Liquid-Metal Core–Shell Particles Coated with Folate and Phospholipids for Targeted Drug Delivery and Photothermal Treatment of Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Liquid Metal Nanoparticles
2.3. Characterization
2.4. DOX-Loading Efficiency and In Vitro Drug Release Test
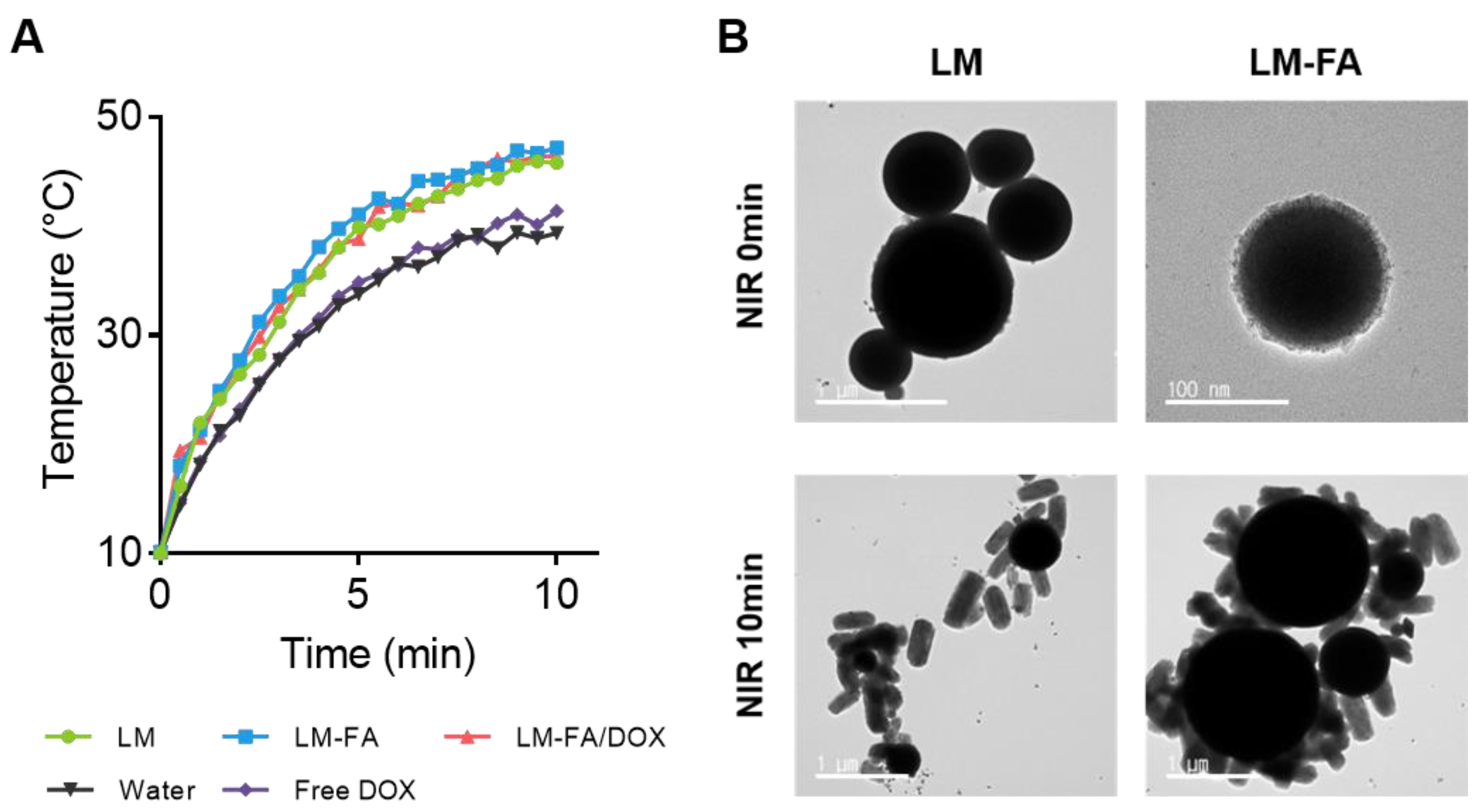
2.5. NIR Treatment
