The Role of Silica Nanoparticles in Promoting the Germination of Tomato (Solanum lycopersicum) Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Seeds Viability Test
2.2. Preparation of Nanoparticle Suspensions
2.3. Dispersity and Size Distribution of Nanoparticle Suspensions
2.4. Seed Germination
2.5. Germination Parameters
2.6. Harvesting and Measurement of Seedling Weight
2.7. Elemental Analysis of Seedlings
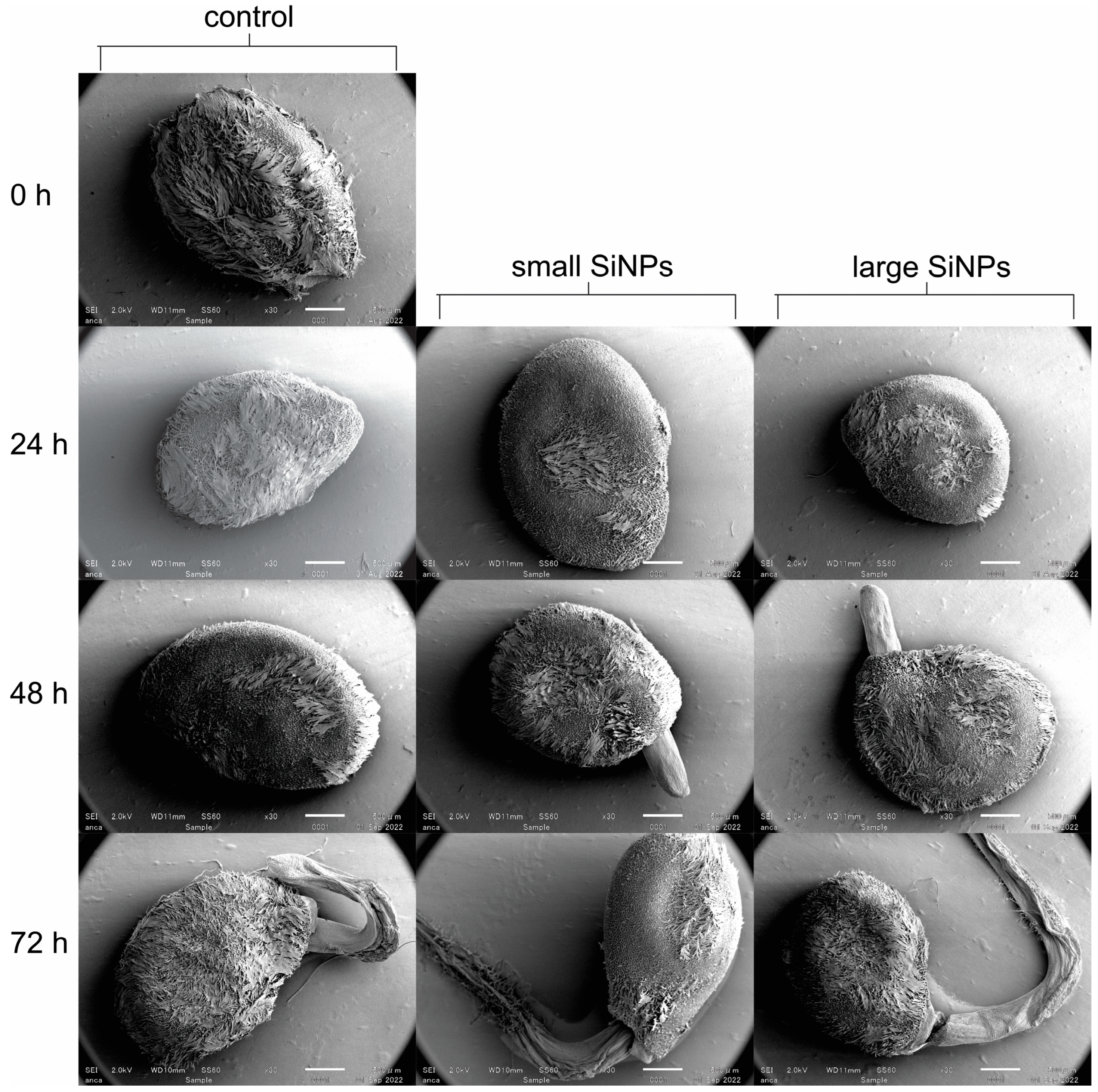
2.8. Imaging of Seed Surface
2.9. Isolation and Quantification of Bacillus sp. from Germination Media
2.10. Statistical Analysis
3. Results
3.1. Characteristics of Nanoparticles
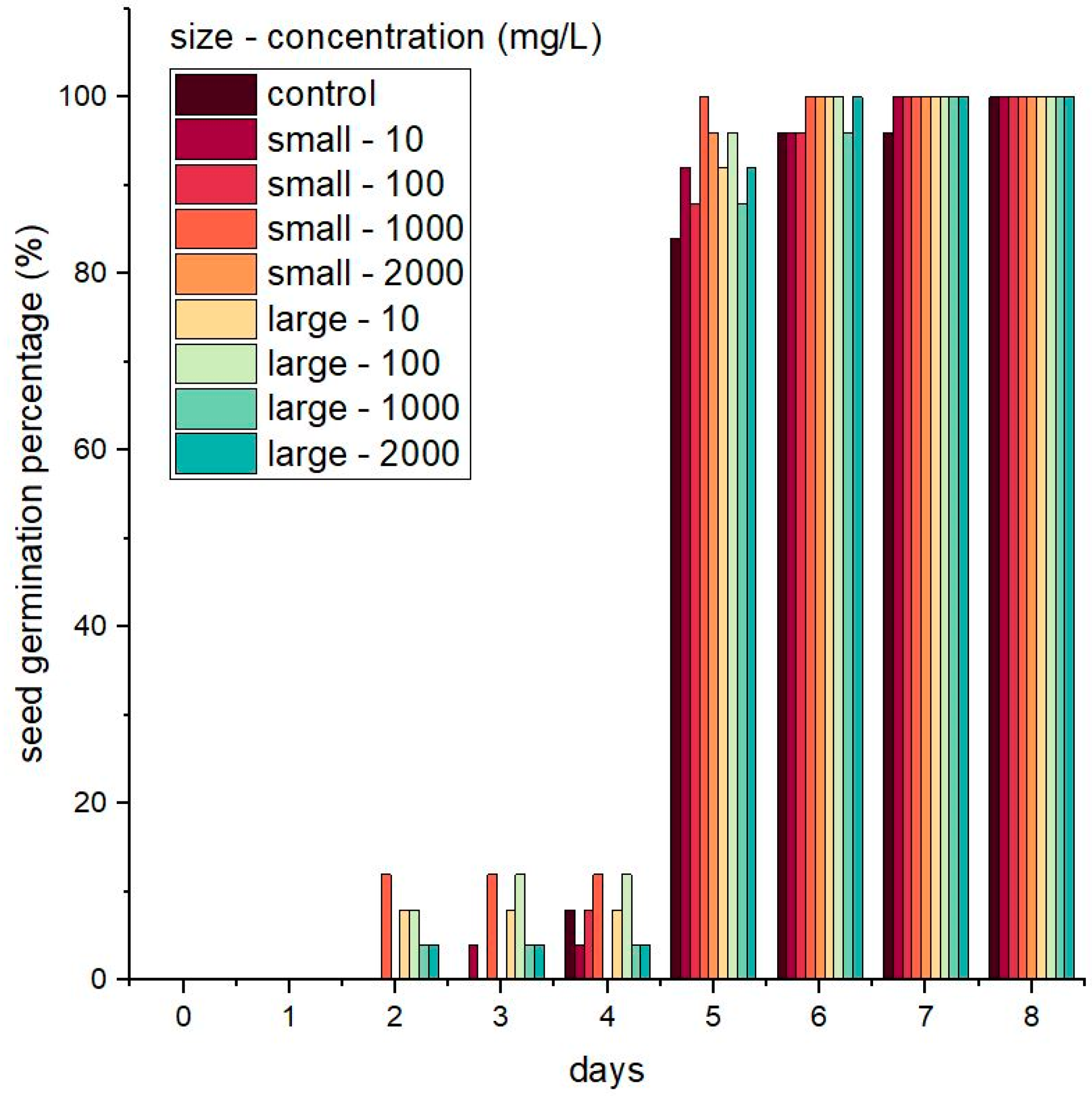
3.2. Seed Germination and Seedling Growth in the Presence of Nanoparticles
3.3. Elemental Analysis of Seedlings during Germination
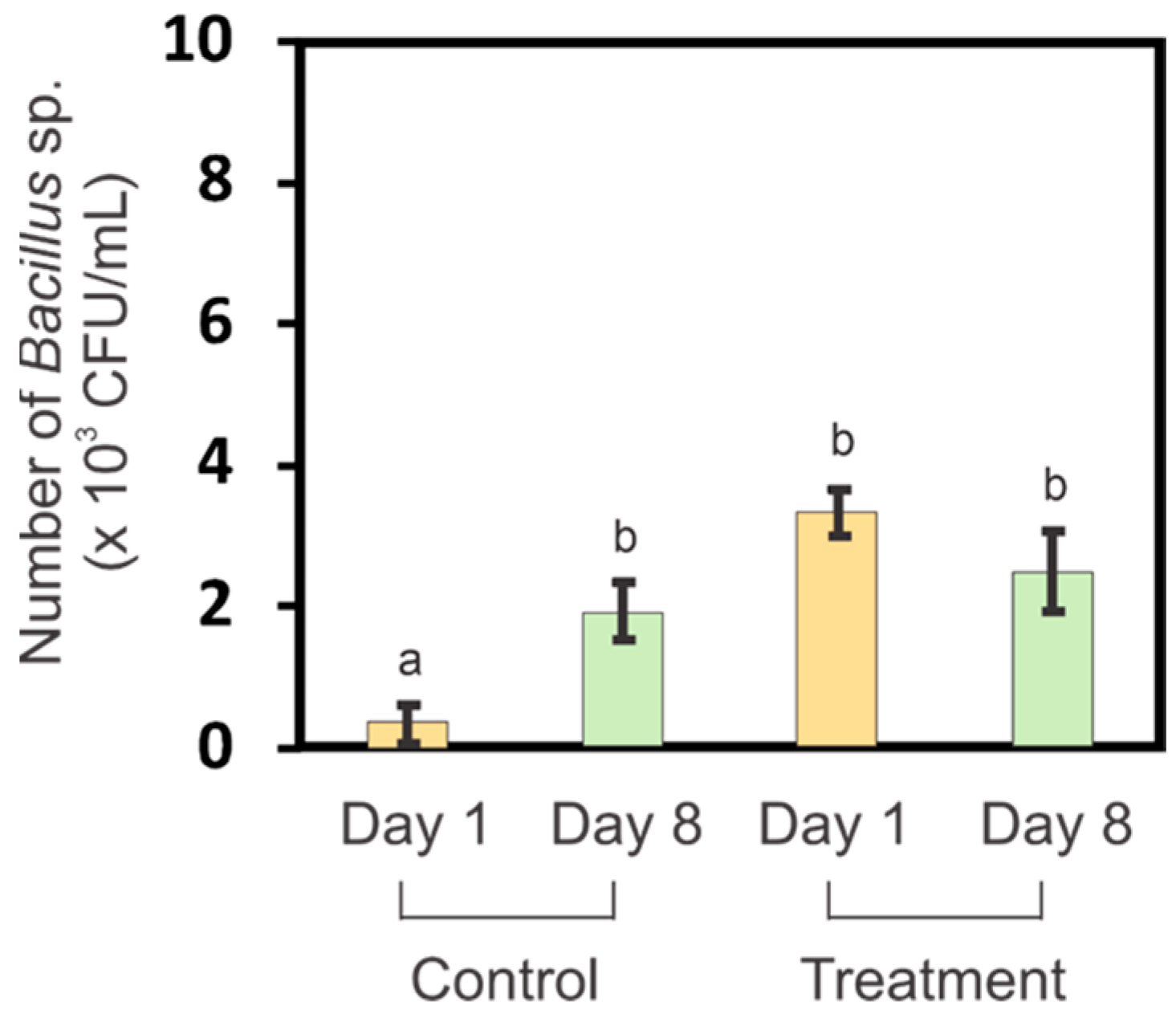
3.4. Bacillus sp. Levels in Germination Media before and after Germination
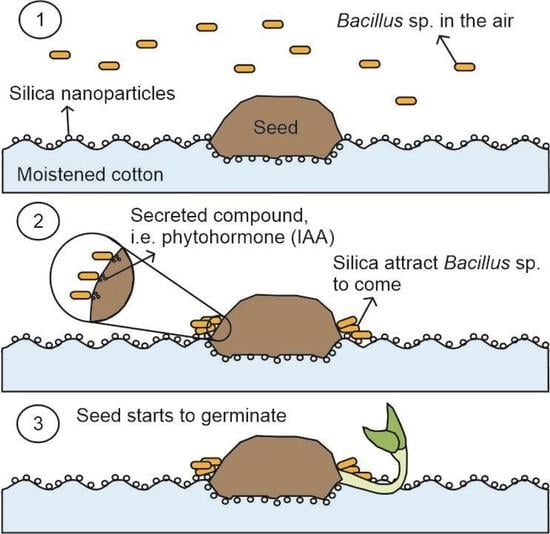
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wojtyla, Ł.; Lechowska, K.; Kubala, S.; Garnczarska, M. Different modes of hydrogen peroxide action during seed germination. Front. Plant Sci. 2016, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Gioria, M.; Pyšek, P.; Osborne, B.A. Timing is everything: Does early and late germination favor invasions by herbaceous alien plants? J. Plant Ecol. 2018, 11, 4–16. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.). Saudi J. Biol. Sci. 2014, 21, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; He, J.; Xie, H.; Wang, W.; Bose, S.K.; Sun, Y.; Hu, J.; Yin, H. Effects of chitosan nanoparticles on seed germination and seedling growth of wheat (Triticum aestivum L.). Int. J. Biol. Macromol. 2019, 126, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, H.; Lal, R. Effects of stabilized nanoparticles of copper, zinc, manganese, and iron oxides in low concentrations on lettuce (Lactuca sativa) seed germination: Nanotoxicants or nanonutrients? Water Air Soil Pollut. 2016, 227, 1–14. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Hernandez-Viezcas, J.A.; Zhao, L.; Diaz, B.C.; Ge, Y.; Priester, J.H.; Holden, P.A.; Gardea-Torresdey, J.L. Cerium dioxide and zinc oxide nanoparticles alter the nutritional value of soil cultivated soybean plants. Plant Physiol. Biochem. 2014, 80, 128–135. [Google Scholar] [CrossRef]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 8263. [Google Scholar] [CrossRef]
