Advancing Thermoelectric Materials: A Comprehensive Review Exploring the Significance of One-Dimensional Nano Structuring
Abstract
1. Introduction
1.1. Historical Background
1.2. Basics of Thermoelectric Materials
2. State-of-the-Art TE Materials
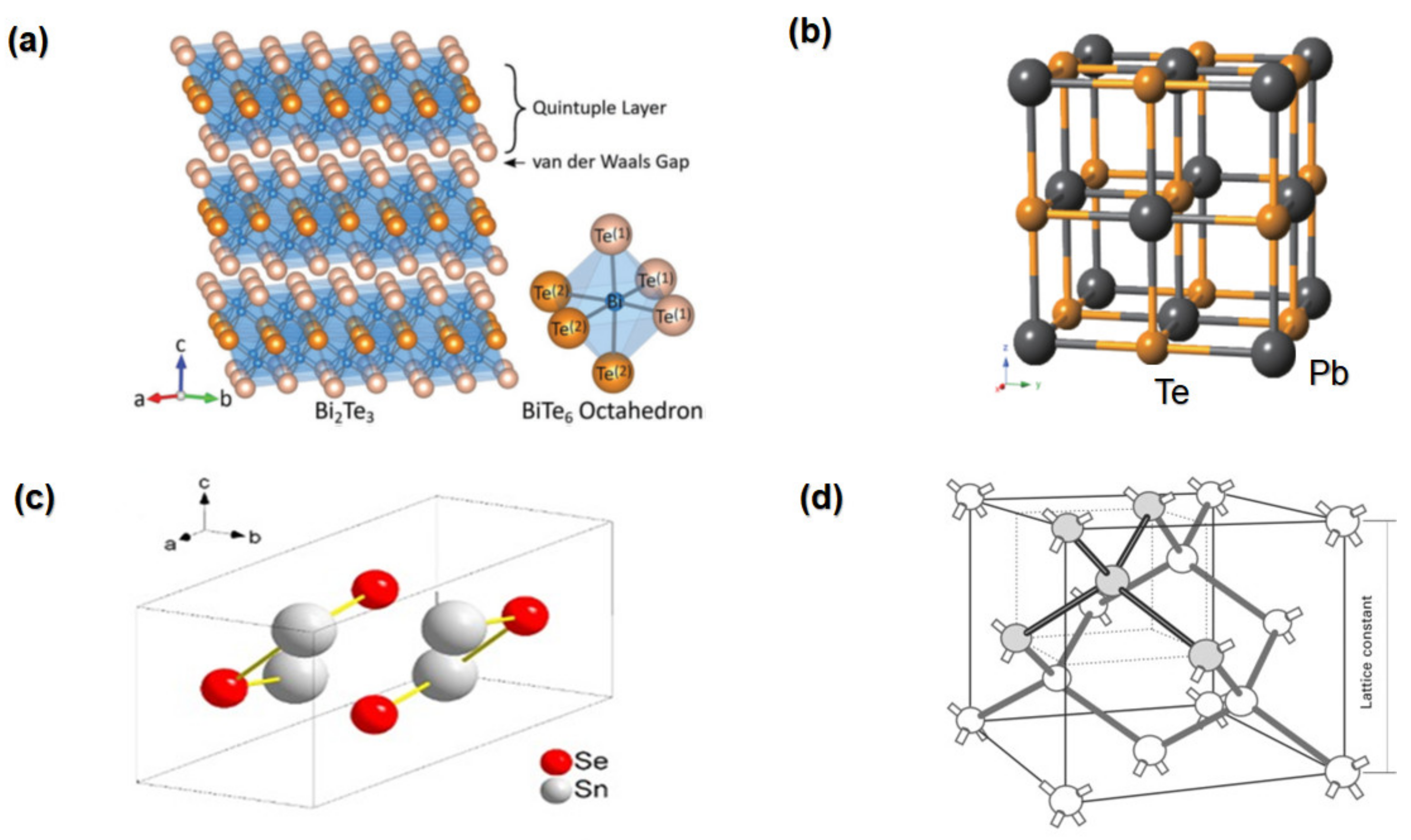
2.1. Bismuth Telluride
2.2. Lead Telluride
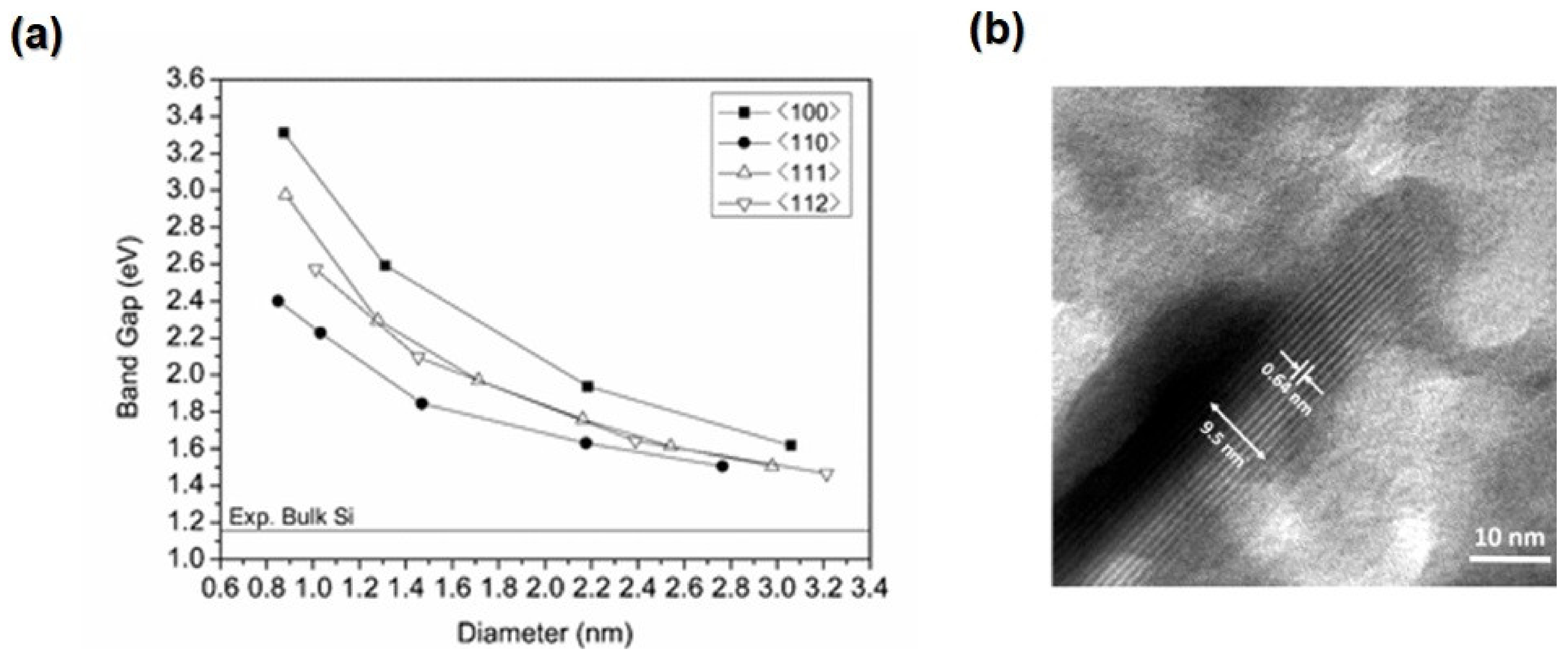
2.3. Tin Selenide
2.4. Silicon-Germanium System
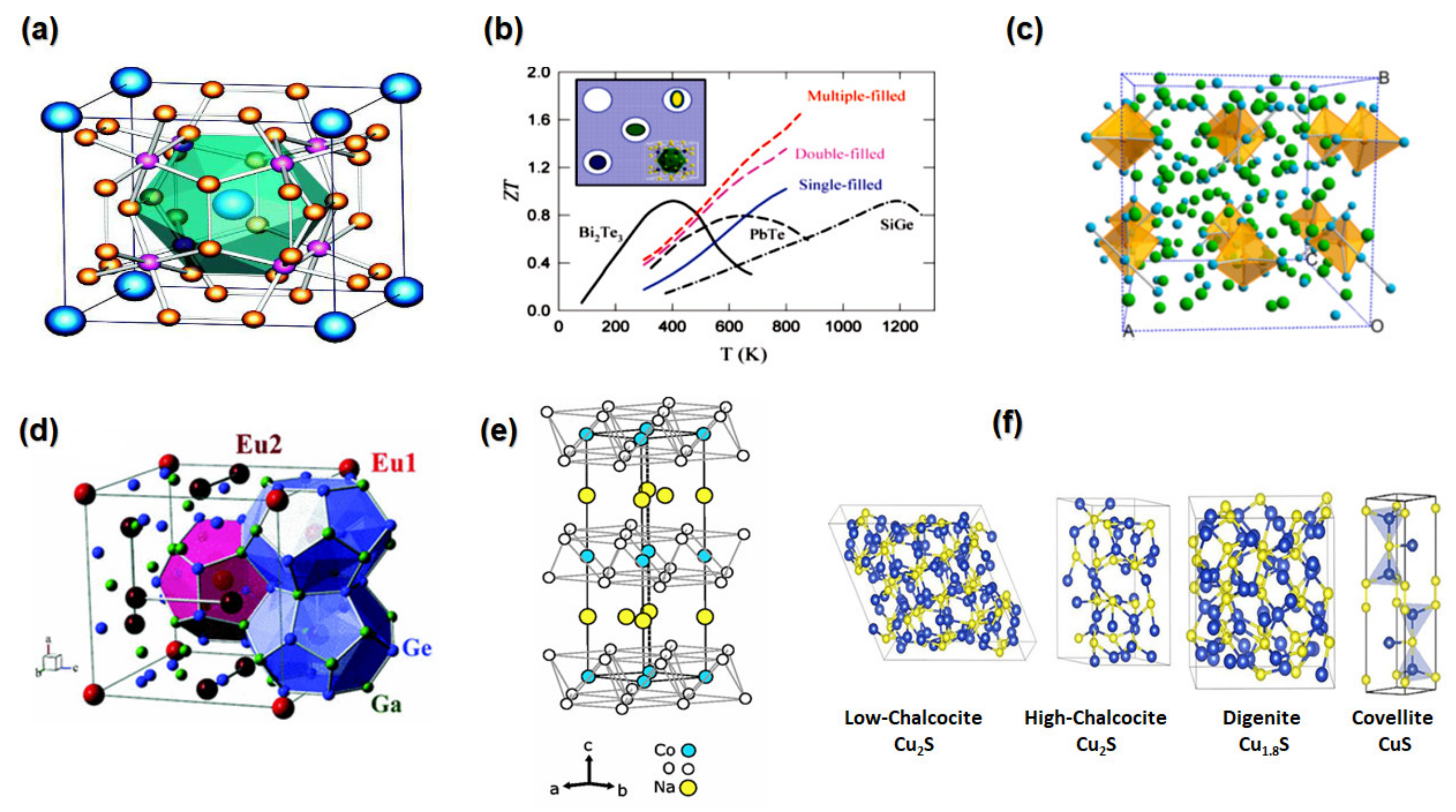
2.5. Skutterudites
2.6. Zintl Phase
2.7. Clathrates
2.8. Oxides
2.9. Metal Sulphides
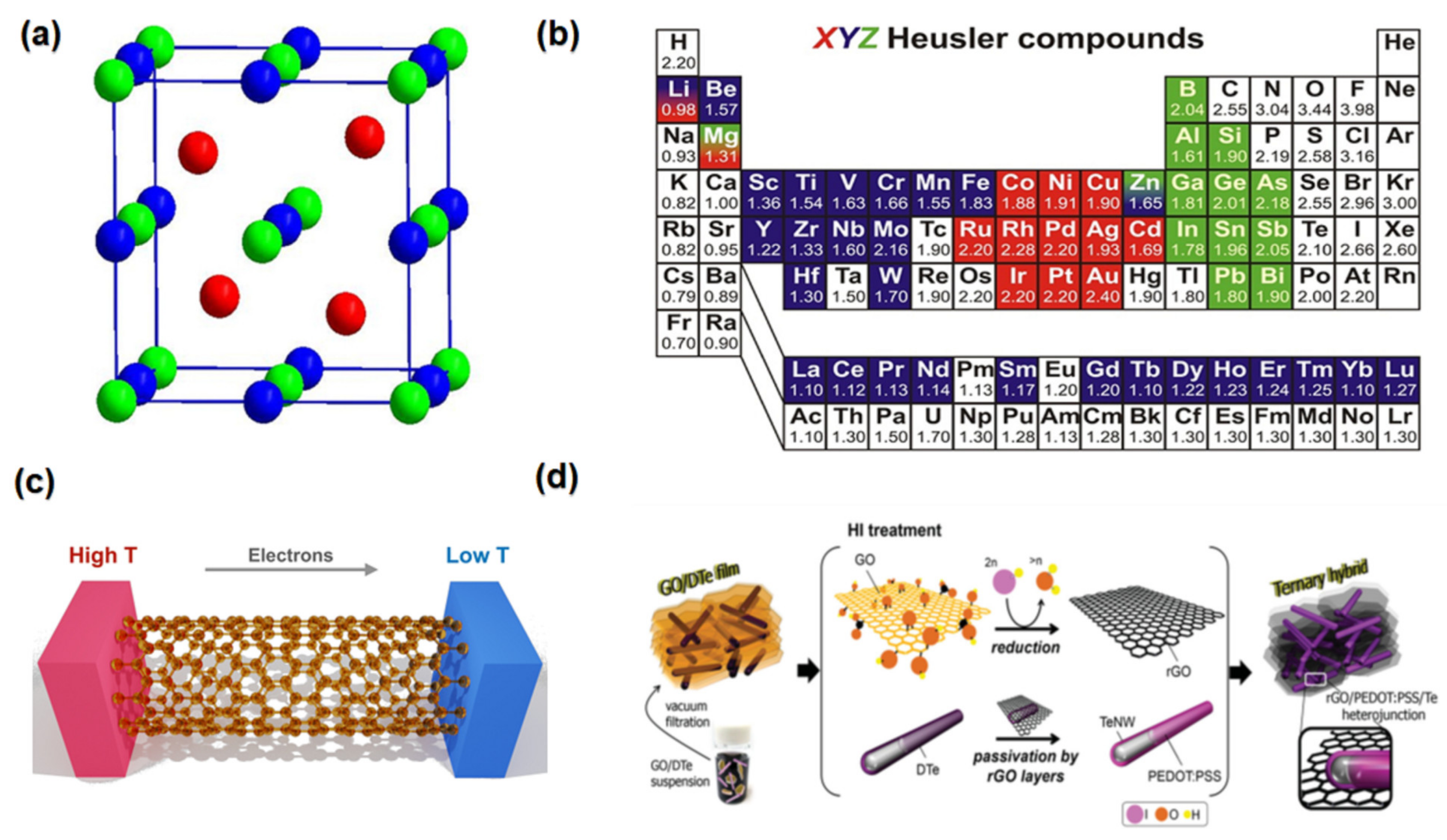
2.10. Half-Heusler Compounds
2.11. Carbon-Based Materials
2.12. Ternary Thermoelectric Composites
2.13. Perovskite Materials
3. Improvement Strategies for Thermoelectric Materials Performance
3.1. Alloying and Doping Approach
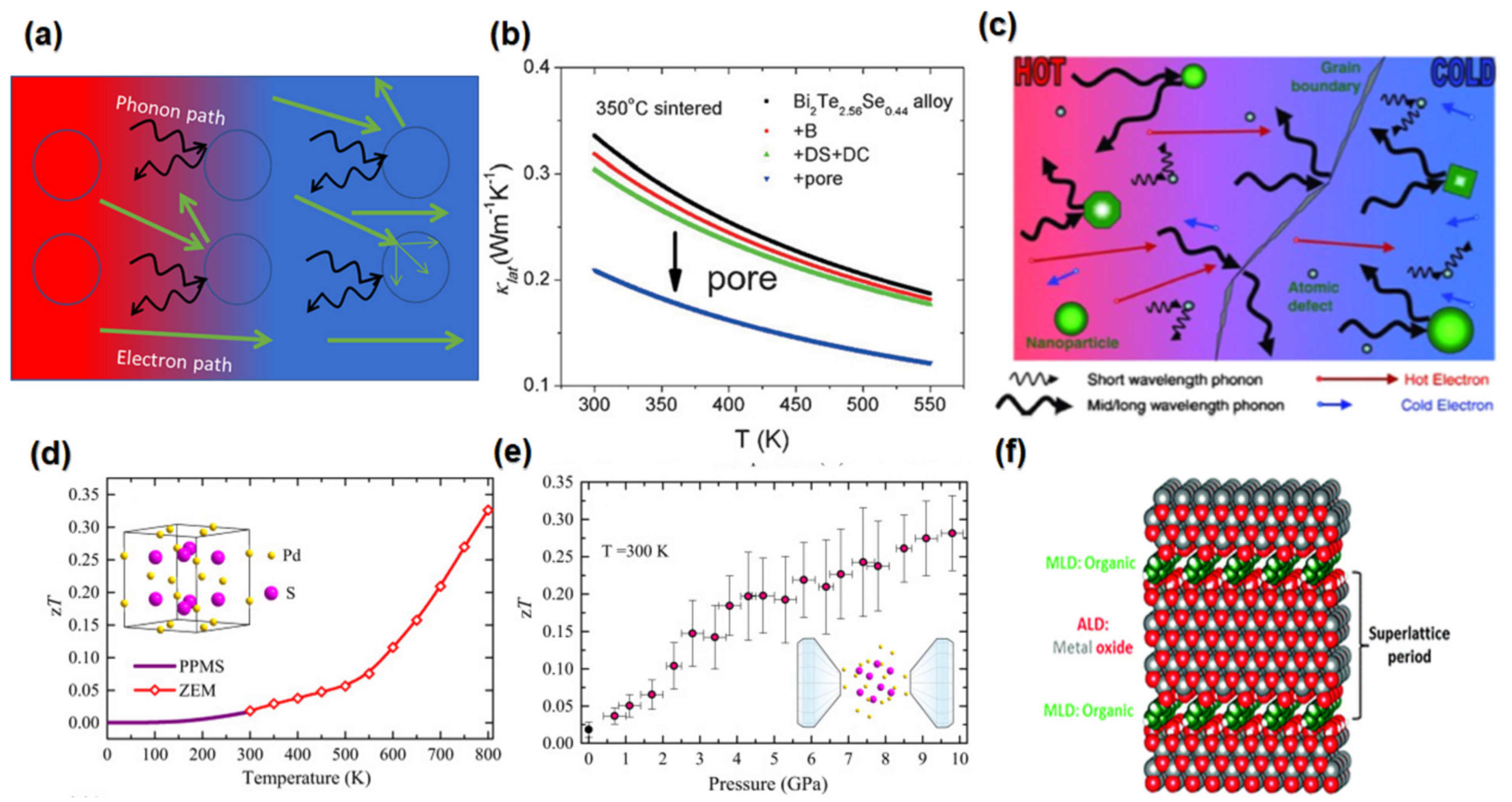
3.2. Porosity Incorporation
3.3. Heavy Element Addition
3.4. Band Engineering Approaches
3.5. Phonon-Glass Electron-Crystal (PGEC)
3.6. Pressure-Induced Approach
3.7. Superlattice Approach
3.8. Phase Transition
3.9. Nanostructuring Approach
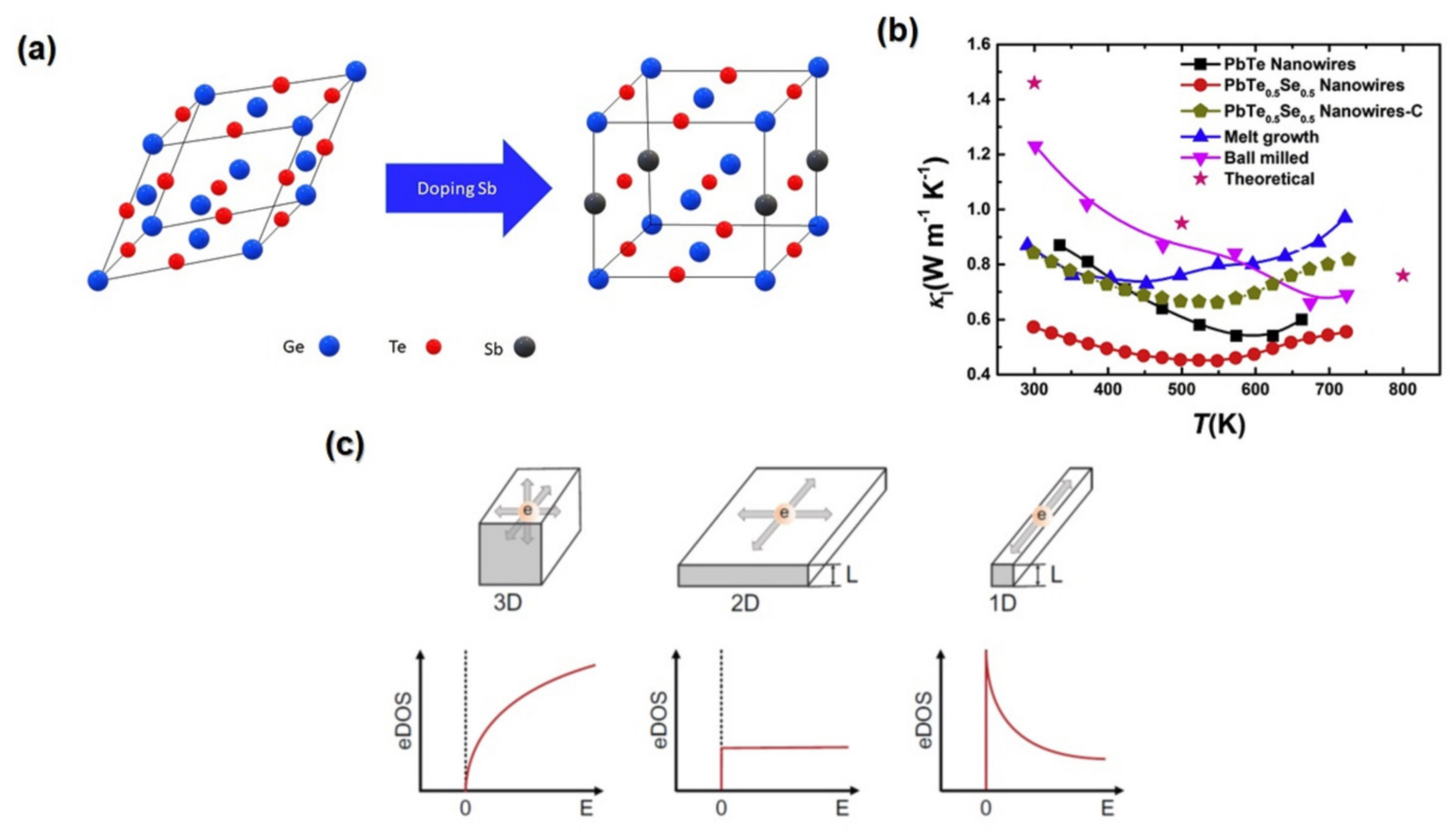
4. One-Dimensional Thermoelectric Materials
