.; Ji, L.; Liu, J.; Li, Y.; Xu, M.; Wang, H.Z.; Qiao, Z.Y.; Zhang, J.T. Engineering of plasmonic metal-semiconductor yolk-shell nanostructures for multi-intensified photodynamic- and immuno- therapy against drug resistant bacteria. Nano Today 2023, 49, 101803. [Google Scholar] [CrossRef]
- Overchuk, M.; Weersink, R.A.; Wilson, B.C.; Zheng, G. Photodynamic and Photothermal Therapies: Synergy Opportunities for Nanomedicine. ACS Nano 2023, 17, 7979–8003. [Google Scholar] [CrossRef]
- Wang, W.; Wu, F.; Zhang, Q.; Zhou, N.; Zhang, M.; Zheng, T.; Li, Y.; Tang, B.Z. Aggregation-Induced Emission Nanoparticles for Single Near-Infrared Light-Triggered Photodynamic and Photothermal Antibacterial Therapy. ACS Nano 2022, 16, 7961–7970. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Wei, X.; Zhao, X.; Chen, L.-J.; Yan, X.-P. Near-Infrared Photothermal/Photodynamic-in-One Agents Integrated with a Guanidinium-Based Covalent Organic Framework for Intelligent Targeted Imaging-Guided Precision Chemo/PTT/PDT Sterilization. ACS Appl. Mater. Interfaces 2021, 13, 27895–27903. [Google Scholar] [CrossRef]
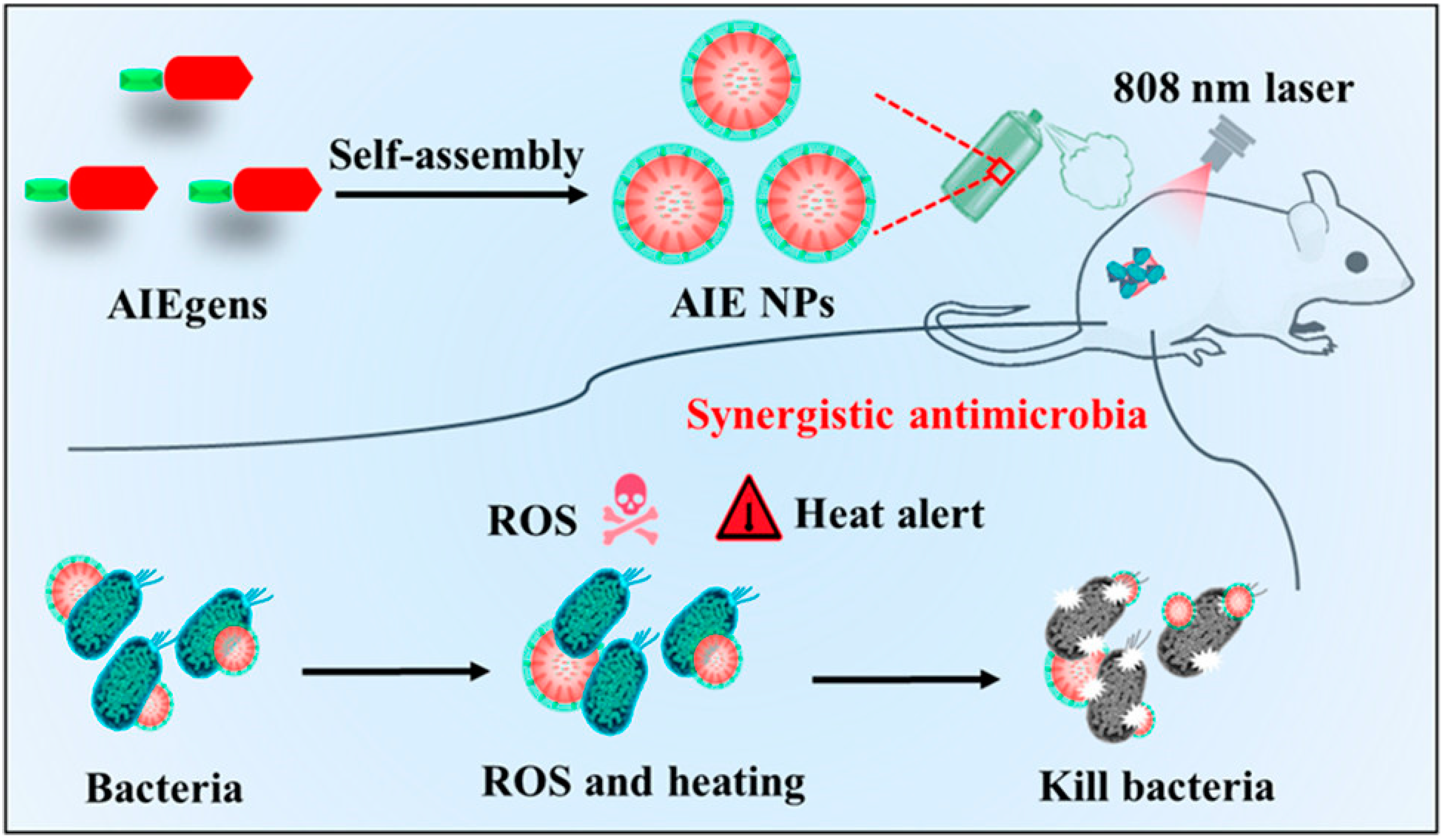
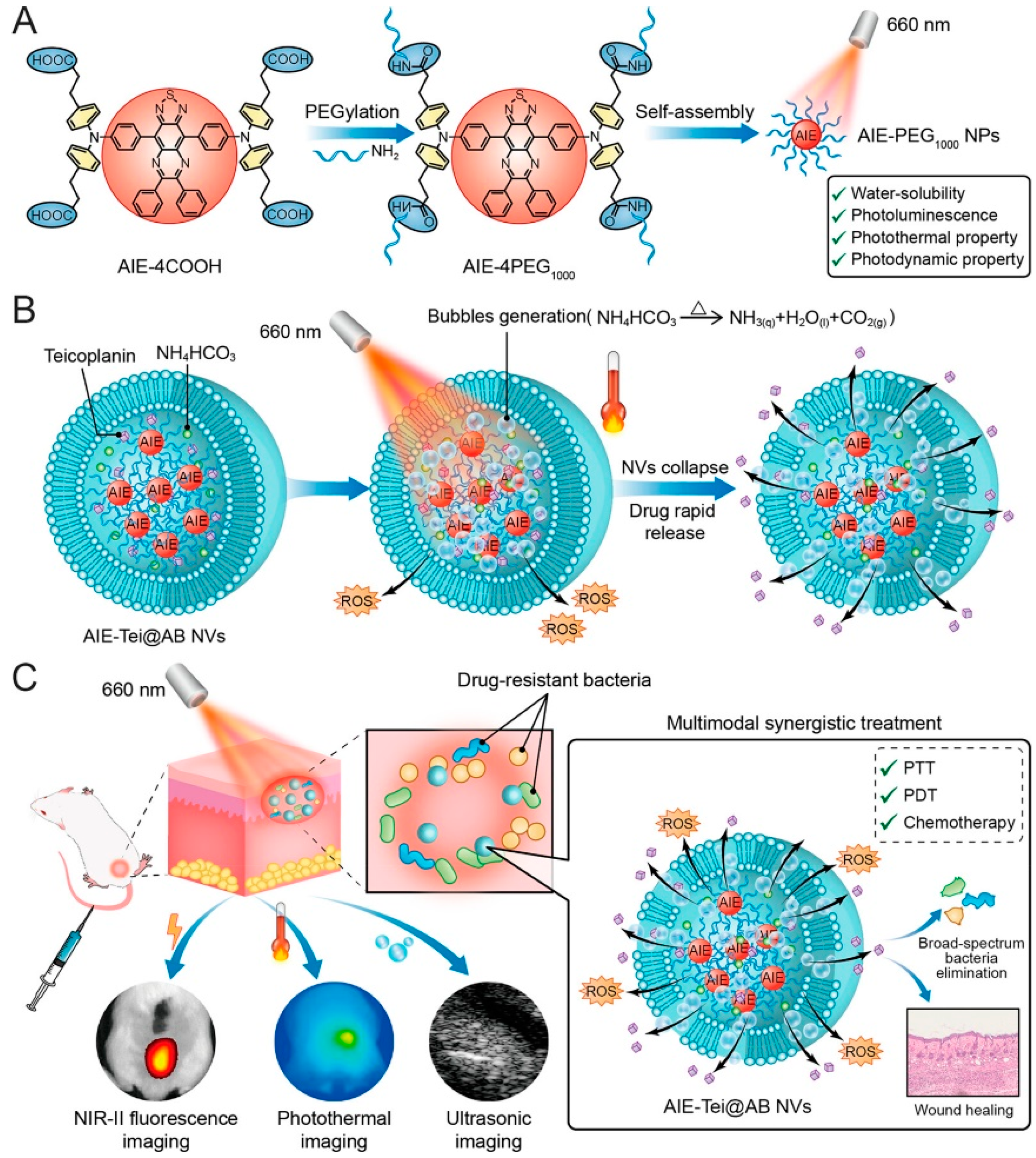
