The Biogenic Synthesis of Bimetallic Ag/ZnO Nanoparticles: A Multifunctional Approach for Methyl Violet Photocatalytic Degradation and the Assessment of Antibacterial, Antioxidant, and Cytotoxicity Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
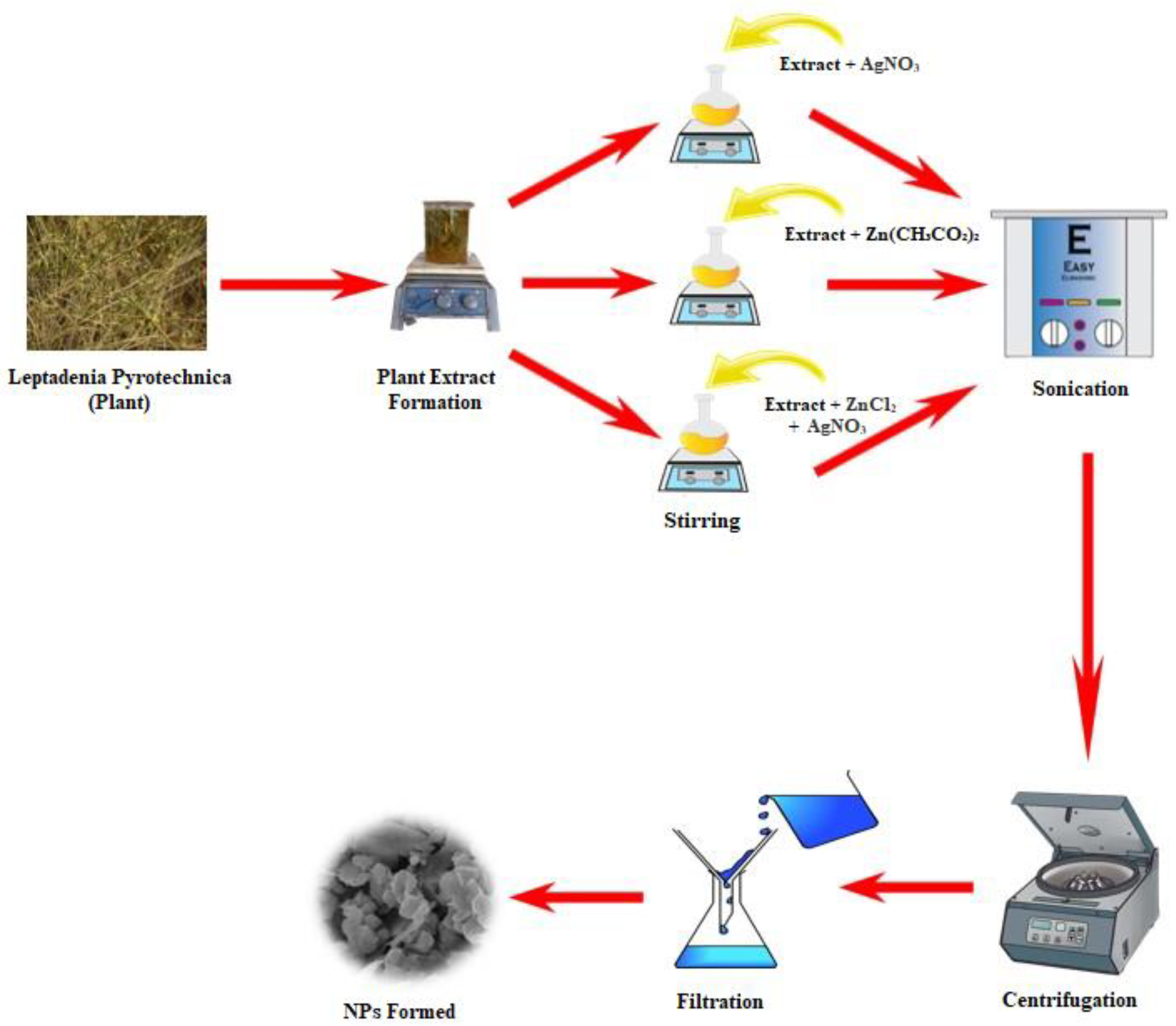
2.2. Preparation of Leaf Extract of L. Pyrotechnica
2.3. Preparation of Monometallic NPs
2.4. Preparation of Bimetallic NPs
2.5. Characterization of MNPs and BNPs
2.6. Antibacterial Activity
2.7. Antioxidant Activity
2.8. Cytotoxicity
2.9. Photocatalytic Activity
3. Results and Discussion
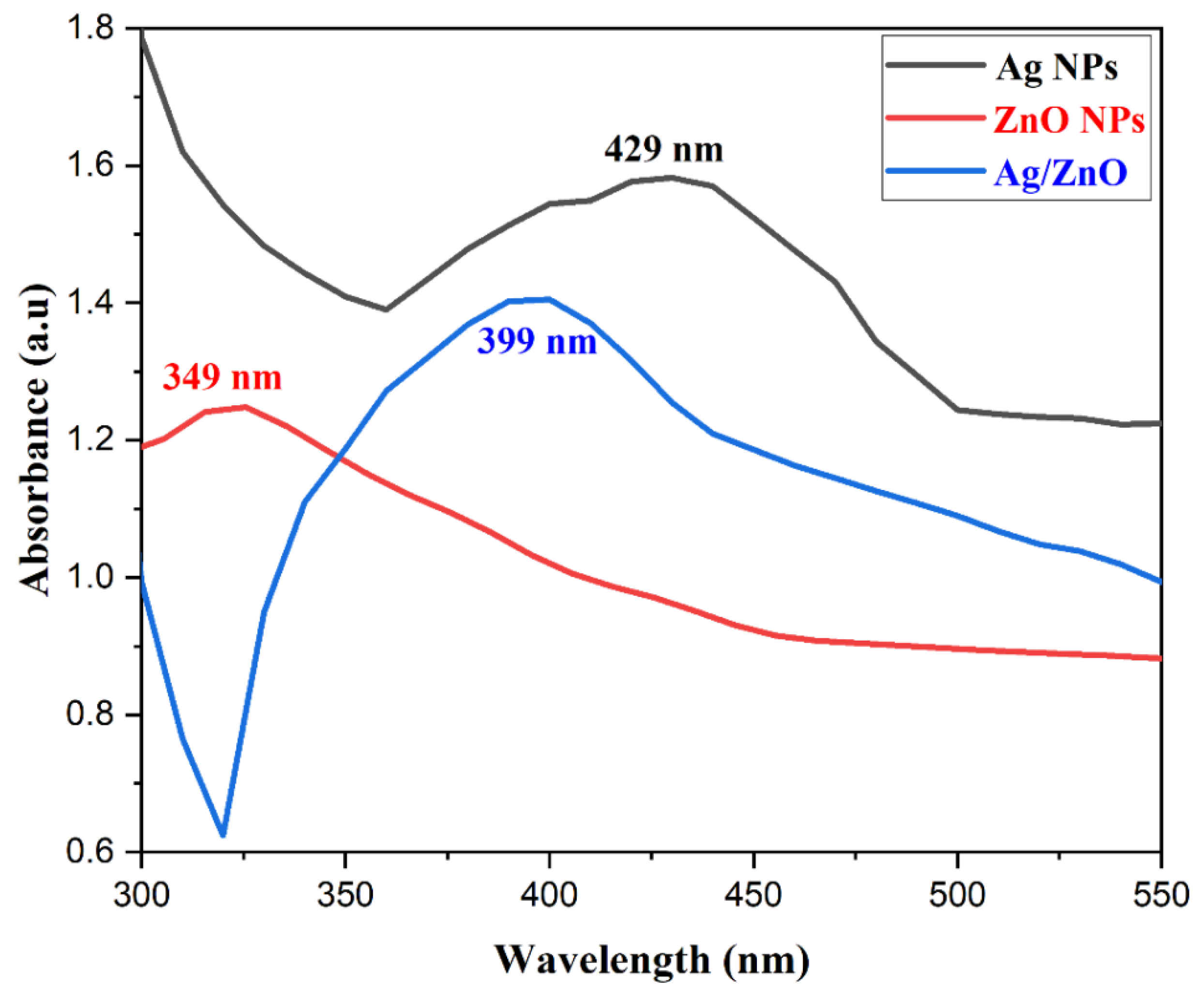
3.1. UV-Vis Monometallic and Bimetallic Nanoparticles
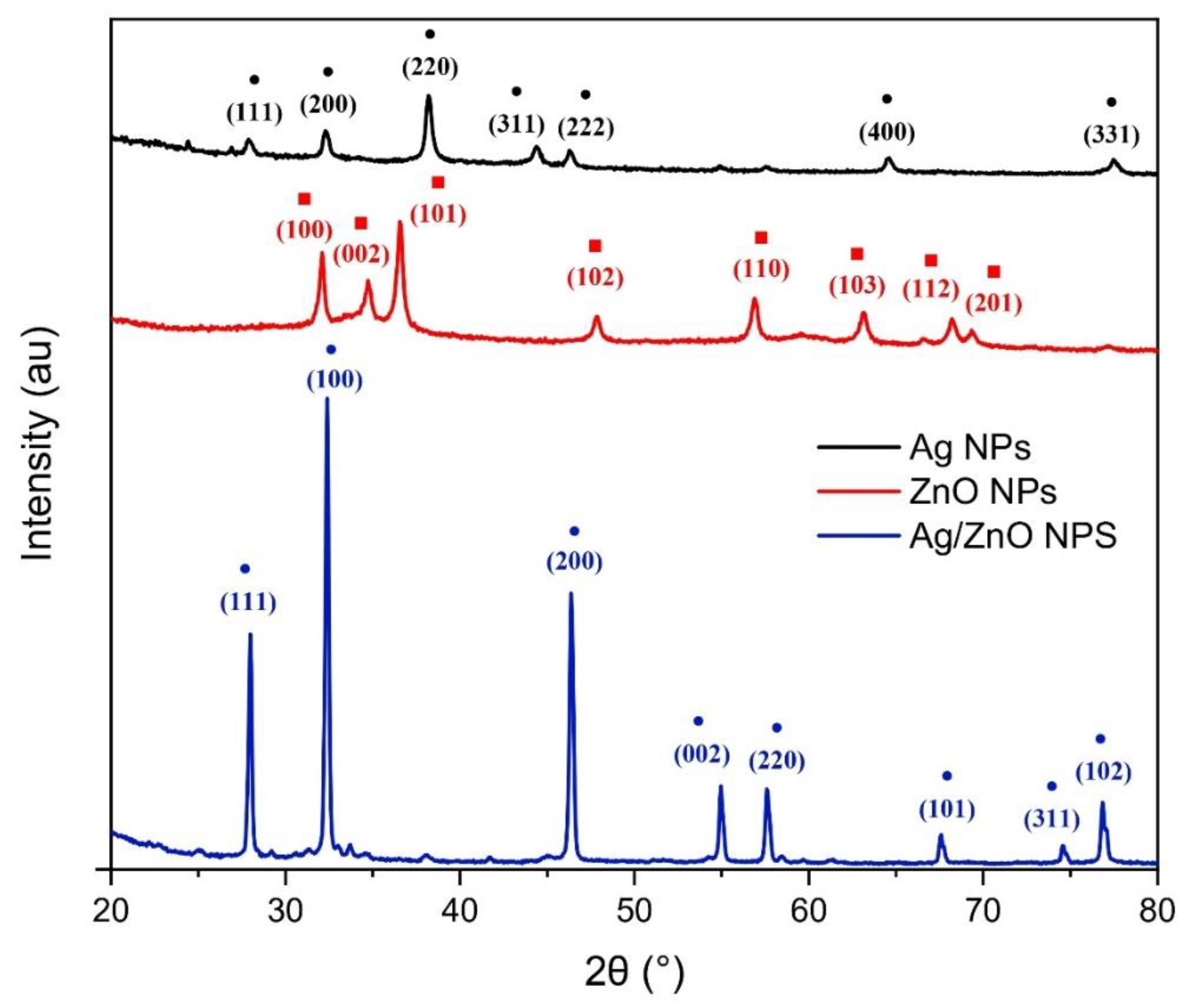
3.2. XRD of Monometallic and Bimetallic Nanoparticles
3.3. SEM of Bimetallic Nanoparticles
3.4. Time-Killing Assay (Antibacterial Activity)
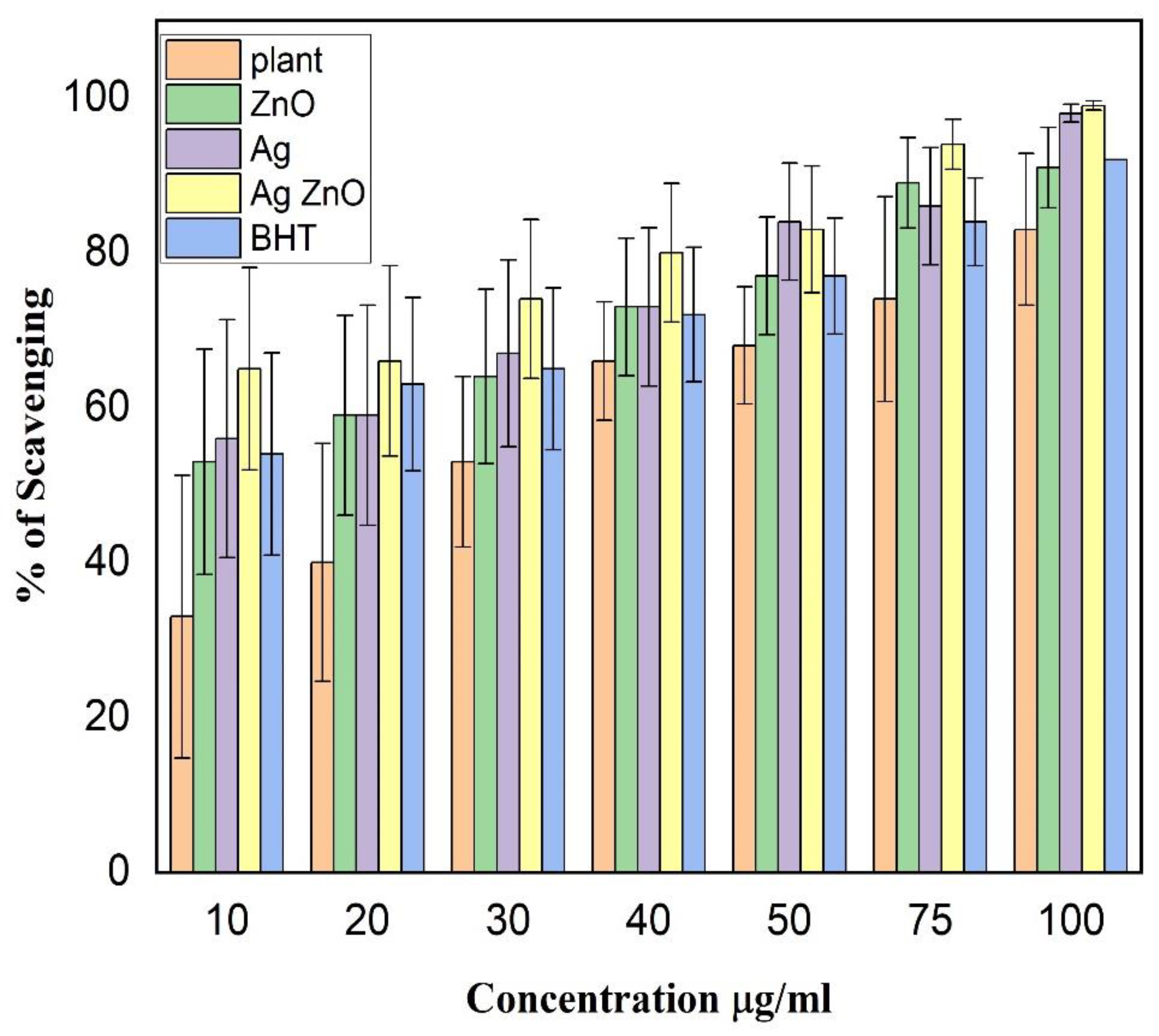
3.5. DPPH Radical Scavenging Activity Assay (Antioxidant Activity)
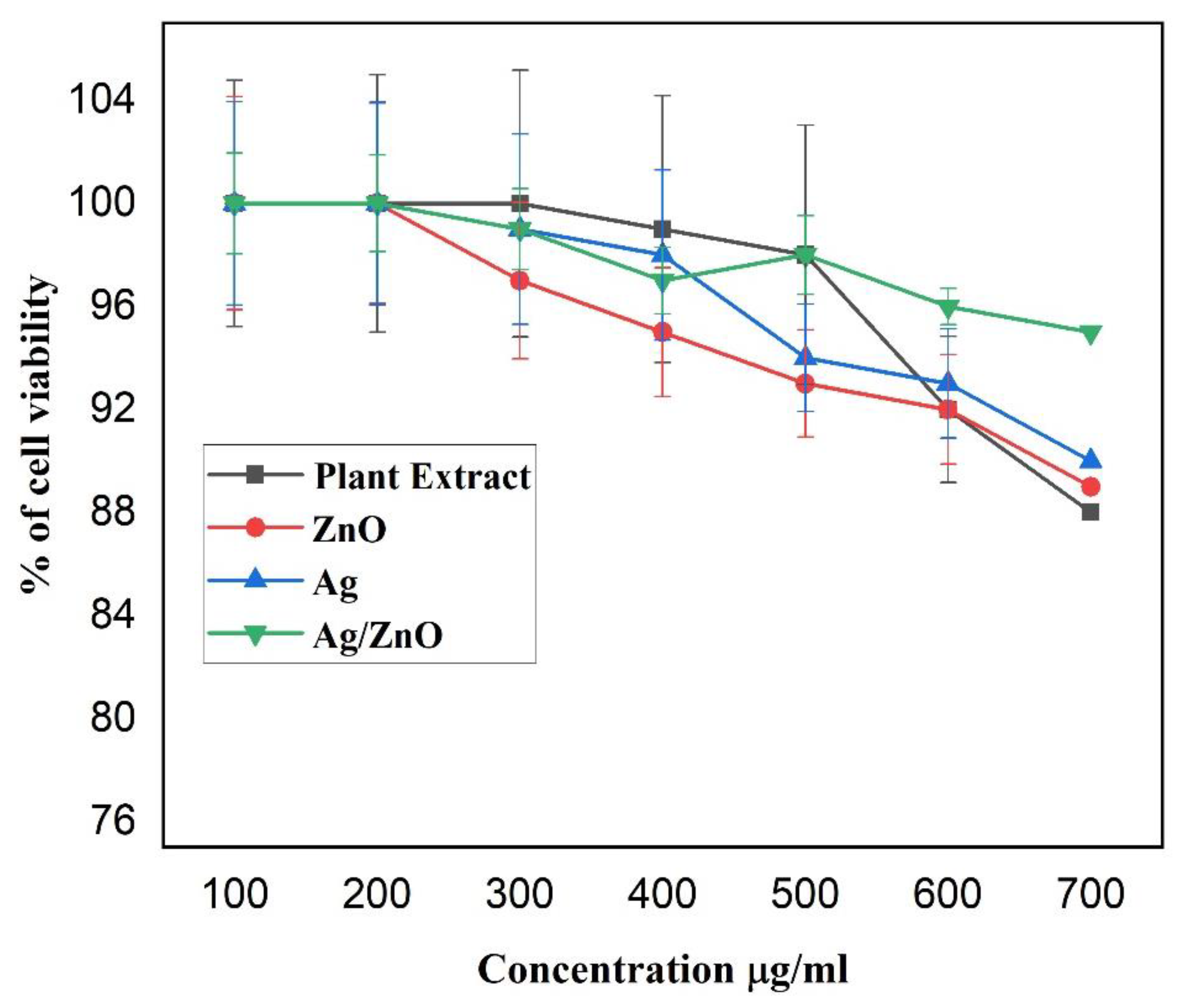
3.6. MTT Assay for Cytotoxicity
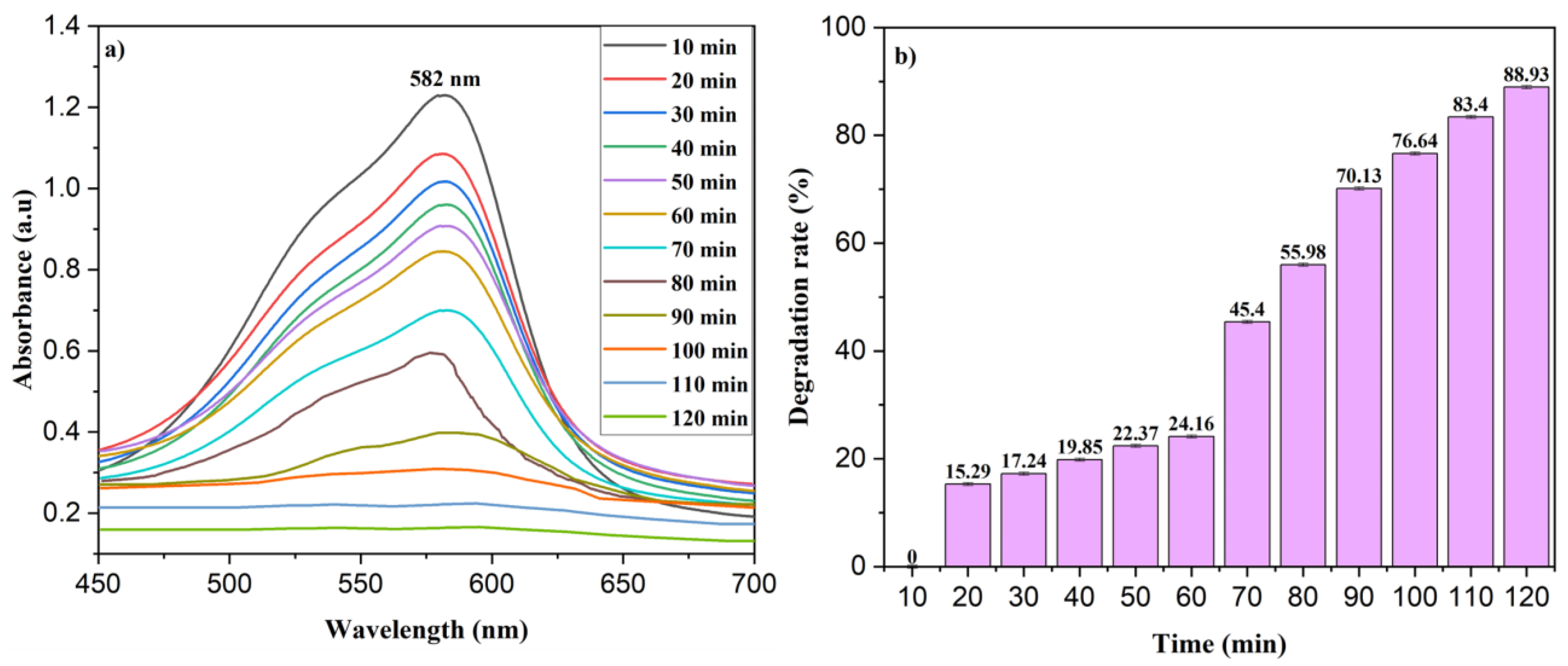
3.7. Photocatalytic Degradation of MV and NPh
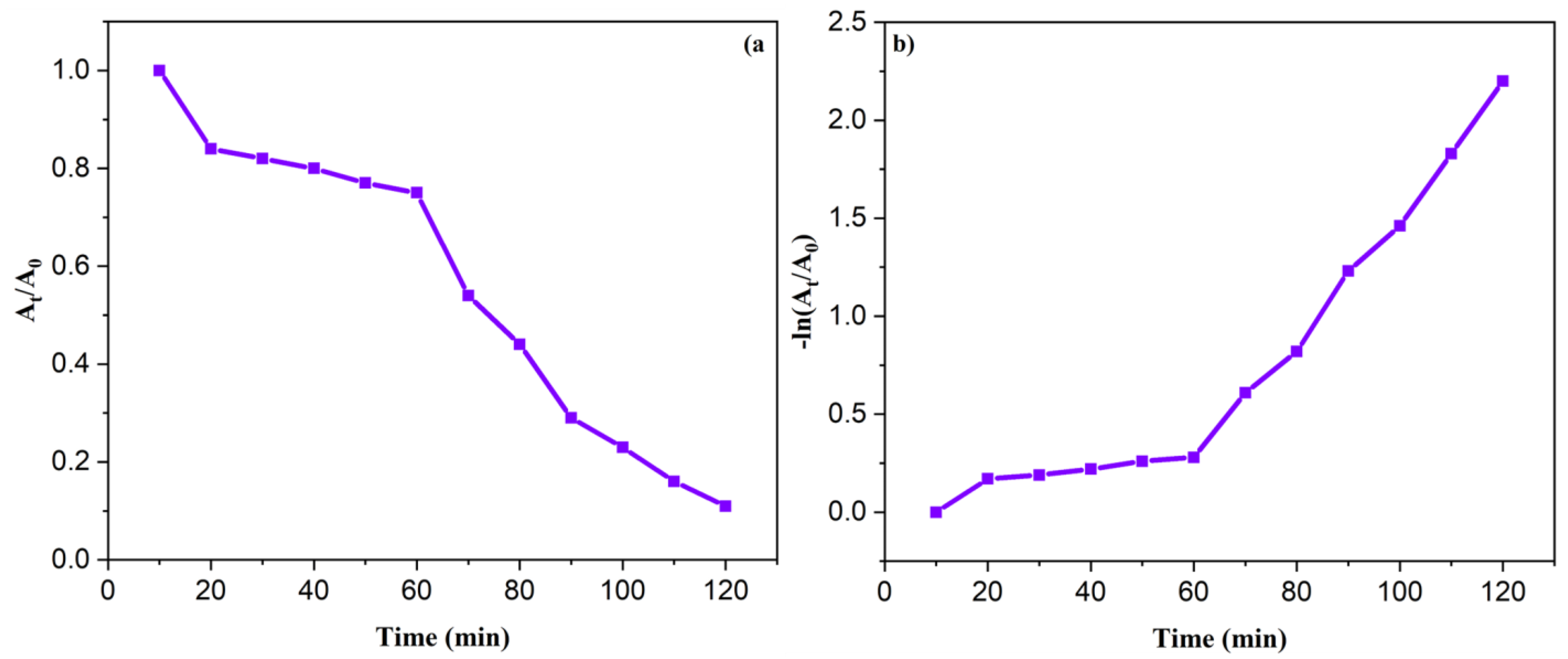
3.8. Kinetic Studies
3.9. Factors Affecting % Removal of MV
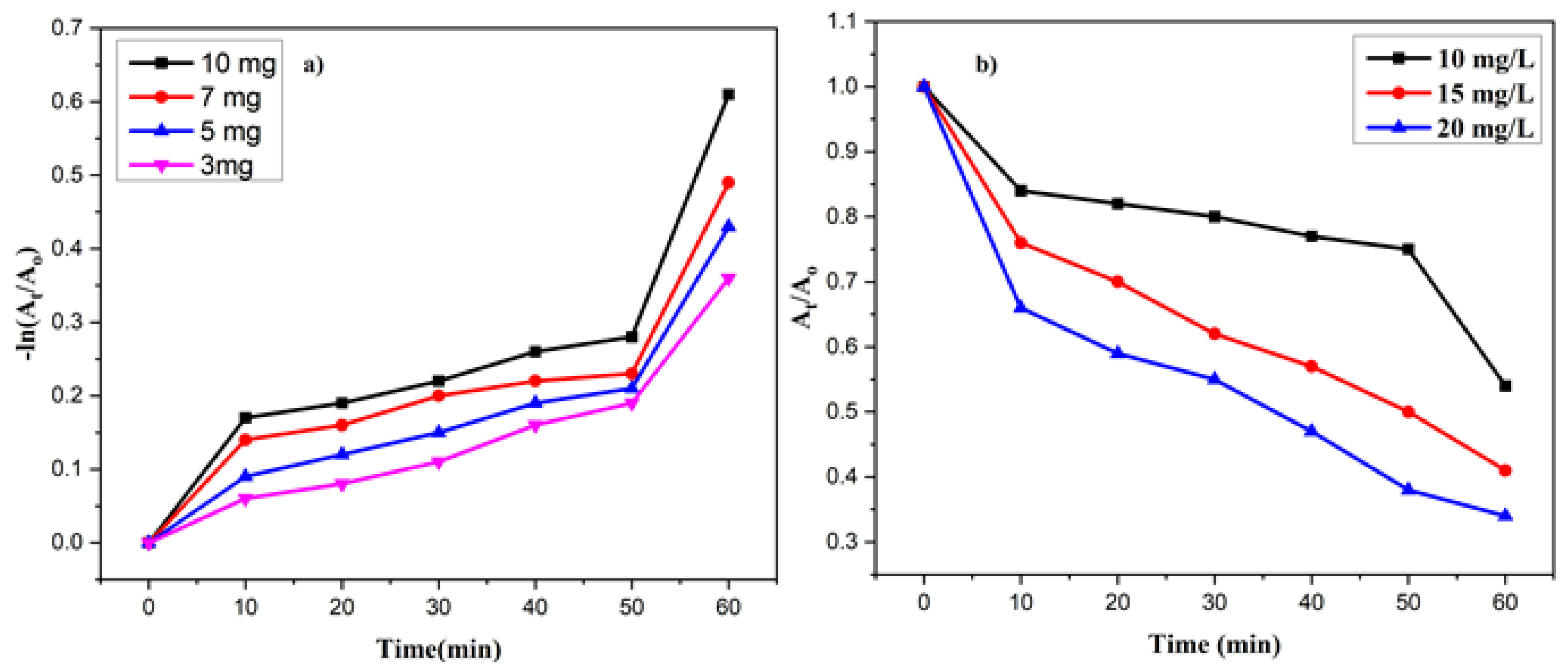
3.9.1. Effect of Catalyst Concentration
3.9.2. Effect of MV Concentration
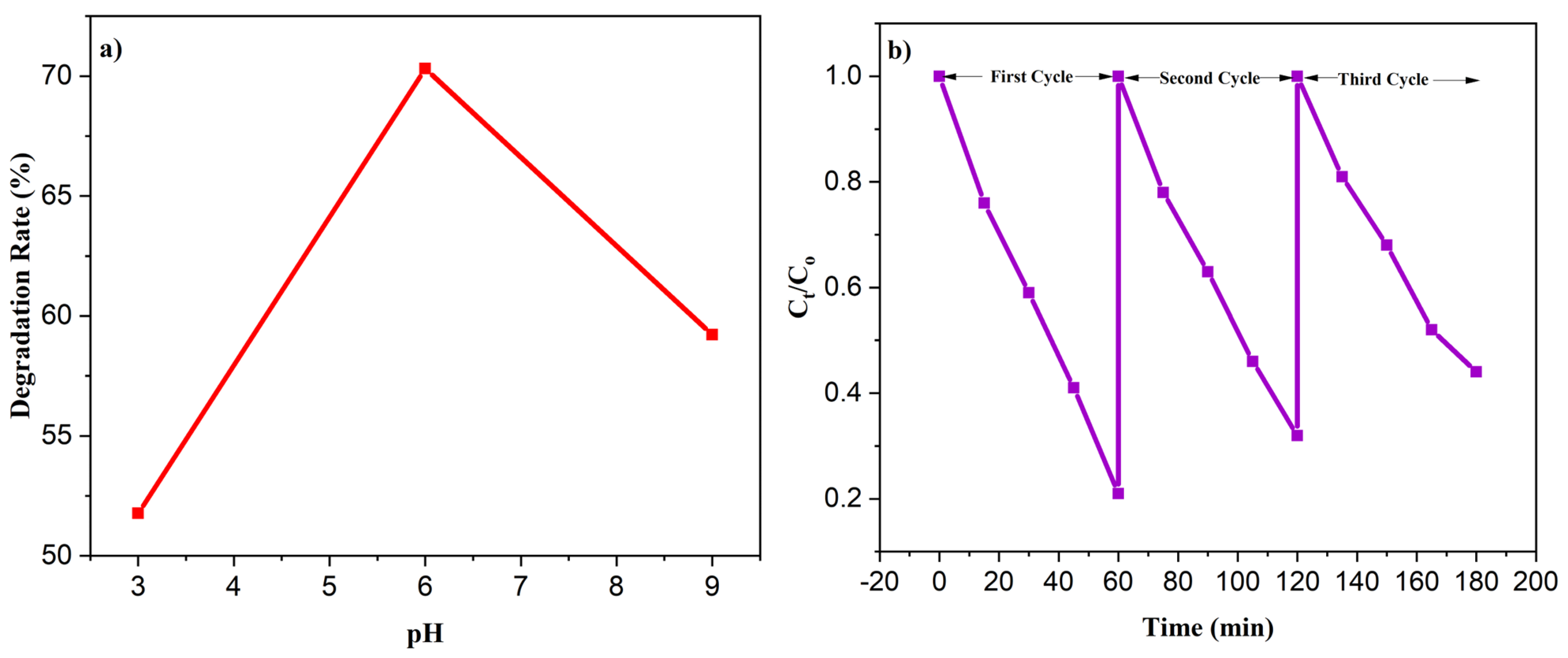
3.9.3. Effect of pH
3.9.4. Reusability of Photocatalyst
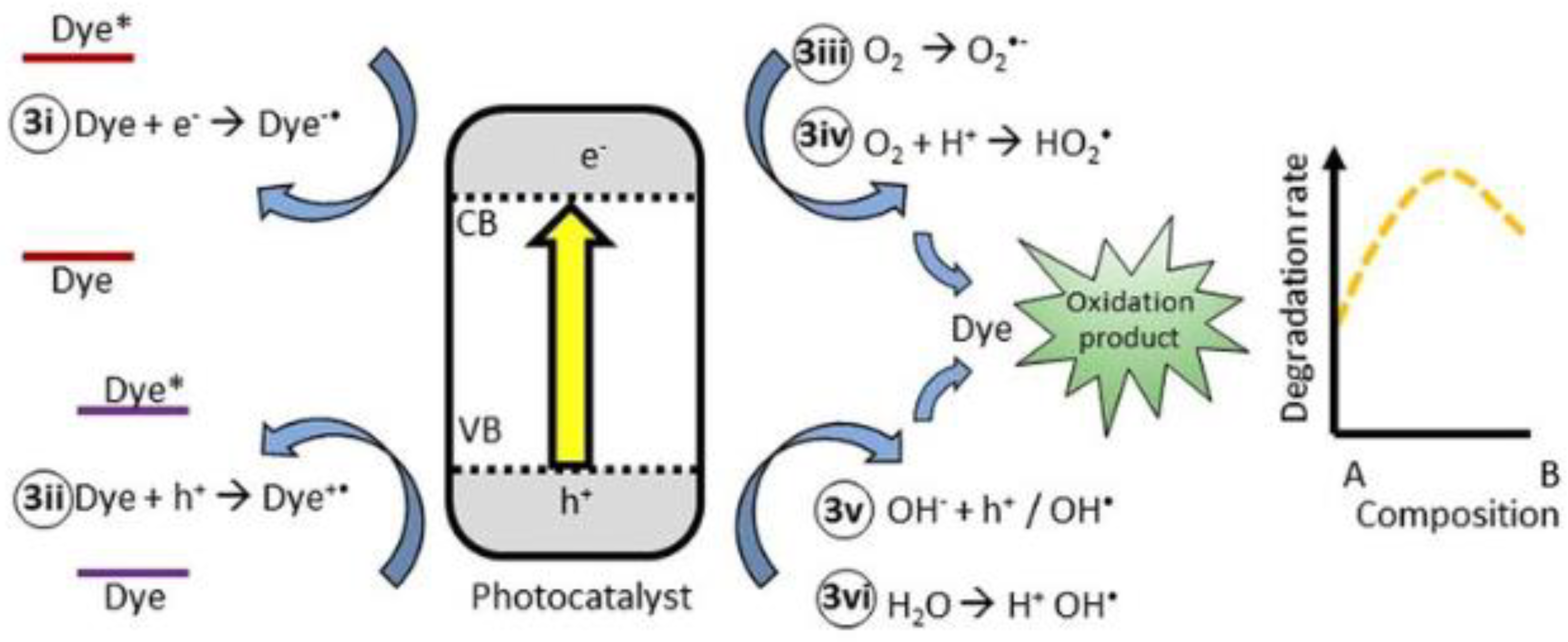
3.10. Mechanism of Photocatalytic Degradation
3.11. Comparison with Literature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samuel, M.S.; Ravikumar, M.; John, J.A.; Selvarajan, E.; Patel, H.; Chander, P.S.; Soundarya, J.; Vuppala, S.; Balaji, R.; Chandrasekar, N. A Review on Green Synthesis of Nanoparticles and Their Diverse Biomedical and Environmental Applications. Catalysts 2022, 12, 459. [Google Scholar] [CrossRef]
- Ehsan, M.; Waheed, A.; Ullah, A.; Kazmi, A.; Ali, A.; Raja, N.I.; Mashwani, Z.-U.-R.; Sultana, T.; Mustafa, N.; Ikram, M.; et al. Plant-Based Bimetallic Silver-Zinc Oxide Nanoparticles: A Comprehensive Perspective of Synthesis, Biomedical Applications, and Future Trends. BioMed Res. Int. 2022, 2022, 1215183. [Google Scholar] [CrossRef] [PubMed]
- Parveen, K.; Banse, V.; Ledwani, L. Green Synthesis of Nanoparticles: Their Advantages and Disadvantages; AIP Publishing: Melville, NY, USA, 2016. [Google Scholar]
- Altammar, K.A. A review on nanoparticles: Characteristics, synthesis, applications, and challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef] [PubMed]
- Naha, P.C.; Liu, Y.; Hwang, G.; Huang, Y.; Gubara, S.; Jonnakuti, V.; Simon-Soro, A.; Kim, D.; Gao, L.; Koo, H.; et al. Dextran-Coated Iron Oxide Nanoparticles as Biomimetic Catalysts for Localized and pH-Activated Biofilm Disruption. ACS Nano 2019, 13, 4960–4971. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, Y.; Pandey, N.K.; Chudal, L.; Amador, E.; Sun, L.; Zhang, S.; Zhang, M.; Chen, W. FeS2 Loaded Porous SiO2 Ball as a Tweezers Recoverable Heterogeneous Fenton Catalyst with Enhanced Recyclability. J. Nanosci. Nanotechnol. 2021, 21, 1474–1482. [Google Scholar] [CrossRef]