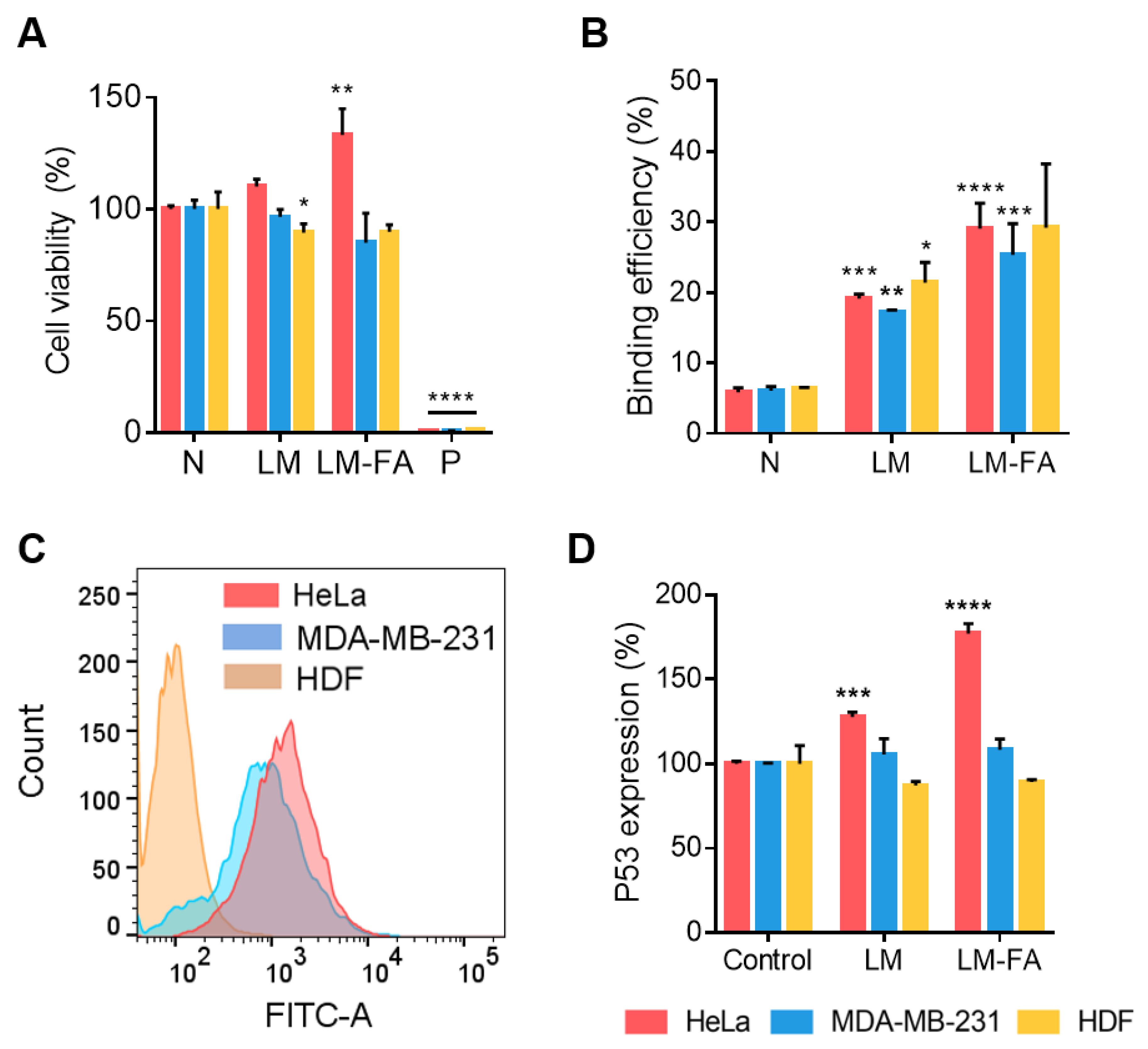
2.6. Cell Cytotoxicity Test
2.7. FACS Measurement
2.8. P53 ELISA
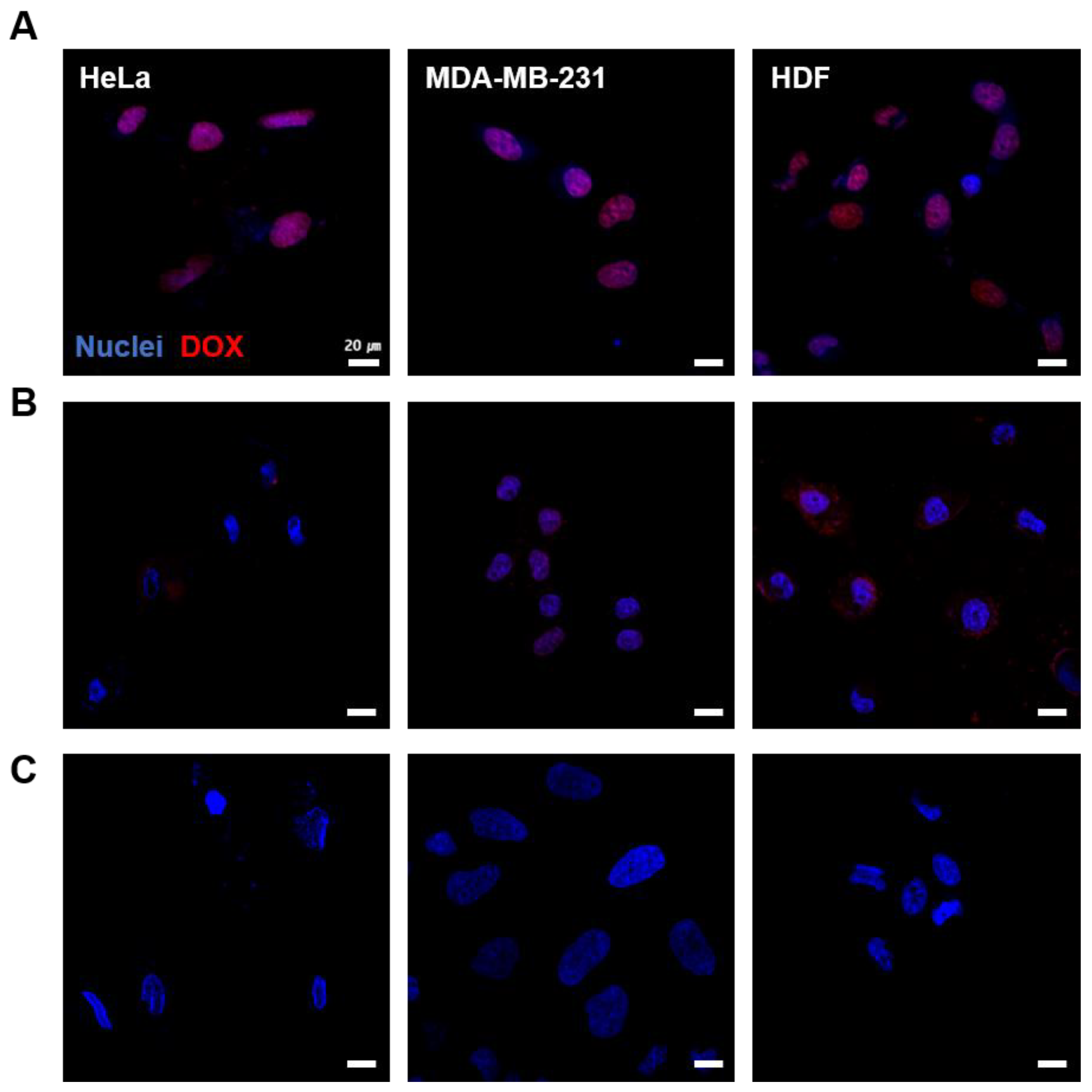
2.9. Cancer Targeting Effect Test
2.10. Cell Viability Test (Live & Dead Assay, Trypan Blue Assay)
2.11. Statistics
3. Results and Discussion
3.1. Liquid Metal Particle Characterization
3.2. Photothermal Characterization by NIR Irradiation
3.3. Cytotoxicity and Cancer Cell Targeting Effect by NIR Irradiation Time
3.4. Cellular Uptake of LM-FA/DOX
3.5. In Vitro Cell Expression by Liquid Metal Particle
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer. 2020. Available online: https://gco.iarc.fr/today (accessed on 28 November 2021).