4.1. Configuration of One-Dimensional Thermoelectric Materials
4.1.1. Quantum Wires
4.1.2. Nanowires and Nanorods
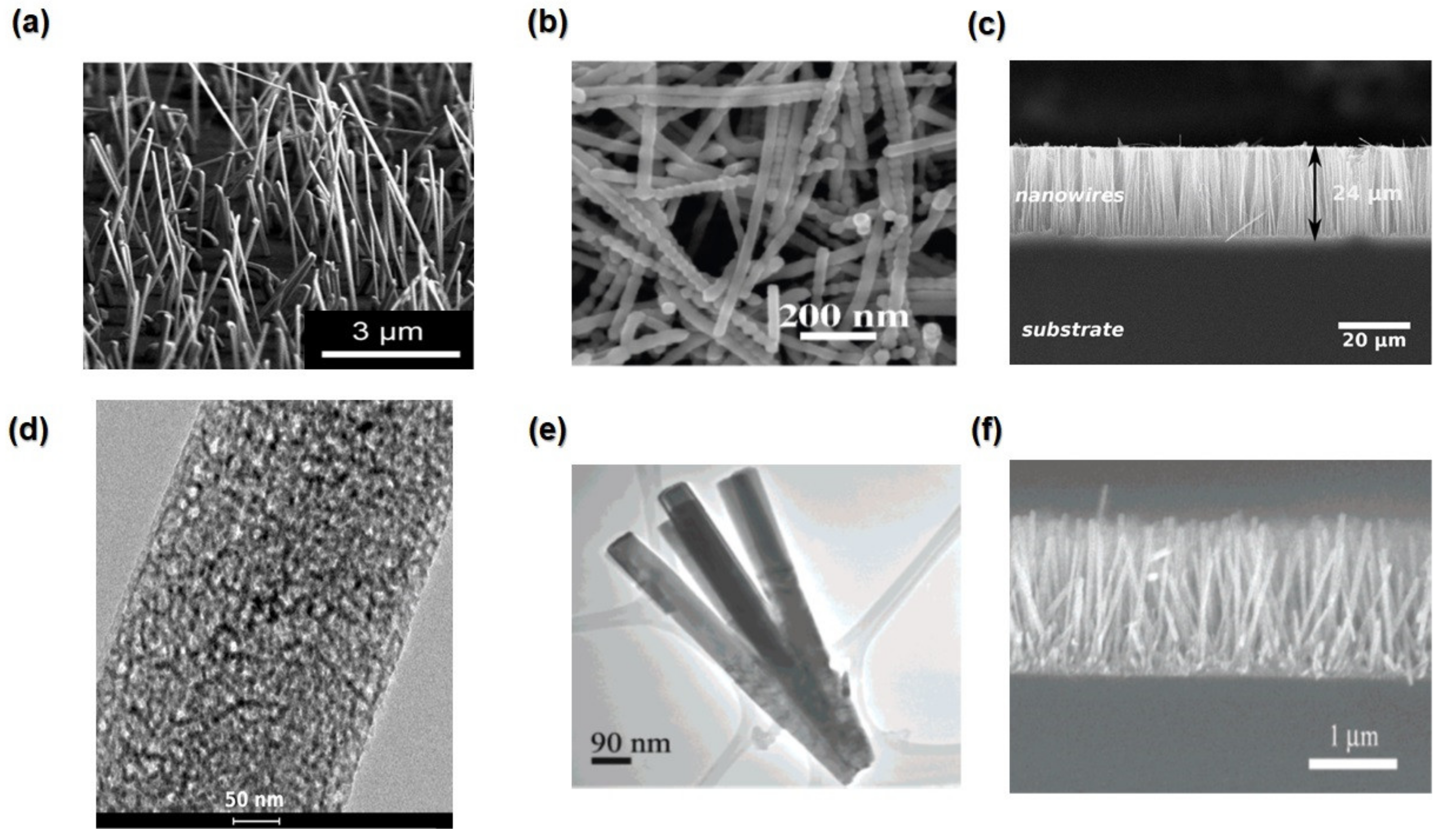
4.1.3. Nanofibers
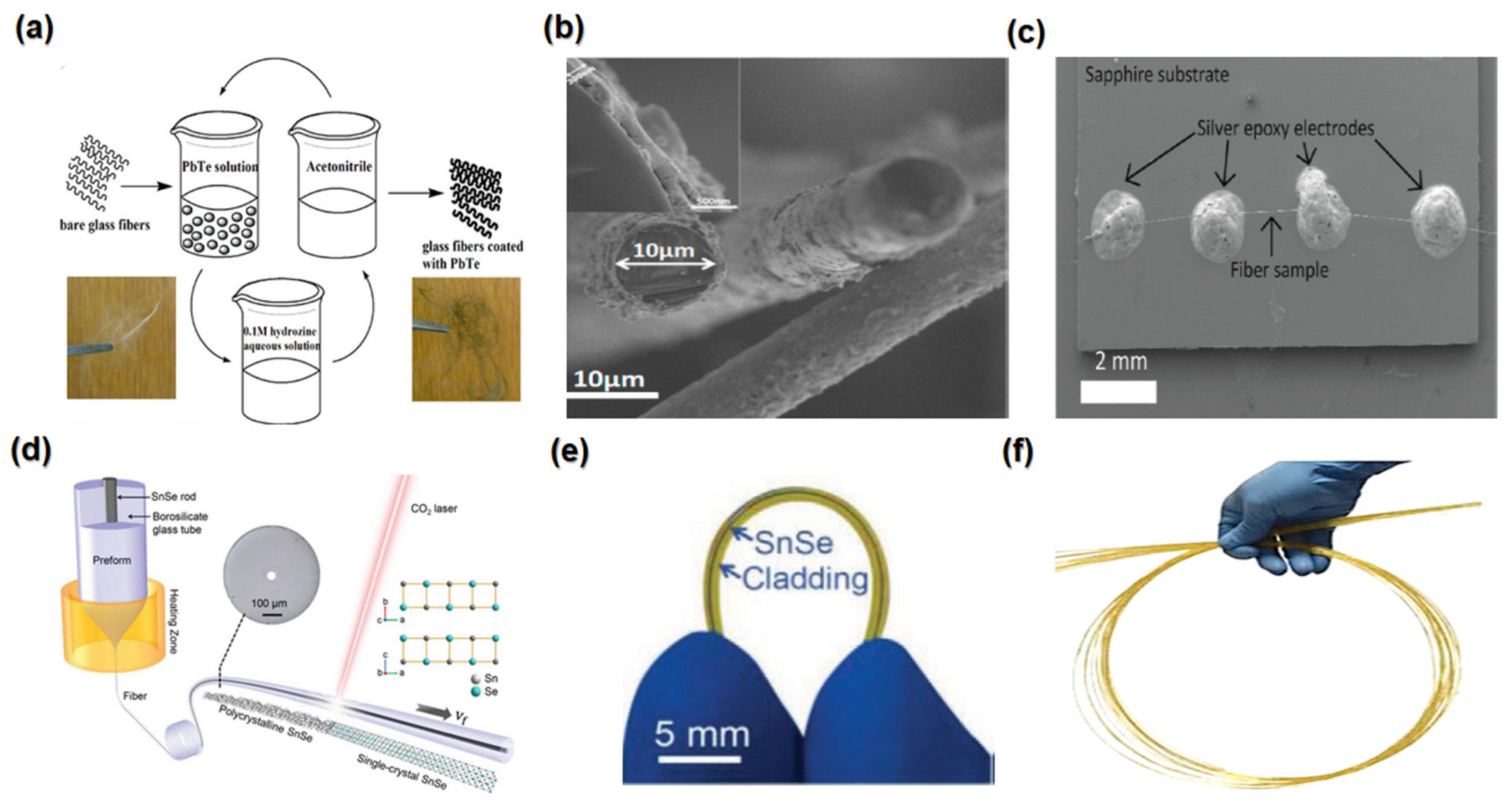
4.1.4. Heterostructures
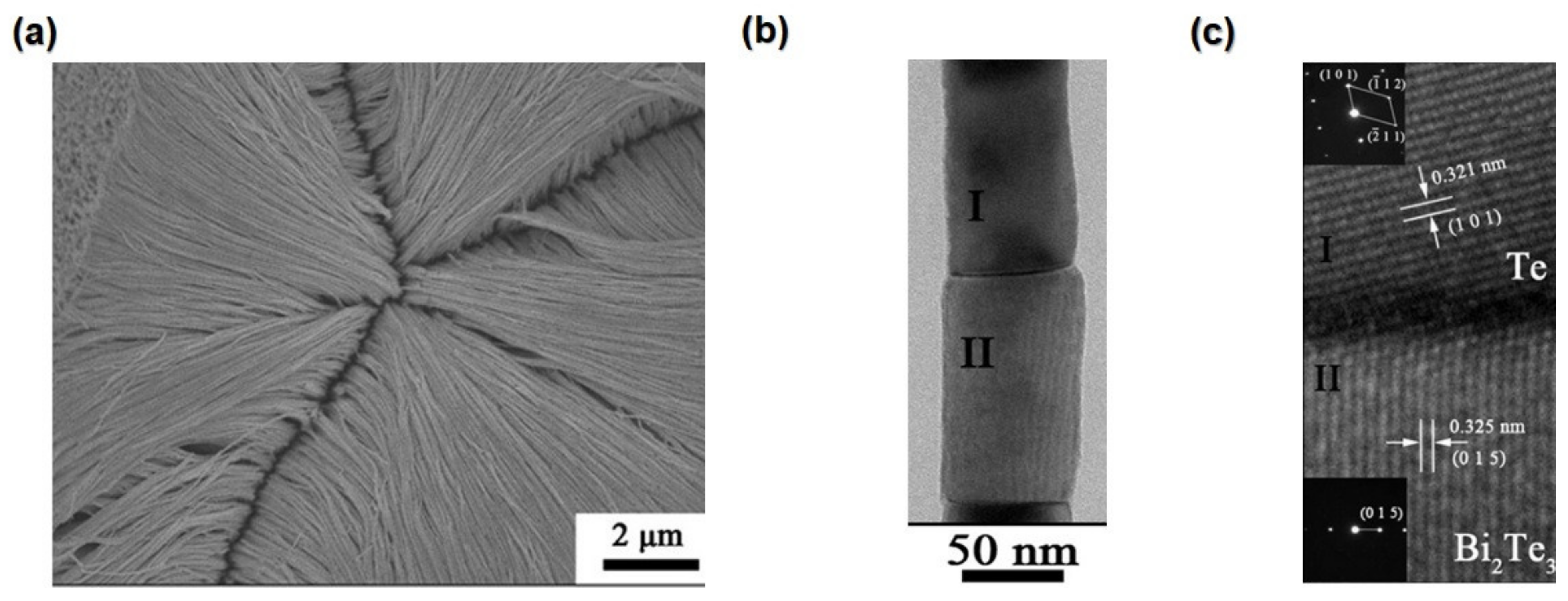
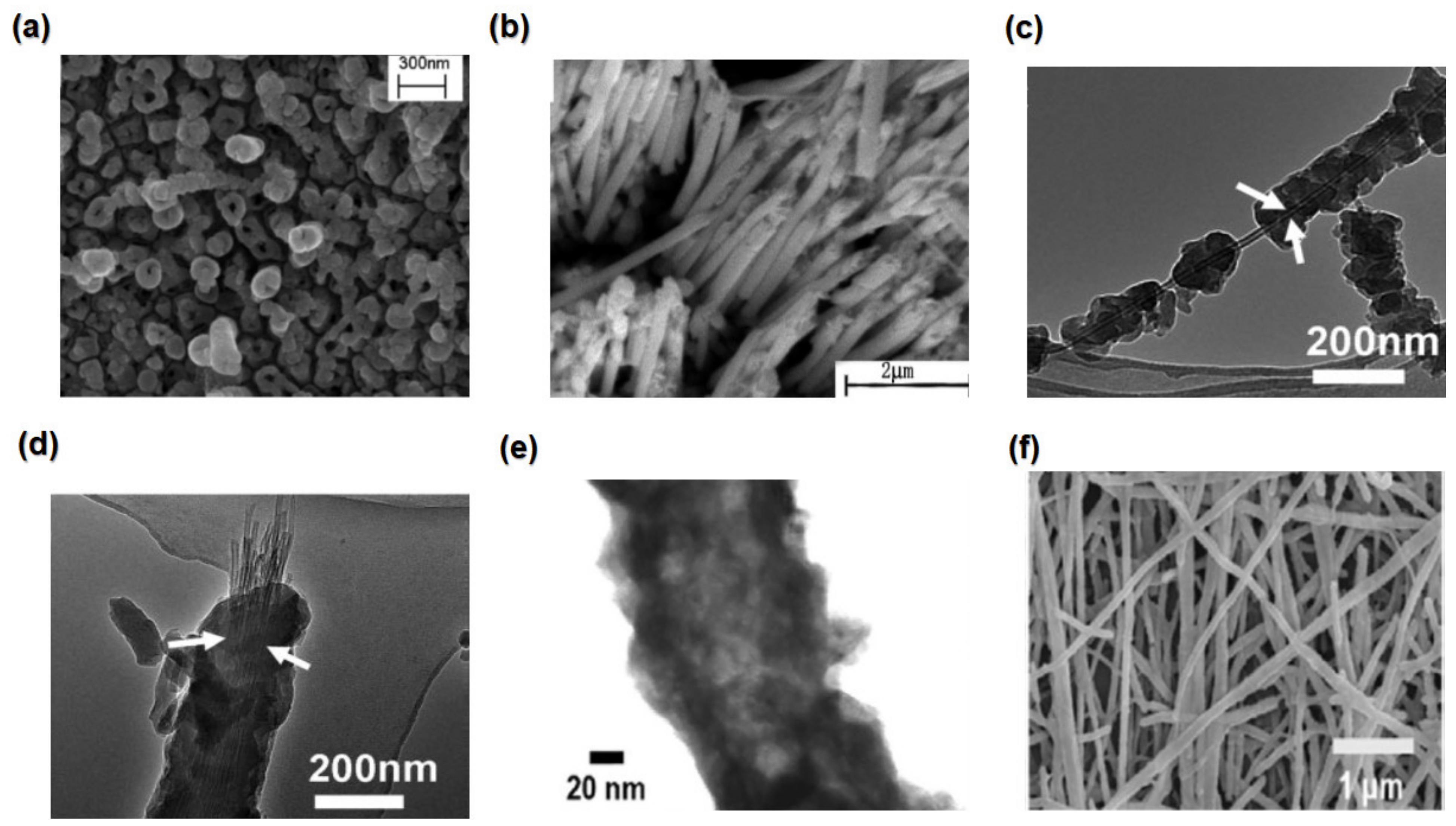
4.1.5. Nanotubes
4.2. The Effect of One-Dimensional Nanostructuring on the Enhancement of TE Generation Performance
4.2.1. Thermal Conductivity
4.2.2. Seebeck Coefficient
4.2.3. Power Factor
4.2.4. Electrical Conductivity
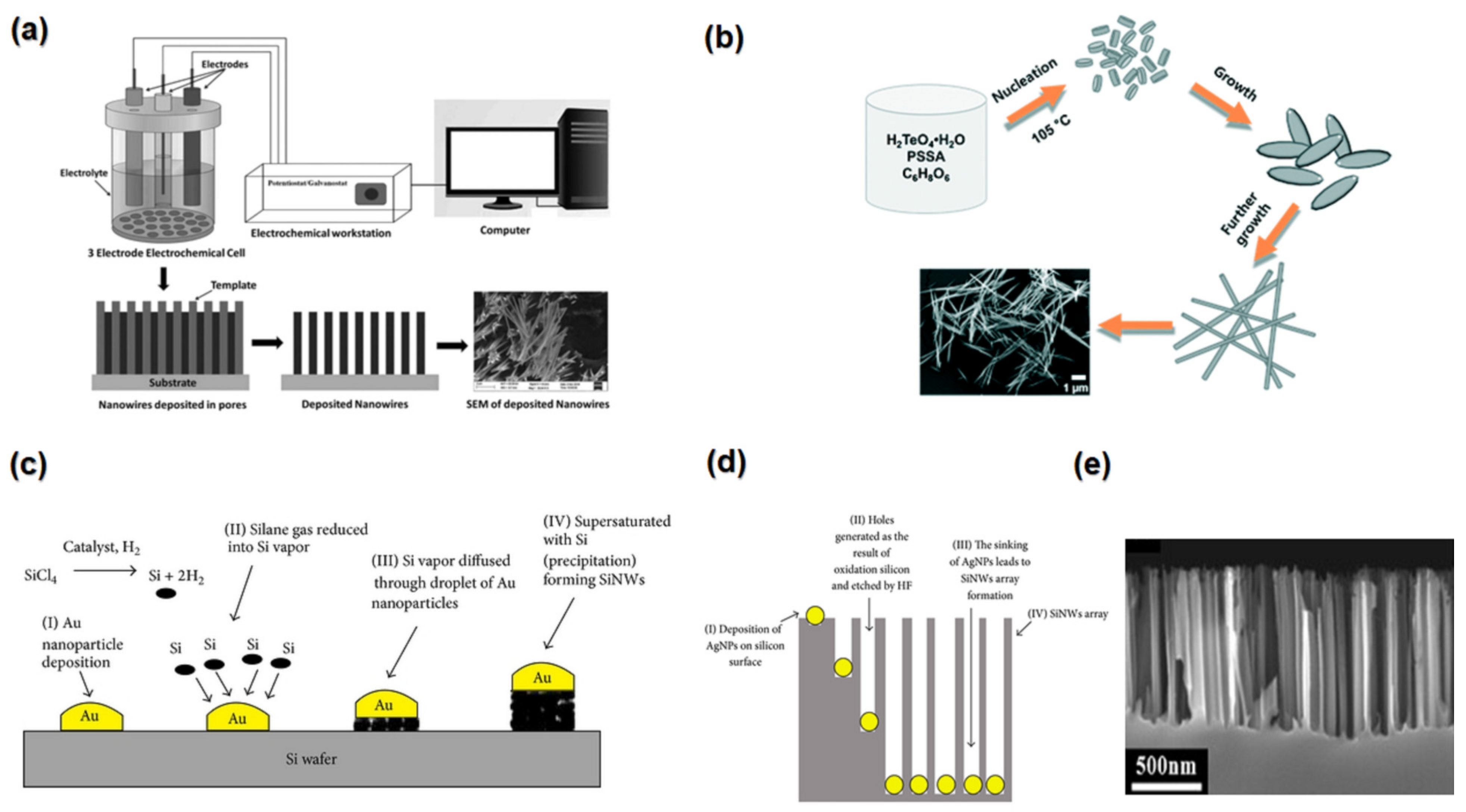
4.3. Synthesis Methods
4.3.1. Electrodeposition
4.3.2. Hydrothermal Synthesis
4.3.3. Vapor Liquid Solid Growth
4.3.4. Electroless Etching Method
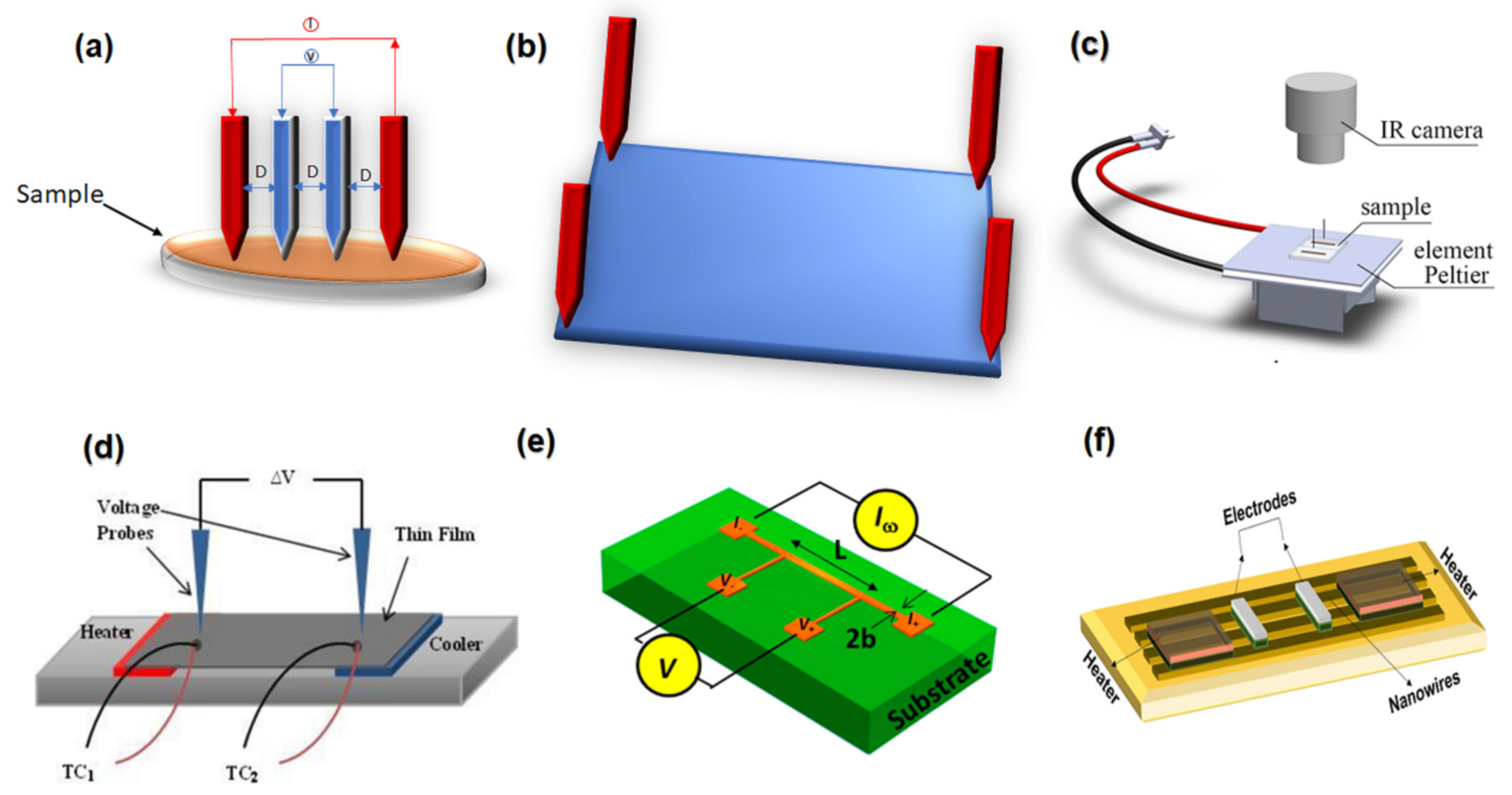
4.4. Performances of Characterizing Thermoelectric Nanowires
5. Summary and Future Scope
- Energy Conversion and Harvesting: TE materials can convert waste heat into useful electrical energy, providing a pathway for enhanced energy efficiency and sustainability. Further research can lead to developing high-performance TE materials that can efficiently harness waste heat from industrial processes, automotive systems, and renewable energy sources.