- Alsaeedi, A.; El-Ramady, H.; Alshaal, T.A.; Almohsen, M. Enhancing seed germination and seedlings development of common bean (Phaseolus vulgaris) by SiO2 nanoparticles. Egypt. J. Soil Sci. 2017, 57, 407–415. [Google Scholar]
- Feizi, H.; Rezvani Moghaddam, P.; Shahtahmassebi, N.; Fotovat, A. Impact of bulk and nanosized titanium dioxide (TiO2) on wheat seed germination and seedling growth. Biol. Trace Elem. Res. 2012, 146, 101–106. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Liu, Y.; She, J. Effects of liquid phase nano titanium dioxide (TiO2) on seed germination and seedling growth of camphor tree. Nanomaterials 2022, 12, 1047. [Google Scholar] [CrossRef]
- Lizzi, D.; Mattiello, A.; Piani, B.; Fellet, G.; Adamiano, A.; Marchiol, L. Germination and early development of three spontaneous plant species exposed to nanoceria (n CeO2) with different concentrations and particle sizes. Nanomaterials 2020, 10, 2534. [Google Scholar] [CrossRef] [PubMed]
- Rai-Kalal, P.; Jajoo, A. Priming with zinc oxide nanoparticles improve germination and photosynthetic performance in wheat. Plant Physiol. Biochem. 2021, 160, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus species in soil as a natural resource for plant health and nutrition. J. Appl. Microbiol. 2020, 128, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Mera, M.U.; Beveridge, T.J. Mechanism of silicate binding to the bacterial cell wall in Bacillus subtilis. J. Bacteriol. 1993, 175, 1936–1945. [Google Scholar] [CrossRef]
- Li, Y.J.; Hu, Q.P. Studying of the promotion mechanism of Bacillus subtilis QM3 on wheat seed germination based on β-amylase. Open Life Sci. 2020, 15, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, R.; Alkhatib, B.; Abdo, N. Effect of Fe3O4 nanoparticles on seed germination in tobacco. Environ. Sci. Pollut. Res. 2021, 28, 53568–53577. [Google Scholar] [CrossRef]
- Azimi, R.; Borzelabad, M.J.; Feizi, H.; Azimi, A. Interaction of SiO2 nanoparticles with seed prechilling on germination and early seedling growth of tall wheatgrass (Agropyron elongatum L.). Pol. J. Chem. Technol. 2014, 16, 25–29. [Google Scholar] [CrossRef]
