Contrasting Properties of Polymeric Nanocarriers for MRI-Guided Drug Delivery
Abstract
1. Introduction
2. Constituents and Morphologies of Polymeric Nanocarriers
2.1. The Nanocarriers Morphologies
2.2. Polymers for Nanocarrier Preparation
2.2.1. Poly(ε-caprolactone) (PCL)
2.2.2. Polyethylene Glycol (PEG)
2.2.3. Poly-L-glutamic Acid (PGA)
2.2.4. Poly(Lactic-co-glycolic Acid) (PLGA)
2.2.5. Poly(α-l-lysine) (PLL)
3. MR Contrast Agents and Their Mechanisms of Action
3.1. Relaxation Contrast Agents

| Chemical Name | Trade Name | Mean r1 at 3.0 T (mM−1s−1) [94,95,96] | Mean r2 at 1.5 T (mM−1s−1) [97,98,99] |
|---|---|---|---|
| Gd-DTPA | MagnevistTM | 3.3–3.7 | - |
| Gd-DOTA | DotaremTM | 3.3–3.5 | - |
| Gd-DO3A-butrol | GadovistTM | 4.9–5.0 | - |
| Gd-EOB-DTPA | PrimovistTM | 5.4–6.2 | - |
| Gd-DTPA-BMA | OmniscanTM | 3.6–4.0 | - |
| Gd-HP-DO3A | ProHanceTM | 3.5–3.7 | - |
| Gd-BOPTA | MultiHenceTM | 5.1–6.3 | - |
| Ferumoxide | FeridexTM | - | 33–129 |
| Ferucarbotran | ResovistTM | - | 95–189 |
| Ferumoxtran | SineremTM | - | 65 |
| Ferumoxytol | FarahemeTM | - | 89 |
3.2. CEST Contrast Agents
3.3. Direct Detection Contrast Agents
4. Polymeric Nanocarriers with MR Contrast Agents
4.1. Nanocarriers with Relaxation Agents
4.1.1. Positive Contrast
4.1.2. Negative Contrast
4.2. Nanocarriers with Chemical Exchange Saturation Transfer CAs
4.3. Nanocarriers with Direct Detection CAs
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rani, S.; Gupta, U. HPMA-Based Polymeric Conjugates in Anticancer Therapeutics. Drug Discov. Today 2020, 25, 997–1012. [Google Scholar] [CrossRef]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current Trends and Challenges in Cancer Management and Therapy Using Designer Nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef]
- Robertson, A.G.; Rendina, L.M. Gadolinium Theranostics for the Diagnosis and Treatment of Cancer. Chem. Soc. Rev. 2021, 50, 4231–4244. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Hwang, H.S.; Na, K. Theranostics and Contrast Agents for Magnetic Resonance Imaging. Biomater. Res. 2018, 22, 20. [Google Scholar] [CrossRef] [PubMed]
- Anani, T.; Rahmati, S.; Sultana, N.; David, A.E. MRI-Traceable Theranostic Nanoparticles for Targeted Cancer Treatment. Theranostics 2021, 11, 579–601. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Top 10 Causes of Death. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 25 December 2022).
- Bhujwalla, Z.M.; Kakkad, S.; Chen, Z.; Jin, J.; Hapuarachchige, S.; Artemov, D.; Penet, M.-F. Theranostics and Metabolotheranostics for Precision Medicine in Oncology. J. Magn. Reson. 2018, 291, 141–151. [Google Scholar] [CrossRef]
- Brito, B.; Price, T.W.; Gallo, J.; Bañobre-López, M.; Stasiuk, G.J. Smart Magnetic Resonance Imaging-Based Theranostics for Cancer. Theranostics 2021, 11, 8706–8737. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, F.; Davies, G.; Williams, G.R. Theranostics for MRI-guided Therapy: Recent Developments. View 2021, 3, 20200134. [Google Scholar] [CrossRef]
- Mena-Giraldo, P.; Orozco, J. Polymeric Micro/Nanocarriers and Motors for Cargo Transport and Phototriggered Delivery. Polymers 2021, 13, 3920. [Google Scholar] [CrossRef]
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric Nanocarriers of Drug Delivery Systems in Cancer Therapy. Pharmaceutics 2020, 12, 298. [Google Scholar] [CrossRef]
- Kothamasu, P.; Kanumur, H.; Ravur, N.; Maddu, C.; Parasuramrajam, R.; Thangavel, S. Nanocapsules: The Weapons for Novel Drug Delivery Systems. Bioimpacts 2012, 2, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Gigliobianco, M.R.; Censi, R.; Di Martino, P. Polymeric Nanocapsules as Nanotechnological Alternative for Drug Delivery System: Current Status, Challenges and Opportunities. Nanomaterials 2020, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-Based Nanocapsules for Drug Delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Gaumet, M.; Vargas, A.; Gurny, R.; Delie, F. Nanoparticles for Drug Delivery: The Need for Precision in Reporting Particle Size Parameters. Eur. J. Pharm. Biopharm. 2008, 69, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, C.; Wang, R. Macrocycle-Surfaced Polymer Nanocapsules: An Emerging Paradigm for Biomedical Applications. Bioconj. Chem. 2022, 33, 2254–2261. [Google Scholar] [CrossRef]
- Karabasz, A.; Bzowska, M.; Szczepanowicz, K. Biomedical Applications of Multifunctional Polymeric Nanocarriers: A Review of Current Literature. IJN 2020, 15, 8673–8696. [Google Scholar] [CrossRef]
- Trindade, I.C.; Pound-Lana, G.; Pereira, D.G.S.; de Oliveira, L.A.M.; Andrade, M.S.; Vilela, J.M.C.; Postacchini, B.B.; Mosqueira, V.C.F. Mechanisms of Interaction of Biodegradable Polyester Nanocapsules with Non-Phagocytic Cells. Eur. J. Pharm. Sci. 2018, 124, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.; Alonso, M.J.; Pinto, M.M.M.; Barbosa, C.M. Development and Characterization of PLGA Nanospheres and Nanocapsules Containing Xanthone and 3-Methoxyxanthone. Eur. J. Pharm. Biopharm. 2005, 59, 491–500. [Google Scholar] [CrossRef]
- Guterres, S.S.; Alves, M.P.; Pohlmann, A.R. Polymeric Nanoparticles, Nanospheres and Nanocapsules, for Cutaneous Applications. Drug Target. Insights 2007, 2, 117739280700200002. [Google Scholar] [CrossRef]
- McMahon, M.T.; Bulte, J.W.M. Two Decades of Dendrimers as Versatile MRI Agents: A Tale with and without Metals. WIREs Nanomed. Nanobiotechnol. 2018, 10, e1496. [Google Scholar] [CrossRef] [PubMed]
- Khemtong, C.; Kessinger, C.W.; Gao, J. Polymeric Nanomedicine for Cancer MR Imaging and Drug Delivery. Chem. Commun. 2009, 24, 3497–3510. [Google Scholar] [CrossRef]
- Sutton, D.; Nasongkla, N.; Blanco, E.; Gao, J. Functionalized Micellar Systems for Cancer Targeted Drug Delivery. Pharm. Res. 2007, 24, 1029–1046. [Google Scholar] [CrossRef]
- Torchilin, V.P. Multifunctional Nanocarriers. Adv. Drug Deliv. Rev. 2006, 58, 1532–1555. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Ahmed, F. Polymersomes. Annu. Rev. Biomed. Eng. 2006, 8, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Shastri, V.P. Non-Degradable Biocompatible Polymers in Medicine: Past, Present and Future. Curr. Pharm. Biotechnol. 2003, 4, 331–337. [Google Scholar] [CrossRef]
- Almeida, J.P.M.; Chen, A.L.; Foster, A.; Drezek, R. In Vivo Biodistribution of Nanoparticles. Nanomedicine 2011, 6, 815–835. [Google Scholar] [CrossRef]
- Ali, I.; Alsehli, M.; Scotti, L.; Tullius Scotti, M.; Tsai, S.-T.; Yu, R.-S.; Hsieh, M.F.; Chen, J.-C. Progress in Polymeric Nano-Medicines for Theranostic Cancer Treatment. Polymers 2020, 12, 598. [Google Scholar] [CrossRef]