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiro, C.L.; Recht, A. Side effects of adjuvant treatment of breast cancer. N. Engl. J. Med. 2001, 344, 1997–2008. [Google Scholar] [CrossRef] [PubMed]
- Ahles, T.A.; Saykin, A.J. Breast cancer chemotherapy-related cognitive dysfunction. Clin. Breast Cancer 2002, 3 (Suppl. 3), S84–S90. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Shin, M.-K.; Han, S.; Oh, I.; Kim, E.; Park, J.; Son, H.Y.; Kang, T.; Jung, J.; Huh, Y.-M.; et al. Magnetic Nanochain-Based Smart Drug Delivery System with Remote Tunable Drug Release by a Magnetic Field. BioChip J. 2022, 16, 280–290. [Google Scholar] [CrossRef]
- Mani, G.; Kim, S.; Kim, K. Development of Folate-Thioglycolate-Gold Nanoconjugates by Using Citric Acid-PEG Branched Polymer for Inhibition of MCF-7 Cancer Cell Proliferation. Biomacromolecules 2018, 19, 3257–3267. [Google Scholar] [CrossRef] [PubMed]
- Byun, M.J.; Lim, J.; Kim, S.-N.; Park, D.-H.; Kim, T.-H.; Park, W.; Park, C.G. Advances in Nanoparticles for Effective Delivery of RNA Therapeutics. BioChip J. 2022, 16, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, S.; Kim, H.; Wooh, S.; Cho, J.; Dickey, M.D.; So, J.-H.; Koo, H.-J. Imbibition-induced selective wetting of liquid metal. Nat. Commun. 2022, 13, 4763. [Google Scholar] [CrossRef]
- Lin, Y.; Genzer, J.; Li, W.; Qiao, R.; Dickey, M.D.; Tang, S.-Y. Sonication-enabled rapid production of stable liquid metal nanoparticles grafted with poly(1-octadecene-alt-maleic anhydride) in aqueous solutions. Nanoscale 2018, 10, 19871–19878. [Google Scholar] [CrossRef] [Green Version]
- Morris, N.J.; Farrell, Z.J.; Tabor, C.E. Chemically modifying the mechanical properties of core-shell liquid metal nanoparticles. Nanoscale 2019, 11, 17308–17318. [Google Scholar] [CrossRef]
- Yan, J.; Lu, Y.; Chen, G.; Yang, M.; Gu, Z. Advances in liquid metals for biomedical applications. Chem. Soc. Rev. 2018, 47, 2518–2533. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; Lee, S.C.; Kim, H.; Lee, C.S. Polydopamine Nanoparticle-Incorporated Fluorescent Hydrogel for Fluorescence Imaging-Guided Photothermal Therapy of Cancers. BioChip J. 2023, 17, 85–92. [Google Scholar] [CrossRef]
- Pinto, A.; Pocard, M. Photodynamic therapy and photothermal therapy for the treatment of peritoneal metastasis: A systematic review. Pleura Peritoneum 2018, 3, 20180124. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Mayyas, M.; Saborio, M.G.; Ghasemian, M.B.; Tang, J.; Daeneke, T.; Han, J.; Esmailpour, A.A.; Allioux, F.-M.; Kalantar-Zadeh, K. Gallium nitride formation in liquid metal sonication. J. Mater. Chem. C 2020, 8, 16593–16602. [Google Scholar] [CrossRef]
- Tang, S.; Qiao, R.; Yan, S.; Yuan, D.; Zhao, Q.; Yun, G.; Davis, T.P.; Li, W. Microfluidic Mass Production of Stabilized and Stealthy Liquid Metal Nanoparticles. Small 2018, 14, e1800118. [Google Scholar] [CrossRef] [Green Version]