- Naganthran, A.; Verasoundarapandian, G.; Khalid, F.E.; Masarudin, M.J.; Zulkharnain, A.; Nawawi, N.M.; Karim, M.; Abdullah, C.A.C.; Ahmad, S.A. Synthesis, Characterization and Biomedical Application of Silver Nanoparticles. Materials 2022, 15, 427. [Google Scholar] [CrossRef]
- Huq, M.A.; Ashrafudoulla, M.; Rahman, M.M.; Balusamy, S.R.; Akter, S. Green Synthesis and Potential Antibacterial Applications of Bioactive Silver Nanoparticles: A Review. Polymers 2022, 14, 742. [Google Scholar] [CrossRef]
- Khane, Y.; Benouis, K.; Albukhaty, S.; Sulaiman, G.M.; Abomughaid, M.M.; Al Ali, A.; Aouf, D.; Fenniche, F.; Khane, S.; Chaibi, W.; et al. Green Synthesis of Silver Nanoparticles Using Aqueous Citrus limon Zest Extract: Characterization and Evaluation of Their Antioxidant and Antimicrobial Properties. Nanomaterials 2022, 12, 2013. [Google Scholar] [CrossRef]
- Tran, T.V.; Nguyen, D.T.C.; Kumar, P.S.; Din, A.T.M.; Jalil, A.A.; Vo, D.-V.N. Green synthesis of ZrO2 nanoparticles and nanocomposites for biomedical and environmental applications: A review. Environ. Chem. Lett. 2022, 20, 1309–1331. [Google Scholar] [CrossRef]
- Ghaderi, R.S.; Adibian, F.; Sabouri, Z.; Davoodi, J.; Kazemi, M.; Jamehdar, S.A.; Meshkat, Z.; Soleimanpour, S.; Daroudi, M. Green synthesis of selenium nanoparticle by Abelmoschus esculentus extract and assessment of its antibacterial activity. Mater. Technol. 2022, 37, 1289–1297. [Google Scholar] [CrossRef]
- Pandey, N.K.; Li, H.B.; Chudal, L.; Bui, B.; Amador, E.; Zhang, M.B.; Yu, H.M.; Chen, M.L.; Luo, X.; Chen, W. Exploration of copper-cysteamine nanoparticles as an efficient heterogeneous Fenton-like catalyst for wastewater treatment. Mater. Today Phys. 2022, 22, 100587. [Google Scholar] [CrossRef]
- Sangami, G.; Nirmala, R.; Kim, H.Y.; Dharmaraj, N. Photodegradation of 4-Nitrophenol Using Cadmium Sulphide Nanoparticles. J. Nanosci. Nanotechnol. 2014, 14, 2299–2306. [Google Scholar] [CrossRef]
- Nicolaou, K.C. Organic synthesis: The art and science of replicating the molecules of living nature and creating others like them in the laboratory. Proc. R. Soc. A Math. Phys. Eng. Sci. 2014, 470, 20130690. [Google Scholar] [CrossRef]
- Ogbodo, J.O.; Arazu, A.V.; Iguh, T.C.; Onwodi, N.J.; Ezike, T.C. Volatile organic compounds: A proinflammatory activator in autoimmune diseases. Front. Immunol. 2022, 13, 928379. [Google Scholar] [CrossRef]
- Kalaimurugan, D.; Sivasankar, P.; Durairaj, K.; Lakshmanamoorthy, M.; Alharbi, S.A.; Al Yousef, S.A.; Chinnathambi, A.; Venkatesan, S. Novel strategy for biodegradation of 4-nitrophenol by the immobilized cells of Pseudomonas sp. YPS3 with Acacia gum. Saudi J. Biol. Sci. 2021, 28, 833–839. [Google Scholar] [CrossRef]
- Awin, L.A.; El-Rais, M.A.; Etorki, A.M.; Abobaker, M.M.; Alzorgani, M.S.; Alnaas, M.M.; Sweesi, M.E.; Ward, A.M. Removal of Methyl Violet from Aqueous solutions using Sr2ANbO5.5 (A = Ca+2, Sr+2 & Ba+2). Rev. CENIC Cienc. Químicas 2021, 26, 7. [Google Scholar]
- Kaci, M.M.; Nasrallah, N.; Djaballah, A.M.; Akkari, I.; Belabed, C.; Soukeur, A.; Atmani, F.; Trari, M. Insights into the optical and electrochemical features of CuAl2O4 nanoparticles and it use for methyl violet oxidation under sunlight exposure. Opt. Mater. 2022, 126, 112198. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Joseph, S.; Mathew, B. Microwave Assisted Biosynthesis of Silver Nanoparticles Using the Rhizome Extract of Alpinia galanga and Evaluation of Their Catalytic and Antimicrobial Activities. J. Nanoparticles 2014, 2014, 967802. [Google Scholar] [CrossRef]