- Sun, D.; Hussain, H.I.; Yi, Z.; Siegele, R.; Cresswell, T.; Kong, L.; Cahill, D.M. Uptake and cellular distribution, in four plant species, of fluorescently labeled mesoporous silica nanoparticles. Plant Cell Rep. 2014, 33, 1389–1402. [Google Scholar] [CrossRef]
- Okubo, T. Determination of the effective charge numbers of colloidal spheres by conductance measurements. J. Colloid Interface Sci. 1988, 125, 380–385. [Google Scholar] [CrossRef]
- Matsumoto, T. Influence of ionic strength on rheological properties of concentrated aqueous silica colloids. J. Rheol. 1989, 33, 371–379. [Google Scholar] [CrossRef]
- Yano, S.; Maeda, H.; Nakajima, M.; Hagiwara, T.; Sawaguchi, T. Preparation and mechanical properties of bacterial cellulose nanocomposites loaded with silica nanoparticles. Cellulose 2008, 15, 111–120. [Google Scholar] [CrossRef]
- Karunakaran, G.; Suriyaprabha, R.; Rajendran, V.; Kannan, N. Influence of ZrO2, SiO2, Al2O3 and TiO2 nanoparticles on maize seed germination under different growth conditions. IET Nanobiotechnol. 2016, 10, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Hoe, P.T.; Mai, N.C.; Lien, L.Q.; Ban, N.K.; Minh, C.V.; Chau, N.H.; Buu, N.Q.; Hien, D.T.; Van, N.T.; Hien, L.T.T.; et al. Germination responses of soybean seeds to Fe, ZnO, Cu and Co nanoparticle treatments. Int. J. Agric. Biol. 2018, 20, 1562–1568. [Google Scholar]
- Kader, M.A. A comparison of seed germination calculation formulae and the associated interpretation of resulting data. J. Proc. R. Soc. N. S. W. 2005, 138, 65–75. [Google Scholar] [CrossRef]
- Sehnal, K.; Hosnedlova, B.; Docekalova, M.; Stankova, M.; Uhlirova, D.; Tothova, Z.; Kepinska, M.; Milnerowicz, H.; Fernandez, C.; Ruttkay-Nedecky, B.; et al. An assessment of the effect of green synthesized silver nanoparticles using sage leaves (Salvia officinalis L.) on germinated plants of maize (Zea mays L.). Nanomaterials 2019, 9, 1550. [Google Scholar] [CrossRef]
- Faizal, A.; Sembada, A.A.; Priharto, N. Production of bioethanol from four species of duckweeds (Landoltia punctata, Lemna aequinoctialis, Spirodela polyrrhiza, and Wolffia arrhiza) through optimization of saccharification process and fermentation with Saccharomyces cerevisiae. Saudi J. Biol. Sci. 2021, 28, 294–301. [Google Scholar] [CrossRef]