- Renewable Energy Integration: As the world moves towards a clean energy future, TE materials can be crucial in integrating renewable energy sources. By converting heat energy from concentrated solar power, geothermal sources, and biomass, TE devices can contribute to renewable energy systems’ overall efficiency and stability.
- Portable and Wearable Electronics: The miniaturization and increasing complexity of electronic devices pose challenges in terms of power supply. TE materials can enable the development of portable and wearable electronics that utilise body heat or ambient temperature variations to generate electricity, eliminating the need for conventional batteries and enhancing the device’s energy autonomy.
- Space Exploration and Remote Sensing: In space missions and remote sensing applications, where power sources are limited and inaccessible, TE materials can provide a reliable and efficient electricity generation. Further advancements in TE materials can significantly enhance the performance and reliability of power systems for space exploration and remote sensing technologies.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Symbols | |
| Specific Heat at constant volume | |
| The specific heat | |
| Thermal diffusivity | |
| e | Carrier charge |
| I | Current |
| Isc | Short circuit current |
| Electron energy | |
| Electron energy at the Fermi level | |
| The density of states | |
| Fermi function | |
| Planck’s constant | |
| Thermal conductivity (W/m·K) | |
| Electron thermal conductivity | |
| Lattice thermal conductivity | |
| Boltzmann constant | |
| L | Lorenz number |
| l | Phonons mean free path |
| 𝑛 | Carrier density |
| Valley degeneracy | |
| 𝑁𝑚* | Effective mass |
| m𝑣b* | Band-mass |
| QH | Thermal energy (hot side) (J) |
| S | Seebeck coefficient |
| T | Temperature (K) |
| Tc | The temperature on the cold side |
| Th | The temperature on the hot side |
| V | Voltage (V) |
| Voc | The open circuit voltage |
| W | Power generated (W) |
| Average Sound velocity | |
| σ | Electrical conductivity (S/m) |
| Electronic conductivity specified in terms of the Fermi energy | |
| η | Energy conversion efficiency |
| μ | Carrier mobility |
| Mass density | |
| Abbreviations | |
| AAO | anodic aluminium oxide |
| CNT | Carbon nanotube |
| MWCNT | Multi-wall carbon nanotube |
| NWs | Nanowires |
| PANI | Polyaniline |
| PEDOT: PSS | Poly(3,4-ethylenedioxythiophene): poly (styrene sulfonate) |
| PDLC | Polymer-dispersed liquid crystals |
| PGEC | Phonon glass electron crystal |
| PTENG | Photo-Thermoelectric Nanogenerator |
| rGO | Reduced graphene oxide |
| TCOs | Transparent conductive oxides |
| TCM | Thermochromic materials |
| TC | Transition temperature |
| TE | Thermoelectric |
| TEGs | Thermoelectric generators |
| TENG | Thermoelectric nanogenerator |
| SWCNT | Single-wall carbon nanotube |
| ZT | Figure of merit |
| 1D | One-dimensional |
References
- Eight Billion People, One Humanity; Development Milestone ‘Testament’ to Power of Health and Science. Available online: https://news.un.org/en/story/2022/11/1130417 (accessed on 16 November 2022).
- Tollefson, J. Carbon emissions hit new high: Warning from COP27. Nature 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Corbyn, Z. A Container-Sized Generator that Converts Waste Heat into Energy. Available online: https://www.theguardian.com/technology/2014/dec/07/1-alphabet-energy-generator-waste-heat-energy-oil-gas (accessed on 16 November 2022).
- Wang, C.; Tang, S.; Liu, X.; Su, G.; Tian, W.; Qiu, S. Experimental study on heat pipe thermoelectric generator for industrial high temperature waste heat recovery. Appl. Therm. Eng. 2020, 175, 115299. [Google Scholar] [CrossRef]
- Zhang, Y.; Cleary, M.; Wang, X.; Kempf, N.; Schoensee, L.; Yang, J.; Joshi, G.; Meda, L. High-temperature and high-power-density nanostructured thermoelectric generator for automotive waste heat recovery. Energy Convers. Manag. 2015, 105, 946–950. [Google Scholar] [CrossRef]
- Pei, Y.; LaLonde, A.; Iwanaga, S.; Snyder, G.J. High thermoelectric figure of merit in heavy hole dominated PbTe. Energy Environ. Sci. 2011, 4, 2085–2089. [Google Scholar] [CrossRef]
- Kim, M.-K.; Kim, M.-S.; Lee, S.; Kim, C.; Kim, Y.-J. Wearable thermoelectric generator for harvesting human body heat energy. Smart Mater. Struct. 2014, 23, 105002. [Google Scholar] [CrossRef]
- Karthick, K.; Suresh, S.; Hussain, M.M.M.; Ali, H.M.; Kumar, C.S. Evaluation of solar thermal system configurations for thermoelectric generator applications: A critical review. Sol. Energy 2019, 188, 111–142. [Google Scholar] [CrossRef]
- Hu, B.; Shi, X.-L.; Zou, J.; Chen, Z.-G. Thermoelectrics for medical applications: Progress, challenges, and perspectives. Chem. Eng. J. 2022, 437, 135268. [Google Scholar] [CrossRef]
- Al-Fartoos, M.M.R.; Roy, A.; Mallick, T.K.; Tahir, A.A. A Short Review on Thermoelectric Glazing for Sustainable Built Environment. Energies 2022, 15, 9589. [Google Scholar] [CrossRef]
- Patyk, A. Thermoelectrics: Impacts on the Environment and Sustainability. J. Electron. Mater. 2010, 39, 2023–2028. [Google Scholar] [CrossRef]
- Finn, P.A.; Asker, C.; Wan, K.; Bilotti, E.; Fenwick, O.; Nielsen, C.B. Thermoelectric Materials: Current Status and Future Challenges. Front. Electron. Mater. 2021, 1, 677845. [Google Scholar] [CrossRef]
- Zulkepli, N.; Yunas, J.; Mohamed, M.A.; Hamzah, A.A. Review of Thermoelectric Generators at Low Operating Temperatures: Working Principles and Materials. Micromachines 2021, 12, 734. [Google Scholar] [CrossRef] [PubMed]
- Vining, C.B. An inconvenient truth about thermoelectrics. Nat. Mater. 2009, 8, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Web of Science Core Collection. Available online: https://clarivate.com/products/scientific-and-academic-research/research-discovery-and-workflow-solutions/webofscience-platform/web-of-science-core-collection/ (accessed on 29 June 2023).
- Korzhuyev, M.; Katin, I. Formal analysis of Galvani and Volta experiments related to thermoelectricity. J. Thermoelectr. 2009, 1, 11–17. [Google Scholar]
- Gogoc, S.; Data, P. Organic Thermoelectric Materials as the Waste Heat Remedy. Molecules 2022, 27, 1016. [Google Scholar] [CrossRef]
- Rowe, D.M. Thermoelectrics Handbook: Macro to Nano; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Zabrocki, C.K.; Goupil, H.; Ouerdane, Y.; Apertet, W.; Seifert, M.E. Continuum Theory and Modeling of Thermoelectric Elements; Goupil, C., Ed.; Wiley-VCH: Berlin, Germany, 2016; pp. 75–156. ISBN 3527687882. [Google Scholar]
- Goupil, C.; Seifert, W.; Zabrocki, K.; Müller, E.; Snyder, G.J. Thermodynamics of Thermoelectric Phenomena and Applications. Entropy 2011, 13, 1481–1517. [Google Scholar] [CrossRef]
- Eucken, A.; Kuhn, G. New Measurement of heat of conductivity of solid crystalline substances at 0 and–190 C. Z. Physik. Chem. A 1928, 134, 193–219. [Google Scholar] [CrossRef]
- Telkes, M. The Efficiency of Thermoelectric Generators. I. J. Appl. Phys. 1947, 18, 1116–1127. [Google Scholar] [CrossRef]
- Slack, G.A.; Rowe, D. CRC Handbook of Thermoelectrics; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Tritt, T.M. Semiconductors and Semimetals; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Nolas, G.S.; Slack, G.A.; Morelli, D.T.; Tritt, T.M.; Ehrlich, A.C. The effect of rare-earth filling on the lattice thermal conductivity of skutterudites. J. Appl. Phys. 1996, 79, 4002. [Google Scholar] [CrossRef]
- Sales, B.C.; Mandrus, D.; Williams, R.K. Filled Skutterudite Antimonides: A New Class of Thermoelectric Materials. Science 1996, 272, 1325–1328. [Google Scholar] [CrossRef]
- Morelli, D.T.; Meisner, G.P. Low temperature properties of the filled skutterudite CeFe4Sb12. J. Appl. Phys. 1995, 77, 3777–3781. [Google Scholar] [CrossRef]
- Hicks, L.D.; Dresselhaus, M.S. Thermoelectric figure of merit of a one-dimensional conductor. Phys. Rev. B 1993, 47, 16631–16634. [Google Scholar] [CrossRef] [PubMed]
- Hicks, L.D.; Dresselhaus, M.S. Effect of quantum-well structures on the thermoelectric figure of merit. Phys. Rev. B 1993, 47, 12727–12731. [Google Scholar] [CrossRef]
- Tohidi, F.; Holagh, S.G.; Chitsaz, A. Thermoelectric Generators: A comprehensive review of characteristics and applications. Appl. Therm. Eng. 2022, 201, 117793. [Google Scholar] [CrossRef]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.; Mahmood, Q.; Rashid, M.; Haq, B.U.; Laref, A.; Ahmad, S. Ab-initio study of thermodynamic stability, thermoelectric and optical properties of perovskites ATiO3 (A = Pb, Sn). J. Solid State Chem. 2018, 263, 115–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Heo, Y.-J.; Park, M.; Park, S.-J. Recent Advances in Organic Thermoelectric Materials: Principle Mechanisms and Emerging Carbon-Based Green Energy Materials. Polymers 2019, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, K.; Takagiwa, Y.; Shi, X. Thermoelectric Materials: Principles and Concepts for Enhanced Properties; De Gruyter: Berlin, Germany, 2020. [Google Scholar]
- Chen, Y.; Zhao, Y.; Liang, Z. Solution processed organic thermoelectrics: Towards flexible thermoelectric modules. Energy Environ. Sci. 2015, 8, 401–422. [Google Scholar] [CrossRef]
- Sootsman, J.R.; Chung, D.Y.; Kanatzidis, M.G. New and Old Concepts in Thermoelectric Materials. Angew. Chem. Int. Ed. 2009, 48, 8616–8639. [Google Scholar] [CrossRef]
- Rowe, D.; Min, G. An alpha-In sigma plot as a thermoelectric material performance indicator. J. Mater. Sci. Lett. 1995, 14, 617–619. [Google Scholar] [CrossRef]
- Edginton, R.S.; Mattana, S.; Caponi, S.; Fioretto, D.; Green, E.; Winlove, C.P.; Palombo, F. Preparation of extracellular matrix protein fibers for Brillouin spectroscopy. JoVE (J. Vis. Exp.) 2016, 115, e54648. [Google Scholar]
- Blakemore, J.S. Solid State Physics; Cambridge University Press: Cambridge, UK, 1985. [Google Scholar]
- Lv, S.; Qian, Z.; Hu, D.; Li, X.; He, W. A Comprehensive Review of Strategies and Approaches for Enhancing the Performance of Thermoelectric Module. Energies 2020, 13, 3142. [Google Scholar] [CrossRef]
- Ravindra, N.; Jariwala, B.; Bañobre, A.; Maske, A. Thermoelectrics: Fundamentals, Materials Selection, Properties, and Performance; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Shi, X.; Zou, J.; Chen, Z.-G. Advanced Thermoelectric Design: From Materials and Structures to Devices. Chem. Rev. 2020, 120, 7399–7515. [Google Scholar] [CrossRef] [PubMed]
- Zakharchuk, K.V.; Tobaldi, D.M.; Xiao, X.; Xie, W.; Mikhalev, S.M.; Martins, J.F.; Frade, J.R.; Weidenkaff, A.; Kovalevsky, A.V. Synergistic effects of zirconium- and aluminum co-doping on the thermoelectric performance of zinc oxide. J. Eur. Ceram. Soc. 2019, 39, 1222–1229. [Google Scholar] [CrossRef]
- Zaferani, S.H.; Ghomashchi, R.; Vashaee, D. Strategies for engineering phonon transport in Heusler thermoelectric compounds. Renew. Sustain. Energy Rev. 2019, 112, 158–169. [Google Scholar] [CrossRef]
- Khan, A.U.; Kobayashi, K.; Tang, D.-M.; Yamauchi, Y.; Hasegawa, K.; Mitome, M.; Xue, Y.; Jiang, B.; Tsuchiya, K.; Golberg, D.; et al. Nano-micro-porous skutterudites with 100% enhancement in ZT for high performance thermoelectricity. Nano Energy 2017, 31, 152–159. [Google Scholar] [CrossRef]
- Kim, W. Strategies for engineering phonon transport in thermoelectrics. J. Mater. Chem. C 2015, 3, 10336–10348. [Google Scholar] [CrossRef]
- Cha, J.; Kim, D.; Hong, H.; Kim, G.; Park, K. Effect of La3+ substitution on the structural and thermoelectric properties of Ca3−xLaxCo4O9+δ. J. Eur. Ceram. Soc. 2019, 39, 3320–3326. [Google Scholar] [CrossRef]
- Liu, W.; Yan, X.; Chen, G.; Ren, Z. Recent advances in thermoelectric nanocomposites. Nano Energy 2012, 1, 42–56. [Google Scholar] [CrossRef]
- Mulla, R.; Rabinal, M.H.K. Copper sulfides: Earth-abundant and low-cost thermoelectric materials. Energy Technol. 2019, 7, 1800850. [Google Scholar] [CrossRef]
- Selvan, K.V.; Hasan, N.; Ali, M.S.M. State-of-the-Art Reviews and Analyses of Emerging Research Findings and Achievements of Thermoelectric Materials over the Past Years. J. Electron. Mater. 2019, 48, 745–777. [Google Scholar] [CrossRef]
- Witting, I.T.; Chasapis, T.C.; Ricci, F.; Peters, M.; Heinz, N.A.; Hautier, G.; Snyder, G.J. The Thermoelectric Properties of Bismuth Telluride. Adv. Electron. Mater. 2019, 5, 1800904. [Google Scholar] [CrossRef]
- Zahid, F.; Lake, R. Thermoelectric properties of Bi2Te3 atomic quintuple thin films. Appl. Phys. Lett. 2010, 97, 212102. [Google Scholar] [CrossRef]
- Fan, S.; Zhao, J.; Guo, J.; Yan, Q.; Ma, J.; Hng, H.H. p-type Bi0.4Sb1.6Te3 nanocomposites with enhanced figure of merit. Appl. Phys. Lett. 2010, 96, 182104. [Google Scholar] [CrossRef]
- Zhou, J.; Jin, C.; Seol, J.H.; Li, X.; Shi, L. Thermoelectric properties of individual electrodeposited bismuth telluride nanowires. Appl. Phys. Lett. 2005, 87, 133109. [Google Scholar] [CrossRef]
- Chen, L.; Liu, R.; Shi, X. Thermoelectric Materials and Devices; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Zhang, Q.; Wang, H.; Liu, W.; Wang, H.; Yu, B.; Zhang, Q.; Tian, Z.; Ni, G.; Lee, S.; Esfarjani, K.; et al. Enhancement of thermoelectric figure-of-merit by resonant states of aluminium doping in lead selenide. Energy Environ. Sci. 2011, 5, 5246–5251. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, L.-D.; Tong, X.; Li, W.; Wu, L.; Tan, Q.; Pei, Y.; Huang, L.; Li, J.-F.; Zhu, Y.; et al. Superior thermoelectric performance in PbTe–PbS pseudo-binary: Extremely low thermal conductivity and modulated carrier concentration. Energy Environ. Sci. 2015, 8, 2056–2068. [Google Scholar] [CrossRef]
- Tan, G.; Shi, F.; Hao, S.; Zhao, L.-D.; Chi, H.; Zhang, X.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Non-equilibrium processing leads to record high thermoelectric figure of merit in PbTe–SrTe. Nat. Commun. 2016, 7, 12167. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Rabin, O.; Hochbaum, A.I.; Fardy, M.; Zhang, M.; Yang, P. Thermoelectric properties of p-type PbSe nanowires. Nano Res. 2009, 2, 394–399. [Google Scholar] [CrossRef]
- Sharma, P.K.; Senguttuvan, T.; Sharma, V.K.; Chaudhary, S. Revisiting the thermoelectric properties of lead telluride. Mater. Today Energy 2021, 21, 100713. [Google Scholar] [CrossRef]
- Kim, H.; Kaviany, M. Effect of thermal disorder on high figure of merit in PbTe. Phys. Rev. B 2012, 86, 045213. [Google Scholar] [CrossRef]
- Chen, Z.-G.; Shi, X.; Zhao, L.-D.; Zou, J. High-performance SnSe thermoelectric materials: Progress and future challenge. Prog. Mater. Sci. 2018, 97, 283–346. [Google Scholar] [CrossRef]
- Kasper, E.; Herzog, H.J. 1—Structural properties of silicon–germanium (SiGe) nanostructures. In Silicon–Germanium (SiGe) Nanostructures; Shiraki, Y., Usami, N., Eds.; Woodhead Publishing: Soston, UK, 2011; pp. 3–25. [Google Scholar]
- Zhou, M.; Snyder, G.J.; Li, L.; Zhao, L.-D. Lead-free tin chalcogenide thermoelectric materials. Inorg. Chem. Front. 2016, 3, 1449–1463. [Google Scholar] [CrossRef]
- Zhao, L.-D.; Lo, S.-H.; Zhang, Y.; Sun, H.; Tan, G.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature 2014, 508, 373–377. [Google Scholar] [CrossRef]
- Erdemir, A. Crystal Chemistry and Solid Lubricating Properties of the Monochalcogenides Gallium Selenide and Tin Selenide. Tribol. Trans. 1994, 37, 471–478. [Google Scholar] [CrossRef]
- Duong, A.T.; Nguyen, V.Q.; Duvjir, G.; Duong, V.T.; Kwon, S.; Song, J.Y.; Lee, J.K.; Lee, J.E.; Park, S.; Min, T.; et al. Achieving ZT=2.2 with Bi-doped n-type SnSe single crystals. Nat. Commun. 2016, 7, 13713. [Google Scholar] [CrossRef]
- Peng, K.; Lu, X.; Zhan, H.; Hui, S.; Tang, X.; Wang, G.; Dai, J.; Uher, C.; Wang, G.; Zhou, X. Broad temperature plateau for high ZT s in heavily doped p-type SnSe single crystals. Energy Environ. Sci. 2016, 9, 454–460. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Lee, H.; Kim, T.; Byun, S.; Lee, Y.K.; Jang, S.; Chung, I.; Chang, H.; Im, J. Data-Driven Enhancement of ZT in SnSe-Based Thermoelectric Systems. J. Am. Chem. Soc. 2022, 144, 13748–13763. [Google Scholar] [CrossRef] [PubMed]
- The Paris Agreement. Available online: https://www.un.org/en/climatechange/paris-agreement (accessed on 4 September 2022).
