Effects of Zwitterions on Structural Anomalies in Ionic Liquid Glasses Studied by EPR
Abstract
1. Introduction
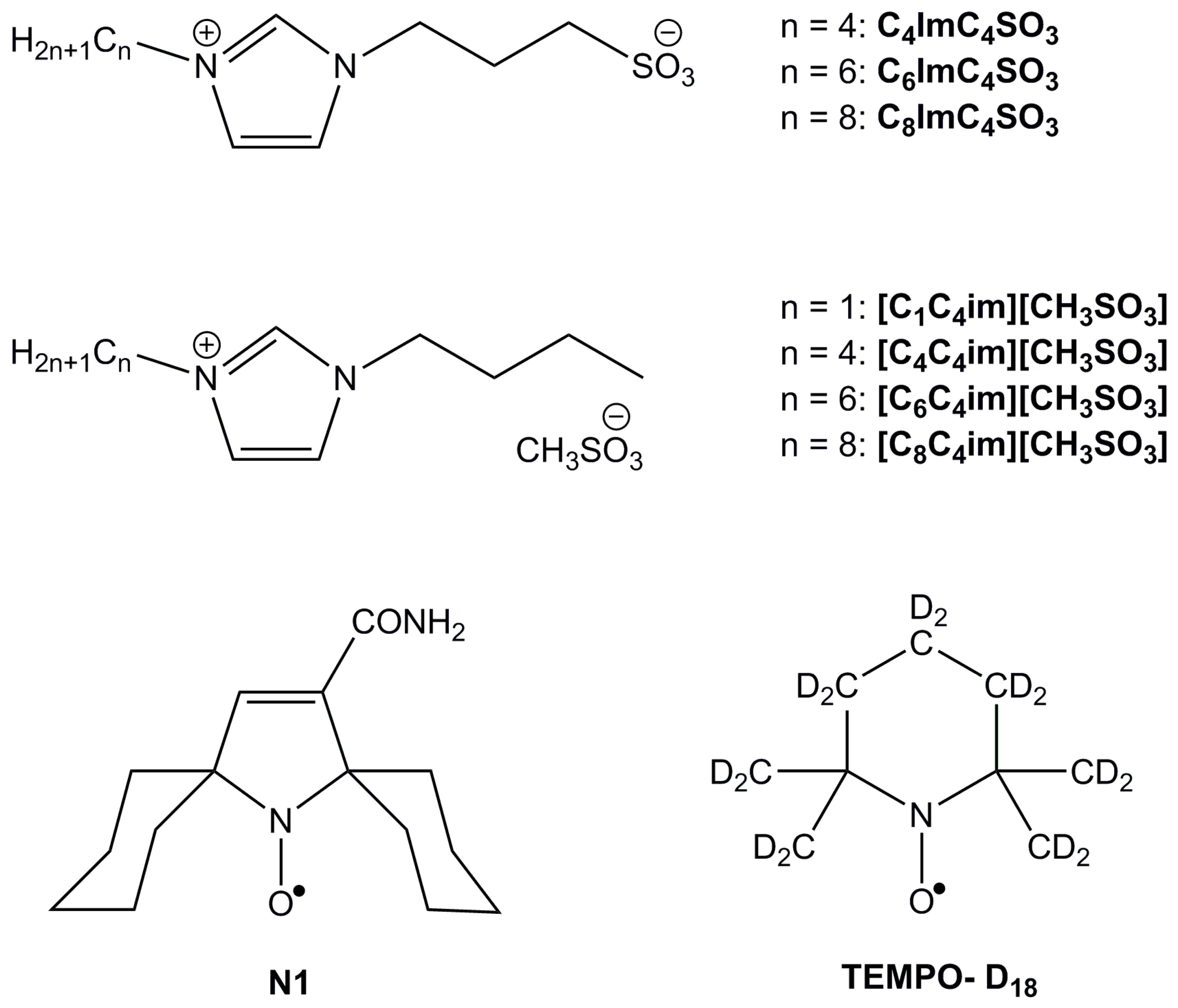
2. Materials and Methods
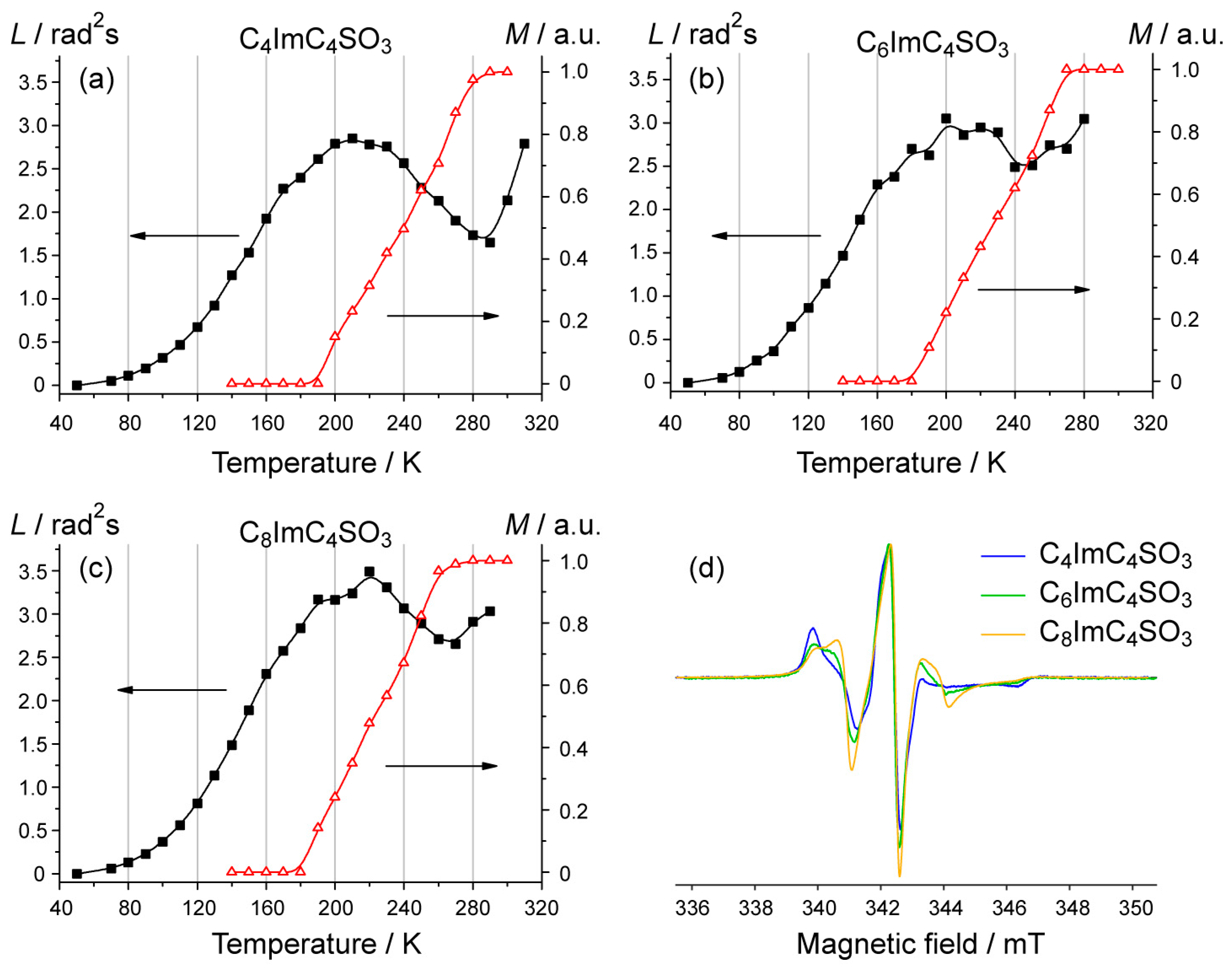
3. Results and Discussion
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Krossing, I.; Slattery, J.M.; Daguenet, C.; Dyson, P.J.; Oleinikova, A.; Weingärtner, H. Why are ionic liquids liquid? A simple explanation based on lattice and solvation energies. J. Am. Chem. Soc. 2006, 128, 13427–13434. [Google Scholar] [CrossRef] [PubMed]
- Walter, K. The Nature of the Glassy State and the Behaviour of Liquids at Low Temperatures–Walter Kauzmann. Chem. Rev. 1948, 43, 219–256. [Google Scholar]
- Pârvulescu, V.I.; Hardacre, C. Catalysis in ionic liquids. Chem. Rev. 2007, 107, 2615–2665. [Google Scholar] [CrossRef] [PubMed]
- Tarasova, N.P.; Smetannikov, Y.V.; Zanin, A.A. Ionic liquids in the synthesis of nanoobjects. Russ. Chem. Rev. 2010, 79, 463–477. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Protic ionic liquids: Properties and applications. Chem. Rev. 2008, 108, 206–237. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, J.; Huang, C.; Lei, Z. Ionic Liquids in Selective Oxidation: Catalysts and Solvents. Chem. Rev. 2017, 117, 6929–6983. [Google Scholar] [CrossRef]
- Dong, K.; Liu, X.; Dong, H.; Zhang, X.; Zhang, S. Multiscale Studies on Ionic Liquids. Chem. Rev. 2017, 117, 6636–6695. [Google Scholar] [CrossRef]
- Ferraz, R.; Branco, L.C.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž. Ionic liquids as active pharmaceutical ingredients. ChemMedChem 2011, 6, 975–985. [Google Scholar] [CrossRef]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of Ionic Liquids to Energy Storage and Conversion Materials and Devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Voth, G.A. Unique spatial heterogeneity in ionic liquids. J. Am. Chem. Soc. 2005, 127, 12192–12193. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and Nanostructure in Ionic Liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef]
- Wang, Y.-L.L.; Li, B.; Sarman, S.; Mocci, F.; Lu, Z.Y.; Yuan, J.; Laaksonen, A.; Fayer, M.D. Microstructural and Dynamical Heterogeneities in Ionic Liquids. Chem. Rev. 2020, 120, 5798–5877. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wu, M.; Kou, Y.; Min, E. Ionic liquids: Applications in catalysis. Catal. Today 2002, 74, 157–189. [Google Scholar] [CrossRef]