- Waheed, A.; Ahmad, M.; Ghufran, M.A.; Jabeen, A.; Ozdemir, F.A.; Zafar, M.; Sultana, S.; Shah, M.A.; Majeed, S.; Khan, A.S. Implication of scanning electron microscopy in the seed morphology with special reference to three subfamilies of Fabaceae. Microsc. Res. Tech. 2021, 84, 2176–2185. [Google Scholar] [CrossRef]
- Travers, R.S.; Martin, P.A.; Reichelderfer, C.F. Selective process for efficient isolation of soil Bacillus spp. Appl. Environ. Microbiol. 1987, 53, 1263–1266. [Google Scholar] [CrossRef]
- Ramaye, Y.; Dabrio, M.; Roebben, G.; Kestens, V. Development and validation of optical methods for zeta potential determination of silica and polystyrene particles in aqueous suspensions. Materials 2021, 14, 290. [Google Scholar] [CrossRef]
- Feng, B.; Ashraf, M.A.; Peng, L. Characterization of particle shape, zeta potential, loading efficiency and outdoor stability for chitosan-ricinoleic acid loaded with rotenone. Open Life Sci. 2016, 11, 380–386. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Yamamoto, T.; Kato, T. Rheological studies of hydrophilic and hydrophobic silica suspensions in the presence of adsorbed poly (N-isopropylacrylamide). Langmuir 1996, 12, 6184–6187. [Google Scholar] [CrossRef]
- Shar, J.A.; Obey, T.M.; Cosgrove, T. Adsorption studies of polyethers: Part II: Adsorption onto hydrophilic surfaces. Colloids Surf. 1999, 150, 15–23. [Google Scholar] [CrossRef]
- Ivani, R.; Sanaei Nejad, S.H.; Ghahraman, B.; Astaraei, A.R.; Feizi, H. Role of bulk and nanosized SiO2 to overcome salt stress during Fenugreek germination (Trigonella foenum-graceum L.). Plant Signal. Behav. 2018, 13, e1044190. [Google Scholar] [CrossRef]
- Widnyana, I.K.; Javandira, C. Activities Pseudomonas spp. and Bacillus sp. to stimulate germination and seedling growth of tomato plants. Agric. Agric. Sci. Procedia 2016, 9, 419–423. [Google Scholar] [CrossRef]
- Szilagyi-Zecchin, V.J.; Ikeda, A.C.; Hungria, M.; Adamoski, D.; Kava-Cordeiro, V.; Glienke, C.; Galli-Terasawa, L.V. Identification and characterization of endophytic bacteria from corn (Zea mays L.) roots with biotechnological potential in agriculture. AMB Express 2014, 4, 1–9. [Google Scholar] [CrossRef]
- Vasanthi, N.; Saleena, L.M.; Raj, S.A. Silica solubilization potential of certain bacterial species in the presence of different silicate minerals. Silicon 2018, 10, 267–275. [Google Scholar] [CrossRef]