- Zhang, Q.; Chere, E.K.; Sun, J.; Cao, F.; Dahal, K.; Chen, S.; Chen, G.; Ren, Z. Studies on Thermoelectric Properties of n-type Polycrystalline SnSe1−xSx by Iodine Doping. Adv. Energy Mater. 2015, 5, 1500360. [Google Scholar] [CrossRef]
- Ge, Z.; Qiu, Y.; Chen, Y.; Chong, X.; Feng, J.; Liu, Z.; He, J. Multipoint Defect Synergy Realizing the Excellent Thermoelectric Performance of n-Type Polycrystalline SnSe via Re Doping. Adv. Funct. Mater. 2019, 29, 1902893. [Google Scholar] [CrossRef]
- Zhou, C.; Lee, Y.K.; Yu, Y.; Byun, S.; Luo, Z.-Z.; Lee, H.; Ge, B.; Lee, Y.-L.; Chen, X.; Lee, J.Y. Polycrystalline SnSe with a thermoelectric figure of merit greater than the single crystal. Nat. Mater. 2021, 20, 1378–1384. [Google Scholar] [CrossRef]
- Hernandez, J.A.; Ruiz, A.; Fonseca, L.F.; Pettes, M.T.; Jose-Yacaman, M.; Benitez, A. Thermoelectric properties of SnSe nanowires with different diameters. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aspan, R.M.; Fatima, N.; Mohamed, R.; Syafiq, U.; Ibrahim, M.A. An Overview of the Strategies for Tin Selenide Advancement in Thermoelectric Application. Micromachines 2021, 12, 1463. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Lee, H.; Lan, Y.C.; Zhu, G.H.; Joshi, G.; Wang, D.Z.; Yang, J.; Muto, A.J.; Tang, M.Y.; Klatsky, J.; et al. Enhanced thermoelectric figure of merit in nanostructured n-type silicon germanium bulk alloy. Appl. Phys. Lett. 2008, 93, 193121. [Google Scholar] [CrossRef]
- Joshi, G.; Lee, H.; Lan, Y.; Wang, X.; Zhu, G.; Wang, D.; Gould, R.W.; Cuff, D.C.; Tang, M.Y.; Dresselhaus, M.S.; et al. Enhanced Thermoelectric Figure-of-Merit in Nanostructured p-type Silicon Germanium Bulk Alloys. Nano Lett. 2008, 8, 4670–4674. [Google Scholar] [CrossRef] [PubMed]
- Vining, C.B. A model for the high-temperature transport properties of heavily doped n-type silicon-germanium alloys. J. Appl. Phys. 1991, 69, 331–341. [Google Scholar] [CrossRef]
- Nozariasbmarz, A.; Roy, P.; Zamanipour, Z.; Dycus, J.H.; Cabral, M.J.; LeBeau, J.M.; Krasinski, J.S.; Vashaee, D. Comparison of thermoelectric properties of nanostructured Mg2Si, FeSi2, SiGe, and nanocomposites of SiGe–Mg2Si, SiGe–FeSi2. APL Mater. 2016, 4, 104814. [Google Scholar] [CrossRef]
- Lee, E.K.; Yin, L.; Lee, Y.; Lee, J.W.; Lee, S.J.; Lee, J.; Cha, S.N.; Whang, D.; Hwang, G.S.; Hippalgaonkar, K.; et al. Large Thermoelectric Figure-of-Merits from SiGe Nanowires by Simultaneously Measuring Electrical and Thermal Transport Properties. Nano Lett. 2012, 12, 2918–2923. [Google Scholar] [CrossRef]
- Ahmad, S.; Singh, A.; Bohra, A.; Basu, R.; Bhattacharya, S.; Bhatt, R.; Meshram, K.; Roy, M.; Sarkar, S.K.; Hayakawa, Y.; et al. Boosting thermoelectric performance of p-type SiGe alloys through in-situ metallic YSi2 nanoinclusions. Nano Energy 2016, 27, 282–297. [Google Scholar] [CrossRef]
- Basu, R.; Singh, A. High temperature Si–Ge alloy towards thermoelectric applications: A comprehensive review. Mater. Today Phys. 2021, 21, 100468. [Google Scholar] [CrossRef]
- Morelli, D.T.; Meisner, G.P.; Chen, B.; Hu, S.; Uher, C. Cerium filling and doping of cobalt triantimonide. Phys. Rev. B 1997, 56, 7376–7383. [Google Scholar] [CrossRef]
- Caillat, T.; Fleurial, J.-P.; Borshchevsky, A. Bridgman-solution crystal growth and characterization of the skutterudite compounds CoSb3 and RhSb3. J. Cryst. Growth 1996, 166, 722–726. [Google Scholar] [CrossRef]
- Bin Masood, K.; Kumar, P.; Singh, R.A.; Singh, J. Odyssey of thermoelectric materials: Foundation of the complex structure. J. Phys. Commun. 2018, 2, 062001. [Google Scholar] [CrossRef]
- Zhou, A.; Liu, L.-S.; Zhai, P.-C.; Zhao, W.-Y.; Zhang, Q.-J. Electronic Structures and Transport Properties of Single-Filled CoSb3. J. Electron. Mater. 2010, 39, 1832–1836. [Google Scholar] [CrossRef]
- Shi, X.; Yang, J.; Salvador, J.R.; Chi, M.; Cho, J.Y.; Wang, H.; Bai, S.; Yang, J.; Zhang, W.; Chen, L. Multiple-Filled Skutterudites: High Thermoelectric Figure of Merit through Separately Optimizing Electrical and Thermal Transports. J. Am. Chem. Soc. 2011, 133, 7837–7846. [Google Scholar] [CrossRef] [PubMed]
- Kauzlarich, S.M.; Zevalkink, A.; Toberer, E.; Snyder, G.J. Chapter 1 Zintl Phases: Recent Developments in Thermoelectrics and Future Outlook. In Thermoelectric Materials and Devices; Nandhakumar, I.N.M.W., Beeby, S., Eds.; The Royal Society of Chemistry: London, UK, 2017; pp. 1–26. [Google Scholar]
- Toberer, E. May AF Snyder GJ Chem. Mater 2010, 22, 624–634. [Google Scholar]
- Toberer, E.S.; Cox, C.A.; Brown, S.R.; Ikeda, T.; May, A.F.; Kauzlarich, S.M.; Snyder, G.J. Traversing the metal-insulator transition in a Zintl phase: Rational enhancement of thermoelectric efficiency in Yb14Mn1−xAlxSb11. Adv. Funct. Mater. 2008, 18, 2795–2800. [Google Scholar] [CrossRef]
- Kastbjerg, S.; Uvarov, C.A.; Kauzlarich, S.M.; Nishibori, E.; Spackman, M.A.; Iversen, B.B. Multi-temperature Synchrotron Powder X-ray Diffraction Study and Hirshfeld Surface Analysis of Chemical Bonding in the Thermoelectric Zintl Phase Yb14MnSb11. Chem. Mater. 2011, 23, 3723–3730. [Google Scholar] [CrossRef]
- Brown, S.R.; Kauzlarich, S.M.; Gascoin, F.; Snyder, G.J. Yb14MnSb11: New High Efficiency Thermoelectric Material for Power Generation. Cheminform 2006, 37, 1873–1877. [Google Scholar] [CrossRef]
- Grebenkemper, J.H.; Hu, Y.; Barrett, D.; Gogna, P.; Huang, C.-K.; Bux, S.K.; Kauzlarich, S.M. High Temperature Thermoelectric Properties of Yb14MnSb11 Prepared from Reaction of MnSb with the Elements. Chem. Mater. 2015, 27, 5791–5798. [Google Scholar] [CrossRef]
- Prytz, O.; Flage-Larsen, E.; Toberer, E.S.; Snyder, G.J.; Taftø, J. Reduction of lattice thermal conductivity from planar faults in the layered Zintl compound SrZnSb2. J. Appl. Phys. 2011, 109, 043509. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Chauhan, N.S.; Misra, D.K. Significantly enhanced thermoelectric figure of merit of p-type Mg3Sb2-based Zintl phase compounds via nanostructuring and employing high energy mechanical milling coupled with spark plasma sintering. J. Mater. Chem. A 2015, 3, 10777–10786. [Google Scholar] [CrossRef]
- Chang, Z.; Ma, J.; Yuan, K.; Zheng, J.; Wei, B.; Al-Fahdi, M.; Gao, Y.; Zhang, X.; Shao, H.; Hu, M.; et al. Zintl Phase Compounds Mg3Sb2−xBix (x = 0, 1, and 2) Monolayers: Electronic, Phonon and Thermoelectric Properties From ab Initio Calculations. Front. Mech. Eng. 2022, 8, 876655. [Google Scholar] [CrossRef]
- Kanno, T.; Tamaki, H.; Sato, H.K.; Kang, S.D.; Ohno, S.; Imasato, K.; Kuo, J.J.; Snyder, G.J.; Miyazaki, Y. Enhancement of average thermoelectric figure of merit by increasing the grain-size of Mg3.2Sb1.5Bi0.49Te0.01. Appl. Phys. Lett. 2018, 112, 033903. [Google Scholar] [CrossRef]
- Liu, K.-F.; Xia, S.-Q. Recent progresses on thermoelectric Zintl phases: Structures, materials and optimization. J. Solid State Chem. 2019, 270, 252–264. [Google Scholar] [CrossRef]
- Okamoto, N.L.; Kim, J.-H.; Tanaka, K.; Inui, H. Splitting of guest atom sites and lattice thermal conductivity of type-I and type-III clathrate compounds in the Ba–Ga–Ge system. Acta Mater. 2006, 54, 5519–5528. [Google Scholar] [CrossRef]
- Mardegan, J.; Fabbris, G.; Veiga, L.; Adriano, C.; Avila, M.; Haskel, D.; Giles, C. Pressure-induced amorphization and collapse of magnetic order in the type-I clathrate Eu8Ga16Ge30. Phys. Rev. B 2013, 88, 144105. [Google Scholar] [CrossRef]
- Hasan, M.N.; Wahid, H.; Nayan, N.; Mohamed Ali, M.S. Inorganic thermoelectric materials: A review. Int. J. Energy Res. 2020, 44, 6170–6222. [Google Scholar] [CrossRef]
- Shi, X.; Yang, J.; Bai, S.; Yang, J.; Wang, H.; Chi, M.; Salvador, J.R.; Zhang, W.; Chen, L.; Wong-Ng, W. On the Design of High-Efficiency Thermoelectric Clathrates through a Systematic Cross-Substitution of Framework Elements. Adv. Funct. Mater. 2010, 20, 755–763. [Google Scholar] [CrossRef]
- Saramat, A.; Svensson, G.; Palmqvist, A.E.C.; Stiewe, C.; Mueller, E.; Platzek, D.; Williams, S.G.K.; Rowe, D.M.; Bryan, J.D.; Stucky, G.D. Large thermoelectric figure of merit at high temperature in Czochralski-grown clathrate Ba8Ga16Ge30. J. Appl. Phys. 2006, 99, 023708. [Google Scholar] [CrossRef]
- Shen, L.; Shen, K.; Ge, W.; Zheng, J.; He, B.; Deng, S. Preparation and Thermoelectric Properties of Type-VIII Single-Crystalline SmxBa8−xGa16Sn30 Clathrate. J. Mater. Eng. Perform. 2022, 31, 3570–3576. [Google Scholar] [CrossRef]
- Dolyniuk, J.-A.; Owens-Baird, B.; Wang, J.; Zaikina, J.V.; Kovnir, K. Clathrate thermoelectrics. Mater. Sci. Eng. R Rep. 2016, 108, 1–46. [Google Scholar] [CrossRef]
- Terasaki, I.; Sasago, Y.; Uchinokura, K. Large thermoelectric power in NaCo2O4 single crystals. Phys. Rev. B 1997, 56, R12685. [Google Scholar] [CrossRef]
- Fujita, K.; Mochida, T.; Nakamura, K. High-temperature thermoelectric properties of NaxCoO2−δ single crystals. Jpn. J. Appl. Phys. 2001, 40, 4644. [Google Scholar] [CrossRef]
- Ohta, H.; Sugiura, K.; Koumoto, K. Recent progress in oxide thermoelectric materials: P-type Ca3Co4O9 and n-type SrTiO3−. Inorg. Chem. 2008, 47, 8429–8436. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Viciu, L.; Li, L.; Wang, Y.; Foo, M.; Watauchi, S.; Pascal Jr, R.; Cava, R.; Ong, N. Large enhancement of the thermopower in NaxCoO2 at high Na doping. Nat. Mater. 2006, 5, 537–540. [Google Scholar] [CrossRef]
- Altin, S.; Aksan, M.; Bayri, A. High temperature spin state transitions in misfit-layered Ca3Co4O9. J. Alloy. Compd. 2014, 587, 40–44. [Google Scholar] [CrossRef]
- Ohta, S.; Nomura, T.; Ohta, H.; Koumoto, K. High-temperature carrier transport and thermoelectric properties of heavily La- or Nb-doped SrTiO3 single crystals. J. Appl. Phys. 2005, 97, 034106. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Su, W.; Liu, J.; Zhao, Y.; Peng, H.; Zhang, J.; Zhao, M.; Li, J.; Yin, N.; et al. Enhancement of thermoelectric figure of merit by doping Dy in La0.1Sr0.9TiO3 ceramic. Mater. Res. Bull. 2010, 45, 809–812. [Google Scholar] [CrossRef]
- Yang, Y.; Pradel, K.C.; Jing, Q.; Wu, J.M.; Zhang, F.; Zhou, Y.; Zhang, Y.; Wang, Z.L. Thermoelectric Nanogenerators Based on Single Sb-Doped ZnO Micro/Nanobelts. ACS Nano 2012, 6, 6984–6989. [Google Scholar] [CrossRef]
- Jood, P.; Mehta, R.J.; Zhang, Y.; Peleckis, G.; Wang, X.; Siegel, R.W.; Borca-Tasciuc, T.; Dou, S.X.; Ramanath, G. Al-Doped Zinc Oxide Nanocomposites with Enhanced Thermoelectric Properties. Nano Lett. 2011, 11, 4337–4342. [Google Scholar] [CrossRef]
- Andrei, V.; Bethke, K.; Rademann, K. Copper(I) oxide based thermoelectric powders and pastes with high Seebeck coefficients. Appl. Phys. Lett. 2014, 105, 233902. [Google Scholar] [CrossRef]
- Andrei, V.; Bethke, K.; Rademann, K. Adjusting the thermoelectric properties of copper(i) oxide–graphite–polymer pastes and the applications of such flexible composites. Phys. Chem. Chem. Phys. 2016, 18, 10700–10707. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.A.; Gorai, P.; Aydemir, U.; Mason, T.O.; Stevanović, V.; Toberer, E.S.; Snyder, G.J. SnO as a potential oxide thermoelectric candidate. J. Mater. Chem. C 2017, 5, 8854–8861. [Google Scholar] [CrossRef]
- Singh, S.; Srivastav, S.K.; Patel, A.; Kumar, A.; Chatterjee, R.; Takeuchi, T.; Pandey, S.K. Enhancement in thermoelectric properties of n-type (La0.7Sr0.3MnO3)0.5.(NiO)0.5: Composite and nano-structure effect. J. Phys. D Appl. Phys. 2021, 55, 065503. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Lan, J.; Nan, C. Oxide Thermoelectric Materials: From Basic Principles to Applications; John Wiley & Sons: Weinheim, Germany, 2019. [Google Scholar]
- Narjis, A.; Outzourhit, A.; Aberkouks, A.; El Hasnaoui, M.; Nkhaili, L. Structural and thermoelectric properties of copper sulphide powders. J. Semicond. 2018, 39, 122001. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, H.; Lou, Y.; Qiu, X.; Zhu, J.; Burda, C. Plasmonic Cu2−xS Nanocrystals: Optical and Structural Properties of Copper-Deficient Copper(I) Sulfides. J. Am. Chem. Soc. 2009, 131, 4253–4261. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Zhao, Y.; Liu, W.-D.; Dai, W.; Wu, T.; Lu, X.; Wu, C.; Luo, W.; Fan, Y.; et al. Carbon-Encapsulated Copper Sulfide Leading to Enhanced Thermoelectric Properties. ACS Appl. Mater. Interfaces 2019, 11, 22457–22463. [Google Scholar] [CrossRef]
- He, Y.; Day, T.; Zhang, T.; Liu, H.; Shi, X.; Chen, L.; Snyder, G.J. High Thermoelectric Performance in Non-Toxic Earth-Abundant Copper Sulfide. Adv. Mater. 2014, 26, 3974–3978. [Google Scholar] [CrossRef]
- Majumdar, D. Recent progress in copper sulfide based nanomaterials for high energy supercapacitor applications. J. Electroanal. Chem. 2021, 880, 114825. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, K.; Fu, J.; Yamaguchi, A.; Li, H.; Pan, H.; Hu, J.; Miyauchi, M.; Liu, M. Recent advances in the utilization of copper sulfide compounds for electrochemical CO2 reduction. Nano Mater. Sci. 2019, 2, 235–247. [Google Scholar] [CrossRef]
- Mansour, B.A.; El Soud, A.M.A.; Tahoon, K.H. Evaluation of Some Physical Quantities in the Two-Phase Region in Copper Sulphides. Phys. Status Solidi (A) 1992, 132, 59–65. [Google Scholar] [CrossRef]
- Chen, Y.-X.; Ge, Z.-H.; Yin, M.; Feng, D.; Huang, X.-Q.; Zhao, W.; He, J. Understanding of the Extremely Low Thermal Conductivity in High-Performance Polycrystalline SnSe through Potassium Doping. Adv. Funct. Mater. 2020, 26, 6836–6845. [Google Scholar] [CrossRef]
- Liang, D.; Ma, R.; Jiao, S.; Pang, G.; Feng, S. A facile synthetic approach for copper iron sulfide nanocrystals with enhanced thermoelectric performance. Nanoscale 2012, 4, 6265–6268. [Google Scholar] [CrossRef]