- Manandhar, S.; Sjöholm, E.; Bobacka, J.; Rosenholm, J.M.; Bansal, K.K. Polymer-Drug Conjugates as Nanotheranostic Agents. JNT 2021, 2, 63–81. [Google Scholar] [CrossRef]
- Larson, N.; Ghandehari, H. Polymeric Conjugates for Drug Delivery. Chem. Mater. 2012, 24, 840–853. [Google Scholar] [CrossRef]
- Allyn, M.M.; Luo, R.H.; Hellwarth, E.B.; Swindle-Reilly, K.E. Considerations for Polymers Used in Ocular Drug Delivery. Front. Med. 2022, 8, 787644. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent Advances in Polymeric Drug Delivery Systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-є-Caprolactone Based Formulations for Drug Delivery and Tissue Engineering: A Review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-Based Biomaterials for Tissue Engineering and Drug Delivery: Current Scenario and Challenges. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Palaniappan, R.; Korang-Yeboah, M.; Gorantla, Y.; Paulos, S.; Sharma, P.; Chaudhary, J. Polycaprolactone/Maltodextrin Nanocarrier for Intracellular Drug Delivery: Formulation, Uptake Mechanism, Internalization Kinetics, and Subcellular Localization. Int. J. Nanomed. 2015, 10, 4763–4781. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer—Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Wei, X.; Gong, C.; Gou, M.; Fu, S.; Guo, Q.; Shi, S.; Luo, F.; Guo, G.; Qiu, L.; Qian, Z. Biodegradable Poly(ɛ-Caprolactone)–Poly(Ethylene Glycol) Copolymers as Drug Delivery System. Int. J. Pharm. 2009, 381, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pitt, C.G.; Chasalow, F.I.; Hibionada, Y.M.; Klimas, D.M.; Schindler, A. Aliphatic Polyesters. I. The Degradation of Poly(ϵ-Caprolactone) in Vivo. J. Appl. Polym. Sci. 1981, 26, 3779–3787. [Google Scholar] [CrossRef]
- Woodward, S.C.; Brewer, P.S.; Moatamed, F.; Schindler, A.; Pitt, C.G. The Intracellular Degradation of Poly(ɛ-Caprolactone). J. Biomed. Mater. Res. 1985, 19, 437–444. [Google Scholar] [CrossRef]
- Sun, H.; Mei, L.; Song, C.; Cui, X.; Wang, P. The in Vivo Degradation, Absorption and Excretion of PCL-Based Implant. Biomaterials 2006, 27, 1735–1740. [Google Scholar] [CrossRef]
- Vert, M. Degradable and Bioresorbable Polymers in Surgery and in Pharmacology: Beliefs and Facts. J. Mater. Sci. Mater. Med. 2009, 20, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as Biomaterial for Bone Scaffolds: Review of Literature. J. Oral Biol. Craniofac. Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Espinoza, S.M.; Patil, H.I.; San Martin Martinez, E.; Casañas Pimentel, R.; Ige, P.P. Poly-ε-Caprolactone (PCL), a Promising Polymer for Pharmaceutical and Biomedical Applications: Focus on Nanomedicine in Cancer. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 85–126. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.; Zhao, M.; Tang, S.; Cheng, X.; Zhang, W.; Li, W.; Liu, X.; Peng, H.; Wang, Q. Effects of Polyethylene Glycol on the Surface of Nanoparticles for Targeted Drug Delivery. Nanoscale 2021, 13, 10748–10764. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S.; El-Sakhawy, M.; Kamel, S. Carboxymethyl Cellulose-Grafted Graphene Oxide/Polyethylene Glycol for Efficient Ni(II) Adsorption. J. Polym. Environ. 2021, 29, 859–870. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Aher, N.; Patil, R.; Khandare, J. Poly(Ethylene Glycol)-Prodrug Conjugates: Concept, Design, and Applications. J. Drug Deliv. 2012, 2012, 103973. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Shiraishi, K.; Yokoyama, M. Toxicity and Immunogenicity Concerns Related to PEGylated-Micelle Carrier Systems: A Review. Sci. Technol. Adv. Mater. 2019, 20, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(Ethylene Glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Hershfield, M.S.; Ganson, N.J.; Kelly, S.J.; Scarlett, E.L.; Jaggers, D.A.; Sundy, J.S. Induced and Pre-Existing Anti-Polyethylene Glycol Antibody in a Trial of Every 3-Week Dosing of Pegloticase for Refractory Gout, Including in Organ Transplant Recipients. Arthritis Res. Ther. 2014, 16, R63. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(Ethylene Glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef]
- Longo, N.; Harding, C.O.; Burton, B.K.; Grange, D.K.; Vockley, J.; Wasserstein, M.; Rice, G.M.; Dorenbaum, A.; Neuenburg, J.K.; Musson, D.G.; et al. Single-Dose, Subcutaneous Recombinant Phenylalanine Ammonia Lyase Conjugated with Polyethylene Glycol in Adult Patients with Phenylketonuria: An Open-Label, Multicentre, Phase 1 Dose-Escalation Trial. Lancet 2014, 384, 37–44. [Google Scholar] [CrossRef]
- Garay, R.P.; El-Gewely, R.; Armstrong, J.K.; Garratty, G.; Richette, P. Antibodies against Polyethylene Glycol in Healthy Subjects and in Patients Treated with PEG-Conjugated Agents. Expert Opin. Drug Deliv. 2012, 9, 1319–1323. [Google Scholar] [CrossRef]
- Ganson, N.J.; Povsic, T.J.; Sullenger, B.A.; Alexander, J.H.; Zelenkofske, S.L.; Sailstad, J.M.; Rusconi, C.P.; Hershfield, M.S. Pre-Existing Anti–Polyethylene Glycol Antibody Linked to First-Exposure Allergic Reactions to Pegnivacogin, a PEGylated RNA Aptamer. J. Allergy Clin. Immunol. 2016, 137, 1610–1613.e7. [Google Scholar] [CrossRef]
- Lipsky, P.E.; Calabrese, L.H.; Kavanaugh, A.; Sundy, J.S.; Wright, D.; Wolfson, M.; Becker, M.A. Pegloticase Immunogenicity: The Relationship between Efficacy and Antibody Development in Patients Treated for Refractory Chronic Gout. Arthritis Res. Ther. 2014, 16, R60. [Google Scholar] [CrossRef]
- Li, C. Poly(l-Glutamic Acid)–Anticancer Drug Conjugates. Adv. Drug Deliv. Rev. 2002, 54, 695–713. [Google Scholar] [CrossRef]
- Drobník, J.; Rypáček, F. Soluble Synthetic Polymers in Biological Systems. In Polymers in Medicine; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 1984; Volume 57, pp. 1–50. ISBN 978-3-540-12796-3. [Google Scholar]
- Li, C.; Ke, S.; Wu, Q.-P.; Tansey, W.; Hunter, N.; Buchmiller, L.M.; Milas, L.; Charnsangavej, C.; Wallace, S. Potentiation of Ovarian OCa-1 Tumor Radioresponse by Poly (L-Glutamic Acid)-Paclitaxel Conjugate. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 1119–1126. [Google Scholar] [CrossRef]
- Milas, L.; Mason, K.A.; Hunter, N.; Li, C.; Wallace, S. Poly(L-Glutamic Acid)-Paclitaxel Conjugate Is a Potent Enhancer of Tumor Radiocurability. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, A.; Perly, B.; Forchioni, A.; Chachaty, C. A Magnetic Resonance Study of the Segmental Motion and Local Conformations of Poly(L-Glutamic Acid) in Aqueous Solutions. Macromolecules 1978, 11, 977–986. [Google Scholar] [CrossRef]