- Sheng, X.F.; Zhao, F.; He, L.Y.; Qiu, G.; Chen, L. Isolation and characterization of silicate mineral-solubilizing Bacillus globisporus Q12 from the surfaces of weathered feldspar. Can. J. Microbiol. 2008, 54, 1064–1068. [Google Scholar] [CrossRef]
- Liu, P.; Sehaqui, H.; Tingaut, P.; Wichser, A.; Oksman, K.; Mathew, A.P. Cellulose and chitin nanomaterials for capturing silver ions (Ag+) from water via surface adsorption. Cellulose 2014, 21, 449–461. [Google Scholar] [CrossRef]
- Wu, C.; Wang, L.; Harbottle, D.; Masliyah, J.; Xu, Z. Studying bubble–particle interactions by zeta potential distribution analysis. J. Colloid Interface Sci. 2015, 449, 399–408. [Google Scholar] [CrossRef]
- Tantra, R.; Schulze, P.; Quincey, P. Effect of nanoparticle concentration on zeta-potential measurement results and reproducibility. Particuology 2010, 8, 279–285. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, K.; Wu, B.; Zhou, J.; Lv, Y. Silver nanoparticles with different particle sizes enhance the allelopathic effects of Canada goldenrod on the seed germination and seedling development of lettuce. Ecotoxicology 2018, 27, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Darlington, T.K.; Neigh, A.M.; Spencer, M.T.; Guyen, O.T.; Oldenburg, S.J. Nanoparticle characteristics affecting environmental fate and transport through soil. Environ. Toxicol. Chem. 2009, 28, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Laghmouchi, Y.; Belmehdi, O.; Bouyahya, A.; Senhaji, N.S.; Abrini, J. Effect of temperature, salt stress and pH on seed germination of medicinal plant Origanum compactum. Biocatal. Agric. Biotechnol. 2017, 10, 156–160. [Google Scholar] [CrossRef]
- Li, R.; Shi, F.; Fukuda, K. Interactive effects of salt and alkali stresses on seed germination, germination recovery, and seedling growth of a halophyte Spartina alterniflora (Poaceae). S. Afr. J. Bot. 2010, 76, 380–387. [Google Scholar] [CrossRef]
- Müller, K.; Tintelnot, S.; Leubner-Metzger, G. Endosperm-limited Brassicaceae seed germination: Abscisic acid inhibits embryo-induced endosperm weakening of Lepidium sativum (cress) and endosperm rupture of cress and Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 864–877. [Google Scholar] [CrossRef]
- Han, C.; Yang, P. Studies on the molecular mechanisms of seed germination. Proteomics 2015, 15, 1671–1679. [Google Scholar] [CrossRef]