- Weingärtner, H. Understanding Ionic Liquids at the Molecular Level: Facts, Problems, and Controversies. Angew. Chem. Int. Ed. 2008, 47, 654–670. [Google Scholar] [CrossRef]
- Moshikur, R.; Goto, M. Application of Ionic Liquids in Drug Delivery; Springer: Singapore, 2021; ISBN 9789811643651. [Google Scholar]
- Ford, L.; Tay, E.; Nguyen, T.H.; Williams, H.D.; Benameur, H.; Scammells, P.J.; Porter, C.J.H. API ionic liquids: Probing the effect of counterion structure on physical form and lipid solubility. RSC Adv. 2020, 10, 12788–12799. [Google Scholar] [CrossRef]
- Claus, J.; Sommer, F.O.; Kragl, U. Ionic liquids in biotechnology and beyond. Solid State Ion. 2018, 314, 119–128. [Google Scholar] [CrossRef]
- Ohno, H.; Yoshizawa-Fujita, M.; Kohno, Y. Design and properties of functional zwitterions derived from ionic liquids. Phys. Chem. Chem. Phys. 2018, 20, 10978–10991. [Google Scholar] [CrossRef]
- Bühler, G.; Zharkouskaya, A.; Feldmann, C. Ionic liquid based approach to nanoscale functional materials. Solid State Sci. 2008, 10, 461–465. [Google Scholar] [CrossRef]
- Kang, X.; Sun, X.; Han, B. Synthesis of Functional Nanomaterials in Ionic Liquids. Adv. Mater. 2016, 28, 1011–1030. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, P.; Yadav, S.; Sadique, M.A.; Khan, R. Nanomaterials for the Electrochemical Biosensors. Biosensors 2021, 11, 414. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, M.; Ohno, H. Anhydrous proton transport system based on zwitterionic liquid and HTFSI. Chem. Commun. 2004, 16, 1828–1829. [Google Scholar] [CrossRef]
- Suzanowicz, A.M.; Mei, C.W.; Mandal, B.K. Approaches to Combat the Polysulfide Shuttle Phenomenon in Li–S Battery Technology. Batteries 2022, 8, 45. [Google Scholar] [CrossRef]
- Di Donato, G.; Ates, T.; Adenusi, H.; Varzi, A.; Navarra, M.A.; Passerini, S. Electrolyte Measures to Prevent Polysulfide Shuttle in Lithium-Sulfur Batteries. Batter. Supercaps 2022, 5, e202200097. [Google Scholar] [CrossRef]