- Banu, H.; Sethi, D.K.; Edgar, A.; Sheriff, A.; Rayees, N.; Renuka, N.; Faheem, S.; Premkumar, K.; Vasanthakumar, G. Doxorubicin loaded polymeric gold nanoparticles targeted to human folate receptor upon laser photothermal therapy potentiates chemotherapy in breast cancer cell lines. J. Photochem. Photobiol. B 2015, 149, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Sathappa, M.; Alder, N.N. Ionization Properties of Phospholipids Determined by Zeta Potential Measurements. Bio Protoc. 2016, 6, e2030. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Lin, J.; Yang, X.; Li, Y.; Wu, S.; Huang, Y.; Ye, S.; Xie, L.; Dai, L.; Hou, Z. Self-Assembled Nanoparticles Based on Amphiphilic Anticancer Drug-Phospholipid Complex for Targeted Drug Delivery and Intracellular Dual-Controlled Release. ACS Appl. Mater. Interfaces 2015, 7, 17573–17581. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, Y.; Chen, Z.; Hu, Q.; Liu, Y.; Yu, S.; Gao, W.; Dickey, M.D.; Gu, Z. Enhanced Endosomal Escape by Light-Fueled Liquid-Metal Transformer. Nano Lett. 2017, 17, 2138–2145. [Google Scholar] [CrossRef]
- Song, H.; Kim, T.; Kang, S.; Jin, H.; Lee, K.; Yoon, H.J. Ga-Based Liquid Metal Micro/Nanoparticles: Recent Advances and Applications. Small 2020, 16, e1903391. [Google Scholar] [CrossRef]
- Soller, B.R.; Micheels, R.H.; Coen, J.; Parikh, B.; Chu, L.; Hsi, C. Feasibility of non-invasive measurement of tissue pH using near-infrared reflectance spectroscopy. J. Clin. Moni. 1996, 12, 387–395. [Google Scholar] [CrossRef]
- Badhe, R.V.; Akinfosile, O.; Bijukumar, D.; Barba, M.; Mathew, M.T. Systemic toxicity eliciting metal ion levels from metallic implants and orthopedic devices—A mini review. Toxicol. Lett. 2021, 350, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, G.; Shimonaka, M.; Ishihara, Y. Differential genotoxicity of chemical properties and particle size of rare metal and metal oxide nanoparticles. J. Appl. Toxicol. 2012, 32, 72–80. [Google Scholar] [CrossRef]
- Bijukumar, D.R.; Segu, A.; Souza, J.C.; Li, X.; Barba, M.; Mercuri, L.G.; Jacobs, J.J.; Mathew, M.T. Systemic and local toxicity of metal debris released from hip prostheses: A review of experimental approaches. Nanomedicine 2018, 14, 951–963. [Google Scholar] [CrossRef]
- Thakur, N.S.; Patel, G.; Kushwah, V.; Jain, S.; Banerjee, U.C. Self-Assembled Gold Nanoparticle-Lipid Nanocomposites for On-Demand Delivery, Tumor Accumulation, and Combined Photothermal-Photodynamic Therapy. ACS Appl. Bio Mater. 2019, 2, 349–361. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Hua, M.; Yu, H.; Wei, S.; Wang, A.; Zhou, J. Photothermal-Triggered Controlled Drug Release from Mesoporous Silica Nanoparticles Based on Base-Pairing Rules. ACS Biomater. Sci. Eng. 2019, 5, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Kullenberg, F.; Degerstedt, O.; Calitz, C.; Pavlović, N.; Balgoma, D.; Gråsjö, J.; Sjögren, E.; Hedeland, M.; Heindryckx, F.; Lennernäs, H. In Vitro Cell Toxicity and Intracellular Uptake of Doxorubicin Exposed as a Solution or Liposomes: Implications for Treatment of Hepatocellular Carcinoma. Cells 2021, 10, 1717. [Google Scholar] [CrossRef]
- Ferjaoui, Z.; Nahle, S.; Schneider, R.; Kerdjoudj, H.; Mertz, D.; Quilès, F.; Ferji, K.; Gaffet, E.; Alem, H. Development of Folate-Superparamagnetic Nanoconjugates for Inhibition of Cancer Cell Proliferation. Adv. Mater. Interfaces 2023, 10, 2202364. [Google Scholar] [CrossRef]
- Xu, L.; Pirollo, K.F.; Chang, E.H. Tumor-targeted p53-gene therapy enhances the efficacy of conventional chemo/radiotherapy. J. Control Release 2001, 74, 115–128. [Google Scholar] [CrossRef]