- Venier, P.; García, C.C.; Cabido, M.; Funes, G. Survival and germination of three hard-seeded Acacia species after simulated cattle ingestion: The importance of the seed coat structure. S. Afr. J. Bot. 2012, 79, 19–24. [Google Scholar] [CrossRef]
- Cleal, C.J.; Shute, C.H. The systematic and palaeoecological value of foliage anatomy in Late Palaeozoic medullosalean seed-plants. J. Syst. Palaeontol. 2012, 10, 765–800. [Google Scholar] [CrossRef]
- Rubin, E.; Baider, A.; Cohen, Y. Phytophthora infestans produces oospores in fruits and seeds of tomato. Phytopathology 2001, 91, 1074–1080. [Google Scholar] [CrossRef]
- Abad, Z.G.; Burgess, T.I.; Redford, A.J.; Bienapfl, J.C.; Srivastava, S.; Mathew, R.; Jennings, K. IDphy: An international online resource for molecular and morphological identification of Phytophthora. Plant Dis. 2023, 107, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Sharma, N.; Verma, O.N.; Singh, V.K.; Tripathi, D.K.; Lee, Y.; Kumar, S.; Rai, P.K.; Gondal, M.A. Application of LIBS to elemental analysis and mapping of plant samples. At. Spectrosc. 2021, 42, 99–113. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Tamindžić, G.; Đorđević, V.; Tintor, B.; Milošević, D.; Ignjatov, M.; Nikolić, Z. Bio-priming of soybean with Bradyrhizobium japonicum and Bacillus megaterium: Strategy to improve seed germination and the initial seedling growth. Plants 2022, 11, 1927. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.L.; Souta, J.F.; Soares, C.P.; Rocha, L.O.; Santos, M.L.C.; Grativol, C.; Roesch, L.F.W.; Olivares, F.L. Insights into the structure and role of seed-borne bacteriome during maize germination. FEMS Microbiol. Ecol. 2021, 97, fiab024. [Google Scholar]
- Pradhan, S.; Tiruwa, B.; Subedee, B.R.; Pant, B. In vitro germination and propagation of a threatened medicinal orchid, Cymbidium aloifolium (L.) Sw. through artificial seed. Asian Pac. J. Trop. Biomed. 2014, 4, 971–976. [Google Scholar] [CrossRef]
- Haider, H.I.; Zafar, I.; Ain, Q.U.; Noreen, A.; Nazir, A.; Javed, R.; Sehgal, S.A.; Khan, A.A.; Rahman, M.M.; Rashid, S.; et al. Synthesis and characterization of copper oxide nanoparticles: Its influence on corn (Z. mays) and wheat (Triticum aestivum) plants by inoculation of Bacillus subtilis. Environ. Sci. Pollut. Res. 2023, 30, 37370–37385. [Google Scholar] [CrossRef]