- Li, B.; Wang, W.; Zhao, L.; Yan, D.Y.; Li, X.X.; Gao, Q.X.; Zheng, J.D.; Zhou, S.T.; Lai, S.S.; Feng, Y.; et al. Multifunctional AIE Nanosphere-Based “Nanobomb” for Trimodal Imaging-Guided Photothermal/Photodynamic/Pharmacological Therapy of Drug-Resistant Bacterial Infections. ACS Nano 2023, 17, 4601–4618. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wang, Y.; Wang, X.; Qi, R.; Yuan, H. Recent Progress of Photothermal Therapy Based on Conjugated Nanomaterials in Combating Microbial Infections. Nanomaterials 2023, 13, 2269. https://doi.org/10.3390/nano13152269
Zhao Y, Wang Y, Wang X, Qi R, Yuan H. Recent Progress of Photothermal Therapy Based on Conjugated Nanomaterials in Combating Microbial Infections. Nanomaterials. 2023; 13(15):2269. https://doi.org/10.3390/nano13152269
Chicago/Turabian StyleZhao, Yue, Yi Wang, Xiaoyu Wang, Ruilian Qi, and Huanxiang Yuan. 2023. "Recent Progress of Photothermal Therapy Based on Conjugated Nanomaterials in Combating Microbial Infections" Nanomaterials 13, no. 15: 2269. https://doi.org/10.3390/nano13152269
APA StyleZhao, Y., Wang, Y., Wang, X., Qi, R., & Yuan, H. (2023). Recent Progress of Photothermal Therapy Based on Conjugated Nanomaterials in Combating Microbial Infections. Nanomaterials, 13(15), 2269. https://doi.org/10.3390/nano13152269