- Miller, W.G. Degradation of Poly-α,L-Glutamic Acid. I. Degradation of High Molecular Weight PGA by Papain. J. Am. Chem. Soc. 1961, 83, 259–265. [Google Scholar] [CrossRef]
- Miller, W.G. Degradation of Synthetic Polypeptides. II. Degradation of Poly-α,L-Glutamic Acid by Proteolytic Enzymes in 0.20 M Sodium Chloride. J. Am. Chem. Soc. 1964, 86, 3913–3918. [Google Scholar] [CrossRef]
- Hayashi, T.; Iwatsuki, M. Biodegradation of Copoly(L-Aspartic Acid/L-Glutamic Acid) in Vitro. Biopolymers 1990, 29, 549–557. [Google Scholar] [CrossRef]
- Hoes, C.J.T.; Potman, W.; Van Heeswijk, W.A.R.; Mud, J.; De Grooth, B.G.; Greve, J.; Feijen, J. Optimization of Macromolecular Prodrugs of the Antitumor Antibiotic Adriamycin. J. Control. Release 1985, 2, 205–213. [Google Scholar] [CrossRef]
- Chiu, H.-C.; Kopečková, P.; Deshmane, S.S.; Kope?ek, J. Lysosomal Degradability of Poly(α-Amino Acids). J. Biomed. Mater. Res. 1997, 34, 381–392. [Google Scholar] [CrossRef]
- Mccormick-Thomson, L.A.; Duncan, R. Poly(Amino Acid) Copolymers as a Potential Soluble Drug Delivery System. 1: Pinocytic Uptake and Lysosomal Degradation Measured In Vitro. J. Bioact. Compat. Polym. 1989, 4, 242–251. [Google Scholar] [CrossRef]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled Ring-Opening Polymerization of Lactide and Glycolide. Chem. Rev. 2004, 104, 6147–6176. [Google Scholar] [CrossRef]
- Xu, Y.; Kim, C.-S.; Saylor, D.M.; Koo, D. Polymer Degradation and Drug Delivery in PLGA-Based Drug-Polymer Applications: A Review of Experiments and Theories: Review on Biodegradation and Drug Delivery from PLGA Polymers. J. Biomed. Mater. Res. 2017, 105, 1692–1716. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-Based Nanoparticles: An Overview of Biomedical Applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.T.C.R.; Cardoso, B.C.O.; Silva, M.E.S.R.E.; Freitas, R.F.S.; Sousa, R.G. Synthesis, Characterization, and Study of PLGA Copolymer in Vitro Degradation. JBNB 2015, 6, 8–19. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef]
- Zheng, M.; Pan, M.; Zhang, W.; Lin, H.; Wu, S.; Lu, C.; Tang, S.; Liu, D.; Cai, J. Poly(α-l-Lysine)-Based Nanomaterials for Versatile Biomedical Applications: Current Advances and Perspectives. Bioact. Mater. 2021, 6, 1878–1909. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, D.M.M. Thirteen Decades of Peptide Synthesis: Key Developments in Solid Phase Peptide Synthesis and Amide Bond Formation Utilized in Peptide Ligation. Amino Acids 2018, 50, 39–68. [Google Scholar] [CrossRef]
- Deming, T.J. Synthesis of Side-Chain Modified Polypeptides. Chem. Rev. 2016, 116, 786–808. [Google Scholar] [CrossRef]
- Shen, Y.; Fu, X.; Fu, W.; Li, Z. Biodegradable Stimuli-Responsive Polypeptide Materials Prepared by Ring Opening Polymerization. Chem. Soc. Rev. 2015, 44, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Wu, L.-P.; Parhamifar, L.; Moghimi, S.M. Differential Modulation of Cellular Bioenergetics by Poly(l-Lysine)s of Different Molecular Weights. Biomacromolecules 2015, 16, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Semenyshyn, R.; Hentschel, M.; Stanglmair, C.; Teutsch, T.; Tarin, C.; Pacholski, C.; Giessen, H.; Neubrech, F. In Vitro Monitoring Conformational Changes of Polypeptide Monolayers Using Infrared Plasmonic Nanoantennas. Nano Lett. 2019, 19, 1–7. [Google Scholar] [CrossRef]
- Rahikkala, A.; Junnila, S.; Vartiainen, V.; Ruokolainen, J.; Ikkala, O.; Kauppinen, E.; Raula, J. Polypeptide-Based Aerosol Nanoparticles: Self-Assembly and Control of Conformation by Solvent and Thermal Annealing. Biomacromolecules 2014, 15, 2607–2615. [Google Scholar] [CrossRef]
- Chen, J.; Zou, X. Self-Assemble Peptide Biomaterials and Their Biomedical Applications. Bioact. Mater. 2019, 4, 120–131. [Google Scholar] [CrossRef]
- Xiao, Y.-D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.-S.; Zhou, S.-K. MRI Contrast Agents: Classification and Application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef]
- Bloembergen, N.; Morgan, L.O. Proton Relaxation Times in Paramagnetic Solutions. Effects of Electron Spin Relaxation. J. Chem. Phys. 1961, 34, 842–850. [Google Scholar] [CrossRef]
- Solomon, I.; Bloembergen, N. Nuclear Magnetic Interactions in the HF Molecule. J. Chem. Phys. 1956, 25, 261–266. [Google Scholar] [CrossRef]
- Solomon, I. Relaxation Processes in a System of Two Spins. Phys. Rev. 1995, 99, 559–565. [Google Scholar] [CrossRef]
- Bloembergen, N. Spin Relaxation Processes in a Two-Proton System. Phys. Rev. 1956, 104, 1542–1547. [Google Scholar] [CrossRef]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Belford, R.L.; Clarkson, R.B. Second-Sphere and Outer-Sphere Proton Relaxation of Paramagnetic Complexes: From EPR to NMRD. J. Phys. Chem. A 1998, 102, 2117–2130. [Google Scholar] [CrossRef]
- Smeraldo, A.; Netti, P.A.; Torino, E. New Strategies in the Design of Paramagnetic CAs. Contrast Media Mol. Imaging 2020, 2020, 4327479. [Google Scholar] [CrossRef] [PubMed]
- Tóth, É.; Helm, L.; Merbach, A.E. Relaxivity of MRI Contrast Agents. In Contrast Agents I; Krause, W., Ed.; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2002; Volume 221, pp. 61–101. ISBN 978-3-540-42247-1. [Google Scholar]
- Verwilst, P.; Park, S.; Yoon, B.; Kim, J.S. Recent Advances in Gd-Chelate Based Bimodal Optical/MRI Contrast Agents. Chem. Soc. Rev. 2015, 44, 1791–1806. [Google Scholar] [CrossRef]
- Geraldes, C.F.G.C.; Laurent, S. Classification and Basic Properties of Contrast Agents for Magnetic Resonance Imaging. Contrast Media Mol. Imaging 2009, 4, 1–23. [Google Scholar] [CrossRef]
- Caspani, S.; Magalhães, R.; Araújo, J.P.; Sousa, C.T. Magnetic Nanomaterials as Contrast Agents for MRI. Materials 2020, 13, 2586. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of Magnetic Nanoparticles in Biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167. [Google Scholar] [CrossRef]
- Shen, Y.; Goerner, F.L.; Snyder, C.; Morelli, J.N.; Hao, D.; Hu, D.; Li, X.; Runge, V.M. T1 Relaxivities of Gadolinium-Based Magnetic Resonance Contrast Agents in Human Whole Blood at 1.5, 3, and 7 T. Investig. Radiol. 2015, 50, 330–338. [Google Scholar] [CrossRef]
- Noebauer-Huhmann, I.M.; Szomolanyi, P.; Juras, V.; Kraff, O.; Ladd, M.E.; Trattnig, S. Gadolinium-Based Magnetic Resonance Contrast Agents at 7 Tesla: In Vitro T1 Relaxivities in Human Blood Plasma. Investig. Radiol. 2010, 45, 554–558. [Google Scholar] [CrossRef]