- Ren, W.; Ma, W.; Zhang, S.; Tang, B. Recent advances in shuttle effect inhibition for lithium sulfur batteries. Energy Storage Mater. 2019, 23, 707–732. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, P.; Liu, Z.; Du, B.; Peng, Z. A Novel Zwitterionic Ionic Liquid-Based Electrolyte for More Efficient and Safer Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2020, 12, 11635–11642. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Hirao, M.; Ito-Akita, K.; Ohno, H. Ion conduction in zwitterionic-type molten salts and their polymers. J. Mater. Chem. 2001, 11, 1057–1062. [Google Scholar] [CrossRef]
- Wu, B.; Kuroda, K.; Takahashi, K.; Castner, E.W. Structural analysis of zwitterionic liquids vs. homologous ionic liquids. J. Chem. Phys. 2018, 148, 193807. [Google Scholar] [CrossRef]
- Biswas, Y.; Ghosh, P.; Mandal, T.K. Chemical Tuning of Zwitterionic Ionic Liquids for Variable Thermophysical Behaviours, Nanostructured Aggregates and Dual-Stimuli Responsiveness. Chem.—A Eur. J. 2018, 24, 13322–13335. [Google Scholar] [CrossRef]
- Hermanutz, F.; Vocht, M.P.; Panzier, N.; Buchmeiser, M.R. Processing of Cellulose Using Ionic Liquids. Macromol. Mater. Eng. 2019, 304, 1800450. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Gräsvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef]
- Sharma, G.; Takahashi, K.; Kuroda, K. Polar zwitterion/saccharide-based deep eutectic solvents for cellulose processing. Carbohydr. Polym. 2021, 267, 118171. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Satria, H.; Miyamura, K.; Tsuge, Y.; Ninomiya, K.; Takahashi, K. Design of Wall-Destructive but Membrane-Compatible Solvents. J. Am. Chem. Soc. 2017, 139, 16052–16055. [Google Scholar] [CrossRef]
- Satria, H.; Kuroda, K.; Endo, T.; Takada, K.; Ninomiya, K.; Takahashi, K. Efficient Hydrolysis of Polysaccharides in Bagasse by in Situ Synthesis of an Acidic Ionic Liquid after Pretreatment. ACS Sustain. Chem. Eng. 2017, 5, 708–713. [Google Scholar] [CrossRef]
- Huet, G.; Araya-Farias, M.; Alayoubi, R.; Laclef, S.; Bouvier, B.; Gosselin, I.; Cézard, C.; Roulard, R.; Courty, M.; Hadad, C.; et al. New biobased-zwitterionic ionic liquids: Efficiency and biocompatibility for the development of sustainable biorefinery processes. Green Chem. 2020, 22, 2935–2946. [Google Scholar] [CrossRef]
- Ivanov, M.Y.; Fedin, M.V. Nanoscale heterogeneities in ionic liquids: Insights from EPR of spin probes. Mendeleev Commun. 2018, 28, 565–573. [Google Scholar] [CrossRef]