- Zhu, C.; Ming, H.; Zhang, J.; Li, D.; Chen, T.; Qin, X. Ultralow Lattice Thermal Conductivity and High Thermoelectric Figure of Merit in Dually Substituted Cu12Sb4S13 Tetrahedrites. Adv. Electron. Mater. 2021, 8, 2200110. [Google Scholar] [CrossRef]
- Guélou, G.; Lemoine, P.; Raveau, B.; Guilmeau, E. Recent developments in high-performance thermoelectric sulphides: An overview of the promising synthetic colusites. J. Mater. Chem. C 2020, 9, 773–795. [Google Scholar] [CrossRef]
- Smith, J.C.; Banerjee, S.; Pardo, V.; Pickett, W.E. Dirac Point Degenerate with Massive Bands at a Topological Quantum Critical Point. Phys. Rev. Lett. 2011, 106, 056401. [Google Scholar] [CrossRef] [PubMed]
- Helme, L.M.; Boothroyd, A.T.; Coldea, R.; Prabhakaran, D.; Stunault, A.; McIntyre, G.J.; Kernavanois, N. Spin gaps and magnetic structure ofNaxCoO2. Phys. Rev. B 2006, 73, 054405. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Q.; Yuan, B.; Lai, X.; Yan, X.; Ren, Z. Recent progress in half-Heusler thermoelectric materials. Mater. Res. Bull. 2016, 76, 107–112. [Google Scholar] [CrossRef]
- Kurosaki, K.; Maekawa, T.; Muta, H.; Yamanaka, S. Effect of spark plasma sintering temperature on thermoelectric properties of (Ti,Zr,Hf)NiSn half-Heusler compounds. J. Alloy. Compd. 2005, 397, 296–299. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Liu, Y.-N.; Ma, H.-K. Effects of the Zr0.5Hf0.5FexCo1−xSb0.8Sn0.2/Fe3Sn2 half-Heusler composites on the ZT value. J. Alloy. Compd. 2015, 621, 324–330. [Google Scholar] [CrossRef]
- Liu, Y.; Poudeu, P.F. Thermoelectric properties of Ge doped n-type TixZr1−xNiSn0.975Ge0.025 half-Heusler alloys. J. Mater. Chem. A 2015, 3, 12507–12514. [Google Scholar] [CrossRef]
- Joshi, G.; Yan, X.; Wang, H.; Liu, W.; Chen, G.; Ren, Z. Enhancement in Thermoelectric Figure-Of-Merit of an N-Type Half-Heusler Compound by the Nanocomposite Approach. Adv. Energy Mater. 2011, 1, 643–647. [Google Scholar] [CrossRef]
- Xia, K.; Hu, C.; Fu, C.; Zhao, X.; Zhu, T. Half-Heusler thermoelectric materials. Appl. Phys. Lett. 2021, 118, 140503. [Google Scholar] [CrossRef]
- Zhu, T.; Fu, C.; Xie, H.; Liu, Y.; Zhao, X. High Efficiency Half-Heusler Thermoelectric Materials for Energy Harvesting. Adv. Energy Mater. 2015, 5, 1500588. [Google Scholar] [CrossRef]
- Yun, J.S.; Choi, S.; Im, S.H. Advances in carbon-based thermoelectric materials for high-performance, flexible thermoelectric devices. Carbon Energy 2021, 3, 667–708. [Google Scholar] [CrossRef]
- Li, J.; Huckleby, A.B.; Zhang, M. Polymer-based thermoelectric materials: A review of power factor improving strategies. J. Materiomics 2021, 8, 204–220. [Google Scholar] [CrossRef]
- Liu, W.-D.; Yu, Y.; Dargusch, M.; Liu, Q.; Chen, Z.-G. Carbon allotrope hybrids advance thermoelectric development and applications. Renew. Sustain. Energy Rev. 2021, 141, 110800. [Google Scholar] [CrossRef]
- Pires, A.L.; Silva, J.A.; Maia, M.M.; Silva, S.; Lopes, A.M.L.; Fonseca, J.; Ribeiro, M.; Pereira, C.; Pereira, A.M. Recent Advances in Functional Thermoelectric Materials for Printed Electronics. In Thin Film and Flexible Thermoelectric Generators, Devices and Sensors; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Hung, N.T.; RT Nugraha, A.; Saito, R. Thermoelectric properties of carbon nanotubes. Energies 2019, 12, 4561. [Google Scholar] [CrossRef]
- Yu, C.; Choi, K.; Yin, L.; Grunlan, J.C. Light-Weight Flexible Carbon Nanotube Based Organic Composites with Large Thermoelectric Power Factors. ACS Nano 2011, 5, 7885–7892. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Duan, W. Thermal and Thermoelectric Properties of Graphene. Small 2014, 10, 2182–2199. [Google Scholar] [CrossRef]
- Sim, D.; Liu, D.; Dong, X.; Xiao, N.; Li, S.; Zhao, Y.; Li, L.-J.; Yan, Q.; Hng, H.H. Power Factor Enhancement for Few-Layered Graphene Films by Molecular Attachments. J. Phys. Chem. C 2011, 115, 1780–1785. [Google Scholar] [CrossRef]
- Anno, Y.; Imakita, Y.; Takei, K.; Akita, S.; Arie, T. Enhancement of graphene thermoelectric performance through defect engineering. 2D Mater. 2017, 4, 025019. [Google Scholar] [CrossRef]
- Barink, M.; Berg, D.v.D.; Yakimets, I.; Giesen, P.; van Dommelen, J.A.; Meinders, E. Flexible electronics: Prediction of substrate deformation during different steps of the lithography process. Microelectron. Eng. 2011, 88, 999–1005. [Google Scholar] [CrossRef]
- Du, Y.; Shen, S.Z.; Cai, K.; Casey, P.S. Research progress on polymer–inorganic thermoelectric nanocomposite materials. Prog. Polym. Sci. 2012, 37, 820–841. [Google Scholar] [CrossRef]
- Choi, J.; Lee, J.Y.; Lee, S.-S.; Park, C.R.; Kim, H. High-Performance Thermoelectric Paper Based on Double Carrier-Filtering Processes at Nanowire Heterojunctions. Adv. Energy Mater. 2016, 6, 1502181. [Google Scholar] [CrossRef]
- Cho, C.; Wallace, K.L.; Tzeng, P.; Hsu, J.-H.; Yu, C.; Grunlan, J.C. Outstanding Low Temperature Thermoelectric Power Factor from Completely Organic Thin Films Enabled by Multidimensional Conjugated Nanomaterials. Adv. Energy Mater. 2016, 6, 1502168. [Google Scholar] [CrossRef]
- Li, X.; Liang, L.; Yang, M.; Chen, G.; Guo, C.-Y. Poly (3,4-ethylenedioxythiophene)/graphene/carbon nanotube ternary composites with improved thermoelectric performance. Org. Electron. 2016, 38, 200–204. [Google Scholar] [CrossRef]
- Chilvery, A.; Palwai, S.; Guggilla, P.; Wren, K.; Edinburgh, D. Perovskite Materials: Recent Advancements and Challenges; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Rose, G. De novis Quibusdam Fossilibus Quae in Montibus Uraliis Inveniuntur; Typis AG Schadii: Berlin, Germany, 1839. [Google Scholar]
- Suzuki, T.; Sakai, H.; Taguchi, Y.; Tokura, Y. Thermoelectric Properties of Electron-Doped SrMnO3 Single Crystals with Perovskite Structure. J. Electron. Mater. 2011, 41, 1559–1563. [Google Scholar] [CrossRef]
- Ravichandran, J.; Siemons, W.; Oh, D.-W.; Kardel, J.T.; Chari, A.; Heijmerikx, H.; Scullin, M.L.; Majumdar, A.; Ramesh, R.; Cahill, D.G. High-temperature thermoelectric response of double-doped SrTiO3 epitaxial films. Phys. Rev. B 2010, 82, 165126. [Google Scholar] [CrossRef]
- Muta, H.; Kurosaki, K.; Yamanaka, S. Thermoelectric properties of reduced and La-doped single-crystalline SrTiO3. J. Alloy. Compd. 2005, 392, 306–309. [Google Scholar] [CrossRef]
- Bouhmaidi, S.; Marjaoui, A.; Talbi, A.; Zanouni, M.; Nouneh, K.; Setti, L. A DFT study of electronic, optical and thermoelectric properties of Ge-halide perovskites CsGeX3 (X = F, Cl and Br). Comput. Condens. Matter 2022, 31, e00663. [Google Scholar] [CrossRef]
- Haque, A.; Nugraha, M.I.; Paleti, S.H.K.; Baran, D. Role of Compositional Tuning on Thermoelectric Parameters of Hybrid Halide Perovskites. J. Phys. Chem. C 2019, 123, 14928–14933. [Google Scholar] [CrossRef]
- Bhumla, P.; Jain, M.; Sheoran, S.; Bhattacharya, S. Vacancy-Ordered Double Perovskites Cs2BI6 (B = Pt, Pd, Te, Sn): An Emerging Class of Thermoelectric Materials. J. Phys. Chem. Lett. 2022, 13, 11655–11662. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhang, J.; Ratnasingham, S.R.; Liscio, F.; Chen, K.; Liu, T.; Wan, K.; Galindez, E.S.; Bilotti, E.; Reece, M.; et al. Substitutional doping of hybrid organic–inorganic perovskite crystals for thermoelectrics. J. Mater. Chem. A 2020, 8, 13594–13599. [Google Scholar] [CrossRef]
- Wu, T.; Gao, P. Development of Perovskite-Type Materials for Thermoelectric Application. Materials 2018, 11, 999. [Google Scholar] [CrossRef] [PubMed]
- Beretta, D.; Neophytou, N.; Hodges, J.M.; Kanatzidis, M.G.; Narducci, D.; Martin-Gonzalez, M.; Beekman, M.; Balke, B.; Cerretti, G.; Tremel, W. Thermoelectrics: From history, a window to the future. Mater. Sci. Eng. R Rep. 2019, 138, 100501. [Google Scholar] [CrossRef]
- Kim, S.I.; Lee, K.H.; Mun, H.A.; Kim, H.S.; Hwang, S.W.; Roh, J.W.; Yang, D.J.; Shin, W.H.; Li, X.S.; Lee, Y.H.; et al. Dense dislocation arrays embedded in grain boundaries for high-performance bulk thermoelectrics. Science 2015, 348, 109–114. [Google Scholar] [CrossRef]
- Choi, H.; Jeong, K.; Chae, J.; Park, H.; Baeck, J.; Kim, T.H.; Song, J.Y.; Park, J.; Jeong, K.-H.; Cho, M.-H. Enhancement in thermoelectric properties of Te-embedded Bi2Te3 by preferential phonon scattering in heterostructure interface. Nano Energy 2018, 47, 374–384. [Google Scholar] [CrossRef]
- Wang, H.; Hsu, J.H.; Yi, S.I.; Kim, S.L.; Choi, K.; Yang, G.; Yu, C. Thermally driven large n-type voltage responses from hybrids of carbon nanotubes and poly (3,4-ethylenedioxythiophene) with tetrakis (dimethylamino) ethylene. Adv. Mater. 2015, 27, 6855–6861. [Google Scholar] [CrossRef]
- Jia, B.; Huang, Y.; Wang, Y.; Zhou, Y.; Zhao, X.; Ning, S.; Xu, X.; Lin, P.; Chen, Z.; Jiang, B.; et al. Realizing high thermoelectric performance in non-nanostructured n-type PbTe. Energy Environ. Sci. 2022, 15, 1920–1929. [Google Scholar] [CrossRef]
- Chang, C.; Wu, M.; He, D.; Pei, Y.; Wu, C.-F.; Wu, X.; Yu, H.; Zhu, F.; Wang, K.; Chen, Y.; et al. 3D charge and 2D phonon transports leading to high out-of-plane ZT in n-type SnSe crystals. Science 2018, 360, 778–783. [Google Scholar] [CrossRef]
- Robert, R.; Bocher, L.; Sipos, B.; Döbeli, M.; Weidenkaff, A. Ni-doped cobaltates as potential materials for high temperature solar thermoelectric converters. Prog. Solid State Chem. 2007, 35, 447–455. [Google Scholar] [CrossRef]
- Li, D.; Li, S.; Li, X.; Yang, B.; Zhong, H. Efficiently synthesized n-type CoSb3 thermoelectric alloys under TGZM effect. Mater. Sci. Semicond. Process. 2021, 123, 105542. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, T.; Wang, Y.; Ruan, X.; Jin, C.; Xia, A. High-performance n-type CoSb3-based thermoelectric material with vortex and strip-shaped grain structures. J. Eur. Ceram. Soc. 2023, 43, 1985–1991. [Google Scholar] [CrossRef]
- Guo, K.; Cao, Q.G.; Feng, X.J.; Tang, M.B.; Chen, H.H.; Guo, X.; Chen, L.; Grin, Y.; Zhao, J.T. Enhanced thermoelectric figure of merit of Zintl phase YbCd2–xMnxSb2 by chemical substitution. Eur. J. Inorg. Chem. 2011, 2011, 4043–4048. [Google Scholar] [CrossRef]
- Chen, X.; Wu, H.; Cui, J.; Xiao, Y.; Zhang, Y.; He, J.; Chen, Y.; Cao, J.; Cai, W.; Pennycook, S.J.; et al. Extraordinary thermoelectric performance in n-type manganese doped Mg3Sb2 Zintl: High band degeneracy, tuned carrier scattering mechanism and hierarchical microstructure. Nano Energy 2018, 52, 246–255. [Google Scholar] [CrossRef]
- Zhang, H.; Borrmann, H.; Oeschler, N.; Candolfi, C.; Schnelle, W.; Schmidt, M.; Burkhardt, U.; Baitinger, M.; Zhao, J.-T.; Grin, Y. Atomic Interactions in the p-Type Clathrate I Ba8Au5.3Ge40.7. Inorg. Chem. 2011, 50, 1250–1257. [Google Scholar] [CrossRef]
- Rathore, E.; Juneja, R.; Culver, S.P.; Minafra, N.; Singh, A.K.; Zeier, W.G.; Biswas, K. Origin of Ultralow Thermal Conductivity in n-Type Cubic Bulk AgBiS2: Soft Ag Vibrations and Local Structural Distortion Induced by the Bi 6s2 Lone Pair. Chem. Mater. 2019, 31, 2106–2113. [Google Scholar] [CrossRef]
- Yu, J.; Fu, C.; Liu, Y.; Xia, K.; Aydemir, U.; Chasapis, T.C.; Snyder, G.J.; Zhao, X.; Zhu, T. Unique Role of Refractory Ta Alloying in Enhancing the Figure of Merit of NbFeSb Thermoelectric Materials. Adv. Energy Mater. 2018, 8, 1701313. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, H.; Fu, C.; Snyder, G.J.; Zhao, X.; Zhu, T. Demonstration of a phonon-glass electron-crystal strategy in (Hf,Zr)NiSn half-Heusler thermoelectric materials by alloying. J. Mater. Chem. A 2015, 3, 22716–22722. [Google Scholar] [CrossRef]
- Torres, M.; Costa, F.; Flahaut, D.; Touati, K.; Rasekh, S.; Ferreira, N.; Allouche, J.; Depriester, M.; Madre, M.; Kovalevsky, A.; et al. Significant enhancement of the thermoelectric performance in Ca3Co4O9 thermoelectric materials through combined strontium substitution and hot-pressing process. J. Eur. Ceram. Soc. 2019, 39, 1186–1192. [Google Scholar] [CrossRef]
- Muthusamy, O.; Singh, S.; Hirata, K.; Kuga, K.; Harish, S.K.; Shimomura, M.; Adachi, M.; Yamamoto, Y.; Matsunami, M.; Takeuchi, T. Synergetic Enhancement of the Power Factor and Suppression of Lattice Thermal Conductivity via Electronic Structure Modification and Nanostructuring on a Ni- and B-Codoped p-Type Si–Ge Alloy for Thermoelectric Application. ACS Appl. Electron. Mater. 2021, 3, 5621–5631. [Google Scholar] [CrossRef]
- Alam, H.; Ramakrishna, S. A review on the enhancement of figure of merit from bulk to nano-thermoelectric materials. Nano Energy 2013, 2, 190–212. [Google Scholar] [CrossRef]
- Adachi, S. Lattice thermal conductivity of group-IV and III–V semiconductor alloys. J. Appl. Phys. 2007, 102, 063502. [Google Scholar] [CrossRef]
- Chung, D.-Y.; Hogan, T.; Brazis, P.; Rocci-Lane, M.; Kannewurf, C.; Bastea, M.; Uher, C.; Kanatzidis, M.G. ChemInform Abstract: CsBi4Te6: A High-Performance Thermoelectric Material for Low-Temperature Applications. Cheminform 2000, 287, 1024–1027. [Google Scholar] [CrossRef]
- Luo, Z.-Z.; Cai, S.; Hao, S.; Bailey, T.P.; Su, X.; Spanopoulos, I.; Hadar, I.; Tan, G.; Luo, Y.; Xu, J. High figure of merit in gallium-doped nanostructured n-type PbTe-x GeTe with midgap states. J. Am. Chem. Soc. 2019, 141, 16169–16177. [Google Scholar] [CrossRef]
- Zide, J.M.; Klenov, D.O.; Stemmer, S.; Gossard, A.C.; Zeng, G.; Bowers, J.E.; Vashaee, D.; Shakouri, A. Thermoelectric power factor in semiconductors with buried epitaxial semimetallic nanoparticles. Appl. Phys. Lett. 2005, 87, 112102. [Google Scholar] [CrossRef]