- Watanabe, K.; Yanagi, U.; Shiraishi, Y.; Harada, K.; Ogino, F.; Asano, K. Bacterial communities in various parts of air-conditioning units in 17 Japanese houses. Microorganisms 2022, 10, 2246. [Google Scholar] [CrossRef]
- Khedher, S.B.; Mejdoub-Trabelsi, B.; Tounsi, S. Biological potential of Bacillus subtilis V26 for the control of Fusarium wilt and tuber dry rot on potato caused by Fusarium species and the promotion of plant growth. Biol. Control 2021, 152, 104444. [Google Scholar] [CrossRef]
- Lastochkina, O.; Garshina, D.; Ivanov, S.; Yuldashev, R.; Khafizova, R.; Allagulova, C.; Fedorova, K.; Avalbaev, A.; Maslennikova, D.; Bosacchi, M. Seed priming with endophytic Bacillus subtilis modulates physiological responses of two different Triticum aestivum L. cultivars under drought stress. Plants 2020, 9, 1810. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, M.; Kushwaha, B.; Nagpure, N.S.; Mani, I.; Lakra, W.S. Molecular characterization of major and minor rDNA repeats and genetic variability assessment in different species of mahseer found in North India. Gene 2013, 527, 248–258. [Google Scholar] [CrossRef]
- Bradford, K.J. Applications of hydrothermal time to quantifying and modeling seed germination and dormancy. Weed Sci. 2002, 50, 248–260. [Google Scholar] [CrossRef]
- Sileshi, G.W. A critique of current trends in the statistical analysis of seed germination and viability data. Seed Sci. Res. 2012, 22, 145–159. [Google Scholar] [CrossRef]

| Silica Type | Concentration (mg/L) | Zeta Potential (mV) | pH | Particle Size Distribution (nm) |
|---|---|---|---|---|
| Snowtex-S (small SiNPs) | 10 | −29.3 ± 0.65 a | 9.94 ± 0.04 | 10.23 ± 0.21 a |
| 100 | −31.87 ± 0.97 bc | 9.97 ± 0.04 | 12.33 ± 0.29 b | |
| 1000 | −32.27 ± 0.81 bc | 9.92 ± 0.03 | 14.49 ± 0.16 c | |
| 2000 | −34.47 ± 0.53 d | 9.91 ± 0.05 | 16.78 ± 0.15 d | |
| Snowtex-ZL (large SiNPs) | 10 | −31.17 ± 0.29 b | 9.92 ± 0.05 | 110 ± 1.33 e |
| 100 | −33.37 ± 0.88 cd | 9.95 ± 0.05 | 113.57 ± 0.79 f | |
| 1000 | −36.67 ± 0.33 e | 9.9 ± 0.05 | 116.03 ± 0.54 g | |
| 2000 | −40.4 ± 0.96 f | 9.92 ± 0.06 | 119.87 ± 0.33 h |
| Silica Size | Concentration (mg/L) | MGT (Days) | GI | CVG |
|---|---|---|---|---|
| - | 0 (control) | 5.24 ± 0.29 b | 18.8 ± 1.47 b | 19.14 ± 1.01 b |