- Ivanov, M.Y.; Prikhod’ko, S.A.; Adonin, N.Y.; Kirilyuk, I.A.; Adichtchev, S.V.; Surovtsev, N.V.; Dzuba, S.A.; Fedin, M.V. Structural Anomalies in Ionic Liquids near the Glass Transition Revealed by Pulse EPR. J. Phys. Chem. Lett. 2018, 9, 4607–4612. [Google Scholar] [CrossRef] [PubMed]
- Bakulina, O.D.; Ivanov, M.Y.; Prikhod’ko, S.A.; Pylaeva, S.; Zaytseva, I.V.; Surovtsev, N.V.; Adonin, N.Y.; Fedin, M.V. Nanocage formation and structural anomalies in imidazolium ionic liquid glasses governed by alkyl chains of cations. Nanoscale 2020, 12, 19982–19991. [Google Scholar] [CrossRef]
- Ivanov, M.Y.; Poryvaev, A.S.; Polyukhov, D.M.; Prikhod’ko, S.A.; Adonin, N.Y.; Fedin, M.V. Nanoconfinement effects on structural anomalies in imidazolium ionic liquids. Nanoscale 2020, 12, 23480–23487. [Google Scholar] [CrossRef]
- Ivanov, M.Y.; Prikhod’ko, S.A.; Bakulina, O.D.; Kiryutin, A.S.; Adonin, N.Y.; Fedin, M.V. Validation of structural grounds for anomalous molecular mobility in ionic liquid glasses. Molecules 2021, 26, 5828. [Google Scholar] [CrossRef]
- Ivanov, M.Y.; Bakulina, O.D.; Alimov, D.V.; Prikhod’ko, S.A.; Veber, S.L.; Pylaeva, S.; Adonin, N.Y.; Fedin, M.V. Inherent heterogeneities and nanostructural anomalies in organic glasses revealed by EPR. Nanoscale 2021, 3, 4973–4978. [Google Scholar] [CrossRef] [PubMed]
- Bakulina, O.D.; Ivanov, M.Y.; Alimov, D.V.; Prikhod’ko, S.A.; Adonin, N.Y.; Fedin, M.V. Active Pharmaceutical Ingredient-Ionic Liquids (API-ILs): Nanostructure of the Glassy State Studied by Electron Paramagnetic Resonance Spectroscopy. Molecules 2022, 27, 5117. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.Y.; Bakulina, O.D.; Polienko, Y.F.; Kirilyuk, I.A.; Prikhod’ko, S.A.; Adonin, N.Y.; Fedin, M.V. Radical ionic liquid: An efficient self-probe to study heterogeneous structure in glassy state using EPR spectroscopy. J. Mol. Liq. 2023, 381, 121830. [Google Scholar] [CrossRef]
- Ivanov, M.Y.; Prikhod’ko, S.A.; Adonin, N.Y.; Fedin, M.V. Structural Anomalies in Binary Mixtures of Ionic Liquid [Bmim]BF 4 with Water Studied by EPR. J. Phys. Chem. B 2019, 123, 9956–9962. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Kattnig, D.R.; Akdogan, Y.; Lieberwirth, I.; Hinderberger, D. Spin probing of supramolecular structures in 1-butyl-3-methyl-imidazolium tetrafluoroborate/water mixtures. Mol. Phys. 2013, 111, 2723–2737. [Google Scholar] [CrossRef]