- Kaur, P.; Bera, C. Effect of alloying on thermal conductivity and thermoelectric properties of CoAsS and CoSbS. Phys. Chem. Chem. Phys. 2017, 19, 24928–24933. [Google Scholar] [CrossRef]
- Xu, B.; Feng, T.; Agne, M.T.; Zhou, L.; Ruan, X.; Snyder, G.J.; Wu, Y. Highly porous thermoelectric nanocomposites with low thermal conductivity and high figure of merit from large-scale solution-synthesized Bi2Te2.5Se0.5 hollow nanostructures. Angew. Chem. Int. Ed. 2017, 56, 3546–3551. [Google Scholar] [CrossRef]
- Shi, X.; Wu, A.; Liu, W.; Moshwan, R.; Wang, Y.; Chen, Z.-G.; Zou, J. Polycrystalline SnSe with Extraordinary Thermoelectric Property via Nanoporous Design. ACS Nano 2018, 12, 11417–11425. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, T.; Pan, R.; Chun, Y.; Zhou, H.; Zhu, W.; Peng, H.; Zhang, Q. Sintering-free preparation of porous ceramsite using low-temperature decomposing pore former and its sound-absorbing performance. Constr. Build. Mater. 2018, 171, 367–376. [Google Scholar] [CrossRef]
- Harman, T.C.; Taylor, P.J.; Spears, D.L.; Walsh, M.P. Thermoelectric quantum-dot superlattices with high ZT. J. Electron. Mater. 2000, 29, L1–L2. [Google Scholar] [CrossRef]
- Hu, H.; Zhuang, H.; Jiang, Y.; Shi, J.; Li, J.; Cai, B.; Han, Z.; Pei, J.; Su, B.; Ge, Z.; et al. Thermoelectric Cu12Sb4 S13 -Based Synthetic Minerals with a Sublimation-Derived Porous Network. Adv. Mater. 2021, 33, 2103633. [Google Scholar] [CrossRef]
- Jood, P.; Mehta, R.J.; Zhang, Y.; Borca-Tasciuc, T.; Dou, S.X.; Singh, D.J.; Ramanath, G. Heavy element doping for enhancing thermoelectric properties of nanostructured zinc oxide. RSC Adv. 2014, 4, 6363–6368. [Google Scholar] [CrossRef]
- Pei, Y.; Bai, S.; Zhao, X.; Zhang, W.; Chen, L. Thermoelectric properties of EuyCo4Sb12 filled skutterudites. Solid State Sci. 2008, 10, 1422–1428. [Google Scholar] [CrossRef]
- Lan, J.-L.; Liu, Y.-C.; Zhan, B.; Lin, Y.-H.; Zhang, B.; Yuan, X.; Zhang, W.; Xu, W.; Nan, C.-W. Enhanced Thermoelectric Properties of Pb-doped BiCuSeO Ceramics. Adv. Mater. 2013, 25, 5086–5090. [Google Scholar] [CrossRef]
- Na, S.-I.; Kim, T.-S.; Oh, S.-H.; Kim, J.; Kim, S.-S.; Kim, D.-Y. Enhanced performance of inverted polymer solar cells with cathode interfacial tuning via water-soluble polyfluorenes. Appl. Phys. Lett. 2010, 97, 223305. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z.; Dargusch, M.S.; Zou, J. High Performance Thermoelectric Materials: Progress and Their Applications. Adv. Energy Mater. 2018, 8, 1701797. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, Y.; Tao, Q.; Yan, Y.; Su, X.; Tang, X. Distinct role of Sn and Ge doping on thermoelectric properties in p-type (Bi, Sb)2Te3-alloys. J. Solid State Chem. 2020, 292, 121722. [Google Scholar] [CrossRef]
- Mott, N.F.; Jones, H.; Jones, H.; Jones, H. The Theory of the Properties of Metals and Alloys; Courier Dover Publications: New York, NY, USA, 1958. [Google Scholar]
- Heremans, J.P.; Jovovic, V.; Toberer, E.S.; Saramat, A.; Kurosaki, K.; Charoenphakdee, A.; Yamanaka, S.; Snyder, G.J. Enhancement of Thermoelectric Efficiency in PbTe by Distortion of the Electronic Density of States. Science 2008, 321, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Banik, A.; Biswas, K. Lead-free thermoelectrics: Promising thermoelectric performance in p-type SnTe1−xSex system. J. Mater. Chem. A 2014, 2, 9620–9625. [Google Scholar] [CrossRef]
- Goldsmid, H.; Douglas, R. The use of semiconductors in thermoelectric refrigeration. Br. J. Appl. Phys. 1954, 5, 386. [Google Scholar] [CrossRef]
- Pei, Y.; Gibbs, Z.M.; Gloskovskii, A.; Balke, B.; Zeier, W.G.; Snyder, G.J. Optimum Carrier Concentration in n-Type PbTe Thermoelectrics. Adv. Energy Mater. 2014, 4, 1400486. [Google Scholar] [CrossRef]
- Takagiwa, Y.; Pei, Y.; Pomrehn, G.; Snyder, G.J. Dopants effect on the band structure of PbTe thermoelectric material. Appl. Phys. Lett. 2012, 101, 092102. [Google Scholar] [CrossRef]
- Pei, Y.; Shi, X.; LaLonde, A.; Wang, H.; Chen, L.; Snyder, G.J. Convergence of electronic bands for high performance bulk thermoelectrics. Nature 2011, 473, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Giri, K.; Wang, Y.-L.; Chen, T.-H.; Chen, C.-H. Challenges and strategies to optimize the figure of merit: Keeping eyes on thermoelectric metamaterials. Mater. Sci. Semicond. Process. 2022, 150, 106944. [Google Scholar] [CrossRef]
- Slack, G.A. The Thermal Conductivity of Nonmetallic Crystals. Solid State Phys. 1979, 34, 1–71. [Google Scholar] [CrossRef]
- Amatya, R.; Ram, R.J. Trend for Thermoelectric Materials and Their Earth Abundance. J. Electron. Mater. 2012, 41, 1011–1019. [Google Scholar] [CrossRef]
- Liang, X.; Chen, C. Ductile inorganic amorphous/crystalline composite Ag4TeS with phonon-glass electron-crystal transport behavior and excellent stability of high thermoelectric performance on plastic deformation. Acta Mater. 2021, 218, 117231. [Google Scholar] [CrossRef]
- Alsaleh, N.M.; Shoko, E.; Arsalan, M.; Schwingenschlögl, U. Thermoelectric materials under pressure. Phys. Status Solidi (RRL)–Rapid Res. Lett. 2018, 12, 1800083. [Google Scholar] [CrossRef]
- Polvani, D.; Meng, J.N.V. Chandra Shekar, I. Sharp, and J. V. Badding. Chem. Mater 2001, 13, 2068. [Google Scholar] [CrossRef]
- Guo, X.; Jia, X.; Jie, K.; Sun, H.; Zhang, Y.; Sun, B.; Ma, H. Investigating the thermoelectric properties of synthesized Bi2Te3 under different synthesis pressures. Chem. Phys. Lett. 2013, 568–569, 190–194. [Google Scholar] [CrossRef]
- Chen, L.-C.; Yu, H.; Pang, H.-J.; Jiang, B.-B.; Su, L.; Shi, X.; Chen, L.-D.; Chen, X.-J. Pressure-induced enhancement of thermoelectric performance in palladium sulfide. Mater. Today Phys. 2018, 5, 64–71. [Google Scholar] [CrossRef]
- Böttner, H.; Chen, G.; Venkatasubramanian, R. Aspects of Thin-Film Superlattice Thermoelectric Materials, Devices, and Applications. MRS Bull. 2006, 31, 211–217. [Google Scholar] [CrossRef]
- Takaki, H.; Kobayashi, K.; Shimono, M.; Kobayashi, N.; Hirose, K. First-principles calculations of thermoelectric properties of TiN/MgO superlattices: The route for an enhancement of thermoelectric effects in artificial nanostructures. J. Appl. Phys. 2016, 119, 014302. [Google Scholar] [CrossRef]
- Niemelä, J.-P.; Karttunen, A.J.; Karppinen, M. Inorganic–organic superlattice thin films for thermoelectrics. J. Mater. Chem. C 2015, 3, 10349–10361. [Google Scholar] [CrossRef]
- Xiong, R.; Yang, C.; Wang, Q.; Zhang, Y.; Li, X. Spectral Phonon Transport Engineering Using Stacked Superlattice Structures. Int. J. Thermophys. 2019, 40, 86. [Google Scholar] [CrossRef]
- Venkatasubramanian, R. Lattice thermal conductivity reduction and phonon localizationlike behavior in superlattice structures. Phys. Rev. B 2000, 61, 3091–3097. [Google Scholar] [CrossRef]
- Ishida, A.; Yamada, T.; Cao, D.; Inoue, Y.; Veis, M.; Kita, T. Seebeck effect in PbTe films and EuTe/PbTe superlattices. J. Appl. Phys. 2009, 106, 023718. [Google Scholar] [CrossRef]
- Vineis, C.J.; Shakouri, A.; Majumdar, A.; Kanatzidis, M.G. Nanostructured Thermoelectrics: Big Efficiency Gains from Small Features. Adv. Mater. 2010, 22, 3970–3980. [Google Scholar] [CrossRef]
- Siyar, M.; Cho, J.-Y.; Youn, Y.; Han, S.; Kim, M.; Bae, S.-H.; Park, C. Effect of annealing temperature on the phase transition, band gap and thermoelectric properties of Cu2SnSe3. J. Mater. Chem. C 2018, 6, 1780–1788. [Google Scholar] [CrossRef]
- Thakur, V.; Upadhyay, K.; Kaur, R.; Goyal, N.; Gautam, S. Investigating phase transition and morphology of Bi-Te thermoelectric system. Mater. Today Adv. 2020, 8, 100082. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, D.; Qiu, Y.; Zhao, L.-D. Boosting the thermoelectric performance of GeTe by manipulating the phase transition temperature via Sb doping. J. Mater. Chem. C 2021, 9, 6484–6490. [Google Scholar] [CrossRef]
- Kumar, A.; Bano, S.; Govind, B.; Bhardwaj, A.; Bhatt, K.; Misra, D.K. A Review on Fundamentals, Design and Optimization to High ZT of Thermoelectric Materials for Application to Thermoelectric Technology. J. Electron. Mater. 2021, 50, 6037–6059. [Google Scholar] [CrossRef]
- Xiang, B.; Liu, J.; Yan, J.; Zhu, H.; Zhang, J.; Li, J.; Chen, L.; Wu, Y. Solution synthesis ultrathin PbTe0.5Se0.5 nanowires and the low lattice thermal conductivity. J. Phys. Chem. Solids 2020, 141, 109370. [Google Scholar] [CrossRef]
- Minnich, A.J.; Dresselhaus, M.S.; Ren, Z.F.; Chen, G. Bulk nanostructured thermoelectric materials: Current research and future prospects. Energy Environ. Sci. 2009, 2, 466–479. [Google Scholar] [CrossRef]
- Su, W.B.; Chang, C.S.; Tsong, T.T. Quantum size effect on ultra-thin metallic films. J. Phys. D Appl. Phys. 2009, 43, 013001. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, S.; Wang, X.; Suwardi, A.; Chua, M.H.; Soo, X.Y.D.; Xu, J. Bottom-Up Engineering Strategies for High-Performance Thermoelectric Materials. Nano-Micro Lett. 2021, 13, 119. [Google Scholar] [CrossRef]
- Szczech, J.R.; Higgins, J.M.; Jin, S. Enhancement of the thermoelectric properties in nanoscale and nanostructured materials. J. Mater. Chem. 2011, 21, 4037–4055. [Google Scholar] [CrossRef]
- Bux, S.K.; Fleurial, J.-P.; Kaner, R.B. Nanostructured materials for thermoelectric applications. Chem. Commun. 2010, 46, 8311–8324. [Google Scholar] [CrossRef]
- Taniguchi, T.; Ishibe, T.; Naruse, N.; Mera, Y.; Alam, M.; Sawano, K.; Nakamura, Y. High Thermoelectric Power Factor Realization in Si-Rich SiGe/Si Superlattices by Super-Controlled Interfaces. ACS Appl. Mater. Interfaces 2020, 12, 25428–25434. [Google Scholar] [CrossRef]
- Chen, Z.-G.; Han, G.; Yang, L.; Cheng, L.; Zou, J. Nanostructured thermoelectric materials: Current research and future challenge. Prog. Nat. Sci. 2012, 22, 535–549. [Google Scholar] [CrossRef]
- Fan, S.; Zhao, J.; Yan, Q.; Ma, J.; Hng, H.H. Influence of Nanoinclusions on Thermoelectric Properties of n-Type Bi2Te3 Nanocomposites. J. Electron. Mater. 2011, 40, 1018–1023. [Google Scholar] [CrossRef]
- Ahmad, S.; Singh, A.; Bhattacharya, S.; Navaneethan, M.; Basu, R.; Bhatt, R.; Sarkar, P.; Meshram, K.N.; Muthe, K.P.; Vitta, S.; et al. Remarkable Improvement of Thermoelectric Figure-of-Merit in SnTe through In Situ-Created Te Nanoinclusions. ACS Appl. Energy Mater. 2020, 3, 7113–7120. [Google Scholar] [CrossRef]
- Hung, N.T.; Saito, R. The Origin of Quantum Effects in Low-Dimensional Thermoelectric Materials. Adv. Quantum Technol. 2021, 4, 2000115. [Google Scholar] [CrossRef]
- Uryupin, O.; Vedernikov, M.; Shabaldin, A.; Ivanov, Y.; Kumzerov, Y.; Fokin, A. Thermoelectric Properties of InSb Nanowires Over a Wide Temperature Range. J. Electron. Mater. 2009, 38, 990–993. [Google Scholar] [CrossRef]
- Vedernikov, M.; Uryupin, O.; Goltsman, B.; Ivanov, Y.; Kumzerov, Y. Experimental study of thermoelectric properties of InSb nanowires. In Proceedings of the ICT2001. 20 International Conference on Thermoelectrics (Cat. No. 01TH8589), Beijing, China, 7 August 2002. [Google Scholar] [CrossRef]
- Ng, M.-F.; Zhou, L.; Yang, S.-W.; Sim, L.Y.; Tan, V.B.C.; Wu, P. Theoretical investigation of silicon nanowires: Methodology, geometry, surface modification, and electrical conductivity using a multiscale approach. Phys. Rev. B 2007, 76, 155435. [Google Scholar] [CrossRef]
- Ma, D.D.D.; Lee, C.S.; Au, F.C.K.; Tong, S.Y.; Lee, S.T. Small-Diameter Silicon Nanowire Surfaces. Science 2003, 299, 1874–1877. [Google Scholar] [CrossRef]
- Imran, M.; Di Stasio, F.; Dang, Z.; Canale, C.; Khan, A.H.; Shamsi, J.; Brescia, R.; Prato, M.; Manna, L. Colloidal Synthesis of Strongly Fluorescent CsPbBr3 Nanowires with Width Tunable down to the Quantum Confinement Regime. Chem. Mater. 2016, 28, 6450–6454. [Google Scholar] [CrossRef]
- Demchenko, O.D.; Heinz, P.D.; Lee, B. Determining factors of thermoelectric properties of semiconductor nanowires. Nanoscale Res. Lett. 2011, 6, 502. [Google Scholar] [CrossRef]
- Li, D.; Hao, S.; Xing, G.; Li, Y.; Li, X.; Fan, L.; Yang, S. Solution Grown Single-Unit-Cell Quantum Wires Affording Self-Powered Solar-Blind UV Photodetectors with Ultrahigh Selectivity and Sensitivity. J. Am. Chem. Soc. 2019, 141, 3480–3488. [Google Scholar] [CrossRef] [PubMed]
- Heremans, J.P.; Thrush, C.M.; Morelli, D.T.; Wu, M.-C. Resistance, Magnetoresistance, and Thermopower of Zinc Nanowire Composites. Phys. Rev. Lett. 2003, 91, 076804. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Ma, Y. Atomistic Design of High Thermoelectricity on Si/Ge Superlattice Nanowires. J. Phys. Chem. C 2011, 115, 20696–20702. [Google Scholar] [CrossRef]
- Poddar, S.; Zhang, Y.; Gu, L.; Zhang, D.; Zhang, Q.; Yan, S.; Kam, M.; Zhang, S.; Song, Z.; Hu, W.; et al. Down-Scalable and Ultra-fast Memristors with Ultra-high Density Three-Dimensional Arrays of Perovskite Quantum Wires. Nano Lett. 2021, 21, 5036–5044. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Sun, X.; Dresselhaus, M. Theoretical investigation of thermoelectric transport properties of cylindrical Bi nanowires. Phys. Rev. B 2000, 62, 4610. [Google Scholar] [CrossRef]
- Moore, A.L.; Pettes, M.T.; Zhou, F.; Shi, L. Thermal conductivity suppression in bismuth nanowires. J. Appl. Phys. 2009, 106, 034310. [Google Scholar] [CrossRef]
- Holtzman, A.; Shapira, E.; Selzer, Y. Bismuth nanowires with very low lattice thermal conductivity as revealed by the 3ω method. Nanotechnology 2012, 23, 495711. [Google Scholar] [CrossRef] [PubMed]
- Mannam, R.S.; Davis, D. High Seebeck Coefficient BiSbTe Nanowires. Electrochem. Solid-State Lett. 2010, 13, P15. [Google Scholar] [CrossRef]