| Small SiNPs | 10 | 5.04 ± 0.27 ab | 19.8 ± 1.33 ab | 19.9 ± 1.07 ab |
| 100 | 4.8 ± 0.34 a | 21 ± 1.67 a | 20.93 ± 1.44 ab | |
| 1000 | 4.64 ± 0.29 a | 21.8 ± 1.47 a | 21.64 ± 1.34 a | |
| 2000 | 5.04 ± 0.08 ab | 19.8 ± 0.4 ab | 19.85 ± 0.31 ab | |
| Large SiNPs | 10 | 4.92 ± 0.2 ab | 20.4 ± 1.02 ab | 20.36 ± 0.86 ab |
| 100 | 4.68 ± 0.27 a | 21.6 ± 1.36 a | 21.44 ± 1.23 a | |
| 1000 | 4.76 ± 0.34 a | 21.2 ± 1.72 a | 21.12 ± 1.58 a | |
| 2000 | 4.96 ± 0.29 ab | 20.2 ± 1.47 ab | 20.24 ± 1.29 ab |
| Silica Size | Concentration (mg/L) | VILENGTH | VIFW | VIDW |
|---|---|---|---|---|
| - | 0 (control) | 946.4 ± 64.79 b | 2.19 ± 0.09 c | 0.2 ± 0.01 c |
| Small SiNPs | 10 | 1082 ± 46.27 a | 2.35 ± 0.08 abc | 0.21 ± 0.01 abc |
| 100 | 1048 ± 68.49 ab | 2.32 ± 0.11 bc | 0.21 ± 0.01 bc | |
| 1000 | 1117.2 ± 81.54 a | 2.52 ± 0.11 a | 0.22 ± 0.01 a | |
| 2000 | 1061.6 ± 66.49 ab | 2.42 ± 0.11 ab | 0.22 ± 0.01 ab | |
| Large SiNPs | 10 | 1088.4 ± 101.76 a | 2.48 ± 0.18 ab | 0.22 ± 0.02 ab |
| 100 | 1102 ± 121.36 a | 2.52 ± 0.11 a | 0.23 ± 0.01 a | |
| 1000 | 1060.4 ± 83.96 ab | 2.46 ± 0.05 ab | 0.22 ± 0.01 ab | |
| 2000 | 1081.2 ± 27.47 a | 2.32 ± 0.1 bc | 0.21 ± 0.01 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sembada, A.A.; Maki, S.; Faizal, A.; Fukuhara, T.; Suzuki, T.; Lenggoro, I.W. The Role of Silica Nanoparticles in Promoting the Germination of Tomato (Solanum lycopersicum) Seeds. Nanomaterials 2023, 13, 2110. https://doi.org/10.3390/nano13142110
Sembada AA, Maki S, Faizal A, Fukuhara T, Suzuki T, Lenggoro IW. The Role of Silica Nanoparticles in Promoting the Germination of Tomato (Solanum lycopersicum) Seeds. Nanomaterials. 2023; 13(14):2110. https://doi.org/10.3390/nano13142110
Chicago/Turabian StyleSembada, Anca Awal, Shinya Maki, Ahmad Faizal, Toshiyuki Fukuhara, Takeshi Suzuki, and I. Wuled Lenggoro. 2023. "The Role of Silica Nanoparticles in Promoting the Germination of Tomato (Solanum lycopersicum) Seeds" Nanomaterials 13, no. 14: 2110. https://doi.org/10.3390/nano13142110
APA StyleSembada, A. A., Maki, S., Faizal, A., Fukuhara, T., Suzuki, T., & Lenggoro, I. W. (2023). The Role of Silica Nanoparticles in Promoting the Germination of Tomato (Solanum lycopersicum) Seeds. Nanomaterials, 13(14), 2110. https://doi.org/10.3390/nano13142110