- Chahal, S.; Rani, N.; Kumar, A.; Kumar, P. UV-irradiated photocatalytic performance of yttrium doped ceria for hazardous Rose Bengal dye. Appl. Surf. Sci. 2019, 493, 87–93. [Google Scholar] [CrossRef]
- Hu, H.; Li, X.; Wu, S.; Yang, C. Sustainable livestock wastewater treatment via phytoremediation: Current status and future perspectives. Bioresour. Technol. 2020, 315, 123809. [Google Scholar] [CrossRef] [PubMed]
- Nagajyothi, P.C.; Vattikuti, S.V.P.; Devarayapalli, K.C.; Yoo, K.; Shim, J.; Sreekanth, T.V.M. Green synthesis: Photocatalytic degradation of textile dyes using metal and metal oxide nanoparticles-latest trends and advancements. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2617–2723. [Google Scholar] [CrossRef]
- Taj, M.B.; Alkahtani, M.D.F.; Raheel, A.; Shabbir, S.; Fatima, R.; Aroob, S.; Yahya, R.; Alelwani, W.; Alahmadi, N.; Abualnaja, M.; et al. Bioconjugate synthesis, phytochemical analysis, and optical activity of NiFe2O4 nanoparticles for the removal of ciprofloxacin and Congo red from water. Sci. Rep. 2021, 11, 5439. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Murthy, H.C.A.; Ahmed, H.; Islam, M.; Prasad, R. Phytogenic Synthesis of Metal/Metal Oxide Nanoparticles for Degradation of Dyes. J. Renew. Mater. 2022, 10, 1911–1930. [Google Scholar] [CrossRef]
- Kumar, S.A.; Jarvin, M.; Inbanathan, S.S.R.; Umar, A.; Lalla, N.P.; Dzade, N.Y.; Algadi, H.; Rahman, Q.I.; Baskoutas, S. Facile green synthesis of magnesium oxide nanoparticles using tea (Camellia sinensis) extract for efficient photocatalytic degradation of methylene blue dye. Environ. Technol. Innov. 2022, 28, 102746. [Google Scholar] [CrossRef]
- Awad, M.A.; Hendi, A.A.; Ortashi, K.M.; Alzahrani, B.; Soliman, D.; Alanazi, A.; Alenazi, W.; Taha, R.M.; Ramadan, R.; El-Tohamy, M.; et al. Biogenic synthesis of silver nanoparticles using Trigonella foenum-graecum seed extract: Characterization, photocatalytic and antibacterial activities. Sens. Actuators A Phys. 2021, 323, 112670. [Google Scholar] [CrossRef]
- Nguyen, T.H.A.; Nguyen, V.-C.; Phan, T.N.H.; Le, V.T.; Vasseghian, Y.; Trubitsyn, M.A.; Nguyen, A.-T.; Chau, T.P.; Doan, V.-D. Novel biogenic silver and gold nanoparticles for multifunctional applications: Green synthesis, catalytic and antibacterial activity, and colorimetric detection of Fe(III) ions. Chemosphere 2022, 287, 132271. [Google Scholar] [CrossRef]
- Abdelbaky, A.S.; Abd El-Mageed, T.A.; Babalghith, A.O.; Selim, S.; Mohamed, A.M.H.A. Green Synthesis and Characterization of ZnO Nanoparticles Using Pelargonium odoratissimum (L.) Aqueous Leaf Extract and Their Antioxidant, Antibacterial and Anti-inflammatory Activities. Antioxidants 2022, 11, 1444. [Google Scholar] [CrossRef]
- Zhen, X.; Chudal, L.; Pandey, N.K.; Phan, J.; Ran, X.; Amador, E.; Huang, X.; Johnson, O.; Ran, Y.; Chen, W.; et al. A powerful combination of copper-cysteamine nanoparticles with potassium iodide for bacterial destruction. Mater. Sci. Eng. C 2020, 110, 110659. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B.M. Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab. J. Chem. 2017, 10, S3029–S3039. [Google Scholar] [CrossRef]