- Akdogan, Y.; Heller, J.; Zimmermann, H.; Hinderberger, D. The solvation of nitroxide radicals in ionic liquids studied by high-field EPR spectroscopy. Phys. Chem. Chem. Phys. 2010, 12, 7874. [Google Scholar] [CrossRef]
- Kattnig, D.R.; Akdogan, Y.; Bauer, C.; Hinderberger, D. High-field EPR spectroscopic characterization of spin probes in aqueous ionic liquid mixtures. Z. Phys. Chem. 2012, 226, 1363–1377. [Google Scholar] [CrossRef]
- Mladenova, B.Y.; Chumakova, N.A.; Pergushov, V.I.; Kokorin, A.I.; Grampp, G.; Kattnig, D.R. Rotational and translational diffusion of spin probes in room-temperature ionic liquids. J. Phys. Chem. B 2012, 116, 12295–12305. [Google Scholar] [CrossRef]
- Mladenova, B.Y.; Kattnig, D.R.; Grampp, G. Room-temperature ionic liquids discerned via nitroxyl spin probe dynamics. J. Phys. Chem. B 2011, 115, 8183–8198. [Google Scholar] [CrossRef]
- Strehmel, V. Radicals in Ionic Liquids. ChemPhysChem 2012, 13, 1649–1663. [Google Scholar] [CrossRef]
- Stoesser, R.; Herrmann, W.; Zehl, A.; Strehmel, V.; Laschewsky, A. ESR spin probes in ionic liquids. ChemPhysChem 2006, 7, 1106–1111. [Google Scholar] [CrossRef]
- Bakulina, O.D.; Ivanov, M.Y.; Prikhod’ko, S.A.; Adonin, N.Y.; Fedin, M.V. EPR study of nanostructuring in protic ionic liquids [PriNH3]NO3 and [BuNH3]NO3. Russ. Chem. Bull. Int. Ed. 2021, 12, 2359–2365. [Google Scholar] [CrossRef]
- Erilov, D.A.; Bartucci, R.; Guzzi, R.; Marsh, D.; Dzuba, S.A.; Sportelli, L. Librational motion of spin-labeled lipids in high-cholesterol containing membranes from echo-detected EPR spectra. Biophys. J. 2004, 87, 3873–3881. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Isaev, N.P.; Dzuba, S.A. Fast Stochastic Librations and Slow Rotations of Spin Labeled Stearic Acids in a Model Phospholipid Bilayer at Cryogenic Temperatures. J. Phys. Chem. B 2008, 112, 13285–13291. [Google Scholar] [CrossRef]
- Syryamina, V.N.; Dzuba, S.A. Dynamical Transitions at Low Temperatures in the Nearest Hydration Shell of Phospholipid Bilayers. J. Phys. Chem. B 2017, 121, 1026–1032. [Google Scholar] [CrossRef]
- Dzuba, S.A. Libration motion of guest spin probe molecules in organic glasses: CW EPR and electron spin echo study. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2000, 56, 227–234. [Google Scholar] [CrossRef]