- Pintaske, J.; Martirosian, P.; Graf, H.; Erb, G.; Lodemann, K.-P.; Claussen, C.D.; Schick, F. Relaxivity of Gadopentetate Dimeglumine (Magnevist), Gadobutrol (Gadovist), and Gadobenate Dimeglumine (MultiHance) in Human Blood Plasma at 0.2, 1.5, and 3 Tesla. Investig. Radiol. 2006, 41, 213–221. [Google Scholar] [CrossRef]
- Rohrer, M.; Bauer, H.; Mintorovitch, J.; Requardt, M.; Weinmann, H.-J. Comparison of Magnetic Properties of MRI Contrast Media Solutions at Different Magnetic Field Strengths. Investig. Radiol. 2005, 40, 715–724. [Google Scholar] [CrossRef]
- Thakor, A.S.; Jokerst, J.V.; Ghanouni, P.; Campbell, J.L.; Mittra, E.; Gambhir, S.S. Clinically Approved Nanoparticle Imaging Agents. J. Nucl. Med. 2016, 57, 1833–1837. [Google Scholar] [CrossRef] [PubMed]
- Na, H.B.; Song, I.C.; Hyeon, T. Inorganic Nanoparticles for MRI Contrast Agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Zijl, P.C.M.V.; Yadav, N.N. Chemical Exchange Saturation Transfer (CEST): What Is in a Name and What Isn’t? Magn. Reson. Med. 2011, 65, 927–948. [Google Scholar] [CrossRef]
- Wu, B.; Warnock, G.; Zaiss, M.; Lin, C.; Chen, M.; Zhou, Z.; Mu, L.; Nanz, D.; Tuura, R.; Delso, G. An Overview of CEST MRI for Non-MR Physicists. EJNMMI Phys. 2016, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, E.; Sherry, A.D.; Lenkinski, R.E. CEST: From Basic Principles to Applications, Challenges and Opportunities. J. Magn. Reson. 2013, 229, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.K.; Schlosser, M.J.; van Zijl, P.C.M.; Pomper, M.G.; Golay, X.; Zhou, J. Amide Proton Transfer Imaging of Human Brain Tumors at 3T. Magn. Reson. Med. 2006, 56, 585–592. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.T.; Gilad, A.A.; DeLiso, M.A.; Cromer Berman, S.M.; Bulte, J.W.M.; van Zijl, P.C.M. New “Multicolor” Polypeptide Diamagnetic Chemical Exchange Saturation Transfer (DIACEST) Contrast Agents for MRI. Magn. Reson. Med. 2008, 60, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.M.; Aletras, A.H.; Balaban, R.S. A New Class of Contrast Agents for MRI Based on Proton Chemical Exchange Dependent Saturation Transfer (CEST). J. Magn. Reson. 2000, 143, 79–87. [Google Scholar] [CrossRef]
- Sherry, A.D.; Woods, M. Chemical Exchange Saturation Transfer Contrast Agents for Magnetic Resonance Imaging. Annu. Rev. Biomed. Eng. 2008, 10, 391–411. [Google Scholar] [CrossRef] [PubMed]
- Parrott, D.; Fernando, W.S.; Martins, A.F. Smart MRI Agents for Detecting Extracellular Events In Vivo: Progress and Challenges. Inorganics 2019, 7, 18. [Google Scholar] [CrossRef]
- Zhou, J.; Heo, H.-Y.; Knutsson, L.; van Zijl, P.C.M.; Jiang, S. APT-Weighted MRI: Techniques, Current Neuro Applications, and Challenging Issues: APTw MRI for Neuro Applications. J. Magn. Reson. Imaging 2019, 50, 347–364. [Google Scholar] [CrossRef]
- Goldenberg, J.M.; Pagel, M.D. Assessments of Tumor Metabolism with CEST MRI. NMR Biomed. 2019, 32, e3943. [Google Scholar] [CrossRef]
- Xu, X.; Chan, K.W.Y.; Knutsson, L.; Artemov, D.; Xu, J.; Liu, G.; Kato, Y.; Lal, B.; Laterra, J.; McMahon, M.T.; et al. Dynamic Glucose Enhanced (DGE) MRI for Combined Imaging of Blood-Brain Barrier Break down and Increased Blood Volume in Brain Cancer: Dynamic Glucose Enhanced (DGE) MRI. Magn. Reson. Med. 2015, 74, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Herz, K.; Lindig, T.; Deshmane, A.; Schittenhelm, J.; Skardelly, M.; Bender, B.; Ernemann, U.; Scheffler, K.; Zaiss, M. T1ρ-based Dynamic Glucose-enhanced (DGEρ) MRI at 3 T: Method Development and Early Clinical Experience in the Human Brain. Magn. Reson. Med. 2019, 82, 1832–1847. [Google Scholar] [CrossRef]
- Chen, Z.; Han, Z.; Liu, G. Repurposing Clinical Agents for Chemical Exchange Saturation Transfer Magnetic Resonance Imaging: Current Status and Future Perspectives. Pharmaceuticals 2020, 14, 11. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Xu, J.; Yadav, N.N.; Chan, K.W.Y.; Luo, L.; McMahon, M.T.; Vogelstein, B.; van Zijl, P.C.M.; Zhou, S.; et al. CEST Theranostics: Label-Free MR Imaging of Anticancer Drugs. Oncotarget 2016, 7, 6369–6378. [Google Scholar] [CrossRef]
- Lock, L.L.; Li, Y.; Mao, X.; Chen, H.; Staedtke, V.; Bai, R.; Ma, W.; Lin, R.; Li, Y.; Liu, G.; et al. One-Component Supramolecular Filament Hydrogels as Theranostic Label-Free Magnetic Resonance Imaging Agents. ACS Nano 2017, 11, 797–805. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, J.; Qi, X.; Li, S.; Liu, G.; Siddhanta, S.; Barman, I.; Song, X.; McMahon, M.T.; Bulte, J.W.M. Furin-Mediated Intracellular Self-Assembly of Olsalazine Nanoparticles for Enhanced Magnetic Resonance Imaging and Tumour Therapy. Nat. Mater. 2019, 18, 1376–1383. [Google Scholar] [CrossRef]
- Yang, X.; Song, X.; Li, Y.; Liu, G.; Ray Banerjee, S.; Pomper, M.G.; McMahon, M.T. Salicylic Acid and Analogues as DiaCEST MRI Contrast Agents with Highly Shifted Exchangeable Proton Frequencies. Angew. Chem. Int. Ed. 2013, 52, 8116–8119. [Google Scholar] [CrossRef]
- Pavuluri, K.D.; Yang, E.; Ayyappan, V.; Sonkar, K.; Tan, Z.; Tressler, C.M.; Bo, S.; Bibic, A.; Glunde, K.; McMahon, M.T. Unlabeled Aspirin as an Activatable Theranostic MRI Agent for Breast Cancer. Theranostics 2022, 12, 1937–1951. [Google Scholar] [CrossRef]
- Song, X.; Yang, X.; Ray Banerjee, S.; Pomper, M.G.; McMahon, M.T. Anthranilic Acid Analogs as Diamagnetic CEST MRI Contrast Agents That Feature an Intramolecular-Bond Shifted Hydrogen: Novel Anthranilic Acid IM-SHY Diacest MRI agents. Contrast Media Mol. Imaging 2015, 10, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jablonska, A.; Li, Y.; Cao, S.; Liu, D.; Chen, H.; Van Zijl, P.C.; Bulte, J.W.M.; Janowski, M.; Walczak, P.; et al. Label-Free CEST MRI Detection of Citicoline-Liposome Drug Delivery in Ischemic Stroke. Theranostics 2016, 6, 1588–1600. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, J.; Lu, J.; Yi, L.; Han, Z.; Zhang, S.; Yang, X.; Liu, G. N-Aryl Amides as Chemical Exchange Saturation Transfer Magnetic Resonance Imaging Contrast Agents. Chem. Eur. J. 2020, 26, 11705–11709. [Google Scholar] [CrossRef] [PubMed]
- Bober, Z.; Aebisher, D.; Ożóg, Ł.; Tabarkiewicz, J.; Tutka, P.; Bartusik-Aebisher, D. 19F MRI As a Tool for Imaging Drug Delivery to Tissue and Individual Cells. Eur. J. Clin. Exp. Med. 2017, 15, 99–109. [Google Scholar] [CrossRef]
- Ruiz-Cabello, J.; Barnett, B.P.; Bottomley, P.A.; Bulte, J.W.M. Fluorine (19F) MRS and MRI in Biomedicine. NMR Biomed. 2011, 24, 114–129. [Google Scholar] [CrossRef]