- Nunez, M.I.; Behrens, C.; Woods, D.M.; Lin, H.; Suraokar, M.; Kadara, H.; Hofstetter, W.; Kalhor, N.; Lee, J.J.; Franklin, W.; et al. High expression of folate receptor alpha in lung cancer correlates with adenocarcinoma histology and EGFR [corrected] mutation. J. Thorac. Oncol. 2012, 7, 833–840. [Google Scholar] [CrossRef] [Green Version]
- Fridman, J.S.; Lowe, S.W. Control of apoptosis by p53. Oncogene 2003, 22, 9030–9040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haupt, S.; Berger, M.; Goldberg, Z.; Haupt, Y. Apoptosis—The p53 network. J. Cell Sci. 2003, 116 Pt 20, 4077–4085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, A.; Bax, H.J.; Josephs, D.H.; Ilieva, K.M.; Pellizzari, G.; Opzoomer, J.; Bloomfield, J.; Fittall, M.; Grigoriadis, A.; Figini, M.; et al. Targeting folate receptor alpha for cancer treatment. Oncotarget 2016, 7, 52553–52574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, G.; Wang, D.; Xu, J.; Shi, G. Molecular markers’ progress of breast cancer treatment efficacy. J. Cancer Res. Ther. 2015, 11 (Suppl. 1), C11–C15. [Google Scholar] [CrossRef] [PubMed]
- Wein, L.; Loi, S. Mechanisms of resistance of chemotherapy in early-stage triple negative breast cancer (TNBC). Breast 2017, 34 (Suppl. 1), S27–S30. [Google Scholar] [CrossRef] [PubMed]
- Strangman, G.; Boas, D.A.; Sutton, J.P. Non-invasive neuroimaging using near-infrared light. Biol. Psychiatry 2002, 52, 679–693. [Google Scholar] [CrossRef]
- Rachim, V.P.; Chung, W.-Y. Wearable-band type visible-near infrared optical biosensor for non-invasive blood glucose monitoring. Sens. Actuators B Chem. 2019, 286, 173–180. [Google Scholar] [CrossRef]
- He, J.; Shi, F.; Wu, J.; Ye, J. Shape Transformation Mechanism of Gallium Indium Alloyed Liquid Metal Nanoparticles. Adv. Mater. Interfaces 2021, 8, 2001874. [Google Scholar] [CrossRef]
- Kim, D.; Hwang, J.; Choi, Y.; Kwon, Y.; Jang, J.; Yoon, S.; Choi, J. Effective Delivery of Anti-Cancer Drug Molecules with Shape Transforming Liquid Metal Particles. Cancers 2019, 11, 1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, S.; Kang, S.H.; Woo, H.; Kim, K.; Koo, H.-J.; Lee, H.-Y.; Choi, Y.; Kang, S.H.; Choi, J. Liquid-Metal Core–Shell Particles Coated with Folate and Phospholipids for Targeted Drug Delivery and Photothermal Treatment of Cancer Cells. Nanomaterials 2023, 13, 2017. https://doi.org/10.3390/nano13132017
Ahn S, Kang SH, Woo H, Kim K, Koo H-J, Lee H-Y, Choi Y, Kang SH, Choi J. Liquid-Metal Core–Shell Particles Coated with Folate and Phospholipids for Targeted Drug Delivery and Photothermal Treatment of Cancer Cells. Nanomaterials. 2023; 13(13):2017. https://doi.org/10.3390/nano13132017
Chicago/Turabian StyleAhn, Suyeon, Seung Hyun Kang, Hyunjeong Woo, Kyobum Kim, Hyung-Jun Koo, Hee-Young Lee, Yonghyun Choi, Shin Hyuk Kang, and Jonghoon Choi. 2023. "Liquid-Metal Core–Shell Particles Coated with Folate and Phospholipids for Targeted Drug Delivery and Photothermal Treatment of Cancer Cells" Nanomaterials 13, no. 13: 2017. https://doi.org/10.3390/nano13132017
APA StyleAhn, S., Kang, S. H., Woo, H., Kim, K., Koo, H.-J., Lee, H.-Y., Choi, Y., Kang, S. H., & Choi, J. (2023). Liquid-Metal Core–Shell Particles Coated with Folate and Phospholipids for Targeted Drug Delivery and Photothermal Treatment of Cancer Cells. Nanomaterials, 13(13), 2017. https://doi.org/10.3390/nano13132017