- Nelyubina, Y.V.; Shaplov, A.S.; Lozinskaya, E.I.; Buzin, M.I.; Vygodskii, Y.S. A New Volume-Based Approach for Predicting Thermophysical Behavior of Ionic Liquids and Ionic Liquid Crystals. J. Am. Chem. Soc. 2016, 138, 10076–10079. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, L.; Di Schino, A.; Giordano, M.; Leporini, D. Evidence of a fractional Debye-Stokes-Einstein law in supercooled o-terphenyl. Europhys. Lett. 1997, 38, 669–674. [Google Scholar] [CrossRef]
- Ivanov, M.Y.; Veber, S.L.; Prikhod’ko, S.A.; Adonin, N.Y.; Bagryanskaya, E.G.; Fedin, M.V.; Prikhod’ko, S.A.; Adonin, N.Y.; Bagryanskaya, E.G.; Fedin, M.V. Probing Microenvironment in Ionic Liquids by Time-Resolved EPR of Photoexcited Triplets. J. Phys. Chem. B 2015, 119, 13440–13449. [Google Scholar] [CrossRef]
- Ivanov, M.Y.; Prikhod’Ko, S.A.; Adonin, N.Y.; Bagryanskaya, E.G.; Fedin, M.V. Influence of C2-Methylation of Imidazolium Based Ionic Liquids on Photoinduced Spin Dynamics of the Dissolved ZnTPP Studied by Time-Resolved EPR. Z. Phys. Chem. 2017, 231, 391–404. [Google Scholar] [CrossRef]
- Rocha, M.A.A.; Neves, C.M.S.S.; Freire, M.G.; Russina, O.; Triolo, A.; Coutinho, J.A.P.; Santos, L.M.N.B.F. Alkylimidazolium based ionic liquids: Impact of cation symmetry on their nanoscale structural organization. J. Phys. Chem. B 2013, 117, 10889–10897. [Google Scholar] [CrossRef] [PubMed]
- Russina, O.; Triolo, A.; Gontrani, L.; Caminiti, R.; Xiao, D.; Hines, L.G.; Bartsch, R.A.; Quitevis, E.L.; Plechkova, N.; Seddon, K.R. Morphology and intermolecular dynamics of 1-alkyl-3-methylimidazolium bis{(trifluoromethane)sulfonyl}amide ionic liquids: Structural and dynamic evidence of nanoscale segregation. J. Phys. Condens. Matter 2009, 21, 424121. [Google Scholar] [CrossRef]
- Triolo, A.; Russina, O.; Bleif, H.J.; Di Cola, E. Nanoscale segregation in room temperature ionic liquids. J. Phys. Chem. B 2007, 111, 4641–4644. [Google Scholar] [CrossRef]
- Avila, J.; Clark, R.; Pádua, A.A.H.; Costa Gomes, M. Porous ionic liquids: Beyond the bounds of free volume in a fluid phase. Mater. Adv. 2022, 3, 8848–8863. [Google Scholar] [CrossRef]
- Durak, O.; Zeeshan, M.; Habib, N.; Gulbalkan, H.C.; Alsuhile, A.A.A.M.; Caglayan, H.P.; Kurtoğlu-Öztulum, S.F.; Zhao, Y.; Haslak, Z.P.; Uzun, A.; et al. Composites of porous materials with ionic liquids: Synthesis, characterization, applications, and beyond. Microporous Mesoporous Mater. 2022, 332, 111703. [Google Scholar] [CrossRef]