- Tirotta, I.; Mastropietro, A.; Cordiglieri, C.; Gazzera, L.; Baggi, F.; Baselli, G.; Bruzzone, M.G.; Zucca, I.; Cavallo, G.; Terraneo, G.; et al. A Superfluorinated Molecular Probe for Highly Sensitive in Vivo19F-MRI. J. Am. Chem. Soc. 2014, 136, 8524–8527. [Google Scholar] [CrossRef] [PubMed]
- Bouvain, P.; Temme, S.; Flögel, U. Hot Spot 19F Magnetic Resonance Imaging of Inflammation. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1639. [Google Scholar] [CrossRef]
- Jirak, D.; Galisova, A.; Kolouchova, K.; Babuka, D.; Hruby, M. Fluorine Polymer Probes for Magnetic Resonance Imaging: Quo Vadis? Magn. Reson. Mater. Phys. 2019, 32, 173–185. [Google Scholar] [CrossRef]
- Rizzo, S.; Padelli, F.; Rinaldi, E.; Gioeni, D.; Aquino, D.; Brizzola, S.; Acocella, F.; Spaggiari, L.; Baggi, F.; Bellomi, M.; et al. 7-T MRI Tracking of Mesenchymal Stromal Cells after Lung Injection in a Rat Model. Eur. Radiol. Exp. 2020, 4, 54. [Google Scholar] [CrossRef]
- Makela, A.V.; Foster, P.J. Preclinical 19F MRI Cell Tracking at 3 Tesla. Magn. Reson. Mater. Phys. 2019, 32, 123–132. [Google Scholar] [CrossRef]
- Fink, C.; Smith, M.; Gaudet, J.M.; Makela, A.; Foster, P.J.; Dekaban, G.A. Fluorine-19 Cellular MRI Detection of In Vivo Dendritic Cell Migration and Subsequent Induction of Tumor Antigen-Specific Immunotherapeutic Response. Mol. Imaging Biol. 2020, 22, 549–561. [Google Scholar] [CrossRef]
- Makela, A.V.; Foster, P.J. Imaging Macrophage Distribution and Density in Mammary Tumors and Lung Metastases Using Fluorine-19 MRI Cell Tracking. Magn. Reson. Med. 2018, 80, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- van Heeswijk, R.B.; De Blois, J.; Kania, G.; Gonzales, C.; Blyszczuk, P.; Stuber, M.; Eriksson, U.; Schwitter, J. Selective In Vivo Visualization of Immune-Cell Infiltration in a Mouse Model of Autoimmune Myocarditis by Fluorine-19 Cardiac Magnetic Resonance. Circ. Cardiovasc. Imaging 2013, 6, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, C.; Yoshihara, H.A.; van Heeswijk, R.B.; Mieville, P.; Helm, L.; Blyszczuk, P.; Kania, G.; Eriksson, U.; Schwitter, J. 19F-Magnetic Resonance Imaging for Tracking Bone- Marrow Macrophages in a Model of Experimental Autoimmune Myocarditis: A Pilot Study. BJSTR 2020, 29, 22419–22428. [Google Scholar] [CrossRef]
- Bönner, F.; Merx, M.W.; Klingel, K.; Begovatz, P.; Flögel, U.; Sager, M.; Temme, S.; Jacoby, C.; Salehi Ravesh, M.; Grapentin, C.; et al. Monocyte Imaging after Myocardial Infarction with 19F MRI at 3 T: A Pilot Study in Explanted Porcine Hearts. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 612–620. [Google Scholar] [CrossRef]
- Vindegaard, N.; Muñoz-Briones, C.; El Ali, H.H.; Kristensen, L.K.; Rasmussen, R.S.; Johansen, F.F.; Hasseldam, H. T-Cells and Macrophages Peak Weeks after Experimental Stroke: Spatial and Temporal Characteristics. Neuropathology 2017, 37, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.; Chapelin, F.; Xu, H.; Acevedo, J.R.; Molinolo, A.; Nguyen, Q.; Ahrens, E.T. Visualization of Macrophage Recruitment in Head and Neck Carcinoma Model Using Fluorine-19 Magnetic Resonance Imaging. Magn. Reson. Med. 2018, 79, 1972–1980. [Google Scholar] [CrossRef]
- Weise, G.; Basse-Luesebrink, T.C.; Wessig, C.; Jakob, P.M.; Stoll, G. In Vivo Imaging of Inflammation in the Peripheral Nervous System by 19F MRI. Exp. Neurol. 2011, 229, 494–501. [Google Scholar] [CrossRef]
- Weibel, S.; Basse-Luesebrink, T.C.; Hess, M.; Hofmann, E.; Seubert, C.; Langbein-Laugwitz, J.; Gentschev, I.; Sturm, V.J.F.; Ye, Y.; Kampf, T.; et al. Imaging of Intratumoral Inflammation during Oncolytic Virotherapy of Tumors by 19F-Magnetic Resonance Imaging (MRI). PLoS ONE 2013, 8, e56317. [Google Scholar] [CrossRef]
- Balducci, A.; Wen, Y.; Zhang, Y.; Helfer, B.M.; Hitchens, T.K.; Meng, W.S.; Wesa, A.K.; Janjic, J.M. A Novel Probe for the Non-Invasive Detection of Tumor-Associated Inflammation. OncoImmunology 2013, 2, e23034. [Google Scholar] [CrossRef]
- He, Z.; Zhang, P.; Xiao, Y.; Li, J.; Yang, F.; Liu, Y.; Zhang, J.-R.; Zhu, J.-J. Acid-Degradable Gadolinium-Based Nanoscale Coordination Polymer: A Potential Platform for Targeted Drug Delivery and Potential Magnetic Resonance Imaging. Nano Res. 2018, 11, 929–939. [Google Scholar] [CrossRef]
- Shalviri, A.; Foltz, W.D.; Cai, P.; Rauth, A.M.; Wu, X.Y. Multifunctional Terpolymeric MRI Contrast Agent with Superior Signal Enhancement in Blood and Tumor. J. Control. Release 2013, 167, 11–20. [Google Scholar] [CrossRef]
- Dong, X.; Tahir, M.A.; Zhang, L.; Schäfer, C.G. Gadolinium-Containing Polymer Microspheres: A Dual-Functional Theranostic Agent for Magnetic Resonance Imaging and Cancer Therapy. New J. Chem. 2019, 43, 5987–5995. [Google Scholar] [CrossRef]
- Lee, S.-M.; Song, Y.; Hong, B.J.; MacRenaris, K.W.; Mastarone, D.J.; O’Halloran, T.V.; Meade, T.J.; Nguyen, S.T. Modular Polymer-Caged Nanobins as a Theranostic Platform with Enhanced Magnetic Resonance Relaxivity and PH-Responsive Drug Release. Angew. Chem. Int. Ed. 2010, 49, 9960–9964. [Google Scholar] [CrossRef]
- Liao, Z.; Wang, H.; Wang, X.; Zhao, P.; Wang, S.; Su, W.; Chang, J. Multifunctional Nanoparticles Composed of A Poly(Dl-Lactide-Coglycolide) Core and A Paramagnetic Liposome Shell for Simultaneous Magnetic Resonance Imaging and Targeted Therapeutics. Adv. Funct. Mater. 2011, 21, 1179–1186. [Google Scholar] [CrossRef]
- Szczęch, M.; Karabasz, A.; Łopuszyńska, N.; Bzowska, M.; Węglarz, W.P.; Warszyński, P.; Szczepanowicz, K. Gadolinium Labeled Polyelectrolyte Nanocarriers for Theranostic Application. Colloids Surf. B Biointerfaces 2019, 183, 110396. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Liu, Y.; Zhou, M.; Wang, W.; Shi, M.; Xing, M.; Liao, W. Theranostic PH-Sensitive Nanoparticles for Highly Efficient Targeted Delivery of Doxorubicin for Breast Tumor Treatment. IJN 2018, 13, 1119–1137. [Google Scholar] [CrossRef]
- Szczepanowicz, K.; Piechota, P.; Węglarz, W.P.; Warszyński, P. Polyelectrolyte Nanocapsules Containing Iron Oxide Nanoparticles as MRI Detectable Drug Delivery System. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 351–356. [Google Scholar] [CrossRef]
- Szczęch, M.; Orsi, D.; Łopuszyńska, N.; Cristofolini, L.; Jasiński, K.; Węglarz, W.P.; Albertini, F.; Kereïche, S.; Szczepanowicz, K. Magnetically Responsive Polycaprolactone Nanocarriers for Application in the Biomedical Field: Magnetic Hyperthermia, Magnetic Resonance Imaging, and Magnetic Drug Delivery. RSC Adv. 2020, 10, 43607–43618. [Google Scholar] [CrossRef]