- Chung, I.M.; Rahuman, A.; Marimuthu, S.; Arivarasan, V.K.; Karunanithi, A.; Padmini, P.; Rajakumar, G. Green synthesis of copper nanoparticles using Eclipta prostrata leaves extract and their antioxidant and cytotoxic activities. Exp. Ther. Med. 2017, 14, 18–24. [Google Scholar] [CrossRef]
- Wu, S.; Rajeshkumar, S.; Madasamy, M.; Mahendran, V. Green synthesis of copper nanoparticles using Cissus vitiginea and its antioxidant and antibacterial activity against urinary tract infection pathogens. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1153–1158. [Google Scholar] [CrossRef]
- Uddin, S.; Safdar, L.B.; Anwar, S.; Iqbal, J.; Laila, S.; Abbasi, B.A.; Saif, M.S.; Ali, M.; Rehman, A.; Basit, A.; et al. Green Synthesis of Nickel Oxide Nanoparticles from Berberis balochistanica Stem for Investigating Bioactivities. Molecules 2021, 26, 1548. [Google Scholar] [CrossRef]
- Ullah, F.; Uzair, M.; Chaudhry, B.A.; Zafar, Z.U. Phytochemical and pharmacological studies of Leptadenia pyrotechnica. Pak. J. Pharm. Res. 2015, 1, 78. [Google Scholar] [CrossRef]
- Idrees, S.; Qureshi, R.; Bibi, Y.; Ishfaq, A.; Khalid, N.; Iftikhar, A.; Shabir, A.; Riaz, I.; Saboon, N.; Ahmad, N. Ethnobotanical and Biological Activities of Leptadenia pyrotechnica (Forssk.) Decne.: A Review. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 88–96. [Google Scholar] [CrossRef]
- El-Fitiany, R.A.; Khasawneh, M.A. Leptadenia pyrotechnica (Forsk.) Decne: From Edibility to Drug Discovery (A Comprehensive Review). Food Rev. Int. 2022, 1–53. [Google Scholar] [CrossRef]
- Olajuyigbe, O.O.; Afolayan, A.J. In Vitro Antibacterial and Time-Kill Evaluation of the Erythrina caffra Thunb. Extract against Bacteria Associated with Diarrhoea. Sci. World J. 2012, 2012, 738314. [Google Scholar] [CrossRef]
- Loo, Y.Y.; Rukayadi, Y.; Nor-Khaizura, M.-A.-R.; Kuan, C.H.; Chieng, B.W.; Nishibuchi, M.; Radu, S. In Vitro Antimicrobial Activity of Green Synthesized Silver Nanoparticles against Selected Gram-negative Foodborne Pathogens. Front. Microbiol. 2018, 9, 1555. [Google Scholar] [CrossRef]
- Mogana, R.; Adhikari, A.; Tzar, M.N.; Ramliza, R.; Wiart, C. Antibacterial activities of the extracts, fractions and isolated compounds from Canarium patentinervium Miq. against bacterial clinical isolates. BMC Complement. Med. Ther. 2020, 20, 55. [Google Scholar] [CrossRef]
- Alharbi, N.S.; Alsubhi, N.S.; Felimban, A.I. Green synthesis of silver nanoparticles using medicinal plants: Characterization and application. J. Radiat. Res. Appl. Sci. 2022, 15, 109–124. [Google Scholar] [CrossRef]
- Thirugnanasambandan, T.; Alagar, M. Electrolytic Synthesis and Characterization of Silver Nanopowder. Nano Biomed. Eng. 2012, 2012, 58–65. [Google Scholar] [CrossRef]
- Yedurkar, S.; Maurya, C.; Mahanwar, P. Biosynthesis of Zinc Oxide Nanoparticles Using Ixora Coccinea Leaf Extract—A Green Approach. Open J. Synth. Theory Appl. 2016, 5, 1–14. [Google Scholar] [CrossRef]