- Barrulas, R.V.; Zanatta, M.; Casimiro, T.; Corvo, M.C. Advanced porous materials from poly(ionic liquid)s: Challenges, applications and opportunities. Chem. Eng. J. 2021, 411, 128528. [Google Scholar] [CrossRef]
- Avila, J.; Červinka, C.; Dugas, P.Y.; Pádua, A.A.H.; Costa Gomes, M. Porous Ionic Liquids: Structure, Stability, and Gas Absorption Mechanisms. Adv. Mater. Interfaces 2021, 8, 2001982. [Google Scholar] [CrossRef]
- Takekiyo, T.; Ishikawa, Y.; Yoshimura, Y. Cryopreservation of proteins using ionic liquids: A case study of cytochrome c. J. Phys. Chem. B 2017, 121, 7614–7620. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Takekiyo, T.; Mori, T. Structural study of lysozyme in two ionic liquids at cryogenic temperature. Chem. Phys. Lett. 2016, 664, 44–49. [Google Scholar] [CrossRef]
- Cai, J.; Liu, J.; Mu, S.; Liu, J.; Hong, J.; Zhou, X.; Ma, Q.; Shi, L. Corrosion inhibition effect of three imidazolium ionic liquids on carbon steel in chloride contaminated environment. Int. J. Electrochem. Sci. 2020, 15, 1287–1301. [Google Scholar] [CrossRef]
- Dupont, J.; Consorti, C.S.; Suarez, P.A.Z.; De Souza, R.F. Preparation of 1-butyl-3-methyl imidszolium-based room temperature ionic liquids. Org. Synth. 2002, 79, 236. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakulina, O.D.; Ivanov, M.Y.; Prikhod’ko, S.A.; Adonin, N.Y.; Fedin, M.V. Effects of Zwitterions on Structural Anomalies in Ionic Liquid Glasses Studied by EPR. Nanomaterials 2023, 13, 2164. https://doi.org/10.3390/nano13152164
Bakulina OD, Ivanov MY, Prikhod’ko SA, Adonin NY, Fedin MV. Effects of Zwitterions on Structural Anomalies in Ionic Liquid Glasses Studied by EPR. Nanomaterials. 2023; 13(15):2164. https://doi.org/10.3390/nano13152164
Chicago/Turabian StyleBakulina, Olga D., Mikhail Yu. Ivanov, Sergey A. Prikhod’ko, Nicolay Yu. Adonin, and Matvey V. Fedin. 2023. "Effects of Zwitterions on Structural Anomalies in Ionic Liquid Glasses Studied by EPR" Nanomaterials 13, no. 15: 2164. https://doi.org/10.3390/nano13152164
APA StyleBakulina, O. D., Ivanov, M. Y., Prikhod’ko, S. A., Adonin, N. Y., & Fedin, M. V. (2023). Effects of Zwitterions on Structural Anomalies in Ionic Liquid Glasses Studied by EPR. Nanomaterials, 13(15), 2164. https://doi.org/10.3390/nano13152164