- Mosafer, J.; Abnous, K.; Tafaghodi, M.; Mokhtarzadeh, A.; Ramezani, M. In Vitro and in Vivo Evaluation of Anti-Nucleolin-Targeted Magnetic PLGA Nanoparticles Loaded with Doxorubicin as a Theranostic Agent for Enhanced Targeted Cancer Imaging and Therapy. Eur. J. Pharm. Biopharm. 2017, 113, 60–74. [Google Scholar] [CrossRef]
- Luque-Michel, E.; Sebastian, V.; Larrea, A.; Marquina, C.; Blanco-Prieto, M.J. Co-Encapsulation of Superparamagnetic Nanoparticles and Doxorubicin in PLGA Nanocarriers: Development, Characterization and in Vitro Antitumor Efficacy in Glioma Cells. Eur. J. Pharm. Biopharm. 2019, 145, 65–75. [Google Scholar] [CrossRef]
- Zhang, Y.; García-Gabilondo, M.; Grayston, A.; Feiner, I.V.J.; Anton-Sales, I.; Loiola, R.A.; Llop, J.; Ramos-Cabrer, P.; Barba, I.; Garcia-Dorado, D.; et al. PLGA Protein Nanocarriers with Tailor-Made Fluorescence/MRI/PET Imaging Modalities. Nanoscale 2020, 12, 4988–5002. [Google Scholar] [CrossRef]
- Jaidev, L.R.; Chellappan, D.R.; Bhavsar, D.V.; Ranganathan, R.; Sivanantham, B.; Subramanian, A.; Sharma, U.; Jagannathan, N.R.; Krishnan, U.M.; Sethuraman, S. Multi-Functional Nanoparticles as Theranostic Agents for the Treatment & Imaging of Pancreatic Cancer. Acta Biomater. 2017, 49, 422–433. [Google Scholar] [CrossRef]
- Situ, J.-Q.; Wang, X.-J.; Zhu, X.-L.; Xu, X.-L.; Kang, X.-Q.; Hu, J.-B.; Lu, C.-Y.; Ying, X.-Y.; Yu, R.-S.; You, J.; et al. Multifunctional SPIO/DOX-Loaded A54 Homing Peptide Functionalized Dextran-g-PLGA Micelles for Tumor Therapy and MR Imaging. Sci. Rep. 2016, 6, 35910. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Su, H.; Jin, R.; Li, D.; Wu, C.; Jiang, X.; Xia, C.; Gong, Q.; Song, B.; Ai, H. Multifunctional Dextran Micelles as Drug Delivery Carriers and Magnetic Resonance Imaging Probes. Sci. Bull. 2015, 60, 1272–1280. [Google Scholar] [CrossRef]
- Wu, L.; Wu, M.; Lin, X.; Zhang, X.; Liu, X.; Liu, J. Magnetite Nanocluster and Paclitaxel-Loaded Charge-Switchable Nanohybrids for MR Imaging and Chemotherapy. J. Mater. Chem. B 2017, 5, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.P.; Thuy, V.T.T.; Kim, D. Integration of Iron Oxide Nanoparticles and Polyaspartamide Biopolymer for MRI Image Contrast Enhancement and an Efficient Drug-Delivery System in Cancer Therapy. Nanotechnology 2020, 31, 335712. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Li, T.; Chen, Z.; Geng, Y.; Xie, X.; Li, S.; Yang, H.; Wu, C.; Liu, Y. Luminescent/Magnetic PLGA-Based Hybrid Nanocomposites: A Smart Nanocarrier System for Targeted Codelivery and Dual-Modality Imaging in Cancer Theranostics. Int. J. Nanomed. 2017, 12, 4299–4322. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, D.; Li, N.; Wang, X.; Ma, Y. Drug-Loaded Poly (ε-Caprolactone)/Fe3O4 Composite Microspheres for Magnetic Resonance Imaging and Controlled Drug Delivery. J. Magn. Magn. Mater. 2018, 456, 316–323. [Google Scholar] [CrossRef]
- Schleich, N.; Sibret, P.; Danhier, P.; Ucakar, B.; Laurent, S.; Muller, R.N.; Jérôme, C.; Gallez, B.; Préat, V.; Danhier, F. Dual Anticancer Drug/Superparamagnetic Iron Oxide-Loaded PLGA-Based Nanoparticles for Cancer Therapy and Magnetic Resonance Imaging. Int. J. Pharm. 2013, 447, 94–101. [Google Scholar] [CrossRef]
- Zhang, Y.; García-Gabilondo, M.; Rosell, A.; Roig, A. MRI/Photoluminescence Dual-Modal Imaging Magnetic PLGA Nanocapsules for Theranostics. Pharmaceutics 2020, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, M.; Zeng, F.; Jin, H.; Xu, Q.; Huang, Y. Dual-Targeting Magnetic PLGA Nanoparticles for Codelivery of Paclitaxel and Curcumin for Brain Tumor Therapy. ACS Appl. Mater. Interfaces 2016, 8, 32159–32169. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Wang, Q.; Chen, K.; Qu, J.; Zhou, Q.; Luo, J.; Lin, J. SPIONs/DOX Loaded Polymer Nanoparticles for MRI Detection and Efficient Cell Targeting Drug Delivery. RSC Adv. 2017, 7, 47715–47725. [Google Scholar] [CrossRef]
- Li, Y.; Qiao, Y.; Chen, H.; Bai, R.; Staedtke, V.; Han, Z.; Xu, J.; Chan, K.W.Y.; Yadav, N.; Bulte, J.W.M.; et al. Characterization of Tumor Vascular Permeability Using Natural Dextrans and CEST MRI. Magn. Reson. Med. 2018, 79, 1001–1009. [Google Scholar] [CrossRef]
- Chen, H.; Liu, D.; Li, Y.; Xu, X.; Xu, J.; Yadav, N.N.; Zhou, S.; van Zijl, P.C.M.; Liu, G. CEST MRI Monitoring of Tumor Response to Vascular Disrupting Therapy Using High Molecular Weight Dextrans. Magn. Reson. Med. 2019, 82, 1471–1479. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, S.; Fujiwara, K.; Zhang, J.; Li, Y.; Liu, J.; van Zijl, P.C.M.; Lu, Z.-R.; Zheng, L.; Liu, G. Extradomain-B Fibronectin-Targeted Dextran-Based Chemical Exchange Saturation Transfer Magnetic Resonance Imaging Probe for Detecting Pancreatic Cancer. Bioconj. Chem. 2019, 30, 1425–1433. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Airan, R.; Han, Z.; Xu, J.; Chan, K.W.Y.; Xu, Y.; Bulte, J.W.M.; van Zijl, P.C.M.; McMahon, M.T.; et al. CT and CEST MRI Bimodal Imaging of the Intratumoral Distribution of Iodinated Liposomes. Quant. Imaging Med. Surg. 2019, 9, 1579591. [Google Scholar] [CrossRef]
- Langereis, S.; Keupp, J.; van Velthoven, J.L.J.; de Roos, I.H.C.; Burdinski, D.; Pikkemaat, J.A.; Grüll, H. A Temperature-Sensitive Liposomal 1H CEST and 19F Contrast Agent for MR Image-Guided Drug Delivery. J. Am. Chem. Soc. 2009, 131, 1380–1381. [Google Scholar] [CrossRef]
- Chan, K.W.Y.; Yu, T.; Qiao, Y.; Liu, Q.; Yang, M.; Patel, H.; Liu, G.; Kinzler, K.W.; Vogelstein, B.; Bulte, J.W.M.; et al. A DiaCEST MRI Approach for Monitoring Liposomal Accumulation in Tumors. J. Control. Release 2014, 180, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wang, C.; Zheng, J.; Lin, G.; Ni, D.; Shen, Z.; Huang, B.; Li, Y.; Guan, J.; Hong, W.; et al. Novel Nanomedicine with a Chemical-Exchange Saturation Transfer Effect for Breast Cancer Treatment in Vivo. J. Nanobiotechnol. 2019, 17, 123. [Google Scholar] [CrossRef]
- Choi, J.; Kim, K.; Kim, T.; Liu, G.; Bar-Shir, A.; Hyeon, T.; McMahon, M.T.; Bulte, J.W.M.; Fisher, J.P.; Gilad, A.A. Multimodal Imaging of Sustained Drug Release from 3-D Poly(Propylene Fumarate) (PPF) Scaffolds. J. Control. Release 2011, 156, 239–245. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, K.; Huang, G.; Takahashi, M.; Sherry, A.D.; Gao, J. A Novel Class of Polymeric PH-Responsive MRI CEST Agents. Chem. Commun. 2013, 49, 6418–6420. [Google Scholar] [CrossRef] [PubMed]
- Janjic, J.M.; Ahrens, E.T. Fluorine-Containing Nanoemulsions for MRI Cell Tracking. WIREs Nanomed. Nanobiotechnol. 2009, 1, 492–501. [Google Scholar] [CrossRef]