- Zhu, H.; Jiang, R.; Xiao, L.; Chang, Y.; Guan, Y.; Li, X.; Zeng, G. Photocatalytic decolorization and degradation of Congo Red on innovative crosslinked chitosan/nano-CdS composite catalyst under visible light irradiation. J. Hazard. Mater. 2009, 169, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Aroob, S.; Carabineiro, S.A.C.; Taj, M.B.; Bibi, I.; Raheel, A.; Javed, T.; Yahya, R.; Alelwani, W.; Verpoort, F.; Kamwilaisak, K.; et al. Green Synthesis and Photocatalytic Dye Degradation Activity of CuO Nanoparticles. Catalysts 2023, 13, 502. [Google Scholar] [CrossRef]
- Liao, Y.-H.B.; Wang, J.X.; Lin, J.-S.; Chung, W.-H.; Lin, W.-Y.; Chen, C.-C. Synthesis, photocatalytic activities and degradation mechanism of Bi2WO6 toward crystal violet dye. Catal. Today 2011, 174, 148–159. [Google Scholar] [CrossRef]
- Saeed, K.; Khan, I.; Gul, T.; Sadiq, M. Efficient photodegradation of methyl violet dye using TiO2/Pt and TiO2/Pd photocatalysts. Appl. Water Sci. 2017, 7, 3841–3848. [Google Scholar] [CrossRef]
- Sanakousar, M.F.; Vidyasagar, C.C.; Jiménez-Pérez, V.M.; Jayanna, B.K.; Mounesh; Shridhar, A.H.; Prakash, K. Efficient photocatalytic degradation of crystal violet dye and electrochemical performance of modified MWCNTs/Cd-ZnO nanoparticles with quantum chemical calculations. J. Hazard. Mater. Adv. 2021, 2, 100004. [Google Scholar] [CrossRef]
- Aroob, S.; Taj, M.B.; Shabbir, S.; Imran, M.; Ahmad, R.H.; Habib, S.; Raheel, A.; Akhtar, M.N.; Ashfaq, M.; Sillanpää, M. In situ biogenic synthesis of CuO nanoparticles over graphene oxide: A potential nanohybrid for water treatment. J. Environ. Chem. Eng. 2021, 9, 105590. [Google Scholar] [CrossRef]
- Sathish, T.; Chandramohan, D.; Kumar, S.D.; Rajkumar, S.; Vijayan, V. A facile synthesis of Ag/ZnO nanocomposites prepared via novel green mediated route for catalytic activity. Appl. Phys. A 2021, 127, 692. [Google Scholar] [CrossRef]
- Subhan, M.A.; Awal, M.; Ahmed, T.; Younus, M. Photocatalytic and antibacterial activities of Ag/ZnO nanocomposities fabricated by co-precipitation method. Acta Metall. Sin. (Engl. Lett.) 2014, 27, 223–232. [Google Scholar] [CrossRef]
- Hosseini, S.; Sarsari, I.A.; Kameli, P.; Salamati, H. Effect of Ag doping on structural, optical, and photocatalytic properties of ZnO nanoparticles. J. Alloys Compd. 2015, 640, 408–415. [Google Scholar] [CrossRef]
- Kumaresan, S.; Vallalperuman, K.; Sathishkumar, S. A Novel one-step synthesis of Ag-doped ZnO nanoparticles for high performance photo-catalytic applications. J. Mater. Sci. Mater. Electron. 2017, 28, 5872–5879. [Google Scholar] [CrossRef]
- Vattikuti, S.P.; Ngo, I.-L.; Byon, C. Physicochemcial characteristic of CdS-anchored porous WS2 hybrid in the photocatalytic degradation of crystal violet under UV and visible light irradiation. Solid State Sci. 2016, 61, 121–130. [Google Scholar] [CrossRef]
- Xin, Z.; Li, L.; Zhang, X.; Zhang, W. Microwave-assisted hydrothermal synthesis of chrysanthemum-like Ag/ZnO prismatic nanorods and their photocatalytic properties with multiple modes for dye degradation and hydrogen production. RSC Adv. 2018, 8, 6027–6038. [Google Scholar] [CrossRef]
- Vasantharaj, S.; Sathiyavimal, S.; Saravanan, M.; Senthilkumar, P.; Gnanasekaran, K.; Shanmugavel, M.; Manikandan, E.; Pugazhendhi, A. Synthesis of ecofriendly copper oxide nanoparticles for fabrication over textile fabrics: Characterization of antibacterial activity and dye degradation potential. J. Photochem. Photobiol. B Biol. 2019, 191, 143–149. [Google Scholar] [CrossRef]