- Shi, J.; Wu, X.; Geng, X.; Hu, L.; Liu, F.; Ao, W.; Zhang, C. Anisotropy engineering in solution-derived nanostructured Bi2Te3 thin films for high-performance flexible thermoelectric devices. Chem. Eng. J. 2023, 458, 141450. [Google Scholar] [CrossRef]
- Soini, M.; Zardo, I.; Uccelli, E.; Funk, S.; Koblmüller, G.; Morral, A.F.I.; Abstreiter, G. Thermal conductivity of GaAs nanowires studied by micro-Raman spectroscopy combined with laser heating. Appl. Phys. Lett. 2010, 97, 263107. [Google Scholar] [CrossRef]
- Lee, K.-M.; Lee, S.-K.; Choi, T.-Y. Highly enhanced thermoelectric figure of merit of a β-SiC nanowire with a nanoelectromechanical measurement approach. Appl. Phys. A 2012, 106, 955–960. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, H.J. Improved Thermoelectric Properties of La1−xSrxCoO3 Nanowires. J. Phys. Chem. C 2010, 114, 13947–13953. [Google Scholar] [CrossRef]
- Bui, C.T.; Xie, R.; Zheng, M.; Zhang, Q.; Sow, C.H.; Li, B.; Thong, J.T.L. Diameter-Dependent Thermal Transport in Individual ZnO Nanowires and its Correlation with Surface Coating and Defects. Small 2012, 8, 738–745. [Google Scholar] [CrossRef]
- Rathnasekara, R.; Mayberry, G.; Hari, P. Thermoelectric, Electrochemical, & Dielectric Properties of Four ZnO Nanostructures. Materials 2022, 15, 8816. [Google Scholar]
- Tai, G.; Guo, W.; Zhang, Z. Hydrothermal Synthesis and Thermoelectric Transport Properties of Uniform Single-Crystalline Pearl-Necklace-Shaped PbTe Nanowires. Cryst. Growth Des. 2008, 8, 2906–2911. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Chen, R.; Diaz Delgado, R.; Liang, W.; Garnett, E.C.; Najarian, M.; Majumdar, A.; Yang, P. Enhanced thermoelectric performance of rough silicon nanowires. Nature 2008, 451, 163–167. [Google Scholar] [CrossRef]
- Elyamny, S.; Dimaggio, E.; Magagna, S.; Narducci, D.; Pennelli, G. High Power Thermoelectric Generator Based on Vertical Silicon Nanowires. Nano Lett. 2020, 20, 4748–4753. [Google Scholar] [CrossRef]
- Seol, J.H.; Moore, A.L.; Saha, S.K.; Zhou, F.; Shi, L.; Ye, Q.L.; Scheffler, R.; Mingo, N.; Yamada, T. Measurement and analysis of thermopower and electrical conductivity of an indium antimonide nanowire from a vapor-liquid-solid method. J. Appl. Phys. 2007, 101, 023706. [Google Scholar] [CrossRef]
- Yazji, S.; Swinkels, M.Y.; De Luca, M.; Hoffmann, A.E.; Ercolani, D.; Roddaro, S.; Abstreiter, G.; Sorba, L.; Bakkers, E.P.A.M.; Zardo, I. Assessing the thermoelectric properties of single InSb nanowires: The role of thermal contact resistance. Semicond. Sci. Technol. 2016, 31, 064001. [Google Scholar] [CrossRef]
- Tian, Y.; Sakr, M.R.; Kinder, J.M.; Liang, D.; MacDonald, M.J.; Qiu, R.L.J.; Gao, H.-J.; Gao, X.P.A. One-Dimensional Quantum Confinement Effect Modulated Thermoelectric Properties in InAs Nanowires. Nano Lett. 2012, 12, 6492–6497. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.C.; Fardy, M.A.; Moore, M.C.; Aloni, S.; Zhang, M.; Radmilovic, V.; Yang, P. Atomic-level control of the thermoelectric properties in polytypoid nanowires. Chem. Sci. 2011, 2, 706–714. [Google Scholar] [CrossRef]
- Liu, M.; Tao, J.; Nam, C.-Y.; Kisslinger, K.; Zhang, L.; Su, D. Surface-Energy Induced Formation of Single Crystalline Bismuth Nanowires over Vanadium Thin Film at Room Temperature. Nano Lett. 2014, 14, 5630–5635. [Google Scholar] [CrossRef]
- Greene, L.E.; Yuhas, B.D.; Law, M.; Zitoun, D.; Yang, P. Solution-Grown Zinc Oxide Nanowires. Inorg. Chem. 2006, 45, 7535–7543. [Google Scholar] [CrossRef]
- Yadav, A.; Pipe, K.; Shtein, M. Fiber-based flexible thermoelectric power generator. J. Power Sources 2008, 175, 909–913. [Google Scholar] [CrossRef]
- Lee, J.A.; Aliev, A.E.; Bykova, J.S.; de Andrade, M.J.; Kim, D.; Sim, H.J.; Lepró, X.; Zakhidov, A.A.; Lee, J.B.; Spinks, G.M. Woven-yarn thermoelectric textiles. Adv. Mater. 2016, 28, 5038–5044. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Yang, H.; Finefrock, S.W.; Wu, Y. Flexible Nanocrystal-Coated Glass Fibers for High-Performance Thermoelectric Energy Harvesting. Nano Lett. 2012, 12, 2140–2145. [Google Scholar] [CrossRef]
- Finefrock, S.W.; Wang, Y.; Ferguson, J.B.; Ward, J.V.; Fang, H.; Pfluger, J.E.; Dudis, D.S.; Ruan, X.; Wu, Y. Measurement of Thermal Conductivity of PbTe Nanocrystal Coated Glass Fibers by the 3ω Method. Nano Lett. 2013, 13, 5006–5012. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Miao, L.; Liu, C.; Wang, X.; Peng, Y.; Wei, X.; Zhou, J.; Chen, Y.; Hashimoto, R.; Asaka, T.; et al. A novel glass-fiber-aided cold-press method for fabrication of n-type Ag2Te nanowires thermoelectric film on flexible copy-paper substrate. J. Mater. Chem. A 2017, 5, 24740–24748. [Google Scholar] [CrossRef]
- Sun, M.; Qian, Q.; Tang, G.; Liu, W.; Qian, G.; Shi, Z.; Huang, K.; Chen, D.; Xu, S.; Yang, Z. Enhanced thermoelectric properties of polycrystalline Bi2Te3 core fibers with preferentially oriented nanosheets. APL Mater. 2018, 6, 036103. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Wang, Z.; Li, C.; Wang, Z.; Li, K.; Huang, X.; Chen, M.; Chen, Z.; Tian, Z.; et al. Single-Crystal SnSe Thermoelectric Fibers via Laser-Induced Directional Crystallization: From 1D Fibers to Multidimensional Fabrics. Adv. Mater. 2020, 32, 2002702. [Google Scholar] [CrossRef]
- Yin, T.; Liu, D.; Ou, Y.; Ma, F.; Xie, S.; Li, J.-F.; Li, J. Nanocrystalline thermoelectric Ca3Co4O9 ceramics by sol− gel based electrospinning and spark plasma sintering. J. Phys. Chem. C 2010, 114, 10061–10065. [Google Scholar] [CrossRef]
- Xu, W.; Shi, Y.; Hadim, H. The fabrication of thermoelectric La0.95Sr0.05CoO3 nanofibers and Seebeck coefficient measurement. Nanotechnology 2010, 21, 395303. [Google Scholar] [CrossRef] [PubMed]
- De Santis, J.; Paolucci, V.; Stagi, L.; Carboni, D.; Malfatti, L.; Cantalini, C.; Innocenzi, P. Bidimensional SnSe2—Mesoporous Ordered Titania Heterostructures for Photocatalytically Activated Anti-Fingerprint Optically Transparent Layers. Nanomaterials 2023, 13, 1406. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, X.; Zhang, T.; Zhang, G.; Jiang, W.; Li, X. Bi2Te3/Te multiple heterostructure nanowire arrays formed by confined precipitation. J. Am. Chem. Soc. 2007, 129, 6702–6703. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, K.; Yu, D.; Wang, Y. Electrodeposition and characterization of thermoelectric Bi2Te2Se/Te multilayer nanowire arrays. Superlattices Microstruct. 2011, 50, 557–562. [Google Scholar] [CrossRef]
- Hu, M.; Giapis, K.P.; Goicochea, J.V.; Zhang, X.; Poulikakos, D. Significant Reduction of Thermal Conductivity in Si/Ge Core−Shell Nanowires. Nano Lett. 2011, 11, 618–623. [Google Scholar] [CrossRef]
- Shelley, M.; Mostofi, A.A. Prediction of high zT in thermoelectric silicon nanowires with axial germanium heterostructures. EPL Europhysics Lett. 2011, 94, 67001. [Google Scholar] [CrossRef]
- Kang, J.; Roh, J.W.; Shim, W.; Ham, J.; Noh, J.-S.; Lee, W. Reduction of Lattice Thermal Conductivity in Single Bi-Te Core/Shell Nanowires with Rough Interface. Adv. Mater. 2011, 23, 3414–3419. [Google Scholar] [CrossRef]
- Lauhon, L.J.; Gudiksen, M.S.; Wang, D.; Lieber, C.M. Epitaxial core–shell and core–multishell nanowire heterostructures. Nature 2002, 420, 57–61. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, W.; Li, X. Enhanced Thermoelectric Properties of Core/Shell Heterostructure Nanowire Composites. Adv. Mater. 2008, 20, 3654–3656. [Google Scholar] [CrossRef]
- Lu, H.; Zheng, F.; Guo, M.; Zhang, M. One-step electrodeposition of single-crystal ZnO nanotube arrays and their optical properties. J. Alloy. Compd. 2014, 588, 217–221. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, Q.; Yao, Z.; Li, X. Large scale highly crystalline Bi2Te3 nanotubes through solution phase nanoscale Kirkendall effect fabrication. Chem. Commun. 2009, 17, 2317–2319. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.D.; Mariano, R.G.; Vu, K.; Nour, N.; Seitz, O.; Chabal, Y.; Balkus Jr, K.J. Hydrothermal synthesis of graphene-TiO2 nanotube composites with enhanced photocatalytic activity. ACS Catal. 2012, 2, 949–956. [Google Scholar] [CrossRef]
- Li, X.-H.; Zhou, B.; Pu, L.; Zhu, J.-J. Electrodeposition of Bi2Te3 and Bi2Te3 derived alloy nanotube arrays. Cryst. Growth Des. 2008, 8, 771–775. [Google Scholar] [CrossRef]
- Zhao, X.B.; Ji, X.H.; Zhang, Y.H.; Zhu, T.J.; Tu, J.P.; Zhang, X.B. Bismuth telluride nanotubes and the effects on the thermoelectric properties of nanotube-containing nanocomposites. Appl. Phys. Lett. 2005, 86, 062111. [Google Scholar] [CrossRef]
- Sevinçli, H.; Cuniberti, G. Enhanced thermoelectric figure of merit in edge-disordered zigzag graphene nanoribbons. Phys. Rev. B 2010, 81, 113401. [Google Scholar] [CrossRef]
- Karamitaheri, H.; Neophytou, N.; Pourfath, M.; Faez, R.; Kosina, H. Engineering enhanced thermoelectric properties in zigzag graphene nanoribbons. J. Appl. Phys. 2012, 111, 054501. [Google Scholar] [CrossRef]
- MacLeod, B.A.; Stanton, N.J.; Gould, I.E.; Wesenberg, D.; Ihly, R.; Owczarczyk, Z.R.; Hurst, K.E.; Fewox, C.S.; Folmar, C.N.; Hughes, K.H.; et al. Large n- and p-type thermoelectric power factors from doped semiconducting single-walled carbon nanotube thin films. Energy Environ. Sci. 2017, 10, 2168–2179. [Google Scholar] [CrossRef]
- Yao, Q.; Chen, L.; Zhang, W.; Liufu, S.; Chen, X. Enhanced Thermoelectric Performance of Single-Walled Carbon Nanotubes/Polyaniline Hybrid Nanocomposites. ACS Nano 2010, 4, 2445–2451. [Google Scholar] [CrossRef]
- Meng, C.; Liu, C.; Fan, S. A Promising Approach to Enhanced Thermoelectric Properties Using Carbon Nanotube Networks. Adv. Mater. 2010, 22, 535–539. [Google Scholar] [CrossRef]
- Choi, J.; Lee, J.Y.; Lee, H.; Park, C.R.; Kim, H. Enhanced thermoelectric properties of the flexible tellurium nanowire film hybridized with single-walled carbon nanotube. Synth. Met. 2014, 198, 340–344. [Google Scholar] [CrossRef]
- Park, K.-R.; Cho, H.-B.; Song, Y.; Kim, S.; Kwon, Y.-T.; Ryu, S.H.; Lim, J.-H.; Lee, W.-J.; Choa, Y.-H. Large-scale synthesis of lead telluride (PbTe) nanotube-based nanocomposites with tunable morphology, crystallinity and thermoelectric properties. Appl. Surf. Sci. 2018, 436, 785–790. [Google Scholar] [CrossRef]
- Park, N.-W.; Kim, H.; Lee, W.-Y.; Kim, G.-S.; Kang, D.Y.; Kim, T.G.; Saitoh, E.; Yoon, Y.-G.; Rho, H.; Lee, S.-K. Giant Thermoelectric Seebeck Coefficients in Tellurium Quantum Wires Formed Vertically in an Aluminum Oxide Layer by Electrical Breakdown. J. Phys. Chem. Lett. 2021, 12, 8212–8219. [Google Scholar] [CrossRef]
- Li, L.; Xu, S.; Li, G. Enhancement of Thermoelectric Properties in Bi-Sb-Te Alloy Nanowires by Pulsed Electrodeposition. Energy Technol. 2015, 3, 825–829. [Google Scholar] [CrossRef]
- Chand, T.; Saxena, M.; Okram, G.S.; Lakhani, A.; Kuo, Y.-K.; Tyagi, S.; Behera, P.; Bhalerao, G.M.; Sathe, V.; Deshpande, U. Enhanced thermoelectric performance of solution-grown Bi2Te3 nanorods. Mater. Today Energy 2021, 21, 100700. [Google Scholar] [CrossRef]
- Ren, F.; Menchhofer, P.; Kiggans, J.; Wang, H. Development of Thermoelectric Fibers for Miniature Thermoelectric Devices. J. Electron. Mater. 2016, 45, 1412–1418. [Google Scholar] [CrossRef]
- Ju, Z.; Crawford, C.; Adamczyk, J.; Toberer, E.S.; Kauzlarich, S.M. Study of the Thermoelectric Properties of Bi2Te3/Sb2Te3 Core–Shell Heterojunction Nanostructures. ACS Appl. Mater. Interfaces 2022, 14, 24886–24896. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Feng, T.; Yang, H.; Ruan, X.; Wu, Y. Synthesis and Thermoelectric Properties of Compositional-Modulated Lead Telluride–Bismuth Telluride Nanowire Heterostructures. Nano Lett. 2013, 13, 2058–2063. [Google Scholar] [CrossRef]
- Lee, E.; Ko, J.; Kim, J.-Y.; Seo, W.-S.; Choi, S.-M.; Lee, K.H.; Shim, W.; Lee, W. Enhanced thermoelectric properties of Au nanodot-included Bi2Te3 nanotube composites. J. Mater. Chem. C 2016, 4, 1313–1319. [Google Scholar] [CrossRef]
- Boukai, A.I.; Bunimovich, Y.; Tahir-Kheli, J.; Yu, J.-K.; Goddard, W.A., III; Heath, J.R. Silicon nanowires as efficient thermoelectric materials. Nature 2008, 451, 168–171. [Google Scholar] [CrossRef]
- Wingert, M.C.; Chen, Z.C.; Dechaumphai, E.; Moon, J.; Kim, J.-H.; Xiang, J.; Chen, R. Thermal conductivity of Ge and Ge–Si core–shell nanowires in the phonon confinement regime. Nano Lett. 2011, 11, 5507–5513. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, G.; Li, B. Remarkable Reduction of Thermal Conductivity in Silicon Nanotubes. Nano Lett. 2010, 10, 3978–3983. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, L.; Kong, L.; Nai, M.H.; Liu, D.; Wu, J.; Liu, Y.; Chiam, S.Y.; Chim, W.K.; Lim, C.T.; et al. Ultralow Thermal Conductivity of Single-Crystalline Porous Silicon Nanowires. Adv. Funct. Mater. 2017, 27, 1702824. [Google Scholar] [CrossRef]
- Heremans, J.P.; Thrush, C.M.; Morelli, D.T.; Wu, M.-C. Thermoelectric Power of Bismuth Nanocomposites. Phys. Rev. Lett. 2002, 88, 216801. [Google Scholar] [CrossRef]
- Tai, G.; Zhou, B.; Guo, W. Structural Characterization and Thermoelectric Transport Properties of Uniform Single-Crystalline Lead Telluride Nanowires. J. Phys. Chem. C 2008, 112, 11314–11318. [Google Scholar] [CrossRef]
- Zhang, G.; Fang, H.; Yang, H.; Jauregui, L.A.; Chen, Y.P.; Wu, Y. Design Principle of Telluride-Based Nanowire Heterostructures for Potential Thermoelectric Applications. Nano Lett. 2012, 12, 3627–3633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, S.; Xu, J.; Zheng, R.; Cheng, G. High thermoelectric figure-of-merits from large-area porous silicon nanowire arrays. Nano Energy 2015, 13, 433–441. [Google Scholar] [CrossRef]