- Boissenot, T.; Fattal, E.; Bordat, A.; Houvenagel, S.; Valette, J.; Chacun, H.; Gueutin, C.; Tsapis, N. Paclitaxel-Loaded PEGylated Nanocapsules of Perfluorooctyl Bromide as Theranostic Agents. Eur. J. Pharm. Biopharm. 2016, 108, 136–144. [Google Scholar] [CrossRef]
- Bo, S.; Yuan, Y.; Chen, Y.; Yang, Z.; Chen, S.; Zhou, X.; Jiang, Z.-X. In Vivo Drug Tracking with 19F MRI at Therapeutic Dose. Chem. Commun. 2018, 54, 3875–3878. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, H.; Chen, K.; Li, Y.; Yang, Z.; Chen, S.; Zheng, X.; Zhou, X.; Jiang, Z.-X. Peptidic Monodisperse PEG “Comb” as Multifunctional “Add-On” Module for Imaging-Traceable and Thermo-Responsive Theranostics. Adv. Healthc. Mater. 2020, 9, 1901331. [Google Scholar] [CrossRef]
- Kolouchova, K.; Cernochova, Z.; Groborz, O.; Herynek, V.; Koucky, F.; Jaksa, R.; Benes, J.; Slouf, M.; Hruby, M. Multiresponsive Fluorinated Polymers as a Theranostic Platform Using 19F MRI. Eur. Polym. J. 2022, 175, 111381. [Google Scholar] [CrossRef]
- Neri, G.; Mion, G.; Pizzi, A.; Celentano, W.; Chaabane, L.; Chierotti, M.R.; Gobetto, R.; Li, M.; Messa, P.; De Campo, F.; et al. Fluorinated PLGA Nanoparticles for Enhanced Drug Encapsulation and 19F NMR Detection. Chem.—A Eur. J. 2020, 26, 10057–10063. [Google Scholar] [CrossRef] [PubMed]
- Szczęch, M.; Łopuszyńska, N.; Tomal, W.; Jasiński, K.; Węglarz, W.P.; Warszyński, P.; Szczepanowicz, K. Nafion-Based Nanocarriers for Fluorine Magnetic Resonance Imaging. Langmuir 2020, 36, 9534–9539. [Google Scholar] [CrossRef]
- Łopuszyńska, N.; Szczepanowicz, K.; Jasiński, K.; Warszyński, P.; Węglarz, W.P. Effective Detection of Nafion®-Based Theranostic Nanocapsules Through 19F Ultra-Short Echo Time MRI. Nanomaterials 2020, 10, 2127. [Google Scholar] [CrossRef] [PubMed]
- Szczęch, M.; Hinz, A.; Łopuszyńska, N.; Bzowska, M.; Węglarz, W.P.; Szczepanowicz, K. Polyaminoacid Based Core@shell Nanocarriers of 5-Fluorouracil: Synthesis, Properties and Theranostics Application. Int. J. Mol. Sci. 2021, 22, 12762. [Google Scholar] [CrossRef]
- Alhaidari, L.M.; Spain, S.G. Synthesis of 5-Fluorouracil Polymer Conjugate and 19F NMR Analysis of Drug Release for MRI Monitoring. Polymers 2023, 15, 1778. [Google Scholar] [CrossRef]
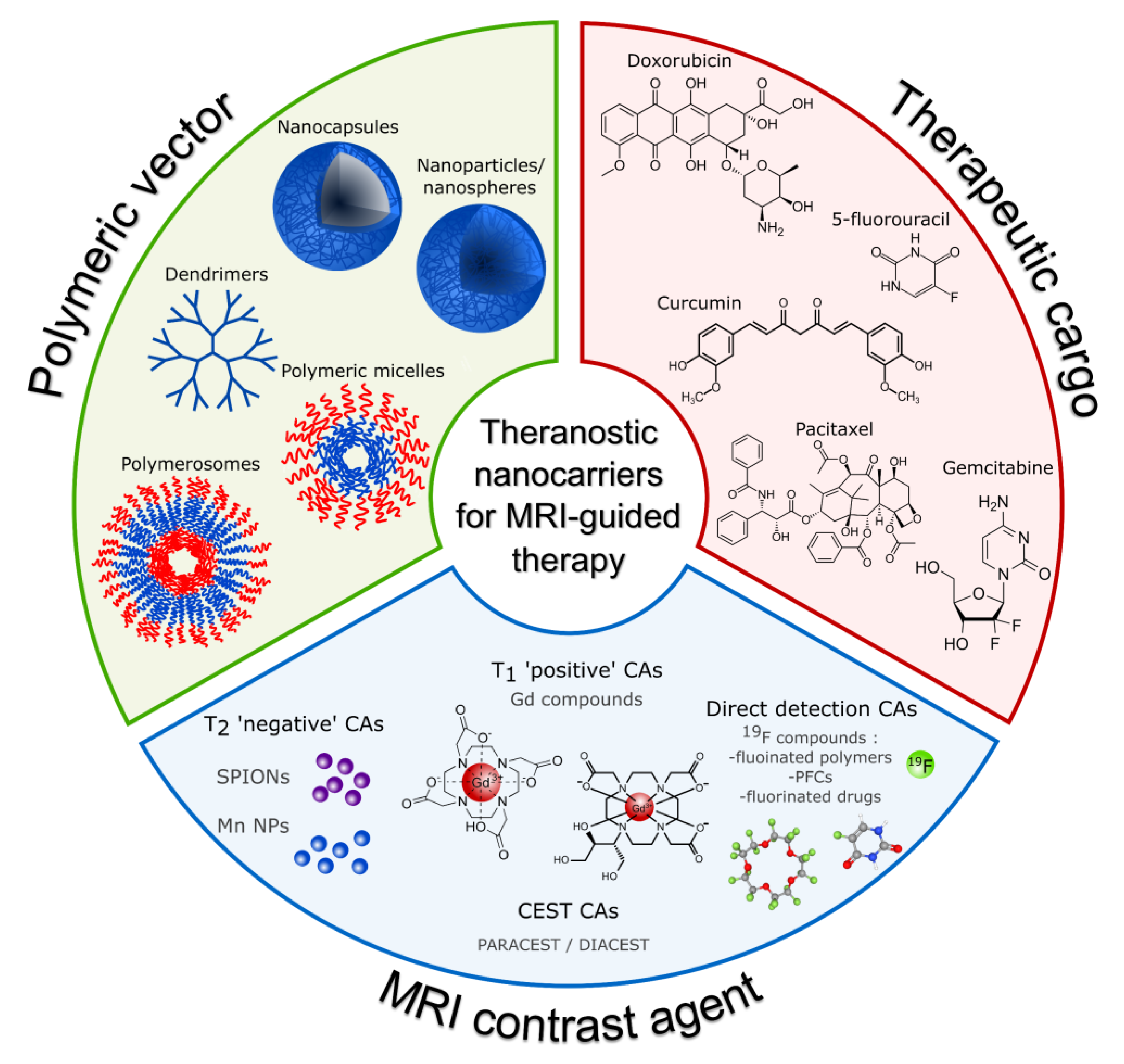
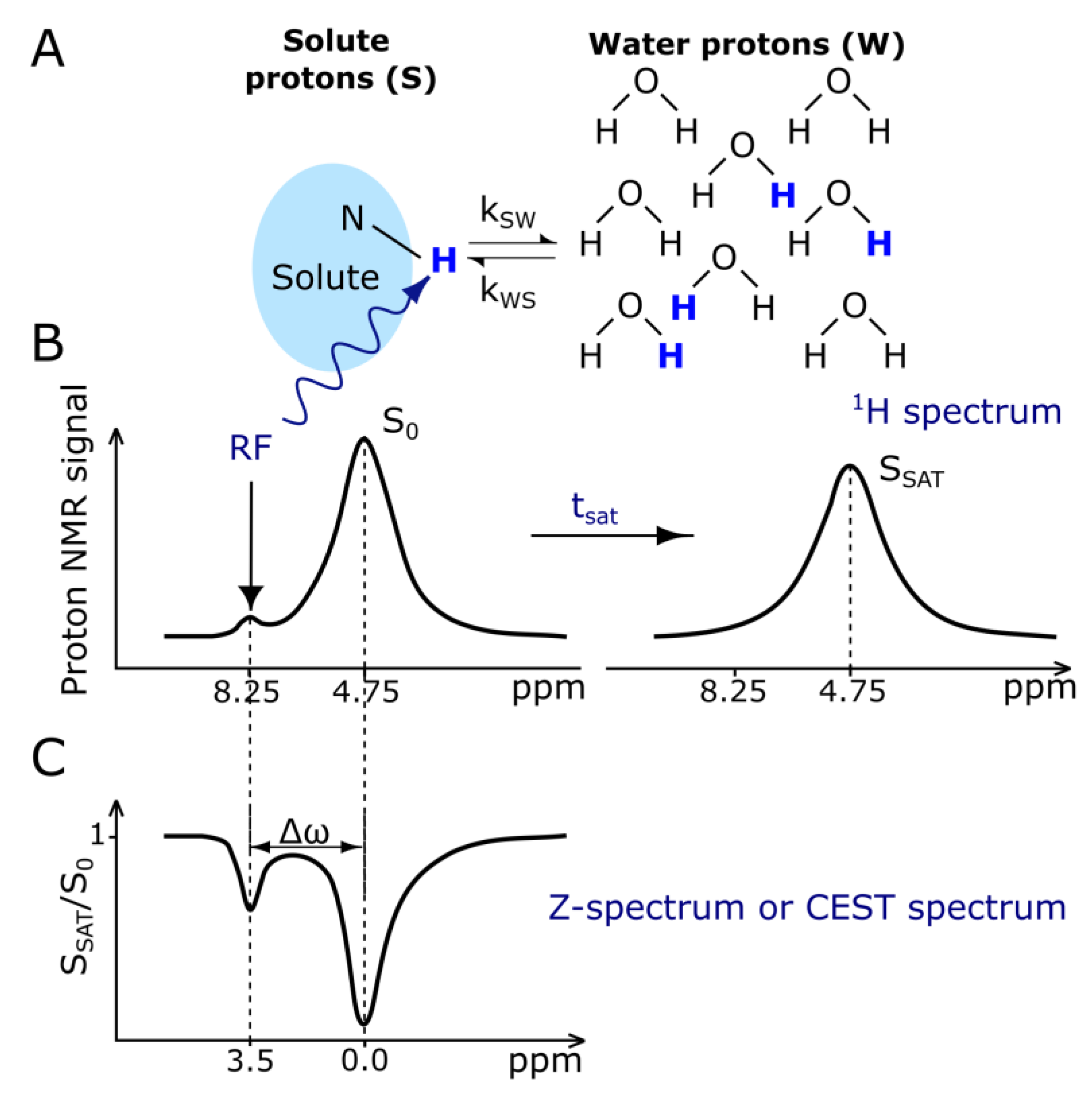
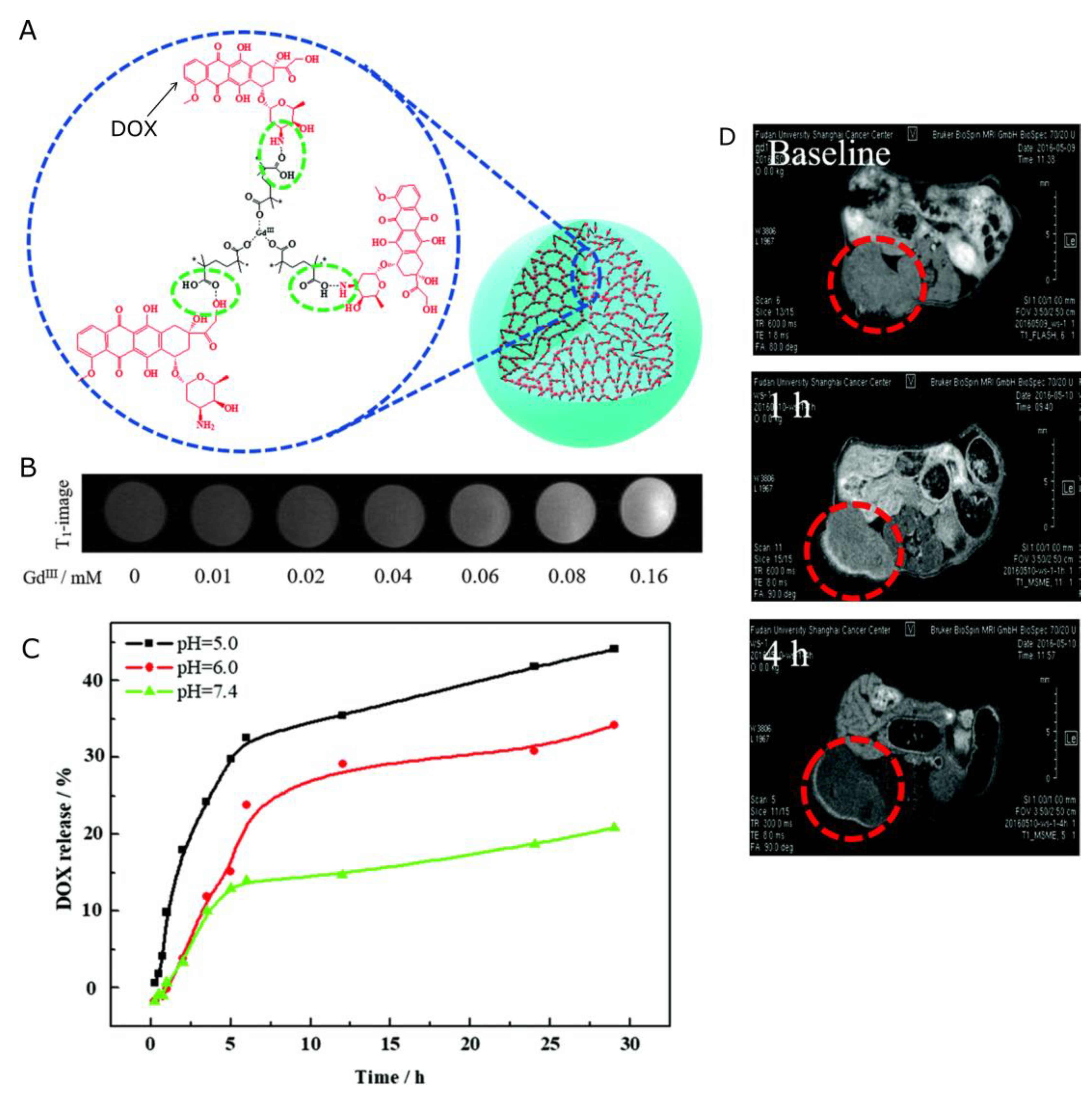

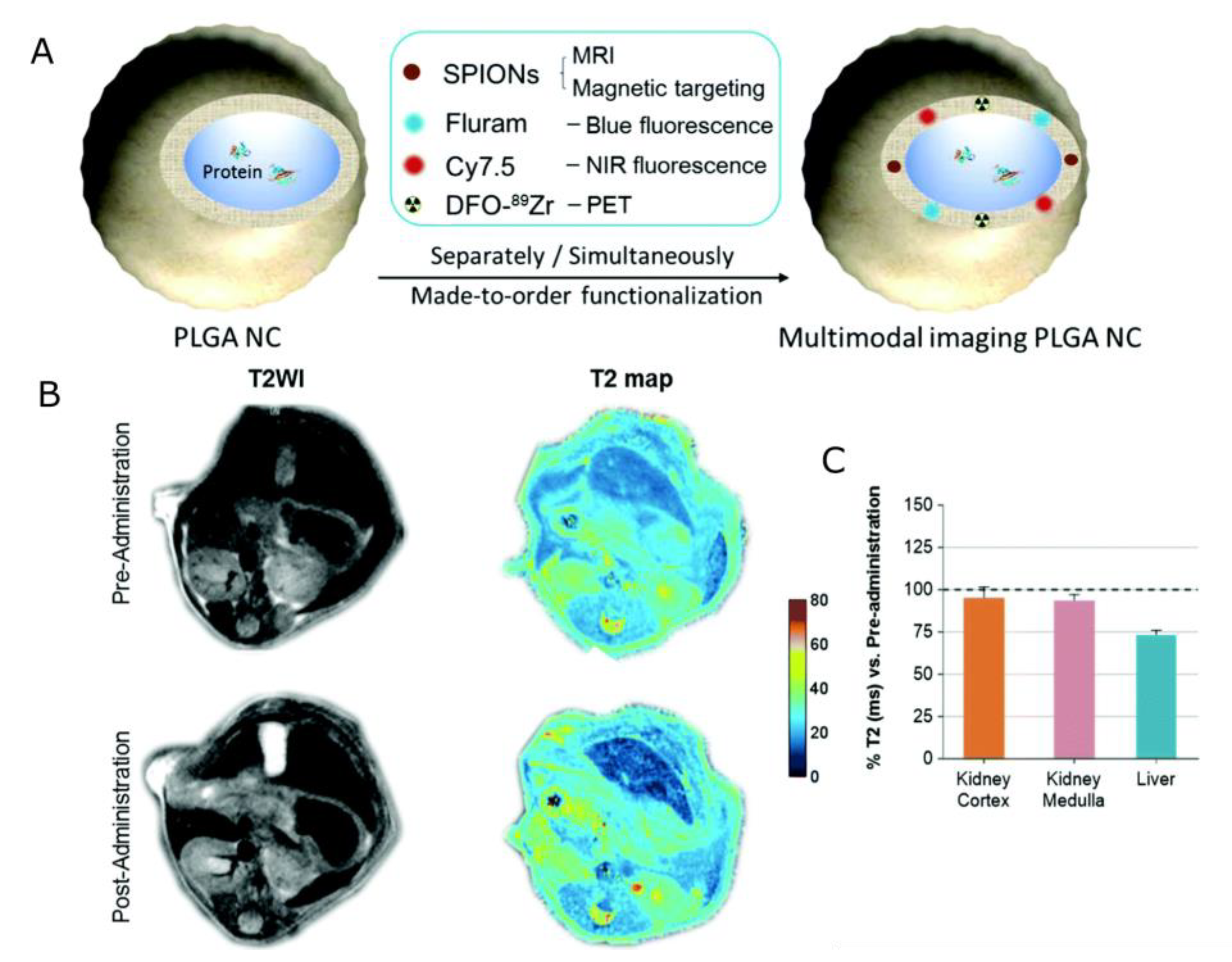

| Structure | Overview | Model Drug 1 | MRI Contrasting Properties | Ref |
|---|---|---|---|---|
| Nanocapsules | Acid-degradable gadolinium-doxorubicin-loaded nanoscale coordination polymer (Gd-Dox NCPs) core and hyaluronic acid shell. | DOX | r1 = 6.58 mM−1s−1 | [138] |
| Nanospheres | The multifunctional terpolymeric system achieved by the polymerization of methacrylic acid and polysorbate onto starch with multiple, chemically bound DTPA groups for gadolinium chelating. | DOX | PolyGd: r1 = 21.8 mM−1s−1 PolyGd-DOX: r1 = 19.2 mM−1s−1 | [139] |
| Nanospheres | Poly(gadolinium methacrylate-co-methacrylic acid) copolymer microspheres. | DOX | r1 = 10.64 mM−1s−1 | [140] |
| Nanocapsules | Gd-loaded liposome core with a polymeric shell of PCL with azide-modified Gd III complexes conjugated to the surface. | G | r1 = 15.9 mM−1s−1 (at a Gd III/lipid ratio of 0.45) | [141] |
| Nanocapsules | Self-assembled, hydrophobic PLGA core and a hydrophilic paramagnetic-folate-coated PEGylated lipid shell with (DTPA-Gd) chelated to the shell layer. | DOX | r1 = 14.38 mM−1s−1 | [142] |
| Nanocapsules | Multilayer shell of biodegradable polyelectrolytes: PLL, PLL-Gd, and PGA. Anticancer drug PTX encapsulated in the nanocarriers; the MRI contrast agent PLL-Gd constituted a part of the NCs shell. | PTX | r1 = 9.90 mM−1s−1 (for nanoemulsion core) r1 = 8.04 mM−1s−1 (for polymeric core) | [143] |
| Structure | Overview | Model Drug 1 | MRI Contrasting Properties | Ref |
|---|---|---|---|---|
| Nanocapsules | The pH-sensitive poly(β-thiopropionate) nanoparticles with a superparamagnetic core and folic acid (FA) conjugation (FA-doxorubicin-iron oxide nanoparticles (FA-DOX@IONPs)). | DOX | In vivo observation of T2-dependent darkening in the tumor site. | [144] |
| Nanocapsules | Polyelectrolyte nanocapsules with multilayer shell containing iron oxide nanoparticles as MRI visible drug delivery system. | - | Nanocapsules with two layers of Fe2O3 in the shell (AOT/PLL/PGA/Fe2O3/PGA/Fe2O3/PGA/PLL/PGA-g-PEG) displayed beneficial T2 − relaxation properties over the pure Fe2O3 suspension. | [145] |
| Nanocapsules | Drug-loaded polymer nanoparticles PCL coated with a multilayer shell of bio-acceptable components: PGA and SPIONs. | PTX | r2 = 850.1 mM−1s−1 | [146] |
| Nanospheres | The SPIONs/DOX co-loaded PLGA-based nanoparticles targeted with AS1411 aptamer. | DOX | In vivo observation of T2-dependent darkening in tumor and liver site | [147] |
| Nanospheres | Surfactant-coated polymer PLGA nanoplatform co-encapsulating (DOX) and SPIONs. | DOX | r2 = 158.03–197.80 mM−1s−1 (depending on surfactant) | [148] |
| Nanocapsules | PLGA NCs with several biocompatible multimodal imaging modalities: Fluram and Cyanine 7.5 as fluorescent probes, 89Zr chelated with DFO as a radio imaging probe, and SPIONs as an MRI contrast agent. | proteins (BSA) | r2 = 336 or 278 mM−1s−1 (for higher and lower SPIONs loading, respectively) | [149] |
| Nanospheres | Fluorescent iron oxide nanoparticles and G-encapsulated PLGA nanospheres, conjugated with HER-PGFIO antibody. | G | r2 = 773 mM−1s−1 | [150] |
| Nanocapsules | The A54 peptide-functionalized PLGA-grafted dextran (A54-Dex-PLGA) micelles with encapsulated DOX and SPIONs. | DOX | The dependences of 1/T2* on Fe concentration presented high slopes. Contrasting properties confirmed in vivo. | [151] |
| Nanocapsules | Micelles formed with Amphiphilic dextran; stearic acid (SA) chains drafted onto the carbohydrate backbone; encapsulating DOX and a cluster of Mn-SPIONs in a hydrophobic core. | DOX | T2 values of the labeled cells decreased from 241.5 to 29.5 ms when the cell number increases from 5 × 104 to 8 × 105. | [152] |
| Nanocapsules | Biocompatible amphiphilic polymer (Pluronic F127) self-assembled with magnetic nanocluster and PTX as the core; hydrophilic stearoyl-polyethylenimine-2,3-dimethylmalefic anhydride (SC-g-PEI-DMMA) shell. | PTX | r2 = 142.68 mM−1s−1 | [153] |
| Nanospheres | Multifunctional biopolymer with PA conjugated with biotin, DOX, and SPIONs. | DOX | r2 not available; However, 1/T2 rates increased gradually vs. the SPION concentrations (in µg/mL of Fe). | [154] |