- Gul, A.; Ullah, R.; Sun, J.; Munir, T.; Bai, S. Synthesis of mesoporous TiO2/BMMs via hydrothermal method and its potential application toward adsorption and photocatalytic degradation of crystal violet from aqueous solution. Arab. J. Chem. 2022, 15, 103530. [Google Scholar] [CrossRef]
- Yang, S.; Xu, Y.; Huang, Y.; Zhou, G.; Yang, Z.; Yang, Y.; Wang, G. Photocatalytic degradation of methyl violet with TiSiW12O40/TiO2. Int. J. Photoenergy 2013, 2013, 191340. [Google Scholar] [CrossRef]
- Araujo, R.; Nascimento, E.; Firmino, H.; Macedo, D.; Neves, G.; Morales, M.; Menezes, R. α-Fe2O3 fibers: An efficient photocatalyst for dye degradation under visible light. J. Alloys Compd. 2021, 882, 160683. [Google Scholar] [CrossRef]
- Franco, P.; Sacco, O.; De Marco, I.; Vaiano, V. Zinc oxide nanoparticles obtained by supercritical antisolvent precipitation for the photocatalytic degradation of crystal violet dye. Catalysts 2019, 9, 346. [Google Scholar] [CrossRef]
- Davis, A.S.; Prakash, P.; Thamaraiselvi, K. Nanobioremediation technologies for sustainable environment. In Bioremediation and Sustainable Technologies for Cleaner Environment; Springer: Cham, Switzerland, 2017; pp. 13–33. [Google Scholar]
- Li, F.; Zhang, D.E.; Tong, Z.W. Facile Synthesis of Co3O4 Cubic Structures and Evaluation of Their Catalytic Properties. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2013, 43, 756–760. [Google Scholar] [CrossRef]
- Messaoudi, Z.A.; Lahcene, D.; Benaissa, T.; Messaoudi, M.; Zahraoui, B.; Belhachemi, M.; Choukchou-Braham, A. Adsorption and photocatalytic degradation of crystal violet dye under sunlight irradiation using natural and modified clays by zinc oxide. Chem. Methodol. 2022, 6, 661–676. [Google Scholar]
- Suthakaran, S.; Dhanapandian, S.; Krishnakumar, N.; Ponpandian, N. Hydrothermal synthesis of SnO2 nanoparticles and its photocatalytic degradation of methyl violet and electrochemical performance. Mater. Res. Express 2019, 6, 0850i3. [Google Scholar] [CrossRef]
- Mostafa, A.M.; Mwafy, E.A. Synthesis of ZnO/CdO thin film for catalytic degradation of 4-nitrophenol. J. Mol. Struct. 2020, 1221, 128872. [Google Scholar] [CrossRef]
- Ramya, E.; Thirumurugan, A.; Rapheal, V.S.; Anand, K. CuO@ SiO2 nanoparticles assisted photocatalytic degradation of 4-nitrophenol and their antimicrobial activity studies. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100240. [Google Scholar] [CrossRef]
- Zakaria, M.A.; Menazea, A.; Mostafa, A.M.; Al-Ashkar, E.A. Ultra-thin silver nanoparticles film prepared via pulsed laser deposition: Synthesis, characterization, and its catalytic activity on reduction of 4-nitrophenol. Surf. Interfaces 2020, 19, 100438. [Google Scholar] [CrossRef]
- Li, N.; Zhang, F.; Wang, H.; Hou, S. Catalytic Degradation of 4-Nitrophenol in Polluted Water by Three-Dimensional Gold Nanoparticles/Reduced Graphene Oxide Microspheres. Eng. Sci. 2019, 7, 72–79. [Google Scholar] [CrossRef]
- Bordbar, M. Biosynthesis of Ag/almond shell nanocomposite as a cost-effective and efficient catalyst for degradation of 4-nitrophenol and organic dyes. RSC Adv. 2017, 7, 180–189. [Google Scholar] [CrossRef]