- He, M.; Ge, J.; Lin, Z.; Feng, X.; Wang, X.; Lu, H.; Yang, Y.; Qiu, F. Thermopower enhancement in conducting polymer nanocomposites via carrier energy scattering at the organic–inorganic semiconductor interface. Energy Environ. Sci. 2012, 5, 8351–8358. [Google Scholar] [CrossRef]
- Zhou, W.; Fan, Q.; Zhang, Q.; Cai, L.; Li, K.; Gu, X.; Yang, F.; Zhang, N.; Wang, Y.; Liu, H.; et al. High-performance and compact-designed flexible thermoelectric modules enabled by a reticulate carbon nanotube architecture. Nat. Commun. 2017, 8, 14886. [Google Scholar] [CrossRef]
- Avery, A.D.; Zhou, B.H.; Lee, J.; Lee, E.-S.; Miller, E.M.; Ihly, R.; Wesenberg, D.; Mistry, K.S.; Guillot, S.L.; Zink, B.L.; et al. Tailored semiconducting carbon nanotube networks with enhanced thermoelectric properties. Nat. Energy 2016, 1, 16033. [Google Scholar] [CrossRef]
- Ramayya, E.B.; Vasileska, D.; Goodnick, S.M.; Knezevic, I. Electron mobility in silicon nanowires. IEEE Trans. Nanotechnol. 2007, 6, 113–117. [Google Scholar] [CrossRef]
- Lu, J.G.; Chang, P.; Fan, Z. Quasi-one-dimensional metal oxide materials—Synthesis, properties and applications. Mater. Sci. Eng. R Rep. 2006, 52, 49–91. [Google Scholar] [CrossRef]
- Curtin, B.M.; Bowers, J.E. Thermoelectric power factor enhancement with gate-all-around silicon nanowires. J. Appl. Phys. 2014, 115, 143704. [Google Scholar] [CrossRef]
- Shin, H.S.; Hamdou, B.; Reith, H.; Osterhage, H.; Gooth, J.; Damm, C.; Rellinghaus, B.; Pippel, E.; Nielsch, K. The surface-to-volume ratio: A key parameter in the thermoelectric transport of topological insulator Bi2Se3nanowires. Nanoscale 2016, 8, 13552–13557. [Google Scholar] [CrossRef] [PubMed]
- Manzano, C.V.; Martin-Gonzalez, M. Electrodeposition of V-VI Nanowires and Their Thermoelectric Properties. Front. Chem. 2019, 7, 516. [Google Scholar] [CrossRef] [PubMed]
- Davydov, A.D.; Volgin, V. Template electrodeposition of metals. Review. Russ. J. Electrochem. 2016, 52, 806–831. [Google Scholar] [CrossRef]
- Verma, S.; Arya, S.; Gupta, V.; Mahajan, S.; Furukawa, H.; Khosla, A. Performance analysis, challenges and future perspectives of nickel based nanostructured electrodes for electrochemical supercapacitors. J. Mater. Res. Technol. 2021, 11, 564–599. [Google Scholar] [CrossRef]
- Prieto, A.L.; Sander, M.S.; Martin-Gonzalez, M.S.; Gronsky, R.; Sands, T.; Stacy, A.M. ChemInform Abstract: Electrodeposition of Ordered Bi2Te3 Nanowire Arrays. Cheminform 2001, 123, 7160–7161. [Google Scholar] [CrossRef]
- Xu, S.H.; Fei, G.T.; Zhu, X.G.; Wang, B.; Wu, B.; De Zhang, L. A facile and universal way to fabricate superlattice nanowire arrays. Nanotechnology 2011, 22, 265602. [Google Scholar] [CrossRef]
- Trahey, L.; Becker, C.R.; Stacy, A.M. Electrodeposited Bismuth Telluride Nanowire Arrays with Uniform Growth Fronts. Nano Lett. 2007, 7, 2535–2539. [Google Scholar] [CrossRef]
- Jin, C.; Xiang, X.; Jia, C.; Liu, W.; Cai, W.; Yao, L.; Li, X. Electrochemical Fabrication of Large-Area, Ordered Bi2Te3 Nanowire Arrays. J. Phys. Chem. B 2004, 108, 1844–1847. [Google Scholar] [CrossRef]
- Lee, J.; Berger, A.; Cagnon, L.; Gösele, U.; Nielsch, K.; Lee, J. Disproportionation of thermoelectric bismuth telluride nanowires as a result of the annealing process. Phys. Chem. Chem. Phys. 2010, 12, 15247–15250. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, Y.W.; Huang, X.H.; Li, G.H.; Ang, R.; Zhang, L.D. Fabrication and electronic transport properties of Bi nanotube arrays. Appl. Phys. Lett. 2006, 88, 103119. [Google Scholar] [CrossRef]
- Taggart, D.K.; Yang, Y.; Kung, S.-C.; McIntire, T.M.; Penner, R.M. Enhanced Thermoelectric Metrics in Ultra-long Electrodeposited PEDOT Nanowires. Nano Lett. 2011, 11, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Guérin, V.-M.; Pauporté, T. From nanowires to hierarchical structures of template-free electrodeposited ZnO for efficient dye-sensitized solar cells. Energy Environ. Sci. 2011, 4, 2971–2979. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, J.; Zhou, X. Controlled synthesis of oriented ZnO nanorod arrays by seed-layer-free electrochemical deposition. Appl. Surf. Sci. 2011, 258, 1491–1494. [Google Scholar] [CrossRef]
- Shi, X.L.; Tao, X.; Zou, J.; Chen, Z.G. High-performance thermoelectric SnSe: Aqueous synthesis, innovations, and challenges. Adv. Sci. 2020, 7, 1902923. [Google Scholar] [CrossRef]
- Han, G.; Chen, Z.-G.; Drennan, J.; Zou, J. Indium Selenides: Structural Characteristics, Synthesis and Their Thermoelectric Performances. Small 2014, 10, 2747–2765. [Google Scholar] [CrossRef]
- Park, H.; Son, W.; Lee, S.H.; Kim, S.; Lee, J.J.; Cho, W.; Choi, H.H.; Kim, J.H. Aqueous chemical synthesis of tellurium nanowires using a polymeric template for thermoelectric materials. Crystengcomm 2015, 17, 1092–1097. [Google Scholar] [CrossRef]
- Zhang, G.; Kirk, B.; Jauregui, L.A.; Yang, H.; Xu, X.; Chen, Y.P.; Wu, Y. Rational Synthesis of Ultrathin n-Type Bi2Te3 Nanowires with Enhanced Thermoelectric Properties. Nano Lett. 2012, 12, 56–60. [Google Scholar] [CrossRef]
- Ho, T.-W.; Hong, F.C.-N. A Novel Method to Grow Vertically Aligned Silicon Nanowires on Si (111) and Their Optical Absorption. J. Nanomater. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Wagner, A.R.; Ellis, S.W. Vapor-liquid-solid mechanism of single crystal growth. Appl. Phys. Lett. 1964, 4, 89–90. [Google Scholar] [CrossRef]
- Schmidt, V.; Wittemann, J.V.; Senz, S.; Gösele, U. Silicon Nanowires: A Review on Aspects of their Growth and their Electrical Properties. Adv. Mater. 2009, 21, 2681–2702. [Google Scholar] [CrossRef] [PubMed]
- Bandaru, P.R.; Pichanusakorn, P. An outline of the synthesis and properties of silicon nanowires. Semicond. Sci. Technol. 2010, 25, 024003. [Google Scholar] [CrossRef]
- Wu, Y.; Cui, Y.; Huynh, L.; Barrelet, C.J.; Bell, D.C.; Lieber, C.M. Controlled Growth and Structures of Molecular-Scale Silicon Nanowires. Nano Lett. 2004, 4, 433–436. [Google Scholar] [CrossRef]
- Rashid, J.I.A.; Abdullah, J.; Yusof, N.A.; Hajian, R. The Development of Silicon Nanowire as Sensing Material and Its Applications. J. Nanomater. 2013, 2013, 1–16. [Google Scholar] [CrossRef]
- Huang, Z.; Geyer, N.; Werner, P.; De Boor, J.; Gösele, U. Metal-assisted chemical etching of silicon: A review: In memory of Prof. Ulrich Gösele. Adv. Mater. 2011, 23, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.-C.; Lin, S.-B.; Hung, S.-C.; Lin, C.-F. Influence of pre-surface treatment on the morphology of silicon nanowires fabricated by metal-assisted etching. Appl. Surf. Sci. 2011, 257, 1829–1834. [Google Scholar] [CrossRef]
- Kolasinski, K.W. Silicon nanostructures from electroless electrochemical etching. Curr. Opin. Solid State Mater. Sci. 2005, 9, 73–83. [Google Scholar] [CrossRef]
- Brahiti, N.; Bouanik, S.-A.; Hadjersi, T. Metal-assisted electroless etching of silicon in aqueous NH4HF2 solution. Appl. Surf. Sci. 2012, 258, 5628–5637. [Google Scholar] [CrossRef]
- Bahk, J.-H.; Favaloro, T.; Shakouri, A. Thin film thermoelectric characterization techniques. In Annual Review of Heat Transfer; BEGELL HOUSE Inc.: Danbury, CT, USA, 2013; Volume 16. [Google Scholar]
- Fahrner, W.R.; Schwertheim, S. Semiconductor Thermoelectric Generators; Trans Tech Publications Ltd.: Bäch, Switzerland, 2009. [Google Scholar]
- Klochko, N.P.; Klepikova, K.S.; Kopach, V.R.; Zhadan, D.O.; Starikov, V.V.; Sofronov, D.S.; Khrypunova, I.V.; Petrushenko, S.I.; Dukarov, S.V.; Lyubov, V.M.; et al. Solution-produced copper iodide thin films for photosensor and for vertical thermoelectric nanogenerator, which uses a spontaneous temperature gradient. J. Mater. Sci. Mater. Electron. 2019, 30, 17514–17524. [Google Scholar] [CrossRef]
- Jaber, W.; Chapuis, P.-O. Non-idealities in the 3ω method for thermal characterization in the low- and high-frequency regimes. AIP Adv. 2018, 8, 045111. [Google Scholar] [CrossRef]
- Yüksel, N. The Review of Some Commonly Used Methods and Techniques to Measure the Thermal Conductivity of Insulation Materials; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Rojo, M.M.; Calero, O.C.; Lopeandía, A.F.; Rodríguez-Viejo, J.; Martín-González, M. Review on measurement techniques of transport properties of nanowires Additions and Corrections. Nanoscale 2013, 5, 11526–11544. [Google Scholar] [CrossRef] [PubMed]
- Fatima, K.; Noor, H.; Ali, A.; Monakhov, E.; Asghar, M. Annealing Effect on Seebeck Coefficient of SiGe Thin Films Deposited on Quartz Substrate. Coatings 2021, 11, 1435. [Google Scholar] [CrossRef]
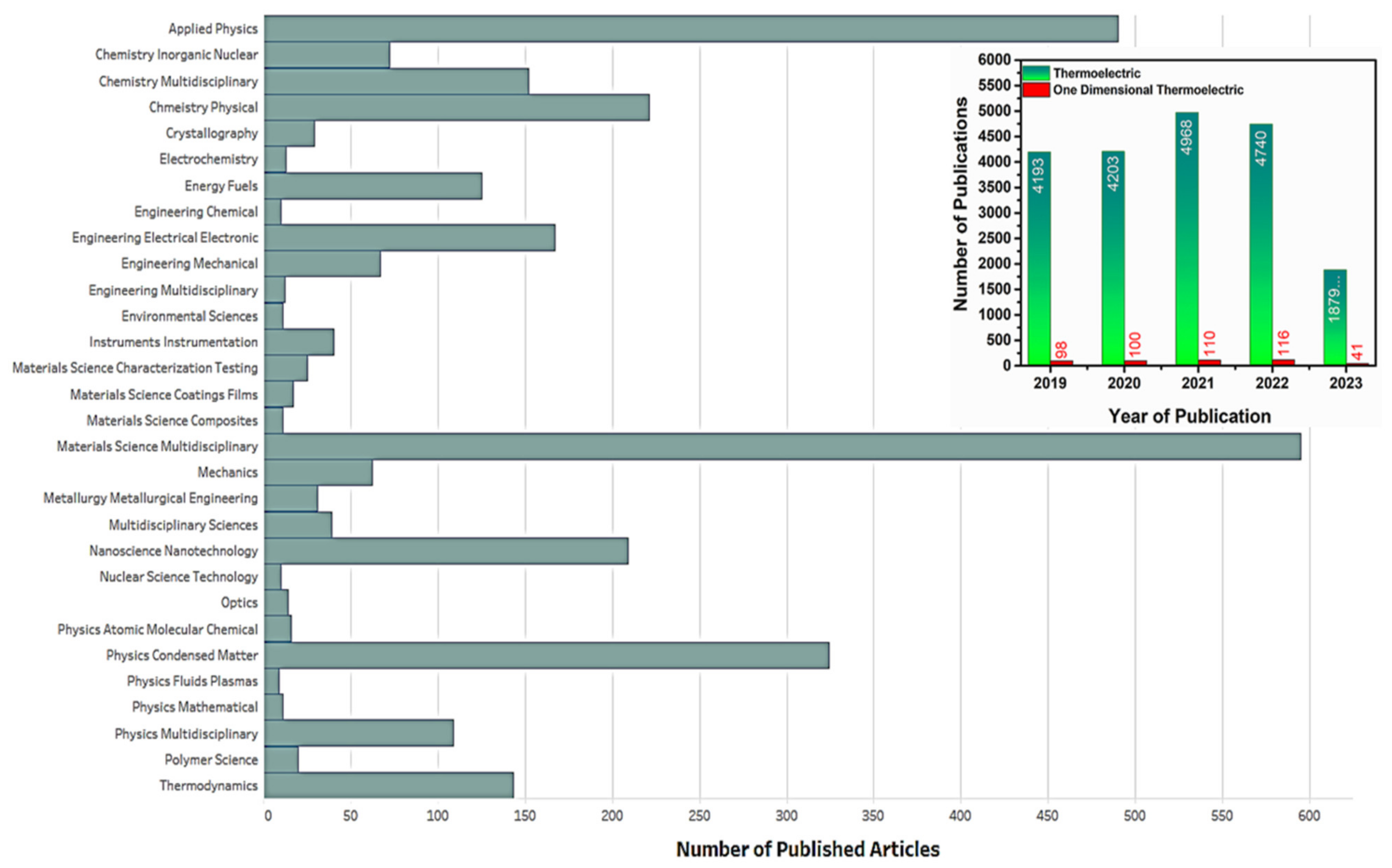

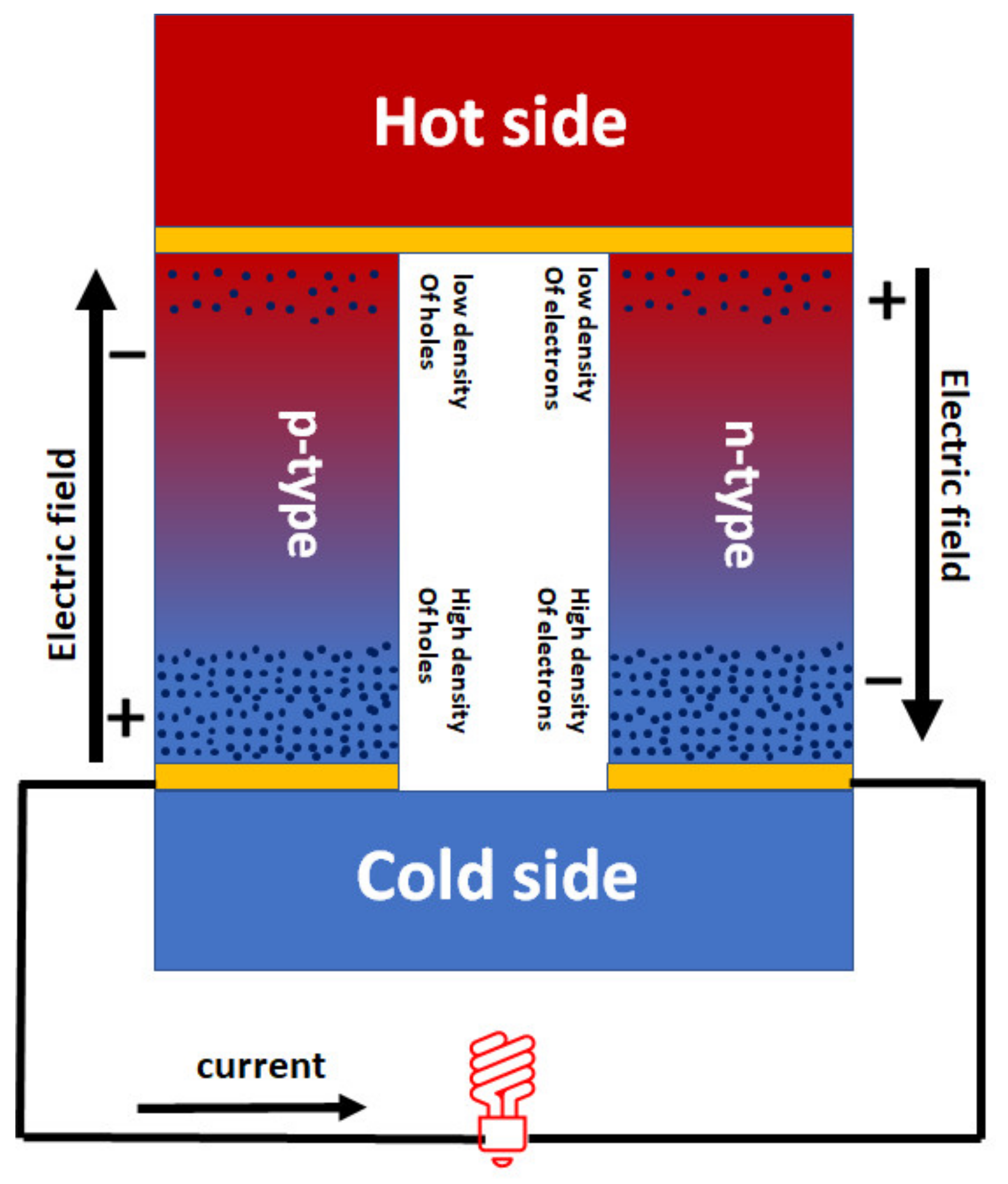
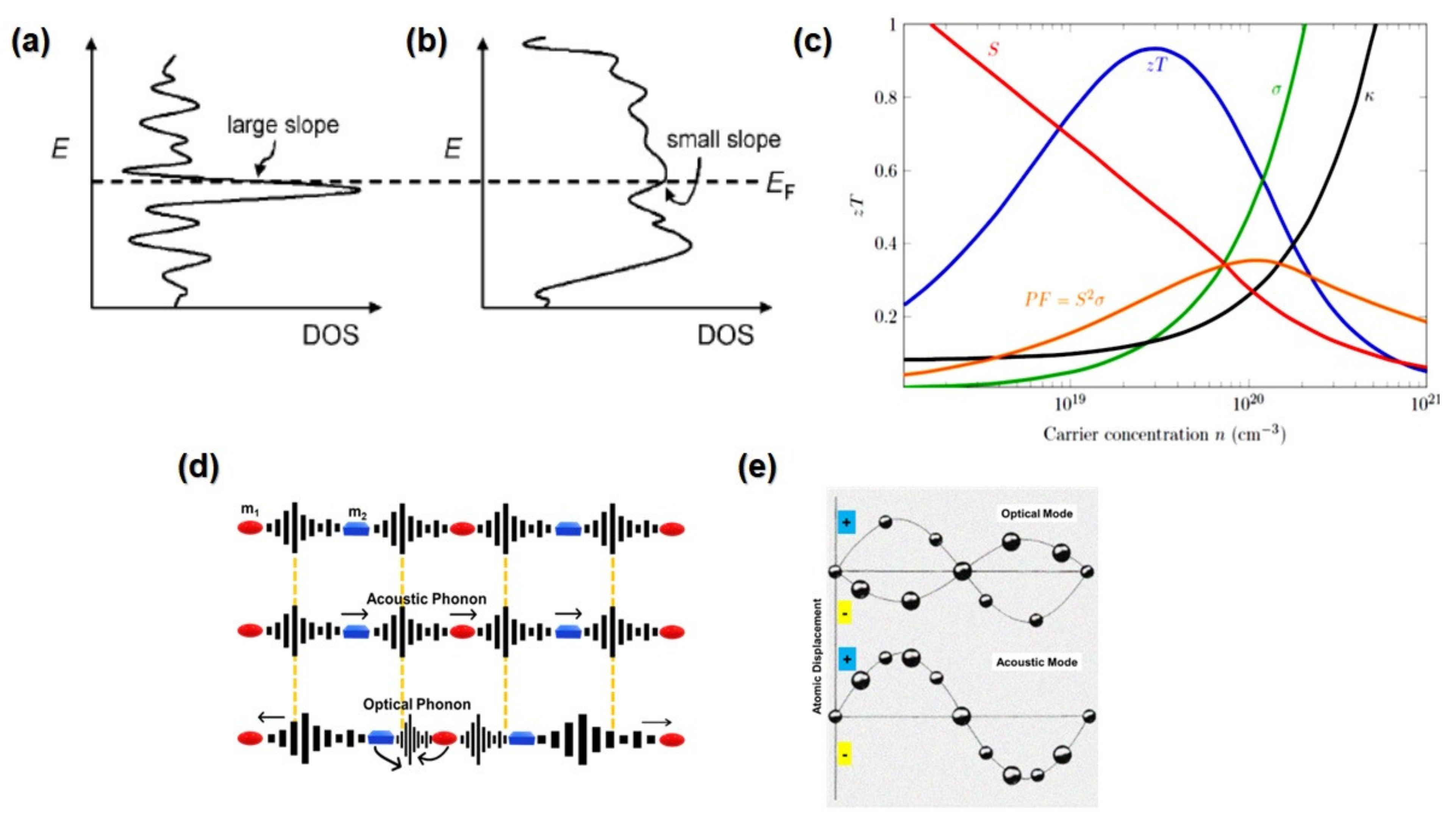
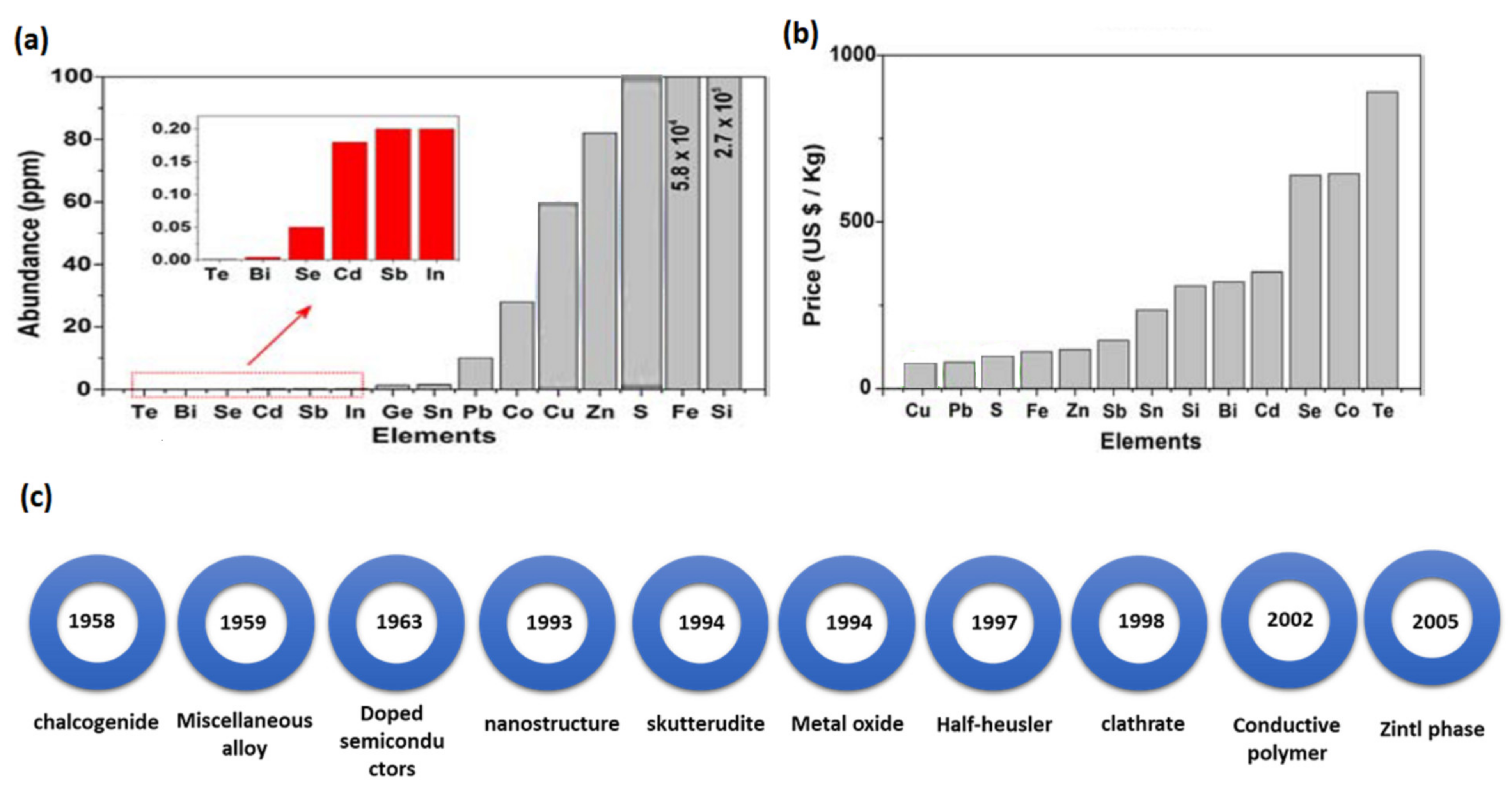

| No | State of Art Material | Material | Conductivity Type | ZT | Working Temperature (K) | Seebeck Coefficient (μVK−1) | Thermal Conductivity (Wm−1K−1) | Electrical Conductivity (S.cm−1) | Stability | Toxic Level | Cost | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bi2Te3 | Bi0.5Sb1.5Te3 | p-type | 1.86 | 320 | 242 | 0.65 | 650 | [165] | |||
| Te-embedded Bi2Te3 | n-type | 2.27 | 375 | −234 | 0.80 | 825 | [166] | |||||
| 2 | Ternary Composites | SWNT/PANI | p-type | 0.12 | 300 | 65 | 0.43 | 769 | [85] | |||
| CNTs/PEDOT | n-type | 0.5 | 300 | −1234 | 0.67 | 6.9 | [167] | |||||
| 3 | PbTe | Pb0.98Na0.02Te–8%SrTe | p-type | 2.5 | 923 | 300 | 0.9 | 250 | [58] | |||
| PbTe0.996S0.001I0.003 | n-type | 1.7 | 800 | −225 | 1.25 | 500 | [168] | |||||
| 4 | SnSe | SnSe | p-type | 2.6 | 923 | 350 | 0.35 | 100 | [65] | |||
| SnSe0.97Br0.03 | n-type | 2.8 | 773 | −480 | 0.25 | 41 | [169] | |||||
| 5 | Perovskite | LaCo0.92Ni0.08O2.9 | p-type | 0.2 | 300 | 220 | 0.35 | 33.3 | [170] | |||
| La-doped SrTiO3 | n-type | 0.27 | 1073 | −300 | 3.1 | 80 | [111] | |||||
| 6 | Skutterudites | Ce0.6Ni1.5Co2.5Sb12 | p-type | 0.45 | 850 | 153 | 1.62 | 600 | [171] | |||
| Ba0.3In0.3Co4Sb12 | n-type | 1.4 | 850 | −190 | 2.5 | 1300 | [172] | |||||
| 7 | Zintl Phase | YbCd1.85Mn0.15Sb2 | p-type | 1.14 | 650 | 250 | 0.4 | 200 | [173] | |||
| Mg3.15Mn0.05Sb1.5Bi0.49Te0.01 | n-type | 1.85 | 723 | −310 | 0.8 | 220 | [174] | |||||
| 8 | clathrates | Ba8Au5.3Ge40.7 | p-type | 0.9 | 680 | 150 | 1 | 50 | [175] | |||
| Ba8Ni0.31Zn0.52Ga13.06Ge32.2 | n-type | 1.2 | 1000 | −200 | 1.5 | 400 | [102] | |||||
| 9 | Metal sulphides | CU1.97S | p-type | 1.7 | 1000 | 300 | 0.5 | 100 | [123] | |||
| AgBiS2 | n-type | 0.7 | 820 | −300 | 0.5 | 120 | [176] | |||||
| 10 | Half-Heusler | (Nb0.6Ta0.4)0.8Ti0.2 | p-type | 1.6 | 1200 | 220 | 1.5 | 550 | [177] | |||
| Zr0.2Hf0.8NiSn0.985Sb0.015 | n-type | 1.1 | 1000 | −190 | 5.5 | 1500 | [178] | |||||
| 11 | Oxides | Ca3Co4O | p-type | 0.29 | 1073 | 155 | 4.4 (at room temp) | 151 | [179] | |||
| ZnO | n-type | 0.44 | 1000 | −300 | 2 | 100 | [114] | |||||
| 12 | Si-Ge alloys | Si0.62Ge0.32Ni0.03B0.03 | p-type | 1.56 | 1000 | 321 | 1.47 | 250 | [180] | |||
| SiGe–Mg2Si | n-type | 1.3 | 950 | −340 | 2.7 | 240 | [79] |
| 1D Nanostructure Configuration | Synthesis Method | Description | Seebeck Coefficient (µV/K) | Electrical Conductivity (S/cm) | Thermal Conductivity (W/m·K) | Power Factor (μW/K2.m) | ZT | Ref. |
|---|---|---|---|---|---|---|---|---|
| Quantum wires | theoretically | 0.5 nm diameter of Bi2Te3 | - | - | - | - | 14 | [28] |
| EBD synthesis method | 1D Te QWs formed locally in the aluminium oxide layer | −800 at 300 K | - | - | - | - | [293] | |
| Nanowires and nanorods | Theoretically | Bi NW | - | - | - | - | 6 at 77 K | [245] |
| Electrochemical Deposition, AAO template | Bi0.5Sb1.5Te3 NWs with 67 nm diameter | −150 | 480 | - | 1080 | - | [294] | |
| Solution-grown | Bi2Te3 nanorods with 40 nm diameter | −180 | - | - | - | - | [295] | |
| Nanofibers | powder-processing | Bi0.5Sb1.5Te3 fibres | −(207–268) | 153–189 | - | 0.3–0.4 | - | [296] |
| Heterostructures | bench top solution method | Bi2Te3/Sb2Te3 core-shell | −110 | - | 0.4 | - | 0.4 at 480 K | [297] |
| solution-phase | PbTe/Bi2Te3 segments NWs achieve | −300 | 110 | 0.51 | 1000 | 1.2 at 600 K | [298] | |
| Nanotubes | hydrothermal synthesis | Bi2Te3 nanotube | - | - | 0.3 | - | - | [285] |
| bottom-up synthesis and SPS | Au-doped Bi2Te3 nanotube | - | - | 0.47 | 2300 | 0.95 at 480 K | [299] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Fartoos, M.M.R.; Roy, A.; Mallick, T.K.; Tahir, A.A. Advancing Thermoelectric Materials: A Comprehensive Review Exploring the Significance of One-Dimensional Nano Structuring. Nanomaterials 2023, 13, 2011. https://doi.org/10.3390/nano13132011
Al-Fartoos MMR, Roy A, Mallick TK, Tahir AA. Advancing Thermoelectric Materials: A Comprehensive Review Exploring the Significance of One-Dimensional Nano Structuring. Nanomaterials. 2023; 13(13):2011. https://doi.org/10.3390/nano13132011
Chicago/Turabian StyleAl-Fartoos, Mustafa Majid Rashak, Anurag Roy, Tapas K. Mallick, and Asif Ali Tahir. 2023. "Advancing Thermoelectric Materials: A Comprehensive Review Exploring the Significance of One-Dimensional Nano Structuring" Nanomaterials 13, no. 13: 2011. https://doi.org/10.3390/nano13132011
APA StyleAl-Fartoos, M. M. R., Roy, A., Mallick, T. K., & Tahir, A. A. (2023). Advancing Thermoelectric Materials: A Comprehensive Review Exploring the Significance of One-Dimensional Nano Structuring. Nanomaterials, 13(13), 2011. https://doi.org/10.3390/nano13132011