| Nanospheres | The encapsulation of quantum dots, SPIONS, and DOX into PLGA polymeric nanocomposites. Coupling of the amine group of polyethyleneimine premodified with PEG acid (PEI-PEG-FA (PPF)) segments and adsorption of vascular endothelial growth factor (VEGF)-targeted small hairpin RNA (shRNA). | DOX | r2 not available. Signal intensity in vitro decreased with the Fe concentration increase. Tumor darkening observed in vivo. | [155] |
| Nanospheres | Polymer (PCL)-based composite microsphere with Fe3O4 nanoparticles and DOX. | DOX | r2 = 7.3 mg mL−1s−1 | [156] |
| Nanospheres | PLGA nanoparticles with oleate-covered iron oxide particles and PTX for AMF heat-induced drug release. | PTX | r2 not available; However, 1/T2 rates increased gradually vs. the SPION concentrations (in µg/mL of Fe). | [157] |
| Nanocapsules | Biodegradable and photoluminescent polyester (BPLP) with the PLGA and SPIONs as a polymeric shell. Functionalized with PEG, providing a hydrophilic surface. | proteins | r2 = 263 and 237 mM−1s−1 for non-PEGylated and PEGylated NCs, respectively. | [158] |
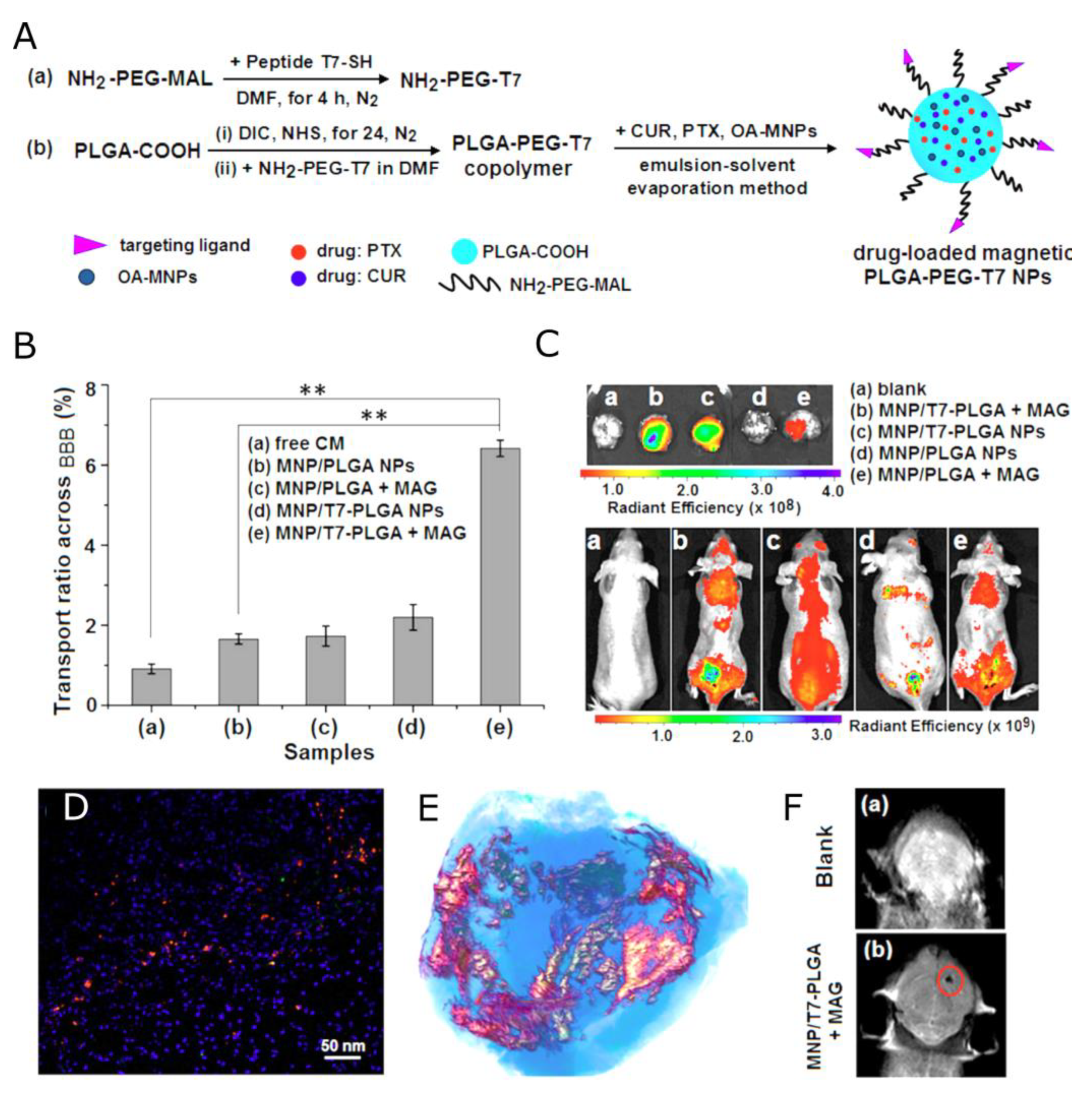
| Nanocapsules | Hydrophobic magnetic nanoparticles entrapped into the PLGA NPs with the human transferrin receptor-binding peptide T7. The water-insoluble drugs, PTX and CUR, loaded into the hydrophobic core. | PTX, CUR | r2 = 281.05 mM−1s−1 | [159] |
| Nanocapsules | DOX-loaded SPIONs@PDA nanoparticles; the shell of the magnetic NP of crosslinked reducible polydopamine and PEG methyl ether methacrylate, with DOPA moiety as an anchor to immobilize SPIONs. | DOX | r2 = 33.53 mM−1s−1 | [160] |
| Structure | Overview | Model Drug 1 | MRI Contrasting Properties | Ref |
|---|---|---|---|---|
| Nanocapsules | Acylamino-containing amphiphilic block copolymer (polyethylene glycol-polyacrylamide-poly acetonitrile, PEG-b-P(AM-co-AN)). New nanomedicine: PEG-PAM-PAN@DOX, based on the copolymer, constructed by nanoprecipitation. | DOX | CEST effect at approximately 0.5 ppm; CEST imaging of NCs at different pH revealed a stronger CEST effect at a weak acid or neutral pH. | [167] |
| Nanospheres | Porous poly(propylene fumarate) (PPF) scaffolds loaded with DOX; the surface of scaffolds modified with three different contrast agents for MRI: iron oxide, manganese oxide, and protamine sulfate (PS-CEST agent). | DOX | CEST signal used for drug release study. The MTRasym at 1.8 ppm showed an increase with incubation time due to the release of PS from the PPF scaffolds. | [168] |
| Nanocapsules | PEG114-b-PDPA116 block copolymers that in physiological pH form micelles, and dissociate in an acidic environment. | - | MTRasym dependent on pH; between pH 5 and 6.5 shows a variable CEST contrast. | [169] |
| Structure | Overview | Model Drug 1 | MRI Contrasting Properties | Ref |
|---|---|---|---|---|
| Nanocapsules | Encapsulation of PTX into core–shell nanocapsules made of a PLGA-PEG shell and PFOB core. | PTX | MRI images obtained by superposition of the 1H and 19F images of a CT-26 tumor-bearing mouse. | [171] |
| Nanocapsules | Fluorinated amphiphile with fluorinated moieties as hydrophobic tails and as a 19F MRI agent, and monodisperse PEG as hydrophilic heads. Formulation of 19F MRI-traceable liposomes with encapsulated DOX based on the fluorinated amphiphile | DOX | In vitro 19F signal intensity evaluation for different concentrations of NCs. In vivo superposition of the 1H and 19F images. | [172] |
| Nanocapsules | Peptidic monodisperse PEG with fluorinated L-lysine side chains and a fluorescent N-terminal modified for 19F MRI and fluorescence dual-imaging traceable and thermo-responsive DOX delivery. | DOX | In vitro 19F signal intensity evaluation for different concentrations of NCs. In vivo superposition of the 1H and 19F images of mice carrying HepG2 tumor. | [173] |
| Nanocapsules | ROS-sensitive core–shell NCs of diblock polymer; the hydrophilic block of poly(methyl-2-oxazoline) (PMeOx) formed a shell; the hydrophobic block of poly(2,2-difluoroethylacrylamide) (PDFEA) provided 19F-NMR signal. | DOX | In vitro 19F signal intensity evaluation for different concentrations of NCs. In vivo superposition of the 1H and 19F images of rat leg after administration of the polymer. | [174] |
| Nanospheres | Fluorinated PLGA co-polymers (F-PLGA) containing an increasing number of magnetically equivalent fluorine atoms. | DEX, LEF | 19F-NMR signal at -72 ppm and -70 ppm; T1 values of 537 ms and 625 ms and T2 values of 122 ms and 60 ms, for F3-PLGA NPs and F9-PLGA NPs, respectively. | [175] |
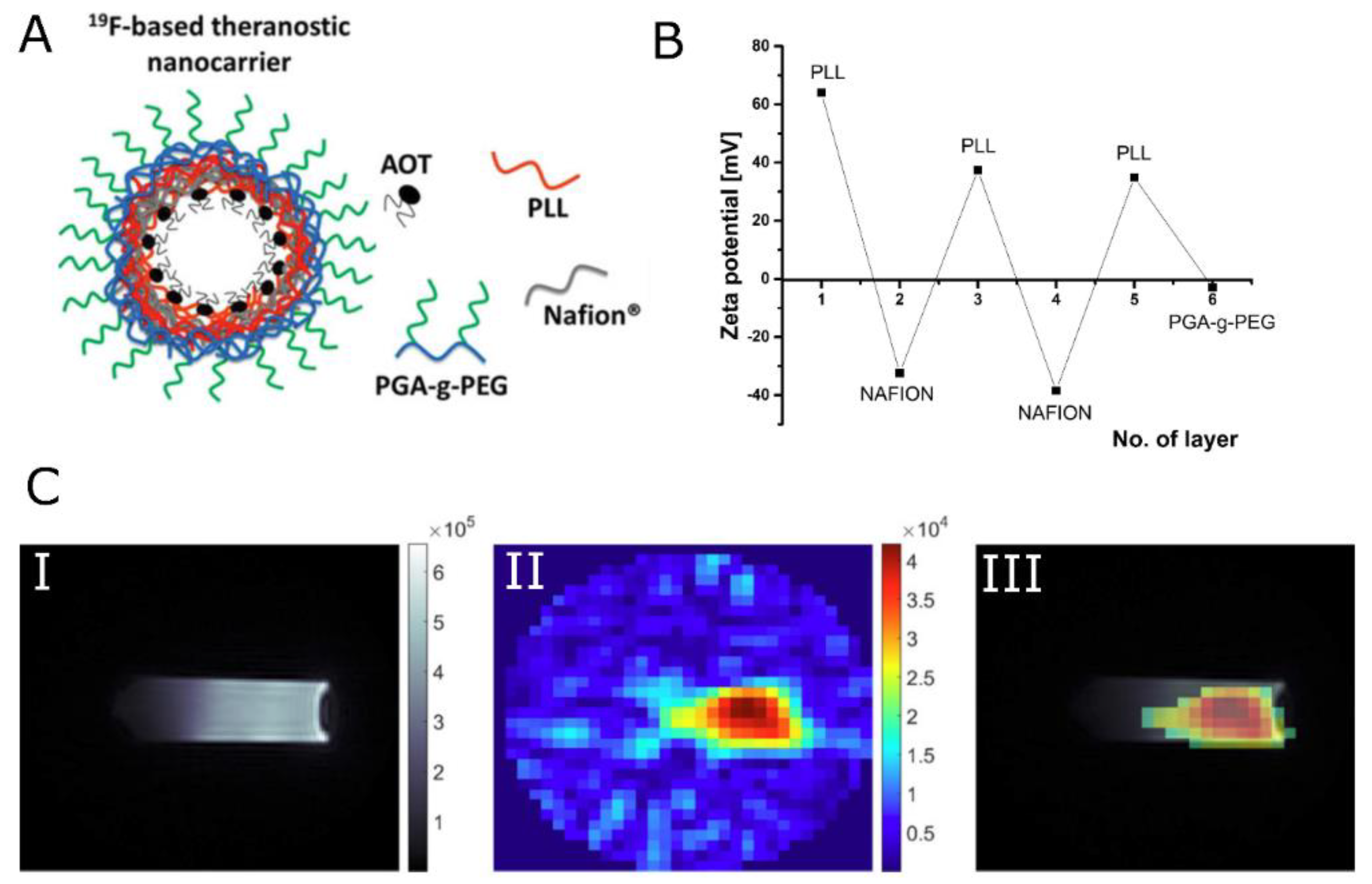
| Nanocapsules | Core–shell nanocapsules formed by LbL technique. Shell is composed of Nafion, the fluorinated ionic polymer, and PLL. The surface modified by the adsorption of pegylated polyanion, PGA. | - | 19F signal arising from Nafion® polymer exhibited multiple resonance lines with T2 values in the range of single milliseconds. In vitro imaging of NCs resulted in SNR = 5 (tacq < 30 min) for 19F concentration as low as 1.53 × 10−2 mM19F g−1. | [176,177] |
| Nanocapsules | The 5-FU loaded nanocapsules. Shell formed with polymers: PLL and PGA. The surface modified with PGA-g-PEG. | 5-FU | In vitro 19F SNR evaluation for the phantom with NCs. SNR = 10 was achieved in tacq of 8 min for the concentration of 982.73 mg/L 5-FU. | [178] |
| Nanospheres | Hyperbranched polymer (Hyperbranched Poly(N,N-dimethylacrylamide)) covalently conjugated to a biodegradable oligopeptide with 5-FU. | 5-FU | Differentiation between attached and released drug states by 19F-NMR. 5-FU release induced a change in 19F peak width. | [179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łopuszyńska, N.; Węglarz, W.P. Contrasting Properties of Polymeric Nanocarriers for MRI-Guided Drug Delivery. Nanomaterials 2023, 13, 2163. https://doi.org/10.3390/nano13152163
Łopuszyńska N, Węglarz WP. Contrasting Properties of Polymeric Nanocarriers for MRI-Guided Drug Delivery. Nanomaterials. 2023; 13(15):2163. https://doi.org/10.3390/nano13152163
Chicago/Turabian StyleŁopuszyńska, Natalia, and Władysław P. Węglarz. 2023. "Contrasting Properties of Polymeric Nanocarriers for MRI-Guided Drug Delivery" Nanomaterials 13, no. 15: 2163. https://doi.org/10.3390/nano13152163
APA StyleŁopuszyńska, N., & Węglarz, W. P. (2023). Contrasting Properties of Polymeric Nanocarriers for MRI-Guided Drug Delivery. Nanomaterials, 13(15), 2163. https://doi.org/10.3390/nano13152163