- Chishti, A.N.; Guo, F.; Aftab, A.; Ma, Z.; Liu, Y.; Chen, M.; Gautam, G.; Chen, C.; Ni, L.; Diao, G. Synthesis of silver doped Fe3O4/C nanoparticles and its catalytic activities for the degradation and reduction of methylene blue and 4-nitrophenol. Appl. Surf. Sci. 2021, 546, 149070. [Google Scholar] [CrossRef]
- Ren, Z.-H.; Li, H.-T.; Gao, Q.; Wang, H.; Han, B.; Xia, K.-S.; Zhou, C.-G. Au nanoparticles embedded on urchin-like TiO2 nanosphere: An efficient catalyst for dyes degradation and 4-nitrophenol reduction. Mater. Des. 2017, 121, 167–175. [Google Scholar] [CrossRef]
- Mostafa, A.M.; Menazea, A. Polyvinyl Alcohol/Silver nanoparticles film prepared via pulsed laser ablation: An eco-friendly nano-catalyst for 4-nitrophenol degradation. J. Mol. Struct. 2020, 1212, 128125. [Google Scholar] [CrossRef]
- Menazea, A.; Mostafa, A.M. Ag doped CuO thin film prepared via pulsed laser deposition for 4-nitrophenol degradation. J. Environ. Chem. Eng. 2020, 8, 104104. [Google Scholar] [CrossRef]
- Bogireddy, N.K.R.; Pal, U.; Gomez, L.M.; Agarwal, V. Size controlled green synthesis of gold nanoparticles using Coffea arabica seed extract and their catalytic performance in 4-nitrophenol reduction. RSC Adv. 2018, 8, 24819–24826. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, N.; Kumar, M.; Jyoti; Agarwal, A.; Mizaikoff, B. Electrochemical sensing and remediation of 4-nitrophenol using bio-synthesized copper oxide nanoparticles. Chem. Eng. J. 2017, 313, 283–292. [Google Scholar] [CrossRef]
- Bordbar, M.; Sharifi-Zarchi, Z.; Khodadadi, B. Green synthesis of copper oxide nanoparticles/clinoptilolite using Rheum palmatum L. root extract: High catalytic activity for reduction of 4-nitro phenol, rhodamine B, and methylene blue. J. Sol-Gel Sci. Technol. 2016, 81, 724–733. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, H.; Tong, Z.; Song, Z.; Chen, N. A facile synthesis of Fe3O4@SiO2@ZnO with superior photocatalytic performance of 4-nitrophenol. J. Environ. Chem. Eng. 2017, 5, 2207–2213. [Google Scholar] [CrossRef]
- Samuel, M.S.; Bhattacharya, J.; Parthiban, C.; Viswanathan, G.; Singh, N.P. Ultrasound-assisted synthesis of metal organic framework for the photocatalytic reduction of 4-nitrophenol under direct sunlight. Ultrason. Sonochem. 2018, 49, 215–221. [Google Scholar] [CrossRef]
- Song, X.; Shi, X. Bioreductive deposition of highly dispersed Ag nanoparticles on carbon nanotubes with enhanced catalytic degradation for 4-nitrophenol assisted by Shewanella oneidensis MR-1. Environ. Sci. Pollut. Res. 2016, 24, 3038–3044. [Google Scholar] [CrossRef]


| Time (min) | Position | Model | Residual | Residual Square | RMSE | R2 | Standard Deviation |
|---|---|---|---|---|---|---|---|
| 10 | 0.17 | 1.068 | −0.898 | 0.806404 | 3.3387 | 0.88766 | 0.286065 |
| 20 | 0.19 | 1.966 | −1.776 | 3.154176 | 3.3185 | ||
| 30 | 0.22 | 2.864 | −2.644 | 6.990736 | 3.2383 | ||
| 40 | 0.26 | 3.762 | −3.502 | 12.264004 | 3.0531 | ||
| 50 | 0.28 | 4.66 | −4.38 | 19.1844 | 2.6977 | ||
| 60 | 0.61 | 5.558 | −4.948 | 24.482704 | 2.0530 | ||
| 70 | 0.64 | 6.456 | −5.816 | 33.825856 | 8.0551 | ||
| 80 | 0.69 | 7.354 | −6.664 | 44.408896 | 7.6972 | ||
| 90 | 0.73 | 8.252 | −7.522 | 56.580484 | 7.2004 | ||
| 100 | 0.81 | 9.15 | −8.34 | 69.5556 | 6.5128 | ||
| 110 | 0.86 | 10.048 | −9.188 | 84.419344 | 5.5519 | ||
| 120 | 0.92 | 10.946 | −10.026 | 100.520676 | 4.0931 |
| Photocatalyst | Synthesis Method | Time for Degradation (min) | Light Source | Degradation Rate (%) | Reference |
|---|---|---|---|---|---|
| Ag/ZnO Bimetallic Nanoparticle | Hydrothermal | 30 | LED lamp | 98.6 | [55] |
| TiSiW12O40/TiO2 | Crystallization | 180 | Xenon lamp | 93.2 | [58] |
| Fe2O3 Nanoparticles | Hydrothermal | 120 | Visible light | 87.6 | [59] |
| ZnO Nanoparticles | Supercritical antisolvent precipitation | 60 | Visible light | 95.4 | [60] |
| Ag Nanoparticles | Chemical reduction | 45 | Xenon lamp | 88.3 | [61] |
| CdS Nanoparticles | Hydrothermal | 50 | Visible light | 96.7 | [54] |
| Co3O4 Nanoparticles | Co-precipitation | 180 | UV lamp | 82.4 | [62] |
| CuO Nanoparticles | Hydrothermal | 90 | Visible light | 94.5 | [56] |
| Ag/ZnO nanoparticles | Co-precipitation | 210 | Mercury lamp | 93 | [51] |
| Ag doped ZnO nanoparticles | Microwave irradiation method | 100 | UV lamp | 77.6 | [53] |
| Ag/ZnO nanocomposites | Co-precipitation | 8 | UV lamp | 97 | [50] |
| Ag doped ZnO nanoparticles | Precipitation | 180 | UV lamp | 95 | [52] |
| ZnO/CNA material | Impregnation method | 120 | Sunlight | 49.7 | [63] |
| SnO2 Nanoparticle | Hydrothermal | 120 | Sunlight | 80 | [64] |
| TiO2 Nanocomposites | Hydrothermal | 180 | Sunlight | 98 | [57] |
| Ag/ZnO nanoparticles | Co-precipitation | 120 | Sunlight | 88.9 | Our work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afzal, M.A.; Javed, M.; Aroob, S.; Javed, T.; M. Alnoman, M.; Alelwani, W.; Bibi, I.; Sharif, M.; Saleem, M.; Rizwan, M.; et al. The Biogenic Synthesis of Bimetallic Ag/ZnO Nanoparticles: A Multifunctional Approach for Methyl Violet Photocatalytic Degradation and the Assessment of Antibacterial, Antioxidant, and Cytotoxicity Properties. Nanomaterials 2023, 13, 2079. https://doi.org/10.3390/nano13142079
Afzal MA, Javed M, Aroob S, Javed T, M. Alnoman M, Alelwani W, Bibi I, Sharif M, Saleem M, Rizwan M, et al. The Biogenic Synthesis of Bimetallic Ag/ZnO Nanoparticles: A Multifunctional Approach for Methyl Violet Photocatalytic Degradation and the Assessment of Antibacterial, Antioxidant, and Cytotoxicity Properties. Nanomaterials. 2023; 13(14):2079. https://doi.org/10.3390/nano13142079
Chicago/Turabian StyleAfzal, Muhammad Asjad, Muhammad Javed, Sadia Aroob, Tariq Javed, Maryam M. Alnoman, Walla Alelwani, Ismat Bibi, Muhammad Sharif, Muhammad Saleem, Muhammad Rizwan, and et al. 2023. "The Biogenic Synthesis of Bimetallic Ag/ZnO Nanoparticles: A Multifunctional Approach for Methyl Violet Photocatalytic Degradation and the Assessment of Antibacterial, Antioxidant, and Cytotoxicity Properties" Nanomaterials 13, no. 14: 2079. https://doi.org/10.3390/nano13142079
APA StyleAfzal, M. A., Javed, M., Aroob, S., Javed, T., M. Alnoman, M., Alelwani, W., Bibi, I., Sharif, M., Saleem, M., Rizwan, M., Raheel, A., Maseeh, I., Carabineiro, S. A. C., & Taj, M. B. (2023). The Biogenic Synthesis of Bimetallic Ag/ZnO Nanoparticles: A Multifunctional Approach for Methyl Violet Photocatalytic Degradation and the Assessment of Antibacterial, Antioxidant, and Cytotoxicity Properties. Nanomaterials, 13(14), 2079. https://doi.org/10.3390/nano13142079







